Abstract
Biosurfactants are surface-active compounds that have sparked interest in recent years because of their environmental advantages over conventional surfactants. The aim of this study was to investigate the production of biosurfactants by soil fungi isolated from the Amazon forest. Fungi colonies were isolated from soil samples and screened for biosurfactant production in submerged fermentation. In addition, the influences of bioprocess factors (carbon source, nitrogen source, pH, and fermentation time) were investigated. Finally, the biosurfactant produced was semipurified and submitted to stability tests. One hundred fungal cultures were obtained from the soil samples, identified by micromorphology, and submitted to screening for biosurfactant production. Sixty-one strains produced biosurfactants. The strain Penicillium 8CC2 showed the highest emulsification index (54.2%). The optimized bioprocess conditions for biosurfactant production by Penicillium 8CC2 were as follows: soybean oil, 20 g/L; yeast extract, 30 g/L; pH 9; duration of 9 days. The semipurified biosurfactant showed stability after heating at 100°C for 60 min and after the addition of 30% NaCl (w/v). Tween 80 (0.2% w/v), a conventional surfactant, was used as the control.
1. Introduction
Surfactants are among the most versatile materials in chemical and process industry. Its amphiphilic nature, containing both hydrophilic and lipophilic functional groups in one molecule, plays an important role in numerous chemical applications (dispersion systems, as emulsions and colloids, personal hygiene, detergents, fabric softeners, emulsions, and paints over food texture) [1, 2]. Recently, literature has been demonstrating the advantages of biosurfactants (surfactant of biological origin) if compared with surfactants from chemical industry: lowest toxicity, higher biodegradability, and possible biological activities [2, 3]. Biosurfactants are a structurally diverse group of compounds produced by organisms that have surface-active properties and emulsification action. Biosurfactant properties are similar to the surfactant; they have industrial applications in relation to detergency, emulsification, lubrication, foaming capacity, ability, solubility, dispersion phases [2–5], and another interesting application in catalysis, biosensing, and electronics using microstructures [6].
Biosurfactants are mainly produced by bacteria and yeast, but, in recent years, studies have highlighted their production by filamentous fungi as well [7, 8]. These microorganisms are potential producers of various substances of biotechnological interest, such as pigment enzymes and antibiotics, and their main sources of isolation are plants and soil [9]. The production of biosurfactants by microorganisms is influenced by several factors, including the nature of the carbon source and the concentrations of nutrients such as nitrogen, phosphorus, magnesium, iron, sulfur, and manganese, as well as the pH, temperature, agitation, and available oxygen [2, 10, 11]. These factors can make the production of biosurfactants more expensive than that of synthetic surfactants [12]. Moreover, several studies have been performed to make the price of the bioprocess more competitive [13].
Despite intensive efforts to study the Amazon, we lack knowledge of the microbial diversity and the production of substances of biotechnological interest by the filamentous fungi that make up this biome. Amazonian bioprospecting studies such as this one can result in the discovery of fungi with high productivity and new biosurfactants. In the current study, we investigated the production of biosurfactants by fungi isolated from soil samples of the Amazonian forest. Analyses were performed related to the following: (i) isolation and identification of biosurfactant-producing fungi, (ii) optimization and kinetic parameters related to the production of the biosurfactant selected, and (iii) comparison of the emulsion stability of the biosurfactant of fungal origin compared to that of a conventional surfactant.
2. Materials and Methods
2.1. Isolation and Identification of Soil Fungi
The samples were collected at six locations in the woods of the National Institute of Amazonian Research (INPA) (3.10′39′′S; 59.96′′77′′W), Amazonas, Brazil. For isolation, 1 g of soil was transferred to a tube containing 9 mL of water, and a 1 : 100 dilution was prepared. Then, 100 μL of the diluted sample was plated on a Petri dish containing Sabouraud agar with chloramphenicol (250 mg L−1). After 72 h of incubation at 25°C, the colonies that developed were transferred to tubes with the same medium; the test was performed in triplicate. A streaking technique was performed to obtain monosporic crops. Fungal colonies were identified by observing macroscopic aspects of the colonies and microscopic features, as described by Lacaz et al. [14] and Barnett and Hunter [15].
