Abstract
Background
We wanted to determine the impact of combined Clinical Pulmonary Infection Score (CPIS) and a spot serum procalcitonin (PCT)-guided protocol to shorten the duration of antibiotic treatment in patients with ventilator-associated pneumonia (VAP), mainly caused by nonfermentative gram-negative bacilli (NF-GNB).
Methods
Patients with VAP who received appropriate antibiotics for 7 days, temperature ⩽ 37.8°C, without shock, and CPIS ⩽ 6 were allocated to the PCT group or conventional group according to the treating physicians’ decisions. In the PCT group, antibiotics were stopped if the PCT level on day 8 < 0.5 ng/ml. In the conventional group, antibiotics were stopped according to physicians’ discretion.
Results
There were 24 patients in the PCT group and 26 patients in the conventional group. NF-GNB were responsible for VAP in 79.2% of the PCT group and 65.4% of the conventional group. PCT group had a greater number of antibiotic-free days alive during the 28 days after VAP onset than the conventional group (14.6 ± 5.4 days versus 5.9 ± 5.7 days, respectively; p <.001). In the multivariate, propensity score-adjusted analysis, the PCT group [coefficient = −9.1 (–12.2 to −6); p <.001] and extrapulmonary infections [coefficient = 6.4 (3.3–9.5); p <.001] were independent predictors of total antibiotic exposure days. There was no relapse in both groups. Meanwhile, 12.5% of the PCT group and 26.9% of the conventional group subsequently developed recurrent VAP compatible with superinfections.
Conclusions
CPIS and a spot serum PCT level appeared effective and safe to guide discontinuation of antibiotic treatment in patients with VAP caused by NF-GNB.
Trial registration:
TCTR20160726002
Keywords: antibiotic discontinuation, clinical pulmonary infection score, mechanical ventilation, nonfermentative gram-negative bacilli, procalcitonin, ventilator-associated pneumonia
Introduction
Ventilator-associated pneumonia (VAP) is responsible for significant morbidity, mortality, and economic costs.1-4 Prompt initiation of appropriate antibiotic is a cornerstone of treatment of VAP.5,6 However, appropriate shortening of the treatment duration is an important aspect of decreasing antibiotic-associated costs, minimizing selection pressures for resistant organisms and improving overall outcomes.6,7
The antibiotic treatment duration for patients with VAP caused by nonfermentative gram-negative bacilli (NF-GNB) remains controversial. A large randomized controlled study showed that patients with NF-GNB VAP who received antibiotics for 8 days had a higher percentage of recurrent pulmonary infections compared with patients who received antibiotics for 15 days.7 However, few recent studies showed that the shorter antibiotic treatment of NF-GNB was not associated with higher recurrence and mortality rates.8,9 Currently, NF-GNB are the most common etiology of VAP in many countries, including the authors’ country,10 and clinicians remain hesitant about prescribing fewer days of antibiotic treatment for this patient subset.
Procalcitonin (PCT) is the precursor molecule of the hormone calcitonin. It is upregulated in severe bacterial infections and sepsis. Several studies showed that a PCT-based algorithm led to fewer antibiotic prescriptions in the intensive care unit.11–15 There has been only one study regarding PCT-based algorithms to stop antibiotics specifically in patients with VAP.16 In this study, physicians in the PCT group were encouraged to withdraw antibiotics when serum PCT was decreased by 80% or more when compared with the first day, or when it was less than 0.5 ng/ml. The PCT group was shown to have more antibiotic-free days alive during the 28 days after VAP diagnosis than the conventional group. Only one third of the patients had VAP due to NF-GNB in this study. In addition, PCT measurements needed to be performed daily for 10 days. Because a serum PCT threshold of more than 0.5 ng/ml on day 7 was shown to be the strongest independent marker of unfavorable outcomes, including death and VAP recurrence.17 In order to reduce the cost of PCT assessment and be more practical in the management of VAP, a clinical study was performed. The authors sought to determine if a spot serum PCT level together with clinical assessment using clinical pulmonary infection score (CPIS) safely shortens the duration of antibiotic treatment in patients with clinically diagnosed VAP in intensive care units with a high prevalence of NF-GNB infections.
