Abstract
Background
Shexiang Baoxin pills (SXBXP), as a Traditional Chinese Medicine, are widely used for chronic heart failure in China. It is essential to systematically assess the efficacy and safety of SXBXP as an adjuvant treatment for chronic heart failure.
Methods
Seven English and Chinese electronic databases (PubMed, Embase, Cochrane Library, CBM, Wanfang, VMIS, and CNKI) were searched from inception to July 2017. The Cochrane Risk of Bias tool was used to evaluate the methodological quality of eligible studies. Meta-analysis was performed by Review Manager 5.3.
Results
A total of 27 RCTs with 2637 participants were included in this review. Compared to conventional treatment, SXBXP combined with conventional treatment showed potent efficacy when it came to the total efficacy rate (OR, 3.88; 95% CI, 2.87, 5.26; P < 0.00001), B-type natriuretic peptide (BNP) (MD = −66.95; 95% CI, −108.57, −25.34; P = 0.002), N-terminal pro-brain natriuretic peptide (NT-ProBNP) (MD = −0.15; 95% CI, −0.21, −0.09; P < 0.00001), six-minute walking distance (6-MWD) (MD = 38.57; 95% CI, 28.47, 48.67; P < 0.00001), cardiac output (CO) (MD = 0.84; 95% CI, 0.68, 0.99; P < 0.00001), and Stroke Volume (SV) (MD = 7.43; 95% CI, 4.42, 10.44, P < 0.00001). The pooled subgroup analysis indicated that there was a significant difference between SXBXP plus conventional treatment and conventional treatment alone in short term course (OR = 3.51; 95% CI, 2.28, 5.40; P < 0.00001), in middle period of treatment (OR = 5.01; 95% CI, 2.61, 9.60; P < 0.00001), and in long-term course (OR = 3.77; 95% CI, 2.13, 6.67; P < 0.00001). No serious adverse events or reactions were mentioned in these RCTs.
Conclusions
As an adjuvant drug, this study suggested that SXBXP provide an obvious efficacy for the treatment of CHF. However, due to small samples and generally low quality studies being applied in this study, more rigorous and well-designed RCTs are needed to confirm these findings.
1. Introduction
In spite of a tremendous advance in pharmacology and therapies, chronic heart failure (CHF) remains the most serious cardiovascular disorder all over the world [1, 2]. There is a significant high mortality in patients with CHF [3], probably an estimated 50% mortality in 5 years [4]. Moreover, with the aging of the population becoming a more and more serious issue, patients with CHF constitute a high proportion of the aging population, and so patients with CHF increase year after year, which highlights the urgent need for effective treatment strategies [5].
CHF is a complex clinical syndrome that results from structural change or functional abnormalities, which may lead to a series of cardiac dysfunctions, such as decrease of cardiac output, increase of intracardiac pressure function, ventricular filling, or impaired ejection at both rest and load conditions [6]. Cardinal manifestations are various, such as dyspnea and fatigue, which may lead to fluid retention due to limited exercise tolerance and which may bring about pulmonary and peripheral edema [7]. CHF is the terminal stage of various heart diseases, often accompanied by high morbidity and high mortality, especially in the elderly [8]. Patients with CHF died within 5 years as well as the survival rate being less than 50% within 1 year [9]. CHF poses a serious challenge to the health of the people of the world, giving the family and society a heavy burden.
Current Western medicine treatment formed with “angiotensin-converting enzyme inhibitor (ACEI) or Angiotensin receptor antagonist (ARB), beta blockers, or Aldosterone receptor antagonist” is the basis of the Golden Triangle treatment program [2]. However, satisfactory results are still difficult to obtain in some patients. A lot of researches show that, in CHF patients with Yang deficiency blood stasis, SXBXP have beneficial Qi Tong Yang Huayu efficacy and played a very effective role in the treatment of CHF. These Western medicine treatments are not only conventional treatments, but also the dominating treatment. However, the existing treatment is not perfect enough [10]. It is well known that long-term use of Western medicine may cause side effects and resistance [11].
SXBXP, as a traditional and complementary medicine, derives from traditional decoction named Suhexiang pills, which is recorded in the Ministry of Health and the benefits of the party side in Song Dynasty. The traditional decoction has been used for more than a thousand years and it includes Moschus, toad, Panax ginseng, Bos taurus domesticus Gmelin, Cinnamomum cassia Presl, and Borneolum [12]. From 1981, since the clinical application, SXBXP was widely used in coronary heart disease, angina, myocardial infarction, and other heart diseases. Most clinical trials showed that SXBXP benefited patients with CHF [13]. Now, a large number of references reported the efficacy of SXBXP on CHF. All the trails did not report obvious side effects and adverse reactions. However, evidence was very limited on the efficacy of SXBXP for CHF [14]. In fact, the previous systematic review did not assess the effects of SXBXP as an adjuvant treatment for CHF, too. Therefore, it is necessary for us to assess the efficacy and safety of SXBXP, which act as an adjuvant treatment with conventional treatment.
2. Materials and Methods
2.1. Search Strategy
Comprehensive searches were conducted in both English and Chinese databases to identify all published RCTs from their inception to July 2017. All relevant RCTs were searched from the following 7 databases including PubMed, Embase, Cochrane Library, CBM, Wanfang, VMIS, and CNKI. The following search terms were used: “Shexiang Baoxin pills” [Title/Abstract] AND “Chronic heart failure” [Title/Abstract] OR “Chronic heart disease” [Title/Abstract]. The literature searches were independently examined by two investigators (Taiwei Dong and Rong Ma) and disagreements were resolved by consensus as well as discussion. The bibliographies of included trials were searched for through references. However, the trials without English abstract would be translated by the investigator Taiwei Dong and checked by the investigator Rong Ma.
2.2. Inclusion Criteria
Two authors (Taiwei Dong and Rong Ma) read the titles and abstracts of trials in all searched databases independently to assess the rationality for inclusion. The full text was further read to evaluate for the inclusion criteria. The inclusion criteria were as follows. (1) Investigative object and intervention: all the randomized controlled trails (RCTs) which combined SXBXP with conventional medical treatment (experimental group) compared with conventional medical treatment (control group) alone in CHF were included. The method of intervention was oral administration. (2) Characteristics of patients: patients diagnosed with CHF with New York Heart Association (NYHA) (The Criteria Committee of the New York Heart Association 1994) classified from II to IV were included. (3) Outcome measures: total efficacy rate. The secondary outcome measures included left ventricular ejection fraction (LVEF), cardiac output (CO), Stroke Volume (SV), B-type natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-ProBNP), and six-minute walking distance (6-MWD). RCTs with one or more outcomes were included.
