Abstract
Monotropa hypopitys is a mycoheterotrophic, nonphotosynthetic plant acquiring nutrients from the roots of autotrophic trees through mycorrhizal symbiosis, and, similar to other extant plants, forming asymmetrical lateral organs during development. The members of the YABBY family of transcription factors are important players in the establishment of leaf and leaf-like organ polarity in plants. This is the first report on the identification of YABBY genes in a mycoheterotrophic plant devoid of aboveground vegetative organs. Seven M. hypopitys YABBY members were identified and classified into four clades. By structural analysis of putative encoded proteins, we confirmed the presence of YABBY-defining conserved domains and identified novel clade-specific motifs. Transcriptomic and qRT-PCR analyses of different tissues revealed MhyYABBY transcriptional patterns, which were similar to those of orthologous YABBY genes from other angiosperms. These data should contribute to the understanding of the role of the YABBY genes in the regulation of developmental and physiological processes in achlorophyllous leafless plants.
1. Introduction
Monotropa hypopitys (syn. Hypopitys monotropa) is a member of the flowering seed plant family Ericaceae, which in turn belongs to the order Ericales splitting from the base of the clade Asterids [1]. This mycoheterotrophic, nonphotosynthetic, achlorophyllous plant acquires carbon from the roots of autotrophic trees through monotropoid mycorrhizal symbiosis [2, 3]. The M. hypopitys root system consists of fleshy roots, on which shoot buds develop, and finer mycorrhizal roots [2]. This plant has a typical aboveground structure, although the stem and leaves can be taken for flowering parts—floral axis and sterile bracts [4, 5]. Similar to extant plants, M. hypopitys forms asymmetrical lateral organs on the flanks of a shoot or inflorescence apical meristem, with adaxial and abaxial surfaces adjacent to or distant from, respectively, the meristem. Paleobotanic studies indicate that such structural asymmetry first appeared in a true leaf (euphyll) transformed from a radially symmetric stem as a consequence of the need to absorb more sunlight [6–9]. Studies of asymmetry in plants indicate that during plant development, cell fate is determined mostly by positional signals. The correct maintenance of the apical meristem and abaxial-adaxial differentiation of lateral organs requires reciprocal signal interaction between the meristem and derived structures [10–12].
It has been shown that the polarity of leaves and floral organs is defined by the network of genes encoding the Class III Homeodomain Leucine Zipper (HD-ZIPIII), ASYMMETRIC LEAVES (AS1/AS2), KANADI (KAN), AUXIN RESPONSE FACTOR (ARF3/ARF4), and YABBY families of transcription factors [8, 13–15]. Among them, the HD-ZIPIII REVOLUTA (REV) is expressed in the adaxial domain of lateral organs, whereas the GARP-family transcription factors KAN1–4 are involved in abaxial differentiation. Together, REV and KAN1 antagonistically regulate the expression of a number of genes encoding auxin signaling and transport components [16, 17].
The YABBY genes originating from the lineage leading to seed plants are identified in various green spermatophyte plant species; they are closely associated with the evolutionary emergence of flat-shaped leaves and are presumably diversified during evolution, which resulted in the appearance of family members with specific functions in the leaf, carpel, and ovule [8, 18–20]. YABBY transcription factors are characterized by their nuclear localization and the presence of the C2C2 zinc-finger and DNA-binding YABBY (High Mobility Group- (HMG-) box-like) domains [21, 22]. In gymnosperms, the YABBY genes are distributed among the A, B, C, and D clades [23]. In extant angiosperms, five YABBY subfamilies, FILAMENTOUS FLOWER (FIL or YAB1)/YABBY3 (YAB3 or AFO), CRABS CLAW (CRC), INNER NO OUTER (INO or YAB4), YAB2, and YAB5 [21] are distinguished by conserved functions in the initiation of lamina outgrowth, polarity maintenance, and establishment of the leaf margin [23–25]. Almeida et al. [26] and Morioka et al. [27] have provided evidence for the involvement of the YAB2 and YAB5 genes in the evolutionary diversification of style and filament morphology. The branching of INO and CRC from other YABBY genes has most likely occurred in parallel with the evolution of the carpel and outer integument via modification of reproductive leaf-like sporophylls [20, 28].
There are current theories related to the history of the YABBY genes in angiosperms. Bartholmes et al. [29] suggested that “vegetative” YABBYs (FIL/YAB3, YAB2 and YAB5) do not form a monophyletic clade and that CRC and FIL evolved from a common ancestor gene, while the INO genes are sisters to that ancestral gene. On the other hand, Finet et al. [23] clustered INO together with clades YAB5 and YAB2. In addition, two alternative evolutionary scenarios, that is, monophyly or paraphyly of the gymnosperm YABBY family towards angiosperm YABBY genes, suggest that all spermatophyte YABBY genes were derived from one or two, respectively, YABBY genes of the last common ancestor of extant seed plants [20, 23, 29]. The reconstruction of YABBY evolution in spermatophytes based on these theories suggests that at least one YABBY predecessor has already functioned as a polarity regulator and that the diversification of the gene family occurred in both angiosperms and gymnosperms [23]. Although the presence of the YABBY genes is presumably restricted to seed plants [19, 20], genomics studies conducted on marine picoeukaryotes revealed YABBY homologs in Chlorophyta [30]. Phylogenetic analysis of identified sequences suggested, with equal probability that either Chlorophyta YAB genes are evolutionarily related to seed plant YABBYs or emerged independently from ancestral HMG-box sequences [23].
The expression data available for the angiosperm YABBY genes suggested that the FIL-, YAB2-, and YAB5-like genes retained a more ancestral expression pattern in both vegetative and reproductive tissues, while the expression of the CRC and INO-like genes is more variable [29]. In Eudicots, FIL, YAB3, YAB2, and YAB5 transcripts were detected in the abaxial side of primordia in all aboveground lateral organs (except ovules) determining the abaxial cell fate [31, 32]. The CRC genes are expressed abaxially in the carpel, placenta, and nectaries promoting the development of the gynoecium and abaxial part of the carpel wall, and terminating the floral meristem [33–38]. INO mRNA is detected in the abaxial epidermis of the outer integument [20, 28, 39, 40]. The YABBY expression pattern differences between cereal monocots and other angiosperms indicate the modification of genetic pathways involving YABBYs during the process of angiosperm diversification [21, 27, 41–46]. It is assumed that FIL, together with REVOLUTA (REV), APETALA1 (AP1), and LEAFY (LFY), corrects the spatial activity of the AGAMOUS (AG), AP3, PISTILLATA (PI), and SUPERMAN (SUP) genes, and, thus, is involved in the initiation of floral organ primordia at the correct position and numbers, defining the fate of appropriate cells [31, 44, 47].
Thus, the bifunctional YABBY transcription factors have an important role in driving the evolution of the leaf and gynoecium, as well as in the initiation, growth, and structural organization of almost all aboveground lateral organs, and in the control of shoot apical meristem organization and activity.
In the present study, we identified and phylogenetically classified seven YABBY members from M. hypopitys and characterized their expression profiles in various tissues during flowering. The structural features and composition of conserved motifs belonging to the predicted MhyYABBY proteins were also analyzed. Our data should further the understanding of possible links between polarity determination and the physiology of achlorophyllous mycoheterotrophic plants.
2. Materials and Methods
2.1. Plants and Transcriptomes
The previous study divided M. hypopitys specimens into a North American cluster and two Eurasian (excluding Russia) sister lineages (Swedish and pan-Eurasian) [48]. Analysis of M. hypopitys from the European part of Russia revealed two types, A and B, which showed 99 and 100% homology with specimens from Japan, Finland, and Great Britain, and with Swedish specimens, respectively [49–51]. The study of H. monotropa specimens from Northern Ireland showed that they occur in small, highly fragmented populations, and exhibit a relatively high level of within-population genetic diversity and a low level of clonality [52].
In the present study, two M. hypopitys plants of type B from one clone of the same genet were used. Flowering plants were collected in a coniferous forest, Kaluga region, Russia, in August, 2015. The individual plant was a 15 cm reproductive axis with bracts and raceme of 10–12 flowers (each of 4 sepals, 4 petals, 8 stamens, and 4 fused carpels), and root system comprised mycorrhizal and fleshy roots with adventitious buds. The annual floral axes arise from adventitious buds on the perennial roots and carry the laminar appendages (there are no flowers in their axils, but they are above the soil level), which are termed sterile bracts (in our study, bracts, for simplicity) [4].
Plants were dissected into flowers, bracts, fleshy roots with adventitious buds, and predominantly haustoria-enriched roots, immediately frozen and homogenized in liquid nitrogen, and stored at −80°C. Total RNA was isolated from tissue of each M. hypopitys bracts (two individual plants), flowers (two individual plants), roots containing buds (individual plant), and haustoria-enriched roots (individual plant) and used for mRNA library preparation, which was sequenced on the Illumina HiSeq2500 platform (Illumina Inc., San Diego, CA, USA). The M. hypopitys RNA-seq data for each of six transcriptomes were assembled into the 98,350 unigenes with a length of 201–12,993 bp [51, 53]. Individual reads were mapped on contigs using Bowtie 2 [54], and protein-coding genes in contigs were identified using TransDecoder (https://transdecoder.github.io/).
2.2. Identification and Bioinformatics Characterization of M. hypopitys YABBY-Coding Sequences
To identify the M. hypopitys genes homologous to the known organ polarity genes, we searched unique transcripts revealed by the RNA-seq against the NCBI database (http://blast.ncbi.nlm.nih.gov/). To predict YABBY transcripts in M. hypopitys, we additionally searched the assembled transcriptomes with the known YABBY-related sequences coding for conserved zinc-finger and HMG-like domains extracted from the NCBI database. Selected YABBY candidates were examined for open reading frames (ORFs), translated using Clone Manager v.7.11 (http://clone-manager-professional.software.informer.com/), and the conserved domains of putative MhyYABBY proteins were identified using the NCBI-CDD analyzer (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and specified according to [21].
To evaluate the overlap between transcriptomes, Venn diagrams were generated using the online program Venny [55]. To illustrate the transcriptome-based gene expression pattern, the data were clustered with the Average linkage method and Spearman rank correlation (distance measurement method), and visualized as a heat map (http://www2.heatmapper.ca/) [56].
Conserved MhyYABBY amino acid motifs were identified using the MEME (Multiple Expectation Maximization for Motif Elicitation) 4.11.2 online analysis (http://meme-suite.org/tools/meme) [57] and used to construct a schematic diagram. To search for motifs, the “Normal” motif discovery mode, the default width range of 6–50 amino acids (aa), and the motif site distribution “zero or one per sequence” were used. The identified motifs were manually compared with previously suggested specific motifs. Initial search was performed on a set of 34 complete sequences, including identified MhyYABBYs, independently of YABBY clade affiliation. In addition, variable regions between conserved domains within the same group of proteins were searched. Since most of the identified motifs were clade-specific, we further analyzed individual YABBY clades.