Materials and methods
Study population
A prospective nonrandomized controlled study was conducted from January 2013 to January 2014 at a university hospital in Bangkok, Thailand. All patients admitted to the critical respiratory care unit, medical intensive care unit and neurosurgical intensive care unit who were intubated and had received mechanical ventilation for at least 48 h were potentially eligible for this study. The patients were enrolled into the study if they met all the following criteria: age > 18 years, clinical suspicion of VAP as defined by the American Thoracic Society (ATS) guidelines6 and simplified version of CPIS (Table 1)18 > 6. Patients were excluded if they were pregnant, were enrolled in another trial, had little chance of survival, had neutropenia, had received immunosuppressants or long-term corticosteroid therapy, had concomitant acquired immunodeficiency syndrome, had a concomitant extrapulmonary infection diagnosed between days 1 and 3 that required prolonged (>8 days) antibiotic treatment, or infection caused by confirmed nonbacterial pathogens. In addition, during the follow-up period, the enrolled patients were excluded if they had not received appropriate antibiotics within 3 days of enrollment, or had unfavorable clinical response after 7 days of appropriate antibiotic treatment as defined by one of the following: body temperature > 37.8°C, shock or use of inotropes or vasopressors, CPIS > 6. At the time this study was performed, serum PCT was not routinely used in the management of VAP in the authors’ institution. The treating physicians were informed of the study protocol on the day of VAP diagnosis (day 0) and offered to join one of the two study groups; the PCT group or conventional group. If the treating physicians joined the PCT group, patients enrolled were assigned to have the duration of antibiotic treatment for VAP determined by the study protocol, including serum PCT level. If the treating physicians selected the conventional group, the duration of antibiotic therapy would be determined by the clinical judgment of the treating physicians.
Table 1.
Simplified version of the clinical pulmonary infection score.18 (from reference 18 with permission)
| Component | Value points | Points |
|---|---|---|
| Temperature °C | ⩾36.5 and ⩽38.4 | 0 |
| ⩾38.5 and ⩽38.9 | 1 | |
| ⩾39.0 and ⩽36.0 | 2 | |
| Blood leukocytes per mm3 | ⩾4000 and ⩽11.000 | 0 |
| <4000 or >11.000 | 1 | |
| Tracheal secretions | Few | 0 |
| Moderate | 1 | |
| Large | 2 | |
| Purulent | +1 | |
| Oxygenation PaO2/FiO2, mm Hg | >240 or presence of ARDS | 0 |
| PaO2/FiO2, mm Hg | ⩽240 and absence of ARDS | 2 |
| Chest radiograph | No infiltrate | 0 |
| Patchy or diffuse infiltrate | 1 | |
| Localized infiltrate | 2 |
Total points for clinical pulmonary infection score varied from 1 to 10 points.
ARDS, acute respiratory distress syndrome; PaO2, partial pressure of oxygen in the arterial blood; FiO2, fraction of inspired oxygen; PaO2/FiO2, ratio of PaO2 to FiO2.
Study design and data collection
Baseline assessment and follow up
At the time of enrollment (day 0), the following data were recorded for each subject: age, sex, length of hospital and ICU stay, number of intubation days prior to enrollment, use of any antibiotics within 14 days of VAP onset, reason for initiating mechanical ventilation, pre-existing chronic diseases, vital signs, CPIS, Acute Physiology and Chronic Health Evaluation II score (APACHE II),19 and microbial etiology of VAP. The organisms were considered to be the potential etiology of VAP if its colonies in endotracheal suction specimens were reported as moderate or in numerous quantities. Multidrug-resistant organisms were defined as non-susceptibility to at least one agent in three or more antimicrobial categories.
During the 28 day follow-up period, the following data were recorded daily: vital signs, antibiotic use, patient’s mechanical ventilation status, and the patient’s status at day 28. The CPIS was monitored after 3 days (day 4) and 7 days (day 8) of appropriate antibiotic treatment. Extreme vigilance for pneumonia recurrence was observed throughout the study. This was defined as an episode of pneumonia that recurred after completion of the initial course of antibiotics. Patients were considered to have microbiologically documented recurrent pneumonia when at least one bacterial species was reported to grow in endotracheal suction specimens with moderate or numerous quantities, together with clinical suspicious of pneumonia and CPIS > 6. Recurrence was considered a relapse if at least one of the initial causative bacterial strains (i.e. same genus and species) grew in moderate or numerous quantities from an endotracheal suction specimen; otherwise, it was considered to be a superinfection.