2.3. Exclusion Criteria
The trials conforming to the following conditions were excluded: (1) reduplicative publications reporting the same trials; (2) nonrandomized controlled trials; (3) nonclinical experiments, reviews, literature research, mechanism research, or animal experiment; (4) controlled interventions combined with any other Chinese herbal medicine or acupuncture in control group or experimental group; (5) unavailable or incorrect data for meta-analysis; (6) patients with unclear functional classification; (7) trials with unclear evaluation indicators or basic data for statistic research.
2.4. Data Extraction
Two investigators (Taiwei Dong and Rong Ma) independently extracted the basic information such as title, published year, total cases, cases of experiment group and control group, interventions, outcome measures, NYHA classification, course of disease, safety evaluation, and ADEs or ADRs to conclusive tables. Relevant disagreements were resolved through discussion with investigator (Jian Wang). Symptom improvement was evaluated according to the Guidance for Clinical Research on New Drugs of TCM, Framingham criteria, American College of Cardiology/American Heart Association (ACC/AHA), or textbook criteria as long as the criteria met the international-used diagnostic criteria [15].
2.5. Risk of Bias Assessment and Quality Assessment
The methodological quality of included RCTs was assessed by Review Manager 5.3 according to the Cochrane Risk of Bias tool. The methodological quality of each trial was evaluated by seven domains including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. The quality of each trial was classified as “high risk,” “unclear risk,” or “low risk.” The trials that had insufficient information available to make a judgment were classified as unclear risk of bias. The trials with low risk of bias represented a good methodological quality and the trials with high risk of bias represented a low methodological quality. Any disagreement was settled through discussion with investigator (Jian Wang).
2.6. Strategy for Data Synthesis and Analysis
The meta-analysis was performed by Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK). For outcome measures, dichotomous variables were presented as odds ratio (OR) with 95% confidence intervals (CI), while continuous outcomes were expressed as mean difference (MD) with 95% CI. As a quantitative measure of inconsistency, the I-square (I2) statistic was used to assess heterogeneity. Fixed effect model was performed with minor heterogeneity when I2 was less than 50%. Random-effect model was applied when I2 was over 50%. A funnel plot was used for assessing the potential publication bias. Furthermore, subgroup analysis was performed due to course of treatment of SXBXP.
3. Results
3.1. Basic Information
3.1.1. Description of Studies
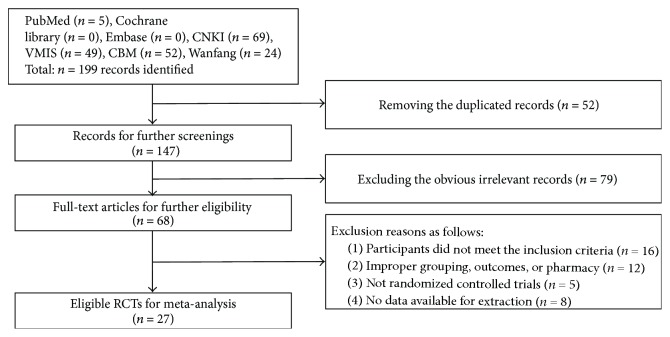
A total of 199 records were identified for preliminary screening after searching English and Chinese databases. All the included trials were conducted in China and published in Chinese. As shown in Figure 1, 147 records were reserved for further screening after removing 52 duplicated publications. For the preserved records, 79 obvious irrelevant literatures were excluded by reading the title and abstract. 68 full-text articles were used for further assessment. After reading the full text, 41 more literatures were excluded for the following reasons: participants not meeting the inclusion criteria (n = 16), improper grouping, outcomes, or pharmacy (n = 12), nonrandomized controlled trials (n = 5), and no data available for extraction (n = 8). Finally, 27 RCTs of SXBXP for CHF were included in this review.
Figure 1.
Flow diagram for searching and selecting study.
3.1.2. Study Characteristics
As shown in Table 1, a total of 27 RCTs with 2637 participants were included in this review. The control group consisted of 1313 patients, while the treatment group consisted of 1324 patients. All trials' sample sizes ranged from 56 to 181; sample size is large enough. The ages of the subjects were over 50 years. Moreover, all the trials included NYHA classification among II~IV. As for the characteristics of intervention, the course of treatment varied from 24 days to 6 months. Only one trial did not mention the course of treatment [16]. The baseline of patients in both groups was balanced.
Table 1.
Principal characteristics of the studies included in the meta-analysis.
| Included research (year) | Sample size (n) | Age (y) | Male (%) | Intervening measure (T/C) | Dosage | Duration | Outcome measures | |||
|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | |||||
| Gu et al. 2007 | 43 | 43 | 67.5 (8.8) | 66.3 (8.2) | 49% | 53% | SXBXW + CT/CT | 135 mg/d | 6 M | LVEF, 6-MWD |
| Yang et al. 2014 | 60 | 60 | 65.9 (16.4) | 64.3 (16.9) | 72% | 68% | SXBXW + CT/CT | 135 mg/d | 6 W | ER, LVEF, CO, SV, BNP |