2.2. Selection of Biosurfactant Producers
The selection of biosurfactant producers was performed in Erlenmeyer flasks (125 mL) containing 25 mL of culture medium (with 40 g L−1 soybean and 20 g L−1 peptone) as previously described by Accorsini et al., 2012; however, the mineral solution was replaced with peptone [16]. The medium was inoculated with spores of the isolates at a final concentration of 1 × 104 spores/mL−1. Then, the flasks were incubated without shaking at room temperature (25 ± 2°C) for 7 days, followed by filtration with a quantitative filter paper (Ø, 12.5 cm; pore size, 28 μm; Quanty®) for removal of fungal cells. The filtrate was separated for use in detection of biosurfactants by the drop collapse method described by Bodour and Miller-Maier [17] and the emulsification index described by Cameron et al. [18].
In the drop collapse test, a polystyrene plate with 96 microwells (8.5 × 12.7 cm) was used. The plate was washed with hot water, ethanol, and distilled water. Then, 5 μL of the filtrate of each sample was inoculated separately into wells prefilled with 2.0 μL of mineral oil previously left at room temperature for 24 h. After 1 min of the reaction, the result was determined visually. The result was considered positive when the drop of mineral oil collapsed. A control was prepared using SDS at a concentration of 25%.
To test the emulsification index (E24), a 4 mL aliquot of the culture filtrate (cell-free) was mixed with 6 mL of toluene in a screw tube. The mixture was shaken vigorously for 2 min on a tube shaker-type vortex (Phoenix®). After 24 h, the ratio of emulsified toluene was compared with the total volume. The emulsification index was calculated using the following formula:
| (1) |
2.3. Influence of Carbon and Nitrogen Sources on Biosurfactant Production
The influences of different carbon (20 g L−1) and nitrogen (10 g L−1) sources on biosurfactant production were evaluated. The experimental conditions were the same as those described in the Selection of Biosurfactant Producers, and the results for each substrate were analyzed using univariate analysis. The carbon sources investigated were soybean oil, starch, sucrose, cellulose, and xylose, and the nitrogen sources investigated were peptone, yeast extract, meat extract, sodium nitrate, and malt.
2.4. Effects of Concentration of Carbon and Nitrogen Sources, pH, and Treatment Time
We evaluated the influence of soybean oil, yeast extract, pH, and bioprocess time through a 2k factorial design (two levels). The chosen design was 24 with four central points (evaluation of experimental error), as described by Neto et al. [19]. The other experimental conditions were similar to those described in the Selection of Biosurfactant Producers.
2.5. Semipurification of Biosurfactant and Stability Analyses
Semipurification of the biosurfactant was performed with 30 repetitions under optimized conditions. The resulting solutions were pooled, filtered, and precipitated with ethanol (1 : 4 v/v, 4°C, 48 h). The mixture was subjected to centrifugation (5000 rev/min for 20 min), and the precipitate obtained was used for stability testing.
The effect of the addition of NaCl (30% w/v) was evaluated. After the addition of salt to the 1% w/v solution of the precipitate containing the biosurfactant, the emulsifying activity was tested using the emulsification index.
The effect of temperature on the biosurfactant activity was investigated by keeping 1% w/v of the precipitate containing the biosurfactant at 100°C in a water bath for 60 min and verifying the emulsification index. The synthetic surfactant Tween 80 (1% w/v) was used as a control substance in these assays.
2.6. Statistical Analysis
Statistical analyses were performed using STATISTICA versions 5.0 and 6.0 (STATGRAPHICS, Statpoint Technologies, Inc., Warrenton, VA, USA). All experiments were performed in triplicate for the calculation of mean and standard deviation.
3. Results
3.1. Isolation and Identification of Fungi
One hundred fungal strains were screened for their biosurfactant production. We obtained colonies from the genera Penicillium (46 isolates), Aspergillus (24 isolates), Fonsecaea, Gliocladium (one isolate), Trichoderma (12 isolates), Fusarium (eight isolates), and Acremonium (two isolates). Additionally, from the phylum Zygomycota, colonies from the genus Mucor (six isolates) of the class Zygomycetes were obtained.
