Abstract
Background
Water-soluble anthocyanin pigments are important ingredients in health-improving supplements and valuable for the food industry. Although great attention has been paid to the breeding and production of crops containing high levels of anthocyanin, genetic variation in red or purple cabbages (Brassica oleracea var. capitata F. rubra) has not yet been characterized at the molecular level. In this study, we identified the mechanism responsible for the establishment of purple color in cabbages.
Results
BoMYBL2–1 is one of the regulatory genes in the anthocyanin biosynthesis pathway in cabbages. It is a repressor whose expression is inversely correlated to anthocyanin synthesis and is not detectable in purple cabbages. Sequence analysis of purple cabbages revealed that most lacked BoMYBL2–1 coding sequences, although a few had a substitution in the region of the promoter 347 bp upstream of the gene that was associated with an absence of BoMYBL2–1 expression. Lack of transcriptional activity of the substitution-containing promoter was confirmed using transgenic Arabidopsis plants transformed with promoter::GUS fusion constructs. The finding that the defect in BoMYBL2–1 expression was solely responsible for purple coloration in cabbages was further demonstrated using genomic PCR and RT-PCR analyses of many other structural and regulatory genes in anthocyanin biosynthesis. Molecular markers for purple cabbages were developed and validated using 69 cabbage lines.
Conclusion
Expression of BoMYBL2–1 was inversely correlated to anthocyanin content, and purple color in cabbages resulted from a loss of BoMYBL2–1 expression, caused by either the promoter substitution or deletion of the gene. This is the first report of molecular markers that distinguish purple cabbages. Such markers will be useful for the production of intraspecific and interspecific hybrids for functional foods, and for industrial purposes requiring high anthocyanin content.
Electronic supplementary material
The online version of this article (10.1186/s12870-018-1290-9) contains supplementary material, which is available to authorized users.
Keywords: Anthocyanin, BoMYBL2–1, Purple cabbage, Promoter substitution, Molecular marker
Background
Anthocyanins synthesized by the flavonoid biosynthetic pathway are water-soluble pigments, responsible for pink, purple, red, and blue colors that are widely distributed in flowering plants [1–3]. Not only do anthocyanins attract animals for pollination and seed dispersal purposes, but they also protect plants from biotic and abiotic stresses, such as UV damage, cold stress, drought stress, and microbial agents [4–7]. Since stress resistance caused by anthocyanins mainly results from their strong antioxidant activity [8–12], anthocyanins are important ingredients in health improvement and prevents the onset of human metabolic syndromes; anthocyanins, for example, can improve visual function [13, 14], reduce the risk of cardiovascular disease [15–17], inhibit obesity and diabetes [9, 18], and exert anti-tumor effects by anti-inflammation and anti-cancer activity [19–21]. In addition to improving health and curing disease, anthocyanins are heavily used in the food industry [22], and thus an increase in anthocyanin content is one of the most important target traits in crop breeding.
Genes involved in anthocyanin biosynthesis can be divided into two groups: early biosynthesis genes (EBGs) and late biosynthetic genes (LBGs) [23, 24]. The EBG group consists of chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), flavanone 3′-hydroxylase (F3’H), and flavonol synthase (FLS) genes, which are regulated by three R2R3-MYB transcription factors (MYB11, MYB12, and MYB111) in the model plant Arabidopsis (Arabidopsis thaliana) [25–29]. The LBGs include dihydroflavonol 4-reductase (DFR), leucoanthocyanidin oxygenase (LDOX), anthocyanidin reductase (ANR), and UDP-glucose: flavonoid 3-O-glucosyltransferase (UD3GT), which is activated by the MYB-bHLH-WD40 (MBW) complex [23, 28–31].
The MBW complex is composed of R2R3-type MYB, bHLH042 (TT8), and bHLH042 (TRANSPARENT TESTA GLABRA 1; TTG1). Its formation is controlled by various environmental factors and repressors, including MYBL2 (MYB-LIKE 2) and SPL (SQUAMOSA PROMOTER BINDING PROTEIN-LIKE) [28, 29, 32]. MYBL2 is designated as one of CPC-like MYB-related genes originated from R2R3-MYBs [33], but it turns out to be a transcriptional repressor which contains two functional motifs, ethylene-responsive element binding factor-associated Amphiphilic Repression (EAR) motif [34] and TLLLER motif [24, 35–37]. It interacts with TT8 in the MBW complex, thereby inhibiting the expression of LBGs [24, 35]. In petunia, an EAR motif-containing R2R3-MYB, MyB27, represses transcription of both anthocyanin pathway genes and an essential component of MBW complex [38]. MYBL2, however, is a positive regulator of brassinosteroid (BR)-regulated plant growth during development; MYBL2 represses BR-repressed gene expression and is stabilized by phosphorylation by BRASSINOSTEROID INSENSITIVE 2 (BIN2), a GSK3-like kinase [39, 40]. Repression of MYBL2 expression increases anthocyanin content in Arabidopsis [41, 42], but the role of MYBL2 in anthocyanin biosynthesis in crop plants has not yet been reported.
In Brassica species, purple or red color by anthocyanin accumulation is closely related to the induction of anthocyanin biosynthetic genes and/or their transcription factors. Increases in expression of TT8 and most anthocyanin biosynthetic genes are associated with purple coloration in pak choi (Brassica rapa var. chinensis) [40] and red mustard (Brassica juncea var. tumida Tsen et Lee) [39]. Similarly, accumulation of anthocyanin is caused by increases of BoMYB2 and bHLH-WD40 expression in purple cauliflower (B. oleracea L. var. botrytis) [44, 45]; of BoMYB2 and BoTT8 expression in red cabbage (B. oleracea var. capitata) [46]; of B. oleracea PRODUCTION OF ANTHOCYANIN PIGMENTATION 1 (BoPAP1/BoMYB2) and downstream genes, such as DFR and anthocyanidin synthase (ANS), under the control of BoPAP1 control in purple kale (B. oleracea var. acephala F. tricolor) [47]; and of most of the anthocyanin biosynthesis genes, as well as BoPAP1 and 2 (BoMYB4), in kohlrabi [48]. Other purple or red crops derived by natural mutations in anthocyanin biosynthetic and regulatory genes have also been reported; for example, red apples resulting from a promoter rearrangement of MdMYB10 [49, 50]; pink onions resulting from mutation in the ANS or DFR genes [51, 52]; beans with a black seed coat caused by deletion of the CHS promoter [53, 54]; purple cauliflower resulting from mutation in the BoMYB2 promoter activating its expression [44]; and purple ornamental kale caused by the deletion of DFR [55]. Red or purple cabbage (Brassica oleracea L. var. capitata f, rubra), a crop native to the Mediterranean region of Europe, is now grown all over the world as a fresh market vegetable [46].
The molecular mechanism responsible for the establishment of heading-type purple cabbages is, however, largely unknown. We found that either the promoter substitution of BoMYBL2–1 or deletion of the entire BoMYBL2–1 gene resulted in the establishment of purple cabbages. Molecular markers to discriminate purple cabbage from green ones were developed based on sequence variation. These markers will be used to develop purple vegetable crops containing high levels of anthocyanin that will be valuable for health improvements and industry use.
Methods
Plant materials
Cabbages used for BoMYBL2–1 cloning and marker validation were B. oleracea var. capitata f, alba or rubra plants from six green inbred lines, 32 green F1 cultivars, two purple inbred lines, two near-isogenic lines (NILs), 16 purple F1 cultivars, and 11 recombinants, as shown in Table 1. Inbred lines of green cabbages and purple cabbages, supplied by Asia Seed Co. (Korea), were selected to distinguish the genetic differences responsible for anthocyanin content.
Table 1.
