Abstract
Aim:
To validate the total illness burden index for prostate cancer (TIBI-CaP) in castration-resistant prostate cancer (CRPC) patients.
Patients & methods:
Baseline comorbidity scores collected using the TIBI-CaP were compared with the baseline patient-reported health-related quality of life using the SF-12v2 and FACT-P questionnaires in 302 patients enrolled in the Treatment Registry for Outcomes in CRPC Patients (TRUMPET).
Results:
Baseline TIBI-CaP scores were negatively correlated with all baseline SF-12v2 domain/composite (p < 0.001) and FACT-P subscale/total (p < 0.020) scores. There was a significant decreasing linear trend in SF12v2 and FACT-P scores over the categories based on TIBI-CaP quartiles of comorbidity burden (from ‘least’ to ‘severe’).
Conclusion:
The TIBI-CaP is a valid measure of comorbidity burden in patients with CRPC in the real world.
Keywords: : castration-resistant prostate cancer, CRPC, TIBI-CaP, total illness burden index for prostate cancer, TRUMPET registry
Lay abstract
This study aimed to validate the total illness burden index for prostate cancer (TIBI-CaP), a patient-reported measure of comorbidity burden, in a cohort of men enrolled in the Treatment Registry for Outcomes in Castration-resistant Prostate Cancer (CRPC) Patients (TRUMPET). Results from this study demonstrate that the TIBI-CaP is a valid measure of comorbidity burden in CRPC patients in the real world and a useful research tool for risk adjustment in CRPC studies.
Treatment for castration-resistant prostate cancer (CRPC) is aimed at slowing disease progression, prolonging survival, improving symptoms and stabilizing/increasing patients’ health-related quality of life (HRQoL). The American Urological Association suggests observation with continuous androgen deprivation therapy for patients with nonmetastatic CRPC and first-generation antiandrogens (flutamide, bicalutamide and nilutamide) or first-generation androgen synthesis inhibitors (ketoconazole plus steroids) for patients with nonmetastatic CRPC who do not accept observation [1]. Treatment options for metastatic CRPC include the oral androgen-receptor inhibitor enzalutamide, the oral CYP17 inhibitor abiraterone acetate plus prednisone, the microtubule inhibitor cabazitaxel, the immunotherapeutic agent sipuleucel-T and the radiopharmaceutical radium-223 [1,2].
Prior research indicates that clinical outcomes may be influenced by the collective comorbidity burden (i.e., the number and severity of comorbid conditions other than prostate cancer) of a patient with prostate cancer [3]. The total illness burden index for prostate cancer (TIBI-CaP) is a patient-reported measure of comorbidity burden that was adapted from the total illness burden index [4] for use in patients with prostate cancer. Previously, the TIBI-CaP has been validated as a measure of comorbidity burden in men with biopsy-proven adenocarcinoma of the prostate in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) registry [5], where an association between the TIBI-CaP and self-reported HRQoL was demonstrated. The TIBI-CaP has also been shown to predict future HRQoL and nonprostate cancer mortality in this patient population [3,5,6]. Consequently, an evaluation of comorbidity burden at diagnosis of CRPC may provide important information about likely clinical and quality-of-life outcomes of treatment.
The TIBI-CaP has not been validated as a measure of comorbidity burden in patients with CRPC; therefore, our objective was to validate the TIBI-CaP in a cohort of men enrolled in the Treatment Registry for Outcomes in CRPC Patients (TRUMPET), a USA-based, prospective, observational multicenter registry (NCT02380274) designed to evaluate treatment patterns, comorbidities and HRQoL outcomes associated with CRPC (nonmetastatic [M0] and metastatic [M1]) in real-world settings [7]. To complete this validation, baseline TIBI-CaP scores were compared with patient-reported baseline generic and disease-specific HRQoL as measured by the short form (SF)-12v2 and the functional assessment of cancer therapy – prostate (FACT-P) questionnaires [2,8,9]. Based on previous validation results in localized prostate cancer, CRPC patients with higher TIBI-CaP scores (greater comorbidity burden) were expected to have worse HRQoL.
Patients & methods
Study population
The TRUMPET registry is studying consented men with CRPC enrolled from community and academic institutions between March 2015 and December 2017, observed for up to 4 years or until death or study discontinuation [7]. A target of approximately 1200 patients (not yet reached) will be enrolled from 150 urology and oncology sites over the study period.
Inclusion criteria for the TRUMPET registry include: male (age ≥18 years) with a confirmed diagnosis of CRPC (defined by a minimum of two rising prostate-specific antigen levels measured at least 7 days apart and serum testosterone level ≤1.73 nmol/l [50 ng/dl] or new evidence of metastatic disease); an estimated life expectancy of ≥6 months; initiating the first course of active treatment for M0 or M1 CRPC (antiandrogens, androgen synthesis inhibitors, chemotherapy, immunotherapy or radiopharmaceutical therapy) within 45 days prior to enrollment; and able to complete HRQoL questionnaires with or without assistance. Exclusion criteria are: currently enrolled in any interventional clinical trial with a nonapproved investigational agent for the primary disease of CRPC; or receiving concomitant treatment for other cancers (excluding basal cell carcinoma and hormone sensitive prostate cancer) within 6 months prior to enrollment [7].
