Abstract
Purpose
This multinational study evaluated the antitumor activity of nivolumab in nasopharyngeal carcinoma (NPC). Tumor and plasma-based biomarkers were investigated in an exploratory analysis.
Patients and Methods
Patients with multiply pretreated recurrent or metastatic NPC were treated with nivolumab until disease progression. The primary end point was objective response rate (ORR) and secondary end points included survival and toxicity. The expression of programmed death-ligand 1 (PD-L1) and human leukocyte antigens A and B in archived tumors and plasma clearance of Epstein-Barr virus DNA were correlated with ORR and survival.
Results
A total of 44 patients were evaluated and the overall ORR was 20.5% (complete response, n = 1; partial response, n = 8). Nine patients received nivolumab for > 12 months (20%). The 1-year overall survival rate was 59% (95% CI, 44.3% to 78.5%) and 1-year progression-free survival (PFS) rate was 19.3% (95% CI, 10.1% to 37.2%). There was no statistical correlation between ORR and the biomarkers; however, a descriptive analysis showed that the proportion of patients who responded was higher among those with PD-L1 positive tumors (> 1% expression) than those with PD-L1-negative tumors. The loss of expression of one or both human leukocyte antigen class 1 proteins was associated with better PFS than when both proteins were expressed (1-year PFS, 30.9% v 5.6%; log-rank P = .01). There was no association between survival and PD-L1 expression or plasma Epstein-Barr virus DNA clearance. There was no unexpected toxicity to nivolumab.
Conclusion
Nivolumab has promising activity in NPC and the 1-year overall survival rate compares favorably with historic data in similar populations. Additional evaluation in a randomized setting is warranted. The biomarker results were hypothesis generating and validation in larger cohorts is needed.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is endemic to parts of Asia and North Africa, and is etiologically associated with the Epstein-Barr virus (EBV). Circulating fragments of EBV-derived DNA can be detected in > 95% of patients with advanced NPC and have been shown to closely reflect tumor burden.1 This virus-associated cancer represents the archetypal “inflamed tumor,” which often exhibits a dense lymphocytic infiltrate and increased programmed death-ligand 1 (PD-L1) expression.2 In a recent study on the whole-exome sequencing (WES) and whole-genome sequencing (WGS) of microdissected NPC primary tumors, researchers found that the mutational load of NPC may be higher than once reported.3,4 A third of primary NPC tumors harbor major histocompatibility complex (MHC) class I gene aberrations, with inactivating mutations and rearrangements in the human leukocyte antigen (HLA) -A and HLA-B genes being the most common, which invariably results in the loss of HLA-A and HLA-B protein expression.4
Given these unique biologic characteristics of NPC, this is, to our knowledge, the first completed report on the activity of the immune-checkpoint inhibitor nivolumab in patients with recurrent or metastatic NPC. To date, there is a lack of prospective data on the biomarkers of response to checkpoint inhibitors in NPC. Therefore, this study also investigated the clinical significance of PD-L1, HLA-A, and HLA-B expression in NPC tumors and plasma EBV DNA.
This study was a multinational trial sponsored by the National Cancer Institute. The protocol was approved by the Central Institutional Review Board of the National Cancer Institute and the institutional ethics committees in Hong Kong and Singapore.
PATIENTS AND METHODS
Patient Selection and Treatment
Eligible patients had histologically or cytologically confirmed NPC that had recurred at locoregional and/or distant sites and were not amenable to curative treatment. The target lesions had to be measurable by the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, criteria. All patients had to receive at least one prior line of platinum-based chemotherapy for recurrent disease and have adequate organ function. They underwent a baseline contrast-enhanced computed tomography of the chest, abdomen, and pelvis, and magnetic resonance imaging or computed tomography scan for locoregional disease. Radiologic assessments were performed every 8 weeks for 6 months and then every 12 weeks thereafter. Archived tumor samples were retrieved and plasma samples were obtained at baseline, then weekly for the first 4 weeks of treatment. Eligible patients were treated with nivolumab at a dosage of 3 mg/kg intravenously every 2 weeks on a 4-week cycle until they experienced disease progression. Patients were allowed to continue treatment beyond RECIST progression occurring during the initial 12 weeks, as long as they satisfied all the criteria set out in the protocol.
Biomarker Study
In a preplanned biomarker study, archived, paraffin-embedded NPC tumors were retrieved for the immunohistochemical analysis of PD-L1 (anti-human PD-L1 antibody, clone 22C3, PD-L1 IHC 22C3; pharmDx assay; Agilent Technologies, Santa Clara, CA) and HLA-B (anti-HLA-B antibody, HLA-N-20; Santa Cruz Biotechnology, Dallas, TX) protein expression. PD-L1 expression in tumor cells and immune cells was scored as the percentage of tumor cells and immune cells with membranous straining, respectively.5 PD-L1 expression of < 1% was regarded as negative and expression of ≥ 1% was regarded as positive. HLA-A and HLA–B expression was scored as the percentage of tumor cells with membranous straining. This is based partly on Garrido et al,6 who used a threshold of ≥ 25% as positive expression and defined HLA positivity as PD-L1 expression in ≥ 20% of the cells and HLA negativity as PD-L1 expression in < 20% of the cells. The results were independently scored by two pathologists who were blinded to the clinical status of the study participants. Plasma EBV DNA level was determined using real-time quantitative polymerase chain reaction and the clearance (half-life) during the first cycle of nivolumab, as previously described.1
Statistics and Sample Size Calculation
The primary end point of this study was objective response by the RECIST criteria (version 1.1), and the secondary end points were overall survival (OS), progression-free survival (PFS), duration of response and toxicity (Common Terminology Criteria for Adverse Events, version 4.0). Time-to-event variables were estimated using the Kaplan-Meier method and survival rates were compared using the log-rank test. Two-sided P values < .05 were considered statistically significant. In an exploratory analysis, Fisher exact test was used to correlate binary clinical data with biomarker data.
