Abstract
Background
Treatment options for patients with non-small cell lung cancer (NSCLC) with brain metastases are limited. Patupilone (EPO906), a blood-brain barrier-penetrating, microtubule-targeting cytotoxic agent, has shown clinical activity in phase I/II studies in patients with NSCLC. This study evaluates efficacy, pharmacokinetics, and safety of patupilone in NSCLC brain metastases.
Methods
Adult patients with NSCLC and confirmed progressive brain metastases received 10 mg/m2 patupilone intravenously every 3 weeks. The primary endpoint of this multinomial 2-stage study combined early progression (EP; death or progression within 3 weeks) and progression-free survival at 9 weeks (PFS9w) to determine drug activity.
Results
Fifty patients with a median age of 60 years (range, 33–74) were enrolled, a majority of whom were men (58%) and had received prior therapy for brain metastases (98%). PFS9w was 36%, and the EP rate was 26%. Patupilone blood pharmacokinetic analyses showed a mean AUC0−τ for cycles 1 and 3 of 1544 and 1978 ng*h/mL, respectively, and a mean steady state distribution volume of 755 L/m2. Grade 3/4 adverse events (AEs), regardless of relationship to study drug, included diarrhea (24%), pulmonary embolism (8%), convulsion (4%), and peripheral neuropathy (4%). All patients discontinued the study drug, 31 (62%) for disease progression and 13 (26%) for AEs. Of 32 deaths, 25 were from brain metastases. Median time to progression and overall survival were 3.2 and 8.8 months, respectively.
Conclusions
This is the first prospective study of chemotherapy for recurrent brain metastases from NSCLC. In this population, patupilone demonstrated activity in heavily-treated patients.
Keywords: brain metastases, patupilone, non-small cell lung cancer, chemotherapy, recurrent metastases
Introduction
Brain metastases are the most common form of adult central nervous system (CNS) tumors significantly outnumbering primary brain tumors, with the majority (40–56%) originating from lung cancer, especially non-small cell lung cancer (NSCLC)1, 2. Brain metastases from NSCLC are common at initial diagnosis or within a year of presentation, but can occur at any time during the course of their disease3. Standard therapies for brain metastases include symptomatic treatments (e.g., corticosteroids to reduce peritumoral edema and anticonvulsants to control seizures), surgery, whole brain radiotherapy (WBRT), stereotactic radiosurgery, and chemotherapy4, 5. Despite aggressive treatment, prognosis is poor with median overall survival of approximately 7 months from diagnosis6, and 4–5 months from brain metastasis recurrence7–9.
While most anticancer agents are ineffective in treating brain tumors due to an inability to cross the blood-brain barrier (BBB), some chemotherapeutic and targeted therapies have demonstrated modest activity in patients with recurrent brain metastases in clinical trials10–12. Epothilones are BBB-penetrating compounds that act as anti-microtubule cytotoxic agents by promoting tubulin polymerization and inducing mitotic cell cycle arrest and apoptosis in human cancer cells13, 14. Similar to taxanes, epothilones bind β-tubulin; however, epothilones have a higher binding affinity to this molecular target than paclitaxel or docetaxel15. Unlike taxanes, epothilones are not substrates for the BBB-associated multi-drug transporter P-glycoprotein, and have been demonstrated to cross the intact BBB in rodents16, 17.
Patupilone is a naturally occurring epothilone B that has shown growth inhibition of intra-cranial human lung tumors in preclinical studies18. In a phase I/II surgical study in recurrent glioblastoma, patients were treated with patupilone 1 week prior to surgical resection, and the concentration of patupilone was found to be 30 times higher in tumor tissue than in plasma clearly demonstrating adequate tumor/brain penetration19.
Patupilone has shown some clinical activity in phase I/II studies in previously-treated patients with metastatic ovariancancer, prostate cancer, and NSCLC20–22. The purpose of this phase II study was to evaluate the activity of patupilone in patients with NSCLC with brain metastases who had progressed after chemotherapy, surgery, and/or radiation to the brain.
Materials and Methods
Study design
This phase II, open-label, single-arm, two-stage, multi-center study evaluated the safety and efficacy of patupilone in patients with NSCLC with progressive brain metastases. The protocol and all amendments were reviewed by each site’s Institutional Review Board. A multinomial stopping rule was used for two-stage enrollment, with 25 patients expected to be enrolled during stage 1 and additional 25 patients during stage 223. The decision to proceed to stage 2 was based on both progression-free survival at 9 weeks (PFS9w, also called response in the protocol) and early progression (EP) as described below.
