Abstract
NLRP1 and NLRP3 inflammasomes might differentially mediate the chronic inflammatory response in abdominal aortic aneurysm (AAA) and aortic occlusive disease (AOD). We measure differential relative gene expression of NLRP1 and NLRP3 inflammasomes in aortic tissues from 30 patients undergoing AAA open repair compared to aortic biopsies from 30 patients undergoing surgery to treat AOD. Aortic wall samples from autopsy without aortic disease were used as controls. NLRP3 was overexpressed in patients with AAA and AOD (RQ 1.185 ± 0.15, and 1.098 ± 0.05, respectively) compared to donors (RQ 1.001 ± 0.08) (OR 2.8, 95% CI 1.2–4.3, p < 0.05 for AAA and OR 2.1, 95% CI 1.1–3.8, p < 0.05 for AOD). NLRP1 gene expression was significantly upregulated in patients with AOD (RQ 1.197 ± 0.09). Meanwhile, NLRP1 was normal expressed in AAA (RQ 1.003 ± 0.07) as well as in autopsy aortic specimens (RQ 1.005 ± 0.11). Enhanced NLRP1 expression in AOD was even significant when compared to AAA (OR 2.3, 95% CI 1.2–3.3, p < 0.05) or controls (OR 2.2, 95% CI 1.1–3.1, p < 0.05). According to our findings, NLRP3 could be involved in the common etiology of AAA and AOD, whereas NLRP1 appears to have a specific role in AOD development.
Keywords: abdominal aortic aneurysm, aortic occlusive disease, inflammasomes, NLRP1, NLRP3
Introduction
Abdominal aortic aneurysm (AAA), a degenerative condition caused by chronic inflammatory processes, represents a common cause of mortality in developed countries.1 So far, the underlying molecular mechanisms of the sterile inflammatory response engaged in AAA formation have long remained elusive,2–4 especially the innate immunity role in the pathogenesis of this disease.4,5
Mounting evidence indicates that some types of these sterile inflammatory cardiovascular responses are mediated by cytosolic molecular complexes termed inflammasomes.6,7 Several different inflammasome complexes have been described to date. The nucleotide-binding oligomerization domain-like receptor (NLR) pyrin domain containing 3 (NLRP3) is the most studied of the inflammasome family members. It has been implicated in vascular inflammation through activation of macrophages in the atherosclerotic arterial wall.8,9 Furthermore, NLRP1 has been involved in the innate immune and inflammatory trigger in arterial endothelial cells implicated in endothelial dysfunction by means of the activation of the caspase-1.10
Very few recent studies have shown the impact of the NLRP3 inflammasome at the beginning of the inflammatory response in AAA formation.11,12 However, no information is available evaluating possible differences between the relative mRNA gene expression of NLRP1 and NLRP3 in human tissues of AAA compared to aortic occlusive samples and healthy aorta at the current time. Identification of different gene expression in aneurysm or occlusive diseased versus healthy states may provide a crucial development in the appraisal of the biologic role of the inflammasomes in the pathogenesis of such conditions.
Further, identifying induction of the cytosolic double-stranded DNA (dsDNA) receptor and inflammasome activator ‘absent in melanoma 2’ (AIM2) in atherosclerotic occlusive plaques and in the wall of aortic aneurysms may suggest a role of AIM2 in vascular inflammation.13
In the present study we hypothesized that such inflammasomes might mediate part of the chronic inflammatory response in AAA. To test this hypothesis, we used a quantitative real-time competitive reverse transcription polymerase chain reaction (qRT-PCR) method to measure the potential differential relative mRNA gene expression of NLRP1 and NLRP3 inflammasomes, and AIM2 in aortic tissues from patients undergoing AAA open repair compared to those from patients undergoing surgery to treat aortic occlusive disease (AOD). Aortic tissues collected from subjects from autopsy without aortic disease were used as controls.