List of the B. oleracea lines and cultivars used in this study
| Classification | Seed source | Name | Characteristics |
|---|---|---|---|
| Green cabbage | Asia Seed Co. (Inbred line) | 337 | High anthocyanin at low temperature |
| 154 | .. | ||
| 2437 | Low anthocyanin at low temperature | ||
| 09WH-45 | .. | ||
| 2409 | Green cabbage (no anthocyanin) | ||
| 842 | .. | ||
| Botanical Interests Inc. | Green A | US cultivar (95–110 days) | |
| Lake Valley Seed | Green B | US cultivar (65 days) | |
| Asia Seed Co. (F1) | Daebakna | F1 hybrid | |
| Japan cultivar | NP-J-4 (Shoshu) | Takii seed (Extreme-early) | |
| NP-J-27 (YR danryu) | Masuda seed (Medium-early) | ||
| NP-J-28 (YR Kinshukyouryoku 152) | Masuda seed (Medium-late) | ||
| NP-J-31 (Saiho) | Takii seed (Early) | ||
| NP-J-34 (YR Uijin) | Nakahara seed (Early) | ||
| NP-J-51 (Kinryoku) | Yamatonoen seed (Early) | ||
| NP-J-90 (Shoshudori) | Nosaki seed (Medium-early) | ||
| NP-J-93 (Hideaki) | Nosaki seed (Early) | ||
| NP-J-110 (Teruyoshi) | Norin seed (Medium) | ||
| NP-J-117 (Shutoku SP) | Takii seed (Early) | ||
| NP-J-135 (Koikaze) | Kaneko seed (Medium-early) | ||
| NP-J-149 (Shogun) | Mikadokyowa seed (Extreme-early) | ||
| NP-J-150 (Kagayaki) | Mikadokyowa seed (Extreme-early) | ||
| NP-J-152 (YR Seinen) | Mikadokyowa seed (Early) | ||
| NP-J-161 (YR Kiyomi) | Snow brand seed (Medium-early) | ||
| NP-J-162 (YR Hatsumi) | Snow brand seed (Medium-early) | ||
| NP-J-172 (YR Akiwase) | Masuda seed (Medium-early) | ||
| NP-J-173 (Akiwase) | Masuda seed (Medium-early) | ||
| NP-J-189 (YR Seitoku) | Takayama seed (Early) | ||
| NP-J-191 (YR Yutoku) | Takayama seed (Medium) | ||
| NP-J-198 (Best) | Watanabenoji (Early) | ||
| NP-J-199 (Ajitama) | Watanabenoji (Early) | ||
| NP-J-200 (Raien) | Watanabenoji (Early) | ||
| NP-J-216 (YR Biminakawase) | Nohara seed (Early) | ||
| NP-J-218 (Natsuboshi) | Nakahara seed (Early) | ||
| NP-J-219 (Takara) | Tama seed (Early) | ||
| NP-J-3 (Shikidori) | Takii seed (Late) | ||
| NP-J-35 (Terunami) | Nakahara seed (Late) | ||
| NP-J-36 (Harunami) | Nakahara seed (Late) | ||
| Purple cabbage | Asia Seed Co. (Inbred line) | 7S4–63 | Purple cabbage |
| 7S4–51 | .. | ||
| HanKook Seed Co. | B90 | NIL from J RED (Late) | |
| HanKook Seed Co. | B98 | NIL from Primero (Early) | |
| Botanical Interests Inc. | Purple A | 110–115 days, Red Acre | |
| Lake Valley Seed | Purple B | Red Acre | |
| Japan cultivar | NP-J-38 (Red ball) | Nakahara seed (Early) | |
| NP-J-126 (Power ruby) | Takii seed (Medium-early) | ||
| NP-J-127 (Nakate ruby ball) | Takii seed (Medium-early) | ||
| NP-J-64 (Red cabbage) | Watanabe seed (Early) | ||
| NP-J-71 (Power acre) | Medium-early | ||
| Takii Seed Co. | Junsaeng Rubia | Purple cabbage (Imported) | |
| SAKATA Seed Co. | Red Jewel | .. | |
| SAKATA Seed Co. | Varna | .. | |
| Bejo Seed Co. | Ranchero | .. | |
| Bejo Seed Co. | Autoro | .. | |
| Bejo Seed Co. | Integro | .. | |
| Bejo Seed Co. | Primero | .. | |
| Nick-Zwaan | Rondale | .. | |
| HanKook Seed Co. | DP60 | F1 | |
| HK-014 | NR (New Recombination) | ||
| HK-016 | BOX-23 | ||
| HK-017 | HKB-035 | ||
| HK-018 N | NR (New Recombination) | ||
| HK-020 | HKB-034 | ||
| HK-032 | HKB-048 | ||
| HK-034 | HKB-059 | ||
| HK-047 | NR (New Recombination) | ||
| HK-058 | NR (New Recombination) | ||
| HK-069 | NR (New Recombination) | ||
| HK-075 | NR (New Recombination) |
Cabbages used for sequence analyses were as follows: inbred lines 337 and 154, selected for high anthocyanin content at low temperature; inbred lines 2437 and 09WH-45, selected for low anthocyanin content; inbred lines 2409 and 842, selected for no anthocyanin content; and the US cultivars Green A and B. The purple cabbages selected were the inbred lines 7S4–51 and 7S4–63, the NILs B90 and B98, and Purple A and B. Additional seeds not listed in Table 1 were purchased at local markets. Most plants were cultivated in a greenhouse at Chungnam National University, Daejeon, Korea, although some leaf samples were obtained from Korean Seed companies.
Cloning of genomic DNA and sequence analysis for BoMYBL2–1
Genomic DNA was isolated from leaf samples using the DNeasy Plant Mini kit (QIAGEN Gmbh, Germany). The DNA sequences around BoMYBL2–1 (Bol016164 = Bo6g112670) available in two databases (http://brassicadb.org/brad/ and http://plants.ensembl.org/Brassica_oleracea/Info/Index) did not match each other. Bo6g112680 of TO1000 [56] was annotated as Bol016161, Bol016162, and Bol016163 of O2–12 [57] (Additional file 1: Table S1). Therefore, DNA sequences from three cultivars, kale, broccoli, and cauliflower, were reanalyzed using four primer sets (Additional file 1: Table S1; Additional file 2: Figure S2A). After that, DNA sequences of heading-type cabbages (B. oleracea var. capitate) were cloned and analyzed.
Genomic PCR was performed under the following conditions: denaturation for 5 min at 94 °C, 30 cycles of amplification (30 s at 94 °C, 30 s at 58 °C, and 2–5 min at 72 °C), and a final extension period of 7 min at 72 °C. PCR products were purified using a LaboPass Gel Extraction kit (Cosmogenetech, Korea) and cloned into the TA-vector using the T&A cloning kit (RBC Bioscience Co., Taiwan). Escherichia coli (DH5α) cells were transformed with plasmid DNA carrying the desired insert. Plasmid DNA was purified using DNA-Spin (Intron Biotech. Inc., Korea) before sequencing. As cabbages might contain multiple MYBL2–1 alleles, at least ten clones from each line were sequenced and analyzed. Any possible PCR and/or sequencing errors were eliminated by aligning independent sequences.
Inverse PCR (iPCR)
To clone DNA sequences from purple cabbages that could not be amplified using any of the primer sets (Additional file 1: Table S1), inverse PCR (iPCR) was performed on Bol010163 gene sequences using the Universal GenomeWalker™ 2.0 (Clonetech, USA). DNA from purple cabbages and control DNA from the kit were digested with DraI, EcoRV, PvuII, and StuI for 16–18 h at 37 °C, and purified using the NuceloSpin Gel kit and PCR Clean-Up kit (Macherey-Nagel GmbH & Co, Germany). After ligation with the GenomeWalker adaptor, primary PCR was performed with a Bol010163 gene-specific primer (5’-AGACGTTGATGAGATCAACGGTTGTGA), followed by secondary PCR with an adaptor primer (5’-CATCCAATAAAGGCGAGCAAGAAAGGA). PCR products were electrophoresed on 1% agarose gels, and the resulting bands were excised, purified, and cloned using the T&A cloning kit (RBC Bioscience Co., Taiwan).
Three sizes of DNA fragments were obtained: 700 bp from the PvuII library, 2.1 kbp from the DraI library, and 4.0 kbp from the StuI library. To minimize sequence error, at least five clones from each library were sequenced and analyzed.
RT-PCR
Leaf samples were collected from at least three individual plants, and total RNA was isolated from liquid nitrogen-ground samples using TRIzol reagent (Invitrogen, USA), and further purified using the NucleoSpin RNA Clean-up Kit (Macherey-Nagel GmbH & Co., Germany). Total RNA (1 μg) was treated with RQ1 RNase-free DNAase (Promega, USA), and cDNA was synthesized using the Ace-α kit with oligo(dT) primers (Toyobo, Japan). Complementary DNA was diluted 10-fold, and 1 μl of diluted cDNA was used in a 20 μl PCR mixture. RT-PCR primers are listed in Additional file 3: Table S2; primers for ACTIN 2 (BoACT2) were used as a control. A standard PCR was performed with a 5 min denaturation at 94 °C, followed by 20–30 cycles of amplification (30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C), and a final extension time of 7 min at 72 °C. PCR products were analyzed following electrophoresis through 1.2% agarose gels.
Marker development and validation
To distinguish green and purple cabbages based on their BoMYBL2–1 sequences, three primer sets were designed following sequence alignment using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) (Table 2). The PCR mix consisted of 10–30 ng of genomic DNA, 5 pmol of each forward and reverse primer, 10 pmol of BoACT2 primers, and 1× HiPi Plus PCR premix buffer in 20 μl. To optimize PCR conditions for each primer pair (BoMYBL2–1w-F and BoMYBL2–1w-R; BoMYBL2–1v-F and BoMYBL2–1v-R; BoMYBL2–1sub-F and BoMYBL2–1sub-R), several annealing temperatures, extension times, and cycle numbers were tested and set. For the primer pair BoMYBL2–1w-F and BoMYBL2–1w-R, used to identify the presence of BoMYBL2–1, the PCR conditions were 5 min at 94 °C, followed by 28 cycles of 30 s at 94 °C, 30 s at 65 °C, and 2 min at 72 °C, with a final extension phase of 5 min at 72 °C. For the primer pair BoMYBL2–1v-F and BoMYBL2–1v-R, used to identify promoter-substituted BoMYBL2–1, the reaction conditions were 5 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 60 °C, and 1 min at 72 °C, and a final extension of 5 min at 72 °C. For the primer pair BoMYBL2–1sub-F and BoMYBL2–1sub-R, used to identify DNA with a substitution of the whole BoMYBL2–1 gene, the reaction conditions were 5 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 65 °C, and 2 min at 72 °C, and a final extension of 5 min at 72 °C.
Table 2.
