Abstract
Aim:
Compound 1-(4-chlorophenyl)-4,4,4-trifluoro-3-hydroxy-2-buten-1-one (compound 1) was identified as a hit against methicillin-resistant Staphylococcus aureus (MRSA) strain MW2.
Methods & results:
The MIC of compound 1 against MRSA was 4 μg/ml. The compound showed enhanced activity at acidic pH by lowering bacterial intracellular pH and exhibited no lysis of human red blood cells at up to 64 μg/ml and its IC50 against HepG2 cells was 32 μg/ml. The compound reduced 1-log10 colony forming units of intracellular MRSA in macrophages and prolonged the survival of MRSA-infected Caenorhabditis elegans (p = 0.0015) and Galleria mellonella (p = 0.0002).
Conclusion:
Compound 1 is a protonophore with potent in vitro and in vivo activity against MRSA and no toxicity in mammalian cells up to 8 μg/ml that warrants further investigation as a novel antibacterial.
Keywords: : antibiotic, Galleria mellonella, macrophages, MRSA infection, protonophore, S. aureus
Staphylococcus aureus, and especially methicillin-resistant S. aureus (MRSA), is a prominent human pathogen [1] that causes skin, soft tissue, blood stream, heart valve and prosthetic device infections [2,3]. Staphylococcal drug resistance is mostly driven by antibiotic-inactivating enzymes, such as β-lactamases, and/or alterations in antibiotic-binding proteins, such as penicillin binding proteins [2]. pH is an important environmental factor and stress inducer that plays a key role in bacterial growth [4]. Staphylococcus aureus adapts to varying acidic conditions, such as those found on human skin and in biological fluids, including blood, saliva and urine [4], and can survive the low intracellular pH (6.0–4.0) within phagocytizing cells or abscesses [5–8]. Bacterial adaptation and reduced activity of conventional antibiotics under acidic conditions have been reported to favor staphylococcal infections [9–11].
Uncouplers, also known as protonophores, are compounds that dissipate proton (H+) gradients across lipid bilayers. Gradient dissipation is generally not mediated via channel proteins, but is instead driven by existing forces, such as membrane potential and pH gradients [12]. Niclosamide (used for tape worm infections) [13] and nitazoxanide (used for Giardia lamblia infections) [14] are drugs that target proton-motive force (PMF) and are used clinically to treat parasitic infections. Given the increasing threat of multidrug-resistant bacterial infections [15], novel agents targeting the PMF warrant investigation as a new antibacterial agent against bacterial infection.
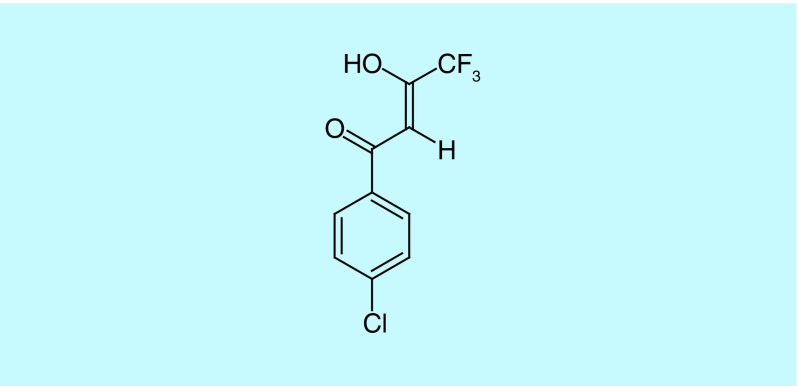
(Z)-1-(4-Chlorophenyl)-4,4,4-trifluoro-3-hydroxy-2-buten-1-one (compound 1, Figure 1) was identified as a hit compound against MRSA in a Caenorhabditis elegans MRSA high-throughput screen [16] that is used to identify anti-MRSA compounds and simultaneously provide an indication of their toxicity to the host. Using C. elegans as a host is more cost effective than rodent models and takes advantage of the conserved innate immune system from nematodes to vertebrates [17]. We now report that compound 1 shows potent activity against MRSA in vitro and in the Galleria mellonella infection model shows low mammalian cell cytotoxicity, and acts via a protonophoric mechanism, with activity increasing at acidic pH.
Figure 1. . Structure of 1-(4-chlorophenyl)-4,4,4-trifluoro-3-hydroxy-2-buten-1-one (compound 1).
Materials & methods
Compound information
(Z)-1-(4-Chlorophenyl)-4,4,4-trifluoro-3-hydroxy-2-buten-1-one (ChemBridge #5404961, referred to herein as compound 1) was purchased from ChemBridge (ChemBridge Corporation, CA, USA). The compound 1 is not soluble in water, hence the stock solutions of compound 1 were prepared by dissolving in DMSO (Sigma-Aldrich, MO, USA) at 10 mg/ml and diluting as required for experiments.