Data for this analysis were obtained from a subset of 355 patients enrolled in the TRUMPET registry from March 2015 to July 2016 or the predefined first interim analysis of the cohort's baseline data. A total of 302 (85.1%) CRPC patients, who had nonmissing TIBI-CaP scores, were available for the interim analysis.
Data elements
Baseline data collected from patients enrolled in the TRUMPET registry included self-reported sociodemographics, clinical history, comorbidities and HRQoL.
Comorbidity data were collected at study entry using the TIBI-CaP, which consists of 81 items in 11 subdimensions, including pulmonary disease, heart disease, stroke and neurologic disease, gastrointestinal conditions, other cancers (excluding prostate), arthritis, foot and leg conditions, eye and vision conditions, hearing problems, hypertension and diabetes. Scores are derived for each subdimension, with higher scores corresponding to greater severity of comorbidities. A composite TIBI-CaP score is calculated by aggregating the 11 individual subdimension scores, as previously described [10].
Patient HRQoL was collected using the SF-12v2 and the FACT-P self-reported questionnaires. The SF-12v2 is a well-known generic measure of HRQoL that has been validated in both healthy and diseased patients suffering from a variety of medical conditions. The SF-12 v2 comprises eight domains (physical functioning, role functioning [physical and emotional], bodily pain, general health, vitality, social functioning and mental health) and two summary measures (the physical component summary [PCS] and the mental component summary [MCS]) [9]. SF-12 v2 domains and summary measures are scored on a scale ranging from 0, representing the lowest level of health, to 100, representing the highest level of health. The FACT-P consists of two sections: the FACT-G (general), a 27-item questionnaire that measures HRQoL in cancer patients and a 12-item prostate cancer subscale [8]. The prostate cancer subscale was designed to measure prostate cancer-specific quality of life. The FACT-P is the most widely used patient-reported outcome instrument in CRPC, whereby changes in the total score by at least 6–10 points on a 0–156-point scale indicate worsening or improving HRQoL [11].
Statistical analysis
Statistical analyses were performed using SAS® software, version 9.4 (NC, USA). Continuous variables are presented as sample size (n), standard deviations (SDs), medians and ranges. Categorical variables are summarized as number and percentage of the total study population. Percentages, by quartile of TIBI-CaP scores, were based on the number of subjects with no missing data.
Patients were placed into four categories based on the quartiles of the composite TIBI-CaP scores: lower quartile (Q1), median (Q2) and upper quartile (Q3). TIBI-CaP scores less than or equal to Q1 were classified by the authors as the ‘least’ degree of comorbidity burden; TIBI-CaP scores falling above Q1 but less than or equal to Q2 were classified as a ‘minimal’ level of comorbidity burden; TIBI-CaP scores falling above Q2 but less than or equal to Q3 were classified as a ‘moderate’ level of comorbidity burden; and the ‘severe’ level was all scores greater than Q3. This classification of the TIBI-CaP quartiles provides simple nomenclature to describe the levels of comorbidity burden experienced by the patients, and the labels of each group (‘least’, ‘minimal’, ‘moderate’ and ‘severe’) are reminiscent of severity classifications considered by Stier et al. [5].
The associations of self-reported comorbidity burden to the two HRQoL questionnaires were analyzed using correlation analysis and analysis of variance models, which included baseline SF-12v2 domain and summary scores, and baseline FACT-P subscale and total scores as response and the four categories, based on TIBI-CaP scores, as predictor. The correlation analysis assessed the strength and direction of association between TIBI-CaP scores and SF-12v2 and FACT-P questionnaires using Pearson correlation coefficients. Given the nature of this study as a correlation analysis (i.e., instrument validation), statistical corrections for multiple comparisons were not conducted. A two-sided p-value of <0.05 was considered statistically significant.
Results
A total of 302 CRPC patients enrolled in the TRUMPET registry through 2016 were included in this study. Among these, mean patient age was 73.7 years (range 47–96 years), 84.7% of the patients were white and 13.9% of the patients were black and 70.3% of patients were covered by Medicare. Mean time since initial prostate cancer diagnosis was 7.4 years and 87.8% of patients had M1 CRPC. Most (69.2%) patients were being treated by urologists (Table 1).