Materials and methods
Study population
Aortic wall samples were collected from 30 consecutive patients who underwent elective open AAA surgery (mean age 71.3 ± 6 years: aneurismal size 6.3 ± 0.5 cm, range 5–11 cm; all were men), from 30 consecutive patients who underwent aortobifemoral bypass for AOD (mean age 68.3 ± 5.2 years; one woman), and from 5 autopsies (mean age 65.2 ± 13.7; four men and one woman) without clinical or macroscopic signs of aorta atherosclerosis or aneurysm.
Patients with mycotic aneurysm, ruptured aneurysm, or urgent surgery were excluded.
Patients with a diagnosis of cancer, autoimmune or rheumatological disease, transplanted or immunosuppressed were excluded to avoid possible bias.
Basic participant demographics are reported in Table 1.
Table 1.
Basic demographics and clinical characteristics.
| Variable | AAA (n = 30) | AOD (n = 30) | Autopsies (n = 5) |
|---|---|---|---|
| Sex, male (%) | 30 (100) | 29 (96) | 4 (80) |
| Age (years) ± SD | 71.3 ± 6 | 68.3 ± 5.2 | 65.2 ± 13.7 |
| Mean aortic diameter (cm) (range) | 6.3 (5–11) | – | – |
| Hypertension (%) | 25 (83) | 20 (67) | 0 |
| Hyperlipidemia (%) | 16 (53) | 17 (56) | 0 |
| Smokers (%) | 8 (27) | 18 (60) | 1 (20) |
| Ischemic heart disease (%) | 11 (37) | 4 (13) | 0 |
| COPD | 4 (13) | 7 (23) | 0 |
| DM (%) | 3 (10) | 15 (50) | 0 |
COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; SD, standard deviation.
All subjects included in this study or the families of organ donors provided written informed consent for both sample collection and inclusion in the study. This study was performed in compliance with the Declaration of Helsinki and was approved by the local ethics committee of Getafe University Hospital (P81/2015).
Aortic samples collection
Tissue samples of AAA were obtained from an area of aneurismal expansion devoid of mural thrombus, routinely removed during AAA open surgery repair. Specimens for AOD were collected from areas of grossly diseased aorta. Control aortic samples were collected from infrarenal aorta grossly free of atherosclerotic disease. Duplicate samples were collected from all research groups. Sample size determination was performed using the GRANMO program (free access). The expected sample size required to detect a difference in inflammasome expression with 5% significance and 80% power for a given variance of these genes’ expression, previously reported,12 was five patients in each group. Given the potential inflammasome transcription heterogeneity in both diseased groups, we decided to include 30 subjects in these groups of the study.
Specimens were stored at −80ºC in RNAlater® solution (Ambion, Austin, USA).
RNA preparation and cDNA synthesis
Total RNA from the samples of aortic artery wall was isolated using RNAeasy Fibrous Mini Kit (Qiagen, Hilden, Germany). Tissue was homogenized with a polytron in Trizol Reagent (U.S Patent No. 5,346,994) and centrifuged. The supernatant was extracted with chloroform and centrifuged. The supernatant was precipitated with isopropyl alcohol overnight at −20ºC and then centrifuged. The RNA pellet was washed with 75% ethanol and dissolved in ribonuclease-free distilled water with 0.1 mmol/l ethylenediamine tetraacetic acid. The purity of RNA was verified according to the ratio of 260/280 nm measurements. Values between 1.8 and 2.1 indicated that the quality of the RNA obtained was optimal and suitable for the qRT-PCR. Then, 10 µg total RNA was digested with DNase FreeTM reagent (Ambion) for 60 min, and then reverse-transcribed with Superscript IITM (Invitrogen, Carlsbad, USA) at 37ºC for 60 min, using random primers (Quiagen). The integrity of each cDNA was checked by amplification of glutaraldhehyde 3-phosphate dehydrogenase (GAPDH) with ExTaq (TaKaRa, Tokyo, Japan).