3.2. Selection of Fungal Producers of Biosurfactants
To select the biosurfactant-producing fungi, we performed analyses of bioprocessing in submerged fermentation and evaluated the emulsification index and drop collapse test (Table 1) of the culture filtrate. Nine isolates showed a high emulsification index (E24 > 40); these belonged to the genera Penicillium (three isolates), Trichoderma (three isolates), and Fusarium (three isolates). The isolated Penicillium 8CC2 showed a high emulsification index (54.2%) and was selected for the remaining stages of the study because its emulsion could be maintained for more than 15 days; others have isolated emulsification rates below 30%.
Table 1.
Fungal cultures isolated from soil samples and results for the best producers of biosurfactants, obtained using the drop collapse test and emulsification index (E24).
| Isolated organism | Drop collapse test | Emulsification index |
|---|---|---|
| Fusarium sp. 87LIV12 | Neg | 64.28 |
| Fusarium sp. 86LV272 | Neg | 58.57 |
| Fusarium sp. 85RV342 | Neg | 57.14 |
| Penicillium sp. 97VV74 | Neg | 57.14 |
| Penicillium sp. 8CC2 | Neg | 54.2 |
| Trichoderma sp. 91BN312 | Neg | 52.85 |
| Trichoderma sp. 60AVR3 | Pos | 51 |
| Trichoderma sp. 16AC2 | Neg | 42.85 |
| Penicillium sp. 62BLC2 | Neg | 40.3 |
3.3. Effect of Carbon and Nitrogen Sources on Biosurfactant Production
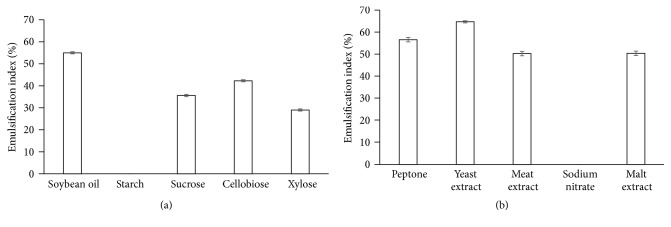
To optimize the production of biosurfactants by the isolated Penicillium 8CC2, we investigated the influence of carbon and nitrogen sources by performing univariate experiments (Figure 1). The carbon and nitrogen sources that enabled the highest production rates were soybean oil (55%) and yeast extract (64,7%), respectively.
Figure 1.
Univariate test measuring the influence of carbon and nitrogen sources in the production of biosurfactants by Penicillium 8CC2. (a) Carbon source and (b) nitrogen source.
3.4. Influence of Soybean Oil, Yeast Extract, pH, and Time on Biosurfactant Production
The influence of soybean oil and yeast extract concentrations, pH, and time on biosurfactant production by Penicillium 8CC2 was investigated using the experimental design 24, as described with four replicates added at the central point (Table 2). All major factors and some interactions (yeast extract × time and pH × time) showed statistical significance (Table 3). An analysis of variance (ANOVA) was performed to validate the mathematical model presented (see (2)); the related information is provided in Table 4. The mathematical model showed a significant regression (91.8%), and its factors showed statistical significance. The lack of fit was not significant (p > 0.05).
| (2) |
Table 2.
Influence of soybean oil, yeast extract, pH, and time on biosurfactant production by Penicillium 8CC2, performed according to a factorial design.
| Soybean oil (g l−1) | Yeast extract (g l−1) | pH | Time (d) |
E24 |
|---|---|---|---|---|
| 40 | 20 | 5 | 7 | 61 |
| 60 | 30 | 4 | 9 | 76 |
| 60 | 10 | 4 | 9 | 53 |
| 40 | 20 | 5 | 7 | 60 |
| 60 | 30 | 6 | 9 | 76 |
| 20 | 30 | 6 | 9 | 80 |
| 20 | 10 | 4 | 5 | 47 |
| 40 | 20 | 5 | 7 | 58 |
| 40 | 20 | 5 | 7 | 62 |
| 60 | 30 | 4 | 5 | 51 |
| 20 | 10 | 6 | 9 | 62 |
| 20 | 10 | 6 | 5 | 56 |
| 60 | 30 | 6 | 5 | 60 |
| 20 | 30 | 6 | 5 | 69 |
| 60 | 10 | 4 | 5 | 48 |
| 20 | 10 | 4 | 9 | 58 |
| 60 | 10 | 6 | 5 | 60 |
| 20 | 30 | 4 | 5 | 62 |
| 20 | 30 | 4 | 9 | 78 |
| 60 | 10 | 6 | 9 | 47 |
Table 3.