The sample size of this study was estimated on the assumption that response rates (RRs) to nivolumab should be around 20%, based on a report that was available at the time this study was planned.7 Furthermore, the RR to noncytotoxic, experimental agents such as pazopanib and cetuximab in similarly pretreated patient cohorts was approximately 5% to 10%.8,9 This study’s design was based on the modified Simon two-stage optimal design (power, 90%; α = 0.09; P0 = .05; P1 = .20; n1 = 20; n = 35 with an additional 10 patients to allow for ineligibility, major protocol violations, or other reasons). Because four responses were observed during the first stage, enrollment was continued until a total of 45 patients was reached.
RESULTS
Efficacy and Tolerability of Nivolumab
A total of 45 patients were enrolled, of whom one patient was ineligible. This study accrued patients across 11 sites; most patients were Asian (83%; Table 1). The enrollment period spanned from October 28, 2015, until June 1, 2016, and the data were frozen on July 10, 2017. The median follow-up was 12.5 months (range, 2.2 to 22.0 months) for the 28 patients who were still alive.
Table 1.
Patient Characteristics
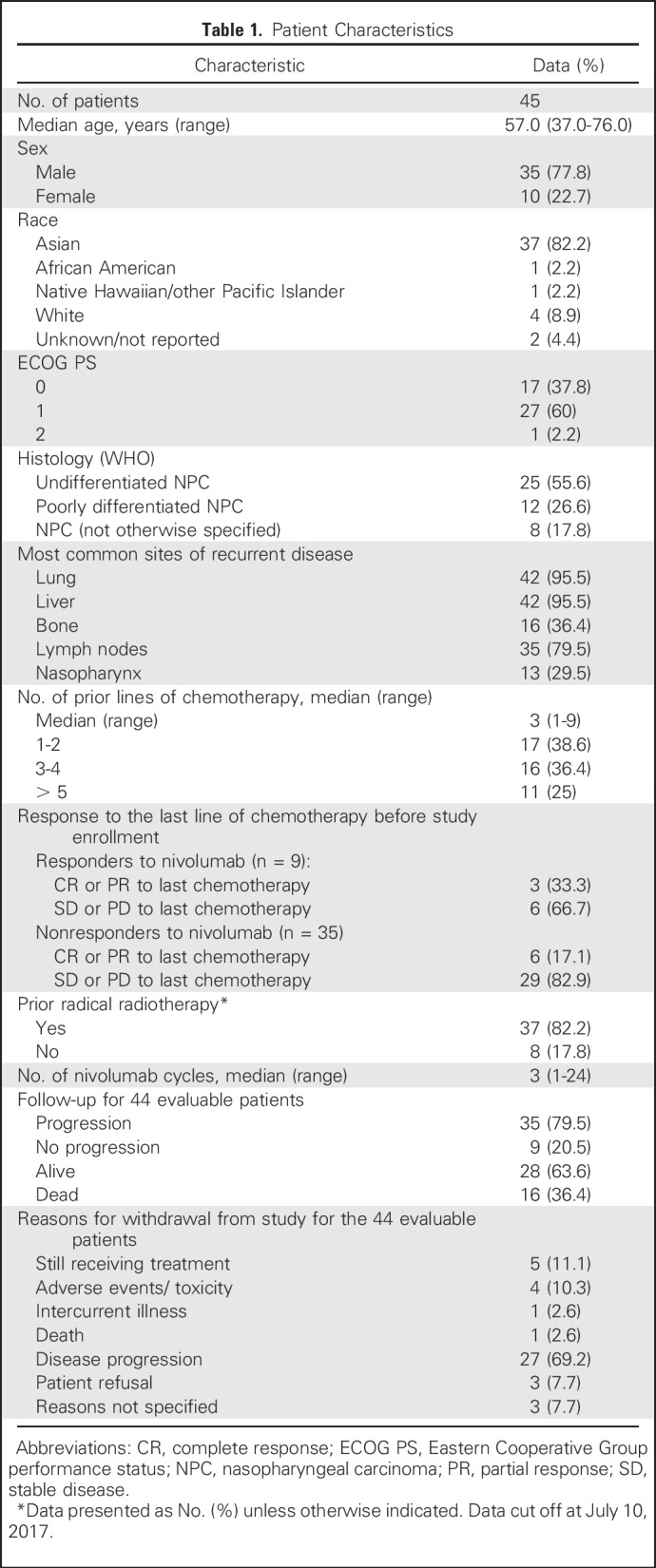
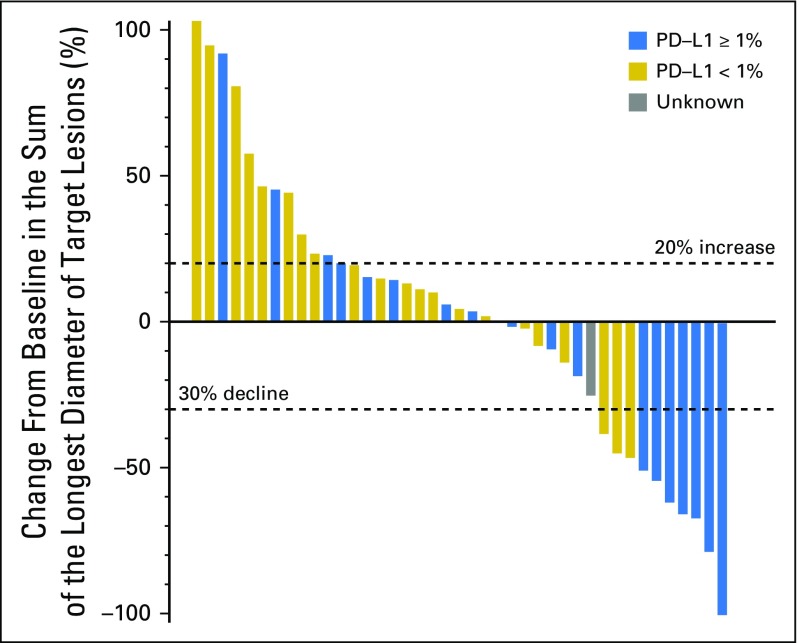
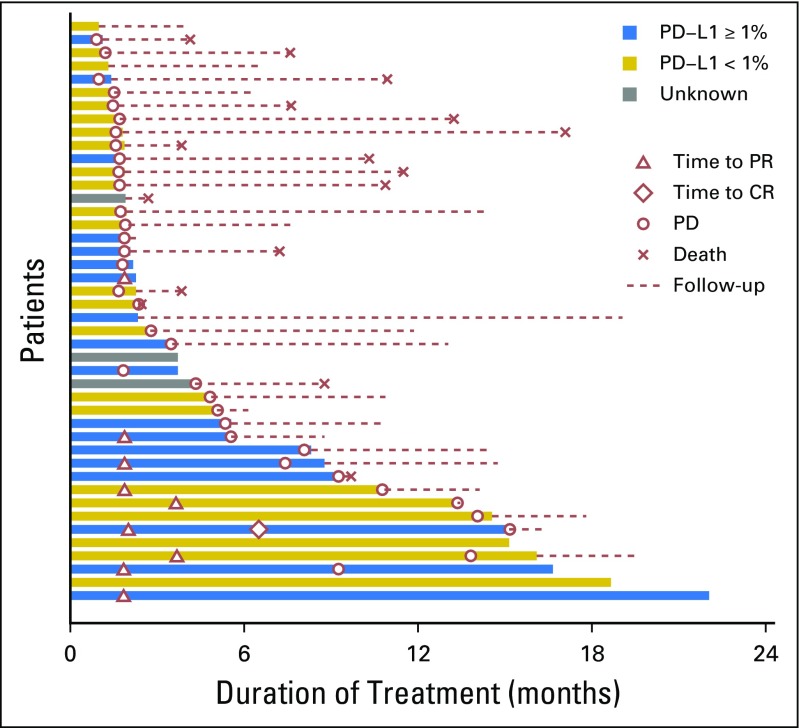
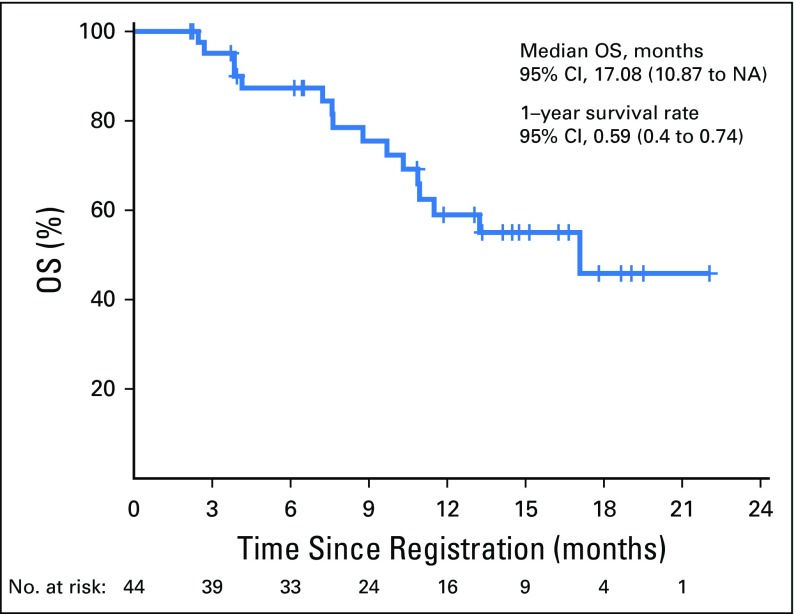
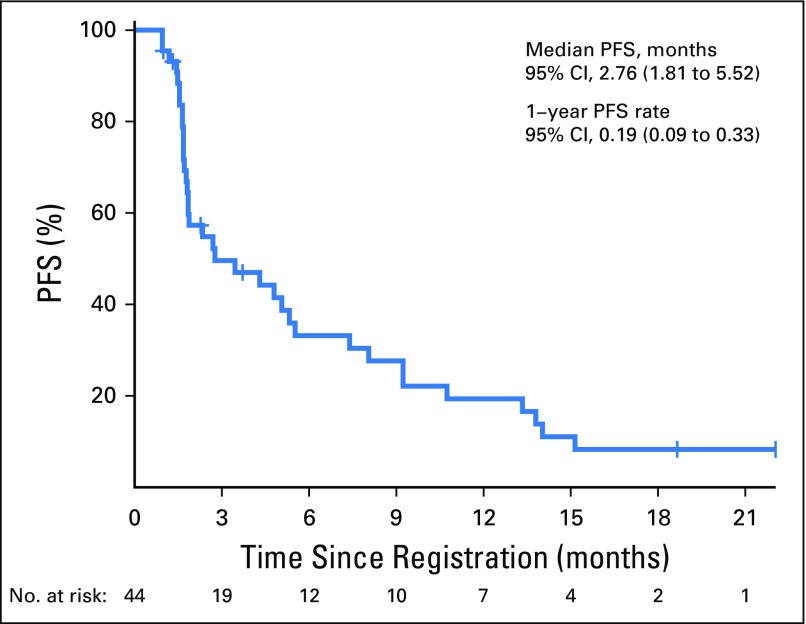
Of the 44 eligible patients, one patient had complete response (CR) lasting > 12 months (2.3%); eight had a partial response (PR; 18.2%; median duration of response, 9.3 months [95% CI, 3.6 to 13.1 months]); 15 had stable disease (SD; 34.1%; three patients had SD > 12 months); 18 had disease progression (40.9%); and two patients (4.5%) were not assessed for response. The overall RR was 20.5% (95% CI, 9.8 to 35.3), and the disease control rate was 54.5% (Fig 1). There was no relationship with the number of prior lines of chemotherapy and the response pattern to the last line of chemotherapy before enrollment (Table 1). The median OS was 17.1 months (95% CI, 10.9 months to not reached) and the 1-year OS rate was 59% (95% CI, 44.3% to 78.5%; Appendix Fig A1, online only). The median progression-free survival (PFS) was 2.8 months (95% CI, 1.8 to 7.4 months) and the 1-year PFS rate was 19.3% (95% CI, 10.1% to 37.2%; Appendix Fig A2, online only). As illustrated in the swimmer plot in Figure 2, most responders achieved a PR at the first radiologic assessment; two patients had a delayed response at the second assessment. All 18 patients whose best response was progressive disease were detected at the first radiologic assessment. Thirteen patients (29.5%) were still receiving treatment 6 months after registration, and nine patients (20.5%) received treatment for > 12 months.
Fig 1.
Waterfall plot. Changes in sum of unidimensional tumor dimension from baseline and Response Evaluation Criteria in Solid Tumors response in individual patients. Partial response was defined as a ≥ 30% decline in tumor dimension. Progressive disease was defined as a > 20% increase in tumor dimensions.
Fig 2.
Swimmer plot. Duration of response and time to response in patients receiving nivolumab. CR, complete response; PD, progressive disease; PR, partial response.