Patients
Patients aged ≥18 years with a WHO performance status of ≤2 and radiographically-confirmed parenchymal brain metastases from histologically-confirmed NSCLC who had progressed after radiotherapy, surgery, and/or chemotherapy were eligible for this study. Patients were required to have at least one recurrent bi-dimensionally measurable intracranial lesion of ≥1 cm and on stable doses of corticosteroids and anticonvulsant agents for at least 1 week before baseline MRI and/or at least 1 week before starting treatment. All patients were required to have adequate hematologic and metabolic function. Patients with leptomeningeal disease, extracranial disease in more than 3 organ sites, >grade 1 peripheral neuropathy, diarrhea of any grade, severe cardiac insufficiency (New York Heart Association III or IV), any serious medical condition, or previously exposed to epothilones were not eligible. Patients were required to stop treatment with any investigational agent within 4 weeks before study enrollment, or with radiotherapy, chemotherapy and/or intracranial surgery at least 3–6 weeks before study entry. All patients provided written informed consent.
Treatment and Evaluation
Patients received patupilone intravenously at 10mg/m2 as a 20-minute intravenous infusion once every 3 weeks (21-day cycle) until disease progression, satisfactory therapeutic response (treating investigator’s discretion), consent withdrawal, loss to follow-up, or unacceptable toxicity occurred. Patupilone was held for grade 3 and 4 hematologic (except anemia) and non-hematologic toxicities until resolution to ≤ grade 1 and the dose of treatment reduced by 25%. It was held for diarrhea of any grade and ≥ grade 2 neuropathy, and the dose reduced by 25% when resolved. Patients were discontinued from study for toxicity or study interruption of >3 weeks beyond the next scheduled dose.
Brain metastases were assessed for overall response as defined in a Neuro-Oncology Criteria of Tumor Response for CNS Tumors in which response was evaluated by assessment of target (up to 5 bi-dimensional measurable enhancing brain tumor lesions) and non-target (additional measurable and evaluable brain as well as extracranial lesions) lesions, appearance of new lesions, neurologic examination scale (score ranging from +2 to −2) and corticosteroid use (Table 1). Tumors were assessed at the completion of every 3-week cycle for the first 4 cycles, at every second cycle beyond cycle 4, and at the end of the study by contrast-enhanced brain MRI and computed tomography (CT) scans of the chest, abdomen, and pelvis.
Table 1.
Evaluation of response using neuro-oncology criteria of tumor response for CNS tumors
| Overall Response |
Target lesion (up to 5 measurable lesions) |
Non Target lesion |
New lesion | Neurologic examination scale |
Corticosteroid use |
|---|---|---|---|---|---|
| Complete response (CR) | None | None | No | +2, +1, 0, −1 | No |
| Partial Response (PR) | ≥ −50% | Non-enhancing, improved, unchanged | No | +2, +1, 0, −1 | Stable (<25% increase from baseline) |
| Progression (PD) | ≥ +25% | Worsened | Yes | −2 | Increase (>25% increase from baseline) |
| Stable disease (SD)* |
SD = Any other disease status not meeting criteria for CR, PR or PD; +2=definitely better, +1=possibly better, 0=unchanged, −1=possibly worse, −2=definitely worse.
AEs were recorded using NCI CTCAEv3.0.
Pharmacokinetic assessments
Blood samples were collected on day 1 immediately prior to and after patupilone infusion, and at 1, 2, 4, 8, 24, 72, 168, 336, and 504 hours (immediately prior to next patupilone dose) during cycle 1 and cycle 3. PK analysis of patupilone was performed using a non-compartmental model. The pharmacokinetic parameters estimated included the peak and minimum drug concentrations after infusion (Cmax and Cmin, respectively), area under the concentration-time cure from time zero to infinity or 504 hours (AUC0−∞ and AUC0−τ, respectively), half-life of the terminal elimination phase (t1/2), clearance of drug in the blood (CL), drug accumulation (AUC0−τ Dose 3/ AUC0−τ Dose 1), and apparent volume of distribution in the body (Vss).
Statistical methods
The primary endpoint was to determine the activity of patupilone with respect to EP and progression-free survival at 9 weeks (PFS9w) for enrolled patients. The EP rate (EPR) was defined as the proportion of patients with progression or death within the first 3 weeks of the study (cycle 1). The PFS9w was defined as the proportion of patients alive without progression after 9 or more weeks of study treatment (cycle 4 day 1 and beyond). The phase 2 multinomial stopping rule was applied to the primary endpoint to determine measures of inactivity (≤ 10% RR and ≥ 60% EPR) and activity (≥ 20% RR and ≤ 40% EPR) at the end of stage 123. Particularly, criteria to consider the drug effective and stop after stage 1 (and reject the inactivity hypothesis) was one of the following: 2–4 responses and ≤10 EP, or 5 responses and ≤11 EP, or ≥6 responses and ≤14 EP.
