Abstract
The recent tremendous successes in clinical trials take cancer immunotherapy into a new era and have attracted major attention from both academia and industry. Among the variety of immunotherapy strategies developed to boost patients’ own immune systems to fight against malignant cells, the pathogen-based and adoptive cell transfer therapies have shown the most promise for treating multiple types of cancer. Pathogen-based therapies could either break the immune tolerance to enhance the effectiveness of cancer vaccines or directly infect and kill cancer cells. Adoptive cell transfer can induce a strong durable antitumor response, with recent advances including engineering dual specificity into T cells to recognize multiple antigens and improving the metabolic fitness of transferred cells. In this review, we focus on the recent prospects in these two areas and summarize some ongoing studies that represent potential advancements for anticancer immunotherapy, including testing combinations of these two strategies.
Keywords: : adoptive cell transfer, CD8 T cell, immunotherapy, pathogen-based vaccine
Despite the fact that cancer has been recognized as one of the most serious health problems worldwide and is responsible for an estimated eight million deaths every year, it remains challenging to treat cancer using traditional approaches [1]. Moreover, the effectiveness of traditional treatments, such as surgery, radiation, chemotherapy or targeted agents can be limited by complications or the development of adverse effects that reduce the quality of life for patients. In addition, many patients develop resistance to treatment or relapse after treatment, resulting in unsatisfactory survival rates. Owing to the recent unprecedented developments in oncoimmunology, immunotherapy holds the promise to transform this situation by stimulating patients’ own anticancer immunity to spur disease eradication.
The immune system is well appreciated as a defense mechanism against pathogens by recognizing foreign antigens and eliminating the infection. It has long been questioned whether such a mechanism exists for malignant cells. In the 1960s, Thomas and Burnet first hypothesized that lymphocytes are capable of recognizing tumor-associated antigens (TAAs) to continually monitor and eliminate the aberrant cells during initial transformation, a process that has come to be known as ‘immunosurveillance’ [2,3]. Further studies have provided strong evidence to support this theory, which fosters the possibility to utilize the immune system for tumor destruction [4,5]. However, these cancer cells develop multiple mechanisms to escape from immune surveillance, including loss of antigenicity, defects in antigen presentation and the upregulation of co-inhibitory molecules that disrupt T-cell signaling (e.g., expression of PD-1), a negative regulator of the immune system [6,7]. These observations represent significant challenges to the field of immunotherapy.
The history of immunotherapy can be traced back to the 1890s when William Coley, a New York physician, observed that some sarcoma patients had spontaneous tumor regression after bacterial infection. Based on this finding, he intentionally infected cancer patients with Streptococcus, hoping to induce a similar response [8]. He achieved a cure rate of over 10% in multiple type of cancers, including sarcoma, lymphoma and testicular carcinoma [8]. Following this, several immunological approaches were developed to fight against cancer, such as therapeutic cancer vaccines and bacteria-based therapy [9]. However, these approaches had minimal efficacy in a clinical setting, in part due to the limited understanding of the interaction between cancer cells and the immune system [10]. It was not until recently that immune checkpoint proteins, such as PD-1 and CTLA-4, were found to be utilized by cancer cells to suppress antitumor immune responses [11]. Since then, treatment with antibodies blocking these immunosuppressive mechanisms has produced significant response rates and improvements in survival in many types of cancer [12]. These remarkable successes have revolutionized the field and reignited enthusiasm for immunotherapeutic approaches to combat cancer.
Alongside the checkpoint inhibitors, other emerging trends in immunotherapy include pathogen-based cancer therapies and adoptive cell transfer therapy (ACT) [11]. Pathogens were historically used to activate the host immune response against cancer cells, but have recently also been used to boost the efficacy of therapeutic cancer vaccination and mediate direct cancer cell lysis. Furthermore, recent advances in ACT include creating T cells with dual specificity or improved metabolic fitness for a more specific and durable treatment. Here we highlight the current landscape and recent advances of these strategies in cancer immunotherapy as well as ongoing challenges.
Pathogen-based cancer therapies
The antitumor effect of bacterial infection has been well observed and practiced throughout history. The first evidence that revealed the link between infection and reduction of cancerous tissue was documented by an Egyptian physician Imhotep 2600 BC [13]. This phenomenon was also independently reported by Busch and Fehleisen in 1867, when they started to intentionally treat cancer patients with streptococcal infection [14]. However, it was not until 40 years later that William Coley first systematically practiced this approach with the hope of stimulating the immune attack on tumors [8]. Since then, much work has been done to demonstrate that bacteria are well suited to serve as anticancer therapy, either as direct oncolytic reagents or potent adjuvants for boosting antitumor immunity (Table 1).
Table 1. . Recent promising examples of pathogen-mediated tumor therapy.
| Pathogen species/strain | Characteristics | Target cancer | Indication |
|---|---|---|---|
| Clostridium novyi-NT | Gram-positive, anaerobic, sporulating | Advanced leiomyosarcoma | Tumor regression after several intratumoral applications of spores |
| Salmonella typhimurium ssp (VP20009 and Ty21a) | Gram-negative, facultative anaerobe, motile, intracellular | Melanoma, pancreatic cancer | Induction of immune response, tumor colonization, insignificant tumor regression |
| BCG | Gram-positive, obligate aerobe, nonmotile, intracellular | Bladder cancer | Approved for bladder cancer management as intravesical BCG therapy, proven to be more effective than intravesical chemotherapy |
| T-VEC | Modified HSV, type 1 | Melanoma | T-VEC has recently been approved for the treatment of advanced of melanoma in the USA, Europe and Australia |
| Listeria monocytogenes (ANZ-100 and CRS-207) | Gram-positive, facultative anaerobic, intracellular | Solid tumors (liver, lung, pancreas, ovary and pancreatic) | Induction of immune response, insignificant tumor regression |
T-VEC: Talimogene laherparepvec.
Oncolytic pathogen-based therapy
Intrigued by Coley's work, the antitumor potential of several genera of bacteria, such as Clostridia sp., Salmonella sp. and Listeria sp. was further explored by many investigators [15]. An advantage of bacteria-based therapy is high tumor specificity. In most of these attempts, bacteria colonization was successfully contained within the tumor without harming the healthy tissue. Furthermore, the high mobility of bacteria allows them to easily move away from the vasculature and penetrate the tumor tissue more deeply than other conventional treatments, including chemotherapy and radiation.
