Abstract
Maintenance pharmacological treatment for stable chronic obstructive pulmonary disease (COPD) is based on inhaled drugs, including long-acting muscarinic receptor antagonists (LAMA), long-acting β2-adrenoceptor agonists (LABA) and inhaled corticosteroids (ICS). Inhaled pharmacological treatment can improve patients’ daily symptoms and reduce decline of pulmonary function and acute exacerbation rate. Treatment with all three inhaled drug classes is reserved for selected, more severe, patients with COPD when symptoms are not sufficiently controlled by dual LABA/LAMA therapy and exacerbations are frequent. This review focuses on the role of single-inhaler triple therapy with once-daily fluticasone furoate/umeclidinium/vilanterol fixed-dose combination, which is in phase III clinical development for maintenance treatment of severe-to-very severe COPD. In this review, we summarize evidence providing the rationale for its use in COPD and discuss the gaps to be filled in this pharmacotherapeutic area.
Keywords: COPD, fluticasone, pharmacotherapy, single-inhaler, triple therapy, umeclidinium, vilanterol
Introduction
Chronic obstructive pulmonary disease (COPD) represents one of the most important health problems, and its prevalence in Europe has been estimated at up to 10%.1 Between 1994 and 2010, about 2.5 million deaths in the European Union were attributed to COPD.2
Management of COPD patients mainly relies on pharmacological treatment to relieve symptoms, improve quality of life and exercise tolerance, and reduce the frequency and severity of exacerbations. Reducing COPD exacerbations is a major objective in COPD control, as they negatively affect patients’ quality of life, increase the decline in lung function and represent an economic burden for society, particularly if the patient requires hospitalization.3 However, despite active research in this pharmacotherapeutic area, there are no clinical trials showing that any existing medications for COPD modify the long-term decline in lung function that characterizes this disease.4 Nevertheless, analysis of the key secondary outcome in the SUMMIT study,5 which failed to meet its primary outcome of improvement in survival with the combination of fluticasone furoate, an inhaled corticosteroid (ICS), and vilanterol, a long-acting β2-adrenoceptor agonist (LABA), in COPD patients with increased cardiovascular risk, shows that fluticasone alone or in combination with vilanterol reduces the rate of forced expiratory volume in 1 s (FEV1) decline compared with vilanterol alone or placebo without increased risk of pneumonia or cardiac adverse events.6
In this review, we summarize the current evidence and discuss future perspectives of single-inhaler therapy with once-daily fluticasone furoate/vilanterol/umeclidium in the management of COPD.
Inhaled treatment for COPD: drug classes and mechanisms of action
Classes of inhaled drugs approved for long-term treatment of COPD include long-acting muscarinic receptor antagonists (LAMA), LABA and ICS.
LAMA-induced bronchodilation is principally due to antagonism at muscarinic acetylcholine M3 receptors (mAChR) expressed on airway smooth muscle cells (Figure 1). M3 receptors are coupled to guanosine triphosphate (GTP)-binding protein (G)q, resulting in an intracellular biochemical cascade that regulates intracellular calcium concentration and calcium-modulated proteins.7 This signaling pathway promotes actin–myosin interaction, leading to airway smooth muscle contraction. The role of M2 receptors, expressed both at presynaptic and postsynaptic levels in airway smooth muscle cells in vivo is less clear. In vitro studies show that antagonism of presynaptic M2 receptors increases acetylcholine release from vagal nerve endings, whereas postsynaptic M2 receptor blockade on airway smooth muscle cells leads to increased relaxation caused by β2 agonists.8,9
Figure 1.
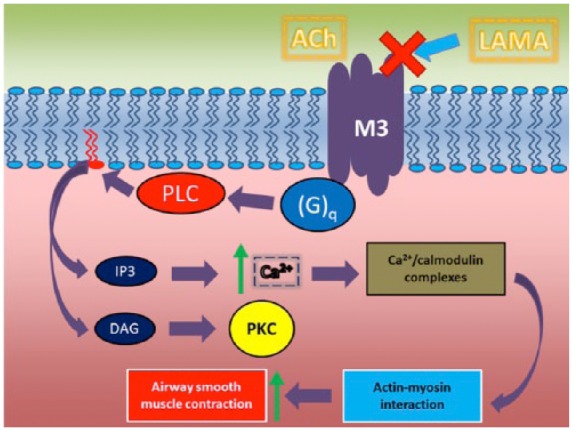
Signal transduction pathways following activation of muscarinic M3 receptors by acetylcholine.
Ach, acetylcholine; DAG, diacylglicerol; (G)q, GTP-binding protein; IP3, inositol-1,4,5-triphosphate; LAMA, long-acting muscarinic receptors antagonist; PKC, protein kinase C; PLC, phospholipase C.
As bronchodilators, LABA act via postsynaptic β2 receptor activation on airway smooth muscle cells with coupling to stimulatory GTP-binding protein (G)s and signaling that leads to reduction in contractility of airway smooth muscle through the inhibition of myosin light chain kinase (MLCK), which, in turn, promotes actin–myosin interaction (Figure 2).
Figure 2.
