Abstract
Background
Coronary artery fluid dynamics may be altered due to the non-physiologic flow seen in continuous-flow left ventricular assist devices (CF-LVADs). Our aim was to study the structure and composition of coronary vessels after CF-LVAD.
Methods and Results
Coronary arteries were collected from patients with heart failure (HF) at the time of transplantation, of whom 15 were supported with a CF-LVAD prior to transplant (HF+LVAD group) and 9 were not (HF Non-LVAD group). In addition, coronary samples were obtained from 5 non-failing age-matched donors (Non-Failing group). Histologic analysis was performed to quantify coronary morphology, composition, vascular fibrosis, and vasa vasorum density. The age and gender mix of the three groups were similar and mean duration of LVAD support was 213 days. Compared to HF patients and non-failing donors, the arteries from HF+LVAD patients had expansion of the adventitia, breakdown of the internal elastic lamina, and increased adventitial collagen deposition and density of vasa vasorum.
Conclusions
Among patients supported with CF-LVADs, the coronary arteries develop marked remodeling with increased adventitial fibrosis. The physiologic consequences of these structural changes are unknown, but it is possible that arterial contractility may be impaired thus limiting coronary flow reserve and promoting myocardial ischemia. This may contribute to CF-LVAD complications such as ventricular arrhythmias and right ventricular failure. As more patients receive CF-LVADs and new pump technology attempts to modulate flow profiles and pulsatility, further research is needed to understand the mechanisms and long-term sequela of these changes in coronary arteries and other vascular beds.
Journal Subject Terms: Heart Failure, Translational Studies, Coronary Circulation, Vascular Biology, Transplantation
Keywords: left ventricular assist device, mechanical circulatory support, vascular remodeling, congestive heart failure, coronary artery
Introduction
Continuous-flow left ventricular assist devices (CF-LVADs) have now replaced the first generation of pulsatile devices and are becoming an increasingly common treatment option for end-stage heart failure (HF).1 Despite the dramatic survival advantage and increased durability of continuous-flow devices, patients living with CF-LVADs face a number of complications, especially with longer durations of support.2 Ventricular arrhythmias and late onset right ventricular failure are particularly common after CF-LVAD implantation and may be related to myocardial ischemia, yet little is known about the influence of non-pulsatile blood flow from CF-LVADs on the coronary arteries.
In normal physiology, myocardial perfusion occurs predominantly in diastole when left ventricular pressures are lower and the coronary arteries are more dilated. The use of CF-LVADs alters this physiology. Unloading with a CF-LVAD lowers left ventricular end-diastolic pressures, but there is no clear mechanical systole and diastole. Additionally, there are alterations in the normal laminar flow patterns in the ascending aorta as a result of the LVAD outflow graft. It is increasingly recognized that there are changes in the structure, collagen content, and elastin content of the aortic wall which result in dynamic changes in vascular distensibility and stiffness.3,4 However, whether such changes contribute to the common CF-LVAD complications of ventricular arrhythmias and right ventricular failure is unknown. The purpose of this study was to examine the structure and composition of coronary vessels immediately after CF-LVAD support.
Methods
Patient Selection and Coronary Artery Tissue Acquisition
The authors declare that all supporting data and analytic methods are available within the article. Additional images of de-identified clinical variables may be obtained by reasonable request via email to the corresponding author for purposes of reproducing the results. Coronary artery tissue samples were collected from patients with end-stage HF at the time of cardiac transplantation at the University of Colorado LVAD-Transplant program during a 2-year period from June 2014 through May 2016. Coronary artery samples were reviewed after hematoxylin and eosin histologic stains to exclude those samples that had co-existing atherosclerotic plaque. This review resulted in coronary samples from a total of 29 patients during this time period, of which 15 patients were supported with a CF-LVAD prior to transplant (HF+LVAD group) and 9 patients did not have an LVAD prior to transplant (HF Non-LVAD group). In addition, age-matched control coronary artery samples were obtained during the same period from 5 non-failing donor hearts that were available for transplant but then unused for non-cardiac reasons (Non-Failing group).