The ideal therapeutic strains of bacteria should preferentially accumulate in the cancerous tissue and induce cell lysis. Thus, Clostridia, an anaerobic bacterium, were selected to develop bacteria-based cancer therapy due to the hypoxic nature of most tumors [16]. After administration, Clostridium selectively invades and multiplies in the hypoxic tumor microenvironment (TME) and reduces tumor burden in a murine model of sarcomas [17]. However, wild-type Clostridium cannot eradicate tumors and exhibits no effect on smaller metastatic masses in clinical trials [16]. Therefore, bacterial engineering and screening were employed to generate a strain with an enhanced antitumor effect, known as Clostridium oncolyticum M-55. However, even the C. oncolyticum M-55 strain failed to produce significant tumor regression in patients [18]. In another attempt to optimize the therapeutic strains, a major virulent factor, α-toxin, was eliminated to produce a strain named Clostridium novyi-NT [19]. This strain induces a strong local oncolytic and inflammatory effect to reduce the tumor burden in both murine and canine models. These promising results led to an ongoing human Phase I clinical trial for patients with treatment-refractory tumors to assess its clinical application (NCT01924689).
Aside from Clostridium, another attempt to increase tumor specificity and restrict bacteria to tumors without dissemination to healthy organs is creating metabolically deficient variants through auxotrophy. The basis of this approach is that the recombinant bacterial strain is dependent on the TME for essential nutrients that are not present in sufficient levels in normal tissue [20]. One of the prominent strains, VNP20009, is a genetically modified strain of Salmonella typhimurium YS72 with attenuated virulence and deletion of the purI genes [21]. This modification makes VNP20009 lack the enzyme for purine synthesis, and thus it depends on external sources for survival. Consequently, this bacterium cannot replicate in normal tissues that lack excess purine, but can still multiply in purine-rich TME. Similar to Clostridium, the significant antitumor activity demonstrated by Salmonella bacterial therapy in mouse models was unable to translate to clinical efficacy in humans. However, these studies did confirm that VNP20009 can safely be injected in humans at large doses with limited toxicity. Additionally, one of the crucial factors contributing to the therapeutic drawbacks is insufficient bacterial colonization in the tumor caused by over-attenuation [22]. Despite all these failures, promising preclinical results of bacteria-based approaches still warrant further investigation for the development and optimization of better-suited therapeutic strains.
Unlike the previous bacterial cancer therapies that faced serious translational challenges, the BCG vaccine, an attenuated strain of Mycobacterium bovis, had remarkable success in clinical settings and has become the standard of care for superficial bladder cancer [23]. This therapeutic strain was initially developed as a vaccine against tuberculosis infection. Its application for cancer treatment was first demonstrated by Morales and Eidinger in 1976 [23]. Later, several independent studies reported that BCG induced superior tumor regression compared with chemotherapy by not only promoting direct cell lysis, but also by eliciting a strong local inflammatory response [24]. After injection into the bladder, BCG can induce antigen presentation of BCG and/or cancer cell peptides, cytokine secretion and T-cell-mediated cytotoxicity, all of which may contribute to the antitumor immune response. A better understating of this therapy may provide guidance for improved design of future approaches.
Despite all of these promising attempts, bacteria-based therapy still faces several challenges. First, many therapeutic strains are developed by deleting virulence factors that are usually required for inducing an immune response and, therefore, might be required for therapeutic effects [9]. Thus, these over-attenuated strains are safe to administer to patients, but fail to produce any meaningful antitumor effect. An ideal strain for effective cancer treatment needs to carefully balance toxicity and therapeutic potential. One way to achieve both attenuation and optimization of therapeutic effect is delayed attenuation. This is based on the principle that the virulence of the bacteria can be fine-tuned after treatment. For example, a virulence factor can be genetically engineered to be expressed under an arabinose inducible promoter PBAD [25]. As a consequence, this strain can be amplified in an in vitro culture with supplemented arabinose. After administration, the level of arabinose is diluted out, leading to halted expression of the virulence factor and the bacteria become attenuated after a few rounds of replication. Recently, such an approach was used for Salmonella to modify the lipopolysaccharide (LPS) structure under the control of PBAD, prompting more vigorous tumor regression compared with an over-attenuated strain [25]. Another problem is that most of these strains were developed using murine models, which do not always mimic human disease. In accordance, the human clinical trial showed that most of the bacteria were cleared from the circulation within 60 min, which is significantly shorter than in the murine model and negatively impacted the therapeutic effect [26]. Despite all of these obstacles, advancements in microbiology and genetic engineering foster the possibility to tailor a safe therapeutic strain with an optimized therapeutic effect.
In addition to bacteria, another innovative use of pathogens is utilizing naive and genetically engineered viruses to induce lysis of cancer cells. Compared with healthy cells, cancer cells are more susceptible to viral infection owing to their prolonged proliferation, inactivation of growth suppressors, and immunosuppressive TME. Thus, oncolytic virus (OV) therapy can specifically target and destroy tumors with minimal toxicity to healthy tissues. In addition to direct tumor lysis, increasing evidence demonstrates the potential of OV therapy to prime the adaptive antitumor responses [27]. Indeed, viral replication provides a source of viral pathogen-associated molecular patterns (PAMPs) that stimulate the production of cytokines, such as type I IFN, TNF-α, IFN-γ and IL-12, which ultimately aid in the maturation of APCs [28]. Together with the release of TAAs from OV-lysed tumor cells, OV therapy could induce a tumor-specific T-cell-mediated antitumor response [27]. Since the initial clinical attempt of OV in the 1950s [29], multiple strains, such HSV-1, vaccine virus, reovirus and adenovirus, have been tested in a range of cancer types [27]. Among these strains, an HSV-1-based OV named talimogene laherparepvec exhibited a durable response rate of 16% in a Phase III clinical trial for late-stage melanoma. Moreover, talimogene laherparepvec became the first US FDA approved OV therapy for the treatment of advanced metastatic melanoma [30]. This revolutionary progress has attracted additional investigations centered on enhancing the OV-mediated antitumor immunity while minimizing pathology.