mRNA NLRP1 and NLRP3 inflammasome, and AIM2 gene analysis
The NLRP1, NLRP3 and AIM2 quantification of the transcription was carried out on the 7500 real-time PCR system using the Taqman® Universal PCR Master Mix and Assays on demand (Applied Biosystems, Carlsbad, USA). Extracted RNA from isolated tissues was initially reverse-transcribed using a High-Capacity cDNA Archive Kit (Applied Biosystems). Primers used to determine inflammasome mRNA levels (forward primer, reverse primer) were 5′-CAACAAGACTTGAACACAACGAG-3′ and 5′-CTCTCAATGACTGTGCTGGGTA-3′ for NLRP1; 5′-GATCTTCGCTGCGATCAACAG-3′ and 5′-CGTGCATTATCTGAACCCCAC-3′ for NLRP3; and 5′-AGCTGACATCTGGAGTTCATAGC-3′ and 5′-CTGCTTAGACCAGTTGGCTTG-3′ for AIM2.
Relative quantification of the NLRP3, NLRP1 and AIM2 expression was carried out using the ΔΔCT (threshold cycle) comparative method.14 The assay was run in triplicate for each case to avoid possible technical variability.
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 (IBM, New York, USA). To establish whether our variables are normally distributed we used the Kolmogorov–Smirnov and Shapiro–Wilk tests. NLRP1, NLRP3 and AIM2 inflammasomes transcription had a Gaussian distribution and was presented as means and standard deviation. Comparison of the transcription of NLRP1 and NLRP3 was provided by odds ratio (OR) and 95% confidence interval (95% CI). A p < 0.05 was considered statistically significant.
Results
Characteristics of patients
In the AAA group, 11 (37%) had suffered from ischemic heart disease, while only 4 (13%) had done so in the AOD group. In addition, 25 (83%) versus 20 (67%) patients had hypertension, 8 (27%) versus 18 (60%) were smokers, and 4 (13%) versus 7 (23%) had chronic obstructive pulmonary disease (COPD). By contrast, all subjects in the control group had no history of COPD, hypertension, hyperlipidemia, or ischemic heart disease. All but one were non-smokers, and all reported no long-term drug abuse (Table 1).
Quality of cDNAs and efficiency of real-time RT-PCR
Amplification of RN 18S1 was equivalent among all the cDNAs synthesized. Fluorescent curves of real-time RT-PCR for NLRP1, NLRP3 and AIM2 and the corresponding working standard exhibited a linear relationship with a slope factor of −2.19 and correlation coefficient of >0.99.
NLRP3 inflammasome gene expression
NLRP3 was overexpressed in aortic samples from aneurysmal disease (RQ 1.185 ± 0.15) and with AOD (RQ 1.098 ± 0.05) compared with autopsy donors (RQ 1.001 ± 0.08) (OR 2.8, 95% CI 1.2–4.3, p < 0.05 for AAA and OR 2.1, 95% CI 1.1–3.8, p < 0.05 for AOD) (Figure 1).
Figure 1.
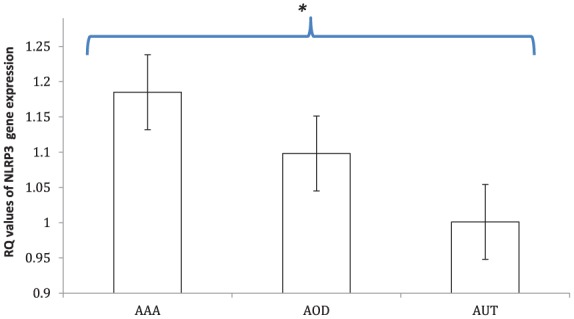
Expression levels of NLRP3 inflammasome. NLRP3 was overexpressed in aorta tissues of patients with abdominal aortic aneurysm (AAA) and aortic occlusive disease (AOD) (RQ 1.185 ± 0.15 and 1.098 ± 0.05, respectively) compared with autopsy donors (AUT) with no cardiovascular risk factors in the control group (RQ 1.001 ± 0.08) (OR 2.8, 95% CI 1.2–4.3, p < 0.05 for AAA and OR 2.1, 95% CI 1.1–3.8, p < 0.05 for AOD).