Compliance with treatment was good, with a median of three cycles of nivolumab administered (range, 1 to 19 cycles). The main reason for permanent discontinuation of the study was disease progression (69.2% of cases), with a minority of cases (10.3%) due to adverse events. Of the 45 patients who were evaluable for toxicities, 10 (22.2%) experienced grade 3 or higher adverse events that were possibly related to nivolumab (Appendix Table A1). Grade 3 or higher toxicities occurred in 22% of patients and included colitis, diarrhea, fatigue, increase in aspartate transaminase or alanine aminotransferase levels, neutropenia, hyponatremia, and lymphopenia. One patient died of pulmonary tuberculosis during treatment.
Correlative Studies With Biomarkers
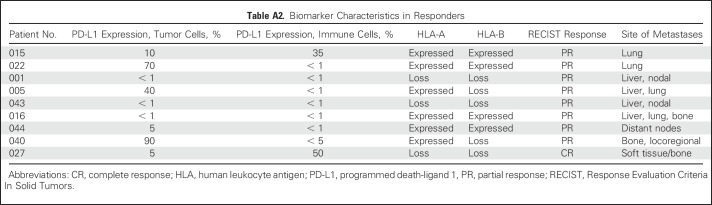
The archived tumors of 42 patients were retrieved and the plasma samples of 43 patients were prospectively collected for biomarker analysis. The results of plasma EBV DNA clearance and expression of PD-L1, HLA-A, and HLA-B are listed in Table 2, and the prognostic value of these markers was investigated. There was no statistical difference between patients with PD-L1–negative versus PD-Ll–positive tumors (expressed in tumor or immune cells) in terms of OS or PFS (Fig 1). In terms of correlation with response, six of 18 patients (33%) with PD-L1–positive tumors responded to nivolumab, whereas only three of 23 patients (13%) with PD-L1–negative tumors responded, but this did not reach statistical significance. The swimmer plot in Figure 2 illustrates the PD-L1 status and duration of response in patients who received nivolumab. In a descriptive summary of objective RRs and different levels of PD-L1 expression (Appendix Fig A3 and Appendix Table A2), a higher proportion of patients with higher levels of PD-L1–expressing tumors responded to nivolumab than those with PD-L1–negative tumors.
Table 2.