Bacterial & nematode stains
All bacteria were from the Mylonakis laboratory collection (Table 1). Strains were grown at 37°C. Staphylococcus aureus (MRSA strain of MW2), Staphylococcus epidermidis and Enterococcus faecium strains were grown in tryptic soy broth (TSB) (BD Biosciences, NJ, USA); Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter aerogenes were grown in Luria-Bertani broth (BD Biosciences). The C. elegans glp-4(bn2);sek-1(km4) double mutant strain was maintained at 15°C on a lawn of Escherichia coli strain HB101 on 10 cm plates, as previously described [16]. The glp-4(bn2) mutation renders the strain unable to produce progeny at 25°C [18] and the sek-1(km4) mutation increases sensitivity to several pathogens [19], thereby reducing assay time.
Table 1. . Antibacterial activity of compound 1 (μg/ml).
| Pathogen | Compound 1 | Vancomycin | Polymyxin B | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | ||
| Staphylococcus aureus MW2 | 4.0 | 16 | 4.0 | 16 | 64 | >64 | 25 |
| Staphylococcus epidermidis 9142 | 4.0 | 16 | 4.0 | 16 | 64 | >64 | 21 |
| Enterococcus faecium (ATCC E007) | 4.0 | 8 | 4.0 | 64 | 64 | >64 | 21 |
| Acinetobacter baumanii (ATCC 17978) | 32 | >64 | >64 | >64 | 4 | 8 | 10 |
| Enterobacter aerogens (EAE 2625) | >64 | >64 | >64 | >64 | 8 | 8 | ND |
| Klebsiella pneumoniae (ATCC 77326) | >64 | >64 | >64 | >64 | 8 | 8 | ND |
| Pseudomonas aeruginosa (PA 14) | >64 | >64 | >64 | >64 | 2 | 4 | ND |
ATCC: American Type Culture Collection; MBC: Minimal bactericidal concentration; ND: Not determined.
Antimicrobial susceptibility testing
In vitro antibiotic susceptibilities were measured using the broth microdilution and disc diffusion methods [20]. Broth microdilution assays were carried out in triplicate using Mueller–Hinton broth (MHB; BD Biosciences) in 96-well plates (BD Biosciences) with a total assay volume of 100 μl. Twofold serial dilutions of compounds were prepared over the concentration range of 0.01– 64 μg/ml. The initial bacterial inoculum was adjusted to OD600 (optical density) = 0.06 and assay solutions were incubated at 35°C for 18 h. To determine the MIC, OD600 was measured after incubation and the lowest concentration of test compound that suppressed bacterial growth was reported as the MIC. Broth culture from the MIC assay (10 μl) was plated onto Mueller–Hinton agar (BD Biosciences) and colony forming units (CFUs) were enumerated after overnight incubation at 37°C. The lowest concentration of compound that resulted in no colonies was reported as the minimal bactericidal concentration.
Disk diffusion assays were performed on Mueller–Hinton agar. Sterile blank disks (BD Biosciences) were impregnated with 25 μg of test compound and air-dried. Bacterial inoculum was spread over the plate and disks were placed on the agar surface. Antimicrobial susceptibility was determined by measuring the diameter of the zone of inhibition after incubating at 35°C for 24 h. All experiments were performed in triplicate.
Time to kill assays
MRSA MW2 was used to probe the bacteriostatic/bactericidal properties of compound 1, as previously described [21]. Assays were carried out in duplicate in 10-ml tubes (BD Biosciences). Briefly, overnight cultures of MRSA MW2 were diluted in fresh TSB to a density of 108 cells/ml. Test compound at 4 × MIC was added and the tubes were incubated at 37°C with shaking. At periodic intervals, aliquots from each tube were serially diluted in TSB and plated onto tryptic soy agar (BD Biosciences). CFUs were enumerated after overnight incubation at 37°C.
Intracellular killing assays
RAW 264.7 macrophages were used to evaluate intracellular killing of MRSA MW2 by compound 1. Assays were carried out in triplicate as described by Schmitt et al., [22]. Briefly, macrophages were cultured and maintained as described under mammalian cell cytotoxicity assays. Cells (50,000) were seeded in 24-well plates 24 h prior to infection. Multiplicity of infection 25 (i.e., 25 bacterial cells per macrophage) of MRSA MW2 were added to macrophages for 2 h to promote phagocytosis. Planktonic bacteria were removed and Dulbecco's Modified Eagle Medium (DMEM) containing 200 μg/ml gentamicin was added for 2 h to inhibit/kill remaining extracellular bacteria. Antibiotic and serum-free DMEM with or without compound 1 was added and the cells were incubated under 5% CO2 for 20 h. Macrophages were lysed by adding Sodium dodecyl sulfate (SDS) to a final concentration of 0.02% (i.e., lyses only macrophages and not ingested bacteria). Cell lysates were diluted serially and CFUs were enumerated by plating on tryptic soy agar plates. Vancomycin (8 μg/ml) was used as a positive control and DMSO at a final concentration of less than 0.1% as the negative control.
Intracellular pH measurements
Intracellular pH measurements were performed in triplicate, as described by Marks et al. [23]. To summarize, MRSA-MW2 cells were washed with phosphate-buffered saline (PBS, pH 7.4). AM-BCECF (membrane-permeable acetoxymethyl ester derivative of the dual-excitation ratiometric pH indicator dye BCECF; 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, Molecular Probes/Thermo Fisher Scientific, ON, Canada, 25 μM) in DMSO was added to the cells and incubated for 30 min at 30°C. Cells were washed with PBS and added to PBS with or without compound 1 (10 μg/ml). The protonophore, carbonyl cyanide m-chlorophenyl hydrazone (10 μg/ml), was used as a positive control. Calibration curves were obtained from cells suspended in PBS at varying pH over the range of 4.0–9.0.