Data in histograms are given as mean ± SD; *p < 0.05. RQ, relative quantification.
NLRP1 inflammasome gene expression
Aortic tissues from patients with AOD showed significantly higher expression of the NLRP1 gene compared to aneurysmal aortic tissues (RQ 1.197 ± 0.09 versus 1.003 ± 0.07) (OR 2.3, 95% CI 1.2–3.3, p < 0.05) or to aortic tissues from controls (RQ 1.197 ± 0.09 versus 1.005 ± 0.11) (OR 2.2, 95% CI 1.1–3.1, p < 0.05) (Figure 2).
Figure 2.
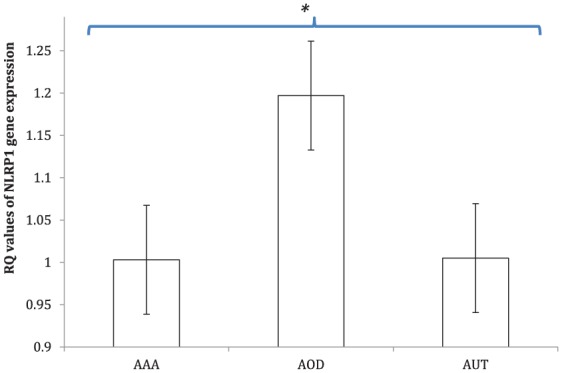
Expression levels of NLRP1 inflammasome. The gene for NLRP1 was significantly upregulated in aorta tissues of patients with aortic occlusive disease (AOD) (RQ 1.197 ± 0.09). Meanwhile, NLRP1 was normally expressed in abdominal aortic aneurysm (AAA) tissue samples (RQ 1.003 ± 0.07) as well as in autopsy aorta specimens (AUT) (RQ 1.005 ± 0.11). Enhanced NLRP1 expression in AOD was even significant when compared to AAA (OR 2.3, 95% CI 1.2–3.3, p < 0.05) or controls (OR 2.2, 95% CI 1.1–3.1, p < 0.05).
Data in histograms are given as mean ± SD; *p < 0.05. RQ, relative quantification.
NLRP1 inflammasome gene expression in aneurysmal aortic tissues was similar to that observed in aortic tissue of autopsies (RQ 1.003 ± 0.07 versus 1.005 ± 0.11, p = 0.8).
AIM2 gene expression
AIM2 was barely detectable in the samples of both AAA and AOD groups, as well as in healthy aortic tissue controls.
Discussion
The present study found overexpression of the NLRP3 gene in AAA and AOD as compared to controls, whereas the NLRP1 gene is upregulated only in AOD as compared to both AAA and normal aortic tissue. Although upregulation of NLRP3 inflammasome in the aorta of patients with ischemic heart disease was previously reported in various clinical studies,15 the novelty of our investigation is that it also demonstrates NLRP3 upregulation in AAA, leading to a potential share of the immune pathogenic pathways with atherosclerotic AOD.
Even more remarkable could be our results concerning the expression of NLRP1, significantly increased in AOD over AAA tissues. Furthermore, NLRP1 expression was not substantially different between AAA and non-diseased aortic tissues. This may be because NLRP1 was upregulated in AOD, just as in endothelial cells in occlusive peripheral disease (PAD) as previously proved,10 but not in endothelial cells or aortic tissue in AAA, where the overexpression of the NLRP1 gene may not play the role it takes in the occlusive condition.