Effect of tested variables on biosurfactant production by Penicillium 8CC2.
| Factors | Effect |
|---|---|
| Average | 61,2 ± 0,381881 |
| A: soybean | −5,125 ± 0,853913∗ |
| B: yeast extract | 15,125 ± 0,853913∗ |
| C: pH | 4,625 ± 0,853913∗ |
| D: time | 9,625 ± 0,853913∗ |
| AB | −1,375 ± 0,853913 |
| AC | −0,875 ± 0,853913 |
| AD | −1,375 ± 0,853913 |
| BC | −0,125 ± 0,853913 |
| BD | 7,375 ± 0,853913∗ |
| CD | −4,625 ± 0,853913∗ |
The effects that showed statistical significance (95% confidence level) are indicated by the symbol ∗.
Table 4.
Analysis of variance for evaluating the statistical significance of the model for biosurfactant production by Penicillium 8CC2 (R2 = 91.8529%).
| Source | Sum of squares | Df | Mean of Squares | F ratio | P value |
|---|---|---|---|---|---|
| A: soybean oil | 105,063 | 1 | 105,063 | 36,02 | 0,0093 |
| B: yeast extract | 915,063 | 1 | 915,063 | 313,74 | 0,0004 |
| C: pH | 85,5625 | 1 | 85,5625 | 29,34 | 0,0123 |
| D: time | 370,563 | 1 | 370,563 | 127,05 | 0,0015 |
| BD | 217,563 | 1 | 217,563 | 74,59 | 0,0033 |
| CD | 85,5625 | 1 | 85,5625 | 29,34 | 0,0123 |
| Lack of fit | 149,075 | 10 | 149,075 | 5,11 | 0,1030 |
| Pure error | 8,75 | 3 | 8,75 | ||
| Total | 1937,2 | 19 |
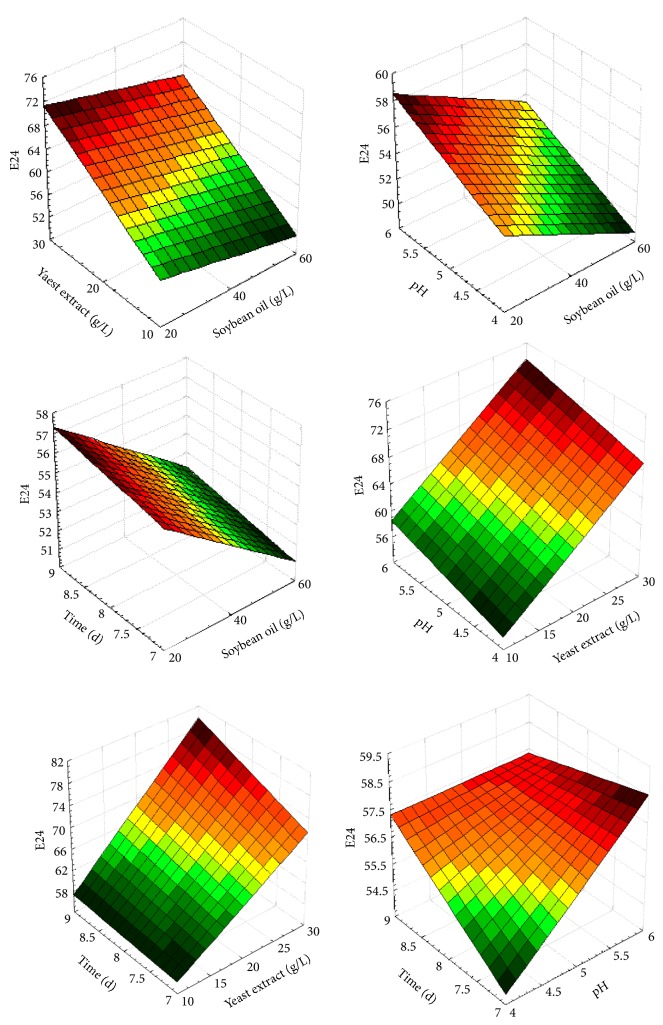
To represent the estimated response (E24), surfaces were prepared (Figure 2) based on the data generated by the model. The surfaces constructed on the basis of the model responses showed that the optimal conditions for maximal biosurfactant production were as follows: soybean oil, 20 g L−1; yeast extract, 30 g L−1; pH 6; and a duration of 9 days. These values were obtained with a theoretical E24 of 79.82%.