Table 1. . Baseline and demographic characteristics.
| Demographics and setting of care | Sample size (n = 302) | |
|---|---|---|
| Age, years | Mean Range |
73.7 47–96 |
| Race (%) | White Black |
84.7 13.9 |
| Ethnicity (%) | Non-Hispanic Hispanic |
94.8 5.2 |
| Marital status (%) | Married | 77.7 |
| Employment status (%) | Retired | 70.5 |
| Household income (%) | US$50,000−74,000 | 22.6† |
| Education level (%) | High school diploma | 28.6† |
| Insurance status (%) | Medicare | 70.3 |
| Pharmacy benefit status (%) | Yes | 89.2 |
| Setting of care (%) | Community based | 84.3 |
| Primary specialty physician (%) | Urologist Oncologist Combined office |
69.2 19.5 11.3 |
| Physical measurements (mean) | Height (cm) Weight (kg) BMI (kg/m2) |
176.41 92.27 29.60 |
| ECOG status (%) | 0 1 2 |
62.0 32.7 4.5 |
| Duration from initial prostate cancer diagnosis (years) | Mean Median |
7.4 5.5 |
| Status at study entry (%) | M0 M1 |
12.2 87.8 |
| First active course of CRPC treatment‡ (%) | Chemotherapy Secondary hormonal treatment: – Enzalutamide – Abiraterone acetate – Bicalutamide Radionuclide Immunotherapy Combination Missing |
3.4 14.1 9.0 10.4 4.5 37.7 5.4 18.6§ |
†Variables with more than 30% of missing data.
‡Percentages/distributions are based on all patients at the time of analysis (interim analysis 2015–2016, n = 355) and include those who had missing TIBI-CaP scores (n = 53).
§Data not cleaned at the time of this study – the majority of these missing data has subsequently been gathered but was not used for the purpose of the TIBI-CaP validation.
BMI: Body mass index; CRPC: Castration-resistant prostate cancer; ECOG: Eastern Cooperative Oncology Group; M0: Nonmetastatic; M1: Metastatic; TIBI-CaP: Total illness burden index for prostate cancer.
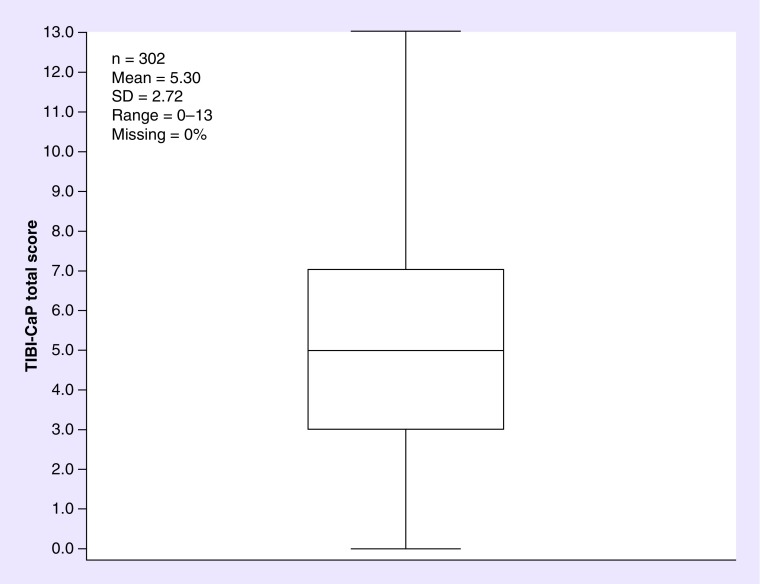
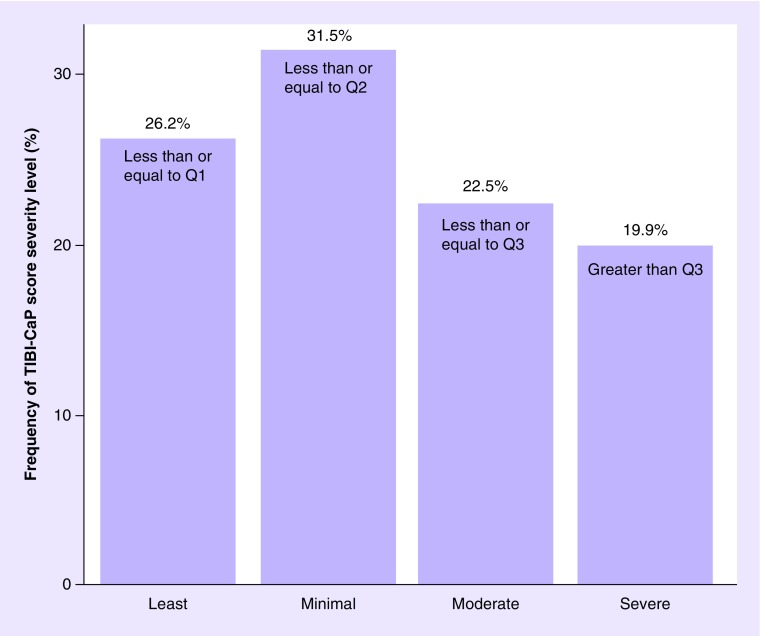
The mean baseline TIBI-CaP score was 5.30 (SD: 2.72; range: 0–13; n = 302) (Figure 1). At baseline, 26.2% of patients were classified as having ‘least’ comorbidity burden, 31.5% of patients were classified as having ‘minimal’ comorbidity burden, 22.5% of patients were classified as having ‘moderate’ comorbidity burden and 19.9% of patients were classified as having ‘severe’ comorbidity burden (Figure 2).