| Sang et al. 2015 | 50 | 50 | 64.73 (5.03) | 65.03 (5.39) | 54% | 44% | SXBXW + CT/CT | 135 mg/d | 24 D | ER, LVEF, 6-MWD |
| Ding et al. 2013 | 48 | 48 | 64.5 (7.2) | 64.5 (7.2) | 58% | 58% | SXBXW + CT/Placebo + CT | 135 mg/d | 24 W | LVEF, 6-MWD, NT-Pro BNP |
| Deng and Zhang 2013 | 65 | 67 | 62 (11) | 62 (11) | 58% | 58% | SXBXW + CT/CT | 135 mg/d | 12 W | LVEF, NT-Pro BNP |
| Zhong et al. 2012 | 42 | 42 | 52.4 (9.6) | 53.1 (10.2) | 52% | 50% | SXBXW + CT/CT | 135 mg/d | 4 W | LVEF, 6-MWD, CO, NT-Pro BNP |
| Ran et al. 2012 | 59 | 57 | 61.5 (12.5) | 64.3 (11.1) | 46% | 60% | SXBXW + CT/CT | 135 mg/d | 3 M | LVEF |
| Fang et al. 2014 | 42 | 40 | 64.35 (13.65) | 65.98 (12.02) | 43% | 48% | SXBXW + CT/CT | 135 mg/d | 6 M | LVEF, NT-Pro BNP |
| Xu 2015 | 70 | 70 | 53.2 (7.3) | 54.2 (6.2) | 47% | 46% | SXBXW + CT/CT | 135 mg/d | 3 M | ER |
| Wang 2015 | 91 | 90 | 48.5 (2.7) | 48.7 (2.6) | 57% | 57% | SXBXW + CT/CT | 202.5 mg/d | NR | ER, LVEF, CO |
| Bao and Liu 2009 | 30 | 28 | 65 (8.1) | 65 (8.1) | 66% | 66% | SXBXW + CT/CT | 135 mg/d | 3 M | ER, LVEF, SV |
| Wang 2013 | 50 | 50 | 64.24 (4.95) | 57.39 (6.21) | 60% | 64% | SXBXW + CT/CT | 67.5 mg/d | 28 D | ER |
| Yang et al. 2007 | 55 | 56 | 60 (8) | 59 (9) | 58% | 54% | SXBXW + CT/CT | 135 mg/d | 6 M | 6-MWD |
| Lv and Zhen 2007 | 40 | 39 | 65.2 | 67.1 | 58% | 51% | SXBXW + CT/CT | 135 mg/d | 3 M | LVEF, CO, SV |
| Wu et al. 2014 | 30 | 30 | 64.2 | 65.7 | 57% | 53% | SXBXW + CT/CT | 135 mg/d | 3 M | ER, LVEF, CO, SV |
| Li et al. 2015 | 50 | 50 | 61.6 (7.1) | 61.8 (7.9) | 52% | 54% | SXBXW + CT/CT | 135 mg/d | 6 W | ER, LVEF, CO, SV, 6-MWD, NT-Pro BNP |
| Han et al. 2010 | 60 | 56 | 63.9 (16.4) | 64.3 (16.9) | 72% | 73% | SXBXW + CT/CT | 135 mg/d | 6 M | ER, LVEF, 6-MWD |
| Niu and Liu 2014 | 31 | 31 | 73.5 | 76.5 | 61% | 71% | SXBXW + CT/CT | 135 mg/d | 30 D | LVEF, SV |
| Sun and Cai 2013 | 45 | 45 | 58.5 (5.7) | 59.2 (6.1) | 67% | 64% | SXBXW + CT/CT | 135 mg/d | 3 M | ER, LVEF, 6-MWD |
| Li 2013 | 28 | 28 | 68.5 | 69.5 | 64% | 57% | SXBXW + CT/CT | 135 mg/d | 6 M | ER, LVEF, CO, SV |
| Wang 2012 | 54/ | 54 | 66 | 67 | 74% | 72% | SXBXW + CT/CT | 202.5 mg/d | 1 M | ER, LVEF, 6-MWD |
| Y. Gao and H. Gao 2013 | 42 | 42 | 52.4 (9.6) | 53.1 (10.2) | 52% | 50% | SXBXW + CT/CT | 135 mg/d | 4 W | ER, LVEF, CO, 6-MWD, NT-Pro BNP, |
| Wang et al. 2010 | 37 | 34 | 58.3 (5.4) | 61.7 (6.5) | 57% | 56% | SXBXW + CT/CT | 135 mg/d | 4 M | LVEF, BNP |
| Zhu and Yang 2012 | 46 | 45 | 60.7 (7.3) | 61.7 (8.1) | 52% | 51% | SXBXW + CT/CT | 135 mg/d | 6 M | LVEF, 6-MWD |
| Dong and Wang 2012 | 56 | 56 | 75.5 (1.2) | 76.5 (1.4) | 41% | 38% | SXBXW + CT/CT | 135 mg/d | 4 W | ER, 6-MWD |
| Guo et al. 2011 | 60 | 62 | 63.9 (16.4) | 64.3 (16.9) | 55% | 55% | SXBXW + CT/CT | 135 mg/d | 6 M | ER, LVEF, 6-MWD |
| Quan and Shi 2013 | 40 | 40 | 61 | 61 | 73% | 73% | SXBXW + CT/CT | 135 mg/d | 6 M | ER, LVEF, 6-MWD, NT-Pro BNP |
Note. NR: not reported; M: month; W: week; D: day; T: trail group; C: conventional group; CT: conventional treatment; SXBXP: Shexiang Baoxin pills.
The treatment group used SXBXP combined with the same conventional treatment as control group; only one trial used Placebo combined with conventional treatment [17]. Two groups of all trails used the dose of 202.5 mg/d, although most doses are 135 mg/d; only one trial used 67.5 mg/d. SXBXP was given through oral administration three times daily in all included trials. The control group used conventional medical treatment alone, including ACEI, ARB, cardiac glycosides, diuretics, β-receptor blockers, antialdosterone drugs, calcium channel blockers, or vasodilators.
None of the included trials reported death. All trails reported NYHA classification. Twenty-two trials reported LVEF. Eighteen trials reported ER and 6 MWT. Four articles reported NT-proBNP. Four articles reported BNP. Eight trails reported CO. Seven trails reported SV.
3.2. Methodological Quality
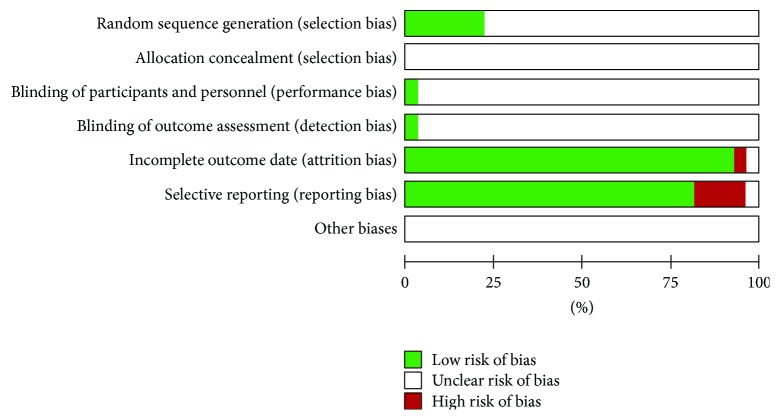
The methodological quality of the included trials was generally poor. Five trials reported that random sequence was generated by a random number table [18] and only one trail generated it by the envelope method [19]; the remaining 21 trials only mentioned random allocation without any description of the method of randomization. There was no allocation concealment mentioned in all the articles. Only one trial mentioned blinding of participants and blinding of outcome assessment [17]. Two articles reported incomplete outcome data and selective reporting [20, 21]. About 20% trials showed high risk of bias in incomplete outcome data. Other potential sources of bias were unclear. Therefore, the quality of all the included trails was graded as high risk of bias. The details of the methodological quality were presented in Figure 2 and Table 2.
Figure 2.
Risk of bias assessment of included studies.
Table 2.