Biomarker Characteristics: Summary

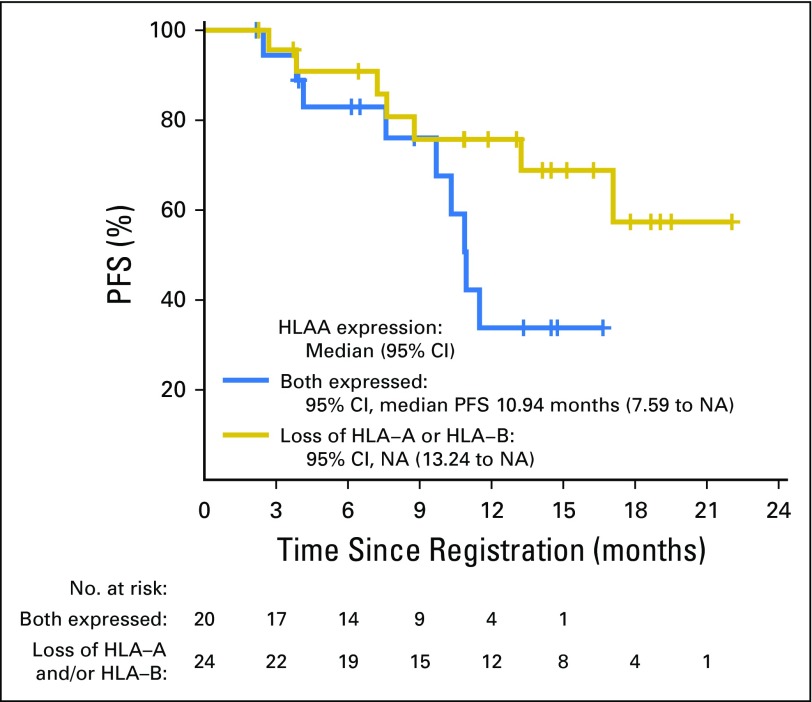
Of the 41 samples that were of sufficient quality for determining HLA expression, a statistical difference in PFS was observed between patients with tumors showing loss of expression of HLA-A and/or HLA-B (1-year PFS, 30.9% [95% CI, 16.2% to 59.1%]; median, 4.8 months [95% CI, 2.7 to 14 months]; log-rank P = .01), and patients with tumors expressing both HLA-A and HLA-B (1-year PFS, 5.6% [95% CI, 0.8% to 37.9%]; median PFS, 1.8 months [95% CI, 1.7 to 7.4 months]; Fig 3). A similar difference in OS was observed between patients with tumors exhibiting loss of expression of HLA-A and/or HLA-B expression (1-year OS, 75.7% [95% CI, 59.2% to 96.8%]; median OS was not reached) and patients with tumors expressing both HLA proteins (1-year OS, 33.8%; 95% CI, 15.5% to 73.7% median OS was 10.9 [9.7-NE]), though the difference was not statistically significant (log-rank P = .08). There was no association between HLA expression and RR (HLA-A and HLA-B expressed, RR, 22.22%; HLA-A and/or HLA-B loss, RR, 19.23%; Fisher P = 1.0).
Fig 3.
Progression-free survival curves of patients with tumors expressing both HLA-A and HLA-B (blue line), versus loss of HLA-A and/or HLA-B expression (gold line). HLA, human leukocyte antigen; NA, not achieved; PFS, progression-free survival.
Plasma EBV DNA was detectable in 97.8% of patients at baseline (Table 2), which is consistent with the literature of a detection rate > 96%.1 Patients with plasma EBV DNA clearance above or below the median half-life (14.1 days) did not differ statistically in terms of RR and survival. There was no statistical difference in RR and survival between patients with a rising versus falling trend of EBV DNA during the first month of nivolumab.
DISCUSSION
To our knowledge, this is the first completed report on the clinical activity and biomarker of response to nivolumab in patients with recurrent and metastatic NPC. There was no statistical association between PD-L1 expression in tumor cells or immune cells with survival and response to nivolumab; however, there was a higher proportion of patients with PD-L1–positive tumors who responded to nivolumab numerically in a descriptive analysis. Intriguingly, loss of HLA-A and HLA-B was associated with better survival than in patients with HLA-A– and HLA-B–intact tumors.
The pattern of response observed in this study was consistent with that reported with PD-1 inhibitors in other cancers (Fig 4).10 In the swimmer plot (Fig 2), some delayed responses (including a CR) are seen beyond the first 4 months of treatment. Disease in nearly all primary nonresponders progressed within the first 2 months of therapy, with a minority displaying a sharp increase in tumor dimensions. The latter may well represent hyperprogression, a newly reported pattern of response occurring in 9% of patients undergoing treatment with immune-checkpoint inhibitors, but this cannot be confirmed without comparison with tumor growth rate from prior therapy.11
Fig 4.
Spider plot of changes in the sum of unidimensional tumor measurements over time. The dotted blue line represents responders (according to Response Evaluation Criteria in Solid Tumors); solid gold line represents nonresponders. CR, complete response; PR, partial response.
Insights from studies of immune-checkpoint inhibitors have now shown that survival milestones (eg, 1-year survival rates) can better reflect the unique patterns of activity of these agents over other end points such as median survival, because clinical trials of PD-1 inhibitors often exhibit a late separation of survival curves and durable SD without tumor shrinkage.10 The 1-year OS rate of nivolumab (59%; 95% CI, 44.3% to 78.5%) compares favorably with phase II studies of similar populations. In these studies, the 1-year OS rates were consistently reported at approximately 45% to cytotoxic and noncytotoxic drugs.8,12-14 In a recently published phase Ib study of 27 patients with a mixed background of treatment-naïve or pretreated squamous and nonsquamous NPC, treatment with pembrolizumab resulted in an RR of 25.9% and 1-year OS of 63%.15 Compared with those in that phase Ib study,15 the patients in our study were all treatment refractory, nearly all had detectable plasma EBV DNA at baseline, and > 80% had nonkeratinizing NPC that was more reflective of endemic NPC.