Bacterial membrane permeabilization assays
Sytox green (Life Technologies, CA, USA; a nucleic acid stain not permeable to intact membrane) was used to measure membrane permeabilization by test compounds, as previously described [24]. Assays were carried out in duplicate in 96-well plates (Corning, NY, USA) using MRSA MW2. Bacteria were harvested from logarithmically growing cultures by centrifugation at 3724 × g for 5 min, washed twice with PBS (Gibco, NY, USA) and resuspended in PBS to optical density OD595 = 0.5. Sytox green was added to a final concentration of 5 μM and cells were incubated in the dark for 30 min. A total of 50 μl of cell suspensions were added to 50 μl of serially diluted test compound in PBS and the fluorescence intensity (excitation: 485 nm; emission: 530 nm) was measured at various time intervals using a Vmax microplate reader (Molecular Devices, CA, USA).
Multistep emergence of resistance
To generate mutants against the compound 1, MRSA MW2 was grown in MHB at OD600 = 0.06 in the presence of serially diluted compound 1 and incubated overnight. From the highest drug concentration allowing visible growth at sub-MIC concentration, aliquots were used to dilute into fresh medium to inoculate the second set of serial drug dilutions as described by Dalhoff [25]. After overnight incubation, bacteria were passaged sequentially for a period of 25 days.
Human red blood cell hemolysis
Human erythrocytes (Rockland Immunochemicals, PA, USA) were used to test the hemolytic properties of compound 1, as detailed in reference [26]. Briefly, 50 μl of 4% human erythrocytes suspended in PBS were added to 50 μl of test compound that had been serially diluted in PBS in 96-well plates. After incubating at 37°C for 1 h, the plates were centrifuged at 500 × g for 5 min and 50 μl of supernatant from each well was transferred to a second 96-well plate. Visual inspection and absorbance at 540 nm were used to measure hemolysis.
Mammalian cell cytotoxicity assays
HepG2 cells were used to probe mammalian cell cytotoxicity of compound 1, as described by Kwon et al. [27]. Cells were grown in DMEM (Gibco) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco) and maintained at 37°C in 5% CO2. After harvesting and resuspension in DMEM, 100 μl of cells were added to wells of a 96-well plate at 5 × 104 cells/well. Compounds were serially diluted in serum and antibiotic-free DMEM, added to the monolayer and the plates were incubated at 37°C in 5% CO2 for 24 h. At 4 h prior to the end of the incubation period, 10 μl of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H-tetrazolium (WST-1) solution (Roche, Mannheim, Germany) was added to each well. WST-1 reduction was monitored at 450 nm using a Vmax microplate reader. Assays were performed in triplicate and the percentage survival was calculated by DMSO-treated vehicle controls.
Mutagenesis studies
The mutagenic properties of compound 1 were examined using the plate incorporation method with Salmonella typhimurium, as described in reference [28]. In brief, S. typhimurium strains TA 1535 and TA 1538 were purchased from American Type Culture Collection (ATCC, VA, USA). Overnight culture of Salmonella strain (0.1 ml), 5 μl of compound 1 (10, 20, 30 and 40 μg per plate) or 5 μl of negative control or 5 μl of positive control and 0.5 ml of metabolic activation mix (S9, S-2067, Sigma-Aldrich; final concentration of 5% S9) were added to sterile tubes. The mixture was incubated for 15 min, added to 2 ml of top molten agar containing traces of histidine/biotin. After gentle shaking, the mixture was poured on the minimal glucose agar plates. Plates were incubated at 37°C for 24–48 h and revertant colonies were counted. 4-Nitro-o-phenylenediamine (20 μg/plate) was used as a positive control and DMSO as the negative control.
Caenorhabditis elegans MRSA infection assay
The C. elegans MRSA infection assay has been described previously [16]. In brief, adult sterile C. elegans glp-4(bn2);sek-1(km4) worms were grown at 25°C and harvested with M9 buffer. MRSA MW2 was grown overnight at 37°C in TSB under aerobic conditions and moved to anaerobic conditions at 37°C. Bacteria were added to the wells of 384-well assay plates (Corning plate 3712) containing serially diluted test compound at a final OD600 of 0.04. Worms were added to each well using a Complex Object Parameter Analyzer and Sorter (Union Biometrica, MA, USA) and the plates were incubated for 5 days at 25°C. Plates were washed to remove bacteria with a microplate washer and the worms were stained with Sytox orange (Life Technologies). After overnight incubation at 25°C, the plates were imaged using an Image Xpress Micro automated microscope (Molecular devices), capturing both transmitted light and tetramethylrhodamine isothiocyanate (TRITC) channel (535 nm excitation, 610 nm emission) fluorescent images with a 2× objective. Images were processed using the open source image analysis software CellProfiller [42]. The ratio of Sytox worm area to bright field worm area for each well was converted to percentage survival by the software. Assays were completed in duplicate.