The accumulated data confirms that inflammation is a major cause of the progression of AAA. Indeed, the innate immune system has a major contribution to the sterile inflammation involved in the pathogenesis of atherosclerosis,16 namely aneurysm or occlusive diseases. Recent investigations have clarified the importance of inflammasomes in this etiology.9,17 Interestingly, cholesterol crystals have been related to inducement of the disease by triggering NLRP3-mediated stimulation of the inflammasome, initiating as well the release of both IL-1β and IL-18.9,17 Moreover, IL-1β induces the expression of adhesion molecules, endothelin-1, and inducible nitric oxide synthase in endothelial cells.18–20
In addition, NLRP3 inflammasome activation has been linked to AAA formation through the angiotensin II pathway in adventitial macrophages. The resulting inflammatory process promotes the activation of matrix metalloproteinases (MMPs) and induces disrupted elastic lamellae, thereby leading to the onset of AAA formation.11 We previously demonstrated that NLRP1, but not NLRP3, is responsible for triggering the immune-inflammatory processes in arterial endothelial cells involving endothelial activation and stress in PAD.10
Even though it seems plausible that NLRP1 and NLRP3 may play a role in atherosclerosis, involvement of NLRP1 and NLRP3 inflammasomes in this inflammatory disease does not automatically mean these inflammasomes are identically implied in aneurysmal or occlusive disease.
NLRP1 and NLRP3 both showed overexpression in aortic occlusive tissue but not in non-diseased specimens, although only NLRP3 gene expression in AAA was significantly higher than that in non-dilated samples. Therefore, one might speculate that NLRP3 could be involved in the common pathogenesis of aneurysmal and occlusive disease, although NLRP1 appears to have a specific role in occlusive atherosclerotic development in addition to NLRP3 that is not activated in AAA formation.
Recently, AIM2 was identified as a novel inflammasome-activating protein that acts as a cytosolic DNA sensor in macrophages.21,22 AIM2 belongs to the HIN200 family of hematopoietic, interferon-inducible, nuclear proteins and appears to be essential for host defense against DNA viruses and certain cytosolic bacteria.23 Overexpression or induction of AIM2 by interferon-gamma in tumor cells resulted in reduced cell proliferation, increased cell migration, and induction of interferon-gamma-stimulated target genes. The role of AIM2 in nonmyeloid vascular cells, however, has not yet been investigated.
The AIM2 protein was immunohistochemically detected in human vascular cells of healthy and pathologic macrovascular tissues. Moreover, the proinflammatory cytokines TNF-α and interferon-gamma, as well as dsDNA, were identified as triggers to stimulate AIM2 expression in aortic endothelial cells (ECs) and vascular smooth muscle cells, respectively.24 Similar to other nonmyeloid cells, such as keratinocytes25 and certain tumor cells,26 aortic ECs might express AIM2 as a component of inflammasomes. Inflammasome activity is regulated by expression of several proteins, including NLRP3, NLRC4, NLRP6, and AIM2, which act as sensors for microbial infection of cellular damage. Alternatively, AIM2 may be involved in activation of other intracellular inflammatory pathways in these cell types.
Unfortunately, we were unable to detect AIM2 in our samples. This protein, which is clearly associated with inflammasome activity, is usually released from cells upon activation, usually restricted to the vasa vasorum and, to a minor extent, to local plaque-infiltrating cells, and may thus have been lost during tissue processing.
Endogenous AIM2 is known to be expressed at very low levels and to be rather unstable, which might result in fast degradation.
The strongest expression of AIM2 was detected within the vascular-associated lymphoid tissue and tertiary lymphoid organs of the AAA samples.13 AIM2 was detected in both compartments of tertiary lymphoid organs and in the majority of lymphocytes clustering at the adventitia media border of AAA-derived aortic tissue.