The proximal right coronary, left main, left anterior descending, and left circumflex arteries with their surrounding epicardial fat were dissected from myocardial tissue, fixed overnight with 4% paraformaldehyde and processed for paraffin embedding and transverse artery sections. Hematoxylin and eosin stained coronary sections were reviewed and selected for analysis if they lacked significant atherosclerotic plaque or evidence of >250 μ of intimal hyperplasia in order to lessen any concurrent effects of atherosclerosis on artery structure and to allow for comparisons between non-failing donors as well as patients with ischemic and non-ischemic etiologies of HF. A total of 57 coronary segments without atherosclerotic plaque or intimal hyperplasia were selected for analysis which included 25 vessels from HF+LVAD patients, 17 vessels from HF Non-LVAD patients, and 15 vessels from Non-Failing patients. The Colorado Multicenter Institutional Review Board approved the protocol for the collection, storage, and analysis of human tissue.
In addition, a trained physician retrospectively reviewed medical records and recorded de-identified clinical variables for each patient in a securely stored REDCap database. The clinical data were collected from the time closest to cardiac transplantation and included medications, echocardiography, and hemodynamics. The data obtained from HF+LVAD group reflect the LVAD device settings that were clinically indicated at that time. As was the standard of care for CF-LVAD management during this time period, anti-hypertensive medical therapy was titrated to maintain a goal mean arterial blood pressure of less than 90 mmHg throughout the duration of CF-LVAD support.
Coronary Artery Morphology
The structure and composition of coronary arteries was evaluated with Russel-Movat’s Pentachrome staining, which allows for the differentiation of the tissue components including collagen (yellow), elastin fibers (black), mucin (blue), muscle or fibrinous deposits (red), and nuclei (dark blue). The areas of the intima, media, and adventitia layers were measured for each coronary artery segment and normalized to the lumen area to adjust for vessel caliber. In addition, the adventitia area was normalized by media dimensions to adjust for the amount of hypertrophy that occurs in the media, such that values >1 indicate adventitia layers that had a larger size and proportionally greater remodeling than their same vessel media counterparts. The percent area of the internal elastic lamina (IEL) and external elastic lamina (EEL) were measured relative to the vessel media area. Image analysis was performed with Image J software (National Institutes of Health, USA).
Measurement of Coronary Adventitial Collagen Content and Fibrosis
In order to quantify the degree of fibrosis within the vessel adventitia, we measured the quantity and density of collagen using two additional techniques: PicroSirius Red (PSR) staining and second harmonic generation (SHG) microscopy. PSR staining and darkfield polarized light microscopy allows for the detection of thin collagen fibrils that are detected poorly with traditional histologic Masson Trichrome and Movat’s Pentachrome stains.5,6 In addition, collagen fibers were detected by SHG microscopy that images fibrillar collagen based on its physical and structural properties and does not require special staining.7 While both PSR staining and SHG signals show a strong spatial correlation within tissue, the modalities have some differences. PSR values tend to be higher, while SHG microscopy is more sensitive to fibrillar collagen and does not detect type IV collagen.7,8 Thus, both modalities provide complementary information and were therefore utilized to accurately assess for differences in fibrosis between each study group.
The PSR signals in polarized light microscopy images were selected by Image J threshold parameters, which measured the area and the percent content of PSR birefringence within the area of the adventitia. Values were combined from non-overlapping 4x images of the entire coronary artery wall. In order to control for differences in vessel size, the PSR birefringence signal in the adventitia was normalized to the media area and comparisons were made between the study groups.
For SHG imaging, paraffin-sections of artery segments were rehydrated and imaged via multiphoton excitation using a Zeiss LSM780 light microscope equipped with a femtosecond pulsed Ti Sapphire laser (Chameleon Ultra; Coherent, Santa Clara) and ZEN software (2012 SP1 Black Edition). Images were acquired at 1024 x 1024 pixels (708.49 μ x 708.49 μ) using a Zeiss C-Apochromat 20X objective. The excitation source was tuned for 800nm to generate a SHG 400nm signal that was collected via a 390 nm-410 nm emission filter. Besides the SHG signal, the auto-fluorescence was also observed via a wide range visible filter (420nm-700nm). The area and percent content of SHG+ collagen in the adventitia was measured in four non-overlapping 20x images of the coronary artery. As with other analyses, the SHG+ collagen in the adventitia was normalized to the media area in order to control for differences in vessel size.