To investigate the evolutionary relationship of the MhyYABBY genes, the MhyYABBY proteins and YABBY homologs from other species available in NCBI were aligned using ClustalX [58]. For analysis, full-size amino acid sequences, as well as conserved regions consisting of the zinc-finger and HMG-like domains were used. Evolutionary divergence between the genes and proteins was estimated using the maximum composite likelihood and equal input models, respectively, in MEGA7 [59–62]. The phylogenetic tree topology was estimated using the maximal likelihood method based on the JTT matrix-based model in MEGA7 [62].
2.3. Analysis of Tissue-Specific Gene Expression
The MhyYABBY gene expression was calculated in each transcriptome. Transcript quantification based on RNA-seq data was performed without a reference genome using the RSEM [63] and Bowtie 2 [54] programs, including normalization of transcripts per kilobase of exon per million fragments mapped (FPKM) values and between samples.
To perform quantitative real-time PCR (qRT-PCR), the first strand cDNA was synthesized from 1 μg of each mixture of two-root, two-bract, and two-flower RNA preparations using the Reverse Transcription System (Promega, Madison, WI, USA) and an oligo-dT primer, and quantified using the Qubit® Fluorometer.
Based on the identified YABBY-like transcripts and corresponding draft genomic sequences (our unpublished data), gene-specific primers separated by at least one big intron were designed to amplify parts of gene-coding sequences (Supplementary Table 1). The qRT-PCR was performed in three technical replicates using 2.5 ng of cDNA and an SYBR Green and ROX RT-PCR mixture (Syntol, Moscow, Russia) at the following cycling conditions: initial denaturation at 95°C for 5 min, 40 cycles of denaturation at 95°C for 15 sec, and annealing/synthesis at 60°C for 40 sec. Obtained PCR-fragments were additionally purified and sequenced to confirm certain gene specificities. Gene expression levels were normalized to those of the reference pinesap Actin5, Actin3, and SAND genes (primers are provided in Supplementary Table 1), which transcripts were evenly represented in six transcriptomes [51]. Normalized expression data were statistically evaluated using GraphPad Prism version 7.02 (San Diego, CA, USA; https://www.graphpad.com/scientific-software/prism/). Three values (3 technical replicates) were used for SD calculation. The error bars were generated based on mean with SD calculation. Significance of the qRT-PCR data within the same tissue between species was estimated by unequal variance Welch's t-test and additionally treated with Bonferroni's correction: if any of the t-tests in the list had p ≤ 0.05/number of t-tests in the list, then the null hypothesis was rejected; that is, the difference between samples was recognized as significant.
3. Results
3.1. M. hypopitys Organ-Polarity Genes
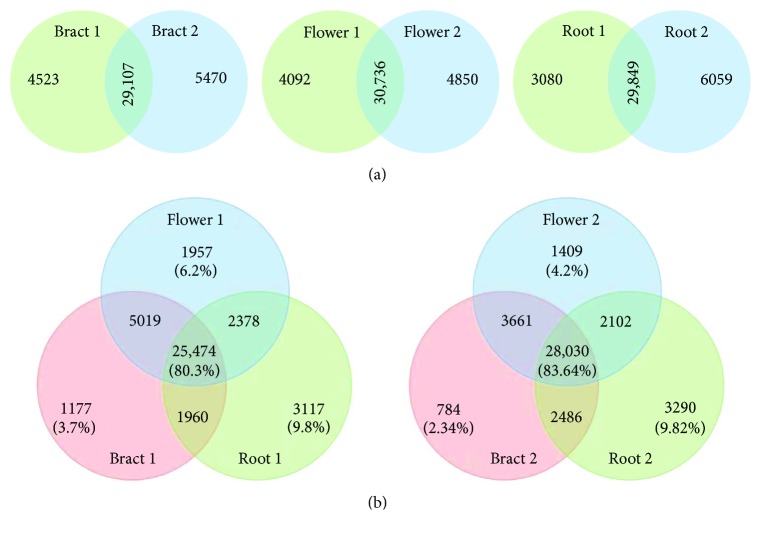
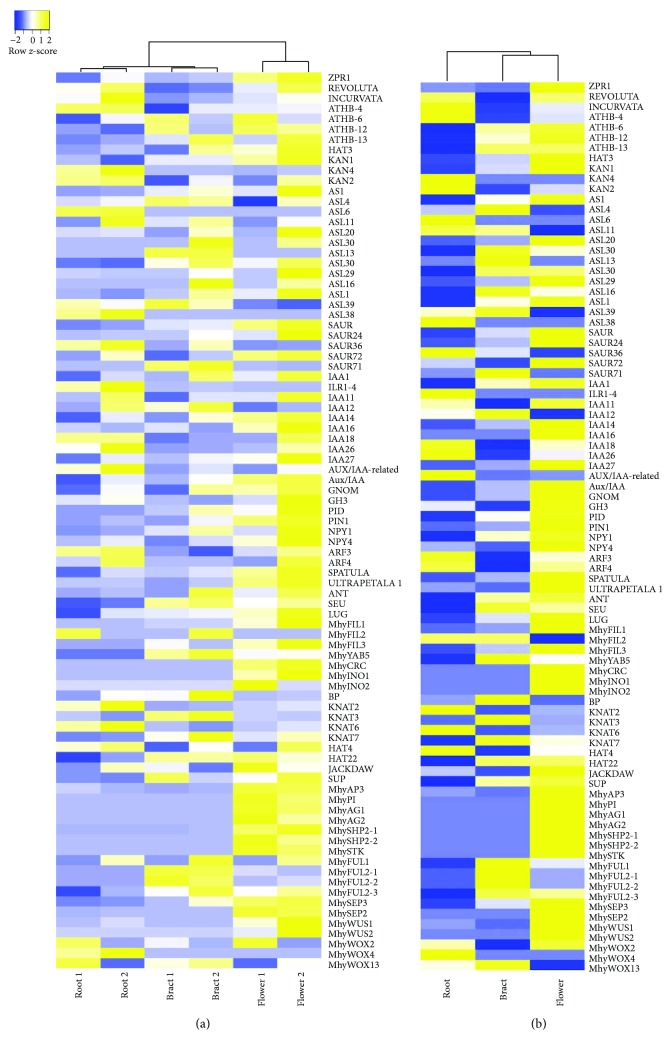
The obtained six transcriptomes [53] showed significant overlap between the whole set of reads of paired libraries (74–77%), and in three libraries for each of the two plants (>80%) (Figure 1), and reflected a number of the known plant organ polarity genes that are being expressed in different tissues of flowering pinesap (Supplementary Table 2). Among them, the genes of the KAN and REV transcription factors, responsible for the abaxial and adaxial cell identity in lateral organs, respectively, and their common targets (ARF, SAUR, Aux/IAA, PID, PIN, NPY, GH3, etc.) involved in auxin biology [17] were identified. The genes encoding SEUSS (SEU) and LEUNIG (LUG) involved in petal polarity determination along the adaxial/abaxial axis [64], AP2-like transcription factor AINTEGUMENTA (ANT) which contributes to organ polarity [65], the ADP ribosylation factor guanine nucleotide exchange factor GNOM essential for basal polarity establishment in A. thaliana [66], ULTRAPETALA1 (ULT1) which acts antagonistically with KAN1 to pattern the adaxial-abaxial polarity axis but jointly to pattern the apical-basal axis restricting the expression domain of the SPATULA gene [67], and HD-ZIPIII transcription factors HAT and ATHB positively regulated by REV [68] were also found (Supplementary Table 2). Heat map-based clustering of transcriptomic reads associated with organ polarity genes revealed similarities between pair libraries (according to the column dendrogram in Figure 2(a)). For most genes, the expression levels in flowers were higher than those in bracts (Figure 2(b)).
Figure 1.
Venn diagrams of the overlap between the whole set of reads in paired transcriptomes (a) and in three transcriptomes for each of the two plants analyzed (b).
Figure 2.
Heat maps of the organ polarity gene expression in six transcriptomes (a) and the mean expression of these genes in paired transcriptomes (b).
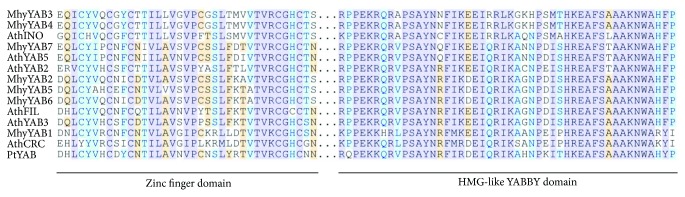
All six transcriptomes of bracts, flowers and roots contained seven unique YABBY-like transcripts, which were considered as putative MhyYABBYs (MhyYAB1–MhyYAB7). The size of putative MhyYABBY ORFs varied from 502 to 673 bp, and the length of predicted proteins was from 166 to 224 aa. One transcript, MhyYAB4, had a partial 3′-truncated coding sequence (CDS), while the remaining six mRNAs contained complete CDSs. Putative MhyYABBY proteins included both a conserved N-terminal 37-aa C2C2 zinc finger-like domain and a C-terminal 48-aa helix-loop-helix domain resembling a part of an HMG box (Figure 3) [21, 33]. A cluster of amino acids at the beginning of the HMG-like domain could potentially serve as a nuclear localization signal [33, 69].
Figure 3.
Alignment of conserved zinc-finger and HMG-like domains from putative M. hypopitys YABBY proteins 1–7, known as A. thaliana YABBYs (AthINO, AF195047; AthCRC, AF132606; AthFIL, AF136538; AthYAB3, AF136540; AthYAB5, NP_850081; AthYAB2, AF136539), and Pinus taeda PtYAB (DR100835; gymnosperms).
Among MhyYABBYs, we distinguished two groups (comprising MhyYAB2, MhyYAB5, and MhyYAB6; and MhyYAB3 and MhyYAB4) based on high identity outside the conserved domains (Supplementary Figures 1a, 1b). The estimated low evolutionary pairwise divergence in the nucleotide and amino acid sequences between the members of each group compared to that between the members of different groups suggested the presence of two sets of paralogs (Supplementary Tables 4 and 5). MhyYAB1 showed high pairwise divergence with other MhyYABBYs, which indicates the affiliation of MhyYAB1 to the separate clade.