Figure 1. . Box plot of total illness burden index for prostate cancer scores (at baseline).
SD: Standard deviation; TIBI-CaP: Total illness burden index for prostate cancer.
Figure 2. . Classification of total illness burden index for prostate cancer score by severity level (at baseline).
Q1: 3.0; Q2: 5.0 (median); Q3: 7.0.
Q: Quartile; TIBI-CaP: Total illness burden index for prostate cancer.
Baseline TIBI-CaP scores had statistically significant (p < 0.001) negative correlations with all baseline SF-12v2 domain and composite scores. Baseline TIBI-CaP scores were strongly associated with the HRQoL physical domains of the SF-12v2, with correlation estimates of -0.46 for the PCS and -0.23 for the MCS (Table 2). Baseline TIBI-CaP scores had statistically significant (p < 0.020) negative correlations with all baseline FACT-P subscale and total scores, including correlation estimates of -0.45 for the prostate cancer subscale and -0.44 for the FACT-P total score (Table 3).
Table 2. . Correlations of baseline total illness burden index for prostate cancer scores with baseline SF-12 domain and summary scores.
| SF-12 composite/domain variables | Estimated correlation (r) | Sample size (n)† |
|---|---|---|
| Physical component summary | -0.46 | 290 |
| Mental component summary | -0.23 | 290 |
| Physical functioning | -0.45 | 290 |
| Role − physical | -0.41 | 291 |
| Bodily pain | -0.37 | 290 |
| General health | -0.38 | 290 |
| Vitality | -0.44 | 291 |
| Social functioning | -0.26 | 291 |
| Role – emotional | -0.30 | 291 |
| Mental health | -0.22 | 291 |
All statistically significant; p < 0.0002.
†Missing values are excluded from the analysis.
Table 3. . Correlations of baseline total illness burden index for prostate cancer scores with baseline functional assessment of cancer therapy – prostate subscale and total scores.
| FACT-P/subscale variables | Estimated correlation (r) | Sample size (n)† |
|---|---|---|
| Patient wellbeing | -0.39 | 243 |
| Social/family wellbeing | -0.15 | 243 |
| Emotional wellbeing | -0.25 | 244 |
| Functional wellbeing | -0.37 | 244 |
| Prostate cancer subscale | -0.45 | 242 |
| FACT-P TOI score | -0.47 | 241 |
| FACT-G total score | -0.40 | 243 |
| FACT-P total score | -0.44 | 241 |
All statistically significant; p < 0.02.
†Missing values are excluded from the analysis.
FACT-G: Functional assessment of cancer therapy – general; FACT-P: Functional assessment of cancer therapy – prostate; TOI: Trial outcome index.
F-tests of all baseline SF-12v2 and FACT-P scores were significant in detecting inequality across the four severity categories of the baseline TIBI-CaP scores indicative of comorbidity burden (all p < 0.050). Notably, all mean baseline SF-12v2 and FACT-P scores showed a significant decreasing linear trend across the four severity categories of baseline TIBI-CaP scores. Specifically, baseline SF-12v2 PCS and MCS scores (n = 290) decreased from 49.95 (SD: 9.68) and 55.42 (SD: 7.73) in patients with least comorbidity burden to 35.46 (SD: 10.44) and 49.41 (SD: 9.53) in patients with severe comorbidity burden (p < 0.001 for both), respectively. Baseline prostate cancer subscale (n = 242) and FACT-P (n = 241) scores decreased from 36.85 (SD: 6.04) and 129.27 (SD: 15.11) in patients with least comorbidity burden to 26.33 (SD: 7.91) and 100.51 (SD: 23.12) in patients with severe comorbidity burden (both p < 0.001), respectively (Table 4).