Sequences of marker primers used to distinguish between green and purple cabbages
| Use | Forward primer | Reverse primer | ||
|---|---|---|---|---|
| Name | Sequence | Name | Sequence | |
| Wild-type BoMYBL2–1 (947 bp) Variant BoMYBL2–1 (1148 bp) Substituted BoMYBL2–1 (2075 bp) Actin 2 (144 bp) | BoMYBL2–1w-F | 5’-CTACCAGTCTCTCCTTTGAAGAAGAC | BoMYBL2–1w-R | 5’-GAGTTTTCCTTGATCTCACAGTACATTTCT |
| BoMYBL2–1v-F | 5’-TGTCCACTATCAACAAAAGATGATC | BoMYBL2–1v-R | 5’-CAAACTCACCCGTGCAAATGTACAT | |
| BoMYBL2–1sub-F | 5’-GTTCAAGCTCATTGTGAACGGA | BoMYBL2–1sub-R | 5’-GGACCACCGTGAGAGAGAGA | |
| BoACT2-F | 5’-TACGGTAACATCGTGCTCAGTG | BoACT2-R | 5’-GATCCAGACACTGTACTTCCTC | |
Presence of genes associated with anthocyanin biosynthesis
To examine whether additional genes were defective in purple cabbages, the presence of genes associated with anthocyanin biosynthesis was studied using genomic PCR. The genes selected were transcriptional activators and repressors (Additional file 3: Table S2). PCR conditions were 5 min at 94 °C, followed by 20–30 cycles of 30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C, and a final extension time of 5 min at 72 °C.
Promoter analysis
To confirm whether the substituted promoter found in purple cabbages retained promoter activity, the wild-type and substituted versions of the BoMYBL2–1 promoter were fused to the GUS reporter gene. Arabidopsis plants were transformed with the reporter constructs, and examined for GUS activity and expression. The MYBL2–1 promoter regions from green and purple B. oleracea (1926 bp upstream from the ATG start codon for green B. oleracea and 1804 bp upstream for purple B. oleracea) were amplified by PCR using the specific forward primers (5’-GATCAGGATCCAAGAACACATGAACTT for green cabbage and 5’-GATCAGGATCCAAGAACCAGTGTTC for purple cabbage) and the same reverse primer (5’-GTGAGCCATGGTACGAGAAGCA). These primers contain the BamHI and NcoI restriction sites (underlined).
The amplified fragments were inserted into the T&A cloning vector (Real Biotech Co., Taiwan), and the presence of the MYBL2–1 promoter sequence was confirmed by sequencing. The fragments were liberated by digestion with BamHI and NcoI, and subcloned into the pCambina3301-GUS binary vector, digested with the same enzymes. The resulting constructs were transformed into Arabidopsis plants using the Agrobacterium tumefaciens-mediated floral dip procedure [58]. Transformed plants were selected using 0.1% BASTA herbicide, and their identity was confirmed by PCR analysis of genomic DNA. T3 homozygous lines containing a T-DNA insertion at a single locus, determined by a 3:1 ratio of segregation of basta-resistance: sensitivity, were selected for the assay of promoter activity.
Arabidopsis thaliana wild-type (Col-0) and transgenic plants were grown in a growth chamber under 16 h light/8 h dark photoperiods at 22 °C and a light intensity of 100 μmol m− 2 s− 1. For plate culture, seeds were surface-sterilized with 30% bleach containing 0.1% Triton X-100, stratified for 3 days at 4 °C, and plated onto solidified half-strength Murashige and Skoog (MS) medium, plus or minus 90 mM sucrose. Three plants were sampled for RT-PCR study of GUS expression, and another three plants were used to analyze GUS expression.
For GUS expression studies, plants were incubated in GUS staining solution (1 mM X-GlucA in 100 mM sodium phosphate, pH 7.0, containing 5 mM Na2EDTA, 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, and 0.1% Triton X-100) for 16 h at 37 °C. After staining, the plants were washed in 95% ethanol at room temperature until wild-type (Col-0) Arabidopsis appeared clear.
Quantification of total anthocyanin content
Total anthocyanin content was determined in cabbage leaves using methanol containing 1% HCl [59]; three independent biological replicates from three individual plants were used in each experiment. The tissues were ground under liquid nitrogen, and the powder was resuspended in a 1.5 ml tube containing methanol (1% HCl) at room temperature and centrifuged at 14,000 rpm for 10 min at 4 °C. The absorbance of the supernatants was determined spectrophotometrically at 530 nm and 657 nm. Total anthocyanin content was quantified as
where Q = total anthocyanins; A530 = absorption at 530 nm; A657 = absorption at 657 nm; FW = fresh weight of tissues (g).
Results
Selection of BoMYBL2–1 gene
To dissect the association of BoMYBL2 with anthocyanin biosynthesis, expression of selected key genes in the pathway was examined in six inbred lines of green cabbage (337, 154, 2477, 09WH-45, 2409, and 842) and two inbred lines of purple cabbage (7S4–51 and 7S4–63). Two-month-old cabbage plants were grown for a month, either under greenhouse (G) conditions, which were non-inductive for anthocyanin accumulation, or outside (C), which was inductive for anthocyanin accumulation because it exposed the plants to low temperature (Fig. 1).
Fig. 1.
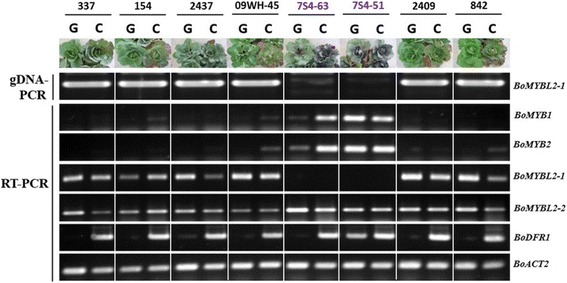
Analysis of anthocyanin biosynthesis-related genes from cabbage plants grown either in a greenhouse (G) or exposed to low temperature conditions outside (C). Plants were grown for 1 month between Oct. 13 and Nov. 12, 2014. Average temperatures ranged between 20 and 30 °C in the greenhouse and between 6 and 18 °C outside
Expression of BoMYB1 (Bol042409 = Bo3g081880), BoMYB2 (Bol012528 = Bo6g100940), BoDFR1 (Bol035269 = Bo9g058630), BoMYBL2–1 (Bol016164 = Bo6g112670), and BoMYBL2–2 (Bol034966 = Bo2g070770) was analyzed. Two BoMYBL2 genes (one on chromosome 6 and the other on chromosome 2) matched Arabidopsis MYBL2 (At1G71030), and BoMYBL2 designations were given according to the level of amino acid sequence identity with the Arabidopsis counterpart. As shown in Fig. 1, the expression of BoDFR1 correlated with anthocyanin accumulation (Additional file 4: Figure S1). BoMYB1 and 2 were highly expressed in purple cabbages, whereas BoMYBL2–2 expression was detected in all samples. BoMYBL2–1 expression was not detected in purple cabbages, and attempts to amplify the gene by PCR from the genomic DNA of purple cabbages failed, suggesting either a high level of sequence variation or deletion of BoMYBL2–1.
To investigate whether BoMYBL2–1 had been deleted from the genome of purple cabbages, we examined the presence and expression of the gene in additional cultivars (Fig. 2). Both the BoMYBL2–1 gene and its transcripts were detected in all the green cabbages tested. Two different types of result were obtained from two purple cabbages: (1) no amplification product from either genomic DNA or cDNA templates; and (2) amplification product from genomic DNA but not from cDNA. More specifically, the BoMYBL2–1 transcript could not be detected in the purple B cultivar, although presence of the gene was confirmed from analysis of genomic DNA. These data suggest that two distinct mechanisms for the loss of BoMYBL2–1 expression were responsible for the color of the two purple cabbages tested.
Fig. 2.
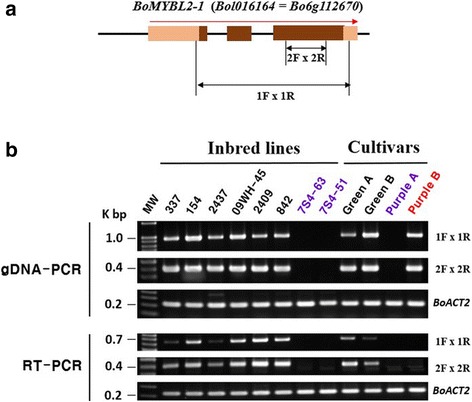
Presence and expression of BoMYBL2–1 in various lines of cabbage. a: Genomic organization of BoMYBL2–1 genes and positions of the primers used in PCR (1F, 2F, 2R, and 1R). b: Results of genomic DNA-PCR and RT-PCR
Cloning and sequence analysis of BoMYBL2–1 from various B. oleracea species
Genomic cloning of BoMYBL2–1 was undertaken using six primer pairs (Additional file 2: Figure S2; Additional file 1: Table S1) and eight inbred lines of cabbages obtained from the Asia Seed Co. (Korea). An initial comparison of PCR results revealed that high levels of sequence variation were present around BoMYBL2–1, as observed in the distinct reference sequences previously obtained from two different varieties of B. oleracea [56, 57] (Additional file 1: Table S1). We therefore re-established a sequence assembly for kale (B. oleracea var. sabellica), broccoli (B. oleracea var. italica), and cauliflower (B. oleracea var. botrytis) cultivars using one allele from each variety (Additional file 5). Based on this new sequence information, sequencing and analysis were extended to inbred lines and cultivars of green cabbages, as well as purple cabbages (B. oleracea var. capitata) (Additional file 5). Nonetheless, no PCR product could be amplified from several lines of purple cabbages, including 7S4–51, 7S4–63, and B98. We used iPCR with primers designed around Bol016163 to obtain sequence information for these plants.