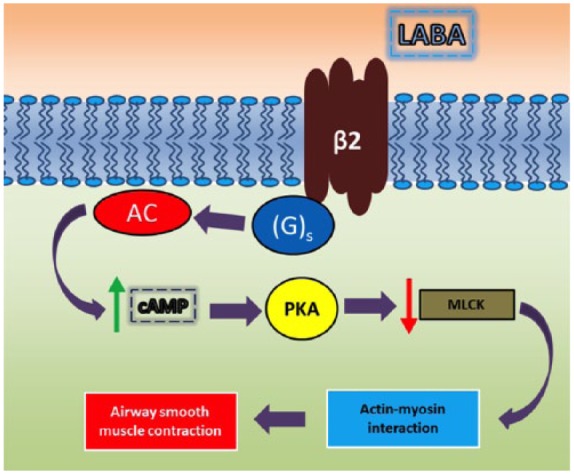
Signal transduction pathways following activation of β2 adrenergic receptors by LABA.
AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; (G)s, stimulatory GTP-binding protein; LABA, long-acting β2-adrenoceptor agonist; MLCK, myosin light chain kinase; PKA, protein kinase A.
The rationale for ICS use in COPD patients involves their anti-inflammatory effects. Mechanisms of action of glucocorticoids include transcription and transduction regulation of genes encoding anti-inflammatory and immune-suppressive mediators.10
Despite their effective anti-inflammatory effects, inflammation due to neutrophils and alveolar macrophages is generally resistant to glucocorticoid treatment.11 By contrast, in COPD patients with peripheral eosinophilia, an ICS, in a fixed-dose combination (FDC) with a LABA, can be effective in improving clinical outcomes, particularly in terms of reduction in moderate and severe exacerbations.12,13
The role of triple inhaled therapy for COPD in Global Initiative for Obstructive Lung Disease recommendations
The Global Initiative for Obstructive Lung Disease (GOLD) recommendations suggest triple inhaled therapy with a LAMA plus a LABA plus an ICS as a step-up pharmacotherapeutic approach in COPD patients with symptoms and history of frequent exacerbations who continue to experience exacerbations notwithstanding dual LABA/LAMA therapy.14,15 However, evidence on the clinical benefits of triple inhaled therapy are not consistent,15 particularly regarding its effect on the COPD exacerbation rate.16 Patients in Group D of the GOLD guidelines, who are not adequately controlled with a combination of LAMA plus a LABA in terms of exacerbations, might be a target COPD population for triple ICS/LABA/LAMA. Likewise, patients who show peripheral blood eosinophil counts might benefit from ICS treatment.15,17
The lack of a well-defined indication for triple ICS/LABA/LAMA therapy for COPD translates into marked differences in real-life prescription patterns.14 At present, prescription and overprescription of triple inhaled therapy seems to rely mostly on an empirical basis rather than on a rational subphenotyping approach.17
Triple inhaled therapy for COPD: evidence from prospective randomized clinical trials, systematic reviews and meta-analyses
Triple inhaled therapy for COPD might be an effective pharmacological strategy in selected COPD phenotypes, including asthma COPD overlap syndrome (ACOS), eosinophilic and frequent exacerbator phenotype, particularly when of a severe degree.18–20 However, further randomized clinical trials are required to clarify efficacy and define the target COPD population(s) of triple inhaled therapy. Different results have been reported according to patients’ selection, follow-up duration, quality of life outcome measures, definition of COPD exacerbations and various active medications.
The CLIMB study was performed to investigate the efficacy and tolerability of budesonide/formeterol added to tiotropium compared with tiotropium alone in patients with a pre-bronchodilator FEV1 < 50%.21 In this 12-week, randomized, double bind multicenter study including a total of 660 patients, improvements in lung function, morning activities, reliever use, and exacerbations rate, including severe exacerbations, were reported.21 Likewise, the 12-week, randomized, multicenter, open-label SECURE 1 study, including 578 patients with severe or very severe COPD, aimed to investigate efficacy and tolerability of budesonide/formeterol added to tiotropium compared with tiotropium alone.22 Compared with monotherapy, triple inhaled therapy was associated with significant improvements in FEV1, health-related quality of life assessed using St. George’s Respiratory Questionnaire (SGRQ) and lower COPD exacerbation rates.22 In both studies, no between-group differences in adverse event rate were observed.21,22
Different results were obtained with a combination of fluticasone propionate/salmeterol plus tiotropium compared to tiotropium alone.16 In a randomized, 52-week, double-bind, placebo-controlled trial including 449 patients with moderate to severe COPD comparing tiotropium plus fluticasone/salmeterol FDC, tiotropium plus salmeterol, and tiotropium alone, triple therapy did not result in a reduction in the proportion of patients who experienced a COPD exacerbation (primary outcome measure) compared with tiotropium plus placebo, although improvements in lung function, quality of life and hospitalization rate were observed.16 Similar results were reported in a 24-week, randomized, open-label, multicenter two-arm parallel study including 479 COPD patients who were treated with either fluticasone propionate/salmeterol FDC plus tiotropium or tiotropium alone.23 Compared with tiotropium, triple therapy was associated with increased pre-bronchodilator FEV1 from baseline to week 24 (primary outcome), whereas no between-group differences in the number of patients with COPD exacerbations were observed.23
A meta-analysis evaluating benefits of adding fluticasone propionate/salmeterol to tiotropium showed that triple inhaled therapy was associated with a significant increase in mean change in trough FEV1 from baseline to the end of follow up and a lower proportion of at least one COPD exacerbation compared with tiotropium monotherapy.24 In the SUMMIT study, an event-driven, randomized, double-blind, placebo-controlled, parallel-group, four-arm study, including 16,485 patients with moderate COPD and increased cardiovascular risk and aiming to assess whether a single-inhaler combination of fluticasone furoate and vilanterol, could reduce all-cause mortality compared with placebo, the primary outcome was not met.5 However, decreased decline in FEV1 (secondary outcome) without increased pneumonia risk was reported in the group receiving fluticasone furoate.6,25 These findings have potential clinical implications for COPD management and could result in a guideline change for ICS treatment for patients with moderate COPD and increased cardiovascular risk.
