Abstract
Background
Glycogen synthase kinase 3 (GSK3) regulates many cell fate decisions in animal development. In multicellular structures of the group 4 dictyostelid Dictyostelium discoideum, GSK3 promotes spore over stalk-like differentiation. We investigated whether, similar to other sporulation-inducing genes such as cAMP-dependent protein kinase (PKA), this role of GSK3 is derived from an ancestral role in encystation of unicellular amoebas.
Results
We deleted GSK3 in Polysphondylium pallidum, a group 2 dictyostelid which has retained encystation as an alternative survival strategy. Loss of GSK3 inhibited cytokinesis of cells in suspension, as also occurs in D. discoideum, but did not affect spore or stalk differentiation in P. pallidum. However, gsk3− amoebas entered into encystation under conditions that in wild type favour aggregation and fruiting body formation. The gsk3− cells were hypersensitive to osmolytes, which are known to promote encystation, and to cyst-inducing factors that are secreted during starvation. GSK3 was not itself regulated by these factors, but inhibited their effects.
Conclusions
Our data show that GSK3 has a deeply conserved role in controlling cytokinesis, but not spore differentiation in Dictyostelia. Instead, in P. pallidum, one of many Dictyostelia that like their solitary ancestors can still encyst to survive starvation, GSK3 promotes multicellular development into fruiting bodies over unicellular encystment.
Electronic supplementary material
The online version of this article (10.1186/s13227-018-0101-6) contains supplementary material, which is available to authorized users.
Keywords: Encystment, Sporulation, Stress response, Polysphondylium, Life cycle choice, Glycogen synthase kinase 3, Cell-type specialization, Amoebozoa, Dictyostelia
Background
Many unicellular protists, including Amoebozoa, survive adverse conditions by shutting down metabolism and differentiating into a walled cyst. Cysts are extremely resilient, which, in case of amoeba pathogens, prevents their eradication by immune clearance or antibiotics [1, 2]. The multicellular Dictyostelia, members of Amoebozoa, evolved an additional strategy to survive starvation stress, in which amoebas aggregate to form a multicellular fruiting structure and differentiate into walled spores and stalk cells. Dictyostelia can be subdivided into four major groups, and while many species in groups 1–3 have retained encystation as an alternative survival strategy, it was lost in group 4, which contains the model organism Dictyostelium discoideum [3, 4]. In D. discoideum, both secreted and intracellular cyclic AMP (cAMP) play major roles in regulating the multicellular developmental programme. Secreted cAMP, acting on G-protein-coupled cAMP receptors (cARs), acts as a chemoattractant to coordinate aggregation and morphogenesis, and additionally induces prespore differentiation, while inhibiting stalk differentiation. Intracellular cAMP, acting on PKA, triggers the maturation of spore and stalk cells and keeps spore dormant in the fruiting body. cAMP is synthesized by the adenylate cyclases ACA, ACR and ACG, but intracellular levels are critically regulated by the cAMP phosphodiesterase RegA. RegA is activated/inhibited by sensor histidine kinases/phosphatases, which are the targets for signals that control timely spore and stalk maturation and spore germination [5, 6].
Comparative functional analysis of PKA, ACR, ACG and RegA in the group 2 Dictyostelid Polysphondylium pallidum and the solitary amoebozoan pathogen Acanthamoeba castellani revealed that the intracellular role of cAMP in spore and stalk maturation and spore dormancy is evolutionary derived from a second messenger role in stress-induced encystation [7–11]. PKA, ACR and RegA are deeply conserved in Amoebozoa, and their sequenced genomes contain many sensor histidine kinase/phosphatases, which could act as food/stress sensors, respectively, to regulate RegA [12, 13].
While PKA is required for both the spore and stalk cell differentiation pathways, glycogen synthase kinase 3 (GSK3), a component of the wnt/wingless pathway that regulates many cell fate decisions in metazoa [14, 15], is in D. discoideum considered to selectively promote prespore over prestalk differentiation as target for secreted cAMP, which activates GSK3 [16, 17]. We are interested in expanding the range of encystation-inducing proteins that could act as therapeutic targets to prevent encystation of pathogens. We therefore investigated whether, similar to ACR, RegA and PKA, GSK3′s role in sporulation was also evolutionary derived from a role in encystation.
To address this issue we deleted the GSK3 gene of P. pallidum, which, in addition to fruiting body formation, has retained encystation as an alternative survival strategy. Surprisingly, loss of GSK3 had no negative effect on P. pallidum sporulation and promoted instead of inhibited encystation.
Methods
Growth and development
Polysphondylium pallidum (Pp), strain PN500, was routinely grown in association with Escherichia coli or Klebsiella aerogenes on lactose-peptone (LP) agar. For multicellular development, Pp cells were harvested in 20 mM K/K-phosphate, pH 6.5 (KK2), washed free from bacteria and incubated at 106 cells/cm2 and 21 °C on non-nutrient agar. To determine growth rate, Pp cells were inoculated at 105 cells/ml in KK2 with autoclaved Klebsiella aerogenes at OD600 = 15.
Amplification of a Pp GSK3 ortholog
The Pp GSK3 gene was amplified by PCR from genomic DNA, using redundant primers GSKredF and GSKredR (Additional file 1: Table 1), which are complementary to amino-acid sequences CHRDIKP and GTPTE/R/KQ, respectively, that are conserved in eukaryote GSK3 proteins. The PCR products were subcloned, and their DNA sequence was determined from 3 independent clones. The complete 1350-bp coding sequence of the Pp GSK3 with 3003-bp 5′ and 1579-bp 3′ UTR was obtained by inverse PCR with primer pair GSKINV1 and GSKINV2 (Additional file 1: Table 1), using religated HindIII or BglII-digested Pp gDNA as template, respectively. All PCR products were subcloned in pBluescript II KS (-) (Stratagene) or pCR4-XL-TOPO (Invitrogen) and sequenced.
To determine the nucleotide sequence of the Pp GSK3 mRNAs, polyA+ RNA was isolated from Pp cells. Full-length cDNAs were subsequently synthesized by RNA-ligation-mediated rapid amplification of 5′ and 3′ cDNA ends (RLM-RACE) and RT-PCR using the GeneRacer kit (Invitrogen) according to the manufacturer’s instructions.