The simplified structure of the BoMYBL2–1 genes and upstream nucleotide sequences from all of the lines used in our analysis (Additional file 5), which confirmed high levels of sequence variation in this region, are shown in Fig. 3. Kale and cauliflower were both heterozygous for Bol016163 alleles, one with complete and the other with deleted versions, while broccoli appeared to have homozygous alleles. All heading-type cabbages and two purple cabbages (Purple B and B90) contained the deleted version of Bol016163; the other purple cabbages contained a complete version of Bol016163.
Fig. 3.
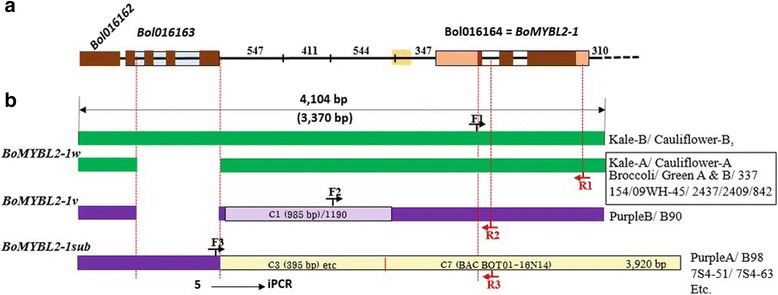
Schematic representation of BoMYML2–1 genomic DNA from various B. oleracea plants showing the positions of the molecular markers. a: Schematic representation of the BoMYBL2–1 genomic sequence based on the BRAD database (http://brassicadb.org/brad; Liu et al. 2014). The yellow block upstream of BoMYBL2–1 represents a 159 bp repeat sequence. b: Schematic representation of BoMYBL2–1 genomic DNA sequences identified in this study. iPCR indicates sequences obtained using inverse-PCR. BoMYBL2–1w, BoMYBL2–1v, and BoMYBL2–1sub represent the wild-type version, the BoMYBL2–1 variant, and the BoMYBL2–1 substitution, respectively. F1, F2, F3: forward primers 1, 2, 3; R1, R2, R3: reverse primers (see Table 2)
As shown in Fig. 3, all heading-type green cabbages carried BoMYBL2–1 (1208 bp) plus approximately 1900 bp of upstream sequences. By contrast, two types of DNA sequence were observed in purple cabbages: BoMYBL2–1 with a substituted promoter containing 1190 bp of sequence, also found on chromosome 1, 347 bp upstream of the start codon (called BoMYBL2–1 variant or BoMYBL2–1v), and a substitution (or deletion) type of the entire BoMYBL2–1 plus regulatory regions with putative chromosome 3 and chromosome 7 (called BoMYBL2–1 substitution or BoMYBL2–1sub). Most purple cabbages contained BoMYBL2–1sub. To distinguish the wild-type or intact BoMYBL2–1 sequence from BoMYBL2–1v, the wild-type version was designated BoMYBL2–1w. Interestingly, both BoMYBL2–1w and BoMYBL2–1v contained 159 bp repeat sequences upstream of the ATG start codon, which included seven ACCCGA repeats, 11 CGAA repeats, seven AAAT repeats, and so on. The MYB core motif GGATA [55] was detected in this repeat.
Promoter analysis
To test whether the promoter region of BoMYBL2–1v had any transcriptional activity, 1926 bp from BoMYBL2–1w and 1804 bp from BoMYBL2–1v upstream of the ATG start codon were fused to the GUS reporter gene and the constructs were used to transform Arabidopsis plants. ß-glucuronidase (GUS) activity in Arabidopsis transgenic plants was evaluated using both histochemical staining and levels of expression of GUS transcript (Figs. 4 and 5). Two independent pCambia3301 transgenic plants were used as positive controls. Comparable levels of GUS staining (Fig. 4a) and GUS transcript expression (Fig. 4b) were shown by seven independent pMYBL2–1w::GUS transgenic plants. On the other hand, neither GUS staining nor GUS transcripts were detected in wild-type Arabidopsis (Col-0) or in seven independent pMYBL2–1v::GUS transgenic plants. These results indicated that the pMYBL2–1v promoter was non-functional under normal growth conditions.
Fig. 4.
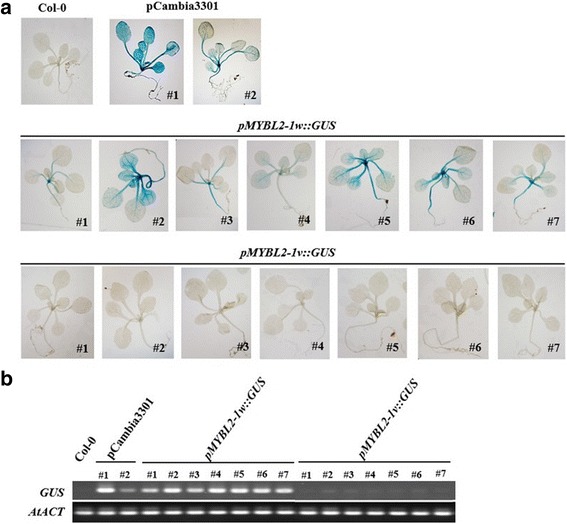
Expression analysis of BoMYBL2–1 reporter constructs in transgenic Arabidopsis. Arabidopsis plants were transformed with reporter constructs containing GUS under the control of two different BoMYBL2–1 promoters: pMYBL2–1-w::GUS and pMYBL2–1v::GUS. a: Histochemical analysis of 10-day-old transgenic Arabidopsis seedlings. Seedlings were stained for 18 h at 37 °C using X-Gluc (Sigma–Aldrich, St. Louis, MO, USA) and washed in 95% ethanol. Non-transgenic plants (Col-0) and pCambia3301 were used as negative and positive controls for GUS expression, respectively. b: Expression analysis of GUS transcripts in transgenic and control plants. ACTIN (AtACT) was used as a normalization control
Fig. 5.
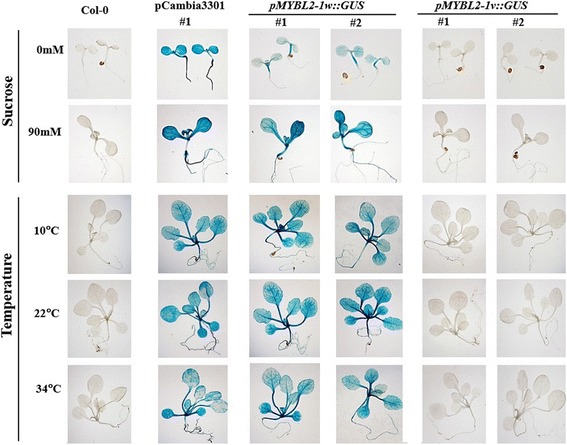
GUS expression in transgenic Arabidopsis under conditions of sucrose or temperature stress. For sucrose stress, Col-0 and mutant seedlings were grown in 0.5× MS medium without sucrose (0 mM) or with 90 mM sucrose. Arabidopsis seedlings were grown for 10 days after sowing at a light intensity of 100 μmol− 2 s− 1 under long-day conditions. For temperature stress, Col-0 and mutant plants were grown on soil for 12 days at 22 °C with light intensity of 100 μmol− 2 s− 1 under long-day conditions. They were transferred to incubators at either 10 °C (low temperature) or 34 °C (high temperature) with a light intensity of 100 μmol− 2 s− 1 and incubated for 19 h. GUS staining was as described in Fig. 4
Since anthocyanin biosynthesis is induced by various environmental factors, GUS expression in transgenic Arabidopsis plants was also tested under conditions of sucrose and temperature (low and high temperature) stress. As shown in Fig. 6, GUS staining was not detectible in pMYBL2–1v::GUS transgenic plants, even when the plants were subjected to the stress of 90 mM sucrose or exposure to high or low temperature. Taken together, these data strongly suggest that the promoter substitution found in BoMYBL2–1v resulted in the loss of promoter activity and BoMYBL2–1 expression in some types of purple cabbages. In others, the change of color from green to purple may be explained by the complete deletion of the BoMYBL2–1 coding sequence.
Fig. 6.
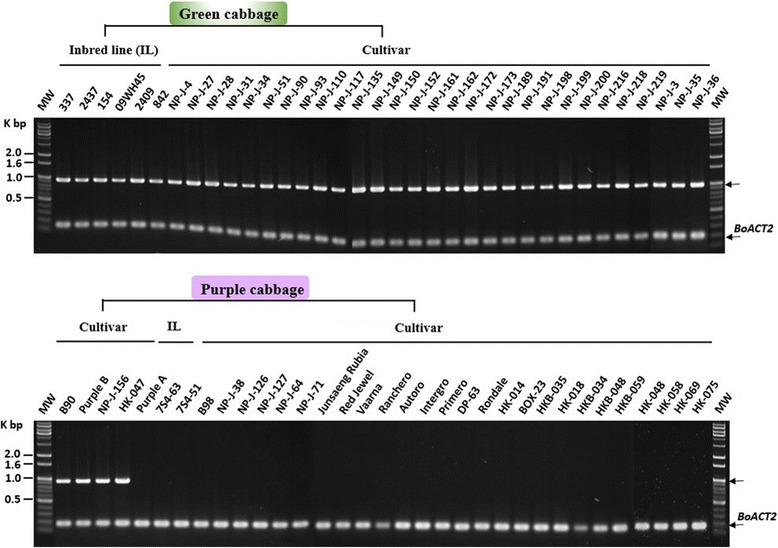
PCR analysis using the F1 and R1 primer pair to identify the presence of the complete coding region of BoMYBL2–1. Arrow indicates bands corresponding to MYBL2–1. Only four purple cabbages contain the coding region
Confirmation of a unique deletion of BoMYBL2–1 in purple cabbages
To determine whether the color change to purple in B. oleracea var. capitata f rubra is attributable to the loss of other important genes regulating anthocyanin biosynthesis, we investigated the presence of regulatory genes, including BoMYBL2–1, associated with anthocyanin biosynthesis. BoMYBD, BoSPL9, BoMYBH, and BoMYBL2–1 are negative regulators of anthocyanin biosynthesis, while BoMYB1–4, BoMYB11, and BoMYB12 are transcriptional activators. BoANL (Bo9g002480/Bol011495) is a homeobox-leucine zipper gene equivalent to ANTHOCYANINLESS 2 (AT4G00730).