Adventitial Vasa Vasorum Density Measurements
Normal coronary arteries develop vasa vasorum (VV) that are confined to the outer media and adventitia but show dramatic proliferation and a less ordered structure in the presence of vascular diseases such as atherosclerosis and vasculitis.9–11 To determine whether the density of VV were altered in association with non-pulsatile LVAD support, VV profiles were identified and counted from Movat’s Pentachrome stained tissue sections by the presence of a vessel lumen containing red blood cells or leukocytes and a border of red-stained pericytes. Additional slides were stained with an antibody reactive to CD34 (QBEND-10 clone, ThermoFisher) that is expressed by mature endothelial cells lining small and large caliber vessels12 as well as adventitia vascular progenitor cells as previously described.13–15 The density of adventitia VV was calculated as the number of VV vessels per mm2 area of adventitia. The relative number of VV was also normalized to the media area to control for differences in the size of coronary arteries.
Statistical Analysis
Data were analyzed using PRISM 5 (GraphPad Software, Inc.) and presented as the mean ± standard deviation. The D ’Agostino and Pearson omnibus tests were performed to assess normality. For normal data, a one-way ANOVA was used to determine if the overall P-value was significant and then followed by Bonferroni’s multiple comparison to determine differences between the three (HF+LVAD, HF Non-LVAD, and Non-Failing) groups. For non-normal data, a Kruskal-Wallis test was used to compare the three groups followed by Dunn’s multiple comparison tests. For comparisons between two groups, the unpaired t-test for normal data and the Mann-Whitney test for non-normal data were used. Statistical significance was defined as a 2-tailed P value of <0.05.
Results
The mean age of individuals in the Non-Failing (48±23 years, 60% female), HF Non-LVAD (46±14 years, 33% female), and HF+LVAD (46±13 years, 33% female) groups was similar. Sex mix was similar for HF Non-LVAD and HF+LVAD groups, but there was a higher proportion of females in the Non-Failing group as female donor organs have a higher chance of not being utilized for transplant and being collected for research purposes. Of the 10 HF+LVAD patients with nonischemic cardiomyopathy, the specific etiologies included idiopathic dilated cardiomyopathy (6 patients), sarcoid cardiomyopathy (2 patients), chemotherapy-related cardiomyopathy (1 patient), and familial/genetic cardiomyopathy (1 patient). Of the 8 HF Non-LVAD patients with nonischemic cardiomyopathy, the specific etiologies included idiopathic dilated cardiomyopathy (3 patients), familial/genetic cardiomyopathy (3 patients), congenital heart disease (1 patient), and hypertrophic cardiomyopathy (1 patient).
Among the HF+LVAD group, 9 (60%) patients were O blood type, compared to only 1 (11%) patient with O blood type in the HF Non-LVAD group. These differences in blood type and anticipated regional transplant listing wait times largely determined whether a patient received a bridge to transplant LVAD. The mean duration of LVAD support among HF+LVAD patients was 213±209 days (median 142, range of 27 to 661 days). There were expected differences in ejection fraction, natriuretic peptide levels, some hemodynamic parameters, and medications related to a non-failing heart and unloading with an LVAD as summarized in Table 1. Importantly, the mean arterial blood pressure was similar between the groups.
Table 1.