There is significant variability in the literature on the prevalence and prognostic significance of PD-L1 expression in NPC, probably because of the differences in the assays and scoring methods used across studies.2,16-18 The predictive utility of PD-L1 expression could also depend on the differential expression in immune cells versus tumor cells.19 Studies that had made such a distinction found that PD-L1 was expressed in 24% to 33% of tumor cells and 40% to 75% of immune cells.17,18 This study was powered based on objective response to nivolumab but not biomarker end points; thus, the lack of statistical correlation with PD-L1 expression could be due to the limited sample size. Interestingly, as shown in Appendix Fig A3, a higher proportion of objective responses was observed in patients with PD-L1–positive tumors (≥ 1% expression) than those with PD-L1–negative tumors. In Checkmate 141 (Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck), a numerically higher (but nonstatistical) benefit in survival was reported in patients with PD-L1–positive tumors who received nivolumab than in those who received chemotherapy.20 In the Pembrolizumab for patients with PD-L1–Positive Advanced Gastric Cancer (Keynote 012) study, patients with PD-L1–positive tumors had higher RR to pembrolizumab than those with PD-L1–negative tumors.21 Notably, the complete responder in this study also possessed the highest level of PD-L1 expression in the immune cells (Appendix Table A2). It is also possible that the clinical significance of PD-L1 expression in NPC may be contingent on the disease stage or other factors in the tumor microenvironment that are yet to be identified, such as the intratumor level of CD3+ cells.18 We used archived (as opposed to fresh) tumor samples for the determination of PD-L1 expression, and tissue fixation and storage may potentially undermine the detection of PD-L1 protein, as reported in renal cell cancers.22 However, archived tumors have been used effectively to determine the eligibility of enrollment in some studies of PD-1 inhibitors in lung cancer.23
Genetic alterations in MHC class 1 genes are found in 30% of NPC primary tumors and have been linked to inferior OS and disease-free survival.4 Approximately 50% of these alterations were gene rearrangements and inactivating mutations in HLA-A and HLA-B genes, and this invariably would result in varying loss of the respective protein expression in NPC tumors.4 In this study, HLA-A and HLA-B expression did not predict response to nivolumab; however, there was a statistical association between loss of expression of HLA-A and/or HLA-B and PFS. This finding is intriguing in light of the result of our WGS/WES study, in which patients with somatic alterations of MHC class I genes had poorer outcomes.4 This difference could be partly explained by the differences in the patient’s clinical stage and origin of the tumor specimens. The WGS/WES study involved mainly the primary tumors of patients with nonmetastatic disease, whereas our study focused on recurrent and/or metastatic NPC and using a mixture of archived primary and metastatic tumors. The heterogeneity of HLA class 1 expression in solid tumors was highlighted by Garrido et al,6 who described a progressive transition of tumor cells from an MHC class 1–positive “permissive” phase during early carcinogenesis, to a “nonpermissive” phase in a later state, as typified by growing populations of MHC class 1–negative cancer cells that have escaped T-cell killing. Thus, the frequency of MHC class 1 downregulation could be higher in metastatic than in primary tumors.24
In the literature, loss of HLA class 1 expression in solid tumors has mostly been associated with poorer prognosis and a more immune-suppressive microenvironment.6,25 However, the literature is lacking on the clinical significance of MHC class 1 downregulation in the tumors of patients undergoing anti-PD-1/PD-L1 therapy.26,27 In melanoma, a nonstatistical association between response to PD-L1 inhibitor and intact MHC-1 gene expression has been found,28,29 whereas another study found an association with increased HLA-A mRNA expression.26 Although the presence of an intact MHC antigen–presenting machinery may be a prerequisite for successful therapy with PD-1 inhibitors in most cancers, some tumors, such as Hodgkin lymphoma, often exhibit total loss of MHC class 1 expression in > 50% of patients and are responsive to PD-1 inhibitors.30 In our study, the favorable prognostic significance of loss of HLA-A and/or HLA-B may raise speculation about whether there could be alternative mechanisms of action of PD-1 inhibitors in heavily pretreated NPC besides reversing T-cell exhaustion, such as via natural killer (NK) cells.31 For instance, preclinical studies have found that PD-1/ PD-L1 blockade may affect the functional exhaustion of NK cells in ovarian cancer and Kaposi sarcoma cells by potentiating NK-cell cytotoxicity.32,33 Additional studies are needed to elucidate the mechanism of action of PD-1 blockade in NPC.
Cell-free plasma EBV DNA exists as DNA fragments and its release into the patient’s circulation may arise from apoptosis of cancer cells after cytotoxic therapy.34 Although the data were not shown, seven of the eight responders with detectable baseline plasma EBV DNA had a decreasing trend for this marker during the first month of therapy. It is possible that the sample size of this study was inadequate to demonstrate a statistical significance.