3.2. Phylogeny of the M. hypopitys YABBY Family
Previous phylogenetic studies considered only conserved YABBY domains; however, it has also been shown that variable protein regions contain clade-specific conserved motifs of potential functional importance [29]. In transcription factors, variable regions are often essential for their activity and/or formation of multimeric protein complexes, as it has been shown for MADS-box transcription factors [70]. We aligned complete amino acid sequences and only conserved domains of MhyYABBYs. For the group comprising MhyYAB2, MhyYAB5, and MhyYAB6 paralogs, two different results within the same clade were obtained. The full-size proteins were orthologous to FIL, while the conserved domains were orthologous to YAB3, another member of the FIL/YAB3 clade. We decided to use complete sequences to increase the sensitivity of phylogenetic analysis.
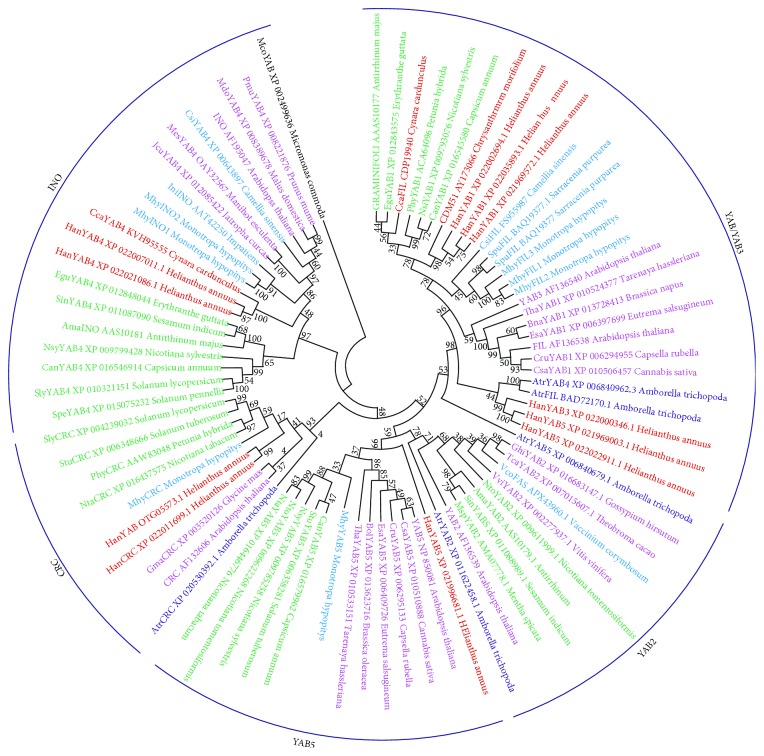
The generated tree, rooted with the Micromonas commode (Chlorophyta) YABBY-like protein, classified MhyYABBY1–7 by comparing them with YABBY-like proteins of A. thaliana and other angiosperm species belonging to Asterids and Rosids. As a result, MhyYABBY transcripts were distributed into the FIL, INO, CRC, and YAB5 clades and renamed accordingly as MhyCRC (MhyYAB1), MhyINO1 and MhyINO2 (MhyYAB3 and MhyYAB4, resp.), MhyYAB5 (MhyYAB7), and MhyFIL1, MhyFIL2, and MhyFIL3 (MhyYAB5, MhyYAB6, and MhyYAB2, resp.). It should be noted that no YABBY2-like transcripts were found in all six transcriptomes. The sequences have been deposited in GenBank (KX12839–KX12841, KX12843–KX12846; Supplementary Table 3). The maximum likelihood reconstruction of the MhyYABBY family with bootstrap values at tree nodes is shown in Figure 4. Within the individual clades, MhyYABBY sequences are sisters to other YABBY-like sequences from Asterids, and the closest homologs are YABBYs from Ericales.
Figure 4.
A phylogenetic tree of MhyYABBY proteins was generated with 83 full-size YABBY-like amino acid sequences from M. hypopitys, A. thaliana, and other angiosperm species. The M. commode (Chlorophyta) YABBY-like protein was used as outgroup. The Amborella trichopoda, Ericales, Rosids, Lamiids (euAsterids), and Campanulids (euAsterids) sequences are colored in dark blue, blue, burgundy, green, and red, respectively. The numbers next to the nodes represent bootstrap values from 1000 replicates.
3.3. MhyYABBY-Specific Motifs Identified outside of the YABBY Domains
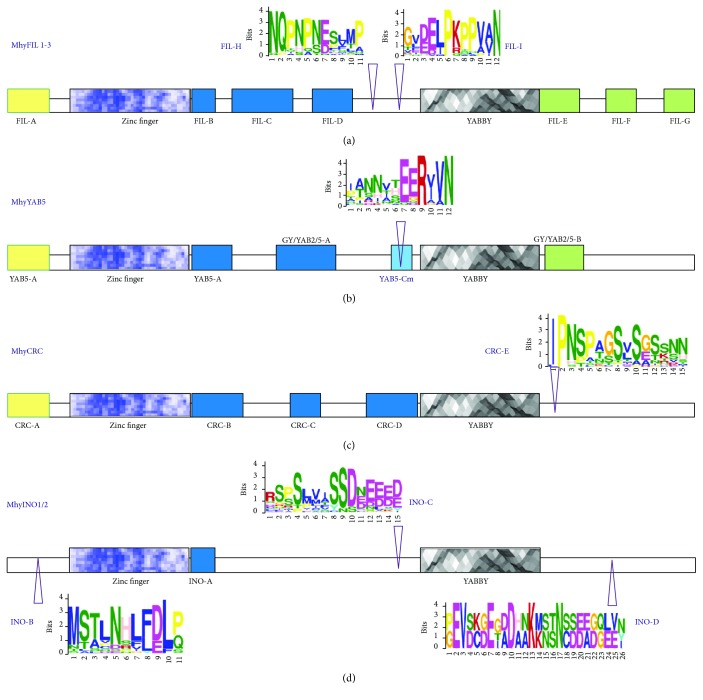
To further investigate the structural divergence of pinesap YABBY proteins, we searched for the motifs conserved within individual clades or the whole MhyYABBY group. Comparison of complete sequences of YABBY orthologs (Supplementary Table 6) revealed two major domains, zinc-finger and YABBY, specific to all YABBY proteins. The number of motifs in variable regions was 4 to 9 with the length from 5 to 26 residues. The obtained data were compared with previously defined motifs specific to individual YABBY clades [29].
In MhyINO1/2, the known INO-A motif was found immediately after the zinc-finger domain. Comparison of MhyINO1/2 with other INO-like sequences in the NCBI database revealed novel putative INO-specific motifs. At the N-terminus and between the zinc-finger and YABBY domains, a highly conserved 11-aa INO-B motif and an Eudicot-specific 15-aa INO-C motif were identified. In addition, we found a C-terminal 26-aa INO-D motif specific to MhyINO1/2 and INO-like proteins in Solanaceae. All previously predicted CRC-specific motifs, CRC-A, CRC-B, CRC-C, and CRC-D, were found in MhyCRC. In addition, we identified a putative conserved Eudicot-specific C-terminal 15-aa CRC-E motif. In MhyYAB5, the presence of the known YAB5-specific motifs YAB5-A, GY/YAB2/5-A, YAB5-B, and GY/YAB2/5-B was confirmed. The YAB5-D motif was not detected, while a modified YAB5-C sequence was identified before the YABBY domain as a 12-aa YAB5-Cm motif. In MhyFIL1/2/3, we found all previously predicted motifs, FIL-A, FIL-B, FIL-C, FIL-D, FIL-E, FIL-F, and FIL-G. In addition, we suggested two motifs, 12-aa FIL-I (monocot- and eudicot-specific) and 11-aa FIL-H (eudicot-specific) located between the zinc-finger and YABBY domains, as candidates for conserved FIL-specific motifs.
Thus, we characterized MhyYABBY proteins by sequence conservation on the clade level. The motifs identified in MhyYABBYs were shown in Figure 5 and sequences of the predicted novel motifs are presented in Supplementary Figure 2).
Figure 5.
Amino acid clade-specific motifs predicted in M. hypopitys YABBY proteins. MhyFIL1/2/3 (a); MhyYAB5 (b); MhyCRC (c); MhyINO1/2 (d). Previously suggested clade-specific motifs [29] are shown as boxes. MEME-predicted novel motifs are marked by arrowheads and shown as letter sequences.
3.4. Expression Pattern of “Vegetative” and Flower-Specific M. hypopitys YABBY Genes
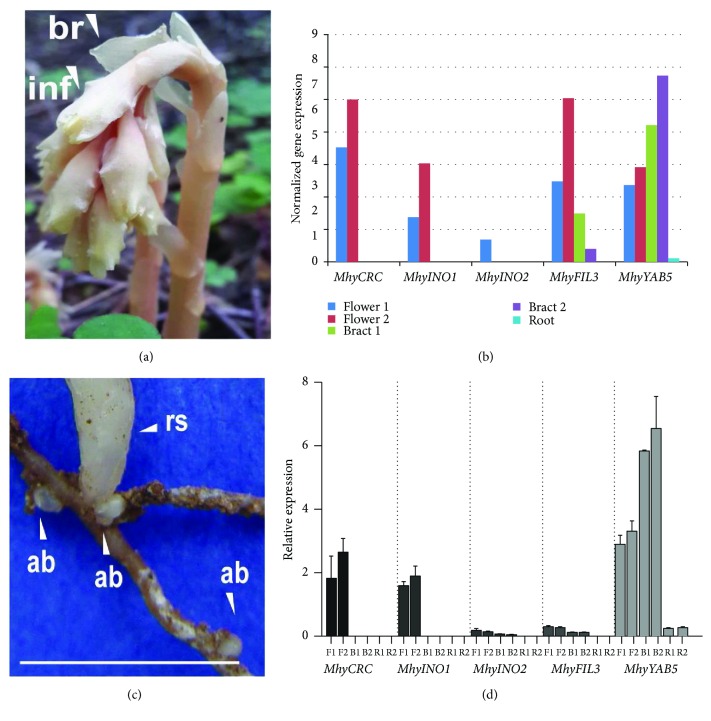
The transcriptome-based data on MhyYABBY expression in the roots and buds, haustoria-enriched roots, bracts, and flowers (Figure 6, Supplementary Table 3) showed that the MhyYABBY mRNAs (except MhyFIL2) were present in the flowers. Except for INO- and CRC-like MhyYABBYs, all other five transcripts were detected in the bracts. The highest bract-specific expression was observed for MhyYAB5, while the remaining four genes were transcribed at similarly low levels. Finally, in roots and buds, only MhyYAB5 and MhyFIL2 mRNAs were expressed at very low levels, while in the haustoria-enriched roots none of genes were expressed. The MhyFIL1 and MhyFIL3 transcripts were increased from the bract to the flower, maintaining the same expression profile. In contrast with this, the number of MhyYAB5 transcripts was decreased from the bract to the flower.