As shown in Additional file 6: Figure S3, all these genes other than BoMYBL2–1, which was lost in several lines of purple cabbage, were present in various varieties of B. oleracea. This strongly suggested that, at least for some purple cabbages (B. oleracea var. capitata f, rubra), the deletion of BoMYBL2–1 was responsible for the development of purple coloration.
Since the presence of a gene does not guarantee its expression, expression of key biosynthetic (BoCHS1, BoCHS2, BoF3H, BoF3’H, and BoDFR1) and regulatory genes affecting anthocyanin accumulation was examined in 20 different purple cabbage (Additional file 6: Figure S3). Transcript levels of most genes did not differ between these purple cabbages, but expression levels of several genes (BoCHS1, BoF3H, BoDFR1, and BoMYB2) were higher in those plants than in the green cabbage Daebakna. All the genes tested, other than BoMYB4, were expressed at relatively high levels, possibly as a result of defective BoMYBL2–1 activity.
And validation of molecular markers to distinguish purple cabbage
Markers that are associated with a particular horticultural trait, such as purple coloration, are very important in developing new B. oleracea varieties with the desired trait from crosses of different subspecies. To develop molecular markers able to identify purple B. oleracea var. capitata carrying defective BoMYBL2–1, we designed three sets of primer pairs: (1) to amplify the BoMYBL2–1 coding sequence; (2) to amplify the promoter-substituted variant BoMYBL2–1 (MYBL2v); and (3) to amplify the DNA sequence that replaced the entire BoMYBL2–1 gene (BoMYBL2–1sub) (Table 2; Fig. 3). The optimal PCR conditions for each pair are described above (Methods). The validity of these primer pairs was tested using the 35 green and 33 purple cabbages listed in Table 1.
As shown in Fig. 6, the band corresponding to BoMYBL2–1 coding sequence was amplified by the F1 and R1 primers (BoMYBL2w-F and BoMYBL2w-R) from all the green cabbages tested, as well as from four purple cabbages (B90, Purple B, NP-J-156, and-047). All of the purple cabbages tested, however, other than B90, contained DNA that could be amplified using the F3 and R3 primers (BoMYBL2sub-F and BoMYBL2sub-R) (Fig. 7), implying that these plants contained the substituted version of the entire BoMYBL2–1 gene, including the promoter and coding regions. In addition, only four purple cabbages contained DNA that could be amplified using the F2 and R2 primers (BoMYBL2v-F and BoMYBL2v-R), which were specific for the promoter-substituted BoMYBL2–1 variant (BoMYBL2–1v) (Fig. 8). Taken together, we concluded that purple cabbages contain either BoMYBL2–1v or BoMYBL2–1sub or both. Our data indicated that most of the purple cabbages in this study were homozygous for BoMYBL2–1sub, although B90 was homozygous for BoMYBL2–1v. Purple B, NP-J-0156, and HK-047 were heterozygous, carrying both the BoMYBL2–1v and BoMYBL2–1sub alleles.
Fig. 7.
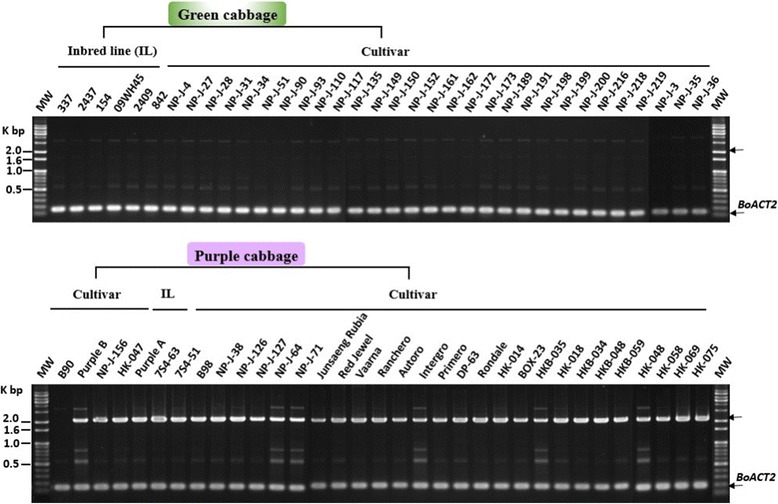
PCR analysis using the F3 and R3 primer pair to amplify the substituted region of BoMYBL2–1 (upper arrow). All purple cabbages other than B90 (left hand side) contain the substituted DNA region
Fig. 8.
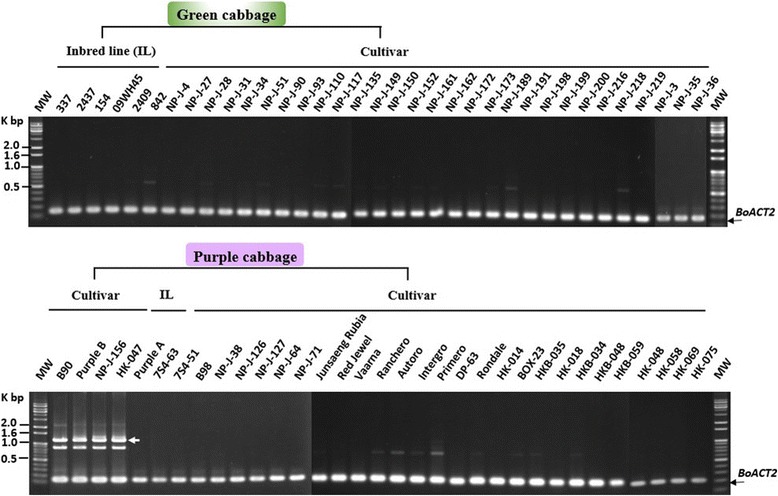
Results of PCR analysis using the F2 and R2 primer pair (BoMYBL2v-F and BoMYBL2v-R) to amplify BoMYBL2–1v, which contains the substituted region of the promoter. The white arrow indicates the position of the predicted PCR product. Three purple cabbages (Purple B, NP-J-156, and HK-047) were heterozygous (BoMYBL2–1v/BoMYBL2–1sub), but B90 was homozygous for BoMYBL2–1v. All other purple cabbages were homozygous for BoMYBL2–1sub
Discussion
Purple or red color in Brassica species due to anthocyanin accumulation is usually related to the activation of anthocyanin biosynthesis genes and/or their transcriptional activators. Purple or red color in B. oleracea is associated with an increase in expression of transcriptional activators, such as BoMYB2 and BoTT8 (BobHLH) [44–46], and BoPAP1 and BoPAP2 [47, 48]. To date, only two studies have reported natural mutations that lead to an increase in anthocyanin biosynthesis: mutation of the BoMYB2 promoter to activate its expression in purple cauliflower [44], and deletion of the DFR gene in purple ornamental kale [55]. There is, however, no information available on the origin of red or purple cabbage (Brassica oleracea L. var. capitate f, rubra), a native of the Mediterranean region of Europe that is now grown as a fresh market vegetable all over the world [46]. The present study found that purple color in heading-type cabbages resulted from defective expression of BoMYBL2–1 caused by two different mechanisms: either a substitution in the BoMYBL2–1 promoter or a deletion of the entire gene. This is the first report showing that purple plants can be spontaneously generated by a defective repressor, rather than by alteration of activator expression by transposon insertion.
MYBL2, a small MYB protein that has two functional transcriptional repressor motifs, EAR and TLLLER motifs, interacts with TT8, a bHLH protein that is a component of the MBW complex, and represses expression of anthocyanin biosynthesis genes (LBGs) [24, 29, 33, 35–37]. MBW complexes control the flavonoid biosynthesis pathway at the transcriptional level developmentally and environmentally, mainly by activating expression of flavonoid LBGs [29]. MYBL2 and other R2R3-MYBs interact with bHLH proteins in a competitive manner; thus other R2R3-MYBs prevent the formation of the MBW complex and so negatively regulate anthocyanin production [24, 28, 35]. Repression or sequestering of MYBL2 activates anthocyanin biosynthesis in Arabidopsis [41–43].
The B. oleracea var. capitata genome contains two MYBL2 genes, BoMYBL2–1 and BoMYBL2–2; however, only BoMYBL2–1 appears to be closely associated with anthocyanin production in cabbages, as a decrease in BoMYBL2–1 expression was observed in some cultivars of purple cabbages (Fig. 1). These expression data, together with studies of BoMYBL2–1 promoter activity in transgenic Arabidopsis (Figs. 5 and 6), supported the conclusion that the absence of BoMYBL2–1 expression, as a result of either promoter substitution or whole gene deletion, was responsible for the production of purple color in cabbage. However, we cannot rule out the possibility that other negative regulators are also involved in the generation of other purple cabbages not included in the current study, as has been observed in poplar [60, 61].