Risk of bias assessment of included studies.
| Included research (year) | Random sequence generation | Allocation concealment |
Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other biases |
|---|---|---|---|---|---|---|---|
| Gu et al. 2007 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Yang et al. 2014 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Sang et al. 2015 | Low | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Ding et al. 2013 | Unclear | Unclear | Low | Low | Low | Low | Unclear |
| Deng and Zhang 2013 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Zhong et al. 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Low | Unclear |
| Ran et al. 2012 | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Unclear |
| Fang et al. 2014 | Low | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Xu 2015 | Low | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Wang 2015 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Bao and Liu 2009 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Wang 2013 | Low | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Yang et al. 2007 | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear |
| Lv and Zhen 2007 | Low | Unclear | Unclear | Unclear | High | Low | Unclear |
| Wu et al. 2014 | Low | Unclear | Unclear | Unclear | Low | High | Unclear |
| Li et al. 2015 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Han et al. 2010 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Niu and Liu 2014 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Sun and Cai 2013 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Li 2013 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Wang 2012 | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear |
| Y. Gao and H. Gao 2013 | Unclear | Unclear | Unclear | Unclear | Low | High | Unclear |
| Wang et al. 2010 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Zhu and Yang 2012 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Dong and Wang 2012 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Guo et al. 2011 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Quan and Shi 2013 | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
Unclear: unclear risk; Low: low risk; High: high risk.
3.3. Publication Bias
Publication bias was assessed using a funnel plot based on the total efficacy rate reported in 18 trials. The funnel plot was asymmetrical, which indicated that the potential publication bias might influence the results of this review. The bias might result from these reasons: small sample size, poor quality, and a high proportion of positive results (Figure 3).
Figure 3.
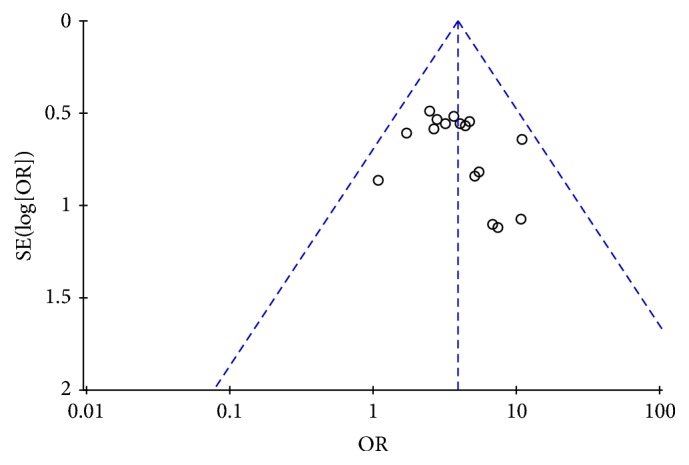
Funnel plot of SXBXP plus conventional treatment versus conventional treatment on total efficacy rate in patients with CHF.
3.4. Effects of Interventions
3.4.1. Primary Outcome Measures
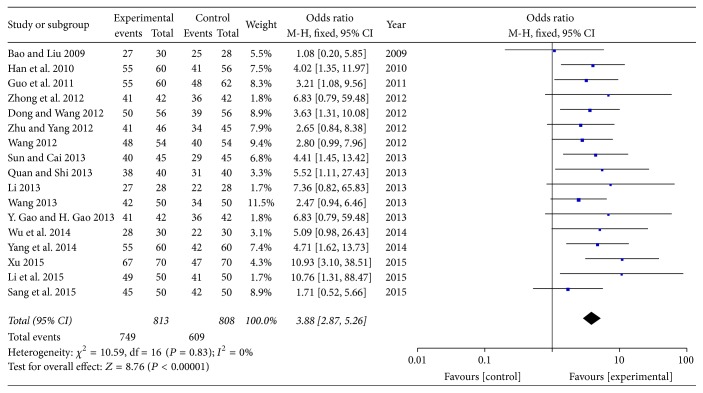
A total of 18 trials with 1735 patients investigated the total efficacy rate of SXBXP plus conventional treatment in improving NYHA classification in patients with chronic heart failure [18–20, 22–35]. There were 831 patients in experiment group and 904 in control group. The result showed that there was no heterogeneity (P = 0.83, I2 = 0%) and the fixed effect model was adopted for analysis. As shown in forest plot, there was a statistically significant difference between SXBXP plus conventional treatment and conventional treatment alone in the total efficacy rate (OR, 3.88; 95% CI, 2.87, 5.26; P < 0.00001) (Figure 4).
Figure 4.
Forest plot of SXBXP plus conventional treatment versus conventional treatment on total efficacy rate in patients with CHF. I2 and P are the criteria for the heterogeneity test, ◆ is pooled mean difference; —■— is mean difference and 95% CI.
3.4.2. Secondary Outcome Measures
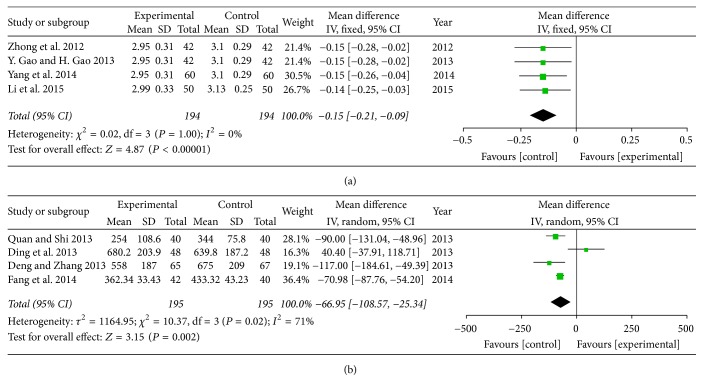
(1) 1B-Type Natriuretic Peptide (BNP) and N-Terminal Pro-Brain Natriuretic Peptide (NT-Pro BNP). Four trails with 390 patients assessed the therapeutic efficacy of NT-Pro BNP [17, 31, 36, 37]. Four trails with 388 participants assessed the effect of SXBXP plus conventional treatment in decreasing BNP in patients with chronic heart failure [27, 33–35]. It has considerably high heterogeneity in BNP (P = 0.02, I2 = 71%), and there is no significant difference between SXBXP plus conventional treatment and conventional treatment alone on NT-Pro BNP (P = 1.00, I2 = 0%). The trials reported that SXBXP plus conventional treatment was superior to conventional treatment alone to reduce NT-Pro BNP (MD = −0.15; 95% CI, −0.21, −0.09; P < 0.00001) (Figure 5(a)) and BNP (MD = −66.95; 95% CI, −108.57, −25.34; P = 0.002) (Figure 5(b)).
Figure 5.
Forest plot of SXBXP plus conventional treatment versus conventional treatment in the decrease of BNP and NT-Pro BNP. I2 and P are the criteria for the heterogeneity test; ◆ is pooled mean difference; —■— is mean difference and 95% CI. (a) is forest plot of NT-Pro BNP; (b) is forest plot of BNP.