A systematic review assessing the effects of the ICS and LABA combination in addition to tiotropium versus tiotropium alone or the combination alone in patients with COPD reported moderate-quality evidence that triple therapy reduces hospital admissions compared with tiotropium plus placebo, whereas low-quality evidence suggests that triple therapy was associated with improved disease-specific quality of life.26 Compared with tiotropium alone, no significant increases in adverse effects were reported with ICS/LABA plus tiotropium.26 Due to excessive variability in inclusion criteria, definition of exacerbation and follow-up duration, the effect on COPD exacerbations was heterogeneous and was not taken into consideration.26
Efficacy and safety of a single-inhaler beclomethasone dipropionate/formoterol fumarate/glycopyrronium bromide FDC was evaluated in two randomized, parallel-group, double-blind, active-controlled trials: TRILOGY and TRINITY.24,25 The TRILOGY trial, aiming to compare efficacy of a beclomethasone/formoterol/glycopyrronium FDC in COPD patients (n = 687) with beclometasone/formeterol FDC (n = 681), showed superiority of triple therapy over dual therapy in improving co-primary endpoints including pre-dose FEV1, 2-h post-dose FEV1 and transition dyspnea index (TDI) focal score at week 26.27 The annualized rate of exacerbations was significantly lower in the group of COPD patients who received triple therapy, with a 23% reduction in exacerbations. No between-group differences in adverse events were reported.27
In the TRINITY study, aiming to compare beclomethasone dipropionate/formoterol fumarate/glycopyrronium bromide FDC (fixed triple therapy) with beclomethasone dipropionate/formoterol fumarate plus tiotropium (open triple therapy) and tiotropium alone in COPD patients with at least one exacerbation in the previous year, a baseline post-bronchodilator FEV1 <50% of predicted value, and a COPD assessment test score of at least 10, fixed triple therapy was superior to tiotropium alone in reducing moderate to severe exacerbation rate and noninferior to open triple therapy at 52 weeks (primary outcome). Incidence of adverse events was similar across treatments.28
Fluticasone, umeclidinium and vilanterol for COPD
Umeclidinium is a potent anticholinergic agent with a prolonged duration of action in in vitro and in vivo animal models.29 Once-daily umeclidinium at a dose of 62.5 µg via a dry powder inhaler (DPI) is approved for maintenance pharmacological treatment of COPD either alone or in an FDC with vilanterol, a LABA, at a dose of 25 µg.
In a 12-week, randomized, double-blind, placebo-controlled, parallel-group study aiming to assess efficacy and safety of once-daily umeclidinium at two different doses (62.5 µg and 125 µg) in patients with moderate-to-very severe COPD, umeclidinium at both doses significantly improved mean change from baseline in trough FEV1 on day 84 (primary outcome) compared with placebo.30 Improvements in dyspnea and health status were also observed with umeclidinium, without any increase in incidence of adverse events over placebo.30
A pooled meta-analysis including phase III clinical trials and comparative studies in patients with moderate-to-very severe COPD shows that umeclidinium is superior to vilanterol and placebo in improving FEV1 and reducing SGRQ score, and more effective that placebo in improving TDI and prolonging the time to first COPD exacerbation.31
Vilanterol, a LABA with inherent 24-h activity, was developed with fluticasone furoate, a once-daily ICS, in an FDC for asthma and COPD, and with a umeclidinium, a LAMA, in an FDC for COPD. In preclinical models, vilanterol showed a significantly faster onset of action than salmeterol and a longer duration of action than both salmeterol and formoterol,32 suggesting its potential for once-daily pharmacological treatment that may lead to increasing patients’ compliance and adherence to therapy.
