Abstract
We have isolated a myosin (referred to as 170-kD myosin) from lily pollen tubes, which consists of 170-kD heavy chain and calmodulin (CaM) light chain and is responsible for cytoplasmic streaming. A 170-kD polypeptide that has similar antigenicity to the 170-kD myosin heavy chain of lily pollen tubes was also present in cultured tobacco (Nicotiana tabacum) Bright Yellow-2 (BY-2) cells, and possessed the ability to interact with F-actin in an ATP-dependent manner. In addition to this myosin, we identified biochemically another kind of myosin in BY-2 cells. This myosin consisted of a CaM light chain and a 175-kD heavy chain with antigenicity different from the 170-kD myosin heavy chain. In the present study, we referred to this myosin as 175-kD myosin. This myosin was able to translocate rhodamine-phalloidin (RP)-labeled F-actin at an average velocity of about 9 μm/s in the motility assay in vitro. In contrast, the sliding velocity of RP-labeled F-actin translocated by fractions containing the 170-kD myosin was 3 to 4 μm/s. The velocity of cytoplasmic streaming in living BY-2 cells ranged from 2 to 9 μm/s. The motile activity of 175-kD myosin in vitro was inhibited by Ca2+ at concentrations higher than 10−6 m. Immunoblot analyses using an antiserum against the heavy chain of 170- or 175-kD myosin revealed that in tobacco plants, the 175-kD myosin was expressed in leaf, stem, and root, but not in germinating pollen, while 170-kD myosin was present in all of these plant parts and in germinating pollen. These results suggest that the two types of myosins, 170 and 175 kD, presumably participate in cytoplasmic streaming in BY-2 cells and other somatic cells of tobacco plants.
Myosin is a well-characterized molecular motor responsible for the actin-based motility observed in eukaryotic cells. On the basis of the primary structure determined by sequence analyses of the heavy-chain genes, myosins are divided into at least 12 classes. Despite the divergence, all heavy chains of myosins identified thus far have three domains in common: the conserved NH2-terminal head domain with actin-binding and ATP-hydrolysis sites, the IQ motif repeat domain for binding calmodulin (CaM) or proteins related to it, and the COOH-terminal tail domain that provides different functions to each class of myosins (Mooseker and Cheney, 1995; Sellers and Goodson, 1995; Cope et al., 1996; Sellers et al., 1996).
In plant cells, myosin is believed to participate as a motor in intra-cellular transport of organelles and vesicles (cytoplasmic streaming; Williamson, 1976; Kamiya, 1981; Staiger and Schliwa, 1987; Shimmen and Yokota, 1994; Li et al., 1997; Taylor and Hepler, 1997) and of generative cells and vegetative nuclei in pollen tubes (Li et al., 1997; Taylor and Hepler, 1997). Immunofluorescence microscopy using antibodies against myosin heavy chains of non-plant cells revealed the association of myosin with the organelles, vesicles, generative cells, and vegetative nuclei in pollen grains and tubes (Heslop-Harrison and Heslop-Harrison, 1989; Tang et al., 1989; Miller et al., 1995; Tirlapur et al., 1995), and with organelles and nuclei in Characean cells (Grolig et al., 1988; Qiao et al., 1989; Braun, 1996).
As shown by electron microscopy, organelles are tethered to actin filament cables through projections or filamentous structures in Characean cells (Nagai and Hayama, 1979; Williamson, 1979). Cell organelles isolated from Chara cells possess the ability to move along actin filament cables in tonoplast-free Nitella cells in which endoplasmic factors are inactivated by N-ethylmaleimide (Shimmen and Tazawa, 1982). By a similar reconstitution procedure, it has been shown that organelles isolated from lily pollen tubes also move along Characean actin filament cables (Kohno and Shimmen, 1988a; Kohno et al., 1990). These observations strongly suggest that myosin associated with organelles and vesicles acts as a motor for cytoplasmic streaming in plant cells.
Compared with non-plant cells, information is limited about biochemical properties, molecular structures, and localization of plant myosins, which is needed to elucidate their functions in the cells. Some efforts have been made to biochemically isolate myosin from plant cells including Nitella (Kato and Tonomura, 1977), Egeria (Ohsuka and Inoue, 1979), tomato (Vahey et al., 1982), and pea (Ma and Yen, 1989), however, none of these results have been reproduced. On the other hand, several genes encoding myosin heavy chains are identified and, as a result, complete or partial sequence data for myosin heavy chains of Arabidopsis (Knight and Kendrick-Jones, 1993; Kinkema and Schifelbein, 1994; Kinkema et al., 1994), other higher plants (Plazinski et al., 1997), and Anemia phyllitidis (Moepps et al., 1993) are available. However, little information is available with respect to localization and function of these myosins in the plant cell.
We have isolated 170-kD myosin composed of 170-kD heavy chain and CaM light chain from lily pollen tubes (Yokota and Shimmen, 1994; Yokota et al., 1999). From immunocytochemical studies using antibodies against the 170-kD heavy chain, we have also determined that this myosin is distributed generally in higher plant cells, in germinating pollen of Tradescantia and tobacco (Nicotiana tabacum), and in cultured cells of Arabidopsis, Catharanthus, and the tobacco cv Bright Yellow-2 (BY-2), and is localized on organelles and the peri-nuclear region in these cells (Yokota et al., 1995a, 1995b). Together with the result showing that the 170-kD myosin is capable of translocating rhodamine-phalloidin (RP)-labeled F-actin in vitro with a velocity consistent with cytoplasmic streaming in living cells (Yokota and Shimmen, 1994; Yokota et al., 1995b), these localization studies suggest that the 170-kD myosin acts as a motor responsible for cytoplasmic streaming in higher plant cells. A myosin composed of a 225- to 230-kD heavy chain has been identified in Characean cells (Yamamoto et al., 1994; Higashi-Fujime et al., 1995). On the basis of its sliding velocity in vitro (Yamamoto et al., 1994; Higashi-Fujime et al., 1995) and its binding ability to vesicles of phospholipids (Yamamoto et al., 1995), it has been predicted that this myosin also functions in cytoplasmic streaming in Characean cells.
On the other hand, Miller et al. (1995) reported that at least three classes of myosins (I, II, and V) were involved in the transport of organelles, vesicles, generative cells, and vegetative nuclei in germinating pollen. In Arabidopsis, molecular biological studies revealed the presence of eight genes encoding different myosin heavy chains (Kinkema et al., 1994). Furthermore, immunocytochemical studies have suggested that myosin also participates not only in cytoplasmic streaming and nuclear transport, but also in other actin-based processes such as contraction of the cytoplasm in Ernodesmis (La Claire II, 1991) and plasma membrane dynamics in cress and maize roots (Reichelt et al., 1996). From these studies, it is suggested that in plant cells, various classes of myosins presumably have overlapping and different functions in numerous actin-based processes, as in non-plant cells (Mooseker and Cheney, 1995; Titus, 1997; Mermall et al., 1998). Therefore, it is important to elucidate the localization and function of the different classes of myosins within the cells in a single species. In the present study, we have identified biochemically and immunocytochemically two types of myosins, each of which is composed of a 170- or 175-kD heavy chain, in cultured tobacco BY-2 cells.