DNA constructs and transformation
Vectors for GSK3 gene disruption
Partial GSK3 sequence with 2.2-kb 5′ UTR and 2.9-kb 3′ UTR was amplified by inverse PCR from EcoRI-digested and religated Pp gDNA, using primers GSKINV3 and GSKINV4 (Additional file 1: Table 1) which contain KpnI sites. The KpnI-digested PCR product was cloned into KpnI-digested pLoxNeoII∆EcoRI, which was generated from pLoxNeoII [10] by destroying its EcoRI site by digestion with EcoRI, fill-in with Klenow and self-ligation with T4 ligase. This yielded vector pPp-GSK3-KO, which was linearized by EcoRI digestion and transformed into Pp cells as described previously [18]. The gene disruption was confirmed by Southern blot analysis (Additional file 1: Fig. 1). To remove the Neo cassette, the knockout cells were transformed with pA15NLS.Cre for transient expression of Cre-recombinase [10] and G418-sensitive clones were selected.
Complementation of Pp gsk3− with GSK3
The GSK3 coding sequence was amplified from cDNA by RT-PCR using primers Pp-GSK3-S51 and Pp-GSK3-E31E (Additional file 1: Table 1) containing BglII and EcoRI sites, respectively. After cloning into pCR4-TOPO (Invitrogen) the PCR product was validated by sequencing, digested with BglII and EcoRI and cloned into BglII- and EcoRI-digested vector pDdNYFP [19], yielding vector pPp-A15GSK3-OE. To express GSK3 from its own promoter, the promoter region was amplified by PCR using primers Pp-GSK3-51 and Pp-GSK3-31 (Additional file 1: Table 1), cloned into pCR4-TOPO (Invitrogen) and sequenced. After digestion with SpeI and BglII, the 1.5-kb fragment, which contains the GSK3 promoter region, was cloned into NheI- and BglII-digested pPp-A15GSK3-OE. This yielded vector pPp-GSK3-OE, which was introduced into gsk3− cells.
Encystation assay
For quantification of encystation, Pp cells were grown in a suspension of autoclaved K. aerogenes in KK2, until cell proliferation reached stationary phase. Cells were washed free of bacteria, resuspended in KK2 at 107 cells/ml and shaken at 180 rpm and 21 °C for 48 h. Aliquots of 0.1 ml were sampled at regular intervals and supplemented with 1 µl 0.1% Calcofluor (which reacts to cellulose in the cyst wall). Total amoeba and cyst numbers were determined by counting cells in a haemocytometer under phase contrast and UV illumination, respectively. 300–500 cells were counted for each time point.
GSK3 kinase assay
GSK3 kinase activity was measured in cell lysates as described previously [17]. In short, Pp cells were resuspended at 5x107 cells/ml in ice-cold lysis buffer (0.5% NP40, 10 mM NaCl, 20 mM PIPES, pH 7.0, 5 mM EDTA, 50 mM NaF, 0.1 mM Na3VO4, 0.05% 2-mercaptoethanol, 5 µg/ml benzamidine, 5 µg/ml aprotinin) and cleared by centrifugation at 10,000 × g. 5 µl cell extract was incubated for 8 min at 22 °C with 15 µl assay buffer (50 mM HEPES, pH 7.5, 4 mM MgCl2, 0.5 mM EGTA, 2 mM DTT, 100 µM ATP) containing 20 µg phosphoglycogen synthase peptide-2 (Upstate) and [γ-32P]ATP to 8–16 Bq/pmole. After the addition of 20 µl 15 mM phosphoric acid, [γ-32P]ATP incorporation was measured by binding to P81 phosphocellulose paper (Whatman) and scintillation counting, after extensive washing with 7.5 mM phosphoric acid. To measure non-specific phosphorylation, 50 mM LiCl (a GSK3 inhibitor) was added to the assay buffer.
Results
Isolation and disruption of a GSK3 homologue in Pp
To identify the role of GSK3 in Pp development, we first amplified a full-length GSK3 gene from Pp gDNA by combining PCR with degenerate primers and inverse PCR. The 2124-bp coding region contained several introns and to elucidate the gene model, we determined mRNA sequence by RT-PCR and RLM-RACE. This revealed that the GSK3 gene consists of 5 exons and 4 introns and encodes 449 amino acids. Pp GSK3 shared 92% sequence identity to D. discoideum (Dd) GSK3 and 60–70% identity with GSK3s from plants, animals and other Amoebozoa (Additional file 1: Figs. 2, 3). Query of Dictyostelid genomes with Pp GSK3 and phylogenetic inference from alignments of the closest hits shows that Pp GSK3 is orthologous to Dd GSK3.
To disrupt the GSK3 gene in Pp by homologous recombination, we transformed Pp with a construct in which the floxed A15neoR cassette is flanked by two fragments of the GSK3 gene, and obtained two gsk3 null clones from about 1000 G418-resistant clones (Additional file 1: Fig. 1). To confirm that the phenotype of the gsk3− mutant was due to the loss of GSK3, the G418 resistance cassette of the mutant was removed by transformation with Cre-recombinase and a complementation vector, which contains Pp GSK3 inclusive of its promoter, was introduced into the disruptant.