Table 4. . Baseline SF-12v2 and functional assessment of cancer therapy – prostate.† .
| Variables | Sample size (n) | Least‡ | TIBI-CaP severity classification | F§ (p-value) | F¶ (p-value) | ||
|---|---|---|---|---|---|---|---|
| Minimal‡ | Moderate‡ | Severe‡ | |||||
| SF-12 composite/domain variables | |||||||
| Physical component summary | 290 | 49.95 (9.68) | 46.27 (9.25) | 41.42 (11.04) | 35.46 (10.44) | 25.81 (<0.0001) | 76.86 (<0.0001) |
| Mental component summary | 290 | 55.42 (7.73) | 51.62 (7.14) | 52.97 (8.38) | 49.41 (9.53) | 6.47 (0.0003) | 14.04 (0.0002) |
| Physical functioning | 290 | 50.47 (9.66) | 46.59 (10.48) | 42.77 (12.06) | 34.81 (9.79) | 26.16 (<0.0001) | 76.96 (<0.0001) |
| Role − physical | 291 | 49.68 (10.15) | 44.77 (9.73) | 41.68 (10.69) | 36.60 (9.86) | 19.58 (<0.0001) | 58.43 (<0.0001) |
| Bodily pain | 290 | 53.40 (8.30) | 49.87 (10.06) | 45.61 (11.27) | 42.18 (11.68) | 15.21 (<0.0001) | 45.19 (<0.0001) |
| General health | 290 | 51.86 (9.17) | 49.27 (8.99) | 46.79 (10.69) | 40.80 (9.75) | 15.73 (<0.0001) | 45.77 (<0.0001) |
| Vitality | 291 | 54.71 (9.99) | 48.75 (8.94) | 47.22 (10.64) | 41.94 (8.40) | 20.21 (<0.0001) | 58.08 (<0.0001) |
| Social functioning | 291 | 53.11 (7.48) | 50.28 (9.49) | 49.12 (9.31) | 45.71 (11.96) | 6.74 (0.0002) | 19.88 (<0.0001) |
| Role − emotional | 291 | 52.47 (8.69) | 48.54 (9.72) | 47.43 (11.52) | 41.49 (12.69) | 11.99 (<0.0001) | 34.56 (<0.0001) |
| Mental health | 291 | 55.95 (8.34) | 52.13 (8.26) | 53.72 (8.46) | 50.20 (8.76) | 5.68 (0.0009) | 11.43 (0.0008) |
| FACT-P/subscale variables | |||||||
| FACT-P TOI | 241 | 85.34 (12.53) | 76.36 (14.50) | 72.82 (16.97) | 61.93 (18.11) | 21.64 (<0.0001) | 63.52 (<0.0001) |
| FACT-G | 243 | 92.42 (11.20) | 84.98 (15.02) | 85.81 (13.67) | 73.76 (17.65) | 15.39 (<0.0001) | 40.47 (<0.0001) |
| FACT-P | 241 | 129.27 (15.11) | 117.74 (20.14) | 116.20 (20.79) | 100.51 (23.12) | 19.29 (<0.0001) | 54.39 (<0.0001) |
| Prostate cancer subscale | 242 | 36.85 (6.04) | 32.59 (6.78) | 30.26 (8.47) | 26.33 (7.91) | 20.53 (<0.0001) | 60.74 (<0.0001) |
| Physical wellbeing | 243 | 25.39 (3.52) | 23.52 (4.77) | 22.22 (5.47) | 19.21 (6.78) | 14.12 (<0.0001) | 41.83 (<0.0001) |
| Social/family wellbeing | 243 | 24.07 (4.12) | 22.18 (5.10) | 23.66 (4.37) | 21.31 (5.96) | 3.84 (0.0103) | 5.32 (0.0219) |
| Emotional wellbeing | 244 | 19.86 (2.75) | 19.10 (4.00) | 19.70 (3.54) | 16.94 (5.17) | 6.08 (0.0005) | 12.21 (0.0006) |
| Functional wellbeing | 244 | 23.10 (5.28) | 20.17 (5.46) | 20.19 (5.77) | 16.30 (6.73) | 12.80 (<0.0001) | 34.90 (<0.0001) |
†F-tests showed statistically significant differences across the four severity categories.
‡Estimated sample mean with sample standard deviation within the parenthesis.
§F-statistic for testing equality of means across severity classification – displayed values are the F-statistic and p-value.
¶F-statistic for linear treatment contrast for linear trends across severity classification – displayed values are the F-statistic and p-value.
FACT-G: Functional assessment of cancer therapy – general; FACT-P: Functional assessment of cancer therapy – prostate; SF: Short form; TIBI-CaP: Total illness burden index for prostate cancer; TOI: Trial outcome index.
Discussion
Results from this observational study that included analyses of data collected from patients with CRPC at baseline entry into the TRUMPET registry, validate the TIBI-CaP by demonstrating the relationship between TIBI-CaP scores and patient-reported generic and disease-specific HRQoL. Findings showed a statistically significant negative association between baseline TIBI-CaP scores and all baseline SF-12v2 domain/summary and FACT-P subscale/total scores. Notably, there was a decreasing linear trend in all SF12v2 and FACT-P scores over the TIBI-CaP severity categories, with scores decreasing by the same absolute amount between each quartile rather than by the same relative amount. Our results demonstrate that the TIBI-CaP correlates the impact of comorbidity burden on mental and physical functioning in patients with CRPC, as reported in the TRUMPET registry.