Baseline clinical characteristics at the time of cardiac transplantation.
| Non-Failing (5 patients) | HF Non-LVAD (9 patients) | HF+LVAD (15 patients) | P value | |
|---|---|---|---|---|
| Clinical Data | ||||
| Age (years) | 48 ± 23 | 46 ± 14 | 46 ± 13 | 0.980 |
| Female gender | 3 (60%) | 3 (33%) | 5 (33%) | 0.535 |
| Duration of LVAD support (days) | – | – | 213 ± 209 | – |
| Ischemic cardiomyopathy | – | 1 (11%) | 5 (33%) | 0.351 |
| Nonischemic cardiomyopathy | – | 8 (89%) | 10 (67%) | 0.351 |
| Prior sternotomy | – | 4 (44%)* | 15 (100%)* | 0.003 |
| Ejection Fraction (%) | 67 ± 13† | 27 ± 15† | 27 ± 9† | 0.021 |
| Left ventricular internal dimension diastole (cm) | – | 6.5 ± 1.5* | 5.5 ± 1.5* | 0.029 |
| Left ventricular end-diastolic volume (mL) | – | 208 ± 122* | 152 ± 144* | 0.042 |
| Brain natriuretic peptide (pg/mL) | NA | 639 ± 533* | 265 ± 206* | 0.022 |
| Hemodynamics | ||||
| Heart rate (beats/minute) | 92 ± 9 | 90 ± 15 | 92 ± 18 | 0.862 |
| Systolic blood pressure (mmHg) | 118 ± 15 | 112 ± 8 | NA | 0.494 |
| Diastolic blood pressure (mmHg) | 63 ± 10 | 70 ± 9 | NA | 0.177 |
| Mean arterial pressure (mmHg) | 81 ± 8 | 84 ± 8 | 81 ± 11 | 0.644 |
| Right atrial pressure (mmHg) | 7 ± 3 | 6 ± 3 | 8 ± 5 | 0.932 |
| Mean pulmonary artery pressure (mmHg) | 27 ± 8 | 28 ± 7 | 22 ± 7 | 0.110 |
| Pulmonary capillary wedge pressure (mmHg) | 15 ± 2 | 17 ± 6* | 11 ± 7* | 0.037 |
| Cardiac index (L/minute/m2) | 4.4 ± 0.8† | 2.7 ± 1.4† | 2.4 ± 0.4† | 0.009 |
| Medications and Interventions before transplant (during LVAD support if applicable)ǂ | ||||
| Aspirin | 0 (0%) | 1 (11%) | 14 (93%) | 0.001 |
| HMG-CoA Reductase Inhibitor | 1 (20%) | 3 (33%) | 6 (40%) | 0.715 |
| ACE inhibitor or ARB | 0 (0%) | 1 (11%) | 6 (40%) | 0.191 |
| β-blocker | 0 (0%) | 2 (22%) | 9 (60%) | 0.105 |
| Aldosterone antagonist | 0 (0%) | 8 (89%) | 11 (73%) | 0.615 |
| Hydralazine/nitrates | 0 (0%) | 5 (56%) | 0 (0%) | 0.003 |
| Calcium channel blocker | 0 (0%) | 0 (0%) | 3 (20%) | 0.266 |
| Diuretic | 0 (0%) | 8 (89%) | 7 (47%) | 0.080 |
| Digoxin | 0 (0%) | 5 (56%) | 0 (0%) | 0.003 |
| Intravenous inotrope | 0 (0%) | 4 (44%) | 1 (7%) | 0.047 |
| Intravenous vasodilator | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Intravenous vasopressor | 1 (20%) | 0 (0%) | 0 (0%) | – |
| Intra-aortic balloon pump | 0 (0%) | 0 (0%) | 0 (0%) | – |
P<0.05 for comparison of HF Non-LVAD vs. HF+LVAD
P<0.05 for comparison of Non-Failing vs. HF Non-LVAD and P<0.05 for comparison of Non-Failing vs. HF+LVAD
P values denote comparisons of HF Non-LVAD vs. HF+LVAD
Expansion of the Coronary Artery Adventitia with CF-LVADs
Quantification of coronary artery vessel morphology revealed no significant differences in the mean intima-to-lumen areas among the groups: Non-Failing 0.59±0.29 μ, HF Non-LVAD 0.62±0.37 μ, and HF+LVAD 0.52±0.25 μ, P=NS. The mean media-to-lumen areas among the three groups were also similar: Non-Failing (1.00±0.43), HF Non-LVAD (1.21±0.64), and HF+LVAD patients (1.06±0.41), P=NS. However, the most dramatic changes were observed in the adventitia layer of HF+LVAD coronaries as shown in Figure 1. The ratios of adventitia-to-lumen areas from HF+LVAD patients (2.35±0.97) were significantly greater than Non-Failing (0.97±0.50) and HF Non-LVAD (1.18±0.60) patients, P<0.001 for both comparisons. Furthermore, the relative adventitia area normalized to the media area was also significantly higher among HF+LVAD patients (1.51±0.53) compared to Non-Failing (1.06±0.24, P<0.01) and HF Non-LVAD (1.14±0.37, P< 0.05).