Growth phenotype of the Pp gsk3 null mutant
The D. discoideum (Dd) gsk3− mutant becomes multinucleate due to a cytokinesis defect, when grown in suspension in axenic medium, but not when grown on agar with bacteria as food source [20]. Pp grows poorly in axenic medium, but can be grown in suspension on autoclaved bacteria. We first tested whether Pp gsk3− shows a similar cytokinesis defect as Dd gsk3−. When grown in suspension with autoclaved Klebsiella aerogenes, the Pp gsk3− cells seemed larger than wild-type cells. DAPI staining revealed that most gsk3− cells contained multiple nuclei, while most wild-type cells contained a single nucleus (Fig. 1a). Complementation of gsk3− with GSK3 restored the mononucleate phenotype. When the Pp gsk3− mutant was grown with bacteria on agar, cells remained mononucleate and had the same size as wild-type cells (Fig. 1a). Counting of the number of nuclei per cell showed that less than 5% of wild-type, gsk3−/GSK3 or gsk3− cells grown on agar had more than one nucleus per cells, while 72% of gsk3− cells grown in suspension had two or more nucleo (Fig. 1b). The cytokinesis defect also reduced the doubling time of gsk3− cells grown in suspension from 6.4 h to 4.3 h as well as the cell density reached at stationary phase (Fig. 1c). However, proliferation of gsk3− cells on solid substratum was normal, as judged from the increase in plaque size of gsk3− cells grown clonally on agar with bacterial lawns (Fig. 1d). Evidently, like Dd gsk3−, Pp gsk3− shows defective cytokinesis when grown in suspension, but not on solid substratum, indicating that the requirement of GSK3 for proper cytokinesis is conserved in Dictyostelia.
Fig. 1.
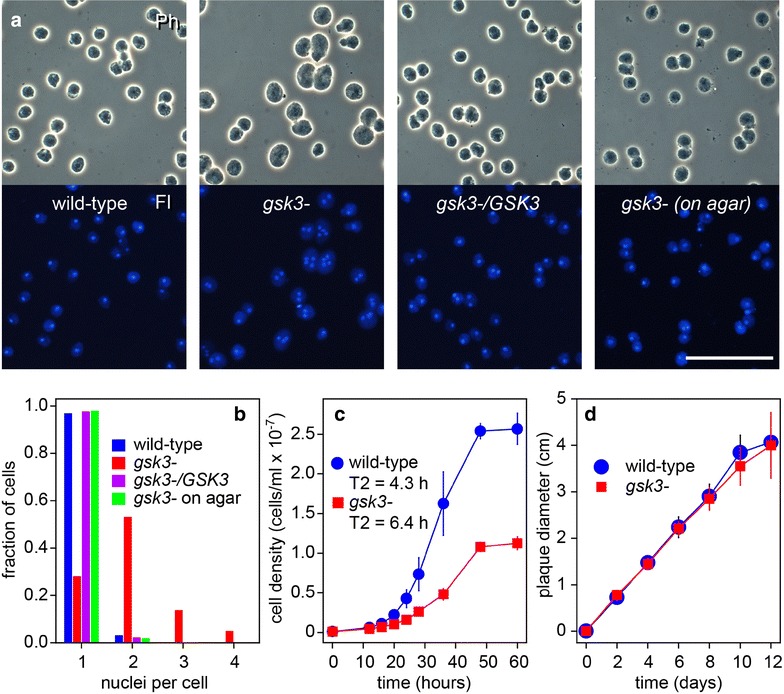
Effect of deletion of Pp GSK3 on cell division. a Cell division. P. pallidum wild-type, gsk3− and gsk3−/GSK3 cells were inoculated at 105 cells/ml in KK2 with autoclaved K. aerogenes and shaken at 22 °C until amoebas had reached late log phase. Cells were then stained with 0.01% DAPI and photographed under phase contrast (Ph, upper panels) and epifluorescence (Fl, lower panels). gsk3− cells were also grown on agar plates with live bacteria and then stained with DAPI (right panels). Bar: 50 µM. b Nuclei per cell. For about 150 cells per strain, the number of nuclei per cell was counted by comparing phase contrast and DAPI fluorescence images, and the fraction of cells with 1, 2, 3 or 4 nuclei was calculated. c Growth in suspension. Wild-type and gsk3− cells were grown in suspension on autoclaved K. aerogenes as described above. At the indicated time points, the cell density was determined and doubling times (T2) were calculated from the exponential phase of the growth curve. Means and SD of 4 experiments are presented. d Growth on agar. Wild-type and gsk3− spores were clonally plated on nutrient agar with live bacteria, and the diameter of emerging plaques in the bacterial lawn was measured with a ruler at 2-day intervals. Means and SD of 4 experiments
Developmental phenotype of the Pp gsk3 null mutant
Pp development differs from that of Dd in several aspects (Fig. 2A). There is no migrating slug stage with prestalk cells as in Dd. Pp cells first all differentiate into prespore cells, only to dedifferentiate into stalk cells at the tips of primary and secondary sorogens. There are also no basal disc cells to support the stalk. However, while Dd fruiting bodies are unbranched, Pp forms regular whorls of side branches out of cell masses that pinch off from the rear of the primary sorogen. Additionally, Pp has retained the ancestral survival strategy of encystation of individual amoebas under conditions that are unfavourable for aggregation, which is lost in Dd.
Fig. 2.