MATERIALS AND METHODS
Tobacco BY-2 Cells
Tobacco (Nicotiana tabacum) cv BY-2 cells were cultured by the method of Nagata et al. (1981). Cells from 4- or 5-d-old cultures were used for isolation of myosin and for immunocytochemical studies.
Preparation of Protoplasts
Protoplasts were prepared according to a method described previously (Sonobe, 1990; Jiang et al., 1992) with some modifications. BY-2 cells were collected by centrifugation at 1,000 rpm for 30 s, and a total of 150 to 200 g (wet weight) of cells was suspended in an enzyme solution containing 2% (w/v) Sumizyme C, 0.2% (w/v) Sumizyme AP-2 (Shin-Nihon Kogaku Industries, Anjyo, Japan), and 0.45 m mannitol, and then were incubated for 90 to 120 min at 30°C. After washing two times with 0.6 m mannitol solution, protoplasts were used to isolate myosin. The pH of the enzyme solution and the 0.6 m mannitol solution was adjusted to 5.5 with HCl.
Isolation of 175-kD Myosin
Unless otherwise noted, each procedure described below was carried out at 0°C to 4°C. In each isolation step, the fraction containing myosins was monitored by a motility assay in vitro or an immunoblot assay using an antiserum against the 170-kD myosin heavy chain as described below.
BY-2 cells (150–200 g) were suspended in 400 mL of 0.25 m Suc, 1% (w/v) casein, 20 mm EGTA, 6 mm MgCl2, 100 μg/mL leupeptin, 0.5 mm PMSF, 2 mm DTT, and 30 mm PIPES-KOH (pH 7.0). This suspension was homogenized by 10 to 15 strokes with a motor-driven glass-glass homogenizer. When protoplasts were used instead of intact cells, homogenization was with a motor-driven glass-Teflon homogenizer by three strokes. After centrifugation at 10,000g for 10 min, the supernatant was further centrifuged at 100,000g for 30 min. The second supernatant was used as a crude extract. F-actin (final concentration at 0.1 mg/mL) prepared from chicken breast muscle was added to this crude extract. The mixture was kept standing on ice for 30 min. After centrifugation at 100,000g for 30 min, the pellet was resuspended in 20 mL of EMP solution (5 mm EGTA, 6 mm MgCl2, 0.5 mm PMSF, 50 μg/mL leupeptin, 1 mm DTT, and 30 mm PIPES-KOH [pH 7.0]) and kept standing for 30 min on ice. After centrifugation at 100,000g for 30 min, the pellet was extracted with 20 mL of EMP solution supplemented with 10 mm ATP and 5 mm potassium phosphate buffer (pH 7.0) for 10 min on ice and then centrifuged at 100,000g for 30 min.
The resultant supernatant (ATP extract) was applied directly to a hydroxylapatite column (column volume, 4 mL) pre-equilibrated with EMP solution supplemented with 5 mm potassium phosphate buffer (pH 7.0). The adsorbed material was eluted with a discontinuous gradient of 5, 150, and 300 mm potassium phosphate buffer (pH 7.0) in EMP solution. Fractions eluted with 5 mm potassium phosphate buffer were pooled and dialyzed against an EMP solution supplemented with 5 mm KCl on ice for 6 h. This dialysate was applied to an ion-exchange column (DE-52, Whatman BioSystems, Kent, UK; column volume, 2 mL) pre-equilibrated with the same solution used for dialysis. The adsorbed material was eluted with 40 mL of a linear gradient of 5 to 300 mm KCl in EMP solution. Fractions possessing motile activity were pooled and F-actin (final concentration at 0.1 mg/mL) was added. The mixture was kept standing on ice for 30 min and then centrifuged at 100,000g for 30 min. The pellet was extracted with a solution containing 0.1 m KCl, 10 mm ATP, 5 mm potassium phosphate buffer (pH 7.0), 5 mm EGTA, 6 mm MgCl2, 50 μg/mL leupeptin, 1 mm DTT, 0.5 mm PMSF, and 30 mm PIPES-KOH (pH 7.0) on ice for 10 min. After centrifugation at 100,000g for 30 min, the supernatant was used as isolated 175-kD myosin for the various experiments described below.
There was no significant difference between the isolation of 175-kD myosin from intact BY-2 cells and from protoplasts of BY-2 cells (data not shown).
Motility Assay in Vitro
A motility assay in vitro was performed according to the method described previously (Yokota and Shimmen, 1994; Yokota et al., 1999). A solution containing 30 mm KCl, 5 mm EGTA, 6 mm MgCl2, 1 mm ATP, 0.3 μg/mL RP-labeled F-actin, 0.216 mg/mL Glc oxidase (Wako Pure Chemical, Osaka), 36 μg/mL catalase (Wako), 4.5 mg/mL Glc, 0.6% (w/v) methyl cellulose, 100 mm DTT, and 30 mm PIPES-KOH (pH 7.0) was used for the assay. RP-labeled F-actin that moved continuously along its long axis for a distance longer than 5 μm was judged to be translocated by myosin. The effect of Ca2+ on the motile activity was measured and evaluated under the same conditions as described in the preceding paper (Yokota et al., 1999).
Preparation of Antiserum and Affinity-Purified Antibody
The 175-kD myosin was subjected to SDS-PAGE using a 6% polyacrylamide gel. After slight staining of the gel with Coomassie Brilliant Blue, the 175-kD band was excised and then homogenized in PBS supplemented with adjuvant (no. 10, Gerbu Biotechnik, Gaiberg, Germany). The homogenate was injected into male ICR mice. A total of five boosts were given at 2-week intervals. The animals were bled 2 weeks after the final injection. Antisera were incubated at 57°C for 30 min and stored frozen at −80°C until use.
Antibody purification from the antisera (AS-175) was carried out according to a method described previously (Yokota et al., 1995a) using the 175-kD polypeptide electrophoretically transferred onto the nitrocellulose membrane. The isolated 175-kD myosin was subjected to SDS-PAGE on 6% (w/v) polyacrylamide gels, and then electrophoretically transferred to a PVDF-nitrocellulose membrane sheet (Immobilon, Millipore, Bedford, MA) according to the method of Towbin et al. (1979). Membrane strips containing the 175-kD polypeptide band that had been ascertained from Ponceau S staining were excised and exposed to PBS supplemented with 2% (w/v) BSA (Wako) for 1 h. The strips were then incubated with AS-175 diluted 10-fold with PBS supplemented with 1% (w/v) BSA and 0.05% (v/v) Tween 20 for 1 h. After washing four times in PBS supplemented with 0.05% (v/v) Tween 20, the strips were incubated in 0.1 m Gly (pH 2.5) for 5 min. After the removal of the strips, 50 mm Tris, 0.2% BSA, and 5 mm NaN3 were added to the solution containing the eluted IgG.