A sample size of 25 patients each in the 1st and 2nd stages (total n=50) would yield a target power of 85% with 10% target level of significance.
Due to the poor prognosis of these patients, 9 weeks of progression-free survival (PFS) was chosen as a measure of response, and EPR was given equal importance in the multinomial approach, which limited the number of patients required for this study.
Secondary endpoints included overall response rate (complete response [CR], PR), time to disease progression (TTP) and duration of SD of the brain metastases, OS, safety and tolerability, and patupilone blood pharmacokinetics (PK). TTP and OS were measured from start of study treatment until disease progression and death, respectively. Kaplan-Meier estimate was used for survival analysis.
Results
Patient characteristics
A total of 50 patients (25 during stage 1 and 25 during stage 2) were enrolled in this study from 11/2005 through 07/2009 (Table 2). The median age was 60 years (range, 33–74) and 58% were men. A majority of patients had a WHO performance status of 0 or 1 (90%). The time since initial diagnosis of brain metastases was < 3 years in 78% of patients. All patients had received prior therapy for NSCLC and 49/50 patients, for brain metastases (Table 2).
Table 2.
Patient demographic and baseline characteristics
| Characteristic | N = 50 |
|---|---|
| Age, years, median (range) | 60 (33 – 74) |
| Sex, Men/Women, n (%) | 29 (58)/21 (42) |
| WHO performance status, n (%) | |
| 0 | 16 (32) |
| 1 | 29 (58) |
| 2 | 5 (10) |
| Time since initial diagnosis of lung cancer, years, n (%) | |
| < 1 | 13 (26) |
| 1 – 3 | 23 (46) |
| ≥ 3 | 13 (26) |
| Missing | 1 (2) |
| Time since initial diagnosis of brain metastases, years, n (%) | |
| < 1 | 21 (42) |
| 1 – 3 | 18 (36) |
| ≥ 3 | 7 (14) |
| Missing | 4 (8) |
| Prior antineoplastic therapy for brain metastases, n (%) | |
| Radiotherapy | 49 (98) |
| Surgery | 22 (44) |
| Chemotherapy | 16 (32) |
Treatment
Patients were treated with a median of 2 cycles of the study drug (range, 1–13 cycles) and remained on treatment for a median of 8 weeks (range, 3–42 weeks). All patients discontinued the study drug; 62% for disease progression, 26% AEs, 8% consent withdrawal for patient preference (reasons other adverse events, etc), 2% administrative issues and 2% abnormal test results. Forty-six percent of the patients required dose adjustment or interruption for AEs, including grade 1–3 diarrhea (28%), grade 2 peripheral neuropathy (4%) and grade 2 dehydration (4%).
Pharmacokinetics
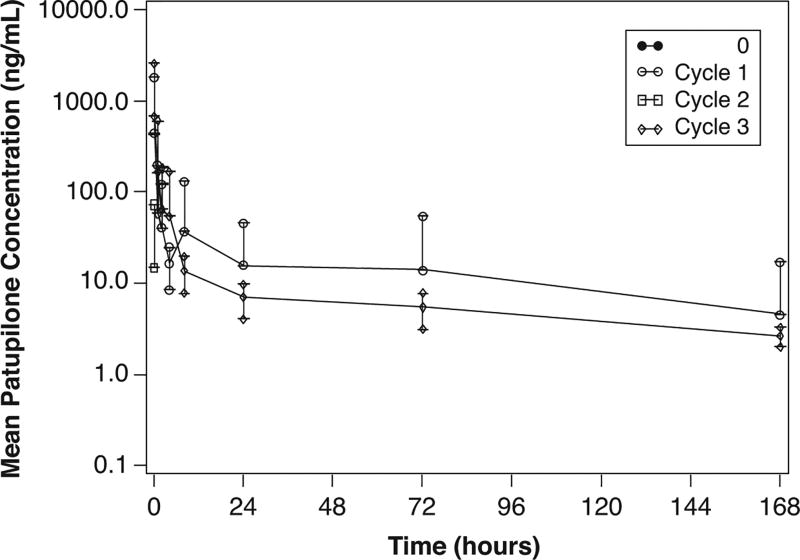
Pharmacokinetics was assessed in 28 patients at cycle 1 and 11 patients at cycle 3 (Table 3). Patupilone blood concentration-time profiles declined rapidly following the 10 mg/m2 dose (Figure 1). The mean AUC0−ταυ for cycles 1 and 3 were 1544 and 1978 L/m2, respectively and the mean steady state volume of distribution (Vss) was 755 L/m2, suggesting an extensive distribution of patupilone to tissues. The low mean blood clearance of patupilone of approximately 7 L/h/m2, coupled with the large Vss, was consistent with the long mean terminal t1/2 for patupilone of about 103 hours. The mean ratio of AUC (cycle 1/cycle 3) was approximately 0.97, indicating a lack of patupilone accumulation with this dosing schedule.