The loss of promoter function in the substituted promoter of BoMYBL2–1 present in several purple cabbage cultivars was supported by an analysis of GUS expression in transgenic Arabidopsis (Figs. 5 and 6). As shown in Fig. 9, the BoMYBL2–1w promoter harbors three types of MYB binding motifs: one MYB core motif (GGATA) [59], two MYB recognition sequences (A/TAACCA), and two MYB recognition sequences (C/TAACG/TG) [62]. By contrast, the upstream sequences of the BoMYBL2–1v coding sequence found in two purple cabbages contain only one MYB core motif, which is located in a 159 bp repeat sequence. This implies that one MYB core motif alone is not sufficient for the initiation of BoMYBL2–1v expression, at least in B. oleracea and Arabidopsis. It is not yet clear when this allele was first introduced to B. oleracea and whether the loss of BoMYBL2–1 has any adaptive value.
Fig. 9.
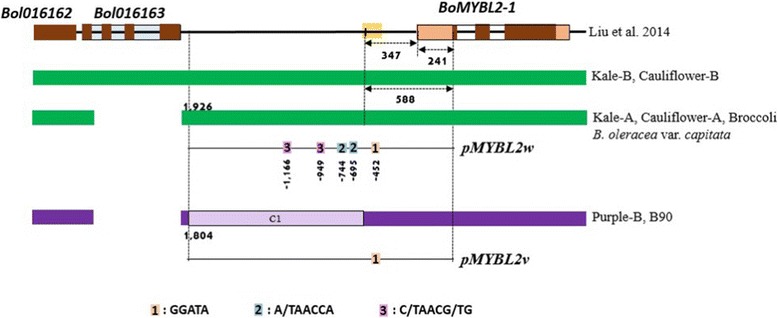
Schematic representation comparing the promoter sequences and cis-elements in BoMYBL2w and BoMYBL2v. The BoMYBL2w promoter harbors one MYB core motif GGATA (1; Baranowskij et al. 1994), two MYB recognition sequences (2; A/TAACCA), and two MYB recognition sequences (3; C/TAACG/TG; Abe et al. 2003). The upstream sequence of BoMYBL2v contains only one MYB core motif
We detected high levels of sequence variation around BoMYBL2–1 and the surrounding region (Fig. 3; Additional file 7: Figure S5). This may be due to genome assembly errors in the ‘02–12’ reference genome of cabbage (http://brassicadb.org) [57] and the TO1000 sequence (http://plants.ensembl.org/Brassica_oleracea) [57]. Similar assembly errors in cabbage reference genomes have been identified and corrected for mapping of clubroot resistance [63] and yellow-green leaf trait [64]. We provide the sequences flanking BoMYBL2–1 in chromosome 6 obtained in this study, which suggest that Bol016163 is absent from B. oleracea var. capitata (Additional file 5; Additional file 7: Figure S5).
As shown in Additional file 6: Figure S3 and Additional file 8: Figure S4, it is clear that all purple cabbages (B. oleracea var. capitata f, rubra) retain most of the genes associated with anthocyanin biosynthesis and regulation. Most studies of the development of purple or red coloration in crops suggest that it results from activation of anthocyanin biosynthesis genes and/or regulatory genes, and is largely due to mutation, especially mutations within a promoter that confer constitutive expression [44, 49–55]. The present study demonstrates, however, that mutation within repressor genes provides another route to the establishment of anthocyanin-enriched plants in nature, and suggests that similar types of mutation may be present in crops with high anthocyanin content.
We developed a method for using PCR-based molecular markers that could quickly identify and distinguish purple cabbages that contain a substituted promoter or a complete deletion of BoMYBL2–1 (Figs. 6, 7 and 8). These markers will facilitate the intraspecific and interspecific breeding of B. oleracea to produce anthocyanin-rich crops. Unfortunately, these markers do not predict the anthocyanin levels or profiles of different purple cabbages, even though a previous study reported genotype-dependent variations in anthocyanin profiles [22]. A more detailed understanding of anthocyanin biosynthesis in cabbages is required to develop additional markers to select purple-colored cabbages possessing more desirable anthocyanin profiles.
Conclusion
B. oleracea var. capitata plants possess two MYBL2 genes: BoMYBL2–1 and BoMYBL2–2. Expression levels of BoMYBL2–1 are inversely correlated to anthocyanin content, particularly in purple cabbage cultivars with defective expression of BoMYBL2–1. We found that most purple cabbages do not have an entire BoMYBL2–1 gene, due to deletion of the coding sequence and the substitution of the regulatory region with other DNA sequences, while others contain a version of the BoMYBL2–1 promoter that lacks transcription activity. We used sequence variations of BoMYBL2–1 to develop and validate molecular markers that distinguish purple cabbage cultivars with defective BoMYBL2–1. These markers will be useful in the production of intraspecific and interspecific hybrids that contain high anthocyanin levels useful for functional foods and industrial use.
Additional files
Table S1. Primers used to clone BoMYBL2. (DOCX 19 kb)
Figure S2. Schematic illustration of the strategy used to clone BoMYBL2–1 from various B. oleracea species. A: Genomic region around the BoMYBL2–1 based on information obtained from two different databases (upper: http://brassicadb.org/brad; Liu et al. 2014; lower: http://plants.ensembl.org/Brassica_oleracea/Info/Index; Parkin et al. 2014). B: Illustration showing the positions of the fragments, amplified using different combinations of primer sets, used to assemble the entire promoter and coding sequence of BoMYBL2–1. The yellow block represents a 159 bp repeat sequence. All primer sets are listed in Table 2. BoLPR2 is B. oleracea multicopper oxidase LPR2; BoPUB10 is B. oleracea U-box domain-containing protein 10. (DOCX 72 kb)
Table S2. List of primer sequences used in RT-PCR and genomic PCR analyses of BoMYBL2. (DOCX 32 kb)
Figure S1. Total anthocyanin content (A) of samples of the cabbages (B) shown in Fig. 1. (DOCX 109 kb)
DNA sequences of BoMYBL2–1 plus the front region or corresponding region of the gene. Gene names represented plant names descirbed in Table 1. TO1000 indicates reference sequence (Parkins et al. 2014). (TXT 86 kb)
Figure S3. Results of genomic DNA-PCR preformed to detect the presence or absence of genes regulating anthocyanin biosynthesis other than BoMYBL2–1. A: B. oleracea var. capitata F. alba or rubra varieties with contrasting characteristics in anthocyanin accumulation were selected for analysis; varieties with green and purple colors are indicated in green or purple. B: PCR analysis of other varieties of B. oleracea. The color of each variety is indicated above each lane. (DOCX 293 kb)
Figure S5. Comparison of different BoMYBL2–1 nucleotide sequences obtained from cabbages. Shaded regions indicate exon sequences. Sequences corresponding to Bol016162 from B. oleracea var. capitata were omitted. (DOCX 31 kb)
Figure S4. Expression of genes associated with anthocyanin biosynthesis in various purple cabbages. Daebakna is a green cabbage used as a reference. (DOCX 387 kb)
Funding
This research work was financially supported by the Golden Seed Project (Center for Horticultural Seed Development, Grant no. 213007–05-2-SB620) of the Ministry of Agriculture, Food and Rural affairs in the Republic of Korea (MAFRA).
Abbreviations
- ANR
Athocyanidin reductase
- bHLH
Basic helix-loop-helix
- bHLH042
TT8
- BIN2
BRASSOSTERROIS INSENSITIVE 2
- BoMYBH
MYBD homolog
- CHI
Chalcone isomerase
- CHS
Chalcone synthase
- CHS
Chalcone synthase (TT4)
- DFR
Dihydorflavonol 4-reductase (TT3)
- DFR
Dihydroflavonol 4-reductase
- EBGs
Early biosynthesis genes
- EGL3
Enhancer of glabra 3
- EGL3
ENHANCER OF GLABRA3/AtMYC-2 MYB123 (TT2)
- F3’H
Flavanone 3′-hydroxylase (TT7)
- F3’H
Flavonoid 3′-hydroxylase
- F3H
Flavanone 3-hydorxylase/TT6
- F3H
Flavanone 3-hydroxylase
- FLS
Flavono synthase
- GL1
GLABROUS1
- GL3
Glabra3
- GL3
GLABROUS3
- GST
Glutathione S-transferase
- GUS
ß-glucuronidase
- iPCR
Inverse PCR
- LBGs
Late biosynthetic genes
- LDOX
Leucoanthocyanidin dioxygenase
- MBW
MYB-bHLH-WD40 complex
- MYB12
PRODUCTION OF FLAVONOL GLYCOSIDES 1
- MYBL2
MYB-like 2
- MYN11
SQUAMOSA PROMOTER BINDING PROTEIN-LIKE9
- PAP1
Production of anthocyanin pigment 1
- PAP2
Production of anthocyanin pigment 1
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SPL
SQUAMOSA PROMOTER BINDING PROTEIN-LIKE9
- TT2
TRANSPARENT TESTA 2
- TT8
Transparent testa 8
- TTG1
Transparent testa glabra 1
- UD3GT
UDP-glucose: flavonoid 3-O-glucosyltransferase,
- UFGT
UDP-flavonoid glucosyl transferase
Authors’ contributions
HS, JP and YH designed the experiments. HS, ML, JL and SK performed the experiments. HK,IN and YH analyzed the data. HY, CH, IN and YH wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12870-018-1290-9) contains supplementary material, which is available to authorized users.