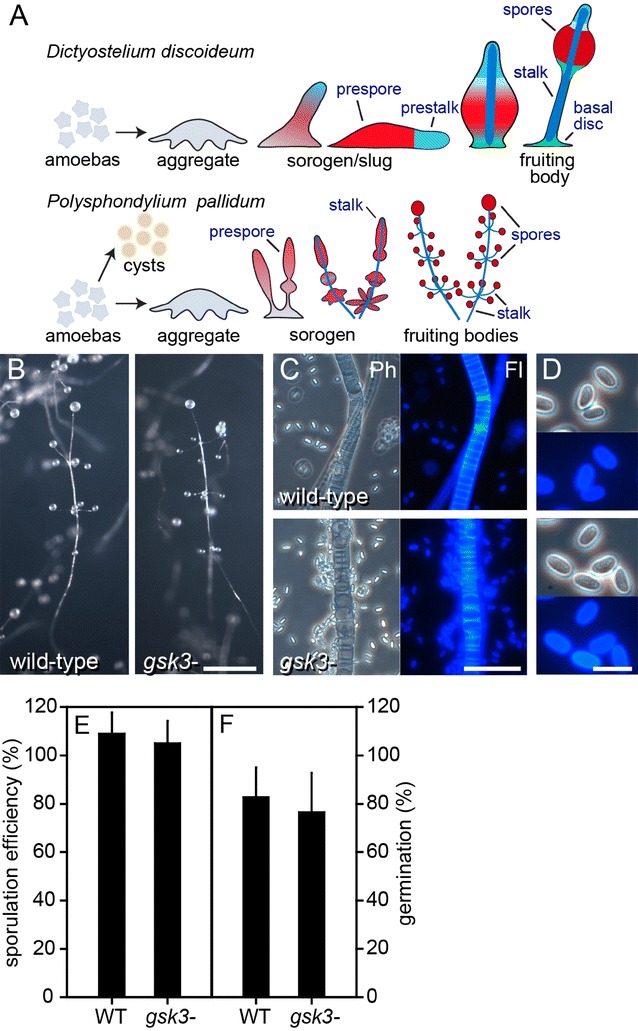
Developmental phenotype of the Pp gsk3− mutant. A Cartoon highlighting differences between Pp and Dd development. B Fruiting bodies. Pp wild-type and gsk3− cells were freed from bacteria and developed for 24 h on non-nutrient agar at 106 cells/cm2 and photographed. Bar: 0.5 mm. C Stalk cells. The fruiting bodies were picked up, deposited in 0.001% Calcofluor on a slide glass and photographed under phase contrast (Ph) and epifluorescence (Fl) Bar: 50 µm. D Spores. Prepared as in panel B, but photographed at higher magnification. Bar: 10 µm. E Sporulation efficiency. 4 × 106 freshly harvested Pp amoebas were plated on 2 × 2 cm2 nitrocellulose membranes, supported by non-nutrient agar. After completion of fruiting body formation, membranes were vortexed in 0.1% Triton-X100 and spore numbers were counted and expressed as percentage of plated cell numbers. Means and SD from 3 independent experiments. F Spore viability. Pp spores were harvested from 7-day-old fruiting bodies, treated for 10 min with 0.1% Triton-X100 to remove amoebas, counted and clonally plated on LP agar with E. coli. Emerging Pp colonies were counted after 4–5 days. Means and SD from 4 independent experiments. There were no significant differences between wild-type (WT) and gsk3− cells in panels D and E (t test, P > 0.5)
The Dd gsk3− mutant forms abnormal fruiting bodies with a large basal mass of stalk-like cells and relatively few spores [16]. However, Pp gsk3− cells appeared to form normal fruiting bodies with a neat array of stalk cells and the multiple spore heads that are common to this species (Fig. 2B). Both the spore and stalk walls stained positively with Calcofluor White, a compound that fluoresces when interacting with cellulose, indicating that they had properly reached their mature cellulose-encapsulated state (Fig. 2C). The elliptical spores of the Pp gsk3− mutant were morphologically indistinguishable from those of wild-type cells (Fig. 2D). We determined sporulation efficiency of the gks3− mutant by counting the number of spores differentiating from a known number of amoebas. Figure 2E shows that the Pp gsk3− cells sporulated as efficiently as wild-type cells. Spore numbers for both exceeded that of plated amoebas, which is due to some cell division still occurring after plating the cells. Spore viability was also normal in Pp gsk3−, since Pp gsk3− spores germinated as efficiently as wild-type spores (Fig. 2F).
Using antispore serum, which apart from spores also detects vesicles with prefabricated spore coat components in prespore cells [20], we assessed whether Pp gsk3− cells normally differentiate in prespore cells. Figure 3 shows that both wild-type and gsk3− cells show the same pattern of spore antigen expression, with only the utmost tip of the sorogen and the stalk devoid of spore antigen as is the norm for this species [4] (Fig. 2a). These experiments indicate that GSK3 is not required for either prespore or spore differentiation in Pp.
Fig. 3.
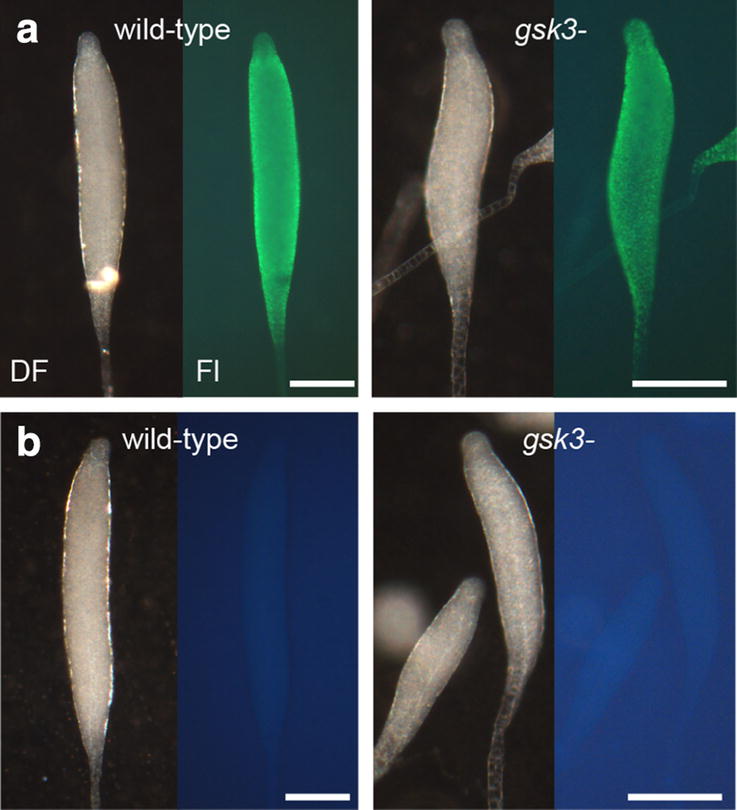
Prespore differentiation in sorogens. Pp wild-type and gsk3− sorogens were fixed and stained with rabbit antispore antibodies and FITC-conjugated antirabbit-IgG [39] (a) or only with FITC-conjugated antirabbit-IgG (b). Structures were photographed under dark field (DF) and epifluorescence (Fl), with prolonged exposure for the structures in b. Bar: 0.1 mm
Encystation of gsk3 null mutant
When, as in the experiments above, gsk3− cells are freed from bacteria and then plated on non-nutrient agar, the greater majority of amoebas aggregate and become incorporated into fruiting bodies with similar timing as wild-type cells. However, when cells are left on the culture plate after the bacteria have been eaten, we observed that many gsk3− cells did not aggregate and develop into fruiting bodies, but remained on the agar surface. This occurred particularly when plates were kept in the dark, where wild-type cells still developed normally, leaving very few cells behind on the agar surface (Fig. 4Aa–c). The prostrate gsk3− cells (the large opaque area in Fig. 4Ad) were round and refractile (Fig. 4Ae), and Calcofluor White staining (Fig. 4Af) revealed that they had cellulose walls and were actually microcysts.