Figure 2.
Response surface analysis for biosurfactant production by Penicillium 8CC2, with soybean oil, yeast extract, pH, and production time.
3.5. Stability Studies
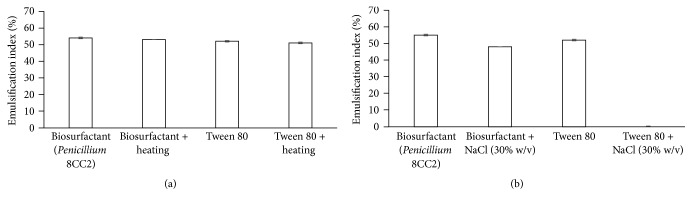
The stability of the new biosurfactant was evaluated under different physical conditions (Figure 3). Heating (100°C for 60 min) did not influence the biosurfactant activity. A high NaCl concentration caused a reduction of 12.7% in the E24 value of the biosurfactant produced by Penicillium 8CC2 and completely inhibited the emulsion formation by the commercial surfactant Tween 80.
Figure 3.
Stability of biosurfactants from Penicillium 8CC2 under different physical conditions: (a) after heating 100°C (60 min) and (b) with the addition of NaCl (30% w/v). The synthetic surfactant Tween 80 (1% w/v) was used as a control substance in these assays.
4. Discussion
We found Penicillium 8CC2 (strain isolated from Amazonian soil) produced biosurfactant with high emulsification index (EI24 > 50). In addition, we found that the biosurfactant from Penicillium 8CC2 has similar thermal-stability and higher NaCl-stability if compared to the surfactant Tween 80. The present work was the first to screen the potential of fungus obtained from soil samples of the Amazon forest to produce biosurfactant and the selected microorganisms and their biosurfactant have industrial potential.
Among the fungi obtained from soil, the genera that showed a strong ability to emulsify toluene were Penicillium, Trichoderma, and Fusarium. The potential of Penicillium sp. to produce biosurfactants has already been demonstrated [20, 21], but few studies have described the biosurfactant potentials of Trichoderma [22] and Fusarium.
Carbon sources for microbial production of biosurfactant can be obtained from carbohydrates, vegetable oils, hydrocarbons, and waste frying oils [23, 24]. In this study, soybean oil was the best carbon source for biosurfactant production. Soybean oil was also described as a suitable carbon source for fungal production of biosurfactant by Accorsini et al. [16]; these last ones investigated more affordable culture medium to produce biosurfactant by yeasts. We believe that triglycerides composition is a natural inducer of the biosurfactant production, since the microorganisms produce biosurfactant to increase the access to lipid degradation and it is clear that the triglycerides can be directly used to anabolize the production of the surfactant. Starch, despite being used by other microorganisms as a carbon source [25], does not appear to be a suitable substrate for biosurfactant production by Penicillium 8CC2.
Biosurfactants are produced when there is nitrogen limitation during the stationary phase of growth of biomass [26–28]. In the present work, yeast extract was found to be the most suitable nutrient source for the production of the metabolite of interest, followed by peptone, meat extract, and malt. The mineral nitrogen source sodium nitrate was not suitable for production of the biosurfactant. Another study showed similar results; it was observed that Torulopsis bombicola produced biosurfactants using yeast extract but was not able to produce them using sodium nitrate.