Table 3.
Patupilone pharmacokinetic parameters in Cycles 1 and 3
| Cycle 1 | Cycle 3 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| PK parameter | n | Mean | CV% mean |
n | Mean | CV% mean |
| Cmax (ng/mL) | 27 | 160.66 | 144.19 | 11 | 198.27 | 183.41 |
| Cmin(ng/mL) | 9 | 0.376 | 91.50 | 8 | 0.342 | 105.13 |
| AUC0−∞ (ng*h/mL) | 27 | 1415.57 | 73.87 | - | - | - |
| AUC0−ταυ (ng*h/mL) | 28 | 1543.83 | 63.80 | 11 | 1977.76 | 100.14 |
| CL (L/h/m2) | 28 | 6.857 | 74.78 | - | - | - |
| Vss (L/m2) | 28 | 754.97 | 88.33 | - | - | - |
| t1/2(hours) | 28 | 102.69 | 42.11 | 10 | 108.08 | 23.46 |
| R | - | - | - | 11 | 0.970 | 6.11 |
CV% mean= = coefficient of variation(%) = square-root(exp(variance for log transformed data)−1)*100, CL = clearance, Vss=steady state volume of distribution, R=drug accumulation by AUC0−ταυ Dose 4/ AUC0−ταυ Dose 1
Figure 1.
Mean blood concentration-time profiles for patupilone for Cycles 0–3. Cycles 0 and 2 only have one data point at time 0. n = 28 patients. Error bars represent standard deviation.
Response
The primary endpoint was evaluated at the end of stage 1. Among 25 patients, there were 7 (28%) who were progression-free at 9 weeks, 8 (32%) EPs (PD within the first cycle), and 8 patients neither PFS9w nor EP. In 2 patients, an assessment could not be made. This result met the statistical criteria to stop the study in Stage 1 for activity. Given that there were only a moderate number of patients without progression at 9 weeks (28%) and early progressors at 32%, a decision was made to continue the trial to the stage 2 in order to obtain more information regarding the activity of patupilone in this population of NSCLC brain metastases. Overall results for stage 1 and stage 2 showed 18 patients progression-free at 9 weeks (36%) (95% CI, 20–50%) and 13 (26%) EPs (95% CI, 10–40%) (Table 4). As statistical boundary for activity crossed at the end of Stage 1, there was no intention to use formal success criteria at the end of Stage 2. Nevertheless, the result at the end of the Stage 2 also crossed the statistical boundary for activity defined in the protocol. These results indicated that patupilone is active in patients with brain metastases from NSCLC. Thirty-one patients eventually progressed in the brain, of whom 7 (22%) had systemic progression and 13 (40%) were stable systemically. The status of systemic disease at the time of PD in brain was unknown in 11 patients. Median duration of stable disease in brain was 72 days (range, 20–301 days).
Table 4.
Overall stage 1 and stage 2 tumor early progression and response
| Primary endpoint | N = 50 | 95% CI |
|---|---|---|
| Early progression (EP), n (%) | 13 (26) | (0.1, 0.4) |
| Progression-free at 9 weeks (PFS9w), n (%) | 18 (36) | (0.2, 0.5) |
| Neither EP nor PFS9w, n (%) | 19 (38) |
EP, disease progression or death within first 3 weeks (cycle 1).
PFS9w, alive without progression up to cycle 4 day 1 and beyond.