Pathogen-boosted cancer vaccines
Another important application of bacteria in cancer treatment is serving as vehicles and adjuvants for therapeutic cancer vaccines, the holy grail of immunotherapy. The ultimate purpose of cancer vaccines is to induce a potent cytotoxic T lymphocyte (CTL) response against a TAA for tumor destruction, thereby preventing tumor development. However, most clinical results from cancer vaccine trials have thus far been disappointing and have yielded less impressive results than other forms of immunotherapy such as ACT and checkpoint blockade. Despite extensive studies in this area, there are still two major obstacles that must be overcome for improved efficacy: cancer-induced tolerance and an immunosuppressive TME [31].
As discovered by Thierry Boon, the immune system is capable of recognizing and responding to TAAs, most of which are usually endogenous antigens bearing strong homology to self-proteins [32]. Thus, the antigen presentation process of TAAs often occurs without the co-stimulatory and/or inflammatory signals required for proper T-cell activation and expansion, thereby facilitating the development of immune tolerance [33]. Another important factor that needs to be considered when designing effective cancer vaccines is the immunosuppressive TME created by cancer cells via various intrinsic and extrinsic mechanisms [34]. Intrinsically, cancer cells express abundant ligands for inhibitory receptors on T cells (e.g., PD-L1) and various soluble factors (e.g., galectin 1 and galectin 3) to directly inhibit T-cell activation [34]. In addition, multiple anti-inflammatory cytokines, such as TGF-β and IL-10, are also secreted by tumor cells to inhibit the antitumor immunity within the TME [34]. Extrinsically, tumors produce chemokines and cytokines to create an environment that favors the recruitment and differentiation of immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCs) and Treg [35]. There is compelling evidence that these immunoregulatory cells effectively curtail the vaccine-induced T-cell response and thus serve as critical targets for improving cancer vaccines.
Among all the approaches tested for enhancing immunogenicity of cancer vaccines, bacteria exhibit the greatest adjuvant activity. Unlike the TAAs, infectious bacteria are naturally capable of evoking vigorous innate and adaptive immune response. Molecules conserved among many types of pathogens, known as PAMPs, are recognized by pattern recognition receptors, including toll-like receptors, NOD-like receptors, C-type lectin receptors and RIG-I-like receptors, to initiate the nonspecific innate immune response [36]. This engagement will recruit Toll/IL-1 receptor domain-containing adaptor proteins, such as MyD88 and TRIF, which result in activation of NF-κB and MAP kinases to induce the production of inflammatory cytokines, such as IL-12, IL-1, IL-6, IFN-γ and TNF-α. All of these act to promote phagocytosis, dendritic cell maturation and upregulation of MHC and co-stimulatory molecules on dendritic cells for effective priming of CD4 and CD8 T cells [37]. Apart from providing essential requirements for initiating T-cell immunity, bacteria can also effectively disrupt the immunosuppressive TME [38]. As one of the most prominent bacterial strains utilized as an adjuvant, Listeria monocytogenes (LM) based vaccines not only reduce the number of tumor-infiltrating MDSCs, but also reduce their suppressive activity [39]. The LM infection creates a pro-inflammatory environment, which repolarizes the MDSCs from an immunosuppressive M2 phenotype to an immunostimulatory, classical M1 phenotype [40,41]. In addition, LM suppression of Treg recruitment has been previously reported in various tumor models. Paterson's lab demonstrated that the recombinant LM expressing a TAA could induce tumor regression and Treg reduction, while an isogenic LM-based vaccine that lacks the bacterial product listeriolysin O actually increased the number of Tregs within the TME and demonstrated reduced antitumor efficacy [42]. Therefore, it is possible that the PAMP activity of LM is a major contributor to the reduction of tumor-associated immunosuppression by LM-based vaccines. In addition, LM infection effectively promotes potent Th1 and Th17 responses, which may also suppress Treg differentiation [43]. All of these strong adjuvant-like features of bacteria constitute it as a powerful platform to deliver TAA for the induction of potent antitumor immune response.
Due to all these advantages, an LM-based cancer vaccine was constructed with Her2, an EGFR family protein that is overexpressed in breast cancer [44]. Despite the fact that the majority of Her2 cancer vaccines were used for prophylaxis, this LM-Her2 cancer vaccine prompted a robust CTL response that resulted in tumor regression in various mouse and rat models of breast cancer [45]. This significant therapeutic effect is derived from the ability of the LM-Her2 vaccine to induce a greater repertoire of Her2-specific CTLs than conventional vaccination strategy. These preclinical results led to several additional vaccines being developed, such as ADXS-cHER2, which is currently undergoing Phase I clinic trials [46]. However, single TAA-targeted vaccines still face the challenge of tumor immune escape due to mutation of a single targeted TAA epitope. In a recent attempt to solve this problem, multiple TAAs, such as oncogenic HBV, alpha-fetoprotein and MAGE-A, were introduced into LM to create a therapeutic vaccine against hepatocellular carcinoma [47]. This LM-based cancer vaccine expressed multiple peptide fusing genes (MPFG) and significantly enhanced the infiltration and CTL activity of MPFG-specific CD8 T cells [47]. Consistent with previous findings, this LM-MPFG vaccine also decreased the number of intratumoral Tregs and their production of TGF-β and IL-10 [47]. Collectively, these features led to further development of LM-based immunotherapy to deliver several other TAAs including prostate-specific antigen, tyrosinase and HPV-16 E7 in mouse models of prostate, melanoma and cervical cancer, respectively [48–50].
All of these promising developments stem from Coley's success, which largely depends on the ability of bacteria to provoke a vigorous innate immune response, thereby making bacteria an attractive anticancer agent. However, the ultimate goal of cancer therapy is to eradicate of the primary tumor and provide long-term protection against recurrence, which usually requires a potent adoptive T-cell response targeting tumor cells. Therefore, rational combinations of multiple modalities targeting distinct aspects of immune pathways will likely be essential to achieve durable antitumor effects and more effective therapeutic outcomes. Many recent promising immunotherapeutic modalities, such as ACT and immune checkpoint blockade antibodies (e.g., PD-1), target tumor-specific adaptive T-cell responses. Therefore, it is feasible and attractive to combine pathogen-based agents with these approaches to synergistically enhance antitumor immunity and/or engage complementary antitumor responses, thereby facilitating additional improvements in clinical outcomes.