Immunoblotting
After SDS-PAGE, proteins were electrophoretically transferred to a PVDF-nitrocellulose membrane sheet. Antigens on the nitrocellulose membrane were detected according to the method described previously (Yokota et al., 1995a). An antiserum against the 170-kD myosin heavy chain (AS-170) isolated from lily pollen tubes (Yokota and Shimmen, 1994), spinach CaM (AS-CaM; Muto and Miyachi, 1984), AS-175, or alkaline phosphatase-conjugated anti-rabbit IgG or anti-mouse IgG (Sigma, St. Louis, MO) was diluted 1,000-, 4,000-, 1,000-, or 3,000-fold, respectively, with PBS supplemented with 1% (w/v) BSA and 0.05% (v/v) Tween 20. Affinity-purified antibodies against the 175-kD myosin heavy chain (AAB-175) were diluted 20-fold and used for immunoblot analyses.
Crude protein samples from germinating pollen, leaf, stem, and root of tobacco plants for immunoblot analyses were prepared using the TCA precipitation procedure (Yokota and Shimmen, 1994).
Other Methods
SDS-PAGE was performed according to the method of Laemmli (1970). Gels were stained with Coomassie Brilliant Blue or a silver-staining kit (Wako). Protein concentrations were determined by the method of Bradford (1976) using BSA as a standard. Muscle actin was purified from chicken breast muscle according to the method of Kohama (1981).
RESULTS
Presence of 170-kD Myosin in BY-2 Cells
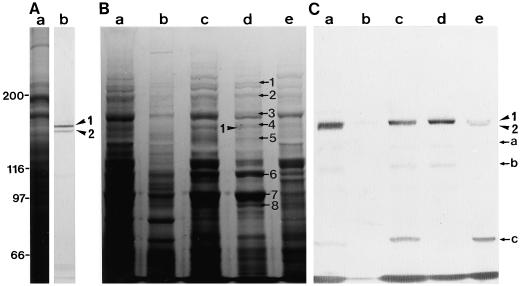
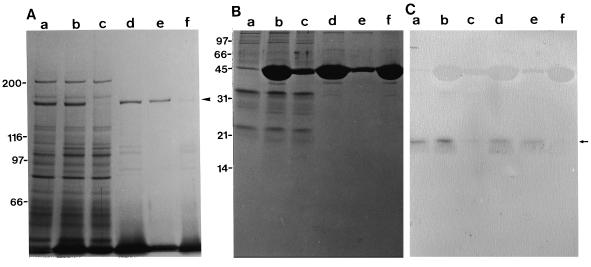
Two polypeptides with molecular masses of about 170 kD (arrowhead 1) and 165 kD (arrowhead 2), which were recognized by AS-170, were present in BY-2 cells (lane b in Fig. 1A). In the crude extract of BY-2 cells, both polypeptides bound to F-actin (lane a in Fig. 1C), and remained associated with F-actin in the absence of ATP (lane c in Fig. 1C). The 170-kD polypeptide was dissociated from F-actin by ATP treatment (lane d in Fig. 1C); however, the 165-kD polypeptide remained bound to F-actin (lane e in Fig. 1C). The other immunoreactive bands with AS-170, the 135-, 120-, and 72-kD bands marked by arrows a, b, and c, respectively, in Fig. 1C, were also found in the fractions obtained from the co-precipitation experiments. The intensity of these bands increased gradually with the progression of this procedure (compare lane a with lanes c and d in Fig. 1C). Furthermore, these bands were not detected in an immunoblot assay of the crude protein sample, as shown in Figure 1A. These results suggest that the three polypeptides are proteolytic fragments of the 170- or 165-kD polypeptides. The 135- and 120-kD polypeptides showed ATP-dependent binding to F-actin (lane d in Fig. 1C) that was similar to the 170-kD polypeptide, whereas the 72-kD polypeptide remained associated with F-actin even in the presence of ATP (lane e in Fig. 1C), like the 165-kD polypeptide. Therefore, we suggest that the 135- and 120-kD or the 72-kD polypeptide are proteolytic fragments of the 170- or 165-kD polypeptide, respectively.
Figure 1.
Identification of 170-kD myosin in BY-2 cells. A, Immunoblotting of a crude protein sample from BY-2 cells. Lane a, Coomassie Brilliant Blue staining of a 6% (w/v) SDS-polyacrylamide gel; lane b, immunoblotting using AS-170. The arrowheads 1 and 2 indicate the 170- and 165-kD polypeptides, respectively. B and C, Coomassie Brilliant Blue staining of a 6% (w/v) SDS-polyacrylamide gel of representative fractions from co-precipitation steps with F-actin and immunoblotting of the same fractions with AS-170, respectively. Co-precipitant with F-actin (lane a) after centrifugation of a mixture of a crude cell extract and F-actin. Supernatant (lane b) and pellet (lane c) after the co-precipitant shown in lane a was treated with EMP solution. Supernatant (lane d) and pellet (lane e) after ATP extraction of the pellet shown in lane c. The arrows 1 to 8 indicate polypeptides that appear to be dissociated from F-actin in an ATP-dependent manner. The arrowheads 1 and 2 indicate the 170- and 165-kD polypeptide, respectively. Arrows a to c indicate proteolytic fragments of the 170-kD myosin heavy chain or 165-kD polypeptide (see the text). The molecular masses (×103) of standard proteins are indicated on the left of A in kilodaltons.
Fractionation of the ATP Extract of Co-Precipitant with F-Actin
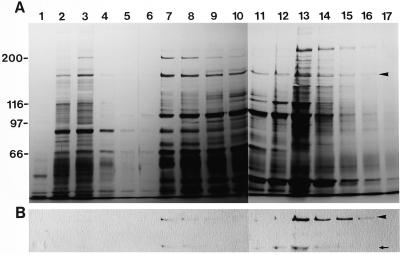
In addition to the 170-kD myosin heavy chain (arrowhead 1 in lane d in Fig. 1B), several polypeptides such as the 230-, 200-, 185-, 175-, 145-, 110-, 98-, 91-, and 86-kD polypeptides, which are marked by arrows 1, 2, 3, 4, 5, 6, 7, and 8, respectively, in lane d in Fig. 1B, appeared to be specifically released to some extent from F-actin by the addition of ATP and were contained in an ATP extract of the co-precipitant with F-actin in the crude extract. The ATP extract had the ability to translocate RP-labeled F-actin in vitro with an average velocity of 2 μm/s (data not shown). When the ATP extract was chromatographed on a hydroxylapatite column followed by elution with a discontinuous gradient of potassium phosphate buffer, a significant amount of 170-kD myosin was detected in the 300 mm potassium phosphate buffer eluate (arrowhead in Fig. 2B). A small amount of this myosin was also found in the 150 mm potassium phosphate buffer eluate (Fig. 2B).
Figure 2.
Hydroxylapatite column chromatography of the ATP extract. A, Silver staining of a 6% (w/v) SDS-polyacrylamide gel. B, Immunoblotting of the same fractions using AS-170. Numbers on the top of gels indicate fraction numbers. After the application of the ATP extract shown in Figure 1B on a hydroxylapatite column, the adsorbed materials were eluted with a discontinuous gradient of 5 mm (fraction 1–5), 150 mm (fraction 6–11), and 300 mm (fraction 12–17) potassium phosphate buffer. The arrowheads and arrow indicate 170-kD myosin and the 120-kD degradation product of the 170-kD heavy chain, respectively.