The negative correlation between TIBI-CaP scores and mental and physical wellbeing observed in patients with CRPC in the TRUMPET registry, and the negative association with the HRQoL physical domains of the SF12v2 is in accordance with previous reports on the TIBI-CaP. In men with localized prostate cancer sampled from the CaPSURE registry, Stier et al. [5] found a statistically significant single point association between TIBI-CaP scores and scores for seven domains of the SF-36, with the strongest association between TIBI-CaP scores and physical functioning. In the same patient population, Daskivich et al. [3] reported that worse TIBI-CaP scores reported within 24 months of treatment with radical prostatectomy or radiation therapy (external beam or brachytherapy) were associated with significantly lower HRQoL at baseline and at 6, 12, 18 and 24 months post-treatment, as measured by the SF-36 and University of California Los Angeles Prostate Cancer Index. The results of the current TIBI-CaP study from the TRUMPET registry emphasize the association of baseline comorbidity with HRQoL in patients with CRPC in real-world settings of care.
Published evidence also suggests that a high comorbidity burden is associated with increased nonprostate cancer mortality rates in men with localized prostate cancer while the risk of prostate cancer mortality remains lower [3,6,11–13]; although mortality rates can vary depending on patient age and stage of disease. Litwin et al. [6] reported that the TIBI-CaP predicted nonprostate cancer mortality over the 3.5 years following its administration in patients enrolled in the CaPSURE registry. Men with the highest TIBI-CaP scores, indicative of high comorbidity, had greater 3.5-year nonprostate cancer mortality than men with the lowest TIBI-CaP scores. Similarly, Daskivich et al. [14] found that nonprostate cancer-specific mortality was significantly different among comorbidity groups, with a significantly larger proportion of patients with the highest TIBI-CaP scores (≥12) dying of causes other than prostate cancer compared with those with the lowest scores (0–2).
Accordingly, the study by Albertsen et al. [11] on the impact that comorbidity burden, determined using the Charlson Comorbidity Index, had on survival of men with localized prostate cancer identified from Medicare insurance program files linked to the population-based Surveillance, Epidemiology and End Results cancer registries, supports the use of the TIBI-CaP for risk adjustment in CRPC studies and to aid in therapeutic decision-making.
The validation of the TIBI-CaP in patients with CRPC is necessary to account for potential measured variation in treatment outcomes within real-world settings of care. Other clinical assessments of comorbidity are available [3,11–13,15,16]; however, these rely on abstraction of data from patients’ medical records, a process that is associated with several limitations. Manual medical record review may be time consuming and expensive and in CRPC, important data elements can be omitted as patients are treated by urologists who may not routinely track nonurologic comorbidities [5]. In contrast, the TIBI-CaP is a patient-reported measure, accounting for the presence and severity of comorbidities. Importantly, evidence suggests that a patient-centered approach to gathering comorbidity data is a reasonable approach for research purposes in population-based cohorts of patients with prostate cancer [17].
Finally, we believe that the TIBI-CaP is a unique, patient-reported measure of comorbidity burden and severity in prostate cancer, providing a more robust measurement of possible confounding beyond what can be assessed by other patient-reported outcome measures, such as the SF-12 and FACT-P. Consequently, the TIBI-CaP should be seen as a clinical measure, derived from what physicians do and what they understand. The TIBI-CaP should be related to the patient's health status measure, as it is in this study, but used and interpreted as a clinical representation of health. The TIBI-CaP may therefore be used to control for bias in an analysis of treatment patterns and outcomes from TRUMPET.
Limitations
This study evaluated the relationship between baseline TIBI-CaP scores and baseline scores on self-reported HRQoL measures in patients with CRPC. Data were obtained from a subset of patients enrolled in the TRUMPET registry under usual care settings. The TRUMPET registry is unique as it provides a patient-centered response and reflects real-world care. However, the study was associated with some limitations. First, the TIBI-CaP questionnaire has 81 items and may represent a substantial administrative burden. Second, this was a cross-sectional study that measured TIBI-CaP and HRQoL scores at a single time point. Future longitudinal studies are required to determine the influence of TIBI-CaP scores on survival and patient-reported outcomes. Third, a high proportion of community-based urology practices contributed patients at the time the interim analysis was conducted on the TRUMPET cohort. In this particular setting, there does appear to be a large proportion of patients with CRPC who initiated their CRPC treatment with sipuleucel-T. Further research into the relationship of these baseline patient characteristics and initial treatment decisions is warranted. Inherent to the observational study design, results may also be affected by selection bias and unmeasured confounding variables.
Conclusion
This analysis of the TRUMPET registry data showed that baseline TIBI-CaP scores were negatively correlated with baseline functional status as measured by the SF-12v2 and FACT-P questionnaires in a cohort of patients with CRPC. Baseline TIBI-CaP scores were strongly associated with SF-12v2 HRQoL physical subscales. This analysis demonstrates the validity of the TIBI-CaP in a cohort of CRPC patients included in a prospective, observational registry. Consequently, the TIBI-CaP represents a useful research tool that can assist in risk adjustment studies of clinical and economic outcomes in advanced prostate cancer. Future analysis of this cohort will focus on describing the patterns of care for patients with CRPC.