Study protocol
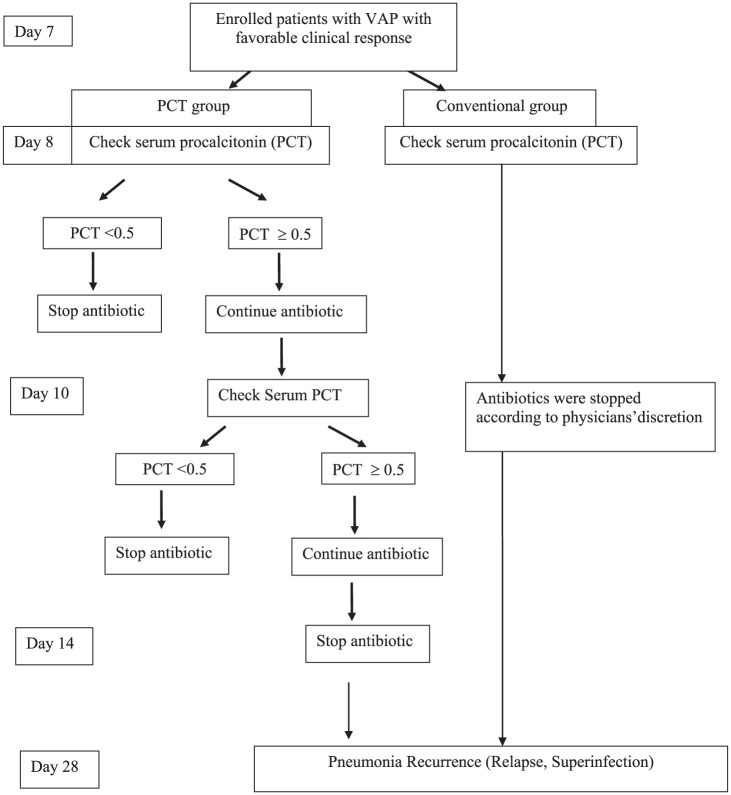
Antibiotic selection was left to the discretion of the treating physicians. The study protocol is shown in Figure 1. In both groups, serum PCT level was first assessed after the enrolled patients received appropriate antibiotic for 7 days (day 8) and revealed favorable clinical response within 7 days of appropriate antibiotic treatment, including: body temperature ⩽ 37.8°C; without shock or use of inotropes or vasopressors; and CPIS ⩽ 6. In the conventional group, serum PCT level was assessed once. However, the treating physicians were not informed the serum PCT result and antibiotic discontinuation would be determined according to the treating physicians’ discretion.
Figure 1.
Flow diagram for the study protocol.
PCT, procalcitonin; VAP, ventilator-associated pneumonia.
The study protocol was approved by Siriraj Ethics Committee on Human Research no. SIRB 373/2555(EC4). Informed consent for inclusion in the study was obtained from each patient or the patient’s next of kin. Trial registration: TCTR20160726002. Thai Clinical Trials Registry (www.clinicaltrials.in.th).
Measurement of serum procalcitonin
Measurements were done using cobas® Elecsys BRAHMS PCT (Roche Diagnostics, Mannheim, Germany) which is an electrochemiluminescence immunoassay (ECLIA). The measuring range was 0.02–100 ng/ml.
Outcome measures
The primary outcome was the number of antibiotic-free days alive assessed 28 days after enrollment into the study (day of diagnosis of VAP, day 0). Any antibiotic exposure from the time of enrollment to day 28, that is, total antibiotic exposure days, regardless of indication, was taken into account for antibiotic exposure analyses. Secondary outcomes were the relapse or superinfection rate, the number of mechanical ventilation-free days from the time of enrollment to day 28, and the overall mortality at 28 days.
Statistical analyses
All statistical analysis was performed using SPSS Statistics software version 20 (SPSS, Inc., Chicago, IL, USA) and STATA version 14 (Stata Corporation, College Station, Texas). Discrete variables are expressed as counts (%) and continuous variables as mean ± standard deviation or median [interquartile range (IQR)]. Comparison of the conventional group and the PCT group was done by using the chi-squared test or Fisher’s exact test for categorical variables and nonparametric Mann–Whitney U test or unpaired t tests for continuous variables, as appropriate.
Multivariate analysis by linear regression was performed to evaluate the influence of the two treatment groups, as well as a list of predefined covariables, on total antibiotic exposure days. Because of the nonrandomized nature of this study, there could be baseline imbalances between the two study groups. The probability that a patient has been allocated to the PCT group was assessed with multivariate analysis, including the factors that might influence the treating physicians’ decision to join the PCT group, including age and sex, and the factors that might affect the outcome of treatment of VAP, including: duration of intubation before VAP (⩽14 days or >14 days), previous antibiotic treatment within 14 days of VAP onset, inappropriate empirical antibiotics during the initial 3 days of treatment, APACHE II score on the day VAP was diagnosed, septic shock, CPIS on the day VAP was diagnosed, and whether or not the causative organisms were NF-GNB. This multivariate model was used to create a propensity score for each patient. A multivariate linear regression model was derived with total antibiotic exposure days as the dependent variable, and the propensity score, PCT group, and extrapulmonary infections as potential confounding variables. Statistical significance was defined as p < 0.05. All reported p values are two tailed.
Based on previous study,16 the median (IQR) antibiotic-free days alive 28 days after VAP onset was 13 days (2–21 days) in the procalcitonin guided treatment group versus 9.5 days (1.5–17 days) in the control group. Then, 27 samples are required in each group to detect significant difference between groups with a power of 90% and an α error of 0.05.