In a DPI FDC with umeclidinium at a dose of 62.5 µg, vilanterol at a dose of 25 µg is approved for once-daily maintenance treatment of COPD.33 In a DPI FDC with fluticasone furoate 100 µg, vilanterol 25 µg is approved for once-daily maintenance treatment of COPD.34 For once-daily treatment of persistent asthma, vilanterol 25 µg is approved in two strengths of fluticasone furoate (100 µg and 200 µg). Unlike other once-daily LABA, including indacaterol and olodaterol, vilanterol was not developed for monotherapy of COPD.33,34
Using two separate DPIs, efficacy of triple therapy with vilanterol/fluticasone plus umeclidinium was evaluated in two 12-week, double-blind, placebo-controlled, multicenter studies.35 COPD patients were randomized 1:1:1 to receive once-daily blinded umeclidinium 62.5 µg, umeclidinium 125 µg or placebo added to open-label fluticasone/vilanterol (100/25 µg). In both studies, trough FEV1 on day 85 (primary outcome measure) was significantly improved with fluticasone/vilanterol plus umeclidinium (62.5 μg and 125 μg) compared to fluticasone/vilanterol plus placebo.35 In both studies, safety profiles, including cardiovascular adverse events and pneumonia, were similar across treatments.35
Pharmacokinetics and pharmacodynamics parameters and safety of fluticasone furoate/vilanterol/umeclidinium FDC triple therapy via a single DPI compared with different FDC of dual therapies was investigated in healthy subjects in two phase I single-center, four-way, single-dose, crossover studies.36 In particular, study CTT116415 [ClinicalTrials.gov identifier: NCT01691547] evaluated fluticasone furoate/vilanterol/umeclidinium at a single dose of 400/100/500 µg versus single doses of fluticasone furoate/vilanterol 400/100 µg, fluticasone furoate/umeclidinium at 400/500 µg or umeclidinium/vilanterol at 500/100 µg; study 200587 [ClinicalTrials.gov identifier: NCT01894386] compared single doses of 400/100/500 µg or 400/100/250 µg fluticasone furoate/vilanterol/umeclidinium with a single dose of umeclidinium/vilanterol 250/100 µg or fluticasone furoate/vilanterol 400/100 µg. A total of 88 healthy subjects were randomized to receive four consecutive inhalations (each administered as a single dose) via a single inhaler. Following single doses, similar total systemic exposure to fluticasone furoate, vilanterol and umeclidinium was observed when drugs were administered as triple FDC by a single DPI or as dual FDC therapy with fluticasone/vilanterol or umeclidinium/vilanterol. Adverse effect rate was low and similar across treatments.36
The FULFIL trial was the first study evaluating single-inhaler triple therapy with fluticasone furoate/vilanterol/umeclidinium versus budesonide/formeterol dual therapy in GOLD Group D patients with COPD.37 This randomized, double-blind, double-dummy trial included 1810 patients according to the intent-to treat population, and showed that change from baseline in trough FEV1 and change from baseline in SGRQ total score at week 24 (co-primary endpoints) were significantly improved after single-inhaler triple therapy compared with single-inhaler dual therapy. Regarding secondary outcome measures, the annualized rate of moderate to severe COPD exacerbations was significantly reduced after single-inhaler fluticasone furoate/vilanterol/umeclidinium FDC compared with budesonide/formoterol FDC [–35%; 95% confidence interval (CI), 14–51%; p = 0.002]. No between-group differences in adverse events were reported.37 These data, showing efficacy and safety of fluticasone furoate/vilanterol/umeclidinium FDC in patients with very severe COPD, suggest that once-daily single-inhaler triple therapy might be a valid pharmacological strategy in these patients, also in view of the potential increase in adherence to therapy and reduction in inhaler device use errors (Table 1).37
Table 1.
Characteristics of studies evaluating triple inhaled therapy with fluticasone furoate, umeclidinium and vilanterol.
| Study | Single inhaler | Randomization | Study type | Treatment arms | Number of subjects | Duration | Primary outcome | Secondary outcome and other parameters evaluated |
|---|---|---|---|---|---|---|---|---|
| Siler and colleagues study A35 | No | Yes | Multicenter | • UMEC (62.5 µg) + FF/VI (100/25 µg) • UMEC (125 µg) + FF/VI (100/25 µg) • Placebo + FF/VI (100/25 µg) |
727 COPD patients | 12 week | change from baseline in trough FEV1 | 0–6 h post-dose weighted mean FEV1 at day 84 SGRQ score Adverse events |
| Siler and colleagues study B35 | No | Yes | Multicenter | • UMEC (62.5 µg) + FF/VI • UMEC (125 µg) + FF/VI (100/25 µg) • Placebo + FF/VI (100/25 µg) |
730 COPD patients | 12 week | Change from baseline in trough FEV1 | 0–6 h post-dose weighted mean FEV1 at day 84 SGRQ score Adverse events |
| Brealey and colleagues study A36 | Yes | Yes | Single center | • FF/UMEC/VI (400/500/100 µg) FF/VI (400/100 µg) • FF/UMEC (400/500 µg) • UMEC/VI (500/100 µg) |
44 healthy subjects | 48 h | PK/PD parameters | Adverse events, serious adverse events, laboratory values, vital sign, physical examination |
| Brealey and colleagues study B36 | Yes | Yes | Single center | • FF/UMEC/VI (400/500/100 µg or 400/250/100 µg) • UMEC/VI (250/100 µg) • FF/VI (400/100 µg) |
44 healthy subjects | 24 h | PK parameters | Adverse events, serious adverse events, laboratory values, vital sign, physical examination |
| Lipson and colleagues37 | Yes | Yes | Multicenter | • FF/UMEC/VI • Budesonide/formeterol |
1810 COPD patients | 24 weeks A subgroup remained on blinded treatment for up to 52 week |
Composite: Change from baseline in trough FEV1 Change from baseline in SGRQ total score |
Efficacy and safety, including exacerbation rate, adverse events, pneumonia, cardiovascular events, bone fractures |
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FF, fluticasone furaoate; PD, pharmacodynamics; PK, pharmacokinetics; SGRQ, St. George’s Respiratory Questionnaire; UMEC, umeclidinium; VI, vilanterol.