The results of this study showed that the factors studied (soybean oil, peptone, pH, and time) and some of their interactions had a significant effect on the response variable (biosurfactant production). The validated mathematical model demonstrated that an E24 of 79.82 could be obtained using 20 g L−1 soybean oil, 30 g L−1 yeast extract, pH 6, and a 9-day duration of the bioprocess. Maximum biosurfactant production occurred in the optimal pH range employed for microorganism growth. During the production of this biosurfactant, as well as during any chemical reaction, the pH directly affects the microbial activity because of the effects of H+ ions on cell permeability and enzyme activity [29]. Kiran et al. [30] reported that an Aspergillus ustus isolate showed optimal biosurfactant production at pH 7. Mucor circinelloides was found to maximally produce biosurfactant at pH 8 using apple peel, vegetable oil, and corn water as substrates [31]. The time required for maximum biosurfactant production by Penicillium sp. 8CC2 in the current study was 9 days. Aspergillus fumigatus was found to produce biosurfactant in 112 h in solid-state fermentation [32]. Aspergillus ustus isolated from a marine sponge showed optimized production at 120 h in Sabouraud dextrose broth [30].
The stability of the biosurfactant was evaluated at high temperatures and high ionic strengths. After subjecting the biosurfactant to a temperature of 100°C for 60 min, there was no verified change in biosurfactant activity. This result demonstrates the potential utility of the biosurfactant in the food, pharmaceutical, and cosmetic industries where heat sterility is of great importance [32, 33]. In the case of ionic strength, there was a small reduction in the emulsification ability of Penicillium 8CC2 when NaCl (30%) was added, but a stable emulsion could still be maintained. The commercial emulsifier Tween 80, used as a control, did not remain stable when NaCl was added. This result demonstrates that the biosurfactant produced by Penicillium 8CC2 can be used in high salt-concentration conditions, for example, in tertiary oil recovery activities.
This study verified stability parameters for only one fungus from the 61 potential biosurfactant-producing fungi, thus ignoring possible potentiality in many others, including some with high levels of emulsification. In the stability tests, we could have assessed the influence of pH. The results demonstrate that biotechnological approaches can be used to improve biosurfactant production by Penicillium 8CC2. The current results suggest the potential usefulness of the biosurfactant produced by a filamentous fungus isolated from soil samples from the Amazon region.
The data from the present work is important since a significative number of isolates (100 cultures isolated from soil samples from the Amazon) were investigated for the production of biosurfactant. The analytical methodology used in the screening of biosurfactant producers did not quantify the biosurfactant directly in the culture filtrate; nowadays, the screening methods have been developed that rely on the interfacial activity of the biosurfactants but do not measure it directly. We used methodology that quantifies the ability of the biosurfactant present in the culture filtrate to produce emulsification with toluene “emulsification index” as classically described by Mullingan et al. [34]. Unfortunately, as a work limitation, during the experimental conditions, it was not possible to identify the species of Penicillium 8CC2 and the chemical composition of the biosurfactant. Literature does not have description of chemical composition of the biosurfactants produced by Penicillium sp. and further works are necessary to elucidate it.