Results
Characteristics of the patients
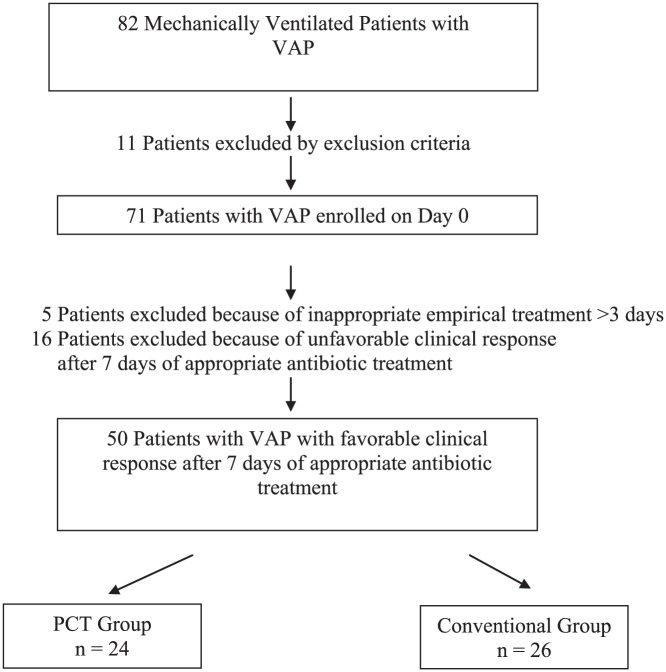
A total of 71 patients treated with antibiotics for presumed VAP were enrolled (day 0). Finally, 50 patients were included in the analysis, of which 24 patients were allocated to the PCT group and 26 to the conventional group, as shown in Figure 2. The clinical characteristics, comorbidities, severity and factors that might affect VAP treatment outcome of both groups on the day of enrollment were not different, as shown in Table 2.
Figure 2.
Patient flow diagram.
PCT, procalcitonin; VAP, ventilator-associated pneumonia.
Table 2.
Characteristics of the cohorts at entry into the study.*
| Characteristics | PCT group (n = 24) |
Conventional group (n = 26) |
p value |
|---|---|---|---|
| Age, years | 74.4 ± 15.8 | 66 ± 18.2 | 0.15 |
| Female, n (%) | 14 (58.3) | 10 (38.5) | 0.16 |
| Comorbidities, n (%) | |||
| Cardiovascular disease | 14 (58.3) | 15 (57.7) | 0.96 |
| COPD | 8 (33.3) | 8 (30.8) | 0.85 |
| Chronic kidney disease | 8 (33.3) | 7 (26.9) | 0.62 |
| Chronic liver disease | 6 (25) | 5 (19.2) | 0.62 |
| Neurological disease | 15 (62.5) | 17 (65.4) | 0.83 |
| Diabetes | 11 (45.8) | 8 (30.8) | 0.27 |
| Underlying malignancy | 1 (4.2) | 2 (7.7) | 0.6 |
| Reason for mechanical ventilation, n (%) | |||
| Cardiovascular failure | 3 (12.5) | 1 (3.8) | 0.34 |
| Respiratory failure | 11 (45.8) | 16 (61.5) | 0.27 |
| Neurologic failure | 8 (33.3) | 9 (34.6) | 0.9 |
| Sepsis | 2 (8.3) | 0 | 0.13 |
| Severity and factors that might affect VAP treatment outcomes | |||
| Duration of intubation prior to VAP > 14 days, n (%) | 8 (33.3) | 13 (50) | 0.23 |
| Previous antibiotic treatment, n (%) | 21 (85.7) | 19 (72) | 0.2 |
| APACHE II score | 20.1 ± 3.6 | 18.9 ± 4.6 | 0.3 |
| Septic shock, n (%) | 5 (20.8) | 4 (15.4) | 0.62 |
| Clinical pulmonary infection score | 7.4 ± 0.9 | 7.15 ± 1.2 | 0.38 |
| Inappropriate empirical antibiotics during the initial 3 days of treatment, n (%) | 6 (25) | 9 (34.6) | 0.46 |
| NF-GNB, n (%) | 19 (79.2) | 17 (65.4) | 0.28 |
Data are presented as mean ± SD or n (%) unless otherwise indicated.
APACHE II, Acute Physiology and Chronic Health Evaluation II score; COPD, chronic obstructive pulmonary disease; NF-GNB, nonfermentative gram-negative bacilli; SD, standard deviation; VAP, ventilator-associated pneumonia.
Causative organisms
Pathogens considered responsible for VAP are listed in Table 3. The most common pathogen was Acinetobacter baumanii. NF-GNB were responsible for VAP in 79.2% of the PCT group and 65.4% of the conventional group.
Table 3.
Pathogens associated with ventilator-associated pneumonia.*
| Pathogens | PCT Group (n = 24) |
Conventional group (n = 26) |
p value |
|---|---|---|---|
| Pseudomonas aeruginosa | 11 (45.8%) | 7 (26.9 %) | 0.16 |
| Acinetobacter baumannii | 9 (37.5%) | 12 (46.1%) | 0.54 |
| Klebsiella pneumoniae | 4 (16.7%) | 3 (11.5%) | 0.7 |
| Stenotrophomonas maltophilia | 1 (4.2%) | 2 (7.7%) | 0.48 |
| Mixed gram-negative rod | 1 (4.2%) | 3 (11.5%) | 0.61 |
| Methicillin-resistant Staphylococcus aureus |
1 (4.2%) | 8 (30.8%) | 0.02 |
| Methicillin-sensitive Staphylococcus aureus |
1 (4.2%) | 0 | 0.48 |
| Multiple pathogens | 6 (25%) | 5 (19.2%) | 0.62 |
| Multidrug-resistant pathogens§ | 14 (58.3%) | 17 (65.4%) | 0.61 |
Data are presented as n (%).