Fig. 4.
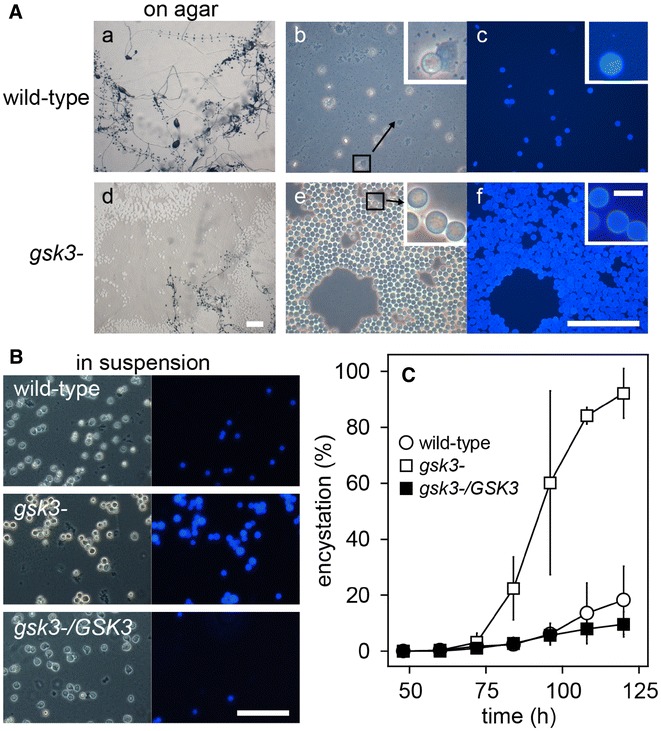
Development on nutrient agar and in suspension. A On agar. Wild-type and gsk3− cells were cultured in darkness with live E. coli on nutrient agar until, about 24 h after clearing bacteria, fruiting bodies had fully formed. Plates were photographed at low magnification (left, Bar: 1 mm). The agar surfaces were then wetted with 0.001% Calcofluor and photographed under phase contrast (centre) and epifluorescence (right). Bar: 100 µm, inset bar: 10 µm. B In suspension. Wild-type, gsk3− and gsk3−/GSK3 cells were incubated in suspension with autoclaved K. aerogenes for 120 h, with bacteria being cleared at 50–60 h. Cells were then stained with Calcofluor and photographed under phase contrast and UV illumination. Bar: 100 µm. C Quantitation. From the experiment shown in B, cells were sampled at the indicated time points and stained with Calcofluor. The numbers of fluorescent cysts and unstained amoebas were counted under UV and phase contrast illumination, respectively, and the percentage of cysts over total cells was calculated. Means and SD of 3 independent experiments, each counting 300–500 cells/sample, are presented
We also observed that gsk3− cells formed microcysts more readily after consumption of autoclaved K. aerogenes when grown in suspension (Fig. 4B). Both wild-type and gsk3− cells reach stationary phase after about 48 h in suspension culture (Fig. 1c) with all bacteria being consumed within 60 h. Both wild-type and gsk3− cells started to encyst around 60–72 h (Fig. 4C). After 120 h only 20% of wild-type cells had encysted as opposed to 90% of gsk3−. Complementation of gsk3− cells with GSK3 reduced their ability to encyst to that of wild type, indicating that loss of GSK3 potentiates encystation.
Production of and response to cyst-inducing factors
The increased ability of gsk3− cells to encyst could either be due to gsk3− cells producing more of an encystation-inducing factor or to being more sensitive to such a factor. To test the possibility that gsk3− cells produce more of an encystation-inducing factor, we prepared supernatants from suspension cultures of either wild-type or gsk3− cells at 60 h of culture, when bacterial food is just depleted. Supernatants prepared from both wild-type and gsk3− cultures strongly induced encystation of gsk3− cells, but were much less effective in wild-type cells. Incubation with water as control was ineffective to induce encystation of gsk3− cells (Fig. 5a). Also phosphate buffer and medium prepared from heat-killed K. aerogenes were ineffective to induce encystation (data not shown). These results indicate that gsk3− cells responded more strongly to encystation-inducing factor(s), rather than secreting more of such factors and that their encystation tendency is cell autonomous.
Fig. 5.
