Abstract
Parkinson's disease (PD) is a multifactorial progressive neurological disorder. Pathological hallmarks of PD are characterized by the presence of α-synuclein (αSyn) aggregates known as Lewy bodies. αSyn aggregation is one of the leading causes for the neuronal dysfunction and death in PD. It is also associated with neurotransmitter and calcium release. Current therapies of PD are limited to only symptomatic relief without addressing the underlying pathogenic factors of the disease process such as aggregation of αSyn. Consequently, the progression of the disease continues with the current therapies. Therefore, the modulation of αSyn aggregation is an emerging approach as a novel therapeutic target to treat PD. There are two major aspects that might be targeted therapeutically: first, protein is prone to aggregation, therefore, anti-aggregative or compounds that can break the pre-existing aggregates should be helpful. Second, there are number of molecular events that may be targeted to combat the disease.
Keywords: : α-synuclein, D-520, Lewy body, molecular tweezers, Parkinson's disease, polyphenols
Parkinson's disease (PD) is a multifactorial progressive neurological disorder that results from the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNPc) region of the brain [1]. There is plethora of findings suggesting a crucial role of α-synuclein (αSyn) in the genesis of PD. The common observations for the involvement of αSyn aggregation in PD are: first, αSyn is a component of Lewy bodies (LBs) and Lewy neurites, an intracytoplasmic eosinophilic hyaline inclusions, the cardinal hallmark of PD pathology present in the different brain regions in the PD patients [2]. Incidentally LBs and Lewy neurites have been reported in normal healthy individuals as well [3]. Second, familial early onset of PD is caused by overexpression of αSyn due to triplication of αSyn gene (SNCA) locus. Three missense mutations in αSyn can increase the aggregation propensity of this protein, which may lead to early onset of PD [4,5]. Third, αSyn forms toxic oligomers or fibrils species, which could lead to cell death. Currently, it is not known how the aggregation of αSyn triggers cell death, but it has been postulated that soluble oligomeric form of αSyn, known as protofibrils, are the major culprit. These protofibrils can cause cell death via their interaction on various cellular targets [6]. Further, the secreted αSyn may migrate from one neuron to another neuron and may contribute to the disease propagation. αSyn aggregates can increase oxidative stress in the SNPc region of the PD brain which can lead to vesicular dopaminergic leakage. Abundance of iron in the SNPc region catalyzes oxidative stress which might also promote αSyn misfolding and aggregation in the cytoplasm [7,8]. αSyn can directly interact with dopamine (DA) transporters, which eventually promotes the DA transporters mediated DA uptake and cellular apoptosis [9].
PD is also associated with mitochondrial dysfunction and oxidative stress. Overexpression of αSyn in mice causes mitochondrial dysfunction, which can lead to the formation of reactive oxygen species (ROS) and eventually cell death [10]. Elevated level of αSyn can also damage lysosomes and golgi apparatus. Finally, increased human αSyn expression in transgenic flies (Drosophila) is associated with loss of dopaminergic neurons, formation of intracellular inclusions and locomotor dysfunction [11].
In human, there are three synuclein family members namely α-, β-, γ- and all the synuclein genes are relatively well conserved both within and between species. Primate αSyn sequence differs from other vertebrate's synuclein by substitutions of alanine for a threonine at position 53. Synucleins have overall 44% identity among all three members, β-synuclein shares 78% identity with αSyn and γ-synuclein shares 60% identity with αSyn [13]. All three members of synuclein family are presynaptic neuronal protein, there are some evidences of involvement of extra nigral β and γ synuclein in synulceinopathies [14]. αSyn is expressed at high level in the brain but it is also found, in erythrocytes and platelets [15]. αSyn, a 140 amino acid protein encoded through SNCA gene, forms α-helical structure on binding to the lipids and β-sheet structure on prolong period of incubation in in vitro.
Primary structure of αSyn can be divided into three regions. First, the N-terminus region with a conserved motif KTKEGV, this part confers the propensity to form α-helical structure on membrane binding. Second, the central 61–95 amino acids also known as nonamyloid β component (NAC), is associated with an increased fibril forming potential. The presence of hydrophobic amino acid residues, GAVVTGVTAVA, in this region plays an important role in initiation of aggregation. Third, the highly conserved C-terminal region from 96–140 amino acids imparts chaperone property to αSyn [13].
The functions of αSyn are unclear, but due to its association with synaptic vesicles, the main function seems to be the release of neurotransmitter through the effect on soluble NSF (N-ethylmaleimide sensitive fusion protein) attachment protein receptor (SNARE) complex [6]. It has been postulated that αSyn can play a role in synaptic plasticity, and it regulates specific pool of synaptic vesicles to modulate synaptic function in the brain [16,17]. αSyn may act as a protein chaperone to protect the cell against the stress-induced death [18], and it may be associated with the non-motor symptoms of PD [19].
Theories of αSyn propagation: αSyn is present not only in intracellular compartment but it has been detected in the extracellular fluids including plasma and cerebrospinal fluid (CSF) [20,21]. αSyn, either monomer or in aggregated form can leave cells via multiple mechanisms such as secretion, exocytosis, impairment of autophagy–lysosome pathway or via exosomes. Once αSyn leaves the donor cell, it can reach to the recipient cell either via direct cell-to-cell transfer or through the tunneling nanotubes [22]. It can gain access to the recipient cell by directly interacting with lipid and membranes, or by passive diffusion. Due to the nucleation or seeding activity of the released αSyn, it can lead to the impairment of the proteasome activity in the recipient cell. In PD, αSyn aggregates in the CNS appears first in the lower brain stem nuclei, and spreads sequentially into the mid brain, followed by mesocortical and neocortical regions [23]. This progression can either be due to aggregation in the different regions of brain independent of each other. Alternatively, protein aggregates formed in different areas of the brain during the earlier stages of PD may be transmitted to the other regions by a mechanism similar to prion propagation. αSyn is a cytosolic protein as opposed to prion, therefore, it has to cross the membrane barriers for the transmission to the neighboring cells. Therefore, energy-dependent mechanisms are required for the transmission of αSyn from donor to recipient neurons. However, release of cytoplasmic αSyn aggregates can happen through passive mechanisms such as the membrane disruption [24]. The release of αSyn via exocytosis can increase under the stress condition.
Pathogenic effects of αSyn: overexpression of αSyn can lead to the neurotoxicity in cell culture and in in vivo animal models [25]. Further, it inhibits neurotransmitter release by reducing the size of synaptic vesicle recycling pool, and by remarkable changes in SNARE protein [6,25]. PD can result from high activity of L-type Ca2+ channel, that increases cytoplasmic Ca2+, which can upregulate DA synthesis, and further interaction of DA with αSyn leading to the formation of toxic oligomeric species [26]. At lower concentrations both wild-type and mutant αSyn oligomers can change the permeability of vesicular membrane by forming pore-like structures on the membrane which may cause excessive influx of calcium [27]. Overexpression of αSyn in PC12 cells can change vesicular pH which leads to the release of excessive neurotransmitter such as DA, and may cause the oxidative stress induced cell death [28].
Double labeling immunofluorescence revealed that, tubulin colocalized with αSyn in LB, infact, tubulin accelerate αSyn aggregation [29]. αSyn can enhance the tau phosphorylation because of its interaction with tubulin protein. αSyn can also affect cellular trafficking and synaptic functions. Both wild-type (WT) and mutant αSyn can cause proteasomal and lysosomal dysfunction, furthermore, WT αSyn can reduce autophagy [6]. αSyn interacts with the histone protein in the nucleus, and reduces the histone acetylation.