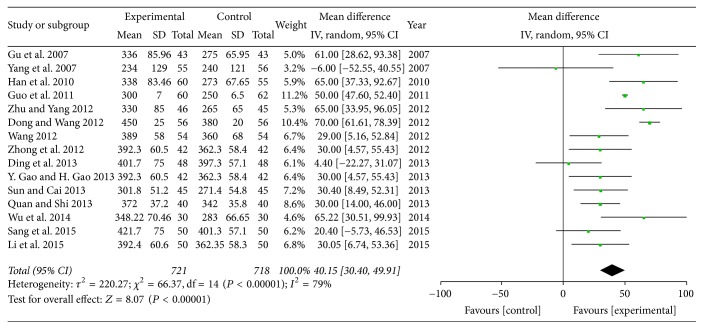
(2) The Comparison of 6-Minute Walking Distance (6-MWD). A total of 15 studies with 1439 subjects reported the level of 6-MWD [17, 18, 24–31, 33, 34, 38, 39]. There was considerable heterogeneity (P < 0.00001, I2 = 79%) and random-effect model was conducted for analysis. The result showed that SXBXP could substantially increase the level of 6-MWD compared with conventional treatment (MD = 40.15; 95% CI, 30.40, 49.91; P < 0.00001) (Figure 6). It indicated a significant improvement of SXBXP for CHF in exercise endurance.
Figure 6.
Forest plot of SXBXP plus conventional treatment versus conventional treatment in increasing 6-MWD. I2 and P are the criteria for the heterogeneity test; ◆ is pooled mean difference; —■— is mean difference and 95% CI.
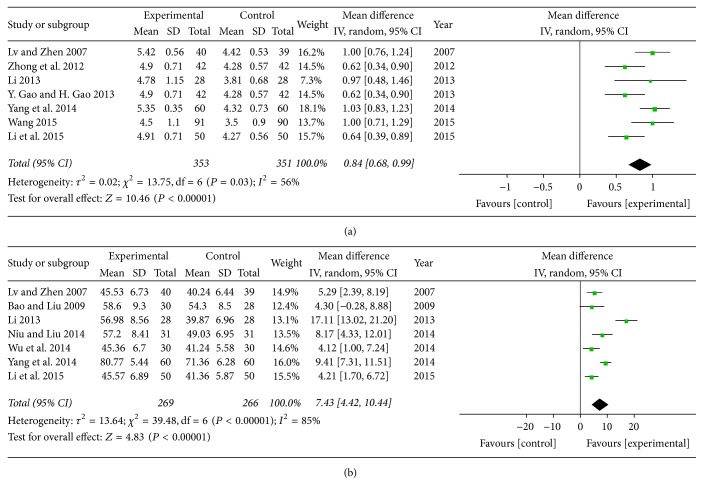
(3) Cardiac Output (CO) and Stroke Volume (SV). Seven trials with 704 participants [16, 17, 32–35, 40] and seven trials with 535 individuals [20, 23, 32, 34, 35, 40, 41] assessed the therapy on cardiac function of SXBXP plus conventional treatment in CO and SV, respectively. There was considerable heterogeneity in CO (P = 0.03, I2 = 56%) and SV (P < 0.00001, I2 = 85%) in trials. Meta-analysis with a random-effect model showed that, compared with conventional treatment, SXBXP plus conventional treatment significantly enhanced the cardiac function. The pooled analysis indicated that there was a statistically significant difference between SXBXP plus conventional treatment and conventional treatment alone on CO (MD = 0.84; 95% CI, 0.68, 0.99; P < 0.00001) (Figure 7(a)) and SV (MD = 7.43; 95% CI, 4.42, 10.44, P < 0.00001) (Figure 7(b)).
Figure 7.
Forest plot of SXBXP plus conventional treatment versus conventional treatment in increasing CO and SV. I2 and P are the criteria for the heterogeneity test; ◆ is pooled mean difference; —■— is mean difference and 95% CI. (a) is forest plot of CO; (b) is forest plot of SV.
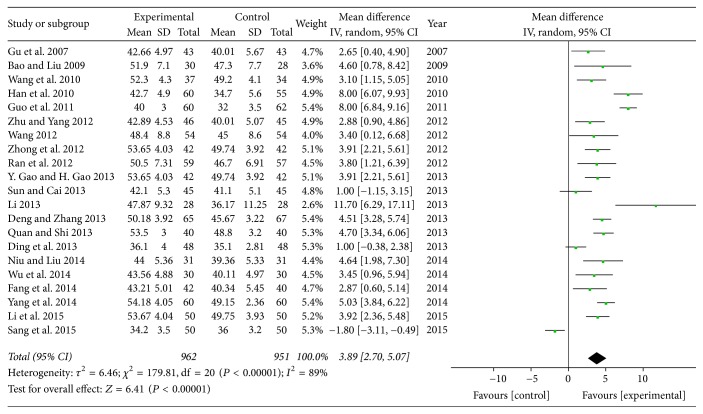
(4) Left Ventricular Ejection Fraction (LVEF). A total of 21 trials evaluated LVEF and were pooled with a random model [17, 18, 20, 21, 23–27, 29–38, 41, 42]. The heterogeneity of the LVEF study was considerably high (P < 0.00001, I2 = 89%). Pooled comparisons demonstrated that SXBXP plus conventional treatment had a statistically significant beneficial effect compared to conventional treatment alone in terms of LVEF (MD = 3.89; 95% CI, 2.70, 5.07, P < 0.00001) (Figure 8). As an adjuvant drug, SXBXP improve the cardiac function of patients with CHF.
Figure 8.
Forest plot of SXBXP plus conventional treatment versus conventional treatment in increasing LVEF. I2 and P are the criteria for the heterogeneity test; ◆ is pooled mean difference; —■— is mean difference and 95% CI.