According to our data, it may be hypothesized that simultaneous upregulation of both NLRP1 and NLRP3 may have promoted an aggravation of atherosclerotic AOD, whereas aneurismal degeneration of the abdominal aorta was driven by targeted gene overexpression of NLRP3 without simultaneous NLRP1 activation.
There is currently great interest in the pathogenesis of AAA focused on identifying targets for the development of novel drug therapies to limit early-stage AAA progression.27 A better understanding of the underlying mechanisms involved in AAA formation and atherosclerosis progression, and the divergences between different clinical entities, may help us to identify new therapeutic targets that could decrease the aneurysmal progression and therefore reduce the risk of rupture.
The clinical perspective of the present research is to bring forward the knowledge of the exact role of NLRP in the processes of aortic diseases in order to find an effective, reliable, and feasible pathway to modify the NLRP expressions involved in the pathogenesis of such diseases. Unfortunately, so far there are no potential therapeutics proved to be effective in influencing the NLRP expression pathway. Further research is required to identify new drugs acting on this target.
The present study has several limitations. First, the control group was difficult to complete as aortic samples can rarely be collected from healthy humans. Thus, we selected aorta specimens from autopsies as the control group. Another limitation is the small size of the study population, which limits the statistical power. Nevertheless, the data drawn from the tissues collected show an over-activation of NLRP3 expression in the aneurysmatic aortas and an over-activation of NLRP1 in aortas from patients with AOD, which covers the shortage to a certain extent. Finally, we assessed the mRNA expression of NLRP1 and NLRP3 by qRT-PCR analysis, but did not determine the protein gene activities. Assessing the expression of NLRP3 and NLRP1 at protein levels by using immunohistochemistry or western blotting would have added relevant information. Moreover, semi-quantitative analyses of the gene transcription could provide additional information; however, it may still be difficult to extract these intracytosolic molecular complexes and to quantitatively assay enzyme activities. Therefore, the evaluation of mRNA expression of NLRP1 and NLRP3 by the present qRT-PCR method should be reliable for evaluation of the relative production of NLRP1 and NLRP3 in clinical tissue samples in this particular case. Even though, western blots of NLRP are required to complete the data and better understand the role of inflammasomes in these aortic diseases, histological analyses are also necessary to localize the overexpression of NLRP. Other markers of inflammation, such as interleukines (IL-1 β, IL-18, IL-γ) or NF-kappa-β, have to be analyzed in order to validate and complete our results.28
Additional investigation of the regulatory mechanism of NLRP1 and NLRP3 genes in which physiological and pathological stimulation upregulates an ever broader spectrum of inflammasomes will provide a clue to the pathogenesis and to devising novel therapeutic agents for aneurysmal or occlusive aortic disease, even at an established stage.
Conclusion
According to our findings our results indicate that NLRP3 inflammasome might be involved in the common etiology of AAA and occlusive disease, whereas NLRP1 inflammasome could have a specific role in occlusive atherosclerotic development.
Footnotes
Authors’ note: The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Silvia Bleda  https://orcid.org/0000-0002-8142-1063
https://orcid.org/0000-0002-8142-1063
Contributor Information
Carmen Gonzalez-Hidalgo, Angiology and Vascular Surgery Department of Getafe University Hospital, Getafe, Madrid, Spain.
Joaquin De Haro, Angiology and Vascular Surgery Department of Getafe University Hospital, Getafe, Madrid, Spain.
Silvia Bleda, Angiology and Vascular Surgery Department of Getafe University Hospital, Carretera de Toledo km 12.500, 28905 Getafe, Madrid, Spain.
Cristina Cañibano, Angiology and Vascular Surgery Department of Getafe University Hospital, Getafe, Madrid, Spain.
Ignacio Michel, Angiology and Vascular Surgery Department of Getafe University Hospital, Getafe, Madrid, Spain.
Francisco Acin, Angiology and Vascular Surgery Department of Getafe University Hospital, Getafe, Madrid, Spain.