Aggregation potential of αSyn: it is not yet completely known whether intrinsically αSyn exists in monomeric or in tetrameric form [30,31]. WT and mutants (A53T and A30P) αSyn can undergo self-aggregation at higher concentrations. The mutant form, particularly A53T, can aggregate at rapid speed in in vitro as the mutation causes disruption of the α-helical structure [32]. The fibrillation is a nucleation polymerization process, which can be divided into an initial lag phase, followed by the exponential growth phase, and an equilibrium phase. During this process initially soluble oligomeric species known as protofibrils, can take various shapes such as spherical or ring. The insoluble protofibrils associate with each other into fibril ranging in the size from 10 nm and above. Some of these intermediate species can be observed on SDS/polyacrylamide gel while other forms can be separated on native gel or by size exclusion chromatography. There is no general consensus on which species, in other words, soluble or insoluble, is more neurotoxic but a general hypothesis is that early soluble species are more toxic than the fibril. Fibrillation rate mainly depends on concentration, presence of other metals, pH and temperature.
It has been shown in the literature that fibril generated in in vitro from both the WT and mutant αSyn, possesses very similar features like amyloid fibril [33,34]. The morphology of αSyn aggregates was characterized by using electron microscopy, congo red and thioflavin T (ThT), Fourier transform-IR and circular dichroism (CD) spectrum. WT as well as mutant αSyn, upon incubation forms fibrils in size range of 8–10 nm in height and 10 nm in width as well as some spherical species of 4 nm in height. The size of fibril may be similar for both WT and mutant proteins but the morphology can vary from one type of αSyn to another type. These fibrills can be stained with the antibody preparation stain LB in PD patients. The disappearance and appearance of minimum at 200 and 220 nm, respectively, in CD indicates the formation of β-sheet structures. The antiparallel β-sheet structure can be confirmed by amide I band at 1626 cm-1 and amide II band at 1693 cm−1 in IR spectroscopy. The fibril structure of αSyn can be further confirmed by its binding to the dye congo red and ThT. The absorption spectrum of congo red shifts from 490 to 540 nm in presence of fibril α-SN, while fibril of αSyn can be detected by strong increase in ThT signal at 490 nm upon excitation at 450 nm.
Several mechanisms have been proposed for the αSyn aggregation but oxidative stress and ubiquitin proteasome system (UPS) inhibition are the most validated till now [35,36]. Metabolism of DA in the nigral neurons produces ROS and other highly reactive species such as DA-quinone. DA-quinone can interact with specific amino acid of αSyn, probably with lysine, and inhibit further conversion of protofibril to mature fibril both in vitro and in vivo. Artificially designed αSyn variants that form oligomers in in vitro have shown enhanced neurotoxicity in cell culture and in vivo animal model over the mature fibrils forming αSyn variant [34,37]. Overexpression of mutant version of αSyn can lead to the DA neuronal cell death due to excessive generation of intracellular ROS. This dopaminergic-specific neurotoxicity of αSyn can be inhibited by the specific tyrosine hydroxylase inhibitor, α-methyl-p-tyrosine, in the cultured dopaminergic neurons [38]. It has been shown in the literature that WT αSyn overexpression in cells may regulate the DA and melanin synthesis by acting on several enzymes such as tyrosine hydroxylase or dihydroxyphenylalanine (DOPA) decarboxylase [39]. UPS is the one of the major biochemical pathways responsible for degradation of normal and abnormal intracellular proteins. It has been shown in the literature that impaired ubiquitin-dependent protein degradation is one of the major factors responsible for the neurodegenerative disease such as PD, which leads to the accumulation of polyubiquitinted chains of LB-ubiquitin in the SNPc region of brain [40,41].
There are literature evidences that the altered metal homeostasis can lead to the loss of dopaminergic neurons in the SN region of brain [42]. In this regard, iron is the central point of attention because it is the most abundant metal of the body and it has been found that total nigral iron level is increased in PD brain compared with the controls. Metals, especially iron can lead to the fibrilization of αSyn either via the release of long-range interaction between N- and C-terminus region of αSyn or metals such as iron can lead to generation of hydroxyl radicals by Fenton reaction which can further cause oxidation of αSyn known as metal catalyzed oxidation. Interestingly, phosphorylation at Tyr125 or at Ser129 can increase trivalent metal binding to the C-terminus of αSyn.
αSyn can bind to Cu2+ via several binding sites. The Cu2+ complex with αSyn promotes formation of αSyn oligomers that are cytotoxic in nature toward the SHSY-5Y neuroblastoma cells. Due to the low redox potential of αSyn-Cu2+ complex, it can oxidize certain cellular reductants such as ascorbic acid, GSH, which leads to generation of H2O2 [43]. DA cannot be oxidized by αSyn–Cu2+ complex but it is the H2O2 which can oxidize DA into the corresponding quinone.
Modulation of αSyn aggregation as a therapeutic target to treat PD
αSyn aggregation is one of the leading causes of neuronal dysfunction and death in PD. The modulation of its aggregation is emerging as a novel therapeutic target to treat PD. Assuming that the toxicity arises due to the aggregated form of αSyn, possible therapeutic strategies are depicted in Figure 1. There are two major aspects that might be targeted therapeutically: first, protein is prone to aggregate, therefore, anti-aggregative or compounds that can break the pre-existing aggregates may be helpful. Second, there are number of molecular events such as aggregation propagation or accumulation of aggregates that may be targeted therapeutically.
Figure 1. . Possible therapeutic pathways to modulate α-synuclein aggregation.
Small molecules as a possible modulator of αSyn aggregation
Effect of various polyphenolic compounds on αSyn aggregation: in the last decade small organic molecules specifically polyphenols have been extensively tested for their ability to inhibit αSyn aggregation. It has been clearly shown that certain polyphenols can dramatically inhibit cell death induced by αSyn aggregates [44–46]. Fruits such as berries, black and green tea and red wine are rich sources of polyphenols. Most of the polyphenols are salubrious due to their potent antioxidant nature. These agents can neutralize free radical via hydrogen atom abstraction as shown in Figure 2.
Figure 2. . Mechanism of free radical quenching ability of polyphenols.
Where POH indicates polyphenols, PO. and R. represent free radicals. The PO-free radical can further react with a second radical leading to the formation of stable quinone structure as shown in Figure 2. In general, the radical scavenging ability depends on the molecular structure and the substitution pattern of hydroxyl group. Besides the radical scavenging ability of the polyphenols, they can also bind to the metal ion which may further enhance their antioxidant activity. Dietary intake of berries can reverse cognitive and motor deficit in rats and that can lead to lower incidence of dementia. epigallocatechin-3-gallate, an antioxidant and metal-chelating polyphenol from green tea has been shown to be neuroprotective in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced animal model of PD [47].
Meng et al. evaluated 48 flavonoids belonging to different classes with structurally different in ring substitutions and in the nature/extent of alkylation for their ability to inhibit in vitro fibrillation of αSyn [48]. The different flavonoids affected the αSyn fibrillation to a varying extent which was confirmed by lower ThT fluorescence intensity for the samples containing these flavonoids [49]. The analysis of various species of αSyn formed in absence or presence of flavonoids was based on transmission electron microscopy (TEM), dynamic light scattering (DLS), size-exclusion chromatography (SEC) and SDS-PAGE. The inhibition of αSyn fibril formation may be due to either inhibition of nucleus formation occurring during lag time or the inhibition of fibril elongation. When the most potent flavonoids in this series were added during different time points starting from 0, 12, 21 and 33 h, they were shown to inhibit the fibril formation completely. It seems that the vicinal dihydroxylphenyl group of the flavonoids is responsible for inhibition of αSyn fibrillation. Moreover, the difference in the number of the vicinal dihydroxyl group and the number of individual hydroxyl group also lead to variation in the inhibitory activities of the flavonoids. Generally higher the number of hydroxyl groups, the stronger is the αSyn fibril inhibitor as illustrated in Figure 3.