Summary points.
The total illness burden index for prostate cancer (TIBI-CaP) is a patient-reported measure of comorbidity burden specifically adapted for research use in patients with castration-resistant prostate cancer (CRPC).
The aim of this study was to validate the TIBI-CaP in 302 patients with CRPC enrolled in the TRUMPET registry.
The TRUMPET registry is a USA-based, prospective, observational multicenter registry (NCT02380274) designed to evaluate treatment patterns, comorbidities and health-related quality of life (HRQoL) outcomes associated with CRPC (nonmetastatic and metastatic) in real-world settings.
Baseline TIBI-CaP comorbidity scores were compared with baseline patient-reported HRQoL measured using the SF-12v2 and functional assessment of cancer therapy – prostate (FACT-P) questionnaires.
Baseline TIBI-CaP scores were negatively correlated with all baseline SF-12v2 domain/composite (p < 0.001) and FACT-P subscale/total (p < 0.020) scores.
There was a strong association between baseline TIBI-CaP scores and baseline HRQoL physical domains of the SF-12v2.
There was a significant decreasing linear trend in all SF12v2 and FACT-P scores over the TIBI-CaP severity categories.
The TIBI-CaP is a valid measure of comorbidity burden in real-world CRPC patients.
The TIBI-CaP is a patient-centered research tool that may be utilized in risk adjustment studies of clinical and economic outcomes in CRPC.
Footnotes
Financial & competing interests disclosure
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors take full responsibility for the scope, direction and content of the manuscript, and have approved the submitted manuscript. The authors received no compensation related to the development of the manuscript.
S Flanders is an employee of Astellas Pharma, Inc. and owns stock holdings from Johnson and Johnson, Abbott Labs, and AbbVie. J Kim, S Wilson and J Braziunas are employees of Astellas Pharma, Inc. S Greenfield collaborates with Astellas Pharma, Inc. and Pfizer on scientific projects. J Billimek is a consultant for Rubicon Biotechnology and Lyntek Medical. S Lechpammer is an employee of Medivation, Inc., which was acquired by Pfizer, Inc. in September 2016 and owns stock holdings from Jazz Pharmaceuticals and Tesaro, Inc. L Karsh is a consultant and speaker at Astellas Pharma, Inc., Pfizer, Janssen, Bayer, Sanofi, and Spectrum and owns stock holdings from Swan Valley Medical. DI Quinn has provided remunerated advisory board and education services for Astellas Pharma, Inc. and Pfizer. D Shevrin has provided services to Sanofi and Astellas Pharma, Inc. ND Shore is a consultant for Astellas Pharma, Inc., Bayer, Ferring, Janssen, Sanofi, Tolmar, Valeant, and Medivation, Inc., which was acquired by Pfizer, Inc. in September 2016. J Symanowski has provided consulting and advisory services to Antigen Express, Astellas Pharma, Inc., Endocyte, Eli Lilly, ProNAi, and Ra Pharma. D Penson collaborates with Astellas Pharma, Inc., Medivation, Inc., which was acquired by Pfizer, Inc. in September 2016, and Dendreon. This study was funded by Astellas Pharma, Inc. and Medivation, Inc., which was acquired by Pfizer, Inc. in September 2016, the co-developers of enzalutamide. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors wish to acknowledge the medical writing assistance of J Kondejewski of SNELL Medical Communication, Inc. and the editorial assistance provided by L Smith of Complete HealthVizion, both funded by the study sponsors.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. No formal consent was required for this retrospective study and data was de-identified to protect patients’ confidentiality.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Cookson MS, Roth BJ, Dahm P, et al. Castration-resistant prostate cancer: AUA guidelines. 2015. www.auanet.org/common/pdf/education/clinical-guidance/Castration-Resistant-Prostate-Cancer.pdf [DOI] [PubMed]
- 2.Nussbaum N, George DJ, Abernethy AP, et al. Patient experience in the treatment of metastatic castration-resistant prostate cancer: state of the science. Prostate Cancer Prostatic. Dis. 2016;19(2):111–121. doi: 10.1038/pcan.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daskivich TJ, van de Poll-Franse LV, Kwan L, Sadetsky N, Stein DM, Litwin MS. From bad to worse: comorbidity severity and quality of life after treatment for early-stage prostate cancer. Prostate Cancer Prostatic. Dis. 2010;13(4):320–327. doi: 10.1038/pcan.2010.33. [DOI] [PubMed] [Google Scholar]; • The total illness burden index for prostate cancer (TIBI-CaP) was used to evaluate the impact on comorbidity severity on longitudinal health-related quality of life (HRQoL) in men treated with radical prostatectomy or radiation therapy. Although comorbidity groups experienced similar long-term declines from baseline HRQOL after treatment, men with more severe comorbidity had significantly lower baseline scores and therefore poorer long-term HRQoL.