The IMPACT trial, a 52-week phase III, randomized, double-blind, three-arm, parallel-group, global multicenter study comparing the rate of moderate and severe exacerbations (primary outcome measure) between single-inhaler fluticasone furoate/umeclidinium/vilanterol and single-inhaler fluticasone furoate/vilanterol or umeclidinium/vilanterol in 10,357 patients with severe-to-very severe COPD was completed in July 2017 and publication of results is awaited [ClinicalTrials.gov identifier: NCT02164513].38 Secondary outcome measures include changes from baseline in FEV1 and SGRQ total score, and the annual rate of exacerbations in patients with peripheral blood eosinophilia.38 The IMPACT trial is potentially pivotal in view of the large number of patients included, the choice of exacerbation rate as primary outcome measure, and the comparison of single-inhaler triple ICS/LABA/LAMA therapy with single-inhaler dual LAMA/LABA therapy. The last point is of particular relevance, as the lack of similar studies represents an unmet need in pharmacological treatment of COPD.39
Regulatory aspects
On 18 September 2017, once-daily single DPI fluticasone furoate/umeclidinium/vilanterol trifenatate FDC (100 µg/62.5 µg/25 µg predispended dose corresponding to 92 µg/55 µg/22 µg delivered dose) (Trelegy Ellipta® GSK, Brentford, United Kingdom) was approved in the USA for the long-term, once-daily, maintenance treatment of patients with COPD, who are on an FDC of fluticasone furoate and vilanterol for airflow obstruction and reducing exacerbations in whom additional treatment of airflow obstruction is required or for patients who are already receiving umeclidinium and an FDC of fluticasone furoate and vilanterol.40
Following this approval by the US Food and Drug Administration (FDA), single DPI fluticasone furoate/umeclidinium/vilanterol trifenatate FDC will soon be available in the USA. Regulatory applications have been submitted and are undergoing assessment in a number of other countries, including Australia and Canada.
The European Commission granted a marketing authorization valid throughout the European Union for twice-daily single pressurised metered dose inhaler (pMDI) beclomethasone dipropionate/formoterol fumarate/glycopyrronium FDC (100 µg/6 µg/10 µg) (metered dose corresponding to 87 µg/5 µg/9 µg delivered dose) (Trimbow®, Chiesi Farmaceutici, Parma, Italy) on 17 July 201741 and for once-daily single DPI fluticasone furoate/umeclidinium/vilanterol FDC (100 μg/62.5 μg/25 μg predispended dose corresponding to 92 μg/55 μg/22 μg delivered dose) (Trelegy Ellipta®) on 15 November 2017.42
These triple single-inhaler therapies are indicated as a maintenance treatment in adult patients with moderate to severe COPD who are not adequately treated by a combination of an ICS and a LABA.41,42
Critical issues for inhaled triple therapy for COPD
At present, there is a discrepancy between GOLD recommendations on inhaled triple therapy and real-world prescription patterns in patients with COPD.14,15
Current GOLD guidelines are cautious on triple therapy as they emphasize that more evidence is required to draw conclusions on benefits of triple LABA/LAMA/ICS FDC compared with available dual FDC, mainly dual LAMA/LABA.15
The TRILOGY27 and FULFIL trials37 have shown that single-inhaler ICS/LABA/LAMA FDC are more effective than single-inhaler dual ICS/LABA therapy in improving clinical outcome measures in patients with COPD, whereas the TRINITY trial28 reported the superiority of single-inhaler triple therapy over tiotropium monotherapy. Healthcare resource utilization data and associated costs from the FULFIL trial show that, in a clinical trial setting over a 24- or 52-week timeframe, nondrug costs for single-inhaler fluticasone furoate/umeclidinium/vilanterol triple therapy are lower than those for twice-daily budesonide/formoterol.43 However, total annualized cost over 24 weeks for triple inhaled therapy was slightly greater than that for budesonide/formoterol.43
The FLAME study showed that, compared with an ICS/LABA FDC, a LABA/LAMA FDC reduced COPD exacerbation rate (primary outcome measure) and improved both lung function and health-related quality of life with a significant lower rate of pneumonia in patients with COPD.44 However, comparative prospective randomized clinical trials aiming to assess single-inhaler triple therapy containing an ICS versus dual LAMA/LABA therapy in COPD are currently not available. These studies are required to define the therapeutic role of single-inhaler triple FDC in COPD management.39 While the central role of bronchodilators in COPD is clearly established, the response to ICS seems to be highly variable in the general population of COPD patients.45 Moreover, phenotypes of COPD patients including frequent exacerbators and ACOS are most likely to respond to these drugs.17 In this context, selecting the ‘right’ target population(s) seems to be pivotal when planning studies aiming to compare triple ICS/LABA/LAMA therapy versus dual LABA/LAMA therapy.
Despite guidelines recommendations,15,46 triple inhaled therapy is widely prescribed in patients with COPD.14 Analysis of prescribing pathways in COPD patients without asthma attending 387 primary-care practices across the United Kingdom from 2002 to 2010 shows that, during the study period, 32% of patients received triple therapy.14 After a diagnosis of COPD, patients in GOLD Group A, B, C and D progressed to triple inhaled therapy in 19%, 28%, 37% and 46% of cases, respectively.14 A major factor involved in progression to triple inhaled therapy seems to be the number of exacerbations.14
These data show a trend toward triple inhaled therapy prescriptions as COPD severity increases. In this study, about 50% of patients with most severe COPD ended up having a prescription for ICS/LABA/LAMA, despite the lack of prospective randomized clinical trials showing the superiority of this therapeutic option over dual bronchodilator FDC. Even more interestingly, 19% of Group A COPD patients who would require a single bronchodilator according to GOLD recommendations ended up having triple inhaled therapy.