Figure 3. . Comparison of potent and weak α-synuclein aggregation modulators.
The molecular mechanism underlying the flavonoids induced inhibition of αSyn fibrillation may be due to the combination of noncovalent binding of flavonoids to αSyn, and the covalent modification by the flavonoids. This may lead to the restriction of the conformational changes in this natively unfolded protein, or the stabilization of soluble flavonoid-modified species of αSyn.
Substantial evidences revealed that flavonoids are salubrious and neuroprotective in vivo; however, little is known about their mechanism [50–52]. According to one model, antioxidant property of flavonoids is more important rather than their ability to modulate cell signaling pathways [48].
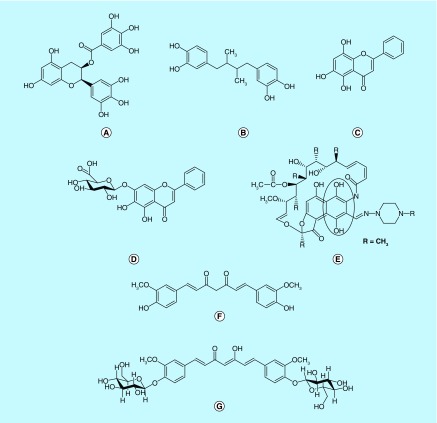
Using confocal single molecular fluorescence spectroscopy, Caruana et al. studied the effect of naturally occurring polyphenolic compounds on αSyn oligomers formation [53]. This technique allows using nanomolar and low micromolar concentrations of αSyn and inhibitors. αSyn was fluorescently labeled using Alexa Fluor dye, and it was incubated at 20–30 nM concentration, either alone or in presence of iron. Further, the atomic force microscopy, and confocal single-molecular fluorescence were used to characterize the aggregates. It was found that most of the polyphenols were able to inhibit the aggregation of αSyn either alone or in presence of iron. The selected polyphenols were tested for their ability to disaggregate the preformed oligomers. Interestingly, epigallocatechin-3-gallate and nordihydroguaiaretic acid (NDGA) exhibited 260 and 20 nM, respectively, IC50 for disaggregation of existing αSyn aggregates (Figure 4A & B). Overall, the potency of compounds to inhibit and disaggregate αSyn aggregates could be correlated to the number of hydroxyl groups present on single phenyl group, and it was observed that potency follows this order trihydroxyl > dihydroxyl > monohydroxyl compounds. In this aggregation model, the compounds with three adjacent hydroxyl groups on the same phenyl ring were more potent compared with the compounds with total number of three hydroxyl groups distributed on different rings. To establish the hypothesis that position of hydroxyl groups plays a vital role in αSyn aggregation modulation, authors compared the effect of apigenin (Figure 4C), scutellarein (Figure 4D) and baicalein on αSyn aggregation [53].
Figure 4. . Polyphenols with ability to modulate αSyn aggregation.
(A) epigallocatechin gallate (EGCG); (B) nordihydroguaiaretic acid (NDGA); (C) apigenin; (D) scutellarein; (E) rifampicin; (F) curcumin; and (G) curcumin-glucoside.
αSyn: Alpha-synuclein.
Interestingly, scutellarein and baicalein turned out to be equally effective in the inhibition and disaggregation of preformed oligomers, however, apigenin did not show promising activity in this assay. In this series, NDGA (Figure 4B) showed exceptional activity probably due to symmetrical structure it can conformationally orient with αSyn in such a way that it can inhibit aggregation to a greater extent.
αSyn aggregation plays a pivotal role in the pathogenesis of various diseases including PD, dementia with Lewy bodies (DLB) and multiple system atrophy. Oxidative stress has been implicated in pathogenesis of PD due to generation of highly reactive species such as free radicals and superoxide [54–56]. In this regard, antioxidant compounds can prevent or reduce the rate of progression of the disease by acting as free radical scavengers. The fluorescence and electron microscopy were used to study the effect of major natural antioxidant compounds including NDGA, curcumin, rifampicin and tetracycline on αSyn aggregation [57]. The effect was observed on the formation and destabilization of preformed αSyn fibril in presence of CSF of the patients with DLB (DLB-CSF). Interestingly, the antifibrillogenic and fibril destabilizing activities were in agreement with antioxidant potency of flavanoids used in this study. Curcumin (Figure 4F) a polyphenolic compound, has antioxidant and anti-inflammatory properties. Wang et al. tested curcumin against αSyn induced cytotoxicity in SH-SY5Y neuroblastoma cell line [58]. Extracellular incubation of SH-SY5Y cells with oligomeric but not the monomeric or fibril form of αSyn can induce significant cytotoxicity. Curcumin can significantly reduce the cytotoxicity of preformed αSyn oligomeric species by reducing the ROS and inhibiting caspase-3 activity. It can also protect the cells against intracellular αSyn toxicity induced by overexpressing αSyn in transient transfected SH-SY5Y cells. Effect of both acute and chronic curcumin treatments in transgenic mice overexpressing human GFP tagged WT αSyn was studied using multiphoton imaging [59]. In this study, moderate dose of curcumin increased the level of phosphorylated αSyn at cortical presynaptic terminal. Curcumin-treated mice also showed significant improvement in various motor behavior tests. A series of biophysical techniques, in other words, fluorescence, and 2D-NMR were applied to find out the mechanism of action of curcumin [60]. Curcumin, upon binding to the preformed oligomer and fibril alters the hydrophobic surface area of αSyn aggregates which eventually reduces the level of toxic oligomeric species. Through various biochemical-, biophysical- and cell-based assays, it was shown that pyrazole derivatives of curcumin could significantly reduce the toxicity of both WT and mutant αSyn (A53T) [61].
Recently, novel curcumin-glucoside (curc-gluc, Figure 4G) derivatives were synthesized, characterized and evaluated for anti-aggregation potential of αSyn [62]. Curc-gluc inhibited oligomers and fibril formation in a dose-dependent manner. The binding efficacy of curc-gluc toward monomeric and oligomeric αSyn was characterized by microcalorimeter titration. The results indicated that the low- and high-heat values of curc gluc for monomeric and oligomeric αSyn, respectively. Curcumin suffers from poor bioavailability and it also undergoes rapid degradation at physiological pH into ferulic acid, vanillin and dehydrozingerone. The ability of dehydrozingerone and its analogs to modulate αSyn aggregation was studied using various biophysical techniques [63]. The C-2 symmetric dimer analogs of dehydrozingerone are able to interact with αSyn with high affinity and also showed potent antioxidant property. Rifampicin, a semisynthetic derivative of rifamycins, obtained from Nocardia mediterranei is commonly used for treatment of leprosy (Figure 4). Patients on the treatment with rifampicin are less prone to develop senile dementia [64]. It has been shown that rifampicin and its analogs, inhibited Aβ1–42 aggregation and neurotoxicity in vitro [65]. Based on these observations and napthoquinoe core in the structure of rifampicin, Li et al., investigated it for αSyn aggregation inhibitory property [66]. ThT, and far-UV CD spectra has supported that rifampicin can inhibit and disaggregate α-SN fibrillation in a dose-dependent manner. After incubation of αSyn at 50 μM in presence of 100 μM rifampicin for 42 h, the SEC profile indicated large amount of monomer of αSyn left over compared with the incubation of αSyn alone [66]. In anaerobic condition in presence of antioxidant, the inhibitory effect of rifampicin reduced significantly which indicated the oxidized quinone form was majorly responsible for its activity. Quinones are susceptible to nucelophilic attack via Michael addition to form imine with the lysine side chain of αSyn leading to covalent modification. The spectrum of αSyn oligomers stabilized by rifampicin showed the evidence for such covalent modifications, but it was not confirmed by mass spectrum.