Figure 6.
Expression profiles of M. hypopitys YABBY genes in the bract, flower, root and bud, and haustoria-enriched root tissues. Expression was estimated as the transcript number per million equal to the sum of total transcripts in the tissue. The M. hypopitys adult plant; br—bract, fl—flower (a); transcriptome-based expression pattern of the MhyFIL1, MhyFIL2, MhyFIL3, MhyYAB5, MhyCRC, MhyINO1, and MhyINO2 genes in pinesap tissues (b); the M. hypopitys roots with adventitious buds; ab—adventitious bud, rs—growing reproductive stem; scale bar = 1 cm (c); relative expression (qRT-PCR) of the pattern of the MhyCRC, MhyINO1, MhyINO2, MhyFIL3, and MhyYAB5 genes in pinesap tissues, F—flower, B—bract, R—root (d).
Quantitative (q) RT-PCR data on MhyFIL3, MhyYAB5, MhyINO1, MhyINO2, and MhyCRC expression are represented at Figure 6(d) and Supplementary Table 7. The relative expression of MhyYABBY genes was estimated in the flowers, bracts, and roots and buds. All analyzed genes were expressed in the flowers with the highest MhyYAB5 level, and the lowest MhyINO2 and MhyFIL3 levels. In the bracts, the MhyYAB5 gene was also highly expressed, but only traces of the MhyINO2 and MhyFIL3 mRNAs were observed. In the roots and buds, the only MhyYAB5 mRNA was detected at low level. The difference in the MhyYAB5 gene expression between the pinesap tissues was statistically significant. The flower-specific expression of MhyCRC and MhyINO1 was significantly different from that in bracts and roots. The expression of MhyINO2 and MhyFIL3 was similar between tissues (Supplementary Table 7). All the analyzed gene expression modes were the same as it was shown in their transcriptome-based patterns, except for MhyINO2. In the flower, the measured qRT-PCR expression of this gene was equally low. Given transcriptomic data, MhyINO2 was absent in flower 2, which may be due to the quality of the libraries or their sequencing. Also, considering the low evolutionary pairwise divergence in the INO1 and INO2 sequences, a higher level of INO1 expression compared to the level of INO2 may indicate that INO1 may be more required than INO2 during plant development.
4. Discussion
The emergence of photosynthesis has become the most significant event in the evolution of plants. The majority of extant plants are autotrophic, except for about 1% of flowering heterotrophic plants. Among the latter, obligate mycoheterotrophs are the results of replicated deevolutionary events of the photosynthetic ability loss, triggering the degradation of both cytoplasmic and nuclear genomes [71]. Full mycoheterotrophs demonstrate a wide range of deevolutionary outcomes such as abrupt morphophysiological changes [2], genome rearrangements, and massive gene loss [71, 72].
In the large and diverse eudicot family Ericaceae with a nearly worldwide distribution, two of nine subfamilies, Pyroloideae and Monotropoideae, contain partial and full mycoheterotrophs, respectively [73]. In Monotropoideae, M. hypopitys represents a unique obligate mycoheterotroph. Recent studies on the M. hypopitys plastid genome and its comparison with that of photosynthetic relative Pyrola rotundifolia indicated that this plant is at the final stages of plastome degradation, which is expressed in highly reduced size and content, dramatic structural rearrangements, and acceleration of nucleotide substitutions in all protein-coding genes [74–76]. Furthermore, the coordinated loss of photosynthesis-related functions in both plastome and nuclear genomes of M. hypopitys is a sign of ongoing changes in the nuclear genome of this mycoheterotrophic plant [51].
It is generally accepted that mycoheterotrophic plants have evolved from photosynthetic mycorrhizal lineages, as mycoheterotrophy helps to succeed in the low-light conditions of the forest [77]. It has been established that dark-induced leaf senescence leads to a significant chlorophyll loss and photosynthesis inactivation [78, 79]. During evolution, a M. hypopitys ancestor (already with megaphylls) growing in shaded habitats lost the genetic ability to photosynthesize due to symbiosis with fungi, which provided a sufficient amount of carbon to pinesap from the roots of autotrophic trees. It is shown that the loss of photosynthetic ability and full heterotrophy are linked to the degradation and/or modification of vegetative structures [77]. It is believed that in M. hypopitys, an elongated raceme emerges instead of a true stem, developing directly from the adventitious bud on the roots [4].
The photosynthetic ability is closely related to the origin of asymmetrical leaves (providing the absorption of sufficient light energy by seed plants [80, 81]), in particular, due to the YABBY genes' evolutionary duplication and diversification [6–9, 25, 82, 83]. Although the role of the YABBY genes in plant evolutionary adaptation to light perception is established and they have been systematically studied in model and nonmodel species [27, 84–87], up to now, no YABBY genes have been described in mycoheterotrophic plants. It was interesting to figure out, if these genes and, therefore, the conserved mechanism of leaf polarity determination, were exposed to the adaptive deevolution in leafless mycoheterotroph M. hypopitys. Therefore, in this study we focused on the diversity and expression profile of the YABBY genes in a M. hypopitys that may further the understanding of the development and evolution of this plant group.
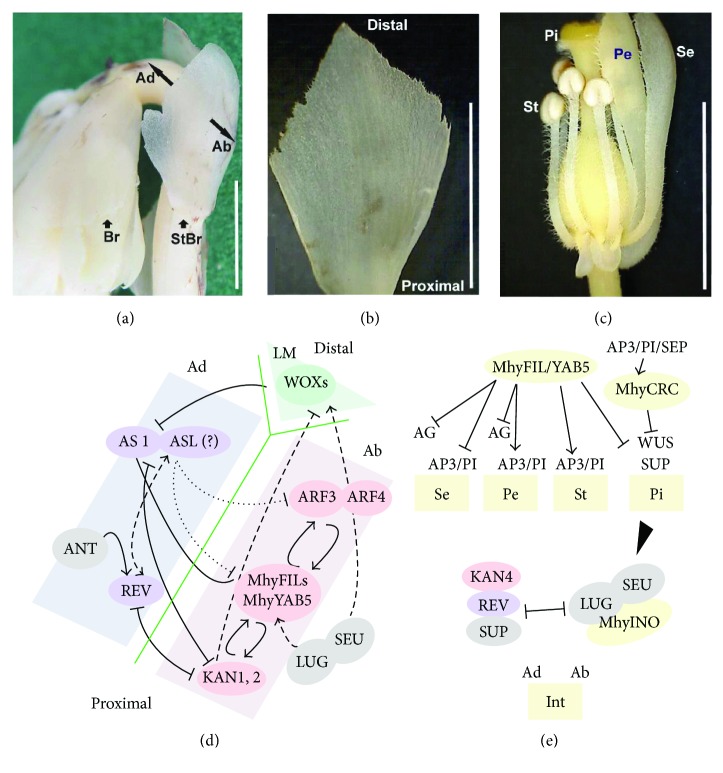
Peripheral cells of the shoot apical meristem give rise to the leaves that develop along three axes and acquire the adaxial-abaxial and proximal-distal asymmetry and the mediolateral symmetry [88]. The elongated M. hypopitys raceme carries bracts below flowers and leaf-like sterile bracts [4]. Bracts are thin, 8–15 mm long, 3–15 mm broad, ovate, and expanding to the top, with irregularly toothed margins [4], rudimentary midvein, and parallel veins of similar thickness (Figures 7(a) and 7(b)). Interestingly, sterile bracts are not exactly leaves as they have the genetic signatures of reproductive organs, which are manifested in the expression of the floral organ identity MADS-box genes [89] (Figure 2).
Figure 7.
A hypothetical scenario for the functioning of the MhyYABBYs during the development of M. hypopitys bracts and flowers. (a) M. hypopitys raceme. (b) M. hypopitys sterile bract. (c) M. hypopitys flower with a partially removed perianth. (d) Scheme of possible relations between “abaxial” and “adaxial” factors in the M. hypopitys bracts. (e) Scheme of possible interactions of MhyYABBYs in the M. hypopitys flowers. Br—bract, StBr—sterile bract, Ad—adaxial side, Ab—abaxial side, LM—leaf margin, Se—sepal, Pe—petal, St—stamen, Pi—pistil, Int—integument. Scale bar = 0.5 cm.
The lack of leaves in M. hypopitys may correlate with possible changes in the conserved genetic network of lateral organ polarity. In pinesap transcriptomes, we found the number of genes associated with this network, including seven YABBY-like sequences encoding proteins, which contain conserved domains and nuclear localization signals characteristic for YABBY transcription factors.
Initially, “adaxial” and “abaxial” genes are expressed throughout the leaf primordium, and, as the leaf develops, their expression becomes restricted to their respective domains due to the mutually exclusive actions of their protein products [90]. YABBYs are “abaxial” genes involved in stimulation of the cellular division during lamina outgrowth in all aboveground lateral organs, vegetative or reproductive (e.g., [25, 91–93]). M. hypopitys flowers do not show any visible abnormalities compared to those of other eudicots. Thence, MhyYABBYs may play common roles in the proper development of the floral meristem into a mature flower. However, the question arises how did the lack of leaves affect the function of the “vegetative” MhyFIL1–3 and MhyYAB5 genes.
In A. thaliana leaves and sepals, FIL and YAB3 genes are upregulated by KAN1 and ARF4, and, in turn, FIL and YAB3 stimulate the expression of ARF4, KAN1, and AS1 [92, 94, 95], and besides, in complex with LUG and SEU promote not only organ polarity, but embryonic shoot apical meristem initiation and maintenance [93]. “Abaxial” KAN represses the “adaxial” HD-ZIPIII genes [90, 96, 97]. At the boundary between adaxial and abaxial tissues, the FIL/YAB3 and KAN, respectively, up- and downregulate the WOX1 and WOX3 genes that specify redundantly lateral lamina outgrowth and leaf margin cell fate [98]. The YAB5, KAN2, ARF3, and ARF4 genes are repressed by the “adaxial” AS1 and AS2 implicated in the proper leaf formation along all three axes [99–101].