Survival
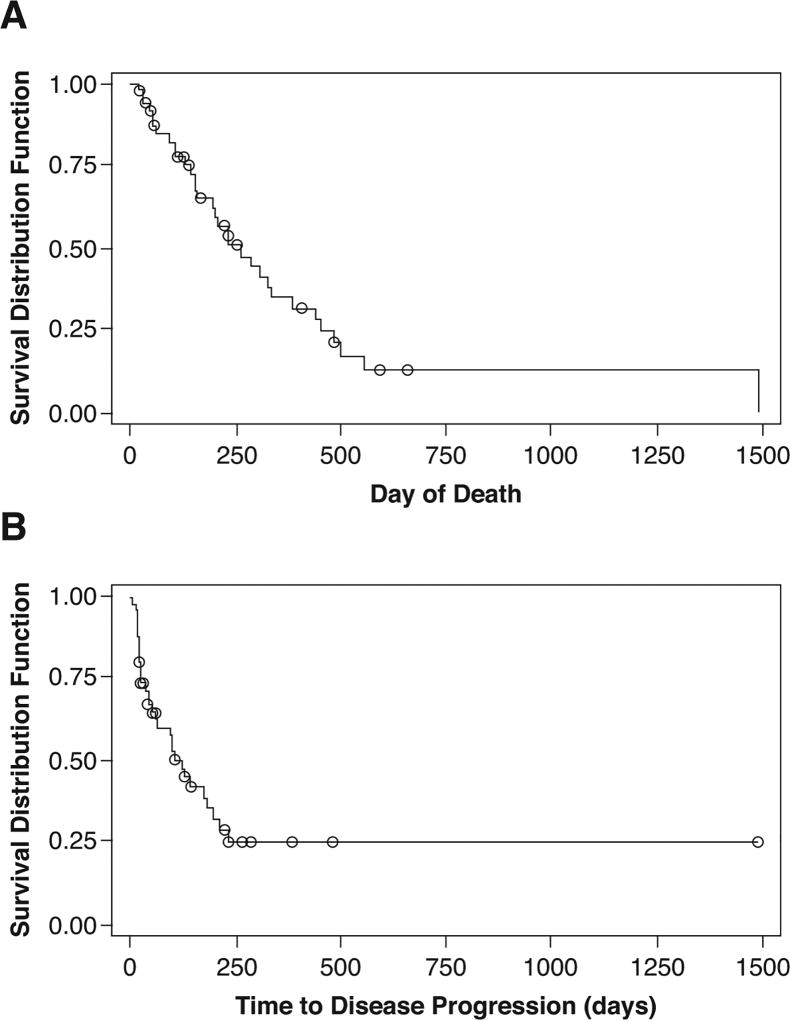
The median follow-up was 163 days (5.4 months). Median OS was 264 days (8.8 months) (95% CI, 158–386 days), with a 6-month OS rate of 65% (95% CI, 48% to 77%) of patients (Figure 2A). The median TTP was 96 days (3.2 months) (95% CI, 42–125 days), with a 6-month TTP rate of 21% (95% CI, 8% – 37%) (Figure 2B). Thirty-two patients had died; 25 from brain metastases, 4 from systemic disease, 2 from infections and 1 from unknown causes.
Figure 2.
Overall survival (A) and time to disease progression (B). N = 50 patients. Circles indicate censored events.
Safety and adverse events (AE)
All patients experienced at least one AE during the study (Table 5), and 88% of reported AEs that were suspected to be treatment-related. The most common drug-related AEs (all grades), included diarrhea (76%), fatigue (26%), peripheral neuropathy (20%), nausea (20%) and dehydration (10%). Grade 3/4 AEs were reported in 60% of patients, which most frequently included diarrhea (24%), hypokalemia (8%), pulmonary embolism (8%), dehydration (8%), and peripheral neuropathy (4%). No patient had grade 4 diarrhea or peripheral neuropathy. Hematologic and liver function abnormalities were mostly grade 1 events and no grade 4 events noted.
Table 5.
Incidence of adverse events (>10% of all grades), regardless of study drug relationship
| Adverse event | Grade 3/4 n (%) |
All grades n (%) |
|---|---|---|
| Any | 30 (60) | 50 (100) |
| Diarrhea | 12 (24) | 38 (76) |
| Fatigue | 1 (2) | 25 (50) |
| Neuropathy peripheral | 2 (4) | 14 (28) |
| Nausea | 0 | 14 (28) |
| Headache | 1 (2) | 11 (22) |
| Constipation | 0 | 9 (18) |
| Muscular weakness | 0 | 8 (16) |
| Dehydration | 3 (6) | 7 (14) |
| Insomnia | 0 | 7 (14) |
| Vomiting | 0 | 7 (14) |
| Convulsion | 2 (4) | 6 (12) |
| Edema peripheral | 1 (2) | 6 (12) |
| Dyspnea | 0 | 6 (12) |
| Rash | 0 | 6 (12) |
Serious AEs (SAEs) were experienced by 25 patients (50%) of whom 20 had grade 3–4 AEs, including diarrhea (20%), dehydration (10%), pulmonary embolism (8%), and convulsion (6%). Majority of the SAEs, other than diarrhea, were thought to be unrelated to the study drug.
Three patients died within 28 days of the last dose of the study drug, unrelated to treatment; one due to brain metastases, and 2 due to peritonitis and pneumonia. There were no study drug-related deaths.