Inappropriate adoption of triple inhaled therapy exposes patients to avoidable side effects. In particular, LABA can produce resting sinus tachycardia and precipitate cardiac rhythm disturbances in susceptible patients. Also, metabolic side effects could occur, especially in patients with chronic heart failure47 or being treated with thiazide diuretics;48 less frequent are muscle tremors, nervousness and occasional insomnia. Under treatment with LAMA, polar quaternary ammonium compounds with low lipid solubility, incidence of systemic side effects is generally low because of poor absorption, which limits systemic exposure. One of the most common side effects is dry mouth.49 In a nested case–control study including 284,220 LABA–LAMA–naïve patients with COPD, initiation of treatment with LAMA or LABA was associated with about 1.5-fold increase in cardiovascular risk, irrespective of history of cardiovascular disease or COPD exacerbations.50 LAMA dosage forms, individual LABAs and concomitant COPD therapeutic regimens did not differ in the cardiovascular risks.50
A systematic review and meta-analysis of 42 randomized trials including about 50,000 patients with COPD raised concerns about tiotropium soft mist inhaler as increased risk of overall mortality compared with other medications, including LABA/ICS and tiotropium DPI, was reported.51 The TIOSPIR study, a randomized, double-blind, parallel-group trial involving 17,135 patients with COPD which was specifically designed to address this safety issue showed that tiotropium soft mist inhaler at a dose of 5 μg once daily was noninferior to tiotropium DPI at a dose of 18 μg once daily with respect to the risk of death (hazard ratio, 0.96; 95% CI, 0.84 to 1.09).52
Adverse events associated with the use of ICS include increased risk of any pneumonia and pneumonia requiring hospitalization.53 A pooled analysis of trials comparing ICS/LABA versus placebo shows that treatment with dual active component inhalers is associated with higher risk of pneumonia.54 Other potential side effects of ICS include increased risk of cataracts,55 pulmonary tuberculosis,56 diabetes onset and diabetes progression,57 and nonvertebral fractures.58
However, safety issues are not the only concern related to use/overuse of ICS as lack of efficacy of these drugs in the general COPD population was reported. A retrospective general practice cohort study in the United Kingdom has shown that the rate of exacerbations requiring hospitalizations did not change, notwithstanding the increased number of prescriptions for LABA plus ICS combinations.59
In a 52-week, double-blind, parallel-group study including 2485 patients with a history of COPD exacerbation who received tiotropium DPI (at a dose of 18 μg once daily), salmeterol DPI (50 μg twice daily) and the fluticasone propionate DPI (500 μg twice daily) during a 6-week run-in period and were then randomized to continued triple therapy or withdrawal of fluticasone propionate in three steps over a 12-week period, the time to the first moderate or severe COPD exacerbation (primary outcome measure) was similar in the two study groups (hazard ratio, 1.06; 95% CI, 0.94 to 1.19), whereas the adjusted mean reduction from baseline in the trough FEV1 was 38 ml greater in the glucocorticoid-withdrawal group than in the glucocorticoid-continuation group.60
Unlike the general COPD population, subphenotypes of COPD patients might have a better response to ICS.
A post-hoc analysis of data from two 52-week, parallel, randomized, controlled trials, aiming to compare three doses of fluticasone furoate (50 μg, 100 μg and 200 μg) added to vilanterol 25 μg with vilanterol alone 25 μg in 3177 patients with moderate to severe COPD with at least one exacerbation in the previous year, showed that, across all ICS doses, reduction in COPD exacerbation rates was significant in those patients with peripheral blood eosinophil cell counts ⩾2% (–29%, p < 0.0001), but not in patients with peri-pheral blood eosinophil cell counts <2% (–10%, p = 0.28).12 Of note, in COPD patients with the eosinophilic phenotype, the extent of reduction in exacerbation rate was correlated with baseline peripheral blood eosinophil cell counts.12 These findings suggest that blood eosinophilia might predict response to ICS in patients with COPD.12 However, large controlled prospective studies are required to formally address this issue.
Conclusion and future perspectives
At present, the choice of triple inhaled therapy seems to rely mostly on an empirical basis rather than on a rational subphenotyping approach.17 ICA/LABA/LAMA overprescription patterns likely reflect the attitude of physicians to provide COPD patients with the ‘maximal’ level of pharmacological treatment in the attempt to slow down the rapid decline in lung function that characterizes this disease. However, in the lack of a demonstrated response to ICS in the individual subject, large-scale prescription of FDC containing an ICS should be avoided due to poor long-term tolerability of these drugs, particularly at the high doses indicated for COPD. As pointed out by GOLD and other guidelines, triple inhaled therapy should be limited to patients with more severe COPD who are not adequately controlled with dual LABA/LAMA therapy. In these patients, single-inhaler triple therapy, including fluticasone furoate/vilanterol/umeclidinium FDC, seems to be an attractive and convenient pharmacological strategy for maintenance COPD treatment. Attempts should be made to identify patients with COPD phenotypes most likely to respond to the ICS component of triple inhaled therapy (e.g. eosinophilic, ACOS and frequent exacerbators). Single-inhaler triple therapy with fluticasone furoate, umeclidinium and vilanterol, which is in phase III development, seems to be an effective and well-tolerated therapeutic option in patients with COPD. The FULFIL trial has shown the superiority of this triple inhaled FDC over inhaled budesonide/formoterol, but comparative studies with LABA/LAMA are currently lacking.
The key question is whether fluticasone furoate/vilanterol/umeclidinium is superior to vilanterol/umeclidinium in patients with severe-to-very severe COPD and if there are differences in drug response based on phenotypes. Results from the IMPACT study, a large phase III study aiming at comparing efficacy and safety of triple fluticasone furoate/vilanterol/umeclidinium with either vilanterol/umeclidinium or fluticasone furoate/vilanterol in different COPD phenotypes/endotypes, will likely fill the gap and answer this question.