During flowering, YABBYs are required to establish a correctly developed flower primordium through the interaction with REV, KAN4, SEU, LUG, ANT, SUP, LFY, and the floral homeotic MADS-box genes [28, 47, 64, 65, 91, 93]. The SEU and LUG are needed to promote and maintain the FIL/YAB3 and HD-ZIPIII expression [64]. In turn, FIL in combination with ANT acts to upregulate the “adaxial” gene PHB and MADS-box gene AP3 [65], and together with REV, AP1, and LFY, spatially regulates the transcription of the SUP and the MADS-box genes AG, AP3, and PI [31, 44, 47]. To maintain the polar development of the ovule outer integument, the INO interacts with SEU and LUG, but its expression is restricted by KAN4, REV, and SUP [28]. CRC, upregulated by AP3/PI/SEP, is involved in the control of radial and longitudinal gynoecium growth, carpel fusion, and nectary location, and participates in the floral meristem termination through the WUS repression [33, 35, 36, 102].
The finding of almost all the above-described polarity genes in the analyzed transcriptomes (Supplementary Table 2; Figure 2) suggests that the polarity of M. hypopitys bracts and floral organs is under the control of conserved mechanisms (Figure 7), with the exception of the absence of transcripts PHB and PHV (HD-ZIPIII), WOX1 and WOX3 (homeodomain protein), and AS2 (LBD domain transcription factor). The PHB and PHV function may be replaced by another member of the HD-ZIPIII family, REV, since these three genes can function redundantly [90]. Similarly, the MhyWOX genes may perform the functions of WOX1 and WOX3 [103]. Although 12 AS2-like (ASL) genes were found in M. hypopitys transcriptomes, the AS2 cannot be functionally replaced by other family members [104]. The lack of AS2 transcripts may contribute to a high level of MhyYAB5 expression compared to other “vegetative” MhyYABBYs, since the AS1/AS2 complex suppresses YAB5 [88]. Moreover, the lack of AS2 activity may be related to the characteristics of M. hypopitys bracts having a plump lamina base, the midvein indistinguishable from parallel veins, and the absence of petioles. This conclusion may be supported by the phenotype of the mutant as2, which has a significantly rudimentary leaf midvein (and several parallel veins of very similar thickness), shortened petioles and leaf blades, and a plump and swelled leaf lamina base [105].
Most of the genes that define organ polarity existed before the emergence of a flat leaf in seed plants. Based on the analyses of the families of organ polarity genes, such as HD-ZIPIII [106], ARFs [23], and ASLs [107], it is assumed that after the ferns' divergence, multiple paralogs arose in the seed-plant common ancestor [23]. Unlike other polarity genes, YABBYs originated in the lineage leading to seed plants, and it is proposed that they are implicated in the transition of an ancestral shoot-specific network into a leaf-specific one [19, 25]. At least four gene duplication events in the YABBY family led to the emergence of at least five YABBY genes with both novel and redundant functions in the last common ancestor of extant flowering plants [23, 29].
The structural and phylogenetic analysis based on comparison with the YABBY orthologs revealed that each MhyYABBY belonged to one of the four highly conserved clades in the angiosperm YABBY family [94]. In the dendrogram, it is possible to single out a cluster consisting of the FIL/YAB3, YAB5, and YAB2 clades (Figure 3). CRC- and INO-orthologs have formed separate clusters, which corresponds to the previously proposed origin of the CRC and INO genes from different ancestors [20, 23]. The tree composition was not completely congruent with the data of other studies with observations of the two clusters CRC/FIL and YAB5/YAB2/INO [23], or the two clusters FIL/CRC/INO and YAB2/YAB5 [20, 29], probably due to the inherent instability of the tree topology depending on the composition of taxa and the mode of analysis.
The obtained tree was consistent with the established phylogenetic relationships among higher plants. The presence of the MhyYABBY paralogs, which are coorthologous to the FIL and INO clades, indicates that the MhyFIL1/MhyFIL2/MhyFIL3 and MhyINO1/MhyINO2 groups could represent allelic variants (for FIL group), alternative splicing variants (for INO group), or may have originated as a result of a recent gene duplication event unique to the Ericales order.
The simplest explanation of the absence of the YABBY2-like transcripts in all analyzed pinesap transcriptomes may be the low abundance and insufficient transcriptome size. It is also possible that YAB2 homologous genes are expressed at the earlier developmental stages during the formation of lateral organ primordia, which were not analyzed. One of three possible evolutionary scenarios explaining the YABBYs' diversification suggests that YAB2 is the result of the earliest duplication of the YABBY ancestor gene [20], and therefore, can be associated with the evolution of a leaf stronger than other YABBYs. The YAB2 ortholog is present in photosynthetic Ericaceae relative species Vaccinum corymbosum. Hence, the absence of the YAB2 gene in M. hypopitys may be due to the loss of the gene during the adaptive evolution (deevolution of the genome) of the autotrophic ancestor of M. hypopitys accompanied by the loss of the leaf. MhyYAB2 functions could be partially complemented through neofunctionalization of MhyYAB5 or MhyFIL paralogs. Studies in Oryza sativa and other plants suggest that within the multicomponent regulatory network composed of homo- and heterodimers formed by “vegetative” FIL/YAB3, YAB2, and YAB5 orthologs, protein substitutions and replacements are possible [29, 108]. It was shown that in O. sativa the loss of the YAB5 genes was complemented by the FIL and YAB2 paralogs [108]. Accordingly, M. hypopitys MhyYAB2 could have been replaced by the MhyYAB5 or the three MhyFILs. It is also known that YAB2 and YAB5 are important for laminar style and filament morphology evolution in angiosperms, when YAB2 expression over a certain threshold disturbs the balance in the regulatory network, leading to radialization of the laminar structure [26, 27]. Therefore, given the M. hypopitys style and filament radial structure and the expression of the MhyYAB5 gene in flower tissue, the absence of MhyYAB2 transcripts may also indicate a possible substitution of YAB2 by YAB5 in M. hypopitys.
Bioinformatics analysis of the MhyYABBY structural organization revealed the presence of 18 previously known conserved motifs [29] and 7 putative novel candidate sequences for clade-specific motifs (Figure 5), which may be used as markers to identify appropriate genes. Given the shared evolutionary history of YABBYs, the novel motifs could be biologically relevant and involved in subfamily-specific functions, which need further investigation.
The MhyYABBY gene orthology data are supported by the MhyYABBY expression patterns (Figure 6). In Arabidopsis, “vegetative” genes FIL, YAB3, and YAB5 are expressed in leaves and leaf-like cotyledons, sepals, petals, stamens, and carpels, whereas expression of CRC and INO is restricted to specific floral organs that are evolutionarily derived from leaves [20]. MhyYABBY transcripts were also found in aboveground tissues (bracts and flowers). In the case of the MhyYAB5 gene, its atypical expression in roots indicates that it may have some roles in the development of the M. hypopitys root system. On the other hand, the perennial plant M. hypopitys commonly develops underground adventitious buds on the roots, which presumably contain an embryonic inflorescence [109], and, thus, the MhyYAB5 gene can be expressed in the buds. Interestingly, in bracts, the expression level of MhyYAB5 is much higher than that of MhyFILs. Given the possible synergy of their action, it can be assumed that the low expression of MhyFILs was compensated by an increase in the expression of the MhyYAB5 gene not only in bracts, but also in roots, or rather in adventitious buds.
The MhyFIL expression profiles indicate the possible subfunctionalization of the paralogs. The MhyFIL3 gene, which according to phylogenetic analysis is at the base of the M. hypopitys FIL clade, is expressed approximately at the same level as MhyFIL1, while the extremely low number of transcripts of the third paralog MhyFIL2 is present only in two of the six transcriptomes (Supplementary Table 3). It is possible that MhyFIL3 and MhyFIL1 may have redundant functions, and MhyFIL2 may be a pseudogene. Given trace amounts of MhyFIL2 transcripts in the root and bud library, similar to MhyYAB5, it is likely that MhyFIL2 may be involved in the development of inflorescence at the early stages after bud dormancy release [110].
The MhyCRC expression was detected only in flower tissue, confirming its potentially conserved roles in carpel fusion, style/stigma and nectary development, and in the floral meristem termination as it was shown for A. thaliana CRC [33, 38, 102], as well as in vascular development, as indicated by a recent report on the functional role of Pisum sativum CRC [111]. Similar to A. thaliana INO [40, 112], MhyINO1 and MhyINO2 may redundantly define and promote the outer ovule integument growth in M. hypopitys, while MhyFIL1/2/3 and MhyYAB5 may influence the abaxial cell fate in all aboveground lateral organs like their corresponding YAB1/3 and YAB5 orthologs [23].
It has recently been shown that “vegetative” YABBYs act as transcriptional activators of jasmonate-triggered responses. Jasmonate-induced degradation releases YABBYs from complexes with JAZ3 to mediate anthocyanin accumulation and chlorophyll breakdown [113]. The analysis of M. hypopitys transcriptome data did not reveal JAZ3-like transcripts, which may be consistent with complete chlorophyll loss in M. hypopitys. Thus, such mechanism may become evolutionarily obsolete in mycoheterotrophic plants.
The current study is the first to identify the YABBY genes in a mycoheterotrophic plant devoid of vegetative leaf-like organs. Seven MhyYABBY members were detected and classified in M. hypopitys, and putative protein structure, conserved motifs, and phylogenetic relationship were systematically analyzed. MhyYABBY transcription profiling in different plant tissues indicated the involvement of MhyYABBY proteins in the regulatory network controlling bract and flower formation. Our findings should further the investigation of YABBY functional roles in the regulation of developmental and physiological processes in achlorophyllous plant species and help to reveal possible differences in generally conserved molecular mechanisms underlying plant development and evolution.
Acknowledgments
This work was supported by the Russian Science Foundation (Grant no. 14-24-00175) and was performed using the experimental climate control facility of the Institute of Bioengineering, Research Center of Biotechnology, RAS. The authors would like to thank Dr. Marina Chuenkova for providing language help.
Abbreviations
- CRC:
CRABS CLAW
- FIL:
FILAMENTOUS FLOWER
- HMG:
High Mobility Group
- INO:
INNER NO OUTER
- MhyYABBY:
M. hypopitys YABBY
- YAB:
YABBY
- qRT-PCR:
Quantitative RT-PCR.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
Konstantin G. Skryabin and Nikolay V. Ravin conceived and designed the research. Elena Z. Kochieva and Mikhail A. Filyushin provided the plant material. Andrey A. Mardanov, Elena Z. Kochieva, Marya A. Slugina, Mikhail A. Filyushin, Olga A. Shulga, and Anna V. Shchennikova performed the transcriptome sequencing and qRT-PCR experiment. Alexey V. Beletsky and Anna V. Shchennikova analyzed the data. Anna V. Shchennikova wrote the paper. All authors read and approved the manuscript.