Galleria mellonella MRSA infection assay
A total of 12 randomly selected G. mellonella larvae (Vanderhorst, Inc., OH, USA) between 300 and 350 mg were used for each group in the experiment [29]. In brief, MRSA-MW2 cells were washed with PBS and diluted to 2 × 106 cells/ml, as determined by optical density at 600 nm, before inoculation into G. mellonella larvae. A 10 μl inoculum was injected into the last left proleg using a 10-μl Hamilton syringe. Compounds were administered at 25 μg/ml into the last right proleg before (prophylactic) or 2 h after (therapeutic) inoculation and the moths were incubated at 37°C. Three groups were used as controls: injected with PBS only; inoculated with MRSA-MW2 but treated with sham injections; and no manipulation. Galleria mellonella survival was evaluated for up to 120 h, and the larvae considered dead if unresponsive to touch. Killing curves and differences in survival were analyzed by the Kaplan–Meier method using GraphPad Prism version 6.04 (GraphPad Software, CA, USA). Statistical analysis (Kruskal–Wallis test) was carried out using the same program and p-values of <0.05 were considered significant.
Results & discussion
Antimicrobial activity
We previously identified compound 1 as a hit compound that prolonged nematode survival in a high-throughput screening assay using the C. elegans MRSA infection model [16]. The compound treatments in the assay recorded Z scores of 34.7 and 18.2, respectively, where compounds producing scores greater than 3 were considered hits. In the current study, the antibacterial activity of compound 1 was evaluated against a test panel of Gram-positive and Gram-negative pathogens. The compound was found to inhibit the growth of MRSA MW2, S. epidermidis, E. faecium and one Gram-negative bacterium, A. baumannii (Table 1). The MIC was 4 μg/ml for the three Gram positives (vancomycin MIC = 4 μg/ml) and 32 μg/ml for A. baumannii (polymyxin B MIC = 4 μg/ml). Three other Gram-negative pathogens, Enterobacter aerogens, K. pneumoniae and P. aeruginosa, showed no growth inhibition at 64 μg/ml compound 1. The MIC remained at ≤4 μg/ml against five clinical isolates of MRSA strains and five methicillin-sensitive S. aureus strains (data not shown). The minimal bactericidal concentration of compound 1 was 16 μg/ml (i.e., 4× MIC) against MRSA MW2 and 8 μg/ml (2× MIC) for E. faecium (Table 1). In disk assays, the zone of inhibition around compound 1 was 25 mm for MRSA MW2 and 21 mm for S. epidermidis strain 9142. For E. faecium and A. baumannii, the zones were 21 mm and 10 mm, respectively (Table 1).
Compound 1 is bacteriostatic against MRSA MW2
‘Time to kill’ assays were carried out to evaluate whether compound 1 is bactericidal or bacteriostatic. When exposed to compound 1 at 4× MIC (16 μg/ml), MRSA-MW2 cell CFU/ml counts were reduced by 1-log10 compared with the initial inoculum (Figure 2A), demonstrating that the compound is bacteriostatic.
Figure 2. . In vitro killing of methicillin-resistant Staphylococcus aureus MW2 cells by compound 1.
(A) Time to kill assays: MRSA MW2 (108 cells/ml) were treated with DMSO or compound 1 (16 μg/ml, 4× MIC) or gentamicin (8 μg/ml, 4× MIC) and CFUs enumerated after incubating for various times. (B) Killing of MRSA MW2 in RAW 264.7 macrophages. Macrophages were exposed to MRSA-MW2 cells (MOI = 25) for 2 h. Extracellular bacteria were killed by treatment with gentamicin (200 μg/ml) for 2 h and the macrophages exposed to 2× MIC compound 1 (8 μg/ml), 2× MIC vancomycin (8 μg/ml) or DMSO control for 20 h. Cells were lysed and the bacteria were plated and counted. Data represent the mean ± SD (n = 3).
CFU: Colony forming unit; MOI: Multiplicity of infection; MRSA: Methicillin-resistant Staphylococcus aureus.
Killing of MRSA MW2 inside macrophages
It is well established that S. aureus can survive as an intracellular pathogen [30]. In the next experiments, RAW 264.7 macrophages were exposed to MRSA-MW2 cells and compound 1 at 8 μg/ml (2× MIC), vancomycin (8 μg/ml, 2× MIC) or DMSO control. It was found that compound 1 reduced intracellular bacteria by 1-log10 CFU/ml (Figure 2B), while vancomycin reduced nearly 1.5 log10 CFU/ml.
Acidic pH enhances antibacterial activity of compound 1
Acidic environments can reduce the efficacy of some antibiotics, for example gentamicin, oxacillin, ciprofloxacin [11,31]. Staphylococcus aureus is able to tolerate a wide range of pH conditions (pH 5–9) and, importantly, staphylococcal infections in acidic environments, such as the skin, urinary tract and blood [10], can result in abscess formation and accumulation of pus [32] that ranges in pH between 6.0 and 7.3 [6].