Footnotes
Funding: Catholic University of the Sacred Heart, Academic Grants (Fondi di Ateneo) 2016–2017. Fondazione Roma, Grant 2014.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Mario Malerba, Department of Translational Medicine-Respiratory Medicine, University of Piemonte Orientale, Novara/Vercelli, Italy.
Matteo Nardin, Department of Internal Medicine, University of Brescia, Brescia, Italy.
Giuseppe Santini, Department of Pharmacology, Catholic University of the Sacred Heart, Agostino Gemelli University Hospital Foundation, Rome, Italy.
Nadia Mores, Department of Pharmacology, Catholic University of the Sacred Heart, Agostino Gemelli University Hospital Foundation, Rome, Italy.
Alessandro Radaeli, Department of Emergency Medicine, ASST Spedali Civili di Brescia, Brescia, Italy.
Paolo Montuschi, Department of Pharmacology, Faculty of Medicine, Catholic University of the Sacred Heart, Agostino Gemelli, University Hospital Foundation, Largo Francesco Vito, 1 – 00168, Rome, Italy.
References
- 1. Miravitlles M, Soriano JB, García-Río F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64: 863–868. [DOI] [PubMed] [Google Scholar]
- 2. López-Campos JL, Ruiz-Ramos M, Soriano JB. Mortality trends in chronic obstructive pulmonary disease in Europe, 1994–2010: a joinpoint regression analysis. Lancet Respir Med 2014; 2: 54–62. [DOI] [PubMed] [Google Scholar]
- 3. Hutchinson A, Brand C, Irving L, et al. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Intern Med J 2010; 40: 364–371. [DOI] [PubMed] [Google Scholar]
- 4. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008; 359: 1543–1554. [DOI] [PubMed] [Google Scholar]
- 5. Vestbo J Anderson JA Brook RD et al.;. SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet 2016; 387: 1817–1826. [DOI] [PubMed] [Google Scholar]
- 6. Calverley PMA, Anderson JA, Brook RD, et al. ; SUMMIT (Study to Understand Mortality and Morbidity) Investigators. Fluticasone furoate, vilanterol, and lung function decline in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. Am J Respir Crit Care Med 2018; 197: 47–55. [DOI] [PubMed] [Google Scholar]
- 7. Pera T, Penn RB. Crosstalk between β2-adrenoceptor and muscarinic acetylcholine receptors in the airway. Curr Opin Pharmacol 2014; 16: 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belmonte KE. Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2: 297–304. [DOI] [PubMed] [Google Scholar]
- 9. Kume H, Fukunaga K, Oguma T. Research and development of bronchodilators for asthma and COPD with a focus on G protein/KCa channel linkage and β2-adrenergic intrinsic efficacy. Pharmacol Ther 2015; 156: 75–89. [DOI] [PubMed] [Google Scholar]
- 10. Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 2013; 131: 636–645. [DOI] [PubMed] [Google Scholar]
- 11. Jiang Z, Zhu L. Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease. Pulm Pharmacol Ther 2016; 37: 1–8. [DOI] [PubMed] [Google Scholar]
- 12. Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435–442. [DOI] [PubMed] [Google Scholar]
- 13. Pavord ID, Lettis S, Locantore N, et al. Blood eosinophils and inhaled corticosteroid/long-acting β2 agonist efficacy in COPD. Thorax 2016; 71: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis 2015; 10: 2207–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Global initiative for chronic obstructive pulmonary disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, http://goldcopd.org (2018, accessed 18 January 2018).
- 16. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomised trial. Ann Intern Med 2007; 146: 545–555. [DOI] [PubMed] [Google Scholar]
- 17. Montuschi P, Malerba M, Santini G, et al. Pharmacological treatment of chronic obstructive pulmonary disease: from evidence-based medicine to phenotyping. Drug Discov Today 2014; 19: 1928–1935. [DOI] [PubMed] [Google Scholar]
- 18. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish COPD guidelines (GesEPOC): pharmacological treatment of stable COPD. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol 2012; 48: 247–257. [DOI] [PubMed] [Google Scholar]
- 19. Kankaanranta H, Harju T, Kilpeläinen M, et al. Diagnosis and pharmacotherapy of stable chronic obstructive pulmonary disease: the Finnish guidelines. Basic Clin Pharmacol Toxicol 2015; 116: 291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miravitlles M, Vogelmeier C, Roche N, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J 2016; 47: 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180: 741–750. [DOI] [PubMed] [Google Scholar]
- 22. Lee S-D, Xie C, Yunus F, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: a randomised, multicentre study in East Asia. Respirology 2016; 21: 119–127. [DOI] [PubMed] [Google Scholar]
- 23. Jung KS, Park HY, Park SY, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomised controlled study. Respir Med 2012; 106: 382–389. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Shi H, Sun X, et al. Benefits of adding fluticasone propionate/salmeterol to tiotropium in COPD: a meta-analysis. Eur J Intern Med 2014; 25: 491–495. [DOI] [PubMed] [Google Scholar]
- 25. Crim C, Calverley PMA, Anderson JA, et al. ; SUMMIT investigators. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: the SUMMIT trial. Respir Med 2017; 131: 27–34. [DOI] [PubMed] [Google Scholar]
- 26. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting β2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016; 6: CD008532. DOI: 10.1002/14651858.CD008532.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 2016; 388: 963–973. [DOI] [PubMed] [Google Scholar]