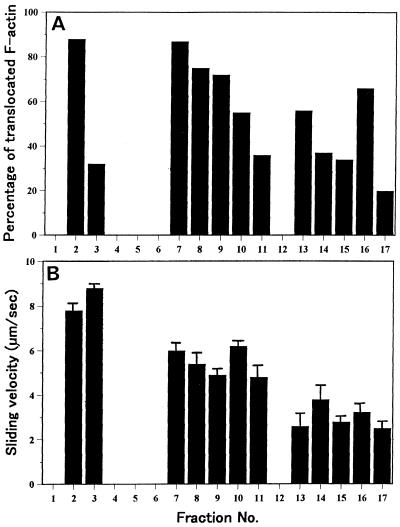
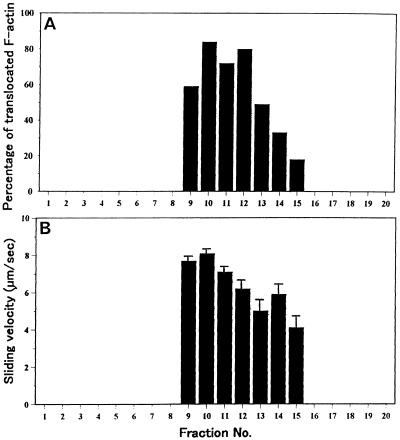
Of the proteolytic fragments of the 170-kD heavy chain, a 120-kD polypeptide was also co-fractionated together with 170-kD myosin (arrow in Fig. 2B). A motile activity in vitro was observed not only in the fractions containing 170-kD myosin, but also in the 5 mm potassium phosphate buffer eluate (Fig. 3), in which no reactive band with AS-170 was found (Fig. 2B). In the fractions eluted with 5 mm potassium phosphate buffer, both the percentage and the sliding velocity of RP-labeled F-actin translocated were higher and faster than those obtained by the 300 mm potassium phosphate buffer eluate. This indicates that a myosin(s) distinct from the 170-kD myosin exists in the 5 mm potassium phosphate buffer eluate and is involved in the motile activity. Therefore, we aimed to isolate such a myosin(s) in this fraction.
Figure 3.
Motile activity of hydroxylapatite column fractions shown in Figure 2. A, Percentage of translocated F-actin. B, Sliding velocity.
Isolation of Myosin from Potassium Phosphate Eluate
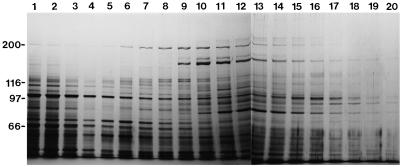
The 5 mm potassium phosphate eluate (fractions 2 and 3 in Fig. 2A) was dialyzed and further chromatographed on a DE-52 ion-exchange column (Fig. 4). Motile activities were present in fractions eluted with 130 to 210 mm KCl (fractions 9–15; Fig. 5). F-actin was added to these fractions, and then the mixture was centrifuged. Little motile activity was detected in the resultant supernatant (lane c in Fig. 6A), concomitant with co-precipitation of the 175-kD polypeptide (arrowhead in Fig. 6A) with F-actin as a main component (lane d in Fig. 6A). This co-precipitant was extracted with a solution containing ATP, and further centrifuged. Almost all of the 175-kD polypeptide was dissociated from F-actin and recovered in the supernatant (lane e in Fig. 6A). Concomitantly, motile activity reappeared in this supernatant: More than 90% of RP-labeled F-actin was translocated. These results demonstrate that the myosin responsible for the motile activity detected in the 5 mm potassium phosphate eluate is composed of a 175-kD heavy chain, which corresponds to the polypeptide marked by arrow 4 in lane d in Figure 1B. The ATP extract shown in lane e in Figure 6A was used as the isolated 175-kD myosin for further experiments described below.
Figure 4.
DE-52 ion-exchange column chromatography of the hydroxylapatite column fractions. Fractions 2 and 3 shown in Figure 2 were applied to a DE-52 column and the adsorbed materials were eluted with a linear gradient of 5 to 300 mm KCl. A 6% (w/v) SDS-polyacrylamide gel was stained with silver. Fraction numbers are shown on the top of the figure.
Figure 5.
Motile activity of each DE-52 column fraction shown in Figure 4. A, Percentage of translocated F-actin. B, Sliding velocity.
Figure 6.
Co-precipitation experiment of polypeptides in DE-52 column fractions with F-actin. A, Silver staining of a 6% (w/v) SDS-polyacrylamide gel. B, Silver staining of a 15% (w/v) SDS-polyacrylamide gel. C, Immunoblotting of the same samples shown in B with AS-CaM. Fractions 9 to 15 shown in Figure 4 were pooled (lanes a) and mixed with F-actin (lanes b). Supernatant (lanes c) and pellet (lanes d) after centrifugation of the mixture shown in lanes b. Supernatant (lanes e) and pellet (lanes f) after ATP treatment of the pellet shown in lanes d. The arrowhead and arrow indicate the 175-kD polypeptide and the 18-kD band, respectively. The percentage of translocated F-actin in motile assay was 79%, 2%, and 92% for fractions a, c, and e, respectively.
Association of CaM with the 175-kD Myosin Heavy Chain
It is well known that all myosins whose primary structures have been determined so far contain at least one repeat of the IQ motif, which represents a binding domain for CaM and CaM-related proteins (Mooseker and Cheney, 1995; Wolenski, 1995). We have also reported that CaM is associated with the 170-kD heavy chain as a light chain and plays an important role in regulating activities of the 170-kD myosin from lily (Yokota et al., 1999). In this study, we examined whether CaM was also associated with the 175-kD myosin heavy chain. In isolated 175-kD myosin, only a few bands with molecular masses below 40 kD were found in a 15% silver-stained polyacrylamide gel (lane e in Fig. 6B). An 18-kD polypeptide reacting with AS-CaM was detected by immunoblot and exhibited remarkably similar behavior to that of the 175-kD heavy chain in the co-precipitation experiments: co-sedimentation with F-actin and then dissociation from F-actin by the addition of ATP (Fig. 6C). Together with the evidence that CaM has not been reported to interact with F-actin in an ATP-dependent manner by itself, these results strongly suggest association of CaM as a light chain with the 175-kD myosin heavy chain. CaM might be insensitive to staining with silver (Fig. 6B; Rochette-Egly and Stussi-Garaud, 1984). Therefore, the possibility remains that another component(s) insensitive to silver staining is also associated with the 175-kD heavy chain as a light chain in the 175-kD myosin molecule.
Effect of Ca2+ on Motile Activity of 175-kD Myosin in Vitro
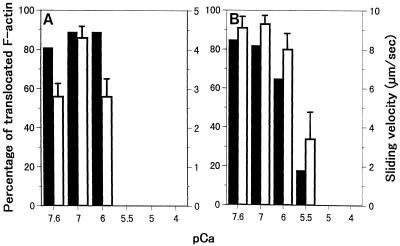
The effect of Ca2+ on motile activities of fraction 13 in Figure 2 containing 170-kD myosin (Fig. 7A) and of isolated 175-kD myosin (Fig. 7B) was examined. In both cases, Ca2+ at concentrations higher than 10−6 m caused a significant reduction in the percentage and the sliding velocity of RP-labeled F-actin translocated. The motile activity that was detected in other fractions containing 175-kD myosin (e.g. the 5 mm potassium phosphate eluate from the hydroxylapatite column and fractions of the DE-52 column) was also inhibited by Ca2+ in the same concentration-dependent manner as that of isolated 175-kD myosin (data not shown).