Supplementary Materials
Tables 1–7: primers used for qRT-PCR analysis of pinesap gene expression (1); assembled transcripts homologous to the known organ polarity genes in six pinesap transcriptomes (2); MhyYABBY characteristics (3); estimates of evolutionary divergence between nucleotide sequences of the MhyYABBY genes (4); estimates of evolutionary divergence between amino acid sequences of the MhyYABBY proteins (5); set of YABBY orthologues from different plant species used for MEME-mediated identification of clade-specific conserved motifs (6); the significance (p-value) of qRT-PCR results for one gene expression between pinesap tissues (7) (in Microsoft Excel worksheet file (.xls)).
Figures 1-2: structural analysis of the MhyYABBY genes and encoded proteins. (1) Alignment of M. hypopitys YABBY genes (a) and encoded putative proteins (b); (2) novel amino acid motifs predicted in the sequence of the MhyYABBY proteins. (a)–(g) Logos created from aligned sequences and copied directly from MEME graphically represent amino acid conservation: FIL-H (a); FIL-I (b); YAB5-Cm (c); CRC-E (d); INO-B (e); INO-C (f); and INO-D (g). The height of the letters in each stack indicates the relative frequency of individual residues at the position (in portable document format (.pdf)).
References
- 1.Anderberg A. A., Rydin C., Kallersjo M. Phylogenetic relationships in the order Ericales s.l.: analyses of molecular data from five genes from the plastid and mitochondrial genomes. American Journal of Botany. 2002;89(4):677–687. doi: 10.3732/ajb.89.4.677. [DOI] [PubMed] [Google Scholar]
- 2.Leake J. R. The biology of myco-heterotrophic (“saprophytic”) plants. New Phytologist. 1994;127(2):171–216. doi: 10.1111/j.1469-8137.1994.tb04272.x. [DOI] [PubMed] [Google Scholar]
- 3.Devers E. A., Teply J., Reinert A., Gaude N., Krajinski F. An endogenous artificial microRNA system for unraveling the function of root endosymbioses related genes in Medicago truncatula . BMC Plant Biology. 2013;13(1):p. 82. doi: 10.1186/1471-2229-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace G. D. Studies of the Monotropoidiae (Ericaceae): taxonomy and distribution. The Wassman Journal of Biology. 1975;33:1–88. [Google Scholar]
- 5.Merckx V. S. F. T., Freudenstein J. V., Kissling J., et al. Taxonomy and classification. In: Merckx V. S. F. T., editor. Mycoheterotrophy. New York: The Biology of Plants Living on Fungi. Springer Science+Buisness Media; 2013. pp. 19–101. [Google Scholar]
- 6.Stewart W. N., Rothwell G. W. Paleobotany and the Evolution of Plants. New York: Cambridge University Press; 1993. [Google Scholar]
- 7.Cronk Q. C. B. Plant evolution and development in a post-genomic context. Nature Reviews. Genetics. 2001;2(8):607–619. doi: 10.1038/35084556. [DOI] [PubMed] [Google Scholar]
- 8.Bowman J. L., Eshed Y., Baum S. F. Establishment of polarity in angiosperm lateral organs. Trends in Genetics. 2002;18(3):134–141. doi: 10.1016/S0168-9525(01)02601-4. [DOI] [PubMed] [Google Scholar]
- 9.Beerling D., Fleming A. Zimmermann’s telome theory of megaphyll leaf evolution: a molecular and cellular critique. Current Opinion in Plant Biology. 2007;10(1):4–12. doi: 10.1016/j.pbi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Snow M., Snow R. The dorsiventrality of leaf primordia. New Phytologist. 1959;58(2):188–207. doi: 10.1111/j.1469-8137.1959.tb05351.x. [DOI] [Google Scholar]
- 11.McConnell J. R., Barton M. K. Leaf polarity and meristem formation in Arabidopsis . Development. 1998;125(15):2935–2942. doi: 10.1242/dev.125.15.2935. [DOI] [PubMed] [Google Scholar]
- 12.Lynn K., Fernandez A., Aida M., et al. The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development. 1999;126(3):469–481. doi: 10.1242/dev.126.3.469. [DOI] [PubMed] [Google Scholar]
- 13.Husbands A. Y., Chitwood D. H., Plavskin Y., Timmermans M. C. P. Signals and prepatterns: new insights into organ polarity in plants. Genes & Development. 2009;23(17):1986–1997. doi: 10.1101/gad.1819909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi T., Nukazuka A., Tsukaya H. Leaf adaxial-abaxial polarity specification and lamina outgrowth: evolution and development. Plant & Cell Physiology. 2012;53(7):1180–1194. doi: 10.1093/pcp/pcs074. [DOI] [PubMed] [Google Scholar]
- 15.Sun X., Feng Z., Meng L. Ectopic expression of the Arabidopsis ASYMMETRIC LEAVES2-LIKE5 (ASL5) gene in cockscomb (Celosia cristata) generates vascular-pattern modifications in lateral organs. Plant Cell, Tissue and Organ Culture (PCTOC) 2012;110(1):163–169. doi: 10.1007/s11240-012-0140-y. [DOI] [Google Scholar]
- 16.Huang T., Harrar Y., Lin C., et al. Arabidopsis KANADI1 acts as a transcriptional repressor by interacting with a specific cis-element and regulates auxin biosynthesis, transport, and signaling in opposition to HD-ZIPIII factors. The Plant Cell. 2014;26(1):246–262. doi: 10.1105/tpc.113.111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merelo P., Paredes E. B., Heisler M. G., Wenkel S. The shady side of leaf development: the role of the REVOLUTA/KANADI1 module in leaf patterning and auxin-mediated growth promotion. Current Opinion in Plant Biology. 2017;35:111–116. doi: 10.1016/j.pbi.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Meyerowitz E. M. Genetic control of cell division patterns in developing plants. Cell. 1997;88(3):299–308. doi: 10.1016/S0092-8674(00)81868-1. [DOI] [PubMed] [Google Scholar]
- 19.Floyd S. K., Bowman J. L. Gene expression patterns in seed plant shoot meristems and leaves: homoplasy or homology? Journal of Plant Research. 2010;123(1):43–55. doi: 10.1007/s10265-009-0256-2. [DOI] [PubMed] [Google Scholar]
- 20.Yamada T., Yokota S.’y., Hirayama Y., Imaichi R., Kato M., Gasser C. S. Ancestral expression patterns and evolutionary diversification of YABBY genes in angiosperms. The Plant Journal. 2011;67(1):26–36. doi: 10.1111/j.1365-313X.2011.04570.x. [DOI] [PubMed] [Google Scholar]
- 21.Bowman J. L. The YABBY gene family and abaxial cell fate. Current Opinion in Plant Biology. 2000;3(1):17–22. doi: 10.1016/S1369-5266(99)00035-7. [DOI] [PubMed] [Google Scholar]
- 22.Kanaya E., Nakajima N., Okada K. Non-sequence-specific DNA binding by the FILAMENTOUS FLOWER protein from Arabidopsis thaliana is reduced by EDTA. The Journal of Biological Chemistry. 2002;277(14):11957–11964. doi: 10.1074/jbc.M108889200. [DOI] [PubMed] [Google Scholar]
- 23.Finet C., Floyd S. K., Conway S. J., Zhong B., Scutt C. P., Bowman J. L. Evolution of the YABBY gene family in seed plants. Evolution & Development. 2016;18(2):116–126. doi: 10.1111/ede.12173. [DOI] [PubMed] [Google Scholar]
- 24.Liu H. L., Xu Y. Y., Xu Z. H., Chong K. A rice YABBY gene, OsYABBY4, preferentially expresses in developing vascular tissue. Development Genes and Evolution. 2007;217(9):629–637. doi: 10.1007/s00427-007-0173-0. [DOI] [PubMed] [Google Scholar]
- 25.Sarojam R., Sappl P. G., Goldshmidt A., et al. Differentiating Arabidopsis shoots from leaves by combined YABBY activities. Plant Cell. 2010;22(7):2113–2130. doi: 10.1105/tpc.110.075853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Almeida A. M. R., Yockteng R., Schnable J., Alvarez-Buylla E. R., Freeling M., Specht C. D. Co-option of the polarity gene network shapes filament morphology in angiosperms. Scientific Reports. 2014;4(1) doi: 10.1038/srep06194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morioka K., Yockteng R., Almeida A. M. R., Specht C. D. Loss of YABBY2-like gene expression may underlie the evolution of the laminar style in Canna and contribute to floral morphological diversity in the Zingiberales. Frontiers in Plant Science. 2015;6 doi: 10.3389/fpls.2015.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley D. R., Skinner D. J., Gasser C. S. Roles of polarity determinants in ovule development. The Plant Journal. 2009;57(6):1054–1064. doi: 10.1111/j.1365-313X.2008.03752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartholmes C., Hidalgo O., Gleissberg S. Evolution of the YABBY gene family with emphasis on the basal eudicot Eschscholzia californica (Papaveraceae) Plant Biology. 2012;14(1):11–23. doi: 10.1111/j.1438-8677.2011.00486.x. [DOI] [PubMed] [Google Scholar]
- 30.Worden A. Z., Lee J.-H., Mock T., et al. Green evolution and dynamic adaptations revealed by genomes of the marine picoeukaryotes Micromonas. Science. 2009;324(5924):268–272. doi: 10.1126/science.1167222. [DOI] [PubMed] [Google Scholar]
- 31.Sawa S., Watanabe K., Goto K., et al. FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes & Development. 1999;13(9):1079–1088. doi: 10.1101/gad.13.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golz J. F., Roccaro M., Kuzoff R., Hudson A. GRAMINIFOLIA promotes growth and polarity of Antirrhinum leaves. Development. 2004;131(15):3661–3670. doi: 10.1242/dev.01221. [DOI] [PubMed] [Google Scholar]
- 33.Bowman J. L., Smyth D. R. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development. 1999;126(11):2387–2396. doi: 10.1242/dev.126.11.2387. [DOI] [PubMed] [Google Scholar]
- 34.Eshed Y., Baum S. F., Bowman J. L. Distinct mechanisms promote polarity establishment in carpels of Arabidopsis . Cell. 1999;99(2):199–209. doi: 10.1016/S0092-8674(00)81651-7. [DOI] [PubMed] [Google Scholar]
- 35.Lee J. Y., Baum S. F., Oh S. H., Jiang C. Z., Chen J. C., Bowman J. L. Recruitment of CRABS CLAW to promote nectary development within the eudicot clade. Development. 2005;132(22):5021–5032. doi: 10.1242/dev.02067. [DOI] [PubMed] [Google Scholar]
- 36.Orashakova S., Lange M., Lange S., Wege S., Becker A. The CRABS CLAW ortholog from California poppy (Eschscholzia californica, Papaveraceae), EcCRC, is involved in floral meristem termination, gynoecium differentiation and ovule initiation. The Plant Journal. 2009;58(4):682–693. doi: 10.1111/j.1365-313X.2009.03807.x. [DOI] [PubMed] [Google Scholar]