Baicalein (Figure 3), a Chinese herbal medicine, is a well-known potent antioxidant, free radical scavenger and iron chelator. It can potentially inhibit αSyn oligomerization in both cell-free and cellular systems [67]. El-Agnaf et al., tested eight different compounds for their antioligomeric and antifibrillar activities using oligomeric-specific ELISA and bimolecular fluorescence complementation (BiFC) assays to monitor oligomers formation in cell-free and cellular systems, respectively [67]. Baicalein effectively inhibited αSyn oligomerization at 50 μM in a dose-dependent manner. First time using the oligomeric-specific BiFC assay, authors demonstrated that baicalein inhibited the formation of high molecular weight (HMW) αSyn oligomers in Hela and SH-SY5Y cell lines transfected with GFP-N terminal fraction αSyn and αSyn-GFP-C terminal fraction plasmids [67]. Furthermore, it alleviated the neurotoxicity of αSyn oligomers in SH-SY5Y transfected with GFP-N terminal fraction αSyn and αSyn-GFP-C terminal fraction plasmids by preventing Lactate dehydrogenase (LDH) release. Baicalein can stabilize oligomers that are less toxic as examined by membrane permeability assay. Presence of vicinal dihydroxyl group on the phenyl ring of baicalein could be responsible for its anti-aggregation action. DA analogs have been studied for their effect on the αSyn aggregation [68,69]. αSyn can readily aggregate into the fibril form upon incubation for sufficient period of time and this process can be monitored by using ThT fluorescence assay, and atomic force microscopy technique. Li et al. confirmed the hypothesis that quinone form of polyphenols is efficacious inhibitors of αSyn fibrillation by increase in absorbance in the UV spectra at 280 and 345 nm in SEC profile, ThT assay and SDS-PAGE [69]. Further, mass spectrum analysis revealed a large amount of αSyn adduct dimers in which one protein dimer was attached with several quinones to give a molecular mass mixture of different species. The quinones interacted with lysine residue of αSyn which lead to the inhibition of αSyn fibrilization. The covalent cross-linked adducts formed by DA were majorly HMW oligomers, while most of the catechol formed monomeric or dimeric species with αSyn. Although, DA modification inhibited αSyn fibrilization process, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on PC12 cells revealed that DA-modified high-molecular-weight oligomers of αSyn were significantly more cytotoxic in comparison to low-molecular-weight species of αSyn.
Lee et al., showed that release of αSyn in differentiated SHSY-5Y cells can increase in a dose-dependent fashion by elevation of DA concentration which can lead to the formation of αSyn aggregates within the vesicles and these aggregates can be secreted from the cells [70].
Another small molecule as a possible modulator of αSyn aggregation is selegiline which is a noncompetitive monoamine oxidase B inhibitor and has been widely used to treat PD either alone or in combination with other drugs (Figure 5H). The effect of selegiline on in vitro aggregation of A30P αSyn and WT αSyn has been studied by Braga et al [71] using NMR, CD, ThT and SDS-PAGE gel analysis. It has been shown that selegiline can delay the fibril formation by extending the lag phase of aggregation and forms heterogeneous aggregates of αSyn, which are innocuous to the primary culture enriched in dopaminergic neurons. The mechanism of interference of selegiline with αSyn aggregation is not yet completely known, however, it greatly interferes with earlier nucleation formation and to a lesser extent with fibril elongation process.
Figure 5. . Novel small heterocyclic molecules with ability to modulate αSyn aggregation.
(A) selegiline; (B & C) molecular tweezers; (D & E) tryptophan and napthoquinone derivatives; (F) B2; (G & H) SNX-2112 and analog (I & J) D519 and D520; (K & L) 2-pyridone analogs; and (M) ceftriaxone.
Recently, Prabhudesai et al. discovered the water soluble ‘molecular tweezers’, termed CLR01 specific for lysine as a general inhibitor of aggregation and toxicity of amyloid proteins including αSyn (Figure 5I & J) [72]. CLR01 binds specifically with the lysine residue of αSyn via the hydrophobic and electrostatic forces. It inhibited the aggregation of αSyn into the fibril and caused disaggregation of preformed fibril. It can also stabilize αSyn in the small, nontoxic oligomeric species and inhibits the toxicity of αSyn in the cell culture model dose dependently. Furthermore, CLR01 has been assessed in zebrafish embryo against the αSyn-induced neurotoxicity. Addition of CLR01 to the water in which zebrafish embryo was developed led to dramatic improvement in viability and it maintained αSyn in soluble form by restoring UPS activity. The molecular basis of inhibition of αSyn aggregation through CLR01 has been reported [73]. CLR01 binds to multiple lysine residues, and potentially reconfigures the kinetically controlled aggregation process. Interestingly, αSyn monomers are prone to aggregation, however, CLR01 upon binding can increase the rate of dissociation of αSyn aggregates to monomers [73].
The inhibition properties of tryptophan and napthoquinone derivatives are based on the assumption that there may be a common mechanism of toxicity for the diseases such as PD, AD and Type II diabetes which are characterized by amyloidal species formation (Figure 5K & L). Aromatic amino acids play a critical role in the formation and growth of amyloid fibril through the Π-stacking. Small hybrid compounds with tryptophan and napthoquinone moieties termed as NQTrp and Cl-NQTrpare capable of inhibiting the fibrilization and oligomerization of amyloidogenic proteins including αSyn in in vitro. It has been shown that NQTrp and Cl-NQTrp are able to inhibit the fibril formation of αSyn by using the ThT, TEM and other techniques [74].
It is also possible that compounds that might not inhibit the αSyn inclusion formation but yet can block downstream pathways responsible for the toxicity of αSyn aggregates [75]. One of the lead molecules, B2 (Figure 5M), was tested for its effect on αSyn inclusion formation in the CHO-K1 cells transiently transfected with SynT, a tagged α-SN. After B2 treatment a significant enhancement in the αSyn aggregates was observed. But when it was tested on H4 neuroglioma cells transfected with αSyn a significant reduction in αSyn-mediated adenylate kinase release was observed and it protected against toxicity from overexpression of αSyn.
LB contains αSyn as well as several heat shock proteins (Hsp), which are molecular chaperones. Hsp modulators are protective against αSyn-induced toxicity, and can prevent its aggregation. Stress can lead to Hsp70 upregulation and it refolds pathogenic species of αSyn or can direct the misfolded species toward the proteasomal or lysosomal degradation [76]. The interaction of Hsp70 with overexpressed αSyn is neuroprotective due to its ability to decrease the high-molecular-weight oligomeric species. Hsp90 is critical for folding, stabilization and binding of many proteins including αSyn. Inhibition of Hsp90 leads to activation of the HSF1 and Hsp70.
Currently available Hsp90 inhibitors such as geldanamycin, and its analogs 17-(allylamino)-17-demethoxygeldanamycin, and 17-dimethylaminoethylamino-17-demethoxygeldanamycin suffer from poor bioavailability. While, SNX-2112 and its derivatives are novel orally available potent Hsp90 inhibitors that can rescue αSyn-induced toxicity and oligomerization in in vitro [76]. Using the bioluminescent complementation assay, Putcha et al. measured the effect of novel SNX derivatives on αSyn oligomerization. H4 cells were either pretreated or cotreated with SNX derivatives, and transiently transfected with Syn-Luc1 and Syn-Luc2. The cells were assayed for luciferase activity. Most of the derivatives inhibited αSyn oligomerization by more than 75% but four compounds: SNX-3113, SNX-3723, SNX-8891 and SNX- 0723 were found most potent in this series (Figure 5N & O, SNX-3723 and SNX- 0723, respectively).