Multidrug resistant: nonsusceptible to ≥1 agent in ≥3 antimicrobial categories.
PCT, procalcitonin.
Clinical pulmonary infection score and serum procalcitonin level
There was no difference between groups in the mean initial CPIS. The CPIS was significantly decreased after 7 days of appropriate treatment of VAP, including VAP caused by NF-GNB, as shown in Table 4. After 7 days of appropriate antibiotic treatment (day 8), most of the patients had a serum PCT level < 0.5 ng/ml.
Table 4.
Clinical pulmonary infection score and procalcitonin level during the study.*
| Result | PCT group (n = 24) |
Conventional group (n = 26) |
p value |
|---|---|---|---|
| CPIS day 0 | 7.4 ± 0.9 | 7.2 ± 1.2 | 0.38 |
| CPIS NF-GNB day 0 | 7.3 ± 0.8 | 7.2 ± 1.2 | 0.81 |
| CPIS day 8 | 3.6 ± 0.7 | 3.7 ± 0.8 | 0.18 |
| CPIS NF-GNB day 8 | 3.6 ± 1 | 3.7 ± 0.8 | 0.77 |
| PCT day 8, median (interquartile range), ng/ml |
0.24 (0.11– 0.4) | 0.2 (0.09–0.6) | 0.82 |
| PCT NF-GNB day 8, median (interquartile range), ng/ml |
0.3 (0.2–0.4) | 0.17 (0.11–0.35) | 0.2 |
| PCT day 8 < 0.5 ng/ml | 19 (79.2%) | 19 (73.1%) | 0.61 |
| PCT NF-GNB day 8 < 0.5 | 15/19 (78.9%) | 14/19 (73.7%) | 0.8 |
| PCT day 10, ng/ml | 0.44 ± 0.04 | – | |
| PCT day 10 < 0.5 ng/ml | 5/5 (100%) | – |
Day 0: the day of diagnosis of VAP; day 8: 7 days after appropriate treatment of VAP.
Data are presented as mean ± SD or n (%) unless otherwise indicated.
CPIS, Clinical Pulmonary Infection Score; CPIS NF-GNB, clinical pulmonary infection score of patients with VAP caused by nonfermentative gram-negative bacilli; PCT, serum procalcitonin level; PCT NF-GNB, serum procalcitonin level of patients with VAP caused by nonfermentative gram-negative bacilli; SD, standard deviation; VAP, ventilator-associated pneumonia.
Outcomes
The study outcomes are shown in Table 5. All patients were clinically cured from their VAP episodes. All of the patients in the PCT group adhered to the study protocol. Duration of VAP treatment in the conventional group was as following: 20 patients (76.9%) were treated for ⩾14 days; 3 patients (11.5%) were treated for 12 days; and 3 patients (11.5%) were treated for ⩽8 days. Multivariate analysis revealed that the PCT group was an independent predictor of fewer total antibiotic exposure days, and extrapulmonary infections was an independent predictor of greater total antibiotic exposure days during the 28 days after diagnosis of VAP. The propensity score-adjusted regression model also identified the PCT group and extrapulmonary infections as independent predictors of fewer and greater total antibiotic exposure days, respectively, as shown in Table 6.
Table 5.
Clinical outcome measures.*
| Result | PCT group (n = 24) |
Conventional group (n = 26) |
p
value |
|---|---|---|---|
| Clinical cure, n (%) | 24 (100) | 26 (100) | 1 |
| Duration of antibiotic treatment for VAP, days | 8.7 ± 1.5 | 13.3 ± 2.5 | <0.001§ |
| Total antibiotic exposure days up to 28 days after VAP onset, n (%) |
<0.001§ | ||
| 1–8 days | 8 (33.3) | 0 | |
| 9–15 days | 7 (29.2) | 5 (19.2) | |
| 16–21 days | 7 (29.2) | 5 (19.2) | |
| 22–28 days | 2 (8.3) | 16 (61.5) | |
| antibiotic-free days alive up to 28 days after VAP onset, days | 14.6 ± 5.4 | 5.9 ± 5.7 | <0.001§ |
| Recurrent pulmonary infection, n (%) | |||
| Relapse | 0 | 0 | 1 |
| Superinfection | 3 (12.5) | 7 (26.9) | 0.29 |
| Extrapulmonary infection, n (%) | 8 (33.3) | 6 (23.1) | 0.4 |
| Mechanical ventilator-free days up to 28 days after VAP onset, median (interquartile range), days |
7 (0–23) | 0 (0–13.2) | 0.055 |
Data are presented as n (%) or mean ± SD unless otherwise indicated.