- 37.Li H., Liang W., Hu Y., et al. Rice MADS6 interacts with the floral homeotic genes SUPERWOMAN1, MADS3, MADS58, MADS13, and DROOPING LEAF in specifying floral organ identities and meristem fate. Plant Cell. 2011;23(7):2536–2552. doi: 10.1105/tpc.111.087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun W., Huang W., Li Z., Lv H., Huang H., Wang Y. Characterization of a Crabs Claw gene in basal eudicot species Epimedium sagittatum (Berberidaceae) International Journal of Molecular Sciences. 2013;14(1):1119–1131. doi: 10.3390/ijms14011119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker S. C., Robinson-Beers K., Villanueva J. M., Gaiser J. C., Gasser C. S. Interactions among genes regulating ovule development in Arabidopsis thaliana . Genetics. 1997;145(4):1109–1124. doi: 10.1093/genetics/145.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villanueva J. M., Broadhvest J., Hauser B. A., Meister R. J., Schneitz K., Gasser C. S. INNER NO OUTER regulates abaxial-adaxial patterning in Arabidopsis ovules. Genes & Development. 1999;13(23):3160–3169. doi: 10.1101/gad.13.23.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juarez M. T., Twigg R. W., Timmermans M. C. P. Specification of adaxial cell fate during maize leaf development. Development. 2004;131(18):4533–4544. doi: 10.1242/dev.01328. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W., Su H. Y., Song J., Zhao X. Y., Zhang X. S. Ectopic expression of TaYAB1, a member of YABBY gene family in wheat, causes the partial abaxialization of the adaxial epidermises of leaves and arrests the development of shoot apical meristem in Arabidopsis . Plant Science. 2006;170(2):364–371. doi: 10.1016/j.plantsci.2005.09.008. [DOI] [Google Scholar]
- 43.Dai M., Hu Y., Zhao Y., Zhou D. X. Regulatory networks involving YABBY genes in rice shoot development. Plant Signaling & Behavior. 2007;2(5):399–400. doi: 10.4161/psb.2.5.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q., Atkinson A., Otsuga D., Christensen T., Reynolds L., Drews G. N. The Arabidopsis FILAMENTOUS FLOWER gene is required for flower formation. Development. 1999;126(12):2715–2726. doi: 10.1242/dev.126.12.2715. [DOI] [PubMed] [Google Scholar]
- 45.Cong B., Barrero L. S., Tanksley S. D. Regulatory change in YABBY-like transcription factor led to evolution of extreme fruit size during tomato domestication. Nature Genetics. 2008;40(6):800–804. doi: 10.1038/ng.144. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama H., Yamaguchi T., Tsukaya H. Expression patterns of AaDL, a CRABS CLAW ortholog in Asparagus asparagoides (Asparagaceae), demonstrate a stepwise evolution of CRC/DL subfamily of YABBY genes. American Journal of Botany. 2010;97(4):591–600. doi: 10.3732/ajb.0900378. [DOI] [PubMed] [Google Scholar]
- 47.Sawa S., Ito T., Shimura Y., Okada K. FILAMENTOUS FLOWER controls the formation and development of Arabidopsis inflorescences and floral meristems. Plant Cell. 1999;11(1):69–86. doi: 10.1105/tpc.11.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bidartondo M. I., Bruns T. D. Extreme specificity in epiparasitic Monotropoideae (Ericaceae): widespread phylogenetic and geographical structure. Molecular Ecology. 2001;10(9):2285–2295. doi: 10.1046/j.1365-294X.2001.01358.x. [DOI] [PubMed] [Google Scholar]
- 49.Filyushin M. A., Reshetnikova N. M., Kochieva E. Z., Skryabin K. G. Polymorphism of sequences and the secondary structure of b/c intron of mitochondrial gene nad1 in Monotropa hypopitys and related Ericaceae species. Biology Bulletin. 2016;43(3):271–275. doi: 10.1134/S1062359016030043. [DOI] [Google Scholar]
- 50.Filyushin M. A., Kochieva E. Z., Skryabin K. G. Polymorphism of the chloroplast gene rps2 in parasitic plant Monotropa hypopitys L. from the European Russian populations. Russian Journal of Genetics. 2017;53(3):400–405. doi: 10.1134/S1022795417030061. [DOI] [Google Scholar]
- 51.Ravin N. V., Gruzdev E. V., Beletsky A. V., et al. The loss of photosynthetic pathways in the plastid and nuclear genomes of the non-photosynthetic mycoheterotrophic eudicot Monotropa hypopitys . BMC Plant Biology. 2016;16(Suppl 3):p. 238. doi: 10.1186/s12870-016-0929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beatty G. E., Provan J. High clonal diversity in threatened peripheral populations of the yellow bird’s nest (Hypopitys monotropa; syn. Monotropa hypopitys) Annals of Botany. 2011;107(4):663–670. doi: 10.1093/aob/mcr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beletsky A. V., Filyushin M. A., Gruzdev E. V., et al. De novo transcriptome assembly of the mycoheterotrophic plant Monotropa hypopitys . Genomics Data. 2017;11:60–61. doi: 10.1016/j.gdata.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langmead B., Salzberg S. L. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oliveros J. C., Venny An interactive tool for comparing lists with Venn’s diagrams (2007–2015) http://bioinfogp.cnb.csic.es/tools/venny/index.html.
- 56.Babicki S., Arndt D., Marcu A., et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Research. 2016;44(W1):W147–W153. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bailey T. L., Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proceedings, International Conference on Intelligent Systems for Molecular Biology. 1994;2:28–36. [PubMed] [Google Scholar]
- 58.Larkin M. A., Blackshields G., Brown N. P., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 59.Tajima F., Nei M. Estimation of evolutionary distance between nucleotide sequences. Molecular Biology and Evolution. 1984;1(3):269–285. doi: 10.1093/oxfordjournals.molbev.a040317. [DOI] [PubMed] [Google Scholar]
- 60.Tamura K., Kumar S. Evolutionary distance estimation under heterogeneous substitution pattern among lineages. Molecular Biology and Evolution. 2002;19(10):1727–1736. doi: 10.1093/oxfordjournals.molbev.a003995. [DOI] [PubMed] [Google Scholar]
- 61.Tamura K., Nei M., Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences. 2004;101(30):11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li B., Dewey C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):p. 323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franks R. G., Liu Z., Fischer R. L. SEUSS and LEUNIG regulate cell proliferation, vascular development and organ polarity in Arabidopsis petals. Planta. 2006;224(4):801–811. doi: 10.1007/s00425-006-0264-6. [DOI] [PubMed] [Google Scholar]
- 65.Nole-Wilson S., Krizek B. A. AINTEGUMENTA contributes to organ polarity and regulates growth of lateral organs in combination with YABBY genes. Plant Physiology. 2006;141(3):977–987. doi: 10.1104/pp.106.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doyle S. M., Haeger A., Vain T., et al. An early secretory pathway mediated by GNOM-LIKE 1 and GNOM is essential for basal polarity establishment in Arabidopsis thaliana . Proceedings of the National Academy of Sciences of the United States of America. 2015;112(7):E806–E815. doi: 10.1073/pnas.1424856112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pires H. R., Monfared M. M., Shemyakina E. A., Fletcher J. C. ULTRAPETALA trxG genes interact with KANADI transcription factor genes to regulate Arabidopsis gynoecium patterning. Plant Cell. 2014;26(11):4345–4361. doi: 10.1105/tpc.114.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bou-Torrent J., Salla-Martret M., Brandt R., et al. ATHB4 and HAT3, two class II HD-ZIP transcription factors, control leaf development in Arabidopsis . Plant Signaling & Behavior. 2012;7(11):1382–1387. doi: 10.4161/psb.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raikhel N. Nuclear targeting in plants. Plant Physiology. 1992;100(4):1627–1632. doi: 10.1104/pp.100.4.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Immink R. G. H., Tonaco I. A. N., de Folter S., et al. SEPALLATA3: the “glue” for MADS box transcription factor complex formation. Genome Biology. 2009;10(2):p. R24. doi: 10.1186/gb-2009-10-2-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wicke S., Müller K. F., dePamphilis C. W., Quandt D., Bellot S., Schneeweiss G. M. Mechanistic model of evolutionary rate variation en route to a nonphotosynthetic lifestyle in plants. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(32):9045–9050. doi: 10.1073/pnas.1607576113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Graham S. W., Lam V. K. Y., Merckx V. S. F. T. Plastomes on the edge: the evolutionary breakdown of mycoheterotroph plastid genomes. New Phytologist. 2017;214(1):48–55. doi: 10.1111/nph.14398. [DOI] [PubMed] [Google Scholar]
- 73.Kron K. A., Judd W. S., Stevens P. F., et al. Phylogenetic classification of Ericaceae: molecular and morphological evidence. Botanical Review. 2002;68(3):335–423. doi: 10.1663/0006-8101(2002)068[0335:PCOEMA]2.0.CO;2. [DOI] [Google Scholar]
- 74.Braukmann T. W. A., Broe M. B., Stefanović S., Freudenstein J. V. On the brink: the highly reduced plastomes of nonphotosynthetic Ericaceae. New Phytologist. 2017;216(1):254–266. doi: 10.1111/nph.14681. [DOI] [PubMed] [Google Scholar]
- 75.Gruzdev E. V., Mardanov A. V., Beletsky A. V., Kochieva E. Z., Ravin N. V., Skryabin K. G. The complete chloroplast genome of parasitic flowering plant Monotropa hypopitys: extensive gene losses and size reduction. Mitochondrial DNA part B Resources. 2016;1(1):212–213. doi: 10.1080/23802359.2016.1155090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Logacheva M. D., Schelkunov M. I., Shtratnikova V. Y., Matveeva M. V., Penin A. A. Comparative analysis of plastid genomes of non-photosynthetic Ericaceae and their photosynthetic relatives. Scientific Reports. 2016;6(1, article 30042) doi: 10.1038/srep30042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bidartondo M. I. The evolutionary ecology of myco-heterotrophy. New Phytologist. 2005;167(2):335–352. doi: 10.1111/j.1469-8137.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 78.Buchanan-Wollaston V., Page T., Harrison E., et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis . The Plant Journal. 2005;42(4):567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H., Zhou C. Signal transduction in leaf senescence. Plant Molecular Biology. 2013;82(6):539–545. doi: 10.1007/s11103-012-9980-4. [DOI] [PubMed] [Google Scholar]
- 80.Tsukaya H. Leaf shape: genetic controls and environmental factors. The International Journal of Developmental Biology. 2005;49(5-6):547–555. doi: 10.1387/ijdb.041921ht. [DOI] [PubMed] [Google Scholar]
- 81.Tsukaya H. Mechanism of leaf-shape determination. Annual Review of Plant Biology. 2006;57(1):477–496. doi: 10.1146/annurev.arplant.57.032905.105320. [DOI] [PubMed] [Google Scholar]