- 4.Greenfield S, Sullivan L, Dukes KA, Silliman R, D'Agostino R, Kaplan SH. Development and testing of a new measure of case mix for use in office practice. Med. Care. 1995;33(4 Suppl.):AS47–AS55. [PubMed] [Google Scholar]
- 5.Stier DM, Greenfield S, Lubeck DP, et al. Quantifying comorbidity in a disease-specific cohort: adaptation of the total illness burden index to prostate cancer. Urology. 1999;54(3):424–429. doi: 10.1016/s0090-4295(99)00203-4. [DOI] [PubMed] [Google Scholar]; •• The TIBI-CaP was modified to measure the impact of comorbid illness on the quality of life and functioning among patients with newly diagnosed prostate cancer. This index explained a significant proportion of the variance in the SF-36 physical functioning domain scores at baseline and provides value in real-world research on clinical and economic outcomes of prostate cancer.
- 6.Litwin MS, Greenfield S, Elkin EP, Lubeck DP, Broering JM, Kaplan SH. Assessment of prognosis with the total illness burden index for prostate cancer: aiding clinicians in treatment choice. Cancer. 2007;109(9):1777–1783. doi: 10.1002/cncr.22615. [DOI] [PubMed] [Google Scholar]; • The presence and severity of comorbidities may influence the decision to treat newly diagnosed prostate cancer patients aggressively. The TIBI-CaP, a patient-reported measure of comorbidity, identified patients at high risk for nonprostate cancer mortality. It successfully predicted both mortality and future quality of life in men with newly diagnosed prostate cancer.
- 7.Penson DF, Lin DW, Karsh L, et al. Treatment registry for outcomes in patients with castration-resistant prostate cancer (TRUMPET): a methodology for real-world evidence and research. Future Oncol. 2016;12(23):2689–2699. doi: 10.2217/fon-2016-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• TRUMPET is a USA-based, prospective, observational multicenter registry (NCT02380274) involving patients with castration-resistant prostate cancer and their caregivers. Data from the registry will enable scientific understanding of disease management in terms of HRQoL, clinical outcomes and healthcare utilization in clinical practice for patients with castration-resistant prostate cancer.
- 8.Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy – prostate instrument. Urology. 1997;50(6):920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 9.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Greenfield S, Rogers W, Mangotich M, Carney MF, Tarlov AR. Outcomes of patients with hypertension and noninsulin dependent diabetes mellitus treated by different systems and specialties. Results from the medical outcomes study. JAMA. 1995;274(18):1436–1444. [PubMed] [Google Scholar]
- 11.Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J. Clin. Oncol. 2011;29(10):1335–1341. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daskivich TJ, Kwan L, Dash A, Saigal C, Litwin MS. An age adjusted comorbidity index to predict long-term, other cause mortality in men with prostate cancer. J. Urol. 2015;194(1):73–78. doi: 10.1016/j.juro.2015.01.081. [DOI] [PubMed] [Google Scholar]
- 13.Ng SP, Duchesne G, Tai KH, Foroudi F, Kothari G, Williams S. Support for the use of objective comorbidity indices in the assessment of noncancer death risk in prostate cancer patients. Prostate Int. 2017;5(1):8–12. doi: 10.1016/j.prnil.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daskivich TJ, Chamie K, Kwan L, et al. Improved prediction of long-term, other cause mortality in men with prostate cancer. J. Urol. 2011;186(5):1868–1873. doi: 10.1016/j.juro.2011.07.033. [DOI] [PubMed] [Google Scholar]; • The TIBI-CaP identified men with a high long-term risk of death from causes other than prostate cancer. Men with severe comorbidity as defined by the TIBI-CaP had a higher risk of nonprostate cancer mortality, having a potential impact on choice of treatments for early-stage prostate cancer.
- 15.Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. The impact of comorbidity on life expectancy among men with localized prostate cancer. J. Urol. 1996;156(1):127–132. [PubMed] [Google Scholar]
- 16.Kent M, Vickers AJ. A systematic literature review of life expectancy prediction tools for patients with localized prostate cancer. J. Urol. 2015;193(6):1938–1942. doi: 10.1016/j.juro.2014.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye F, Moon DH, Carpenter WR, et al. Comparison of patient report and medical records of comorbidities: results from a population-based cohort of patients with prostate cancer. JAMA Oncol. 2017;3(8):1035–1042. doi: 10.1001/jamaoncol.2016.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comorbid conditions of patients with cancer affect treatment decisions, which in turn affect survival and HRQoL outcomes. Comparative effectiveness research studies should account for these conditions. Use of patient reports, which are less costly than medical record audits, is a reasonable approach for observational comparative effectiveness research.