3.4.3. Subgroup Analysis
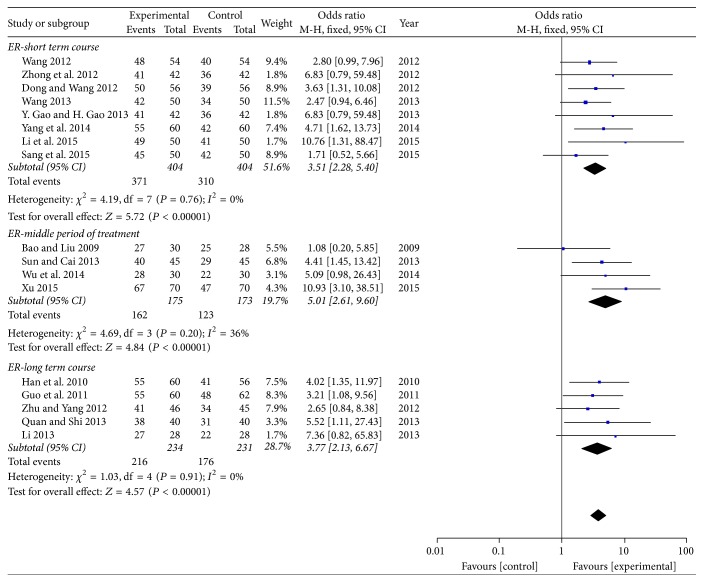
(1) The Total Efficacy Rate with Different Courses. Subgroup analysis was conducted to assess the efficacy of SXBXP plus conventional treatment on total efficacy rate according to different course of treatment. 8 trails with 681 participants were treated within 2 months [18, 19, 26, 28, 29, 33–35], which was regarded as short term course. The middle period of treatment included 4 trails with 285 patients; these participants were treated within 2–4 months [20, 22, 23, 30]. Moreover, the last subgroup contained 5 trails with 392 patients [24–26, 31, 32]; these participants were treated for over 4 months but less than 6 months. The I2 statistic of short term course showed that there was minor heterogeneity among these trials (P = 0.76, I2 = 0%). It also demonstrated similar result of the middle period of treatment (P = 0.20, I2 = 36%) and the long-term course (P = 0.91, I2 = 0%). All trails used fixed effect model to pool the results. The pool analysis presented that there was a significant difference between two therapy methods in short term course (OR = 3.51; 95% CI, 2.28, 5.40; P < 0.00001), in middle period of treatment (OR = 5.01; 95% CI, 2.61, 9.60; P < 0.00001), and in long-term course (OR = 3.77; 95% CI, 2.13, 6.67; P < 0.00001) (Figure 9).
Figure 9.
Forest plot of SXBXP plus conventional treatment versus conventional treatment according to different courses in total efficacy rate. I2 and P are the criteria for the heterogeneity test; ◆ is pooled mean difference; —■— is mean difference and 95% CI.
4. Discussion
The basic pathogenesis of CHF is myocardial pathology “reconstruction.” CHF is the final stage of most heart diseases, but generally there are two processes that ultimately lead to CHF. One is the occurrence of myocardial death, such as myocardial damage caused by myocardial injury and severe myocarditis; the other is the neuroendocrine system caused by overactivation of systemic reactions [43, 44]. SXBXP can improve the heart pump function of CHF patients, reverse ventricular remodeling, and improve activity tolerance. The activation of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system leads to cardiac hypertrophy, which will result in ventricular remodeling and ultimately decompensation [45]. Therefore, delaying this process is particularly important for improving the quality of life and prolonging the survival time of patients with CHF. The indexes such as CO, SV, 6 MWT, LVEF, BNP, and NT-Pro BNP reflect the cardiac function and cardiac output. These indexes can be used to evaluate the cardiac function of patients with CHF and judge the CHF patients' clinical efficacy [46]. This study found that the combination of SXBXP and conventional treatment appeared to be more effective and safer for the treatment of CHF compared with conventional treatment. It indicated that SXBXP was worthy of clinical application and promotion.
SXBXP is a Chinese patent medicine. It is based on the work of Professor Ruihong Dai of Huashan Hospital, who belongs to Fudan University [47]. A group of Western medicine experts used the Western standard research and development of pure Chinese medicine preparations; it contains Moschus 6%, ginseng extract 27%, bezoar 4%, cinnamon 24%, storsin 16%, toad 4%, and Dryobalanops 19% [48]. Basic Research Certificate showed that storsin and Dryobalanops have slowed the heart rate and had the role of the lifting coronary artery spasm; Moschus extract can dilate blood vessels and enhance cardiac function effectively [49]. Ginseng saponins have antioxidant properties and positive muscle force and reduce the role of lipid [50]. Toad has a strong effect with heart [51].
This is the first systematic review to assess the effects of SXBXP as an adjuvant treatment for CHF. In this meta-analysis review, only two trails reported mild adverse reactions, and none of the included trails reported death. Thus, evidence is limited to make a summary of death and adverse reactions. So on the whole long-term use of SXBXP can be considered safe and effective. This result also proves several expert consensuses that the addition of Chinese medicines can improve the clinical symptoms and quality of life in patients with CHF, simultaneously maintain the cardiac function and reduce the rehospitalization rate, and depress mortality of patients with unique comment Western medicine treatment [52].
In this study, SXBXP displayed positive impact on clinical efficacy via increasing levels of LVEF and 6-MWT and reducing levels of NT-pro BNP and BNP. It seemed that SXBXP could ameliorate the cardiac function in patients with CHF according to primary outcomes, secondary outcomes, and subgroup analysis according to different course of treatment in experiment group.
The study implemented strict inclusion and exclusion criteria. However, there were still statistical heterogeneity between some of the outcome indicators of the included trials, due to the main consideration and the limited sample size and the variation in the length of treatment. Five issues still remain in all RCTs from the results: (1) the amount of included trials is small, in addition to the lack of high-quality and large sample study; (2) quality is generally low, random application is less, and blind implementation is unknown; (3) long-term follow-up is lacking; most studies did not mention mortality and readmission rate; (4) most of the studies did not report adverse reactions. Therefore, more high-quality with long-term follow-up RCTs were required to elucidate the effectiveness and security of SXBXP for CHF in the future.
5. Conclusions
In summary, as an adjuvant treatment for chronic heart failure, this study suggested that SXBXP have an obvious efficacy for the treatment of CHF. In the future, more multicenter, large sample size, randomized double-blind, long-term evaluation RCTs are needed to confirm the efficacy and mechanism of SXBXP for CHF.
Acknowledgments
This work was financially supported by grants from National Natural Science Foundation of China (81703725 and 81473371), China Postdoctoral Science Foundation Grant (2017M622987), Chengdu University of Traditional Chinese Medicine Foundation (ZRQN1773), and Sichuan Province Clinical Chinese Pharmacy Science and Technology Innovation Youth Team (2017TD0001).
Abbreviations
- SXBXP:
Shexiang Baoxin pills
- CHF:
Chronic heart failure
- NYHA:
New York Heart Association
- LVEF:
Left ventricular ejection fraction
- BNP:
B-type natriuretic peptide
- NT-ProBNP:
N-terminal pro-brain natriuretic peptide
- 6-MWD:
6-minute walking distance
- CO:
Cardiac output
- SV:
Stroke Volume
- ADEs:
Adverse events
- ADRs:
Adverse drug reactions
- ACEI:
Angiotensin-converting enzyme inhibitors
- ARB:
Angiotensin receptor blocker
- TCM:
Traditional Chinese Medicine
- OR:
Odds ratio
- CI:
Confidence interval
- MD:
Mean difference.