Novel synthetic peptides are based on the native sequence of the protein that can prevent the conversion into the toxic species. El-Agnaf et al. identified the critical binding region in the αSyn molecule responsible for its self-aggregation [77]. Then, the library of small peptides homologous to this region was synthesized and the binding of these peptides was studied using an ELISA assay. αSyn at the 50 μM, either alone or with peptide inhibitors at the molar ration of 2:1, 1:1 and 1:2 was incubated at 37°C for 4 days with continuous shaking at 1000 rpm. Based upon the ELISA assay for binding to αSyn, the NAC terminus region containing amino acid residues from 64 to 86 were found to be mainly responsible for its aggregation. Based on the hydrophobic/hydrophilic nature of amino acid residues and other criteria, novel peptides were designed, synthesized and further evaluated for their αSyn aggregation inhibition properties [77]. The shortest peptide responsible for inhibition of αSyn aggregation has the sequence AVVT which is similar to the central NAC region of αSyn. Furthermore, these peptides were able to inhibit the aggregation of NAC region. The ability of these peptides to cross the blood–brain barrier is yet to be determined.
Other alternative pathways to combat αSyn toxicity
The total amount of αSyn is very critical factor for its aggregation and can increase due to the enhanced transcription or due to reduced degradation of αSyn. Regulator factor for αSyn transcription are not clearly known but intron 1 of SNCA is found to be responsible element for its transcription. Using the siRNA approach, Vekrellis et al. found that zinc finger proliferation 1 as one of the elements at the 5′ end of intron 1 is responsible for αSyn transcription in PC12 cell [78]. Apart from this, extracellular-signal-regulated kinase and PI3K are also important for the regulation of αSyn level. Micro RNAs are also emerging as novel regulator for the αSyn gene expression. Degradation of αSyn occurs via lysosomal pathway of chaperone-mediated autophagy, macro autophagy or by proteasome. Targeting these αSyn degradation pathways are emerging as novel approach to treat PD. Active and passive immunization is another approach emerging as novel strategy to treat the synucleinopathies. Masliah et al. vaccinated transgenic mice with human αSyn which produced relative high-affinity antibodies. A decrease in accumulation of aggregated αSyn in neuronal cell bodies and synapses along with improvement in behavioral deficit was observed [79]. Furthermore, the generated antibody in these mice was able to promote the clearance of intracellular αSyn aggregates. The mechanism of aggregation clearance is not yet known but possibly antibody might have entered in the neurons either by receptor mediated or by endocytosis followed by the antigen–antibody complex formation, and finally promoted the degradation by lysosomal activation. In another study, Ingelsson et al. used transfected H4 neuroglioma cells to study the effect of monoclonal αSyn antibody on dimerization or oligomerization using BiFC assay [80]. After addition of αSyn C-terminal antibody a significant reduction in the GFP fluorescence was observed while the mid region antibody did not reduce the GFP signals which indicated that αSyn oligomerization was not affected by irrelevant monoclonal antibody. Vaccine AFFiRiS PD 01(AFFITOPE), composed of seven amino acids, is under clinical trial. It targets αSyn aggregates responsible for PD by inducing the antibody that recognizes αSyn but not β-synuclein [81].
Withanolide A, constituent of Withania somnifera has been reported for several neuroprotective effects including αSyn aggregation modulation property. Treatment of Caenorhabditis elegans strain (NL5901) with 5 μM of withanolide A exhibited significantly lower expression of αSyn tagged with yellow fluorescent protein compared with control group [82]. A fragment-based approach was developed to screen large database of small molecules to identify inhibitors of intrinsically disordered protein. In the first step, 72 compounds known for binding with αSyn were used to find 139 fragments expected to interfere with αSyn aggregation. Based on these fragments, a large dataset of the compound was screened which resulted in the identification of 14,735 compounds exhibiting necessary feature required for αSyn aggregation modulation [83]. Aggregation of αSyn in the presence and absence of two isomers of inositol namely scyllo and chiro was observed. Interestingly, TEM analysis revealed that active isomer scyllo-inositol inhibited both human and mouse αSyn aggregation to a significant extent, however, chiro-inositol turnout to be inactive in this experiment [84]. Yeast cells expressing GFP-αSyn were pretreated with PEFS for 6 h to determine the cells metabolic capacity and quantification of αSyn expression. The leaves extract of corema album promotes the formation of nontoxic αSyn aggregates in vitro and diminishes the toxicity of the aggregates of αSyn due to the presence of polyphenols. The detail mechanistic analysis revealed the reduction of oxidative stress and increase in autophagic flux from corema album leaf extract treatment [46].
In order to develop multifunctional dopaminergic D2/D3 agonist various dopaminergic agonists were designed, synthesized and biologically evaluated for their ability to cross BBB and modulate αSyn aggregation in vitro and in vivo. One such compound, D520 (Figure 5P), turned out to be a potent agonist towards DA D2 and D3 receptors (Ki, D2 = 41.8, D3 = 0.35 nM; EC50 D2 = 4.73 nM, D3 = 2.18 nM). Furthermore, D520 displayed excellent in vivo activity in PD animal models. Additionally, D520 is able to modulate the toxicity of αSyn aggregates to a significant extent compared with the positive control rifampicin and also provided neuroprotection against 6-OHDA-induced toxicity [85]. Recently, D519 (Figure 5Q), and D520 were tested for their ability to modulate αSyn aggregation in a novel in vitro assay and Drosophila model of synucleinopathy. The αSyn aggregates formed in the presence of D519 and D520 at different time points exhibited less toxicity toward PC-12 cells compared with αSyn alone. Further, both the compounds could rescue the αSyn-induced toxicity in the eyes of Drosophila melanogaster in a dose-dependent manner (Figure 5P & Q) [86]. This results validated a proof of concept for developing multifunctional therapeutic agents with symptomatic and disease modifying effects for treatment of PD.
A series of fused 2-pyridone analogs was synthesized and evaluated for its impact on αSyn aggregation property. Minor modifications in the central ring of pyridone have significant effect on αSyn fibrillization property of the compounds (Figure 5R & S). Lipophilicity, presence of sulfur, and amine groups are critical for αSyn modulation property, however, electronic nature of substituent does not play a vital role in the aggregation inhibition. Interestingly, some compounds could increase fibril formation and others could induce oligomerization of αSyn. It is yet not known, whether, fibrils are toxic or oligomers, therefore, detail studies are required to further confirm the toxic nature of the species [87].
The β-lactam antibiotic ceftriaxone has shown promising nueroprotective effect in in vivo PD animal model (Figure 5T) [88]. The in vivo efficacy of ceftriaxone has been rationalized on the basis of its ability to block αSyn polymerization [89]. Interestingly, it could also rescue PC-12 cells from 6-OHDA induced toxicity. Further, molecular docking studies of ceftriaxone with αSyn revealed the significance of C-terminus tyrosine residues in the inhibition of fibril formation. In another in vitro studies, the authors found that low concentration of trehalose was much effective in stabilization of the αSyn aggregation compared with high concentration of trehalose [90].
Conclusion & future perspective
Protein aggregation is one of the cardinal features of neurodegenerative diseases. αSyn, a small 140 amino acid protein, which plays a pivotal role in the pathogenesis of PD, can undergo aggregation. Currently available treatments for PD provide only symptomatic relief without addressing the pathogenetic factors responsible for the disease process. Furthermore, these treatments are only symptomatic in nature and do not provide any disease-modifying effect. Hence, there is an important unmet need of novel therapies which should not only alleviate the symptoms of the disease but also be able to modify the disease process to slow or stop the progression. In this respect, the design and discovery of novel αSyn aggregation modulators as neuroprotective treatment agents for PD is an emerging trend. In recent years, several studies focusing on elucidation of structural features required for modulation of αSyn aggregation have been reported. Numerous small molecules including the natural products have been explored for their potential to interfere with αSyn aggregation in the PD pathogenesis. Further, studies are going on to elucidate the toxic nature of oligomeric or fibril forms of αSyn. These reports also shed a light on the pathogenesis and possible preventative measures for PD. The newer concept of multifunctional drug development for multifactorial diseases such as PD is likely to change the scenario in the area of design and development of therapeutic agents. These molecules have to undergo rigorous stages of preclinical and clinical trials before coming into the clinic. Current research in the PD area is focused on the development of such agents which will hopefully add disease-modifying properties to the current mostly symptomatic treatment regimen.