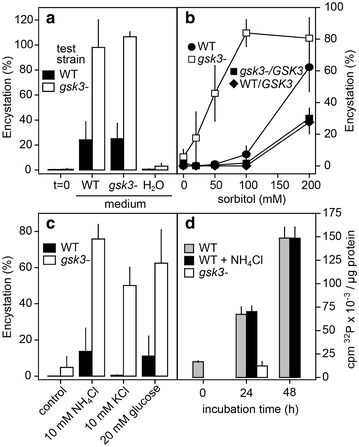
Effects of secreted factors and osmolytes on encystation and GSK3 activity. a Secreted factors. Wild-type and gsk3− cells were grown to stationary phase with autoclaved K.aerogenes, and medium and cells were separated by centrifugation. The cells were subsequently incubated for 60 h in either water, wild-type medium or gsk3− medium, and cyst percentages were determined after staining with Calcofluor. Means and SE of 4 experiments are presented. b Sorbitol. Wild-type, gsk3−, gsk3−/GSK3 and wild-type/GSK3 cells were incubated with the indicated concentrations of the osmolyte sorbitol. After 48 h of incubation, cells were stained with Calcofluor and percentages of fluorescent cysts were determined. Means and SE of 3 experiments. c Osmolyte sensitivity. Wild-type and gsk3− cells were incubated in 10 mM NH4Cl, 10 mM KCl or 20 mM glucose for 48 h. After Calcofluor staining, the percentage of fluorescent cysts was determined. Means and SE of 4 experiments. d GSK3 activity. Wild-type cells were incubated with and without 10 mM NH4Cl and gsk3− cells without NH4Cl only. Cell extracts were prepared at the indicated time points and incubated with [γ-32P]ATP and the GSK3 substrate phosphoglycogen synthase peptide-2 in the presence and absence of the GSK3 inhibitor LiCl. After 8 min, 32P incorporation in the peptide was measured and non-specific 32P incorporation in the presence of LiCl was subtracted [17]. Means and SE of 4 experiments are presented
Osmolytes were previously reported to effectively induce encystation [21], with NH4Cl and KCl being more effective than other ions or solutes [22]. We compared the effects of a range of osmolytes on encystation in gsk3− and wild-type cells. In the presence of 200 mM sorbitol, about 60% of wild-type cells encysted after 48 h of incubation, against 80% of gsk3− cells (Fig. 5b). At 100 mM sorbitol, only 10% of wild-type cells encysted, but still 80% of gsk3− cells, while 50 mM did not induce wild-type encystation anymore, but still caused 50% of gsk3− cells to encyst (Fig. 5b). On the other hand, gsk3− cells complemented with GSK3 and wild-type cells overexpressing GSK3 were even less responsive to sorbitol than wild-type cells. Similar results were obtained with other osmolytes. Both 10 mM of KCl and NH4Cl and 20 mM glucose induced 50–80% encystation of gsk3− cells, compared to 1–13% of wild-type cells (Fig. 5c). These findings indicate that gsk3− cells were much more sensitive to osmolytes than wild-type cells. While the naturally secreted encystation-inducing factor is unknown, NH3/NH4+ is a candidate, because NH3 is produced in large amounts by protein degradation in starving cells.
We next measured whether NH4Cl affects GSK3 activity, measured in cleared lysates of wild-type cells starved in suspension buffer. Figure 5d shows that the ability of GSK3 to phosphorylate phosphoglycogen synthase peptide-2 increases up to fourfold after 24 h of starvation and up to eightfold after 48 h. However, 10 mM NH4Cl had no effect on GSK3 activity. The gsk3− cells showed only 10.8 ± 1.8% of the kinase activity of wild-type cells after 24 h of starvation, indicating that non-specific phosphorylation of phosphoglycogen synthase peptide-2 in this experiment was low. This experiment shows that GSK3 is not itself regulated by NH4Cl, but may inhibit the pathway that mediates NH4Cl-induced encystation.
Discussion
GSK3 has a conserved role in cytokinesis of Dictyostelia
Pp GSK3 null mutant cells were larger than those of wild type and contained multiple nuclei, indicative of a defect in cytokinesis (Fig. 1). When grown on solid substratum, these defects were not observed. This behaviour is also found in the Dd gsk3− mutant [23] and in several Dd mutants in cytoskeletal proteins [24–26]. In both Dd and animals, GSK3 associates with the mitotic spindle during cell division [23, 27] and has for animals been described to phosphorylate microtubule-associated proteins, with the loss of GSK3 activity causing defects in spindle alignment [27, 28]. Apparently, this role of GSK3 is deeply conserved between animals and Dictyostelia. On solid substratum, Dictyostelium cells can also undergo cytokinesis by actin-based traction forces [26], a process which likely does not require GSK3.
Loss of GSK3 does not affect prespore and spore differentiation in Pp
Unlike the Dd gsk3− mutant in strain DH1 [16], the Pp gsk3− mutant showed no defects in prespore or spore differentiation (Figs. 2, 3). In Dd, the cAMP receptor cAR3 and the tyrosine kinases Zak1 and Zak2 are considered to mediate cAMP induction of prespore gene expression by GSK3 by phosphorylating GSK3 at tyrosine residues Y214 and Y220. Conversely, cAMP acting on cAR4 suppresses GSK3 activity by stimulating a tyrosine phosphatase [17, 29–31]. The Dd DH1/gsk3− fruiting bodies consist mostly of a basal mass of stalk-like vacuolated cells and few spores, but this extreme phenotype was not observed in a GSK3 knockout in Dd strain AX2, which forms fruiting bodies with shorter stalks, but normal spores [32]. cAMP-induced prespore gene expression is normal in AX2/gsk3−, but the mutant is hypersensitive to induction of the stalk marker gene ecmB by DIF-1, and like the DH1/gsk3−, car3− and zak1− mutants, does not show cAMP inhibition of ecmB [17, 29, 30, 32]. This suggests that GSK3 indirectly favours the spore pathway in Dd by preventing its inhibition by DIF-1. DIF-1 was originally identified as the stalk-inducing factor of Dd, but deletion of its biosynthetic pathway revealed that it was not required for the differentiation of the stalk, but of the basal disc [33–35]. The basal disc cells are phenotypically identical to stalk cells and also express ecmB [36]. Species outside of group 4, including Pp, do not form a basal disc and have neither cAR3 nor Zak1 or Zak2 in their genomes [10, 37]. It therefore appears that the role of GSK3 in regulating prespore/basal disc proportions newly evolved in group 4.
GSK3 controls the decision between encystation and fructification in Pp
Earlier studies showed that similar to Dd [5], PKA is required for entry into multicellular development and for spore and stalk maturation in Pp, and additionally for entry into encystation [7, 11], suggesting that PKA’s roles in multicellular development are evolutionary derived from a more ancestral role in encystation. On the other hand, GSK3 appears to control the decision to either encyst or aggregate and form fruiting bodies. The Pp gsk3− mutant formed cysts under conditions where wild-type cells normally aggregate and was hypersensitive to secreted factors and osmolytes that induce encystation (Figs. 4, 5). However, GSK3 activity was itself not regulated by these factors. GSK3 was also not obviously regulated at the expression level, since it is already present in feeding amoebas and modestly upregulated during both encystation and multicellular development (Additional file 1: Fig. 2). In Dd, GSK3 is required for the upregulation of about 81 genes and downregulation of 105 others in early development [38]. The hypersensitivity of Pp gsk3− cells to encystation-inducing factors might occur if sensors for these factors or components of their signal transduction pathways were among the Pp genes downregulated by GSK3.