Salvage Treatment
Of 50 patients, 29 received no further treatment. The various salvage regimens used were pemetrexed (4), temozolomide (3), radiation (3), surgical resection (1), topotecan (1), erlotinib (1), irinotecan and bevacizumab (1), bevacizumab (1), gemcitabine and carboplatin (1). There was no available data on salvage treatment in 5 patients.
Discussion
This study is the largest prospective trial of chemotherapy for recurrent brain metastasis reported to date, and the only study soley focusing on NSCLC. It serves as a benchmark, providing reliable historical controls for future trials in unselected NSCLC.
Patupilone, an epothilone B with good blood-brain barrier penetration, was found to have some activity in a heavily pre-treated population of NSCLC patients with brain metastases. However, it was relatively poorly tolerated with a fourth of the patients discontinuing drug due to adverse events. The toxicities were similar to those demonstrated in a recently published breast cancer brain metastases phase 2 trial that did not meet its primary endpoint of 35% 3-month CNS progression-free survival24. Although the patients in our study had relatively favorable prognostic factors in terms of age and performance status, the majority had recurrent brain metastases within < 3 months. They did poorly overall with a median OS of 8.8 months, which is similar to other studies6. We did not have information on the number of brain metastases or extracranial metastases at the time of starting treatment, but the majority died of progressive brain metastases and not systemic disease. This highlights the fact that in addition to brain-penetrating properties, there is a need to develop agents that have improved anti-tumor activity. Another limiting factor other than CNS penetration is the difference in blood-tumor barrier (BTB) permeability. While BTB is thought to be compromised in brain metastases, the extent and magnitude is heterogeneous25. This leads to variable drug uptake which may not reach cytotoxic concentrations within brain metastases. In general, the blood pharmacokinetic data for patupilone was similar to that seen in a previously published phase I study26.
Several chemotherapeutic agents such as cisplatin, carboplatin, vinorelbine, etoposide, pemetrexed and others in various combinations have been tried as first-line treatment in patients with brain metastases from NSCLC with response rates of 23–50%, with best results observed in synchronous brain metastases in chemotherapy-naïve patients27–30. However, traditional chemotherapy is found to be less effective in patients that are pre-treated with chemotherapy or radiotherapy30, and only small rare studies in NSCLC brain metastases are available. Most of the reported experience derives from studies grouping recurrent brain metastases from all histologies with outcomes in NSCLC described as a subgroup7, 8. Temozolomide, both as single-agent and in combinations, has been the most studied agent in this setting, associated with poor objective response of 0–10% in patients and TTP of up to 3.6 months, although some studies have shown relatively higher rates of disease control (including stable disease)7–10, 31; a single agent study specifically focusing on NSCLC that allowed enrollment of brain metastasis patients was terminated early due to lack of efficacy31. Comparison with our results is challenging due to varying response criteria32, 33 and small sample size of previous studies.
Molecularly targeted agents are increasingly used for NSCLC harboring specific genetic alterations. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) such as erlotinib and gefitinib have demonstrated high response rates of over 74–88% in previously untreated patients with brain metastases from EGFR mutant NSCLC12, 34, 35. A prospective trial of gefitinib in pretreated patients achieved disease control of 27% and median progression-free survival of 3 months11. Pulsatile high-dose weekly erlotinib led to an objective response rate of 67% with a median TTP of 2.7 months in patients with EGFR mutant lung cancers majority of whom were previously treated36. In a retrospective pooled analysis of phase II and III trials with crizotinib, an anaplastic lymphoma kinase (ALK) TKI, to evaluate outcomes of ALK-rearranged NSCLC patients with asymptomatic untreated brain metastases, 9% of patients were found to have an objective response and another 70% stable disease37. A more recent phase I/II study of alectinib in crizotinib resistant ALK-positive NSCLC demonstrated an objective response of 52% in 21 patients with brain metastases38. Overall the use of this targeted therapy is limited to the 5–20% of NSCLC patients with EGFR-mutant or ALK positive tumors, but clearly in pre-treated individuals the median TTP is not more than 3 months39. However, median PFS of 8 months has been seen with combination of erlotinib and WBRT, and the initial results with alectinib appear promising38–40.
This study had several limitations. We did not have uniform data on the status of systemic disease in several patients. CSF pharmacokinetics would have been helpful to determine if cytotoxic concentrations of patupilone were attained. We did not have information on the molecular subtypes of the patients to identify if a certain group of patients fared better than others.
In conclusion, patupilone was found to have activity in patients with brain metastases from NSCLC, but was poorly tolerated. Further development of this drug for any indication was discontinued after a recent phase 3 study did not meet its primary endpoint of improved OS in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer, although the overall response rate was higher in the patupilone arm41.
Acknowledgments
Funding
This study was sponsored by Novartis Pharmaceuticals.