Figure 1. Morphologic Changes of Coronary Arteries with Continuous-Flow Left Ventricular Assist Devices.
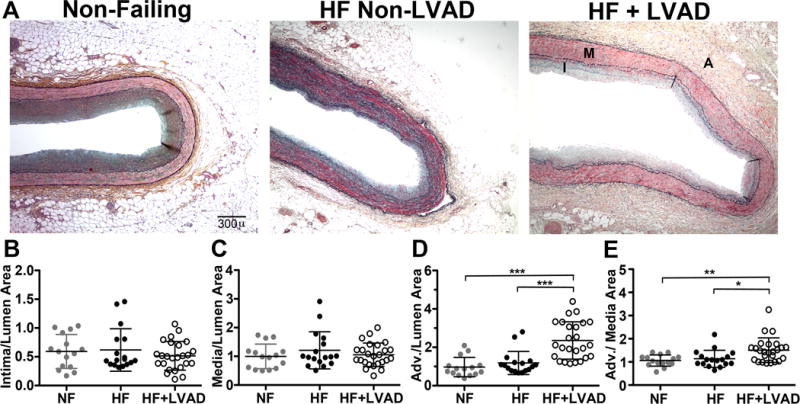
A) Representative coronary artery transverse sections stained with Russel-Movat’s Pentachrome are shown from patients in the Non-Failing (NF, gray circles, N=15 vessels from 5 patients), HF Non-LVAD (HF, black circles, N=17 vessels from 9 patients), and HF with LVAD support (HF+LVAD, open circles, N=25 vessels from 15 patients) groups. The media (M) contains red-stained smooth muscle cells and is bordered by the black-stained internal and external elastic lamina (arrowheads). The adventitia (A) contains more loosely-dispersed cells with dark blue-stained nuclei, collagen fibers (yellow), and mucinous extracellular matrix (green). Perivascular adipose tissue (PAT) seen outside the adventitia was not included in measurements of the adventitia areas. Light microscopy images are shown at 4x magnification. Scale bar represents 300 microns (μ). B) The intima (I), media, adventitia, and lumen areas were measured on low power images that encompassed the full transverse profile of coronaries from each group. The ratios of intima-to-lumen areas were similar for arteries from patients in the NF, HF, and HF+LVAD groups, consistent with the selection criterion for vessels that lacked significant atherosclerosis. C) The ratios of media-to-lumen areas were similar among the groups. D) The mean ratios of adventitia-to lumen areas were 2.3-fold greater in the HF+LVAD group compared to the NF and HF Non-LVAD groups. E) The adventitia areas normalized to the respective media areas were also greater in the HF+LVAD group compared to NF and HF Non-LVAD groups. Error bars represent mean ± standard deviation. Data with overall statistical significance among all 3 groups were tested for pair-wise comparisons between each group and statistical significant P values of ≤0.05, ≤0.01, ≤0.001, and ≤0.0001 are shown as *, **, ***, and ****, respectively for all Figures.
Elastic Lamina Remodeling with CF-LVAD
The IEL runs along the intima-media border and the EEL runs along the media-adventitia border as shown in Figure 2. In normal coronary arteries, the IEL and EEL are similar in thickness as was the case for vessels from the Non-Failing and HF Non-LVAD groups. However, there was significant breakage and attenuation of the IEL compared to the EEL in the HF+LVAD group (2.0±1.1% vs. 4.8±2.1% of the media area, P<0.0001). In addition, there was attenuation of the coronary artery IEL in HF+LVAD patients (2.0±1.1% of the media area) compared to Non-Failing (3.1±0.9%, P<0.05) and HF Non-LVAD patients (3.2±1.3%, P<0.01). Finally, there was thickening of the vessel EEL in HF+LVAD patients (4.8±2.1% of the media area) compared to Non-Failing (3.4±0.9%, P<0.05) and HF Non-LVAD (3.4±1.1%, P<0.05). In contrast, the content of black-stained elastic fibers that are dispersed within the interior of the media and visible at higher magnification was not significantly altered among the three groups.