In conclusion, nivolumab has promising clinical activity in heavily pretreated recurrent and/or metastatic NPC and should be investigated in other clinical settings. This clinical study was primarily designed to investigate the activity of nivolumab in NPC and the biomarker component of this study was intended to be hypothesis generating. The clinical utility of PD-L1 expression on response to nivolumab and the prognostic significance of HLA-A and HLA-B expression in patients treated with nivolumab should be validated in independent cohorts. The possibility of non–T-cell dependent mechanisms of nivolumab in NPC should be explored.
Appendix
Fig A1.
Overall survival (OS) of 44 evaluable patients.
Fig A2.
Profession-free survival (PFS) of 44 evaluable patients.
Fig A3.
Descriptive summary on the proportion of patient with response (defined by Response Evaluation Criteria in Solid Tumors) to nivolumab according to the level of programmed death-ligand 1 (PD-L1) expression in tumor cells.
Table A1.
Selected Adverse Events Possibly Related to Nivolumab

Table A2.
Biomarker Characteristics in Responders
Footnotes
Supported by the Cancer Therapy Evaluation Program, National Cancer Institute; Mayo Clinic Phase 2 Consortium (Grants No. P2C-MN026, HHSN261201100099C, and NCI N01CM-2011-00099); and Theme-Based Research Scheme Grant No. T12-401/13R and General Research Grant No. 14161317 from the University Grants Committee, Hong Kong.
Presented at the 110th Annual Meeting of the American Association of Cancer Research, Washington, DC, April 7, 2017.
Clinical trial information: NCT02339558
AUTHOR CONTRIBUTIONS
Conception and design: Brigette B.Y. Ma, Wan-Teck Lim, Boon-Cher Goh, Nathan R. Foster, Kevin J. Cullen, Ann D. King, Charles Erlichman, Jun Yin, Brian A. Costello, Anthony T.C. Chan
Provision of study materials or patients: Brigette B.Y. Ma, Jonathan W. Riess, Mark Agulnik, Barbara J. Gitlitz
Collection and assembly of data: Brigette B.Y. Ma, Wan-Teck Lim, Boon-Cher Goh, Edwin P. Hui, Kwok-Wai Lo, Adam Pettinger, Nathan R. Foster, Jonathan W. Riess, Mark Agulnik, Alex Y.C. Chang, Akhil Chopra, Julie A. Kish, Christine H. Chung, Douglas R. Adkins, Kevin J. Cullen, Barbara J. Gitlitz, Dean W. Lim, Ka Fai To, K.C. Allen Chan, Ann D. King
Data analysis and interpretation: Brigette B.Y. Ma, Wan-Teck Lim, Boon-Cher Goh, Edwin P. Hui, Kwok-Wai Lo, Adam Pettinger, Nathan R. Foster, Jonathan W. Riess, K.C. Allen Chan, Y.M. Dennis Lo, Ann D. King, Charles Erlichman, Jun Yin, Brian A. Costello, and Anthony T.C. Chan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Brigette B.Y. Ma
Honoraria: Novartis, Merck Sharp & Dohme, Merck Serono, Roche
Consulting or Advisory Role: Novartis, Merck Serono, Merck Sharp & Dohme
Research Funding: Novartis
Travel, Accommodations, Expenses: Amgen
Wan-Teck Lim
Research Funding: Bristol-Myers Squibb
Boon-Cher Goh
No relationship to disclose
Edwin P. Hui
Honoraria: Merck Serono, Merck Sharp & Dohme
Consulting or Advisory Role: Bristol-Myers Squibb
Kwok-Wai Lo
No relationship to disclose
Adam Pettinger
No relationship to disclose
Nathan R. Foster
No relationship to disclose
Jonathan W. Riess
Consulting or Advisory Role: Celgene, ARIAD, Clovis Oncology, Medtronic, Abbvie, Biodesix
Research Funding: Millennium (Inst), Novartis (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst)
Travel, Accommodations, Expenses: Roche
Mark Agulnik
Consulting or Advisory Role: Novartis, Janssen Oncology, Eisai, Eli Lilly
Speakers' Bureau: Bristol-Myers Squibb, Eli Lilly, Janssen Oncology
Alex Y.C. Chang
Consulting or Advisory Role: Bristol-Myers Squibb, Celgene, Pfizer
Akhil Chopra
Consulting or Advisory Role: Bristol-Myers Squibb, Roche India, Bayer, Merck, AstraZeneca, Astellas Pharma, Eli Lilly, MSD Oncology
Travel, Accommodations, Expenses: Astellas Pharma, Bristol-Myers Squibb
Julie A. Kish
No relationship to disclose
Christine H. Chung
Consulting or Advisory Role: AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Vigilant Biosciences, Celgene
Research Funding: AstraZeneca, Bristol-Myers Squibb Eli Lilly, Merck, Regeneron, Ignyta, IRX Therapeutics, Pfizer, Iovance Biotechnologies
Travel, Accommodations, Expenses: AstraZeneca, Bristol-Myers Squibb, Eli Lilly
Douglas R. Adkins
No relationship to disclose
Kevin J. Cullen
No relationship to disclose
Barbara J. Gitlitz
Employment: Roche
Honoraria: Roche, Eli Lilly
Speakers' Bureau: Roche, Eli Lilly
Travel, Accommodations, Expenses: Roche
Dean W. Lim
No relationship to disclose
Ka Fai To
No relationship to disclose
K.C. Allen Chan
Stock or Other Ownership: Grail
Consulting or Advisory Role: Grail
Speakers' Bureau: BioRad
Patents, Royalties, Other Intellectual Property: Patent portfolios on molecular diagnostics, Grail, Illumina, Sequenom
Y.M. Dennis Lo
Stock or Other Ownership: Grail, Sequenom, Xcelom, Cirina
Consulting or Advisory Role: Grail, Decheng Capital, Sequenom
Research Funding: Grail/Cirina (Inst)
Patents, Royalties, Other Intellectual Property: Patent portfolio in molecular diagnostics using circulating nucleic acids
Ann D. King
No relationship to disclose
Charles Erlichman
Research Funding: National Cancer Institute
Jun Yin
No relationship to disclose
Brian A. Costello
Honoraria: GU CONNECT
Research Funding: GlaxoSmithKline/Novartis (Inst)
Anthony T.C. Chan
Research Funding: Bristol-Myers Squibb, Pfizer, MSD Oncology
REFERENCES
- 1.Lo YM, Leung SF, Chan LY, et al. : Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma. Cancer Res 60:2351-2355, 2000 [PubMed] [Google Scholar]