Grant Support: P30 CA008748
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Peter J. Simon, PhD and Amanda L. Kauffman, PhD for medical editorial assistance with this manuscript.
Footnotes
Conflict of interest(s):
LN: Advisory board for Amgen
LMD: Advisory board for CarThera, Celgene
RG: Consulting for Pfizer, Merck, Boehringer Ingelheim, Clovis Oncology, Helsinn Healthcare, Genentech, Abbvie, GlaxoSmith Kline
SG: Personal compensation from Novartis (for activities outside of submitted work)
HIR, KK, JRR, SSR, AM: none
DMP: Research funding from Novartis (for activities outside of submitted work)
MZ, PU: Employee of Novartis
LEA: Employee of Roche
AO: Advisory Board for Novartis (for activities outside of submitted work)
PYW: Research support from Novartis for submitted work. Research support from AbbVie, Agios, Angiochem, Astra Zeneca, Cubist, Exelixis, Genentech/Roche, GlaxoSmith Kine, Karyopharm, Merck, Novartis, Sanofi-Aventis, Vascular Biogenics. Advisory Board for AbbVie, Celldex,Genentech/Roche, Novocure, Sigma Tau, Midatech, Vascular Biogenics. Speakers Bureau for Merck.
Presented in part at the 2008 annual meeting of the American Society of Clinical Oncology, 2013 annual meetings of the American Academy of Neurology and Society for Neuro-Oncology
Authorship Responsibility:
Concept and Design: HIR, RG, SG, KK, JRR, DMP, SSR, MZ, PU, LEA, PYW
Analysis and Interpretation of the data: LN, AM, MZ, LEA, PYW
Draft, Revision and Final Approval of the manuscript, and Agreement to be accountable to all aspects of the work: LN, LMD, HIR, RG, SG, KK, JRR, DMP, SSR, AM, MZ, PU, LEA, AO, PYW
References
- 1.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14:1171–1177. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 3.Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, Davis FG. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol. 2014 doi: 10.1093/neuonc/nou099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist. 2007;12:884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 5.Nabors LB, Ammirati M, Bierman PJ, et al. Central nervous system cancers. J Natl Compr Canc Netw. 2013;11:1114–1151. doi: 10.6004/jnccn.2013.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwamoto FM, Omuro AM, Raizer JJ, et al. A phase II trial of vinorelbine and intensive temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol. 2008;87:85–90. doi: 10.1007/s11060-007-9491-3. [DOI] [PubMed] [Google Scholar]
- 8.Christodoulou C, Bafaloukos D, Kosmidis P, et al. Phase II study of temozolomide in heavily pretreated cancer patients with brain metastases. Ann Oncol. 2001;12:249–254. doi: 10.1023/a:1008354323167. [DOI] [PubMed] [Google Scholar]
- 9.Omuro AM, Raizer JJ, Demopoulos A, Malkin MG, Abrey LE. Vinorelbine combined with a protracted course of temozolomide for recurrent brain metastases: a phase I trial. J Neurooncol. 2006;78:277–280. doi: 10.1007/s11060-005-9095-8. [DOI] [PubMed] [Google Scholar]
- 10.Abrey LE, Olson JD, Raizer JJ, et al. A phase II trial of temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol. 2001;53:259–265. doi: 10.1023/a:1012226718323. [DOI] [PubMed] [Google Scholar]
- 11.Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crino L, Villa E. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol. 2004;15:1042–1047. doi: 10.1093/annonc/mdh276. [DOI] [PubMed] [Google Scholar]
- 12.Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77:556–560. doi: 10.1016/j.lungcan.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 13.Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Cheng KL, Bradley T, Budman DR. Novel microtubule-targeting agents - the epothilones. Biologics. 2008;2:789–811. doi: 10.2147/btt.s3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altmann KH, Wartmann M, O'Reilly T. Epothilones and related structures--a new class of microtubule inhibitors with potent in vivo antitumor activity. Biochim Biophys Acta. 2000;1470:M79–91. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann J, Fichtner I, Lemm M, et al. Sagopilone crosses the blood-brain barrier in vivo to inhibit brain tumor growth and metastases. Neuro Oncol. 2009;11:158–166. doi: 10.1215/15228517-2008-072). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Reilly T, Wartmann M, Brueggen J, et al. Pharmacokinetic profile of the microtubule stabilizer patupilone in tumor-bearing rodents and comparison of anti-cancer activity with other MTS in vitro and in vivo. Cancer Chemother Pharmacol. 2008;62:1045–1054. doi: 10.1007/s00280-008-0695-9. [DOI] [PubMed] [Google Scholar]