Adoptive T-cell therapy
The ACT therapies are based on the principle that tumor reactive T cells can be transferred to patients and induce a robust antitumor response [51,52]. To support this idea, Rosenberg et al. first illustrated the feasibility of ex vivo expansion of tumor-infiltrating T lymphocytes (TILs) and utilization of these cells to treat patients with advanced melanoma [53]. More recently, another form of ACT has attracted increasing interest, where the antigen specificity of T cells is genetically modified to target tumor cells. In such approaches, T cells can be manipulated to express modified T-cell receptors (TCRs) or protein-fusion-derived chimeric antigen receptors (CARs) that have enhanced tumor antigen specificity [52]. These therapies have demonstrated remarkable clinical efficacy for reducing tumor burden and improving survival of patients with B-cell malignancies [54,55]. The success of ACT depends on several factors, such as the magnitude of the T-cell response, the phenotypic and functional attributes of responding T cells and TCR affinity [56]. Despite these unprecedented successes, many obstacles, such as poor tumor specificity and T-cell infiltration, still prevent its application on solid tumors. Much attention has been focused on overcoming these challenges and extending the use of ACT to the treatment of a broader range of cancers.
The major challenges of ACT against solid tumors
Unlike the successes in hematopoietic malignancies, the clinical trials that applied ACT to solid tumors have faced multiple challenges. Many trials either showed no beneficial antitumor effect or induced life-threatening side effects including neurotoxicity [57], cardiac toxicity [58], liver toxicity [59] and severe inflammation on other organs [60]. Several features of solid tumors, such as the immunosuppressive TME, harsh physical barriers and inhibition of T-cell chemotaxis, limit the ability of T cells to infiltrate the tumors and eliminate malignant cells. Therefore, multiple factors need to be considered to design a successful ACT against solid tumors.
First, T cells need to be able to traffic and penetrate into the tumor tissue to access the malignant cells for destruction. After the ACT, the transferred T cells need sufficient chemotaxis to be recruited to the tumor site. Unfortunately, the critical step of this process, adhesion to endothelium, is impeded by downregulation of endothelial cell adhesion molecules, such as ICAM-1, during tumor angiogenesis. The blood vessels of solid tumors express elevated level of endothelin B receptor, which suppresses the expression and surface clustering of ICAM-1 [61]. The endothelin B receptor antagonist BQ-788 can significantly induce ICAM-1-dependent adhesion of T cells to endothelial cells, leading to a strong antitumor immune response [61]. Following migration to the tumor site, T cells encounter the stroma-rich environment and aberrant vasculature within the tumor. The stroma contains fibroblasts and vascular endothelial cells with excessive extracellular matrix, which can sufficiently trap T cells to prevent them from reaching the tumor tissue. The disruption of stromal structure has been shown to markedly promote the intratumoral penetration of T cells [62].
Second, upon arrival of T cells inside the tumor, they must expand and persist sufficiently under the immunosuppressive TME to mediate tumor killing. As mentioned earlier, many inhibitory pathways, such as anti-inflammatory cytokines (TGF-β), immunoregulatory cells and ligands for T-cell inhibitory receptors (PD-L1), are dramatically enriched in the TME [34]. To overcome these barriers, one straightforward method is to engineer the T cells to ignore these inhibitory signals. For example, CRISPR genome editing is being used to delete the inhibitory receptor PD-1 from T cells to prevent them from PD-L1-mediated suppression [63,64]. Similarly, enforcing the expression of dominant-negative TGF-β receptor could also make T cells resistant to the TME and improve their antitumor effect [65]. Another innovative approach transforms the inhibitory signaling to induce stimulatory pathway in T cells. Several recent studies attempted to fuse the extracellular domain of PD-1 with an intracellular co-stimulatory domain, and then overexpress this chimeric receptor in T cells [66]. Consequently, these T cells can interpret the immunosuppressive signals from PD-L1 to trigger co-stimulatory signaling pathways for enhanced T-cell activity [66]. Other effects to equip T cells themselves with the ability to counteract the TME include engineering T cells to produce potent cytokines. For example, armored CAR T cells were developed to constitutively express IL-12, a cytokine with beneficial impacts on both the innate and adaptive immune response [67,68]. Once the CAR T cells are activated, they will produce increasing amount of IL-12 that could shift differentiation of CD4 T cells toward the Th1 phenotype, reprogram the MDSCs and enhance the activity of NK cells and CD8 T cells [69]. All the above actions will alter the TME for a better antitumor response. Instead of improving the design of ACT, another approach is simply combining ACT with other agents that can remodel TME, like PD-1 blockade or OVs expressing the chemokine RANTES and the cytokine IL-15 [70,71]. These combination therapies transform the suppressive TME to a more favorable microenvironment for T cells and can enhance the efficacy of ACT.
The third challenge that CD8 T cells inside the TME have to face is the nutrient restriction that significantly impairs the functional fate of T cells. For example, glucose, the main source of energy for activated T cells, is quickly consumed by fast-growing tumor cells and unavailable to T cells [52]. To support this competition model, several recent studies revealed that TILs isolated from mutant tumors with a highly glycolytic phenotype exhibited reduced IFN-γ production and mTOR activity compared with TILs from wild-type control tumors [72,73]. Furthermore, glucose deprivation induces phosphoenolpyruvate deficiency, leading to impaired antitumor T-cell function that is corrected by overexpression of phosphoenolpyruvate [74]. Collectively, these studies revitalized the field of immunemetabolism and brought attention to the therapeutic potential of metabolic reprogramming of T cells for ACT. As the model of competition for glucose between tumors and T cells has become well appreciated, one evident strategy to improve ACT would be optimizing the in vitro culture conditions of T cells to prepare them for the nutrient-limited TME.