Executive summary.
Parkinson's disease (PD) is a multifactorial progressive neurological disorder characterized by loss of dopaminergic neurons in the substantia nigra pars compacta region of the brain.
The cardinal hallmark of PD pathology is the presence of α-synuclein (αSyn) aggregates in the Lewy bodies (LBs) and Lewy neurites found in the different brain regions in the PD patient.
The complete mechanism of αSyn-induced toxicity toward dopaminergic neurons is not yet known. However, it has been postulated that soluble oligomeric form of αSyn, known as protofibrils, is the major toxic species.
The central nonamyloid β component region of αSyn is mainly responsible for the β-sheet formation upon prolong period of incubation in vitro.
Several mechanisms have been proposed for the αSyn migration from one neuron to other neuron. Overexpression of αSyn can lead to neuronal cell death in vitro and in vivo PD models via multiple mechanisms.
The morphology of αSyn aggregates can be characterized with help of atomic force microscopy, congo red, thioflavin T, Fourier transform-IR and CD spectrum.
The modulation of αSyn aggregation is emerging as leading approach for the neuroprotective treatment of PD. So, compounds having the ability to break existing oligomers and can interfere with molecular mechanisms involved in the αSyn aggregation have been synthesized for effective PD treatment.
In the last decade polyphenols have been extensively tested for their ability to modulate αSyn aggregation.
The difference in the number of the vicinal dihydroxyl group and the number of individual hydroxyl groups on the aromatic ring are one of the major factors responsible for differences in the inhibitory activities of the flavonoids.
The molecular mechanism underlying the flavonoids induced inhibition of αSyn fibrillation may be the combination of noncovalent and covalent interactions of the flavanoids with αSyn.
Oxidative stress has been implicated in pathogenesis of PD, therefore, antioxidant compounds can prevent or reduce the rate of progress of the disease.
Natural and synthetic antioxidant compounds were tested to observe their effect on the formation and destabilization of preformed αSyn fibril in presence of cerebrospinal fluid of the patients with dementia with Lewy bodies.
Curcumin and its novel analogs upon binding to the preformed oligomers and fibril, alters the hydrophobic surface area of αSyn aggregates which eventually reduces the level of toxic oligomeric species.
Selegiline (monoamine oxidase inhibitor) can delay the fibril formation due to interference with earlier nucleation formation by extending the lag phase of aggregation and forms heterogeneous aggregates of αSyn.
Molecular tweezers can specifically interact with lysine residue of αSyn via the hydrophobic and electrostatic forces to act as inhibitor of αSyn aggregation.
Hsp90 inhibitors are also under investigation for the protective effect against αSyn-induced toxicity.
The regulator factor for αSyn transcription is an attractive target to treat the synuclepathies.
Selective dopaminergic multifunctional agonists have shown promising activity in both in vitro and in vivo PD animal models as well as in αSyn aggregation models.
Protein aggregation is one of the critical features responsible for neurodegeneration.
The design and development of novel molecules with ability to provide the symptomatic relief along with modulation of αSyn aggregation as exhibited by D520 should provide significant improvement for the treatment of PD.
Acknowledgements
GP Modi thanks D Kumar for his help during preparation of this manuscript.
Footnotes
Financial & competing interests disclosure
Part of the work cited in this manuscript is supported by the National Institute of Neurological Disorders and Stroke/National Institute of Health (NS047198, AKD). GP Modi and SK Singh are thankful to Indian Institute of Technology (BHU) for providing seed money (SM/2016-17/1198/L) and Department of Biotechnology (BT/PR9624/MED/30/1253/2013 dated 29/11/2014), respectively, to carry out part of this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Przedborski S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017;18(4):251–259. doi: 10.1038/nrn.2017.25. [DOI] [PubMed] [Google Scholar]
- 2.Silva BA, Einarsdottir O, Fink AL, Uversky VN. Biophysical characterization of alpha-synuclein and rotenone interaction. Biomolecules. 2013;3(3):703–732. doi: 10.3390/biom3030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J. Neuropathol. Exp. Neurol. 2009;68(7):816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emanuele M, Chieregatti E. Mechanisms of alpha-synuclein action on neurotransmission: cell-autonomous and non-cell autonomous role. Biomolecules. 2015;5(2):865–892. doi: 10.3390/biom5020865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasuda T, Nakata Y, Mochizuki H. α-Synuclein and neuronal cell death. Mol. Neurobiol. 2013;47(2):466–483. doi: 10.1007/s12035-012-8327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefanis L. Alpha-synuclein in Parkinson's disease. Cold Spring Harb. Perspect. Med. 2012;2(2):a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Jiang H, Song N, Xie J. Oxidative stress partially contributes to iron-induced alpha-synuclein aggregation in SK-N-SH cells. Neurotox. Res. 2011;19(3):435–442. doi: 10.1007/s12640-010-9187-x. [DOI] [PubMed] [Google Scholar]
- 8.Munoz Y, Carrasco CM, Campos JD, Aguirre P, Nunez MT. Parkinson's disease: the mitochondria–iron link. Parkinsons Dis. 2016;2016:7049108. doi: 10.1155/2016/7049108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler B, Saha K, Rana T, et al. Dopamine transporter activity is modulated by alpha-synuclein. J. Biol. Chem. 2015;290(49):29542–29554. doi: 10.1074/jbc.M115.691592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramaniam SR, Vergnes L, Franich NR, Reue K, Chesselet MF. Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol. Dis. 2014;70:204–213. doi: 10.1016/j.nbd.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buttner S, Habernig L, Broeskamp F, et al. Endonuclease G mediates alpha-synuclein cytotoxicity during Parkinson's disease. EMBO J. 2013;32(23):3041–3054. doi: 10.1038/emboj.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988;8(8):2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paleologou KE, Irvine GB, El-Agnaf OM. Alpha-synuclein aggregation in neurodegenerative diseases and its inhibition as a potential therapeutic strategy. Biochem. Soc. Trans. 2005;33(Pt 5):1106–1110. doi: 10.1042/BST20051106. [DOI] [PubMed] [Google Scholar]
- 14.Galvin JE, Uryu K, Lee VM, Trojanowski JQ. Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc. Natl Acad. Sci. USA. 1999;96(23):13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Yu S, Li F, Feng T. Detection of alpha-synuclein oligomers in red blood cells as a potential biomarker of Parkinson's disease. Neurosci. Lett. 2015;599:115–119. doi: 10.1016/j.neulet.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Cheng F, Vivacqua G, Yu S. The role of alpha-synuclein in neurotransmission and synaptic plasticity. J. Chem. Neuroanat. 2011;42(4):242–248. doi: 10.1016/j.jchemneu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Burre J. The synaptic function of alpha-synuclein. J. Parkinsons Dis. 2015;5(4):699–713. doi: 10.3233/JPD-150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witt SN. Molecular chaperones, alpha-synuclein, and neurodegeneration. Mol. Neurobiol. 2013;47(2):552–560. doi: 10.1007/s12035-012-8325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jellinger KA. Synuclein deposition and non-motor symptoms in Parkinson disease. J. Neurol. Sci. 2011;310(1–2):107–111. doi: 10.1016/j.jns.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Tinsley RB, Kotschet K, Modesto D, et al. Sensitive and specific detection of alpha-synuclein in human plasma. J. Neurosci. Res. 2010;88(12):2693–2700. doi: 10.1002/jnr.22417. [DOI] [PubMed] [Google Scholar]