Figure 7.
The effect of Ca2+ on the motile activity of the 300 mm potassium phosphate eluate corresponding to fraction 13 shown in Figure 2 (A) and isolated 175-kD myosin (B). Black bars and white bars represent the percentage of translocated F-actin and sliding velocity, respectively.
Different Antigenicity of 175-kD Myosin Heavy Chain from 170-kD Myosin Heavy Chain
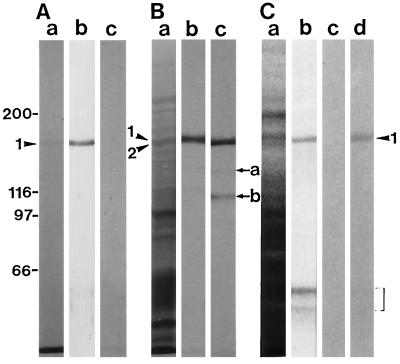
A mouse antiserum against the 175-kD heavy chain was prepared (lane b in Fig. 8A). AS-170 did not recognize the 175-kD myosin heavy chain (lane c in Fig. 8A). To determine whether AS-175 recognized the 170-kD myosin heavy chain, immunoblot assays on the ATP extract of the co-precipitant with F-actin from the crude extract (lane d in Fig. 1B) were carried out with AS-175 and AS-170. Both the 175-kD heavy chain (arrowhead 1 in Fig. 8B) and the 170-kD heavy chain (arrowhead 2 in Fig. 8B) were present in the ATP extract. The electrophoretic mobility of a band immunoreactive with AS-175 was different from and a little slower than that of the AS-170 reactive band (lane b in Fig. 8B). Furthermore, proteolytic fragments of the 170-kD myosin heavy chain, the 135- and 120-kD polypeptides (arrow a and b in Fig. 8B, respectively), showed no cross-reactivity with AS-175 (lane b in Fig. 8B). These results demonstrate that AS-175 does not cross-react with the 170-kD myosin heavy chain. In crude protein samples prepared from BY-2 cells, AS-175 reacted with not only the 175-kD polypeptide, but also with low-molecular-mass polypeptides of around 60 kD marked by the bracket in Figure 8C with similar intensity (lane b in Fig. 8C). However, AAB-175 recognized only the 175-kD polypeptide (lane d in Fig. 8C).
Figure 8.
Cross-reactivity of antibodies against the 175-kD myosin heavy chain. A, Immunoblotting of isolated 175-kD myosin shown in Figure 6. Lane a, Coomassie Brilliant Blue staining of a 6% (w/v) SDS-polyacrylamide gel; lane b, immunoblotting using AS-175; lane c, immunoblotting using AS-170. B, Immunoblotting of the ATP extract of the co-precipitant with F-actin in a crude cell extract. Lane a, Silver staining of a 6% (w/v) SDS-polyacrylamide gel; lane b, immunoblotting using AS-175; lane c, immunoblotting using AS-170. C, Immunoblotting of a crude protein sample from BY-2 cells. Lane a, Coomassie Brilliant Blue staining of a 6% (w/v) SDS-polyacrylamide gel; lane b, immunoblotting using AS-175; lane c, immunoblotting using non-immune serum; lane d, immunoblotting using AAB-175. The arrowheads 1 and 2 indicate the 175- and the 170-kD myosin heavy chains, respectively. The arrows a and b indicate the 135- and the 120-kD degradation product of the 170-kD myosin heavy chain, respectively. The region marked by a bracket shows the low-molecular-mass polypeptides reacting with AS-175.
Expression of the 170- and 175-kD Myosins in Tobacco
Polypeptides immunoreactive with AS-170 and AS-175 were investigated in crude protein samples prepared from leaf (Fig. 9A), root (Fig. 9B), stem (Fig. 9C), and germinating pollen (Fig. 9D) of tobacco plants. Low-molecular-mass polypeptides of 60 to 70 kD in these organs marked by the brackets, which were considered to be analogous to the 60-kD polypeptide in BY-2 cells (lane b in Fig. 8C), were also recognized by AS-175. The 170-kD polypeptide cross-reacting with AS-170 was detected in all organs and germinating pollen. On the other hand, the 175-kD polypeptide cross-reacting with AS-175 was detected in vegetative organs but not in germinating pollen. Although large amounts of protein samples from the germinating pollen were applied onto a SDS gel, we could not detect a band with a molecular mass of around 175 kD cross-reactive to AS-175.
Figure 9.
Immunoblotting of a crude protein sample from leaf (A), root (B), stem (C), and germinating pollen (D) of tobacco plants. Lanes a, Coomassie Brilliant Blue staining of a 6% (w/v) SDS-polyacrylamide gel; lanes b, immunoblotting using AS-170; lanes c, immunoblotting using AS-175. A total of 100, 30, 22, and 155 μg of protein from leaf, root, stem, and germinating pollen, respectively, were applied to SDS gels. The arrows and the arrowheads indicate the 170- and the 175-kD bands, respectively. The region marked by a bracket shows the low-molecular-mass polypeptides reacting with AS-175.
DISCUSSION
In the present study, we succeeded in isolating 175-kD myosin from cultured tobacco BY-2 cells. Cross-reactivity of the 175-kD heavy chain with AS-170 was not found (Fig. 8). Conversely, the 170-kD myosin heavy chain and its proteolytic fragments in the ATP extract of co-precipitant with F-actin from a crude extract did not cross-react with AS-175 (Fig. 8). Furthermore, as mentioned below, no polypeptide with a molecular mass of higher than 100 kD in the extract of the germinating pollen of tobacco plant cross-reacted with AS-175, while we did find a 170-kD band reacting with AS-170 (Fig. 9). These results indicate that 175-kD myosin possesses different antigenicity than 170-kD myosin.
The velocity of cytoplasmic streaming in living BY-2 cells ranged from 2.4 to 8.8 μm/s with an average velocity of 4.9 μm/s at room temperature (data not shown). In the motility assay in vitro, isolated 175-kD myosin caused translocation of RP-labeled F-actin with an average velocity of about 9 μm/s at lower Ca2+ concentrations (Fig. 7B). This value is consistent with the highest velocity of cytoplasmic streaming observed in intact BY-2 cells. Plant myosins that are responsible for cytoplasmic streaming are able to translocate RP-labeled F-actin in vitro with velocity comparable to cytoplasmic streaming in living cells (Yamamoto et al., 1994; Yokota and Shimmen, 1994; Higashi-Fujime et al., 1995), indicating that the velocity of cytoplasmic streaming is reflected by the sliding activity of myosin. Therefore, we suggest that the 175-kD myosin functions as a motor for cytoplasmic streaming in BY-2 cells.