The rationale behind this approach is to inhibit glycolysis during in vitro culture to preferentially promote memory T differentiation with improved longevity after adoptive transfer. To test this idea, 2-deoxyglucose, which inhibits hexokinase and thus glycolysis, was used to treat cultured T cells [75]. Compared with untreated cells, these cells exhibited prolonged longevity without a compromise in effector function and proliferative capacity, thereby resulting in superior antitumor function [75].
A fourth area to consider when designing an ACT protocol is manipulation of key transcriptional factors for metabolism to enhance the activity of transferred cells inside the tumor. This notion is supported by the recent observations that T cells develop a defective mitochondrial phenotype via repressed PGC1α in the TME, which correlates with dampened effector function [72]. Further studies showed that enforcing PGC1α expression in T cells leads to improved effector function of tumor-specific T cells and tumor regression. Additionally, other similar targets include Opa1 and BATF, which are known to be involved in T-cell differentiation and target multiple genes involved in metabolic regulation [76–78]. Collectively, all these studies highlight that T-cell metabolic fitness is an important component of effective ACT.
Finally, one of the most important factors for successful ACT is to minimize the toxicity. In almost all ACT trials, various levels of cross-reaction of T cells and immune attack on bystander normal cells were observed. As reported in CD19 CAR trials, CAR T cells also eliminated nonmalignant CD19-expressing B cells and resulted in B-cell aplasia in many patients, which can be managed by replacement antibody therapy. However, these side effects become more severe and unacceptable when applying ACT to solid tumor. Specifically, one of the common types of cross-reaction is the ON-target OFF-tissue effect caused by the fact that the targeted TAAs cannot offer a clear discrimination between tumor and healthy tissue. Many TAAs are highly expressed on tumor cells, but are also found widely expressed at low but recognizable levels in other normal tissue. Thus the possibility exists where tumor-targeted T cells may also attack normal tissue, which can lead to life-threatening toxicity. For example, one patient in the CAR T trial targeting Her2 developed acute lung inflammation and died from severe side effects caused by T cells attacking the ERBB2-expressing epithelial cells in the lung and other organs [79]. Consistent with this, most patients from another ACT trial targeting MART-1 suffered intolerable adverse effects on the skin, eyes and ears [80]. Currently, no TAAs are exclusively expressed in solid tumors without any detectable level in other normal tissues. Therefore, an emerging approach that equips T cells with the ability to recognize two antigens holds great promise in improving the specificity of ACT and will be discussed in further detail later. Overall, it is important to design an ACT that overcomes multiple challenges simultaneously in order to achieve a vigorous antitumor effect with acceptable side effects.
Dual-specific ACT
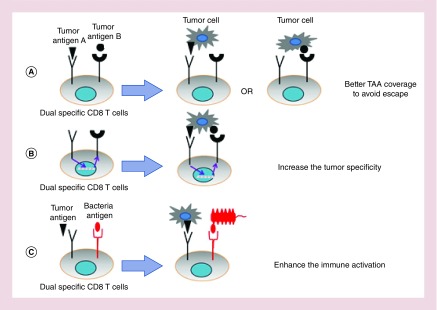
A major challenge of ACT is the lack of well-defined TAAs, which are expressed at high levels in tumor cells and low levels in healthy cells. As a consequence, the T cells engineered to recognize TAAs might also attack healthy cells leading to off-target toxicity [81]. This is the most common side effect of ACT therapies and can be predicted if the expression of target TAAs in various tissues has been profiled. Additionally, these T cells are usually engineered to recognize a single TAA, leading to potential loss of efficacy due to the tumor antigen heterogeneity and immune escape [52]. One of the most promising solutions for these hurdles is the use of dual-specific T cells that are engineered to express two receptors for targeting combinations of antigens. Depending on the purpose, dual-specific ACT can be divided into three types: for covering a wider range of antigens to avoid escape, T cells can be engineered to target two independent TAAs and may respond to either antigen; for increasing the tumor specificity, T cells can be engineered to recognize two TAAs, with both antigens being required to drive T-cell activation; for enhancing overall immune activation, T cells can be engineered to target one TAA and one bacterial antigen (Figure 1).
Figure 1. . The design of dual-specific T-cell therapy.
(A) T cells are engineered to express two TCRs, with each specific to a distinct target (tumor antigen A and B). The tumor cells expressing either antigen A or B will be eliminated by T cells to avoid immune escape. (B) T cells are modified to express two TCRs with different antigen specificities and can only be activated by the presence of both antigens. This approach ensures that T-cell activation only occurs on tumor cells with minimal effect on healthy cells. (C) T cells are generated to respond to a TAA and a bacteria antigen. An intramural bacterial infection will help expand and activate these T cells in TME.
TAA: Tumor-associated antigen; TCR: T-cell receptor; TME: Tumor microenvironment.
Inspired by the discovery of naturally generated T cells expressing two different TCRs, dual-specific T cells were initially developed to overcome the immune escape [82,83]. This is based on the notion that it would be advantageous to equip T cells with multiple specificities to anticipate immune escape by loss of a single antigen. It has been shown that these dual-specific T cells can produce cytokines (such as TNF-α, IL-2 and IFN-γ) and perform CTL activity in response to both antigens separately in vitro [82]. Adoptive transfer of these T cells to tumor-bearing mice led to various results (from a mild effect to tumor regression) depending on the type of tumor [82–87]. In the case of recent CAR T-cell studies, T cells targeting CD19 induced a complete response in approximately 90% of patients. However, in 11% of these patients, leukemic cells lost CD19 expression, which ultimately led to resistance to the therapy and relapse. To overcome this immune escape, two B-cell-specific antigens, CD19 and CD20, were selected as targets to generate CD19-OR-CD20 CAR T cells. The T cells equipped with this CAR exhibited potent cytotoxicity against either CD19- or CD20-expressing B cells. Upon transfer into the mice, these dual-specific CAR T cells can effectively control both wild-type B-cell lymphoma and CD19- mutants. This strategy could significantly improve the therapeutic efficacy of CAR therapy and prevent relapse by counteracting the immune escape. However, further clinical testing is necessary to determine the therapeutic efficacy of this tailored approach.