- 82.Eckardt N. A. YABBY genes and the development and origin of seed plant leaves. Plant Cell. 2010;22(7):p. 2103. doi: 10.1105/tpc.110.220710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mathews S., Kramer E. M. The evolution of reproductive structures in seed plants: a re-examination based on insights from developmental genetics. The New Phytologist. 2012;194(4):910–923. doi: 10.1111/j.1469-8137.2012.04091.x. [DOI] [PubMed] [Google Scholar]
- 84.Siegfried K. R., Eshed Y., Baum S. F., Otsuga D., Drews G. N., Bowman J. L. Members of the YABBY gene family specify abaxial cell fate in Arabidopsis . Development. 1999;126(18):4117–4128. doi: 10.1242/dev.126.18.4117. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y., Huang H., Ma Y., Fu J., Wang L., Dai S. Construction and de novo characterization of a transcriptome of Chrysanthemum lavandulifolium: analysis of gene expression patterns in floral bud emergence. Plant Cell, Tissue and Organ Culture (PCTOC) 2014;116(3):297–309. doi: 10.1007/s11240-013-0404-1. [DOI] [Google Scholar]
- 86.Han H. Q., Liu Y., Jiang M. M., Ge H. Y., Chen H. Y. Identification and expression analysis of YABBY family genes associated with fruit shape in tomato (Solanum lycopersicum L.) Genetics and Molecular Research. 2015;14(2):7079–7091. doi: 10.4238/2015.June.29.1. [DOI] [PubMed] [Google Scholar]
- 87.Kim J., Yang J., Yang R., Sicher R. C., Chang C., Tucker M. L. Transcriptome analysis of soybean leaf abscission identifies transcriptional regulators of organ polarity and cell fate. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Machida C., Nakagawa A., Kojima S., Takahashi H., Machida Y. The complex of ASYMMETRIC LEAVES (AS) proteins plays a central role in antagonistic interactions of genes for leaf polarity specification in Arabidopsis . Wiley Interdisciplinary Reviews: Developmental Biology. 2015;4(6):655–671. doi: 10.1002/wdev.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shulga O. A., Shchennikova A. V., Beletsky A. V., et al. Transcriptome-wide characterization of the MADS-box family in pinesap Monotropa hypopitys reveals flowering conservation in non-photosynthetic myco-heterotrophs. Journal of Plant Growth Regulation. 2017:1–16. doi: 10.1007/s00344-017-9772-9. [DOI] [Google Scholar]
- 90.Emery J. F., Floyd S. K., Alvarez J., et al. Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Current Biology. 2003;13(20):1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 91.Simon M. K., Skinner D. J., Gallagher T. L., Gasser C. S. Integument development in Arabidopsis depends on interaction of YABBY protein INNER NO OUTER with co-activators and co-repressors. Genetics. 2017;207(4):1489–1500. doi: 10.1534/genetics.117.300140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eshed Y., Izhaki A., Baum S. F., Floyd S. K., Bowman J. L. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development. 2004;131(12):2997–3006. doi: 10.1242/dev.01186. [DOI] [PubMed] [Google Scholar]
- 93.Stahle M. I., Kuehlich J., Staron L., von Arnim A. G., Golz J. F. YABBYs and the transcriptional corepressors LEUNIG and LEUNIG_HOMOLOG maintain leaf polarity and meristem activity in Arabidopsis . Plant Cell. 2009;21(10):3105–3118. doi: 10.1105/tpc.109.070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonaccorso O., Lee J. E., Puah L., Scutt C. P., Golz J. F. FILAMENTOUS FLOWER controls lateral organ development by acting as both an activator and a repressor. BMC Plant Biology. 2012;12(1):p. 176. doi: 10.1186/1471-2229-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.La Rota C., Chopard J., Das P., et al. A data-driven integrative model of sepal primordium polarity in Arabidopsis . Plant Cell. 2011;23(12):4318–4333. doi: 10.1105/tpc.111.092619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pekker I., Alvarez J. P., Eshed Y. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell. 2005;17(11):2899–2910. doi: 10.1105/tpc.105.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reinhart B. J., Liu T., Newell N. R., et al. Establishing a framework for the ad/abaxial regulatory network of Arabidopsis: ascertaining targets of class III homeodomain leucine zipper and KANADI regulation. Plant Cell. 2013;25(9):3228–3249. doi: 10.1105/tpc.113.111518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakata M., Matsumoto N., Tsugeki R., Rikirsch E., Laux T., Okada K. Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis . Plant Cell. 2012;24(2):519–535. doi: 10.1105/tpc.111.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Iwakawa H., Iwasaki M., Kojima S., et al. Expression of the ASYMMETRIC LEAVES2 gene in the adaxial domain of Arabidopsis leaves represses cell proliferation in this domain and is critical for the development of properly expanded leaves. The Plant Journal. 2007;51(2):173–184. doi: 10.1111/j.1365-313X.2007.03132.x. [DOI] [PubMed] [Google Scholar]
- 100.Xu B., Li Z., Zhu Y., et al. Arabidopsis genes AS1, AS2, and JAG negatively regulate boundary specifying genes to promote sepal and petal development. Plant Physiology. 2008;146(2):566–575. doi: 10.1104/pp.107.113787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iwasaki M., Takahashi H., Iwakawa H., et al. Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis . Development. 2013;140(9):1958–1969. doi: 10.1242/dev.085365. [DOI] [PubMed] [Google Scholar]
- 102.Prunet N., Morel P., Thierry A. M., et al. REBELOTE, SQUINT, and ULTRAPETALA1 function redundantly in the temporal regulation of floral meristem termination in Arabidopsis thaliana . Plant Cell. 2008;20(4):901–919. doi: 10.1105/tpc.107.053306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shchennikova A. V., Shulga O. A., Kochieva E. Z., et al. Homeobox genes encoding WOX transcription factors in the flowering parasitic plant Monotropa hypopitys . Russian Journal of Genetics: Applied Research. 2017;7(7):781–788. doi: 10.1134/S2079059717070085. [DOI] [Google Scholar]
- 104.Matsumura Y., Iwakawa H., Machida Y., Machida C. Characterization of genes in the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) family in Arabidopsis thaliana, and functional and molecular comparisons between AS2 and other family members. The Plant Journal. 2009;58(3):525–537. doi: 10.1111/j.1365-313X.2009.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Semiarti E., Ueno Y., Tsukaya H., Iwakawa H., Machida C., Machida Y. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development. 2001;128(10):1771–1783. doi: 10.1242/dev.128.10.1771. [DOI] [PubMed] [Google Scholar]
- 106.Prigge M. J., Clark S. E. Evolution of the class III HD-Zip gene family in land plants. Evolution & Development. 2006;8(4):350–361. doi: 10.1111/j.1525-142X.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 107.Chanderbali A. S., He F., Soltis P. S., Soltis D. E. Out of the water: origin and diversification of the LBD gene family. Molecular Biology and Evolution. 2015;32(8):1996–2000. doi: 10.1093/molbev/msv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Toriba T., Harada K., Takamura A., et al. Molecular characterization the YABBY gene family in Oryza sativa and expression analysis of OsYABBY1 . Molecular Genetics and Genomics. 2007;277(5):457–468. doi: 10.1007/s00438-006-0202-0. [DOI] [PubMed] [Google Scholar]
- 109.Vandelook F., Van Assche J. A. Temperature conditions control embryo growth and seed germination of Corydalis solida (L.) Clairv., a temperate forest spring geophyte. Plant Biology (Stuttgart, Germany) 2009;11(6):899–906. doi: 10.1111/j.1438-8677.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- 110.Leake J. R., McKendrick S. L., Bidartondo M., Read D. J. Symbiotic germination and development of the myco-heterotroph Monotropa hypopitys in nature and its requirement for locally distributed Tricholoma spp. New Phytologist. 2004;163(2):405–423. doi: 10.1111/j.1469-8137.2004.01115.x. [DOI] [PubMed] [Google Scholar]
- 111.Fourquin C., Primo A., Martínez-Fernández I., Huet-Trujillo E., Ferrándiz C. The CRC orthologue from Pisum sativum shows conserved functions in carpel morphogenesis and vascular development. Annals of Botany. 2014;114(7):1535–1544. doi: 10.1093/aob/mcu129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Simon M. K., Williams L. A., Brady-Passerini K., Brown R. H., Gasser C. S. Positive- and negative-acting regulatory elements contribute to the tissue-specific expression of INNER NO OUTER, a YABBY-type transcription factor gene in Arabidopsis . BMC Plant Biology. 2012;12(1):p. 214. doi: 10.1186/1471-2229-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boter M., Golz J. F., Giménez-Ibañez S., Fernandez-Barbero G., Franco-Zorrilla J. M., Solano R. FILAMENTOUS FLOWER is a direct target of JAZ3 and modulates responses to Jasmonate. Plant Cell. 2015;27(11):3160–3174. doi: 10.1105/tpc.15.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables 1–7: primers used for qRT-PCR analysis of pinesap gene expression (1); assembled transcripts homologous to the known organ polarity genes in six pinesap transcriptomes (2); MhyYABBY characteristics (3); estimates of evolutionary divergence between nucleotide sequences of the MhyYABBY genes (4); estimates of evolutionary divergence between amino acid sequences of the MhyYABBY proteins (5); set of YABBY orthologues from different plant species used for MEME-mediated identification of clade-specific conserved motifs (6); the significance (p-value) of qRT-PCR results for one gene expression between pinesap tissues (7) (in Microsoft Excel worksheet file (.xls)).
Figures 1-2: structural analysis of the MhyYABBY genes and encoded proteins. (1) Alignment of M. hypopitys YABBY genes (a) and encoded putative proteins (b); (2) novel amino acid motifs predicted in the sequence of the MhyYABBY proteins. (a)–(g) Logos created from aligned sequences and copied directly from MEME graphically represent amino acid conservation: FIL-H (a); FIL-I (b); YAB5-Cm (c); CRC-E (d); INO-B (e); INO-C (f); and INO-D (g). The height of the letters in each stack indicates the relative frequency of individual residues at the position (in portable document format (.pdf)).