Statistically significant difference.
PCT, procalcitonin; SD, standard deviation; VAP, ventilator-associated pneumonia.
Table 6.
Multivariate analysis of independent predictors of total antibiotic exposure days up to 28 days after diagnosis of ventilator-associated pneumonia.
| Variables | Coefficient (95% CI) | p value |
|---|---|---|
| Age | −0.01 (–0.11 to 0.08) | 0.77 |
| Duration of intubation before VAP >14 days | 1.1 (–1.9 to 4.1) | 0.47 |
| Previous antibiotic treatment before VAP | 0.53 (–3.9 to 5) | 0.81 |
| Shock | 2.1 (–1.9 to 6.1) | 0.3 |
| APACHE II on the day VAP was diagnosed | 0.11 (–0.28 to 0.51) | 0.56 |
| NF-GNB | 0.81 (–3.1 to 4.7) | 0.67 |
| Inappropriate empirical antibiotics during the initial 3 days of treatment | 0.24 (–3.1 to 3.6) | 0.89 |
| Extrapulmonary infections | 6.8 (3 to 10.6) | 0.001* |
| PCT group | −9.5 (–12.6 to −6.3) | <0.001* |
| Propensity score-adjusted regression model § | ||
| Propensity score | −0.73 (–7.6 to 6.2) | 0.83 |
| Extrapulmonary infections | 6.4 (3.3 to 9.5) | <0.001* |
| PCT Group | −9.1 (–12.2 to −6) | <0.001* |
Statistically significant difference.
Propensity scores represent the probability of patients being allocated to PCT or conventional group given independent variables in propensity score models. Independent variables in propensity score models: age, sex, duration of endotracheal intubation before enrollment, previous antibiotic use, shock on the day of enrollment, inappropriate empirical antibiotics during the initial 3 days of treatment, NF-GNB causative organisms, APACHE II score, and clinical pulmonary infection score on the day of enrollment.
APACHE II, acute physiology and chronic health evaluation II score; CI, confidence interval; NF-GNB, nonfermentative gram-negative bacilli; PCT, procalcitonin; VAP, ventilator-associated pneumonia.
No relapse was found in either the PCT group or the conventional group. However, seven patients (26.9%) in the conventional group and three patients (12.5%) in the PCT group developed recurrent pulmonary infections compatible with superinfections. The causative organisms of these superinfections are shown in Table 7. Most of these causative organisms were resistant to the previous antibiotics used for the initial VAP. In addition, the patients in the PCT group tended to have higher mechanical ventilation-free days during the 28 days after enrollment than the conventional group, as shown in Table 5. No patients died during the study period.
Table 7.
Pathogens associated with recurrent ventilator-associated pneumonia.
| Pathogens | |||
|---|---|---|---|
| PCT group (n = 3) | Conventional group (n = 7) | ||
| First VAP | Recurrent VAP | First VAP | Recurrent VAP |
| Pseudomonas aeruginosa | Methicillin-resistant Staphylococcus aureus |
Acinetobacter baumannii | Pseudomonas aeruginosa |
| Klebsiella pneumoniae | Pseudomonas aeruginosa | Mixed gram-negative rods | Pseudomonas aeruginosa |
| Pseudomonas aeruginosa | Acinetobacter baumannii | Pseudomonas aeruginosa | Acinetobacter baumannii |
| Mixed gram-negative rods | Acinetobacter baumannii | ||
| Acinetobacter baumannii | Mixed gram-negative rods | ||
| Acinetobacter baumannii |
Stenotrophomonas maltophilia
+Pseudomonas aeruginosa |
||
|
Stenotrophomonas maltophilia
+Pseudomonas aeruginosa |
Acinetobacter baumannii | ||
PCT, procalcitonin; VAP, ventilator-associated pneumonia.
Discussion
This study revealed four main findings: (a) NF-GNB were very common causative organisms of VAP in the authors’ institution; (b) most of the VAP in the authors’ institution was still treated for ⩾14 days; (c) most of the patients with VAP (>70%), including VAP caused by NF-GNB, who received appropriate antibiotic treatment and had favorable clinical response had their serum PCT level < 0.5 ng/ml after 7 days of appropriate antibiotic treatment; (d) CPIS and a spot serum PCT level appeared to safely guide discontinuation of antibiotic treatment in patients with VAP, and increase antibiotic-free days without increasing relapse of VAP during the 28 day follow-up period.