As some recent CAR clinical trials exhibited potentially lethal side effects on the cardiovascular and neurological system [88,89], more attention has recently been brought to designing a safer ACT approach. One potential strategy to attain this is by restricting T-cell responses to the tumor itself without harming healthy cells. To achieve this, Roybal et al. developed a novel dual-specific T-cell system in which T cells can only be activated by antigen engagement of both CARs [90]. As designed, the expression of a second CAR is regulated by the first CAR via hijacking the signal transduction mechanism of the Notch receptor. In this approach, T cells were engineered with a synthetic Notch receptor, whose extracellular domain is replaced with a recognition domain of anti-CD19 CAR, while a mesothelin CAR gene was also inserted into T cells under the control of Notch signaling. Once the CD19 antigen is engaged, the intracellular domain will be cleaved and translocate into the nucleus. Such action will induce the expression of the second mesothelin CAR with a 4–1BBζ costimulatory domain to equip T cells with the ability to recognize a secondary TAA. Further results from this study demonstrated that these T cells were only activated by tumor cells that simultaneously expressed both CD19 and mesothelin, but not those expressing only one of these antigens [90]. This approach of engineering T cells only become activated in the presence of two TAAs, which rarely occurs in healthy tissue, holds great promise to enhance the tumor specificity and minimize the risk of toxicity.
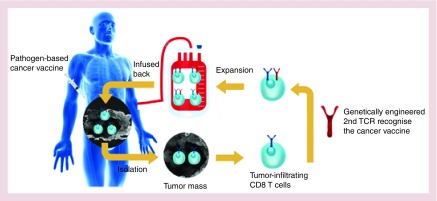
Regardless of all these advancements, ACT still fails to produce adequate clinical responses for solid tumors, which requires a robust T-cell-mediated antitumor response. To overcome several limitations simultaneously, we explored the synergistic effect of combining ACT with pathogen-boosted immunotherapy to heighten specificity and efficacy [91]. To achieve this goal, we produced dual-specific T cells that are capable of responding to both tumor and bacterial antigens, and ACT of these cells was performed while administering intratumoral bacteria vaccination. Our therapy, named re-energized ACT facilitates T-cell penetration and expansion inside the tumor, while reversing the immunosuppressive TME (Figure 2). As expected, reenergized ACT provoked a strong antitumor immune reaction that led to tumor eradication in around 60% of treated mice and also mediated long-term protection [91]. Independently, another group also utilized combinatorial therapy between ACT and viral vaccination for eradication of large orthotopic solid tumors including breast cancer, sarcoma and colon carcinoma [92]. Though this dual specificity TCR strategy needs more thorough examination before clinical testing, these results collectively demonstrate that, in principle, it is possible to use a naturally robust response against foreign infectious agents to help fight against poorly immunogenic tumors.
Figure 2. . The potential application of reenergized adoptive cell transfer.
Tumor-reactive CD8 T cells will be engineered with a second TCR that recognizes an antigen from a cancer vaccine. Following expansion, these dual-specific CD8 T cells will be infused back into patients and a pathogen-based cancer vaccine will be administered to mediate a vigorous antitumor response.
TCR: T-cell receptor.
Conclusion
Recently, bacteria-based therapies, both direct oncolytic reagents and cancer vaccines, have demonstrated significant preclinical and clinical benefits; yet, also highlight potential limitations in treating solid tumors. Meanwhile, despite the impressive clinical results in treating hematopoietic malignancies, ACT also faces several challenges in treating solid tumors. Therefore, combining ACT together with bacteria-based therapies may represent a more promising strategy for promoting long-lasting tumor-specific T responses and tumor elimination. The feasibility and dramatic therapeutic effect of this approach has been revealed in a few preclinical studies. Further investigation will open new avenues to treat solid tumors.
Future perspective
As single-agent treatments, both pathogen-based cancer therapies and ACT have shown antitumor efficacy in many cancer types, but still face multiple challenges. Many attempts to address these problems include improving the oncolytic and adjuvant effect of pathogens and increasing tumor specificity using dual TCR design. Furthermore, the potential synergistic effects between these two approaches have been revealed by several recent studies, which further rationalize the utility of developing a pathogen-boosted ACT therapy. All of these exciting advancements may lead to novel single or combinatorial therapies that can significantly improve the future treatment of cancer and ultimately achieve complete remission.
Executive summary.
Oncolytic pathogen-based therapy
As oncolytic reagents, both bacteria and virus-based therapies show encouraging preclinical results in treating solid tumors, but further clinical optimization is warranted.
Pathogen-boosted cancer vaccines
Bacteria also have been employed as powerful adjuvants in cancer vaccines to overcome cancer-induced tolerance and immunosuppression.
Multiple recombinant bacteria expressing tumor-associated antigens (TAAs) have been developed for numerous malignancies, which have thus far delivered impressive therapeutic efficacy in preclinical and clinical settings.
The major challenges of adoptive cell transfer against solid tumors
The therapeutic efficacy of adoptive cell transfer for treating solid tumor depends on several factors including: T-cell trafficking, T-cell expansion, nutrient restriction inside tumor, manipulation of key transcriptional factors for resistance against immunosuppression and minimization of toxicity.
Dual-specific adoptive cell transfer
The dual-specific T cells are engineered to express two T-cell receptors for targeting combinations of antigens to enhance the specificity, efficacy or safety of adoptive cell transfer.
Dual-specific T cells can be designed to target two different TAAs, which may either help avoid antigen escape or increase the tumor specificity.
Dual-specific T cells designed to target one TAAs and one bacterial antigen can be combined with local bacterial injection for boosting the antitumor immune response.