The acidic character of compound 1, whose pKa (6.25, [33]) is close to physiological pH, led us to speculate that the compound may be eliciting antibacterial effects through a protonophoric mechanism [3]. To test this hypothesis, the MIC of compound 1 against MRSA MW2 was measured in media of varying pH alongside two antibiotics (vancomycin and gentamicin) that do not act as protonophores. At pH 7.0, all three compounds showed very similar activity (Figure 3C). At pH 6.0, the MIC of compound 1 was reduced fourfold to 1 μg/ml and at pH 5.0, an eightfold decrease was observed (MIC = 0.5 μg/ml) (Figure 3A & B). In contrast, the activity of the vancomycin and gentamicin decreased at lower pH, with MICs for both compounds rising to 8 μg/ml and 64 μg/ml at pH 6.0 and 5.0, respectively. These observations were consistent with previous studies showing that vancomycin and gentamicin are less active under acidic conditions [11]. Interestingly, at pH 8.0 the situation was reversed, with vancomycin and gentamicin showing improved potency (MIC = 1 μg/ml) and compound 1 reduced activity (MIC = 8–16 μg/ml) (Figure 3D).
Figure 3. . Effect of pH on antibacterial activity.
The MICs of compound 1, vancomycin and gentamicin were measured against MRSA MW2 at pH: (A) 5.0, (B) 6.0, (C) 7.0 and (D) 8.0. Data represent the mean ± SD (n = 3).
MRSA: Methicillin-resistant Staphylococcus aureus.
Effects of compound 1 on MRSA-MW2 intracellular pH & membrane proton gradients
Bacteria have the ability to maintain internal pH at or near neutral when the external pH is altered [34]. Dissipation of PMF can lead to changes in internal pH, which has been shown to impair bacterial growth [34]. We hypothesized that if compound 1 is acting as a protonophore it should affect the S. aureus proton gradient, leading to intracellular pH changes. To evaluate this hypothesis, MRSA-MW2 cells were preloaded with the pH-sensitive fluorescent probe AM-BCECF and treated with compound 1. In the absence of compound, the internal pH of MRSA MW2 was measured at 7.5. After incubation for 30 min with compound 1, the intracellular pH lowered to 6.5 (Figure 4A), consistent with the compound acting as a protonophore and dissipating the membrane PMF. To corroborate this finding, we tested the well-known protonophore carbonyl cyanide m-chlorophenylhydrazone (pKa 5.95) [35–37] and found that it too reduced intracellular pH of MRSA-MW2 cells to 6.5 after a 30 min incubation. Neither DMSO nor vancomycin-treated cells showed any changes in intracellular pH. In addition, we performed permeabilization assays with the nucleic acid stain Sytox green, which is permeable only when membranes are damaged. No fluorescence was observed (Figure 4B) indicating that compound 1 did not cause damage to the bacterial cell membrane. As reported by Feng et al., US FDA approved protonophoric drugs like niclosamide, nitazoxanide that dissipate the PMF [36], can be detected by changes in intracellular pH, supporting our observation that compound 1 acts as a protonophore. To characterize the exact mechanism of action, we passaged MRSA MW2 in the presence of compound 1 to generate mutants but were unable to generate mutants after continuous passage for 25 days (data not shown).
Figure 4. . Intracellular pH and membrane permeability.
(A) Staphylococcus aureus intracellular pH: MRSA-MW2 cells preloaded with the fluorescence pH indicator probe AM-BCECF were treated with 10 μg/ml of compound 1, vancomycin, CCCP or DMSO (negative control) and fluorescence measurements taken. (B) Membrane permeabilization by compound 1: MRSA-MW2 cells were loaded with the membrane impermeable nucleic acid stain Sytox green and treated with compound 1 (3.125–50 μg/ml) and the fluorescence was measured at regular time interval post treatment. Data represent the mean ± SD (n = 3).
AM-BCECF: Acetoxymethyl ester, 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein; CCCP: Carbonyl cyanide m-chlorophenylhydrazone; MRSA: Methicillin-resistant Staphylococcus aureus.
Mammalian cell cytotoxicity of compound 1
The FDA has previously approved drugs that target the PMF (e.g., niclosamide and clofazimine) for use as anti-infectives [21,36], in spite of their perceived toxicity. Since many antibiotics are delivered intravenously, we evaluated the toxicity of compound 1 toward human erythrocytes. Serial dilutions of the compound showed no hemolysis up to 64 μg/ml (Figure 5A). As many drugs are detoxified by the liver, we also evaluated compound 1 for cytotoxicity toward HepG2 liver cells [21]. The IC50 of compound 1 was found to be in the range of 16–32 μg/ml (Figure 5B) or eightfold higher than the MIC observed in MRSA MW2.
Figure 5. . Hemolysis and cytotoxicity assays.
(A) Hemolytic activity of compound 1: human erythrocytes were treated with various concentrations of compound 1 (0.06–64 μg/ml) or Triton X-100 (0.001 to 1.0%) for 1 h and the supernatant was examined for hemolysis. (B) Cytotoxicity of compound 1 in HepG2 cells: HepG2 cells were treated with increasing concentrations of compound 1 (0.125–64 μg/ml) and incubated for 24, 48 or 72 h. Data represent the mean ± SD (n = 3).