Contributor Information
Jian Wang, Email: jianwang08@163.com.
Xiao Ma, Email: flyinghox@qq.com.
Conflicts of Interest
The authors of this manuscript state that they do not have any conflicts of interest and that there is nothing to disclose.
Authors' Contributions
Taiwei Dong and Xiao Ma designed the study. Taiwei Dong carried out literature research. Taiwei Dong and Rong Ma acquired data. Taiwei Dong and Jianxia Wen carried out statistical analysis. Taiwei Dong and Qian Xie edited the manuscript. Taiwei Dong and Nian Chen revised/reviewed the manuscript. Xiao Ma and Jian Wang approved the final version of the manuscript.
References
- 1.McMurray J. J., Adamopoulos S., Anker S. D., Auricchio A., Böhm M. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Journal of Heart Failure. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 2.Yancy C. W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American college of cardiology foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128(16):1810–1852. doi: 10.1161/cir.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P., Voors A. A., Anker S. D. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronicheart failure of the european society of cardiology (esc) developed with the special contribution ofthe heart failure association (hfa) of the esc. European Heart Journal. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin E. J., Blaha M. J., Chiuve S. E., et al. Heart Disease and Stroke Statistics'2017 Update: A Report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidenreich P. A., Albert N. M., Allen L. A., et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation: Heart Failure. 2013;6(3):606–619. doi: 10.1161/hhf.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A., Lavie C. J., Borer J. S., et al. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. American Journal of Cardiology. 2015;115(10):1428–1434. doi: 10.1016/j.amjcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Berg D., Postuma R. B., Bloem B., et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson's disease. Movement Disorders. 2014;29(4):454–462. doi: 10.1002/mds.25844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lok D. J., Lok S. I., Bruggink-André De La Porte P. W., et al. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clinical Research in Cardiology. 2013;102(2):103–110. doi: 10.1007/s00392-012-0500-y. [DOI] [PubMed] [Google Scholar]
- 9.Sandri M., Viehmann M., Adams V., et al. Chronic heart failure and aging—effects of exercise training on endothelial function and mechanisms of endothelial regeneration: results from the Leipzig Exercise Intervention in Chronic heart failure and Aging (LEICA) study. European Journal of Preventive Cardiology. 2016;23(4):349–358. doi: 10.1177/2047487315588391. [DOI] [PubMed] [Google Scholar]
- 10.Savarese G., Trimarco B., Dellegrottaglie S., et al. Natriuretic peptide-guided therapy in chronic heart failure: a meta-analysis of 2,686 patients in 12 randomized trials. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058287.e58287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu N., Xu L., Yang Y., et al. Tetramethylpyrazine-mediated regulation of CXCR4 in retinoblastoma is sensitive to cell density. Molecular Medicine Reports. 2017;15(5):2481–2488. doi: 10.3892/mmr.2017.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H., Yang Y., Lv C., et al. Pharmacokinetics and tissue distribution of five bufadienolides from the Shexiang Baoxin pill following oral administration to mice. Journal of Ethnopharmacology. 2015;161:175–185. doi: 10.1016/j.jep.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 13.Zhang K. J., Zhu J. Z., Bao X. Y., Zheng Q., Zheng G. Q., Wang Y. Shexiang Baoxin Pills for coronary heart disease in animal models: Preclinical evidence and promoting angiogenesis mechanism. Frontiers in Pharmacology. 2017;8, article no. 404 doi: 10.3389/fphar.2017.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang W., Han L., Huang H., et al. Simultaneous determination of four volatile compounds in rat plasma after oral administration of Shexiang Baoxin Pill (SBP) by HS-SPDE-GC-MS/MS and its application to pharmacokinetic studies. Journal of Chromatography B. 2014;963:47–53. doi: 10.1016/j.jchromb.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 15.Levin R., Dolgin M., Fox C., Gorlin R. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels , lww handbooks. Vol. 9. LWW Handbooks; 1994. The Criteria Committee of the New York Heart Association; pp. 253–256. [Google Scholar]
- 16.Wang K. B. To observe the therapeutic effect of Shexiang Baoxin Pills on adjuvant treatment of chronic heart failure. Cardiovascular Disease Journal of integrated traditional Chinese and Western Medicine. 2015;3(23):85–86. [Google Scholar]
- 17.Ding R., Gui Y. P., Chen J. M., Wu Z. G. Effect of Shexiangbaoxin pills on heart function in patients with chronic ischemia heart failure. Academic Journal of Second Military Medical University (AJSMMU) 2013;34(1):37–40. doi: 10.3724/SP.J.1008.2013.0037. [DOI] [Google Scholar]
- 18.Sang C., Hao H. J., Xu J. Effects of Shexiang Baoxin pills on cardiac function and vascular endothelialfunction in patients with chronic congestive heart failure. China Pharmaceuticals. 2015;24(7):6–8. [Google Scholar]
- 19.Wang B. Study on randomized parallel control of shexiang baoxin pills combined with western medicine in treating chronic heart failure. Journal of Practical Traditional Chinese Internal Medicine. 2013;27(9):36–37. [Google Scholar]
- 20.Wu H. X., Wang C. Q., Feng X. Y., Bai Y., Wang Y. M. Clinical observation on treatment of chronic heart failure with coronary heart disease by Shexiang Baoxin pills. Chinese Journal of Trauma and Disability Medicine. 2014;22(3):112–113. [Google Scholar]
- 21.Ran X., Chen L., Wu W. Effects of Shexiang Baoxin pills on cardiac function and heart rate variability in patients with chronic heart failure. Journal of New Chinese Medicine. 2012;44(7):13–15. [Google Scholar]
- 22.Xu H. G. Clinical Study on the Treatment of Chronic Systolic Heart Failure with Shexiang BaoxinPills. The Asia-pacific Traditional Medicine. 2015;11(12):128–129. [Google Scholar]
- 23.Bao M. Z., Liu Y. G. Effects of Shexiang Baoxin Pills on Chronic Heart Failure in Patients withOld Heart Disease. China Metallurgical Industry Journal of Medicine. 2009;26(2):p. 147. [Google Scholar]