The development for screening microbes from thousands of potentially active organisms and the subsequent evaluation of surface activity holds the key to the discovery of new biosurfactants or production strains.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Raza Z. A., Rehman A., Hussain M. T., et al. Production of rhamnolipid surfactant and its application in bioscouring of cotton fabric. Carbohydrate Research. 2014;391(1):97–105. doi: 10.1016/j.carres.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Banat I. M., Franzetti A., Gandolfi I., et al. Microbial biosurfactants production, applications and future potential. Applied Microbiology and Biotechnology. 2010;87(2):427–444. doi: 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- 3.Pacwa-Płociniczak M., Płaza G. A., Piotrowska-Seget Z., Cameotra S. S. Environmental applications of biosurfactants: recent advances. International Journal of Molecular Sciences. 2011;12(1):633–654. doi: 10.3390/ijms12010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Das P., Mukherjee S., Sen R. Improved bioavailability and biodegradation of a model polyaromatic hydrocarbon by a biosurfactant producing bacterium of marine origin. Chemosphere. 2008;72(9):1229–1234. doi: 10.1016/j.chemosphere.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Nitschke M., Pastore G. M. Biossurfactantes: propriedades e aplicações. Química Nova. 2002;25(5):772–776. doi: 10.1590/S0100-40422002000500013. [DOI] [Google Scholar]
- 6.Rehman A., Raza Z. A., Saif-ur-Rehman, et al. Synthesis and use of self-assembled rhamnolipid microtubules as templates for gold nanoparticles assembly to form gold microstructures. Journal of Colloid and Interface Science. 2010;347(2):332–335. doi: 10.1016/j.jcis.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Castiglioni G. L., Bertolin T. E., Costa J. A. Produção de biossurfactante por Aspergillus fumigatus utilizando resíduos agroindustriais como substrato. Química Nova. 2009;32(2):292–295. doi: 10.1590/S0100-40422009000200005. [DOI] [Google Scholar]
- 8.Velioglu Z., Urek R. O. Biosurfactant production by Pleurotus ostreatus in submerged and solid-state fermentation systems. Turkish Journal of Biology. 2015;39(1):160–166. doi: 10.3906/biy-1406-44. [DOI] [Google Scholar]
- 9.Adrio J. L., Demain A. L. Fungal biotechnology. International Microbiology. 2003;6(3):191–199. doi: 10.1007/s10123-003-0133-0. [DOI] [PubMed] [Google Scholar]
- 10.Fontes G. C., Amaral P. F., Coelho M. A. Produção de biossurfactante por levedura. Química Nova. 2008;31(8):2091–2099. doi: 10.1590/S0100-40422008000800033. [DOI] [Google Scholar]
- 11.Pirôllo M. Estudo da Produção de Biossurfactantes Utilizando Hidrocarbonetos. Sao Paulo, Brazil: Universidade Estadual Paulista; 2006. [Google Scholar]
- 12.Thavasi R., Jayalakshmi S., Balasubramanian T., Banat I. M. Biosurfactant production by Corynebacterium kutscheri from waste motor lubricant oil and peanut oil cake. Letters in Applied Microbiology. 2007;45(6):686–691. doi: 10.1111/j.1472-765X.2007.02256.x. [DOI] [PubMed] [Google Scholar]
- 13.Pattanathu K., Rahman M., Gapke M. Production, characterisation and applications of biosurfactants - Review. Biotechnology. 2008;2:360–370. doi: 10.3923/biotech.2008.360.370. [DOI] [Google Scholar]
- 14.Lacaz C., Porto E., Martins J. Martins, Microbiologia Médica: Fungos, Actinomicetos e Algas de Interesse Médico. 8th. Vol. 33. São Paulo, Brazil: Sarvier; 2001. [Google Scholar]
- 15.Barnett H. L., Hunter B. B. IIlustrated Genera of Imperfect Fungi. 4th. Vol. 64. Burgess Publishing Co: 1998. [Google Scholar]
- 16.Accorsini F. R., Mutton M. J. R., Lemos E. G. M., Benincasa M. Biosurfactants production by yeasts using soybean oil and glycerol as low cost substrate. Brazilian Journal of Microbiology. 2012;43(1):116–125. doi: 10.1590/S1517-83822012000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodour A. A., Miller-Maier R. M. Application of a modified drop-collapse technique for surfactant quantitation and screening of biosurfactant-producing microorganisms. Journal of Microbiological Methods. 1998;32(3):273–280. doi: 10.1016/S0167-7012(98)00031-1. [DOI] [Google Scholar]