Mutagenic properties of compound 1
The mutagenic properties of compound 1 were examined using a variation of the Ames test [28] that uses mutant S. typhimurium strains that can be induced to revert from a histidine requirement back to the parental strain by mutagens [38]. Spontaneous revertant colonies observed with compound 1 at 40, 30, 20 and 10 μg per plate were 4, 8, 22 and 34, respectively, for S. typhimurium strain TA 1535 and 10, 18, 25 and 61 for strain TA 1538 (Table 2). The changes were similar to the carrier control and significantly less than those induced by the positive control mutagen 4-nitro-o-phenylenediamine. Taken together, these findings demonstrate that at concentrations close to MIC, compound 1 shows low mammalian cell toxicity and has no mutagenic properties.
Table 2. . Mutagenic properties of compound 1 determined by Ames test.
| Test compound | Number of His+ revertants/plate | ||
|---|---|---|---|
| Concentration (μg/plate) | Salmonella typhi TA 1535 | Salmonella typhi TA 1538 | |
| DMSO | 5.0 | 22 | 31 |
| Compound 1 | 2.5 | 4 | 10 |
| 5.0 | 8 | 18 | |
| 7.5 | 22 | 25 | |
| 10.0 | 34 | 61 | |
| 4NOP | 5.0 | 735 | 835 |
4NOP: 4-Nitro-o-phenylenediamine.
In vivo infection models
The nematode C. elegans serves as a simple whole animal host for examining the anti-infective properties and toxicity of small molecules [39]. The earlier high-throughput screen using the C. elegans MRSA model identified that compound 1 rescues nematodes from MRSA infection at the single concentration of 2.86 μg/ml (Figure 6A) [16]. The C. elegans MRSA infection assay was repeated here using twofold serial dilutions of compound 1 from 0.5 to 64 μg/ml. The compound was confirmed to rescue C. elegans from MRSA infection (p = 0.0015) at concentrations (0.5 μg/ml) similar to vancomycin, which were below MIC (Figure 6B).
Figure 6. . Whole animal Staphylococcus aureus infection models.
(A) Bright field and Sytox orange stained images of MRSA-MW2 infected Caenorhabditis elegans in the presence of DMSO (1%), compound 1 (2.86 μg/ml) or vancomycin (10 μg/ml). Images were obtained from the original HTS campaign. (B) Rescue of C. elegans nematodes infected with MRSA MW2 by compound 1 over the concentration range of 0.5–64 μg/ml. Data represent the mean ± SD (n = 15). (C) Galleria mellonella survival assay: Compound 1 (25 mg/kg) was administrated into the last right proleg before (prophylactic) or after (therapeutic) bacterial inoculation (2 × 106 cells/ml). Vancomycin (25 mg/kg) and DMSO or no injection were used as positive and negative controls, respectively.
HTS: High-throughput screening; MRSA: Methicillin-resistant Staphylococcus aureus.
In 2011, Desbois and Coote reported a G. mellonella wax moth whole animal model for assessing compounds against S. aureus infections. The simple in vivo model provides a readout of compound efficacy and host toxicity and reduces the need for larger animal experiments [40]. The wax moth model has also been used to evaluate compounds [29] and phage therapies [41] against other pathogens. Compound 1 was tested in G. melonella larvae (n = 12/group) both before (prophylactic) and after (therapeutic) inoculation with 2 × 106 cells of MRSA MW2. In both the prophylactic and therapeutic arms, survival of infected larvae increased significantly (p = 0.0002) in the presence of compound 1 (25 mg/kg) relative to vancomycin (25 mg/kg) control (Figure 6C).
Conclusion
In summary, compound 1 shows bacteriostatic activity against MRSA that is enhanced at acidic pH. The compound is effective against intracellular MRSA and appears to act as a protonophore that dissipates the membrane proton gradient, resulting in decreased intracellular pH. Moreover, it demonstrated low cellular toxicity, is nonmutagenic and shows comparable activity to vancomycin in two whole animal alternative MRSA infection models. Based on these findings, compound 1 warrants further investigation as a novel antibacterial for treating MRSA infections.
Future perspective
MRSA is categorized as a ‘serious threat’ by the Centers for Disease Control and Prevention and is associated with ≥11,000 deaths per year. Our study demonstrates that the compound 1-(4-chlorophenyl)-4,4,4-trifluoro-3-hydroxy-2-buten-1-one (compound 1) is active in vitro and in vivo against MRSA and appears to act through a protonophoric mechanism. In future work, the compound should be advanced to vertebrate models and structural analogs should be prepared to probe structure–activity relationships, especially the relationship between pKa, potency/efficacy and toxicity.
Summary points.
Compound 1-(4-chlorophenyl)-4,4,4-trifluoro-3-hydroxy-2-buten-1-one inhibited the growth of methicillin-resistant Staphylococcus aureus (MRSA) with an MIC of 4.0 μg/ml.
The compound is bacteriostatic.
Importantly, the efficiency of the compound was increased in acidic pH and the MIC was reduced by fourfold (to 1 μg/ml) in pH 6.0 and by eightfold (to 0.5 μg/ml) in pH 5.0.