The PneumA study and two recent systematic review and meta-analysis, raised important concerns regarding risk of recurrence of VAP caused by NF-GNB after short fixed-course therapy (⩽8 days) compared with long-course therapy (10–15 days).7,20,21 Therefore, in practice, many clinicians continue to use extended courses for at least this subset of patients. It is widely accepted that NF-GNB are difficult to eradicate from the respiratory tract and that the risk of relapse is high in such a setting.7,22–24 In the PneumA study, the prevalence of VAP caused by NF-GNB was around 30% and the relapse rate was 32.8% in the 8-day regimen group and 19% in the 15-day regimen group.7 This implied that only a subset of VAP caused by NF-GNB would develop pulmonary infection relapse after a short course of antibiotic treatment. Therefore, instead of a fixed 8-day course of therapy for all patients, the authors developed a strategy incorporating CPIS and a spot serum PCT level to determine the subset of patients with VAP caused by NF-GNB who might need a longer duration of antibiotic treatment.
The clinical pulmonary infection score (CPIS) was developed to objectively diagnose VAP, and assigns points on the basis of clinical and radiographic data.25 Serial measurements of CPIS can define the clinical course of VAP resolution.18 The CPIS can be used to define whether or not a patient is responding to empiric therapy and if the patient is a good candidate for a reduced duration of therapy.26,27 The current study demonstrated that serial measurements of CPIS may also be used to define the resolution of VAP caused by NF-GNB.
Use of a strategy incorporating a PCT-guided antibiotic discontinuation algorithm was associated with a significantly reduced duration of antibiotic therapy.11–16 However, the PCT-based algorithms might have relative difficulty of implementation. The algorithms need serial measurements of serum PCT and physicians were encouraged to withdraw antibiotics when serum PCT was decreased by 80% or more when compared with the first day, or when it was less than 0.5 ng/ml.16 This would result in unnecessary high costs of serial PCT measurements. In the ProVAP study, the PCT measurements needed to be performed daily for 10 days.16 In a study regarding procalcitonin kinetics as a prognostic marker of VAP, a serum PCT threshold of more than 0.5 ng/ml on day 7 was the strongest independent marker of unfavorable outcomes, including death and VAP recurrence.17 Furthermore, physicians may be reluctant to follow the algorithm when there is discordance between some clinical features and the serum PCT level. Infection with NF-GNB was one of the reasons physicians did not follow the algorithm.16 Therefore, application of a variable duration protocol like PCT-based algorithms to guide antibiotic discontinuation may actually lead to a more prolonged course of antibiotics than expected. For example, the duration of antibiotic therapy in the ProVAP study was higher than those advocated in the ATS/Infectious Diseases Society of America (IDSA) guidelines28 (median 10 days in the PCT group versus 15 days in standard therapy group). Therefore, instead of PCT-based algorithms, the authors applied a short-course fixed-duration protocol, incorporated both clinical features (CPIS) and a spot serum PCT level less than 0.5 ng/ml after 7 days of appropriate antibiotic treatment, to guide discontinuation of antibiotic therapy in VAP. It is only possible to reduce duration of antibiotic therapy if clinicians have confidence that it is a safe and effective practice and if they have a protocolized approach for how to do it.
The present study has some limitations that should be mentioned when interpreting the results of this study. First, it was a nonrandomized study. Currently, as shown in the results of this study, most of the physicians in the authors’ country treated VAP caused by NF- GNB for 14 days. Therefore, it is difficult to enroll the patients into the PCT group, as 8 days was perceived to be too short for this group of patients. If a randomized study was performed, the problem of nonadherence to the protocol would definitely occur. The treating physicians who decided to join the PCT group were those who had knowledge and confidence in PCT. We used multivariate analysis and a propensity score analysis to support that the selection bias and confounding bias was minimized. Propensity score analysis has been widely adopted when making causal inferences in nonrandomized experimental trials. In addition, multivariate analysis confirmed that being allocated to the PCT group was the strongest predictor of fewer total antibiotic exposure days. Second, it was a small, single-center study, to ensure that shortening duration of treatment of VAP caused by NF-GNB, guided by CPIS and serum PCT was possible without relapse. However, other important outcomes that require a large number of patients in a study, such as mortality, could not be ascertained from the results of this study.
Conclusion
8 days of appropriate antibiotic treatment may be set as the default course for most patients with VAP, including those caused by NF-GNB. CPIS and a spot serum PCT level appeared effective and safe to guide discontinuation of antibiotic treatment in patients with VAP caused by NF-GNB, increase antibiotic free days without increasing relapse of VAP.
Acknowledgments
The authors would like to express their appreciation to Khemajira Karaketklang for statistical assistance.
Footnotes
List of abbreviations: APACHE II: acute physiology and chronic health evaluation II score
Author contributions: All authors meet the ICMJE authorship criteria. P.W. contributed to the conception, hypothesis, outline, and design of the study; data acquisition; data analysis and interpretation; drafting the manuscript and final approval of the version to be submitted. S. T. contributed to the conception and design of the study; data acquisition; data analysis and interpretation; revising the manuscript critically for important intellectual content and final approval of the version to be submitted.