- 21.Unterberger U, Lachmann I, Voigtlander T, et al. Detection of disease-associated alpha-synuclein in the cerebrospinal fluid: a feasibility study. Clin. Neuropathol. 2014;33(5):329–334. doi: 10.5414/NP300796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner JA, Angot E, Brundin P. A deadly spread: cellular mechanisms of alpha-synuclein transfer. Cell Death Differ. 2011;18(9):1425–1433. doi: 10.1038/cdd.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SJ, Lim HS, Masliah E, Lee HJ. Protein aggregate spreading in neurodegenerative diseases: problems and perspectives. Neurosci. Res. 2011;70(4):339–348. doi: 10.1016/j.neures.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 2010;11(4):301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemani VM, Lu W, Berge V, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65(1):66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosharov EV, Larsen KE, Kanter E, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62(2):218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Scala C, Yahi N, Boutemeur S, et al. Common molecular mechanism of amyloid pore formation by Alzheimer's beta-amyloid peptide and alpha-synuclein. Sci. Rep. 2016;6:28781. doi: 10.1038/srep28781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosharov EV, Staal RG, Bove J, et al. Alpha-synuclein overexpression increases cytosolic catecholamine concentration. J. Neurosci. 2006;26(36):9304–9311. doi: 10.1523/JNEUROSCI.0519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emamzadeh FN. Alpha-synuclein structure, functions, and interactions. J. Res. Med. Sci. 2016;21:29. doi: 10.4103/1735-1995.181989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477(7362):107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fauvet B, Mbefo MK, Fares MB, et al. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J. Biol. Chem. 2012;287(19):15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry. 2000;39(10):2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 33.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013;14(1):38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winner B, Jappelli R, Maji SK, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc. Natl Acad. Sci. USA. 2011;108(10):4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson's disease. J. Parkinsons Dis. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, et al. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J. Neurosci. 2011;31(41):14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karpinar DP, Balija MB, Kugler S, et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson's disease models. EMBO J. 2009;28(20):3256–3268. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA. Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 2002;8(6):600–606. doi: 10.1038/nm0602-600. [DOI] [PubMed] [Google Scholar]
- 39.Pan T, Zhu J, Hwu WJ, Jankovic J. The role of alpha-synuclein in melanin synthesis in melanoma and dopaminergic neuronal cells. PLoS ONE. 2012;7(9):e45183. doi: 10.1371/journal.pone.0045183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Q, Figueiredo-Pereira ME. Ubiquitin/proteasome pathway impairment in neurodegeneration: therapeutic implications. Apoptosis. 2010;15(11):1292–1311. doi: 10.1007/s10495-010-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkin G, Paulson H. Ubiquitin pathways in neurodegenerative disease. Front. Mol. Neurosci. 2014;7:63. doi: 10.3389/fnmol.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Lorenzo F. Iron and Parkinson's disease. Neuro Endocrinol. Lett. 2015;36(1):24–27. [PubMed] [Google Scholar]
- 43.Carboni E, Lingor P. Insights on the interaction of alpha-synuclein and metals in the pathophysiology of Parkinson's disease. Metallomics. 2015;7(3):395–404. doi: 10.1039/c4mt00339j. [DOI] [PubMed] [Google Scholar]
- 44.Caruana M, Vassallo N. Tea polyphenols in Parkinson's disease. Adv. Exp. Med. Biol. 2015;863:117–137. doi: 10.1007/978-3-319-18365-7_6. [DOI] [PubMed] [Google Scholar]
- 45.Ardah MT, Paleologou KE, Lv G, et al. Structure activity relationship of phenolic acid inhibitors of α-synuclein fibril formation and toxicity. Front. Aging Neurosci. 2014;6:197. doi: 10.3389/fnagi.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macedo D, Tavares L, Mcdougall GJ, et al. (Poly)phenols protect from alpha-synuclein toxicity by reducing oxidative stress and promoting autophagy. Hum. Mol. Genet. 2015;24(6):1717–1732. doi: 10.1093/hmg/ddu585. [DOI] [PubMed] [Google Scholar]
- 47.Xu Q, Langley M, Kanthasamy A, Reddy M. Neurorescue effect of EGCG in an animal model of Parkinson's disease. FASEB J. 2016;30(Suppl. 1):1174.1111–1174.1111. [Google Scholar]
- 48.Meng X, Munishkina LA, Fink AL, Uversky VN. Effects of various flavonoids on the alpha-synuclein fibrillation process. Parkinsons Dis. 2010;2010:650794. doi: 10.4061/2010/650794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine H., 3rd Quantification of beta-sheet amyloid fibril structures with thioflavin T. Meth. Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 50.Leonardo CC, Doré S. Dietary flavonoids are neuroprotective through Nrf2-coordinated induction of endogenous cytoprotective proteins. Nutr. Neurosci. 2011;14(5):226–236. doi: 10.1179/1476830511Y.0000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gutierrez-Merino C, Lopez-Sanchez C, Lagoa R, Samhan-Arias AK, Bueno C, Garcia-Martinez V. Neuroprotective actions of flavonoids. Curr. Med. Chem. 2011;18(8):1195–1212. doi: 10.2174/092986711795029735. [DOI] [PubMed] [Google Scholar]
- 52.Ren R, Shi C, Cao J, et al. Nueroprotective effects of a standardized flavonoid extract of safflower against neurotoxin-induced cellular and animal models of Parkinson's disease. Sci. Rep. 2016;6:22135. doi: 10.1038/srep22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caruana M, Hogen T, Levin J, Hillmer A, Giese A, Vassallo N. Inhibition and disaggregation of alpha-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011;585(8):1113–1120. doi: 10.1016/j.febslet.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 54.Hwang O. Role of oxidative stress in Parkinson's disease. Exp. Neurobiol. 2013;22(1):11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson's disease. Front. Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaki GS, Papavassiliou AG. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson's disease. Neuromolecular Med. 2014;16(2):217–230. doi: 10.1007/s12017-014-8294-x. [DOI] [PubMed] [Google Scholar]
- 57.Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro . J. Neurochem. 2006;97(1):105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- 58.Wang MS, Boddapati S, Emadi S, Sierks MR. Curcumin reduces alpha-synuclein induced cytotoxicity in Parkinson's disease cell model. BMC Neurosci. 2010;11:57. doi: 10.1186/1471-2202-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spinelli KJ, Osterberg VR, Meshul CK, Soumyanath A, Unni VK. Curcumin treatment improves motor behavior in alpha-synuclein transgenic mice. PLoS ONE. 2015;10(6):e0128510. doi: 10.1371/journal.pone.0128510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh PK, Kotia V, Ghosh D, Mohite GM, Kumar A, Maji SK. Curcumin modulates alpha-synuclein aggregation and toxicity. ACS Chem. Neurosci. 2013;4(3):393–407. doi: 10.1021/cn3001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahsan N, Mishra S, Jain MK, Surolia A, Gupta S. Curcumin pyrazole and its derivative (N-(3-nitrophenylpyrazole) curcumin inhibit aggregation, disrupt fibrils and modulate toxicity of wild type and mutant alpha-synuclein. Sci. Rep. 2015;5:9862. doi: 10.1038/srep09862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gadad BS, Subramanya PK, Pullabhatla S, Shantharam IS, Rao KS. Curcumin-glucoside, a novel synthetic derivative of curcumin, inhibits alpha-synuclein oligomer formation: relevance to Parkinson's disease. Curr. Pharm. Des. 2012;18(1):76–84. doi: 10.2174/138161212798919093. [DOI] [PubMed] [Google Scholar]