As described in the introduction, the 170-kD myosin acts as a motor for cytoplasmic streaming in higher plant cells (Yokota et al., 1995a, 1995b; Yokota and Shimmen, 1994). In the case of BY-2 cells, the 170-kD myosin exhibited ATP-dependent binding to F-actin (Fig. 1C). Fractions containing 170-kD myosin were able to translocate RP-labeled F-actin at lower Ca2+ concentrations with a velocity comparable to slower ones of cytoplasmic streaming (Fig. 7A), indicating that 170-kD myosin is also involved in cytoplasmic streaming in BY-2 cells. An immunocytochemical study using antibodies against several classes of myosins from non-plant cells has suggested that at least three classes of myosins (I, II, and V) are responsible for the transport or conveyance of organelles, vesicles, generative cells, and vegetative nuclei within pollen tubes in a single species (Miller et al., 1995). The present study also strongly suggests that in BY-2 cells two different types of myosins, of 170 and 175 kD, provide the motive force for cytoplasmic streaming.
In a crude protein sample and a crude extract of BY-2 cells, a 165-kD polypeptide cross-reacted with AS-170 was also included and was associated with F-actin in an ATP-independent manner (arrowhead 2 in Fig. 1). The possibility is suggested that the 165-kD polypeptide is an isoform of 170-kD myosin. It has also been reported that some antibodies against the myosin I heavy chain cross-reacted with not only the myosin I heavy chain but also with actin-binding proteins of Acanthamoeba (Hagen et al., 1986; Rimm and Pollard, 1989). Therefore, it is also possible that the 165-kD polypeptide is an actin-binding protein but not a myosin heavy chain.
It is well established that cytoplasmic Ca2+ serves as a second messenger in many signal transduction processes (Drøbak, 1992; Muto, 1992; Bush, 1995) and modulates various physiological processes in plant cells (Hepler and Wayne, 1985; Takagi and Nagai, 1992; Nagai, 1993; Trewavas and Knight, 1994; Hepler, 1997). On the basis of physiological studies, Ca2+ is also believed to play an essential and crucial role in the regulation of cytoplasmic streaming in plant cells (Nagai, 1993; Shimmen and Yokota, 1994). In Characean cells, cytoplasmic streaming is ceased transiently when the cytoplasmic concentration of Ca2+ is increased by the generation of an action potential (Williamson and Ashley, 1982).
The elevation of Ca2+ concentration using a Ca2+ ionophore in lily pollen tubes (Kohno and Shimmen, 1988b), stamen hair cells of Tradescantia (Dorée and Picard, 1980), trichome cells of tomato (Woods et al., 1984), and leaf cells of Vallisneria (Takagi and Nagai, 1986), or by microinjection of Ca2+ into the cytoplasm of Nitella (Kikuyama and Tazawa, 1982) and stamen hair cells of Tradescantia (Hepler and Callaham, 1987) also causes inhibition of cytoplasmic streaming in these cells. Inhibition of cytoplasmic streaming by Ca2+ is a general phenomenon in plant cells. The movement of pollen tube organelles along Characean actin cables is inhibited by Ca2+ (Kohno and Shimmen, 1988a), suggesting that the myosins responsible for cytoplasmic streaming in plant cells possess inhibitory Ca2+ sensitivity. Indeed, we have shown that activities of an isolated 170-kD myosin from lily pollen is suppressed by Ca2+ at concentrations higher than 10−6 m (Yokota et al., 1999).
The motile activities of isolated 175-kD myosin and of 170-kD myosin in the 300 mm potassium phosphate eluate were suppressed by Ca2+ at concentrations higher than 10−6 m (Fig. 7). At present, the physiological meaning of the inhibitory Ca2+ sensitivity of myosins in BY-2 cells has not been elucidated; it is not known when or where cytoplasmic streaming in BY-2 cells is ceased temporarily or spatially. However, it is known that hypo-osmotic shock and activators of G-proteins and of phospholipase C elevate intracellular Ca2+ up to a micromolar level (Chandra and Low, 1997; Takahashi et al., 1997) in BY-2 cells, comparable to the threshold concentration of Ca2+ that initiated the suppression of myosin activities. Therefore, Ca2+-induced inhibition of the 170- and 175-kD myosin might have physiological meaning.
Several unconventional myosins in non-plant cells have Ca2+ sensitivity, and their motile activities in vitro are modulated by Ca2+ through CaM light chains (Mooseker and Cheney, 1995; Wolenski, 1995). In our preceding paper (Yokota et al., 1999), we suggested that activities of the 170-kD myosin from lily in vitro is inhibited by Ca2+ via the CaM light chain. From the F-actin co-precipitation experiments (Fig. 6), it is suggested that CaM is one of the light chains of 175-kD myosin, although the molar ratio of CaM to the 175-kD heavy chain remained to be established. Therefore, it is possible that, like the lily 170-kD myosin, the CaM light chain provides the Ca2+ sensitivity to the 175-kD myosin.
With respect to myosin heavy chain genes of higher plants, three complete sequences from Arabidopsis (Knight and Kendrick-Jones, 1993; Kinkema and Schiefelbein, 1994) and some partial sequences PCR amplified from Arabidopsis (Kinkema et al., 1994) and other higher plants (Plazinski et al., 1997) have been determined. At least eight genes encoding different myosin heavy chains have been reported in Arabidopsis (Kinkema et al., 1994). Among these genes, transcripts from two genes referred to as ATM2 and MYA3 are shown to be preferentially accumulated in particular organs, ATM2 in flower, leaf, and root, and MYA3 in flower and leaf (Kinkema et al., 1994). The present study revealed that the 175-kD myosin is expressed in the vegetative organs but not in germinating pollen at the protein level (Fig. 9). Therefore, it is likely that the expression or transcription of certain myosin genes is regulated in an organ-specific or -dependent manner. Since an organ is composed of various tissues, the possibility remains that the 170-kD myosin and the 175-kD myosin are expressed in different tissues.
In addition to heavy chains of the 170- and 175-kD myosin, several polypeptides appeared to bind to F-actin in an ATP-dependent manner in a crude extract of BY-2 cells (Fig. 1). Recently, Reichelt et al. (1996) indicated that the translated product of the Arabidopsis ATM1 gene encoding the 135-kD myosin heavy chain seem to be involved in plasma membrane dynamics. Together with observations reported by Miller et al. (1995), it is predicted that other myosins distinct from 170- and 175-kD myosins participate in cytoplasmic streaming and in other actin-based processes in BY-2 cells. At present, we are aiming at biochemical identification of those myosins in BY-2 cells.
ACKNOWLEDGMENT
We thank the National Live Stock Breeding Center Hyogo Station for the gift of chicken breast muscle.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (grant no. 09740602 to E.Y.).