Funding: This study was supported by Siriraj Research Development Fund, Mahidol University, Thailand. The funding source had no involvement in study design, collection, analysis and interpretation of data, writing of the report or decision to submit the article for publication.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Phunsup Wongsurakiat  https://orcid.org/0000-0002-6576-1690
https://orcid.org/0000-0002-6576-1690
Contributor Information
Phunsup Wongsurakiat, Division of Respiratory Disease, Department of Medicine, Siriraj Hospital, Mahidol University, Bangkoknoi, Bangkok 10700, Thailand.
Sirapat Tulatamakit, Department of Medicine, Srinakharinwirot University, Nakhon Nayok, Thailand.
References
- 1. Ling ML, Apisarnthanarak A, Madriaga G. The burden of healthcare-associated infections in southeast Asia: a systematic literature review and meta-analysis. Clin Infect Dis 2015; 60: 1690–1699. [DOI] [PubMed] [Google Scholar]
- 2. Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002; 165: 867–903. [DOI] [PubMed] [Google Scholar]
- 3. Restrepo MI, Anzueto A, Arroliga AC, et al. Economic burden of ventilator-associated pneumonia based on total resource utilization. Infect Control Hosp Epidemiol 2010; 31: 509–515. [DOI] [PubMed] [Google Scholar]
- 4. Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol 2012; 33: 250–256. [DOI] [PubMed] [Google Scholar]
- 5. Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 1999; 115: 462–474. [DOI] [PubMed] [Google Scholar]
- 6. The American Thoracic Society and the Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171: 388–416. [DOI] [PubMed] [Google Scholar]
- 7. Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 2003; 290: 2588–2598. [DOI] [PubMed] [Google Scholar]
- 8. Pugh RJ, Cooke RPD, Dempsey G. Short course antibiotic therapy for gram-negative hospital-acquired pneumonia in the critically ill. J Hosp Infect 2010; 74: 337–343. [DOI] [PubMed] [Google Scholar]
- 9. Hedrick TL, McElearney ST, Smith RL, et al. Duration of antibiotic therapy for ventilator-associated pneumonia caused by non-fermentative gram-negative bacilli. Surg Infect 2007; 8: 589–597. [DOI] [PubMed] [Google Scholar]
- 10. Chung DR Song JH Kim SH et al.;. Asian Network for Surveillance of Resistant Pathogens Study Group. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med 2011; 184: 1409–1417. [DOI] [PubMed] [Google Scholar]
- 11. Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med 2008; 177: 498–505. [DOI] [PubMed] [Google Scholar]
- 12. Schroeder S, Hochreiter M, Koehler T, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg 2009; 394: 221–226. [DOI] [PubMed] [Google Scholar]
- 13. Svoboda P, Kantorová I, Scheer P, et al. Can procalcitonin help us in timing of re-intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology 2007; 54: 359–363. [PubMed] [Google Scholar]
- 14. Hochreiter M, Koehler T, Schweiger AM, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care 2009; 13: R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouadma L Luyt CE Tubach F et al.;. PRORATA Trial Group. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375: 463–474. [DOI] [PubMed] [Google Scholar]
- 16. Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J 2009; 34: 1364–1375. [DOI] [PubMed] [Google Scholar]
- 17. Luyt CE, Guérin V, Combes A, et al. Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med 2005; 171: 48–53. [DOI] [PubMed] [Google Scholar]
- 18. Luna CM, Blanzaco D, Niederman MS, et al. Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med 2003; 31: 676–682. [DOI] [PubMed] [Google Scholar]
- 19. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 20. Pugh R, Grant C, Cooke RP, et al. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2015; 8: CD007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dimopoulos G, Poulakou G, Pneumatikos IA, et al. Short- vs long-duration antibiotic regimens for ventilator-associated pneumonia: a systematic review and meta-analysis. Chest 2013; 144: 1759–1767. [DOI] [PubMed] [Google Scholar]
- 22. Rello J, Mariscal D, March F, et al. Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients: relapse or reinfection? Am J Respir Crit Care Med 1998; 157: 912–916. [DOI] [PubMed] [Google Scholar]
- 23. Combes A, Luyt CE, Fagon JY, et al. Early predictors for infection recurrence and death in patients with ventilator-associated pneumonia. Crit Care Med 2007; 35: 146–154. [DOI] [PubMed] [Google Scholar]
- 24. Rangel EL, Butler KL, Johannigman JA, et al. Risk factors for relapse of ventilator-associated pneumonia in trauma patients. J Trauma 2009; 67: 91–95. [DOI] [PubMed] [Google Scholar]
- 25. Pugin J, Auckenthaler R, Mili N, et al. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and non-bronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 1991; 143: 1121–1129. [DOI] [PubMed] [Google Scholar]
- 26. Singh N, Rogers P, Atwood CW, et al. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit: a proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 2000; 162: 505–511. [DOI] [PubMed] [Google Scholar]
- 27. Micek ST, Ward S, Fraser VJ, et al. A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator-associated pneumonia. Chest 2004; 125: 1791–1799. [DOI] [PubMed] [Google Scholar]
- 28. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63: e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]