- 63.Marchiani A, Mammi S, Siligardi G, et al. Small molecules interacting with alpha-synuclein: antiaggregating and cytoprotective properties. Amino Acids. 2013;45(2):327–338. doi: 10.1007/s00726-013-1503-3. [DOI] [PubMed] [Google Scholar]
- 64.Yulug B, Hanoglu L, Kilic E, Schabitz WR. RIFAMPICIN: an antibiotic with brain protective function. Brain Res. Bull. 2014;107:37–42. doi: 10.1016/j.brainresbull.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Qosa H, Abuznait AH, Hill RA, Kaddoumi A. Enhanced brain amyloid-beta clearance by rifampicin and caffeine as a possible protective mechanism against Alzheimer's disease. J. Alzheimers Dis. 2012;31(1):151–165. doi: 10.3233/JAD-2012-120319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J, Zhu M, Rajamani S, Uversky VN, Fink AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem. Biol. 2004;11(11):1513–1521. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 67.Lu JH, Ardah MT, Durairajan SS, et al. Baicalein inhibits formation of alpha-synuclein oligomers within living cells and prevents Abeta peptide fibrillation and oligomerisation. ChemBioChem. 2011;12(4):615–624. doi: 10.1002/cbic.201000604. [DOI] [PubMed] [Google Scholar]
- 68.Latawiec D, Herrera F, Bek A, et al. Modulation of alpha-synuclein aggregation by dopamine analogs. PLoS ONE. 2010;5(2):e9234. doi: 10.1371/journal.pone.0009234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li HT, Lin DH, Luo XY, et al. Inhibition of alpha-synuclein fibrillization by dopamine analogs via reaction with the amino groups of alpha-synuclein. Implication for dopaminergic neurodegeneration. FEBS J. 2005;272(14):3661–3672. doi: 10.1111/j.1742-4658.2005.04792.x. [DOI] [PubMed] [Google Scholar]
- 70.Lee HJ, Baek SM, Ho DH, Suk JE, Cho ED, Lee SJ. Dopamine promotes formation and secretion of non-fibrillar alpha-synuclein oligomers. Exp. Mol. Med. 2011;43(4):216–222. doi: 10.3858/emm.2011.43.4.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braga CA, Follmer C, Palhano FL, et al. The anti-Parkinsonian drug selegiline delays the nucleation phase of alpha-synuclein aggregation leading to the formation of nontoxic species. J. Mol. Biol. 2011;405(1):254–273. doi: 10.1016/j.jmb.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 72.Prabhudesai S, Sinha S, Attar A, et al. A novel “molecular tweezer” inhibitor of alpha-synuclein neurotoxicity in vitro and in vivo . Neurotherapeutics. 2012;9(2):464–476. doi: 10.1007/s13311-012-0105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Acharya S, Safaie BM, Wongkongkathep P, et al. Molecular basis for preventing alpha-synuclein aggregation by a molecular tweezer. J. Biol. Chem. 2014;289(15):10727–10737. doi: 10.1074/jbc.M113.524520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scherzer-Attali R, Shaltiel-Karyo R, Adalist YH, Segal D, Gazit E. Generic inhibition of amyloidogenic proteins by two naphthoquinone-tryptophan hybrid molecules. Proteins. 2012;80(8):1962–1973. doi: 10.1002/prot.24080. [DOI] [PubMed] [Google Scholar]
- 75.Bodner RA, Outeiro TF, Altmann S, et al. Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington's and Parkinson's diseases. Proc. Natl Acad. Sci. USA. 2006;103(11):4246–4251. doi: 10.1073/pnas.0511256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Putcha P, Danzer KM, Kranich LR, et al. Brain-permeable small-molecule inhibitors of Hsp90 prevent alpha-synuclein oligomer formation and rescue alpha-synuclein-induced toxicity. J. Pharmacol. Exp. Ther. 2010;332(3):849–857. doi: 10.1124/jpet.109.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Agnaf OM, Paleologou KE, Greer B, et al. A strategy for designing inhibitors of alpha-synuclein aggregation and toxicity as a novel treatment for Parkinson's disease and related disorders. FASEB J. 2004;18(11):1315–1317. doi: 10.1096/fj.03-1346fje. [DOI] [PubMed] [Google Scholar]
- 78.Vekrellis K, Stefanis L. Targeting intracellular and extracellular alpha-synuclein as a therapeutic strategy in Parkinson's disease and other synucleinopathies. Expert Opin. Ther. Targets. 2012;16(4):421–432. doi: 10.1517/14728222.2012.674111. [DOI] [PubMed] [Google Scholar]
- 79.Masliah E, Rockenstein E, Adame A, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46(6):857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Nasstrom T, Goncalves S, Sahlin C, et al. Antibodies against alpha-synuclein reduce oligomerization in living cells. PLoS ONE. 2011;6(10):e27230. doi: 10.1371/journal.pone.0027230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schneeberger A, Mandler M, Mattner F, Schmidt W. Vaccination for Parkinson's disease. Parkinsonism Relat. Disord. 2012;18(Suppl. 1):S11–S13. doi: 10.1016/S1353-8020(11)70006-2. [DOI] [PubMed] [Google Scholar]
- 82.Akhoon BA, Pandey S, Tiwari S, Pandey R. Withanolide A offers neuroprotection, ameliorates stress resistance and prolongs the life expectancy of Caenorhabditis elegans . Exp. Gerontol. 2016;78:47–56. doi: 10.1016/j.exger.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 83.Joshi P, Chia S, Habchi J, Knowles TP, Dobson CM, Vendruscolo M. A fragment-based method of creating small-molecule libraries to target the aggregation of intrinsically disordered proteins. ACS Comb. Sci. 2016;18(3):144–153. doi: 10.1021/acscombsci.5b00129. [DOI] [PubMed] [Google Scholar]
- 84.Ibrahim T, Mclaurin J. α-Synuclein aggregation, seeding and inhibition by scyllo-inositol. Biochem. Biophys. Res. Commun. 2016;469(3):529–534. doi: 10.1016/j.bbrc.2015.12.043. [DOI] [PubMed] [Google Scholar]
- 85.Modi G, Voshavar C, Gogoi S, et al. Multifunctional D2/D3 agonist D-520 with high in vivo efficacy: modulator of toxicity of alpha-synuclein aggregates. ACS Chem. Neurosci. 2014;5(8):700–717. doi: 10.1021/cn500084x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yedlapudi D, Joshi GS, Luo D, Todi SV, Dutta AK. Inhibition of alpha-synuclein aggregation by multifunctional dopamine agonists assessed by a novel in vitro assay and an in vivo Drosophila synucleinopathy model. Sci. Rep. 2016;6:38510. doi: 10.1038/srep38510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horvath I, Sellstedt M, Weise C, et al. Modulation of alpha-synuclein fibrillization by ring-fused 2-pyridones: templation and inhibition involve oligomers with different structure. Arch. Biochem. Biophys. 2013;532(2):84–90. doi: 10.1016/j.abb.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 88.Leung TCH, Lui CNP, Chen LW, Yung WH, Chan YS, Yung KKL. Ceftriaxone ameliorates motor deficits and protects dopaminergic neurons in 6-hydroxydopamine-lesioned rats. ACS Chem. Neurosci. 2012;18(3(1)):22–30. doi: 10.1021/cn200072h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruzza P, Siligardi G, Hussain R, et al. Ceftriaxone blocks the polymerization of alpha-synuclein and exerts neuroprotective effects in vitro . ACS Chem. Neurosci. 2014;5(1):30–38. doi: 10.1021/cn400149k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruzza P, Hussain R, Biondi B, et al. Effects of trehalose on thermodynamic properties of alpha-synuclein revealed through synchrotron radiation circular dichroism. Biomolecules. 2015;5(2):724–734. doi: 10.3390/biom5020724. [DOI] [PMC free article] [PubMed] [Google Scholar]