References
- 1. Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med 1997; 126: 441–449. [DOI] [PubMed] [Google Scholar]
- 2. Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscle Thromb Vasc Biol 2006; 26: 987–994. [DOI] [PubMed] [Google Scholar]
- 3. Ludewig B, Zinkernagel RM, Hengartner H. Arterial inflammation and atherosclerosis. Trends Cardiovas Med 2002; 12: 154–159. [DOI] [PubMed] [Google Scholar]
- 4. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blasi C. The autoimmune origin of atherosclerosis. Atherosclerosis 2008; 201: 17–32. [DOI] [PubMed] [Google Scholar]
- 6. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 2006; 6: 508–519. [DOI] [PubMed] [Google Scholar]
- 7. Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 2011; 29: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanno K, Hirata Y, Imai T, et al. Induction of nitric oxide synthase gene by interleukin in vascular smooth muscle cells. Hypertension 1993; 22: 34–39. [DOI] [PubMed] [Google Scholar]
- 9. Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010; 464: 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bleda S, De Haro J, Varela C, et al. NLRP1 inflammasome, and not NLRP3, is the key in the shift to proinflammatory state on endothelial cells in peripheral arterial disease. Int J Cardiol 2014; 172: 282–284. [DOI] [PubMed] [Google Scholar]
- 11. Usui F, Shirasuna K, Kimura H, et al. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler Thromb Vasc Biol 2015;35: 127–136. [DOI] [PubMed] [Google Scholar]
- 12. Roberts RL, Van Rij AM, Phillips LV, et al. Interaction of the inflammasome genes CARD8 and NLRP3 in abdominal aortic aneurysms. Atherosclerosis 2011; 218: 123–126. [DOI] [PubMed] [Google Scholar]
- 13. Dihlmann S, Erhart P, Mehrabi A, et al. Increased expression and activation of absent in melanoma 2 inflammasome components in lymphocytic infiltrates of abdominal aortic aneurysms. Mol Med 2014; 20: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCt) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 15. Zheng F, Xing S, Gong Z, et al. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ 2013; 22: 746–750. [DOI] [PubMed] [Google Scholar]
- 16. Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clin Immunol 2010; 134: 5–24. [DOI] [PubMed] [Google Scholar]
- 17. Rajamäki K, Lappalainen J, Oörni K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 2010; 5: e11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009; 27: 519–550. [DOI] [PubMed] [Google Scholar]
- 19. Wang X, Feuerstein GZ, Gu JL, et al. Interleukin-1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis 1995; 115: 89–98. [DOI] [PubMed] [Google Scholar]
- 20. Herman WH, Holcomb JM, Hricik DE, et al. Interleukin-1 beta induces endothelin-1 gene by multiple mechanisms. Transplant Proc 1999; 31: 1412–1413. [DOI] [PubMed] [Google Scholar]
- 21. Fernandes-Alnemri T, Yu JW, Datta P, et al. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009; 458: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sagulenko V, Thygesen SJ, Sester DP, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ 2013; 20: 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saiga H, Kitada S, Shimada Y, et al. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol 2012; 24: 637–644. [DOI] [PubMed] [Google Scholar]
- 24. Hakimi M, Peters A, Becker A, et al. Inflammation-related induction of absent in melanoma 2 (AIM2) in vascular cells and atherosclerotic lesions suggests a role in vascular pathogenesis. J Vasc Surg 2014; 59: 794–803. [DOI] [PubMed] [Google Scholar]
- 25. Dombrowski Y, Peric M, Koglin S, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 2011; 3: 82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen LC, Wang LJ, Tsang NM, et al. Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol Med 2012; 4: 1276–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Golledge J, Norman PE. Current status of medical management for abdominal aortic aneurysm. Atherosclerosis 2011; 217: 57–63. [DOI] [PubMed] [Google Scholar]
- 28. Johnston WF, Salmon M, Su G, et al. Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol 2013; 33: 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]