Footnotes
Financial & competing interests disclosure
This work is supported by NIH grants AI125741 (W Cui), 5T32HL007209 (R Zander) and DK108557 (D Schauder). G Xin is supported by The Elizabeth Elser Doolittle Postdoctoral Fellowship. D Schauder is a member of the Medical Scientist Training Program at MCW, which is partially supported by a training grant from NIGMS T32-GM080202. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.A.C. Society. Cancer Facts and Figures 2017. 2017. www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html
- 2.Burnet FM. Immunological aspects of malignant disease. Lancet. 1967;1:1171–1174. doi: 10.1016/s0140-6736(67)92837-1. [DOI] [PubMed] [Google Scholar]
- 3.Thomas L. Discussion of cellular and humoral aspects of the hypersensitivity states. In: Lawrence HS, editor. Cellular and Humoral Aspects of Hypersensitivity. Hoeber-Harper Book; ID, USA: 1959. pp. 529–532. [Google Scholar]
- 4.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 5.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J. Natl Cancer. Inst. 1957;18:769–767. [PubMed] [Google Scholar]
- 6.Swann JB, Smyth MJ. Immune surveillance of tumors. J. Clin. Investig. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. Annal. Surg. 1893;18:68–69. [PubMed] [Google Scholar]
- 9.Felgner S, Kocijancic D, Frahm M, Weiss S. Bacteria in cancer therapy: renaissance of an old concept. Int. J. Microbiol. 2016;2016:8451728. doi: 10.1155/2016/8451728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016;13(5):273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvoni F, Ebbell B. The Papyrus Ebers. The greatest Egyptian medical document. Aegyptus. 1940:240–244. [Google Scholar]
- 14.Busch W. Aus der Sitzung der medicinischen Section vom 13. Berlin Klin Wochenschr. 1867;1868(5):137. [Google Scholar]
- 15.Sarotra P, Bikash M. Use of bacteria in cancer therapy. In: Walter W, editor. Current Strategies in Cancer Gene Therapy (Volume 8) Springer International Publishing; NY, USA: 2016. pp. 111–121. [Google Scholar]
- 16.Staedtke V, Roberts NJ, Bai RY, Zhou S. Clostridium novyi-NT in cancer therapy. Genes Dis. 2016;3(2):144–152. doi: 10.1016/j.gendis.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl Acad. Sci. 1975;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey RW, Holland JF, Whang HY, Neter E, Bryant B. Clostridial oncolysis in man. Eur. J. Cancer. 1965;3(1) 37–42, IN5, 43–46. [Google Scholar]
- 19.Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc. Natl Acad. Sci. 2001;98(26):15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57(20):4537–4544. [PubMed] [Google Scholar]
- 21.Low KB, Itensohn M, Lin S, et al. VNP20009, a genetically modified Salmonella typhimurium for treatment of solid tumors. Proc. Am. Assoc. Cancer. Res. 1999;40:87. [Google Scholar]
- 22.Hoffman RM. The preclinical discovery of bacterial therapy for the treatment of metastatic cancer with unique advantages. Expert Opin. Drug Discov. 2012;7(1):73–83. doi: 10.1517/17460441.2012.644534. [DOI] [PubMed] [Google Scholar]
- 23.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 24.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer, a current perspective. Nat. Rev. Urol. 2014;11(3):153–162. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 25.Dai Y, Toley BJ, Swofford CA, Forbes NS. Construction of an inducible cell-communication system that amplifies Salmonella gene expression in tumor tissue. Biotechnol. Bioeng. 2013;110(6):1769–1781. doi: 10.1002/bit.24816. [DOI] [PubMed] [Google Scholar]
- 26.Frahm M, Felgner S, Kocijancic D, et al. Efficiency of conditionally attenuated Salmonella enterica serovar Typhimurium in bacterium-mediated tumor therapy. MBio. 2016;6(2):e00254–e00215. doi: 10.1128/mBio.00254-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14(9):642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 29.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 30.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]; •• Shows the beneficial effect of talimogene laherparepvec in Phase III clinical trial, which resulted in US FDA approval of talimogene laherparepvec for clinical use for melanoma.
- 31.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Cancer. 2016;16(4):219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 32.Van Eynde BD, Hainaut P, Herin M, et al. Presence on a human melanoma of multiple antigens recognized by autologous CTL. Int. J. Cancer. 1989;44(4):634–640. doi: 10.1002/ijc.2910440413. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen SR, Sørensen MR, Buus S, Christensen JP, Thomsen AR. Comparison of vaccine-induced effector CD8 T cell responses directed against self- and non-self-tumor antigens: implications for cancer immunotherapy. J. Immunol. 2013;191(7):3955–3967. doi: 10.4049/jimmunol.1300555. [DOI] [PubMed] [Google Scholar]
- 34.Munn DH, Bronte V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 2016;39:1–6. doi: 10.1016/j.coi.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fearon DT. Immune-suppressing cellular elements of the tumor microenvironment. Ann. Rev. Cancer Biol. 2016;1:241–255. [Google Scholar]
- 36.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Ann. Rev. Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16(4):343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hobohm U, Stanford JL, Grange JM. Pathogen-associated molecular pattern in cancer immunotherapy. Crit. Rev. Immunol. 2008;28(2):95–107. doi: 10.1615/critrevimmunol.v28.i2.10. [DOI] [PubMed] [Google Scholar]
- 39.Wood LM, Paterson Y. Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front. Cell. Infect. Microbiol. 2014;4:51. doi: 10.3389/fcimb.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umemura N, Saio M, Suwa T, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J. Leukocyte Biol. 2008;83(5):1136–1144. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 41.Yang WC, Ma G, Chen SH, Pan PY. Polarization and reprogramming of myeloid-derived suppressor cells. J. Mol. Cell Biol. 2013;5(3):207–209. doi: 10.1093/jmcb/mjt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan ZK, Weiskirch LM, Paterson Y. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res. 1999;59(20):5264–5269. [PubMed] [Google Scholar]
- 43.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 2001;167(11):6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 44.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 45.Singh R, Dominiecki ME, Jaffee EM, Paterson Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J. Immunol. 2005;175(6):3663–3673. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- 46.Petit R, Basu P. ADXS11–001 LM-LLO immunotherapy targeting HPV-E7: preliminary safety and survival data from a Phase II study in Indian women with recurrent/refractory cervical cancer. J. Immunother. 2012;35(9):732–733. [Google Scholar]
- 47.Chen YD, Yang D, Li S, et al. Development of a Listeria monocytogenes-based vaccine against hepatocellular carcinoma. Oncogene. 2012;31(17):2140–2152. doi: 10.1038/onc.2011.395. [DOI] [PubMed] [Google Scholar]
- 48.Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008;26(41):5315–5320. doi: 10.1016/j.vaccine.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruhn KW, Craft N, Nguyen BD, Yip J, Miller JF. Characterization of anti-self CD8 T-cell responses stimulated by recombinant Listeria monocytogenes expressing the melanoma antigen TRP-2. Vaccine. 2005;23(33):4263–4272. doi: 10.1016/j.vaccine.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Wallecha A, Maciag PC, Rivera S, Paterson Y, Shahabi V. Construction and characterization of an attenuated Listeria monocytogenes strain for clinical use in cancer immunotherapy. Clin. Vaccine Immunol. 2009;16(1):96–103. doi: 10.1128/CVI.00274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. 2016;16(9):566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N. Engl. J. Med. 1988;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 54.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor – modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates astonishing clinical result of adoptive T-cell therapy in treating leukemia.