References
- 1.Chandler S, Tanaka Y. Genetic modification in floriculture. Crit Rev Plant Sci. 2007;26:169–197. doi: 10.1080/07352680701429381. [DOI] [Google Scholar]
- 2.Chandler SF, Brugliera F. Genetic modification in floriculture. Biotechnol Lett. 2011;33:207–214. doi: 10.1007/s10529-010-0424-4. [DOI] [PubMed] [Google Scholar]
- 3.Wessinger CA, Rausher MD. Lessons from flower colour evolution on targets of selection. J Exp Bot. 2012;63:5741–5749. doi: 10.1093/jxb/ers267. [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2013;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- 5.Lorenc-Kukuła K, Jafra S, Oszmiański J, Szopa J. Ectopic expression of anthocyanin 5-o-glucosyltransferase in potato tuber causes increased resistance to bacteria. J Agric Food Chem. 2005;53:272–281. doi: 10.1021/jf048449p. [DOI] [PubMed] [Google Scholar]
- 6.Castellarin SD, Pfeiffer A, Sivilotti P, Degan M, Peterlunger E, DI Gaspero G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007;30:1381–1399. doi: 10.1111/j.1365-3040.2007.01716.x. [DOI] [PubMed] [Google Scholar]
- 7.Buer CS, Imin N, Djordjevic MA. Flavonoids: new roles for old molecules. J Integr Plant Biol. 2010;52:98–111. doi: 10.1111/j.1744-7909.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- 8.Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol. 2002;5:218–223. doi: 10.1016/S1369-5266(02)00256-X. [DOI] [PubMed] [Google Scholar]
- 9.He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- 10.Bártiková H, Skálová L, Dršata J, Boušová I. Interaction of anthocyanin with drug-metabolizing and antioxidant enzymes. Curr Med Chem. 2013;20:4665–4679. doi: 10.2174/09298673113209990153. [DOI] [PubMed] [Google Scholar]
- 11.Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014;77:367–379. doi: 10.1111/tpj.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Zhao L, Wang D, Huo Y, Ji B. Anthocyanin-rich extracts from blackberry, wild blueberry, strawberry, and chokeberry: antioxidant activity and inhibitory effect on oleic acid-induced hepatic steatosis in vitro. J Sci Food Agric. 2015;96:2494–2503. doi: 10.1002/jsfa.7370. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr. 2007;16:200–208. [PubMed] [Google Scholar]
- 14.Shim SH, Kim JM, Choi CY, Kim CY, Park KH. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J Med Food. 2012;15:818–823. doi: 10.1089/jmf.2012.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannum SM. Potential impact of strawberries on human health: a review of the science. Crit Rev Food Sci Nutr. 2004;44:1–17. doi: 10.1080/10408690490263756. [DOI] [PubMed] [Google Scholar]
- 16.de Pascual-Teresa S, Moreno DA, Garcia-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11:1679–1703. doi: 10.3390/ijms11041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang PC, Kuo WW, Shen CY, Chen YF, Lin YM, Ho TJ, Padma VV, Lo JF, Huang CY, Huang CY. Anthocyanin attenuates doxorubicin-induced cardiomyotoxicity via estrogen receptor-α/β and stabilizes HSF1 to inhibit the IGF-IIR apoptotic pathway. Int J Mol Sci. 2016;17(9):1588. [DOI] [PMC free article] [PubMed]
- 18.Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. 2015;145:742–748. doi: 10.3945/jn.114.205674. [DOI] [PubMed] [Google Scholar]
- 19.Cerletti C, de Curtis A, Bracone F, Digesù C, Morganti AG, Iacoviello L, de Gaetano G, Donati MB. Dietary anthocyanins and health: data from FLORA and ATHENA EU projects. Br J Clin Pharmacol. 2017;83:103–106. doi: 10.1111/bcp.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Xu J, Liu Y, Yu X, Tang X, Wang Z, Li X. Anthocyanins potentiate the activity of trastuzumab in human epidermal growth factor receptor 2-positive breast cancer cells in vitro and in vivo. Mol Med Rep. 2014;10:1921–1926. doi: 10.3892/mmr.2014.2414. [DOI] [PubMed] [Google Scholar]
- 21.Lin BW, Gong CC, Song HF, Cui YY. Effect of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol. 2017;174:1226–1243. doi: 10.1111/bph.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiczkowski W, Topolska J, Honke J. Anthocyanins profile and antioxidant capacity of red cabbages are influenced by geneotype and vegetation period. J Funct Foods. 2014;7:201–211. doi: 10.1016/j.jff.2014.02.011. [DOI] [Google Scholar]
- 23.Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M. Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol. 2006;57:405–430. doi: 10.1146/annurev.arplant.57.032905.105252. [DOI] [PubMed] [Google Scholar]
- 24.Dubos C, Gourrierec JL, Baudry A, Huep G, Lanet E, Debeaujon I, Routaboul JM, Alboresi A, Weisshaar B, Lepiniec L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J. 2008;55:940–953. doi: 10.1111/j.1365-313X.2008.03564.x. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J. 2004;40:22–34. doi: 10.1111/j.1365-313X.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- 26.Mehrtens F, Kranz H, Bednarek P, Weisshaar B. The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 2005;138:1083–1096. doi: 10.1104/pp.104.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007;50:660–677. doi: 10.1111/j.1365-313X.2007.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S. Transcriptional control of flavonoid biosynthesis: fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal Behav. 2014;9:e27522. doi: 10.4161/psb.27522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu W, Dubos C, Lepiniec L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trend Plant Sci. 2015;20:176–185. doi: 10.1016/j.tplants.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Debeaujon I, Nesi N, Perez P, Devic M, Grandjean O, Caboche M, Lepiniec L. Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development. Plant Cell. 2003;15:2514–2531. doi: 10.1105/tpc.014043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanindin biosynthesis in Arabidopsis thaliana. Plant J. 2004;39:366–380. doi: 10.1111/j.1365-313X.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- 32.Xie Y, Tan H, Ma Z, Huang J. DELLA proteins promote anthocyanin biosynthesis via sequestering MYBL2 and JAZ suppressors of the MYB/bHLH/WD40 complex in Arabidopsis thaliana. Mol Plant. 2016;9:711–721. doi: 10.1016/j.molp.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Yanhui C, Xiaoyuan Y, Kun H, Meihua L, Jigang L, Zhaofeng G, Zhiqiang L, Yunfei Z, Xiaoxiao W, Xiaoming Q, Yunping S, Li Z, Xiaohui D, Jingchu L, Xing-Wang D, Zhangliang C, Hongya G, Li-Jia Q. The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol Biol. 2006;60:107–124. doi: 10.1007/s11103-005-2910-y. [DOI] [PubMed] [Google Scholar]
- 34.Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell. 2001;13:1959–1968. doi: 10.1105/tpc.13.8.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui K, Umemura Y, Ohme-Takagi M. AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J. 2008;55:954–967. doi: 10.1111/j.1365-313X.2008.03565.x. [DOI] [PubMed] [Google Scholar]
- 36.Kagale S, Rozwadowski K. Small yet effective: the ethylene responsive element binding factor-associated amphiphilic repression (EAR) motif. Plant Signal Behav. 2010;5:691–694. doi: 10.4161/psb.5.6.11576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kagale S, Rozwadowski K. EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics. 2011;6:141–146. doi: 10.4161/epi.6.2.13627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albert NW, Davies KM, Lewis DH, Zhang H, Montefiori M, Brendolise C, Boase MR, Ngo H, Jameson PE, Schwinn KE. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell. 2014;26:962–980. doi: 10.1105/tpc.113.122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye H, Li L, Guo H, Yin Y. MYBL2 is a substrate of GSK3-like kinase BIN2 and acts as a corepressor of BES1 in brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci U S A. 2012;109:20142–20147. doi: 10.1073/pnas.1205232109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Chen G, Dong T, Pan Y, Zhao Z, Tian S, Hu Z. Anthocyanin accumulation and transcriptional regulation of anthocyanin biosynthesis in purple box choy (Brassica rapa var. chinensis) J Agric Food Chem. 2014;62:12366–12376. doi: 10.1021/jf503453e. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen NH, Jeong CY, Kang GH, Yoo SD, Hong SW, Lee H. MYBD employed by HY5 increases anthocyanin accumulation via repression of MYBL2 in Arabidopsis. Plant J. 2015;84:1192–1205. doi: 10.1111/tpj.13077. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Wang Y, Song Z, Zhang H. Repression of MYBL2 by both microRNA858a and HY5 leads to the activation of anthocyanin biosynthetic pathway in Arabidopsis. Mol Plant. 2016;9:1395–1405. doi: 10.1016/j.molp.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Xie Q, Hu Z, Zhang Y, Taian S, Wang Z, Zhao Z, Yang Y, Chen G. Accumulation and molecular regulation of anthocyanin in purple tumorous stem mustard (Brassica juncea var. tumida Tsen et lee) J Agric Food Chem. 2014;62:7813–7821. doi: 10.1021/jf501790a. [DOI] [PubMed] [Google Scholar]