Conclusions
Most Dictyostelia can choose between solitary encystment and social sporulation in fruiting bodies when faced with environmental stress. We show that active GSK3 favours sociality over solitary survival.
In contrast to D. discoideum where GSK3 promotes prespore differentiation by inhibiting basal disc differentiation, GSK3 has no effects on prespore or spore differentiation in the encysting Dictyostelid P. pallidum, most likely because they do not form the basal disc.
It is possible that the lack of encystment in D. discoideum and group 4 in general, allowed recruitment of the GSK3 pathway for regulating the differentiation of a novel encapsulated cell type in the group, i.e. the basal disc.
Additional file
Additional file 1. Additional figures 1–3 and additional table 1.
Authors’ contributions
YK, YT and PS designed experiments, YK and TM performed experiments, and YK and PS wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The sequence of the Pp GSK3 genomic region and cDNA has been deposited in GenBank under Accession Number MF186935. All other data generated or analysed during this study are included in this published article and its additional information files.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable to research on micro-organisms.
Funding
This work was funded by BBSRC Project Grant BB/K000799/1 and ERC Grant 742288.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13227-018-0101-6) contains supplementary material, which is available to authorized users.
Contributor Information
Yoshinori Kawabe, Email: y.kawabe@dundee.ac.uk.
Takahiro Morio, Email: morio@sakura.cc.tsukuba.ac.jp.
Yoshimasa Tanaka, Email: tanaka.y@orange.plala.or.jp.
Pauline Schaap, Email: p.schaap@dundee.ac.uk.
References
- 1.Aguilar-Diaz H, Carrero JC, Arguello-Garcia R, Laclette JP, Morales-Montor J. Cyst and encystment in protozoan parasites: optimal targets for new life-cycle interrupting strategies? Trends Parasitol. 2011;27(10):450–458. doi: 10.1016/j.pt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqui R, Aqeel Y, Khan NA. Killing the dead: chemotherapeutic strategies against free-living cyst-forming protists (Acanthamoeba sp. and Balamuthia mandrillaris) J Euk Microbiol. 2013;60(3):291–297. doi: 10.1111/jeu.12026. [DOI] [PubMed] [Google Scholar]
- 3.Schaap P, Winckler T, Nelson M, Alvarez-Curto E, Elgie B, Hagiwara H, Cavender J, Milano-Curto A, Rozen DE, Dingermann T, et al. Molecular phylogeny and evolution of morphology in the social amoebas. Science. 2006;314(5799):661–663. doi: 10.1126/science.1130670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romeralo M, Skiba A, Gonzalez-Voyer A, Schilde C, Lawal H, Kedziora S, Cavender JC, Glockner G, Urushihara H, Schaap P. Analysis of phenotypic evolution in Dictyostelia highlights developmental plasticity as a likely consequence of colonial multicellularity. Proc Biol Sci. 2013;280(1764):20130976. doi: 10.1098/rspb.2013.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loomis WF. Cell signaling during development of Dictyostelium. Dev Biol. 2014;391(1):1–16. doi: 10.1016/j.ydbio.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Q, Kawabe Y, Schilde C, Chen ZH, Schaap P. The evolution of aggregative multicellularity and cell-cell communication in the Dictyostelia. J Mol Biol. 2015;427:3722–3733. doi: 10.1016/j.jmb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawabe Y, Schilde C, Du Q, Schaap P. A conserved signalling pathway for amoebozoan encystation that was co-opted for multicellular development. Sci Rep. 2015;5:9644. doi: 10.1038/srep09644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Q, Schilde C, Birgersson E, Chen ZH, McElroy S, Schaap P. The cyclic AMP phosphodiesterase RegA critically regulates encystation in social and pathogenic amoebas. Cell Signal. 2014;26(2):453–459. doi: 10.1016/j.cellsig.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabe Y, Weening KE, Marquay-Markiewicz J, Schaap P. Evolution of self-organisation in Dictyostelia by adaptation of a non-selective phosphodiesterase and a matrix component for regulated cAMP degradation. Development. 2012;139(7):1336–1345. doi: 10.1242/dev.077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawabe Y, Morio T, James JL, Prescott AR, Tanaka Y, Schaap P. Activated cAMP receptors switch encystation into sporulation. Proc Natl Acad Sci USA. 2009;106(17):7089–7094. doi: 10.1073/pnas.0901617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie AV, van Es S, Fouquet C, Schaap P. From drought sensing to developmental control: evolution of cyclic AMP signaling in social amoebas. Mol Biol Evol. 2008;25(10):2109–2118. doi: 10.1093/molbev/msn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaap P, Barrantes I, Minx P, Sasaki N, Anderson RW, Benard M, Biggar KK, Buchler NE, Bundschuh R, Chen X, et al. The Physarum polycephalum genome reveals extensive use of prokaryotic two-component and metazoan-type tyrosine kinase signaling. Genome Biol Evol. 2015;8(1):109–125. doi: 10.1093/gbe/evv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke M, Lohan AJ, Liu B, Lagkouvardos I, Roy S, Zafar N, Bertelli C, Schilde C, Kianianmomeni A, Burglin TR, et al. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol. 2013;14(2):R11. doi: 10.1186/gb-2013-14-2-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 2007;64(15):1930–1944. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bejsovec A. Wingless/Wnt signaling in Drosophila: the pattern and the pathway. Mol Reprod Dev. 2013;80(11):882–894. doi: 10.1002/mrd.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwood AJ, Plyte SE, Woodgett J, Strutt H, Kay RR. Glycogen synthase kinase 3 regulates cell fate in Dictyostelium. Cell. 1995;80:139–148. doi: 10.1016/0092-8674(95)90458-1. [DOI] [PubMed] [Google Scholar]