Treatment with the compound resulted in a 1-log10 reduction of colony forming unit per milliliter of intracellular MRSA-MW2 bacteria.
- We confirmed the activity of the compound using two alternative model hosts:
- In the Caenorhabditis elegans MRSA infection model, treatment with the compound rescued C. elegans nematodes from S. aureus infection (p = 0.0015).
- In the Galleria mellonella model, the compound, administered before or after inoculation, resulted in prolonged survival (p = 0.0002).
Notably, administration of compound resulted in a reduced intracellular pH (6.5) of MRSA MW2 by dissipating the proton gradient and concentrations up to 64 μg/ml did not cause toxicity to human red blood cells.
This potential protonophore warrants further evaluation for the development of this compound as a novel antibacterial agent.
Footnotes
Financial & competing interest disclosure
This study was supported by NIH grant P01 AI083214 to E Mylonakis. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005;5(12):751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan ME, Murray-Leisure KA, Ribner BS, et al. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am. J. Med. 1993;94(3):313–328. doi: 10.1016/0002-9343(93)90063-u. [DOI] [PubMed] [Google Scholar]
- 3.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015;28(3):603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Truong-Bolduc QC, Bolduc GR, Okumura R, et al. Implication of the NorB efflux pump in the adaptation of Staphylococcus aureus to growth at acid pH and in resistance to moxifloxacin. Antimicrob. Agents Chemother. 2011;55(7):3214–3219. doi: 10.1128/AAC.00289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen MS, Bainton DF. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J. Cell Biol. 1973;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nekoofar MH, Namazikhah MS, Sheykhrezae MS, et al. pH of pus collected from periapical abscesses. Int. Endod. J. 2009;42(6):534–538. doi: 10.1111/j.1365-2591.2009.01550.x. [DOI] [PubMed] [Google Scholar]
- 7.Fraunholz M, Sinha B. Intracellular Staphylococcus aureus: live-in and let die. Front. Cell. Infect. Microbiol. 2012;2:43. doi: 10.3389/fcimb.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rathman M, Sjaastad MD, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect. Immun. 1996;64(7):2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemaire S, Van Bambeke F, Mingeot-Leclercq MP, Glupczynski Y, Tulkens PM. Role of acidic pH in the susceptibility of intraphagocytic methicillin-resistant Staphylococcus aureus strains to meropenem and cloxacillin. Antimicrob. Agents Chemother. 2007;51(5):1627–1632. doi: 10.1128/AAC.01192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes how the Staphylococcus aureus senses and responds to pH stimulus.
- 10.Weinrick B, Dunman PM, Mcaleese F, et al. Effect of mild acid on gene expression in Staphylococcus aureus . J. Bacteriol. 2004;186(24):8407–8423. doi: 10.1128/JB.186.24.8407-8423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falagas ME, Mcdermott L, Snydman DR. Effect of pH on in vitro antimicrobial susceptibility of the Bacteroides fragilis group. Antimicrob. Agents Chemother. 1997;41(9):2047–2049. doi: 10.1128/aac.41.9.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sell TS, Belkacemi T, Flockerzi V, Beck A. Protonophore properties of hyperforin are essential for its pharmacological activity. Sci. Rep. 2014;4:7500. doi: 10.1038/srep07500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson RD, Hewlett EL. Niclosamide therapy for tapeworm infections. Ann. Intern. Med. 1985;102(4):550–551. doi: 10.7326/0003-4819-102-4-550. [DOI] [PubMed] [Google Scholar]
- 14.White CA., Jr Nitazoxanide: a new broad spectrum antiparasitic agent. Expert Rev. Anti Infect. Ther. 2004;2(1):43–49. doi: 10.1586/14787210.2.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Roca I, Akova M, Baquero F, et al. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajamuthiah R, Fuchs BB, Jayamani E, et al. Whole animal automated platform for drug discovery against multi-drug resistant Staphylococcus aureus . PLoS ONE. 2014;9(2):e89189. doi: 10.1371/journal.pone.0089189. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes antimicrobial screening using nematode infection model.
- 17.Sifri CD, Begun J, Ausubel FM, Calderwood SB. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 2003;71(4):2208–2217. doi: 10.1128/IAI.71.4.2208-2217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beanan MJ, Strome S. Characterization of a germ line proliferation mutation in C. elegans . Development. 1992;116(3):755–766. doi: 10.1242/dev.116.3.755. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka-Hino M, Sagasti A, Hisamoto N, et al. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans . EMBO Rep. 2002;3(1):56–62. doi: 10.1093/embo-reports/kvf001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wikler MA. Methods For Dilution Antimicrobial Susceptibility Tests For Bacteria That Grow Aerobically: Approved Standard. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2006. [Google Scholar]
- 21.Rajamuthiah R, Fuchs BB, Conery AL, et al. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus . PLoS ONE. 2015;10(4):e0124595. doi: 10.1371/journal.pone.0124595. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes how to test antimicrobials in macrophages.