- 18.McSheehy PM, Allegrini PR, Ferretti S, Becquet M, Stumm M. Patupilone, the novel microtubule stabilizer, inhibits growth of intra-cranial lung tumors in athymic mice. Proc Amer Assoc Cancer Res. 2006;47 Abs 489. [Google Scholar]
- 19.Oehler C, Frei K, Rushing EJ, et al. Patupilone (epothilone B) for recurrent glioblastoma: clinical outcome and translational analysis of a single-institution phase I/II trial. Oncology. 2012;83:1–9. doi: 10.1159/000339152. [DOI] [PubMed] [Google Scholar]
- 20.Hussain A, DiPaola RS, Baron AD, Higano CS, Tchekmedyian NS, Johri AR. Phase II trial of weekly patupilone in patients with castration-resistant prostate cancer. Ann Oncol. 2009;20:492–497. doi: 10.1093/annonc/mdn665. [DOI] [PubMed] [Google Scholar]
- 21.Osterlind K, Sanchez JM, Zatloukal P, et al. Phase I/II dose escalation trial of patupilone every 3 weeks in patients with non-small cell lung cancer. Journal of Clinical Oncology. 2005;23:16S. Abs 7110. [Google Scholar]
- 22.Ten Bokkel Huinink WW, Sufliarsky J, Smit WM, et al. Safety and efficacy of patupilone in patients with advanced ovarian, primary fallopian, or primary peritoneal cancer: a phase I, open-label, dose-escalation study. J Clin Oncol. 2009;27:3097–3103. doi: 10.1200/JCO.2008.20.4826. [DOI] [PubMed] [Google Scholar]
- 23.Zee B, Melnychuk D, Dancey J, Eisenhauer E. Multinomial phase II cancer trials incorporating response and early progression. J Biopharm Stat. 1999;9:351–363. doi: 10.1081/BIP-100101181. [DOI] [PubMed] [Google Scholar]
- 24.Peereboom DM, Murphy C, Ahluwalia MS, et al. Phase II trial of patupilone in patients with brain metastases from breast cancer. Neuro Oncol. 2014;16:579–583. doi: 10.1093/neuonc/not305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16:5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melichar B, Casado E, Bridgewater J, et al. Clinical activity of patupilone in patients with pretreated advanced/metastatic colon cancer: results of a phase I dose escalation trial. Br J Cancer. 2011;105:1646–1653. doi: 10.1038/bjc.2011.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortes J, Rodriguez J, Aramendia JM, et al. Front-line paclitaxel/cisplatin-based chemotherapy in brain metastases from non-small-cell lung cancer. Oncology. 2003;64:28–35. doi: 10.1159/000066520. [DOI] [PubMed] [Google Scholar]
- 28.Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01) Ann Oncol. 2011;22:2466–2470. doi: 10.1093/annonc/mdr003. [DOI] [PubMed] [Google Scholar]
- 29.Bailon O, Chouahnia K, Augier A, et al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro Oncol. 2012;14:491–495. doi: 10.1093/neuonc/nos004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40:716–722. doi: 10.1016/j.ctrv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Dziadziuszko R, Ardizzoni A, Postmus PE, et al. Temozolomide in patients with advanced non-small cell lung cancer with and without brain metastases. a phase II study of the EORTC Lung Cancer Group (08965) Eur J Cancer. 2003;39:1271–1276. doi: 10.1016/s0959-8049(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 32.Lin NU, Wefel JS, Lee EQ, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013;14:e407–416. doi: 10.1016/S1470-2045(13)70308-5. [DOI] [PubMed] [Google Scholar]
- 33.Lin NU, Lee EQ, Aoyama H, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol. 2013;14:e396–406. doi: 10.1016/S1470-2045(13)70311-5. [DOI] [PubMed] [Google Scholar]
- 34.Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65:351–354. doi: 10.1016/j.lungcan.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;82:282–287. doi: 10.1016/j.lungcan.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Grommes C, Oxnard GR, Kris MG, et al. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crino L, Ahn MJ, Ou SHI, et al. Clinical experience with crizotinib in patients (pts) with advanced ALK+ non-small cell lung cancer (NSCLC) and brain metastases. Eur J Cancer. 2013;49:S800. (Abs 3413) [Google Scholar]
- 38.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014 doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 39.Dienstmann R, Martinez P, Felip E. Personalizing therapy with targeted agents in non-small cell lung cancer. Oncotarget. 2011;2:165–177. doi: 10.18632/oncotarget.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colombo N, Kutarska E, Dimopoulos M, et al. Randomized, open-label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. J Clin Oncol. 2012;30:3841–3847. doi: 10.1200/JCO.2011.38.8082. [DOI] [PubMed] [Google Scholar]