LITERATURE CITED
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun M. Immunolocalization of myosin in rhizoids of Chara globularis Thuill. Protoplasma. 1996;191:1–8. [Google Scholar]
- Bush DS. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Chandra S, Low PS. Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem. 1997;272:28274–28280. doi: 10.1074/jbc.272.45.28274. [DOI] [PubMed] [Google Scholar]
- Cope MJTV, Whisstock J, Rayment I, Kendrick-Jones J. Conservation within the myosin motor domain: implication for structure and function. Structure. 1996;4:969–987. doi: 10.1016/s0969-2126(96)00103-7. [DOI] [PubMed] [Google Scholar]
- Dorée M, Picard A. Release of Ca2+ from intracellular pools stops cytoplasmic streaming in Tradescantia staminal hairs. Experienta. 1980;36:1291–1292. [Google Scholar]
- Drøbak BK. The plant phosphoinositide system. Biochem J. 1992;288:697–712. doi: 10.1042/bj2880697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolig F, Williamson R, Parke J, Miller C, Anderton BH. Myosin and Ca2+-sensitive streaming in the alga Chara: detection of two polypeptides reacting with a monoclonal anti-myosin and their localization in the streaming endoplasm. Eur J Cell Biol. 1988;47:22–31. [PubMed] [Google Scholar]
- Hagen SJ, Kiehart DP, Kaiser DA, Pollard TD. Characterization of monoclonal antibodies to Acanthamoeba myosin-I that cross-reacted with both myosin-II and low molecular mass nuclear proteins. J Cell Biol. 1986;103:2121–2128. doi: 10.1083/jcb.103.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK. Tip growth in pollen tubes: calcium leads the way. Trends Plant Sci. 1997;2:79–80. [Google Scholar]
- Hepler PK, Callaham DA. Free calcium increases during anaphase in stamen hair cells of Tradescantia. J Cell Biol. 1987;105:2137–2143. doi: 10.1083/jcb.105.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Wayne RO. Calcium and plant development. Annu Rev Plant Physiol. 1985;36:397–439. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Myosin associated with the surfaces of organelles, vegetative nuclei and generative cells in angiosperm pollen grains and tubes. J Cell Sci. 1989;94:319–325. [Google Scholar]
- Higashi-Fujime S, Ishikawa R, Iwasawa H, Kagami O, Kurimoto E, Kohama K, Hozumi T. The fastest actin-based motor protein from the green algae, Chara, and its distinct mode of interaction with actin. FEBS Lett. 1995;375:151–154. doi: 10.1016/0014-5793(95)01208-v. [DOI] [PubMed] [Google Scholar]
- Jiang C-J, Sonobe S, Shibaoka H. Assembly of microtubules in a cytoplasmic extract of tobacco BY-2 miniprotoplasts in the absence of microtubule-stabilizing agents. Plant Cell Physiol. 1992;33:497–501. [Google Scholar]
- Kamiya N. Physical and chemical basis of cytoplasmic streaming. Annu Rev Plant Physiol. 1981;32:205–235. [Google Scholar]
- Kato T, Tonomura Y. Identification of myosin in Nitella flexilis. J Biochem. 1977;82:777–782. doi: 10.1093/oxfordjournals.jbchem.a131754. [DOI] [PubMed] [Google Scholar]
- Kikuyama M, Tazawa M. Ca2+ ion reversibly inhibits the cytoplasmic streaming of Nitella. Protoplasma. 1982;113:241–243. [Google Scholar]
- Kinkema M, Schiefelbein J. A myosin from a higher plant has structural similarities to class V myosins. J Mol Biol. 1994;239:591–597. doi: 10.1006/jmbi.1994.1400. [DOI] [PubMed] [Google Scholar]
- Kinkema M, Wang H, Schiefelbein J. Molecular analysis of the myosin gene family in Arabidopsis thaliana. Plant Mol Biol. 1994;26:1139–1153. doi: 10.1007/BF00040695. [DOI] [PubMed] [Google Scholar]
- Knight AE, Kendrick-Jones J. A myosin-like protein from a higher plant. J Mol Biol. 1993;231:148–154. doi: 10.1006/jmbi.1993.1266. [DOI] [PubMed] [Google Scholar]
- Kohama K. Amino acid incorporation rates into myofibrillar proteins of dystrophic chicken skeletal muscle. J Biochem. 1981;90:497–501. doi: 10.1093/oxfordjournals.jbchem.a133497. [DOI] [PubMed] [Google Scholar]
- Kohno T, Chaen S, Shimmen T. Characterization of the translocator associated with pollen tube organelles. Protoplasma. 1990;154:179–183. [Google Scholar]
- Kohno T, Shimmen T. Accelerated sliding of pollen tube organelles along Characeae actin bundles regulated by Ca2+ J Cell Biol. 1988a;106:1539–1543. doi: 10.1083/jcb.106.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Shimmen T. Mechanism of Ca2+ inhibition of cytoplasmic streaming in lily pollen tubes. J Cell Sci. 1988b;91:501–509. [Google Scholar]
- La Claire JW., II Immunolocalization of myosin in intact and wounded cells of the green alga Ernodesmis verticillata (Kützing) Børgesen. Planta. 1991;184:209–217. doi: 10.1007/BF00197949. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Y-Q, Moscatelli A, Cai G, Cresti M. Functional interactions among cytoskeleton, membranes, and cell wall in the pollen tube of flowering plants. Int Rev Cytol. 1997;176:133–199. doi: 10.1016/s0074-7696(08)61610-1. [DOI] [PubMed] [Google Scholar]
- Ma Y-Z, Yen L-F. Acitin and myosin in pea tendrils. Plant Physiol. 1989;89:586–589. doi: 10.1104/pp.89.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermall V, Post PL, Mooseker MS. Unconventional myosins in cell movement, membrane traffic, and signal transduction. Science. 1998;279:527–533. doi: 10.1126/science.279.5350.527. [DOI] [PubMed] [Google Scholar]
- Miller DD, Scordilis SP, Hepler PK. Identification and localization of three classes of myosins in pollen tubes of Lilium longiflorum and Nicotiana alata. J Cell Sci. 1995;108:2549–2563. doi: 10.1242/jcs.108.7.2549. [DOI] [PubMed] [Google Scholar]
- Moepps B, Conrad S, Schraudolf H. PCR-dependent amplification and sequence characterization of partial cDNAs encoding myosin-like proteins in Anemia phyllitidis (L.) Sw. and Arabidopsis thaliana (L.) Heynh. Plant Mol Biol. 1993;21:1077–1083. doi: 10.1007/BF00023604. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Cheney RE. Unconventional myosins. Annu Rev Cell Dev Biol. 1995;11:633–675. doi: 10.1146/annurev.cb.11.110195.003221. [DOI] [PubMed] [Google Scholar]
- Muto S. Intracellular Ca2+ messenger system in plants. Int Rev Cytol. 1992;142:305–345. [Google Scholar]
- Muto S, Miyachi S. Production of antibody against spinach calmodulin and its application to radioimmunoassay for plant calmodulin. Z Planzenphysiol Bd. 1984;114:421–431. [Google Scholar]