- 28. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 2017; 389: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 29. Babu KS, Morjaria JB. Umeclidinium in chronic obstructive pulmonary disease: latest evidence and place in therapy. Ther Adv Chronic Dis 2017; 8: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trivedi R, Richard N, Mehta R, et al. Umeclidinium in patients with COPD: a randomised, placebo-controlled study. Eur Respir J 2014; 43: 72–81. [DOI] [PubMed] [Google Scholar]
- 31. Pleasants RA, Wang T, Gao J, et al. Inhaled umeclidinium in COPD patients: a review and meta-analysis. Drugs 2016; 76: 343–361. [DOI] [PubMed] [Google Scholar]
- 32. Hanania NA, Feldman G, Zachgo W, et al. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomised placebo-controlled trial. Chest 2012; 142: 119–127. [DOI] [PubMed] [Google Scholar]
- 33. Montuschi P, Ciabattoni G. Bronchodilating drugs for chronic obstructive pulmonary disease: current status and future trends. J Med Chem 2015; 58: 4131–4164. [DOI] [PubMed] [Google Scholar]
- 34. Malerba M, Radaeli A, Montuschi P, et al. Vilanterol trifenatate for the treatment of COPD. Expert Rev Respir Med 2016; 10: 719–731. [DOI] [PubMed] [Google Scholar]
- 35. Siler TM, Kerwin E, Sousa AR, et al. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomised studies. Respir Med 2015; 109: 1155–1163. [DOI] [PubMed] [Google Scholar]
- 36. Brealey N, Gupta A, Renaux J, et al. Pharmacokinetics of fluticasone furoate, umeclidinium, and vilanterol as a triple therapy in healthy volunteers. Int J Clin Pharmacol Ther 2015; 53: 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 196: 438–446. [DOI] [PubMed] [Google Scholar]
- 38. Pascoe SJ, Lipson DA, Locantore N, et al. A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. Eur Respir J 2016; 48: 320–330. [DOI] [PubMed] [Google Scholar]
- 39. Lipworth BJ, Jabbal S. FULFIL an unmet need in COPD. Am J Respir Crit Care Med 2017; 196: 1082. [DOI] [PubMed] [Google Scholar]
- 40. Trelegy Ellipta. www.accessdata.fda.gov/drugsatfda_docs/label/2017/209482s000lbl.pdf. 9/2017. (accessed 18 January 2018).
- 41. Trimbow. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004257/human_med_002139.jsp&mid=WC0b01ac058001d124 . 09/08/2017. (accessed 20 January 2018).
- 42. Trelegy Ellipta. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004363/human_med_002191.jsp&mid=WC0b01ac058001d124 . 12/01/2018. (accessed 20 January 2018).
- 43. Ismaila AS, Birk R, Shah D, et al. Once-daily triple therapy in patients with advanced COPD: healthcare resource utilization data and associated costs from the FULFIL trial. Adv Ther 2017; 34: 2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 2016; 374: 2222–2234. [DOI] [PubMed] [Google Scholar]
- 45. Barnes PJ. Inhaled corticosteroids in COPD: a controversy. Respiration 2010; 80: 89–95. [DOI] [PubMed] [Google Scholar]
- 46. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care, https://www.nice.org.uk/guidance/cg12 (accessed 20 October 2018).
- 47. Uren NG, Davies SW, Jordan SL, et al. Inhaled bronchodilators increase maximum oxygen consumption in chronic left ventricular failure. Eur Heart J 1993; 14: 744–750. [DOI] [PubMed] [Google Scholar]
- 48. Lipworth BJ, McDevitt DG, Struthers AD. Hypokalemic and ECG sequelae of combined beta-agonist/diuretic therapy: protection by conventional doses of spironolactone but not triamterene. Chest 1990; 98: 811–815. [DOI] [PubMed] [Google Scholar]
- 49. Disse B, Speck GA, Rominger KL, et al. Tiotropium (Spiriva): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci 1999; 64: 457–464. [DOI] [PubMed] [Google Scholar]
- 50. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case–control study. JAMA Intern Med. Epub ahead of print 2 January 2018. DOI: 10.1001/jamainternmed.2017.7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dong Y-H, Lin H-H, Shau W-Y, et al. Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trials. Thorax 2013; 68: 48–56. [DOI] [PubMed] [Google Scholar]
- 52. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013; 369: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 53. Di Santostefano RL, Sampson T, Le H Van, et al. Risk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: a new-user cohort study. PLoS One 2014; 9: e97149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nannini LJ, Poole P, Milan SJ, et al. Combined corticosteroid and long-acting β2-agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013; 11: CD003794. DOI: 10.1002/14651858.CD003794.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cumming RG, Mitchell P, Leeder SR. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med 1997; 337: 8–14. [DOI] [PubMed] [Google Scholar]
- 56. Kim J-H, Park J-S, Kim K-H, et al. Inhaled corticosteroid is associated with an increased risk of TB in patients with COPD. Chest 2013; 143: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 57. Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med 2010; 123: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 58. Weatherall M, James K, Clay J, et al. Dose–response relationship for risk of non-vertebral fracture with inhaled corticosteroids. Clin Exp Allergy 2008; 38: 1451–1458. [DOI] [PubMed] [Google Scholar]
- 59. Harries TH, Seed PT, Jones S, et al. Chronic obstructive pulmonary disease hospital admissions and drugs: unexpected positive associations – a retrospective general practice cohort study. NPJ Prim care Respir Med 2014; 24: 14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014; 371: 1285–1294. [DOI] [PubMed] [Google Scholar]