- 18.Cameron D. R., Cooper D. G., Neufeld R. J. The mannoprotein of Saccharomyces cerevisiae is an effective bioemulsifier. Applied and Environmental Microbiology. 1988;54(6):1420–1425. doi: 10.1128/aem.54.6.1420-1425.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neto B. B., Scarminio I. S., Bruns R. E. Planejamento e otimização de experimentos. 1995. [Google Scholar]
- 20.Luna-Velasco M. A., Esparza-García F., Cañízares-Villanueva R. O., Rodríguez-Vázquez R. Production and properties of a bioemulsifier synthesized by phenanthrene-degrading Penicillium sp. Process Biochemistry. 2007;42(3):310–314. doi: 10.1016/j.procbio.2006.08.015. [DOI] [Google Scholar]
- 21.Camargo-de-Morais M. M., Ramos S. A., Pimentel M. C., Morais J. Production of an extracellular polysaccharide with emulsifier properties by Penicillium citrinum. World Journal of Microbiology and Biotechnology. 2003;19:191–194. [Google Scholar]
- 22.Askolin S., Nakari-Setälä T., Tenkanen M. Overproduction, purification, and characterization of the Trichoderma reesei hydrophobin HFBI. Applied Microbiology and Biotechnology. 2001;57(1-2):124–130. doi: 10.1007/s002530100728. [DOI] [PubMed] [Google Scholar]
- 23.Raza Z. A., Khan M. S., Khalid Z. M. Evaluation of distant carbon sources in biosurfactant production by a gamma ray-induced Pseudomonas putida mutant. Process Biochemistry. 2007;42(4):686–692. doi: 10.1016/j.procbio.2006.10.001. [DOI] [Google Scholar]
- 24.Kim H.-S., Yoon B.-D., Lee C.-H., et al. Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. Journal of Fermentation and Bioengineering. 1997;84(1):41–46. doi: 10.1016/S0922-338X(97)82784-5. [DOI] [Google Scholar]
- 25.Makkar R. S., Cameotra S. S. Production of biosurfactant at mesophilic and thermophilic conditions by a strain of Bacillus subtilis. Journal of Industrial Microbiology and Biotechnology. 1998;20(1):48–52. doi: 10.1038/sj.jim.2900474. [DOI] [Google Scholar]
- 26.Banat I. M., Desai D. J. Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews. 1997;61(1):47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decesaro A., Rigon M. R., Thomé A., Colla L. M. Produção de biossurfactantes por microrganismos isolados de solo contaminado com óleo diesel. Química Nova. 2013;36(7):947–954. doi: 10.1590/S0100-40422013000700005. [DOI] [Google Scholar]
- 28.Kim H.-S., Jeon J.-W., Kim B.-H., Ahn C.-Y., Oh H.-M., Yoon B.-D. Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, by Candida sp. SY16 using fed-batch fermentation. Applied Microbiology and Biotechnology. 2006;70(4):391–396. doi: 10.1007/s00253-005-0092-9. [DOI] [PubMed] [Google Scholar]
- 29.Castiglioni G. L., Stanescu G., Rocha L. A. O., Costa J. A. V. Acta scientiarum analytical modeling and numerical optimization of the biosurfactants production in solid-state fermentation by aspergillus fumigatus. Acta Scientiarum. 2014;36(1):61–67. doi: 10.4025/actascitechnol.v36i1.17818. [DOI] [Google Scholar]
- 30.Kiran G. S., Hema T. A., Gandhimathi R., et al. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids and Surfaces B: Biointerfaces. 2009;73(2):250–256. doi: 10.1016/j.colsurfb.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Acioly L. M., Silveira A., Anjos M., et al. Biosurfactant production by Mucor circinelloides using apple peel, vegetable oil and corn steep liquor as substrate. In: Mendez-Vilas A., editor. Microbes in Applied Research–Currents Advances and Challenges. New York, NY, USA: World Science; 2012. pp. 344–347. [Google Scholar]
- 32.Monteiro A. S., Bonfim M. R. Q., Domingues V. S., et al. Identification and characterization of bioemulsifier-producing yeasts isolated from effluents of a dairy industry. Bioresource Technology. 2010;101(14):5186–5193. doi: 10.1016/j.biortech.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 33.Khopade A., Ren B., Liu X.-Y., Mahadik K., Zhang L., Kokare C. Production and characterization of biosurfactant from marine Streptomyces species B3. Journal of Colloid and Interface Science. 2012;367(1):311–318. doi: 10.1016/j.jcis.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Mullingan C., Cooper D., Neufeld R. Selection of microbes producing biosurfactants in media without hydrocarbons. Journal of Fermentation Technology. 1984;4:311–314. [Google Scholar]