- Nagai R. Regulation of intracellular movements in plant cells by environmental stimuli. Int Rev Cytol. 1993;145:251–310. [Google Scholar]
- Nagai R, Hayama T. Ultrastructure of the endoplasmic factor responsible for cytoplasmic streaming in Chara internodal cells. J Cell Sci. 1979;36:121–136. doi: 10.1242/jcs.36.1.121. [DOI] [PubMed] [Google Scholar]
- Nagata T, Okada K, Takebe I, Matsui C. Delivery of tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes) Mol Gen Genet. 1981;184:161–165. [Google Scholar]
- Ohsuka K, Inoue A. Identification of myosin in a flowering plant, Egeria densa. J Biochem. 1979;85:375–378. doi: 10.1093/oxfordjournals.jbchem.a132343. [DOI] [PubMed] [Google Scholar]
- Plazinski J, Elliott J, Hurley UA, Burch J, Arioli T, Williamson RE. Myosins from angiosperms, ferns, and alga amplification of gene fragments with versatile PCR primers and detection of protein products with a monoclonal antibody to a conserved head epitope. Protoplasma. 1997;196:78–86. [Google Scholar]
- Qiao L, Grolig F, Jablonsky PP, Williamson RE. Myosin heavy chains: detection by immunoblotting in higher plants and localization by immunofluorescence in the alga Chara. Cell Biol Int Rep. 1989;13:107–117. [Google Scholar]
- Reichelt S, Knight A, Hodge TP, Baluska F, Volkmann D, Kendrick-Jones J. Characterization of a plant myosin. Mol Biol Cell. 1996;7:37a. doi: 10.1046/j.1365-313x.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Pollard TD. Purification and characterization of an Acanthamoeba nuclear actin-binding protein. J Cell Biol. 1989;109:585–591. doi: 10.1083/jcb.109.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochette-Egly C, Stussi-Garaud C. Selective detection of calmodulin in polyacrylamide gels by double staining with Coomassie Blue and silver. Electrophoresis. 1984;5:285–288. [Google Scholar]
- Sellers JR, Goodson HV. Myosin. Protein Profile. 1995;2:1323–1339. [PubMed] [Google Scholar]
- Sellers JR, Goodson HV, Wang F. A myosin family reunion. J Muscle Res Cell Motil. 1996;17:7–22. doi: 10.1007/BF00140320. [DOI] [PubMed] [Google Scholar]
- Shimmen T, Tazawa M. Reconstitution of cytoplasmic streaming in Characeae. Protoplasma. 1982;113:127–131. [Google Scholar]
- Shimmen T, Yokota E. Physiological and biochemical aspects of cytoplasmic streaming. Int Rev Cytol. 1994;155:97–139. [Google Scholar]
- Sonobe S. Cytochalasin B enhances cytokinetic cleavage in miniprotoplasts isolated from cultured tobacco cells. Protoplasma. 1990;155:239–242. [Google Scholar]
- Staiger CJ, Schliwa M. Actin localization and function in higher plants. Protoplasma. 1987;141:1–12. [Google Scholar]
- Takagi S, Nagai R. Intracellular Ca2+ concentration and cytoplasmic streaming in Vallisneria mesophyll cells. Plant Cell Physiol. 1986;27:953–959. [Google Scholar]
- Takagi S, Nagai R. Several aspects of current research into the role of calcium in plant physiology. Bot Mag Tokyo. 1992;105:687–697. [Google Scholar]
- Takahashi K, Isobe M, Knight MR, Trewavas AJ, Muto S. Hypoosmotic shock induces increases in cytosolic Ca2+ in tobacco suspension-culture cells. Plant Physiol. 1997;113:587–594. doi: 10.1104/pp.113.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Hepler PK, Scordilis SP. Immunochemical and immunocytochemical identification of a myosin heavy chain polypeptide in Nicotiana pollen tubes. J Cell Sci. 1989;92:569–574. doi: 10.1242/jcs.92.4.569. [DOI] [PubMed] [Google Scholar]
- Taylor LP, Hepler PK. Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:461–491. doi: 10.1146/annurev.arplant.48.1.461. [DOI] [PubMed] [Google Scholar]
- Tirlapur UK, Cai G, Faleri C, Moscatelli A, Scali M, Casino CD, Tiezzi A, Cresti M. Confocal imaging and immunogold electron microscopy of changes in distribution of myosin during pollen hydration, germination and pollen tube growth in Nicotiana tabacum L. Eur J Cell Biol. 1995;67:209–217. [PubMed] [Google Scholar]
- Titus MA. Unconventional myosins: new frontiers in actin-based motors. Trends Cell Biol. 1997;7:119–123. doi: 10.1016/S0962-8924(97)01019-2. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas AJ, Knight MR. Mechanical signalling, calcium and plant form. Plant Mol Biol. 1994;26:1329–1341. doi: 10.1007/BF00016478. [DOI] [PubMed] [Google Scholar]
- Vahey M, Titus M, Trautwein R, Scordilis S. Tomato actin and myosin: contractile proteins from a higher land plant. Cell Motil. 1982;2:131–147. [Google Scholar]
- Williamson RE. Cytoplasmic streaming in Characean algae. In: Wardlaw IF, Passioura JB, editors. Transport and Transfer Processes in Plants. NY: Academic Press; 1976. pp. 51–58. [Google Scholar]
- Williamson RE. Filaments associated with the endoplasmic reticulum in the streaming cytoplasm of Chara corallina. Eur J Cell Biol. 1979;20:177–183. [PubMed] [Google Scholar]
- Williamson RE, Ashley CC. Free Ca2+ and cytoplasmic streaming in the alga Chara. Nature. 1982;296:647–651. doi: 10.1038/296647a0. [DOI] [PubMed] [Google Scholar]
- Wolenski JS. Regulation of calmodulin-binding myosins. Trend Cell Biol. 1995;5:310–316. doi: 10.1016/s0962-8924(00)89053-4. [DOI] [PubMed] [Google Scholar]
- Woods CM, Polito VS, Reid MS. Response to chilling stress in plant cells. II. Redistribution of intracellular calcium. Protoplasma. 1984;121:17–24. [Google Scholar]
- Yamamoto K, Kikuyama M, Sutoh-Yamamoto N, Kamitsubo E. Purification of actin based motor protein from Chara coralina. Proc Jpn Acad. 1994;70:175–180. [Google Scholar]
- Yamamoto K, Kikuyama M, Sutoh-Yamamoto N, Kamitsubo E, Katayama E. Myosin from alga Chara: unique structure revealed by electron microscopy. J Mol Biol. 1995;254:109–112. doi: 10.1006/jmbi.1995.0603. [DOI] [PubMed] [Google Scholar]
- Yokota E, McDonald AR, Liu B, Shimmen T, Palevitz BA. Localization of a 170-kDa myosin heavy chain in plant cells. Protoplasma. 1995a;185:178–187. [Google Scholar]
- Yokota E, Mimura T, Shimmen T. Biochemical, immunochemical and immunohistochemical identification of myosin heavy chains in cultured cells of Catharanthus roseus. Plant Cell Physiol. 1995b;36:1541–1547. [Google Scholar]
- Yokota E, Muto S, Shimmen T. Inhibitory regulation of higher-plant myosin by Ca2+ ions. Plant Physiol. 1999;119:231–239. doi: 10.1104/pp.119.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Shimmen T. Isolation and chracterization of plant myosin from lily pollen tubes of lily. Protoplasma. 1994;177:153–162. [Google Scholar]