- 24.Han L., Chen J. L., Miao Y. G., et al. Clinical observation on the effects of shexiang baoxin pills on coronary heart disease with heart failure. Chinese Journal of Cardiovascular Rehabilitation Medicine. 2010;19(1):88–90. [Google Scholar]
- 25.Guo F., Hu L., Lin A. M., Jian A., Liu L. Treatment of chronic heart failure with integrated traditional Chinese and western medicine in 122 cases. Journal of Practical Traditional Chinese Medicine. 2011;27(11):758–759. [Google Scholar]
- 26.Zhu Z., Yang F. Effects of Shexiang Baoxin pills on chronic heart failure. Zhejiang J Tradit Chin Med. 2012;47(7):p. 542. [Google Scholar]
- 27.Zhong Y., Lin G. Y., Yang D. Z. Effects of Shexiang Baoxin pills on cardiac function and plasma nt-probnp in patients with chronic heart failure. Chinese Journal of Experimental Traditional Medical Formulae. 2012;18(17):273–275. [Google Scholar]
- 28.Dong Z. C., Wang X. L. Effects of Shexiang Baoxin pills on diastolic heart failure. Chinese Journal of Urban and Rural Industrial Hygiene. 2012;17:123–124. [Google Scholar]
- 29.Wang X. Z. Effects of Shexiang Baoxin pills on chronic heart failure. Guangming Traditional Chinese Medicine. 2012;27(7):1337–1338. [Google Scholar]
- 30.Sun H. F., Cai Y. X. Effects of Shexiang Baoxin Pills On chronic congestive heart failure. Jilin Medical Journal. 2013;34(9):1693–1694. [Google Scholar]
- 31.Quan J. M., Shi Y. C. The effects of combined traditional Chinese and western medicine on the treatment of chronic heart failure. Zhejiang Journal of Traditional Chinese Medicine. 2013;48(2):118. [Google Scholar]
- 32.Li X. F. Effects of Shexiang Baoxin pills on chronic congestive heart failure. Guide of China Medicine. 2013;11(34):505–506. [Google Scholar]
- 33.Gao Y., Gao H. Clinical study on the treatment of chronic heart failure with shexiang baoxin pills. Modern Diagnosis & Treatment. 2013;24(3):542–543. [Google Scholar]
- 34.Li R., TR W. u., Zhu C. H., Huang G. Y., Peng L. H. Clinical Observation on the treatment of chronic heart failure in patients with coronary heart disease by shexiang baoxin pills. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease. 2015;13(15):1704–1706. [Google Scholar]
- 35.Yang R. H., Fang C. G., Liang J. G. H-FABP in Patients with Coronary Heart Disease and Heart Failure. Vol. 35. Jilin Medical; 2014. Effects of Shexiang Baoxin pills on left ventricular function and serum; pp. 5317–5318. [Google Scholar]
- 36.Fang X. J., Qian B. Q., Zhao Q. Effects of Shexiang Baoxin Pills on chronic heart failure. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 2014;24(11):1009–1010. [Google Scholar]
- 37.Deng Y. F., Zhang H. S. Effects of Shexiang Baoxin pills on B-type brain natriuretic peptide and left ventricular ejection fraction in patients with chronic heart failure. Chinese Journal of Modern Drug Application. 2013;7(14):p. 181. [Google Scholar]
- 38.Gu X. L., Chao S. W., Fan J. H., et al. Effects of Shexiang Baoxin pills on rehabilitation of patients with coronary heart disease and heart failure. Chinese Journal of Cardiovascular Rehabilitation Medicine. 2007;16(1):92–94. [Google Scholar]
- 39.Yang F., Cheng X. W., Zhang Y. L. Shexiang Baoxin pills in the treatment of chronic heart failure. Central Plains Medical Journal. 2007;34(7):80–81. [Google Scholar]
- 40.Lv L. Y., Zhen S. Q. Effects of Shexiang Baoxin pill on 40 cases of chronic cardiac insufficiency. Medical Information. 2007;20(6):1031–1032. [Google Scholar]
- 41.Niu C. J., Liu P. Effects of Shexiang Baoxin pills on senile chronic congestive heart failure. West China Medical Journal. 2014;29(2):303–304. [Google Scholar]
- 42.Wang S. K., Yin J. R., Liu D. H., Wu P. Efficacy of Shexiang Baoxin Pills in treating chronic heart failure. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2010;19(16):1990–1991. [Google Scholar]
- 43.Kalter-Leibovici O., Freimark D., Freedman L. S., et al. Disease management in the treatment of patients with chronic heart failure who have universal access to health care: A randomized controlled trial. BMC Medicine. 2017;15(1, article no. 90) doi: 10.1186/s12916-017-0855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang K. J., Zhu J. Z., Bao X. Y., Zheng Q., Zheng G. Q., Wang Y. Shexiang Baoxin Pills for coronary heart disease in animal models: Preclinical evidence and promoting angiogenesis mechanism. Frontiers in Pharmacology. 2017;8:p. 404. doi: 10.3389/fphar.2017.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senni M., Paulus W. J., Gavazzi A., et al. New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. European Heart Journal. 2014;35(40):2797–2811. doi: 10.1093/eurheartj/ehu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maas J. J., Pinsky M. R., De Wilde R. B., De Jonge E., Jansen J. R. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: Interpretation with venous return and cardiac function curves. Critical Care Medicine. 2013;41(1):143–150. doi: 10.1097/CCM.0b013e318265ea64. [DOI] [PubMed] [Google Scholar]
- 47.Peng C., Yang Y., Lv C., et al. Pharmacokinetic study of five ginsenosides using a sensitive and rapid liquid chromatography-tandem mass spectrometry method following single and multiple oral administration of Shexiang Baoxin pills to rats. Biomedical Chromatography. 2015;29(3):425–436. doi: 10.1002/bmc.3293. [DOI] [PubMed] [Google Scholar]
- 48.Liu R. H., Runyon R. S., Wang Y. C., Oliver S. G., Fan T. P., Zhang W. D. Deciphering ancient combinatorial formulas: the Shexiang Baoxin pill. Science. 2015;347(6219):S40–S42. [Google Scholar]
- 49.Cao H., Zhang A., Zhang H., Sun H., Wang X. The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytotherapy Research. 2015;29(2):159–166. doi: 10.1002/ptr.5240. [DOI] [PubMed] [Google Scholar]
- 50.Shin B. K., Kwon S. W., Park J. H. Chemical diversity of ginseng saponins from Panax ginseng. Journal of Ginseng Research. 2015;39(4):287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mccann S., Greenlees M. J., Newell D., Shine R. Rapid acclimation to cold allows the cane toad to invade montane areas within its Australian range. Functional Ecology. 2014;28(5):1166–1174. doi: 10.1111/1365-2435.12255. [DOI] [Google Scholar]
- 52.Tsai M. Y., Hu W. L., Lin C. C., et al. Prescription pattern of Chinese herbal products for heart failure in Taiwan: A population-based study. International Journal of Cardiology. 2017;228:90–96. doi: 10.1016/j.ijcard.2016.11.172. [DOI] [PubMed] [Google Scholar]