- 44.Chiu LW, Zhou X, Burke S, Wu X, Prior RL, Li L. The purple cauliflower arises from activation of a MYB transcription factor. Plant Physiol. 2010;154:1470–1480. doi: 10.1104/pp.110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiu LW, Li L. Characterization of the regulatory network of BoMYB2 in controlling anthocyanin biosynthesis in purple cauliflower. Planta. 2012;236:1153–1164. doi: 10.1007/s00425-012-1665-3. [DOI] [PubMed] [Google Scholar]
- 46.Yuan Y, Chiu LW, Li L. Transcriptional regulation of anthocyanin biosynthesis in red cabbage. Planta. 2009;230:1141–1153. doi: 10.1007/s00425-009-1013-4. [DOI] [PubMed] [Google Scholar]
- 47.Zhang B, Hu Z, Zhang Y, Li Y, Zhou S, Chen G. A putative functional MYB transcription factor induced by low temperature regulates anthocyanin biosynthesis in purple kale (Brassica oleracea var. acephala f. tricolor) Plant Cell Rep. 2012;31:281–289. doi: 10.1007/s00299-011-1162-3. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Hu Z, Zhu M, Zhu Z, Wang Z, Tian S, Chen G. Anthocyanin accumulation and molecular analysis of correlated genes in purple kohlrabi (Brassica oleracea var. gongylodes L.) J Agr Food Chem. 2015;63:4160–4169. doi: 10.1021/acs.jafc.5b00473. [DOI] [PubMed] [Google Scholar]
- 49.Chagné D, Carlisle C, Blond C, Volz R, Whitworth C, Oraguzie N, Crowhurst R, Allan A, Espley R, Hellens R, Gardiner S. Mapping a candidate gene (MdMYB10) for red flesh and foliage colour in apple. BMC Genomics. 2007;8:212. doi: 10.1186/1471-2164-8-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Espley RV, Brendolise C, Chagne D, Kutty-Amma S, Green S, Volz R, Putterill J, Schouten HJ, Gardiner SE, Hellens RP, Allan AC. Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell. 2009;21:168–183. doi: 10.1105/tpc.108.059329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Binzel ML, Yoo KS, Park S, Pike LM. Pink (P), a new locus responsible for a pink trait in onions (Allium cepa) resulting from natural mutations of anthocyanidin synthase. Mol Gen Genomics. 2004;272:18–27. doi: 10.1007/s00438-004-1041-5. [DOI] [PubMed] [Google Scholar]
- 52.Kim S, Park JY, Yang TJ. Characterization of three active transposable elements recently inserted in three independent DFR-A alleles and one high-copy DNA transposon isolated from the Pink allele of the ANS gene in onion (Allium cepa L.) Mol Gen Genomics. 2015;290:1027–1037. doi: 10.1007/s00438-014-0973-7. [DOI] [PubMed] [Google Scholar]
- 53.Tuteja JH, Clough SJ, Chan WC, Vodkin LO. Tissue-specific gene silencing mediated by a naturally occurring Chalcone synthase gene cluster in Glycine max. Plant Cell. 2004;16:819–835. doi: 10.1105/tpc.021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuteja JH, Zabala G, Varala K, Hudson M, Vodkin LO. Endogenous, tissue-specific short interfering RNAs silence the Chalcone synthase gene family in Glycine max seed coats. Plant Cell. 2009;21:3063–3077. doi: 10.1105/tpc.109.069856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu XP, Gao BZ, Han FQ, Fang ZY, Yang LM, Zhuang M, Lv HH, Liu YM, Li ZS, Cai CC, Yu HL, Li ZY, Zhang YY. Genetics and fine mapping of a purple leaf gene, BoPr, in ornamental kale (Brassica oleracea L. var. acephala) BMC Genomics. 2017;18:230. doi: 10.1186/s12864-017-3613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parkin IA, Koh C, Tang H, Robinson SJ, Kagale S, Clarke WE, Town CD, Nixon J, Krishnakumar V, Bidwell SL, Denoeud F, Belcram H, Links MG, Just J, Clarke C, Bender T, Huebert T, Mason AS, Pires JC, Barker G, Moore J, Walley PG, Manoli S, Batley J, Edwards D, Nelson MN, Wang X, Paterson AH, King G, Bancroft I, Chalhoub B, Sharpe AG. Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol. 2014;15:R77. doi: 10.1186/gb-2014-15-6-r77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu S, Liu Y, Yang X, Tong C, Edwards D, Parkin IA, Zhao M, Ma J, Yu J, Huang S, Wang X, Wang J, Lu K, Fang Z, Bancroft I, Yang TJ, Hu Q, Wang X, Yue Z, Li H, Yang L, Wu J, Zhou Q, Wang W, King GJ, Pires JC, Lu C, Wu Z, Sampath P, Wang Z, Guo H, Pan S, Yang L, Min J, Zhang D, Jin D, Li W, Belcram H, Tu J, Guan M, Qi C, Du D, Li J, Jiang L, Batley J, Sharpe AG, Park BS, Ruperao P, Cheng F, Waminal NE, Huang Y, Dong C, Wang L, Li J, Hu Z, Zhuang M, Huang Y, Huang J, Shi J, Mei D, Liu J, Lee TH, Wang J, Jin H, Li Z, Li X, Zhang J, Xiao L, Zhou Y, Liu Z, Liu X, Qin R, Tang X, Liu W, Wang Y, Zhang Y, Lee J, Kim HH, Denoeud F, Xu X, Liang X, Hua W, Wang X, Wang J, Chalhoub B, Paterson AH. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun. 2014;5:3930. doi: 10.1038/ncomms4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 59.Baranowskij N, Frohberg C, Prat S, Willmitzer L. A novel DNA binding protein with homology to Myb oncoproteins containing only one repeat can function as a transcriptional activator. EMBO J. 1994;13:5383–5392. doi: 10.1002/j.1460-2075.1994.tb06873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida K, Ma D, Constabel CP. The MYB182 protein down-regulates proanthocyanidin and anthocyanin biosynthesis in poplar by repressing both structural and regulatory flavonoid genes. Plant Physiol. 2015;167:693–710. doi: 10.1104/pp.114.253674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wan S, Li C, Ma X, Luo K. PtrMYB57 contributes to the negative regulation of anthocyanin and proanthocyanidin biosynthesis in poplar. Plant Cell Rep. 2017;36:1263–1276. doi: 10.1007/s00299-017-2151-y. [DOI] [PubMed] [Google Scholar]
- 62.Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15:63–78. doi: 10.1105/tpc.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J, Izzah NK, Choi BS, Joh HJ, Lee SC, Perumal S, Seo J, Ahn K, Jo EJ, Choi GJ, Nou IS, Yu Y, Tang TJ. Genotyping-by-sequencing map permits identification of clubroot resistance QTLs and revision of the reference genome assembly in cabbage (Brassica oleracea L.) DNA Res. 2016;23:29–41. doi: 10.1093/dnares/dsv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu XP, Yang C, Han FQ, Fang ZY, Yang LM, Zhuang M, Lv HH, Liu YM, Li ZS, Zhang YY. Genetics and fine mapping of a yellow-green leaf gene (ygl-1) in cabbage (Brassica oleracea var. capitata L.) Mol Breed. 2016;36:1–8. doi: 10.1007/s11032-015-0425-z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used to clone BoMYBL2. (DOCX 19 kb)
Figure S2. Schematic illustration of the strategy used to clone BoMYBL2–1 from various B. oleracea species. A: Genomic region around the BoMYBL2–1 based on information obtained from two different databases (upper: http://brassicadb.org/brad; Liu et al. 2014; lower: http://plants.ensembl.org/Brassica_oleracea/Info/Index; Parkin et al. 2014). B: Illustration showing the positions of the fragments, amplified using different combinations of primer sets, used to assemble the entire promoter and coding sequence of BoMYBL2–1. The yellow block represents a 159 bp repeat sequence. All primer sets are listed in Table 2. BoLPR2 is B. oleracea multicopper oxidase LPR2; BoPUB10 is B. oleracea U-box domain-containing protein 10. (DOCX 72 kb)
Table S2. List of primer sequences used in RT-PCR and genomic PCR analyses of BoMYBL2. (DOCX 32 kb)
Figure S1. Total anthocyanin content (A) of samples of the cabbages (B) shown in Fig. 1. (DOCX 109 kb)
DNA sequences of BoMYBL2–1 plus the front region or corresponding region of the gene. Gene names represented plant names descirbed in Table 1. TO1000 indicates reference sequence (Parkins et al. 2014). (TXT 86 kb)
Figure S3. Results of genomic DNA-PCR preformed to detect the presence or absence of genes regulating anthocyanin biosynthesis other than BoMYBL2–1. A: B. oleracea var. capitata F. alba or rubra varieties with contrasting characteristics in anthocyanin accumulation were selected for analysis; varieties with green and purple colors are indicated in green or purple. B: PCR analysis of other varieties of B. oleracea. The color of each variety is indicated above each lane. (DOCX 293 kb)
Figure S5. Comparison of different BoMYBL2–1 nucleotide sequences obtained from cabbages. Shaded regions indicate exon sequences. Sequences corresponding to Bol016162 from B. oleracea var. capitata were omitted. (DOCX 31 kb)
Figure S4. Expression of genes associated with anthocyanin biosynthesis in various purple cabbages. Daebakna is a green cabbage used as a reference. (DOCX 387 kb)