- 22.Schmitt DM, O'dee DM, Cowan BN, et al. The use of resazurin as a novel antimicrobial agent against Francisella tularensis. Front. Cell. Infect. Microbiol. 2013;3:93. doi: 10.3389/fcimb.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks LR, Clementi EA, Hakansson AP. Sensitization of Staphylococcus aureus to methicillin and other antibiotics in vitro and in vivo in the presence of HAMLET. PLoS ONE. 2013;8(5):e63158. doi: 10.1371/journal.pone.0063158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim W, Conery AL, Rajamuthiah R, Fuchs BB, Ausubel FM, Mylonakis E. Identification of an antimicrobial agent effective against methicillin-resistant Staphylococcus aureus persisters using a fluorescence-based screening strategy. PLoS ONE. 2015;10(6):e0127640. doi: 10.1371/journal.pone.0127640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalhoff A. Comparative in vitro and in vivo activity of the C-8 methoxy quinolone moxifloxacin and the C-8 chlorine quinolone BAY y 3118. Clin. Infect. Dis. 2001;32(Suppl. 1):S16–S22. doi: 10.1086/319371. [DOI] [PubMed] [Google Scholar]
- 26.Isnansetyo A, Kamei Y. MC21-A, a bactericidal antibiotic produced by a new marine bacterium, Pseudoalteromonas phenolica sp. nov. O-BC30(T), against methicillin-resistant Staphylococcus aureus . Antimicrob. Agents Chemother. 2003;47(2):480–488. doi: 10.1128/AAC.47.2.480-488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An excellent review of Ames test.
- 27.Kwon B, Kumar P, Lee HK, et al. Aberrant cell cycle reentry in human and experimental inclusion body myositis and polymyositis. Hum. Mol. Genet. 2014;23(14):3681–3694. doi: 10.1093/hmg/ddu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000;455(1–2):29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 29.Gibreel TM, Upton M. Synthetic epidermicin NI01 can protect Galleria mellonella larvae from infection with Staphylococcus aureus . J. Antimicrob. Chemother. 2013;68(10):2269–2273. doi: 10.1093/jac/dkt195. [DOI] [PubMed] [Google Scholar]; •• Describes reduced efficacy of conventional antibiotics in acidic pH.
- 30.Brouillette E, Grondin G, Shkreta L, Lacasse P, Talbot BG. In vivo and in vitro demonstration that Staphylococcus aureus is an intracellular pathogen in the presence or absence of fibronectin-binding proteins. Microb. Pathog. 2003;35(4):159–168. doi: 10.1016/s0882-4010(03)00112-8. [DOI] [PubMed] [Google Scholar]; •• An excellent review of S. aureus abscess.
- 31.Baudoux P, Bles N, Lemaire S, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Combined effect of pH and concentration on the activities of gentamicin and oxacillin against Staphylococcus aureus in pharmacodynamic models of extracellular and intracellular infections. J. Antimicrob. Chemother. 2007;59(2):246–253. doi: 10.1093/jac/dkl489. [DOI] [PubMed] [Google Scholar]
- 32.Cheng AG, Dedent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011;19(5):225–232. doi: 10.1016/j.tim.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones JR, Patel SP. The acidites of weak acids. Part III. Some 3-benzoyl-1, 1, 1-trifluoroacetones. J. Chem. Soc., Perkin Trans. 1975;2(11):1231–1234. [Google Scholar]
- 34.Slonczewski JL, Fujisawa M, Dopson M, Krulwich TA. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 2009;55:1–79. doi: 10.1016/S0065-2911(09)05501-5. 317. [DOI] [PubMed] [Google Scholar]; •• Describes nature of antibiotics targeting proton-motive force.
- 35.Terada H. The interaction of highly active uncouplers with mitochondria. Biochim. Biophys. Acta. 1981;639(3–4):225–242. doi: 10.1016/0304-4173(81)90011-2. [DOI] [PubMed] [Google Scholar]
- 36.Feng X, Zhu W, Schurig-Briccio LA, et al. Antiinfectives targeting enzymes and the proton motive force. Proc. Natl Acad. Sci. USA. 2015;112(51):E7073–E7082. doi: 10.1073/pnas.1521988112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C, Dolla NK, Casadei G, Bremner JB, Lewis K, Kelso MJ. Diarylacylhydrazones: clostridium-selective antibacterials with activity against stationary-phase cells. Bioorg. Med. Chem. Lett. 2014;24(2):595–600. doi: 10.1016/j.bmcl.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ames BN, Mccann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mut. Res. 1975;31(6):347–363. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 39.Moy TI, Conery AL, Larkins-Ford J, et al. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem. Biol. 2009;4(7):527–533. doi: 10.1021/cb900084v. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes about excellent utilization of wax moth for anti-infective agents evaluation.
- 40.Desbois AP, Coote PJ. Wax moth larva (Galleria mellonella): an in vivo model for assessing the efficacy of antistaphylococcal agents. J. Antimicrob. Chemother. 2011;66(8):1785–1790. doi: 10.1093/jac/dkr198. [DOI] [PubMed] [Google Scholar]
- 41.Beeton ML, Alves DR, Enright MC, Jenkins AT. Assessing phage therapy against Pseudomonas aeruginosa using a Galleria mellonella infection model. Int. J. Antimicrob. Agents. 2015;46(2):196–200. doi: 10.1016/j.ijantimicag.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 42.CellProfiler. www.cellprofiller.org/