- 55.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt TM, Stromnes IM, Chapuis AG, Greenberg PD. New strategies in engineering T-cell receptor gene-modified T cells to more effectively target malignancies. Clin. Cancer Res. 2015;21(23):5191–5197. doi: 10.1158/1078-0432.CCR-15-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan RA, Chinnasamy N, Abate-Daga DD, et al. Cancer regression and neurologic toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36(2):133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamers CH, Sleijfer S, Van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 2013;21(4):904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson LA, Morgan RA, Dudley M, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buckanovich RJ, Facciabene A, Kim S, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 2008;14(1):28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 62.Salmon H, Franciszkiewicz K, Damotte D, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012;122(3):899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin. Cancer Res. 2016;23:2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schumann K, Lin S, Boyer E, et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl Acad. Sci. 2015;112(33):10437–10442. doi: 10.1073/pnas.1512503112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foster AE, Dotti G, Lu A, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-β receptor. J. Immunother. 2008;31(5):500–505. doi: 10.1097/CJI.0b013e318177092b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prosser ME, Brown CE, Shami AF, Forman SJ, Jensen MC. Tumor PD-L1 co-stimulates primary human CD8+ cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol. Immunol. 2012;51(3):263–272. doi: 10.1016/j.molimm.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 67.Kerkar SP, Muranski P, Kaiser A, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70(17):6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tugues S, Burkhard SH, Ohs I, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22(2):237–246. doi: 10.1038/cdd.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hillerdal V, Essand M. Chimeric antigen receptor-engineered T cells for the treatment of metastatic prostate cancer. BioDrugs. 2015;29(2):75–89. doi: 10.1007/s40259-015-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishio N, Dotti G. Oncolytic virus expressing RANTES and IL-15 enhances function of CAR-modified T cells in solid tumors. Oncoimmunology. 2015;4(2):e988098. doi: 10.4161/21505594.2014.988098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scharping NE, Menk AV, Moreci RS, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45(2):374–388. doi: 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chamoto K, Chowdhury PS, Kumar A, et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc. Natl Acad. Sci. 2017;114:E761–E770. doi: 10.1073/pnas.1620433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho PC, Bihuniak JD, Macintyre AN, et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell. 2015;162(6):1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sukumar M, Liu J, Ji Y, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 2013;123(10):4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buck MD, O'Sullivan D, Klein Geltink RI, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166(1):63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xin G, Schauder DM, Lainez B, et al. A critical role of IL-21-induced BATF in sustaining CD8-T-cell-mediated chronic viral control. Cell Rep. 2015;13(6):1118–1124. doi: 10.1016/j.celrep.2015.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurachi M, Barnitz RA, Yosef N, et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat. Immunol. 2014;15(4):373–383. doi: 10.1038/ni.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gladow M, Uckert W, Blankenstein T. Dual T cell receptor T cells with two defined specificities mediate tumor suppression via both receptors. Eur. J. Immunol. 2004;34(7):1882–1891. doi: 10.1002/eji.200425041. [DOI] [PubMed] [Google Scholar]
- 83.Kershaw MH, Westwood JA, Hwu P. Dual-specific T cells combine proliferation and antitumor activity. Nat. Biotechnol. 2002;20(12):1221–1227. doi: 10.1038/nbt756. [DOI] [PubMed] [Google Scholar]
- 84.Berdien B, Reinhard H, Meyer S, et al. Influenza virus-specific TCR-transduced T cells as a model for adoptive immunotherapy. Hum. Vaccin. Immunother. 2013;9(6):1205–1216. doi: 10.4161/hv.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heemskerk MH, Hagedoorn RS, van der Hoorn MA, et al. Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR-CD3 complex. Blood. 2007;109(1):235–243. doi: 10.1182/blood-2006-03-013318. [DOI] [PubMed] [Google Scholar]
- 86.Höfflin S, Prommersberger S, Uslu U, et al. Generation of CD8+ T cells expressing two additional T-cell receptors (TETARs) for personalised melanoma therapy. Cancer Biol. Ther. 2015;16(9):1323–1331. doi: 10.1080/15384047.2015.1070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weinhold M, Sommermeyer D, Uckert W, Blankenstein T. Dual T cell receptor expressing CD8+ T cells with tumor-and self-specificity can inhibit tumor growth without causing severe autoimmunity. J. Immunol. 2007;179(8):5534–5542. doi: 10.4049/jimmunol.179.8.5534. [DOI] [PubMed] [Google Scholar]
- 88.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Trans. Med. 2014;6(224):224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics. 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roybal KT, Rupp LJ, Morsut L, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell. 2016;164(4):770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reveals the therapeutic value of pathogen booster adoptive cell transfer therapy in multiple preclinical solid tumor models.
- 91.Xin G, Schauder DM, Jing W, et al. Pathogen boosted adoptive cell transfer immunotherapy to treat solid tumors. Proc. Natl Acad. Sci. USA. 2017;114:740–745. doi: 10.1073/pnas.1614315114. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reveals the therapeutic value of pathogen booster adoptive cell transfer therapy in multiple preclinical solid tumor models.
- 92.Slaney CY, von Scheidt B, Davenport AJ, et al. Dual-specific chimeric antigen receptor T cells and an indirect vaccine eradicate a variety of large solid tumors in an immunocompetent, self-antigen setting. Clin. Cancer Res. 2016;23:2478–2490. doi: 10.1158/1078-0432.CCR-16-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]