- 2.Chen BJ, Chapuy B, Ouyang J, et al. : PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 19:3462-3473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin DC, Meng X, Hazawa M, et al. : The genomic landscape of nasopharyngeal carcinoma. Nat Genet 46:866-871, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Li YY, Chung GT, Lui VW, et al. : Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat Commun 8:14121, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muro K, Chung HC, Shankaran V, et al. : Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol 17:717-726, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Garrido F, Ruiz-Cabello F, Aptsiauri N: Rejection versus escape: The tumor MHC dilemma. Cancer Immunol Immunother 66:259-271, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443-2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim WT, Ng QS, Ivy P, et al. : A Phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin Cancer Res 17:5481-5489, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Chan AT, Hsu MM, Goh BC, et al. : Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol 23:3568-3576, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Hoos A: Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 15:235-247, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Champiat S, Dercle L, Ammari S, et al. : Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23:1920-1928, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Wang CC, Chang JY, Liu TW, et al. : Phase II study of gemcitabine plus vinorelbine in the treatment of cisplatin-resistant nasopharyngeal carcinoma. Head Neck 28:74-80, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Zhao L, Huang P, et al. : Open-label, single-arm phase II study of pemetrexed in the treatment of patients with recurrent or metastatic nasopharyngeal carcinoma who have had prior platinum-based chemotherapy. Cancer Chemother Pharmacol 70:611-615, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Hui EP, Ma BBY, Loong H, et al. : Efficacy, safety and pharmacokinetics of axitinib in nasopharyngeal carcinoma: A preclinical and phase 2 correlative study . Clin Cancer Res 24:5 2018 [DOI] [PubMed] [Google Scholar]
- 15.Hsu C, Lee SH, Ejadi S, et al. : Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: Results of the KEYNOTE-028 Study. J Clin Oncol 35:4050-4056, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Fang W, Zhang J, Hong S, et al. : EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 5:12189-12202, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan OS, Kowanetz M, Ng WT, et al. : Characterization of PD-L1 expression and immune cell infiltration in nasopharyngeal cancer. Oral Oncol 67:52-60, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Zhu Q, Cai MY, Chen CL, et al. : Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. OncoImmunology 6:e1312240, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kluger HM, Zito CR, Turcu G, et al. : PD-L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors. Clin Cancer Res 23:4270-4279, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris RL, Blumenschein G, Jr, Fayette J, et al. : Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856-1867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow LQM, Haddad R, Gupta S, et al. : Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 34:3838-3845, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson RH, Gillett MD, Cheville JC, et al. : Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer 104:2084-2091, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Grigg C, Rizvi NA: PD-L1 biomarker testing for non-small cell lung cancer: Truth or fiction? J Immunother Cancer 4:48, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-Nevot MA, Esteban F, Ferrón A, et al. : HLA class I gene expression on human primary tumours and autologous metastases: Demonstration of selective losses of HLA antigens on colorectal, gastric and laryngeal carcinomas. Br J Cancer 59:221-226, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Šmahel M: PD-1/PD-L1 blockade therapy for tumors with downregulated MHC class I expression. Int J Mol Sci 18:1331, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue H, Park JH, Kiyotani K, et al. : Intratumoral expression levels of PD-L1, GZMA, and HLA-A along with oligoclonal T cell expansion associate with response to nivolumab in metastatic melanoma. OncoImmunology 5:e1204507, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson DB, Estrada MV, Salgado R, et al. : Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun 7:10582, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hugo W, Zaretsky JM, Sun L, et al. : Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 165:35-44, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. : Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375:819-829, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roemer MG, Advani RH, Redd RA, et al. : Classical Hodgkin lymphoma with reduced β2M/MHC class I expression is associated with inferior outcome independent of 9p24.1 status. Cancer Immunol Res 4:910-916, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JM, Chen DS: Immune escape to PD-L1/PD-1 blockade: Seven steps to success (or failure). Ann Oncol 27:1492-1504, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Beldi-Ferchiou A, Lambert M, Dogniaux S, et al. : PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 7:72961-72977, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pesce S, Greppi M, Tabellini G, et al. : Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol 139:335-346.e3, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Chan KC, Chan AT, Leung SF, et al. : Investigation into the origin and tumoral mass correlation of plasma Epstein-Barr virus DNA in nasopharyngeal carcinoma. Clin Chem 51:2192-2195, 2005 [DOI] [PubMed] [Google Scholar]