- 17.Plyte SE, O’-Donovan E, Woodgett JR, Harwood AJ. Glycogen synthase kinase-3 (GSK-3) is regulated during Dictyostelium development via the serpentine receptor cAR3. Development. 1999;126:325–333. doi: 10.1242/dev.126.2.325. [DOI] [PubMed] [Google Scholar]
- 18.Kawabe Y, Enomoto T, Morio T, Urushihara H, Tanaka Y. LbrA, a protein predicted to have a role in vesicle trafficking, is necessary for normal morphogenesis in Polysphondylium pallidum. Gene. 1999;239(1):75–79. doi: 10.1016/S0378-1119(99)00379-0. [DOI] [PubMed] [Google Scholar]
- 19.Meima ME, Weening KE, Schaap P. Vectors for expression of proteins with single or combinatorial fluorescent protein and tandem affinity purification tags in Dictyostelium. Protein Expr Purif. 2007;53(2):283–288. doi: 10.1016/j.pep.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi I. Immunochemical and immunohistochemical studies on the development of the cellular slime mold Dictyostelium mucoroides. Dev Biol. 1963;8:1–26. doi: 10.1016/0012-1606(63)90023-X. [DOI] [PubMed] [Google Scholar]
- 21.Toama MA, Raper KB. Microcysts of the cellular slime mold Polysphondylium pallidum. I. Factors influencing microcyst formation. J Bacteriol. 1967;94(4):1143–1149. doi: 10.1128/jb.94.4.1143-1149.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi AH, O’Day DH. Ammonia and the induction of microcyst differentiation in wild type and mutant strains of the cellular slime mold Polysphondylium pallidum. DevBiol. 1982;92:356–364. doi: 10.1016/0012-1606(82)90181-6. [DOI] [PubMed] [Google Scholar]
- 23.Harwood AJ, Forde-Thomas JE, Williams H, Samereier M, Muller-Taubenberger A. Aberrant spindle dynamics and cytokinesis in Dictyostelium discoideum cells that lack glycogen synthase kinase 3. Eur J Cell Biol. 2013;92(6–7):222–228. doi: 10.1016/j.ejcb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masud Rana AYKM, Tsujioka M, Miyagishima S, Ueda M, Yumura S. Dynamin contributes to cytokinesis by stabilizing actin filaments in the contractile ring. Genes Cells. 2013;18(8):621–635. doi: 10.1111/gtc.12060. [DOI] [PubMed] [Google Scholar]
- 25.Tsujioka M, Yoshida K, Nagasaki A, Yonemura S, Muller-Taubenberger A, Uyeda TQ. Overlapping functions of the two talin homologues in Dictyostelium. Euk Cell. 2008;7(5):906–916. doi: 10.1128/EC.00464-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uyeda TQ, Nagasaki A. Variations on a theme: the many modes of cytokinesis. Curr Opin Cell Biol. 2004;16(1):55–60. doi: 10.1016/j.ceb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Wakefield JG, Stephens DJ, Tavare JM. A role for glycogen synthase kinase-3 in mitotic spindle dynamics and chromosome alignment. J Cell Sci. 2003;116(Pt 4):637–646. doi: 10.1242/jcs.00273. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Noritake J, Kakeno M, Matsui T, Harada T, Wang S, Itoh N, Sato K, Matsuzawa K, Iwamatsu A, et al. Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. J Cell Sci. 2009;122(Pt 16):2969–2979. doi: 10.1242/jcs.046649. [DOI] [PubMed] [Google Scholar]
- 29.Kim L, Liu J, Kimmel AR. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell. 1999;99(4):399–408. doi: 10.1016/S0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- 30.Kim L, Harwood A, Kimmel AR. Receptor-dependent and tyrosine phosphatase-mediated inhibition of GSK3 regulates cell fate choice. Dev Cell. 2002;3(4):523–532. doi: 10.1016/S1534-5807(02)00269-1. [DOI] [PubMed] [Google Scholar]
- 31.Kim L, Brzostowski J, Majithia A, Lee NS, McMains V, Kimmel AR. Combinatorial cell-specific regulation of GSK3 directs cell differentiation and polarity in Dictyostelium. Development. 2011;138(3):421–430. doi: 10.1242/dev.055335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schilde C, Araki T, Williams H, Harwood A, Williams JG. GSK3 is a multifunctional regulator of Dictyostelium development. Development. 2004;131(18):4555–4565. doi: 10.1242/dev.01330. [DOI] [PubMed] [Google Scholar]
- 33.Thompson CR, Kay RR. The role of DIF-1 signaling in Dictyostelium development. Mol Cell. 2000;6(6):1509–1514. doi: 10.1016/S1097-2765(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 34.Saito T, Kato A, Kay RR. DIF-1 induces the basal disc of the Dictyostelium fruiting body. Dev Biol. 2008;317(2):444–453. doi: 10.1016/j.ydbio.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann CS, Walsh CT, Kay RR. A flavin-dependent halogenase catalyzes the chlorination step in the biosynthesis of Dictyostelium differentiation-inducing factor 1. Proc Natl Acad Sci USA. 2010;107(13):5798–5803. doi: 10.1073/pnas.1001681107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jermyn K, Traynor D, Williams J. The initiation of basal disc formation in Dictyostelium discoideum is an early event in culmination. Development. 1996;122:753–760. doi: 10.1242/dev.122.3.753. [DOI] [PubMed] [Google Scholar]
- 37.Glockner G, Lawal HM, Felder M, Singh R, Singer G, Weijer CJ, Schaap P. The multicellularity genes of dictyostelid social amoebas. Nat Commun. 2016;7:12085. doi: 10.1038/ncomms12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strmecki L, Bloomfield G, Araki T, Dalton E, Skelton J, Schilde C, Harwood A, Williams JG, Ivens A, Pears C. Proteomic and microarray analyses of the Dictyostelium Zak1-GSK-3 signaling pathway reveal a role in early development. Eukaryot Cell. 2007;6(2):245–252. doi: 10.1128/EC.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schilde C, Skiba A, Schaap P. Evolutionary reconstruction of pattern formation in 98 Dictyostelium species reveals that cell-type specialization by lateral inhibition is a derived trait. EvoDevo. 2014;5(1):34. doi: 10.1186/2041-9139-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Additional figures 1–3 and additional table 1.
Data Availability Statement
The sequence of the Pp GSK3 genomic region and cDNA has been deposited in GenBank under Accession Number MF186935. All other data generated or analysed during this study are included in this published article and its additional information files.