Figure 2. Medial Elastic Lamina Remodeling.
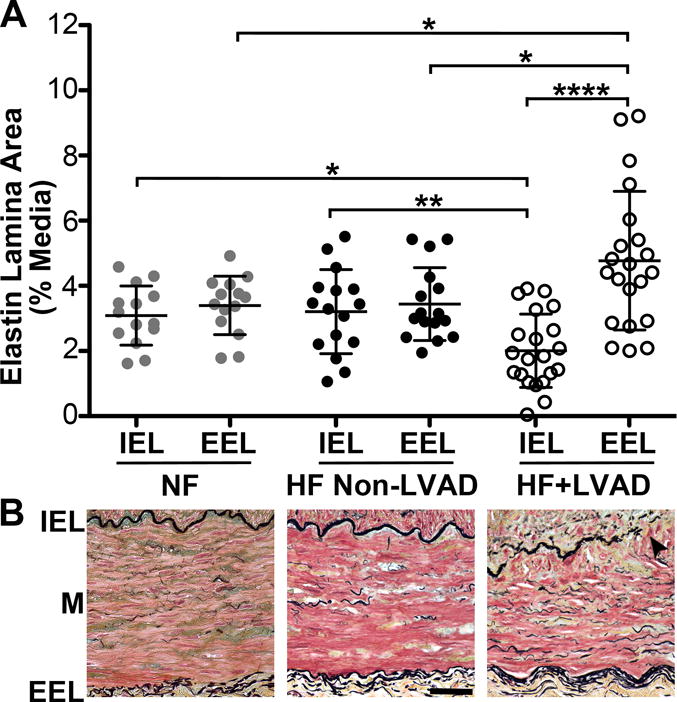
A) The areas of the internal elastic lamina (IEL) and external elastic lamina (EEL) are reported as the percent-area relative to the media area of the transverse coronary artery section. There were significant differences in the areas of both the IEL and EEL between the Non-Failing, HF Non-LVAD, and HF+LVAD groups. In the HF+LVAD group, there was significant attenuation of the IEL and thickening of the EEL. B) Representative high power 40x images of coronary arteries from Non-Failing, HF Non-LVAD, and HF+LVAD patients. The IEL containing black-stained fibers run at the top of the image along the intima-media (M) border and the lower EEL run along the media-adventitia border. Arrowheads show breakage and attenuation of the IEL compared to the thickening of the EEL in HF+LVAD vessels.
Increased Coronary Adventitial Collagen Deposition and Fibrosis with CF-LVADs
Polarized light microscopy images of coronary arteries show intense PSR signals in the adventitia layers, indicative of collagen deposition, as shown in Figure 3. The degree of collagen deposition as assessed by this PSR signal within the adventitia was significantly higher in the HF+LVAD group (2.1±0.8mm2) compared to the Non-Failing (1.4±0.5mm2, P<0.01) and HF Non-LVAD groups (1.0±0.4mm2, P<0.0001). In addition, the density of collagen fibrils in the adventitia as the percent-content of PSR birefringence was significantly higher in the HF+LVAD group (53.1±6.1%) compared to the Non-Failing (46.3±3.0%, P< 0.05) and HF Non-LVAD groups (45.4±8.7%, P<0.01). Finally, after adjusting for differences in vessel size by normalizing the collagen area to the vessel media area, the ratio of adventitia collagen density to media area in the HF+LVAD group (1.3±0.2) remained significantly higher compared to the Non -Failing (0.8±0.2, P<0.0001) and HF Non-LVAD groups (0.7±0.1, P<0.0001).
Figure 3. Changes in Collagen Deposition within the Adventitia of the Coronary Arteries.
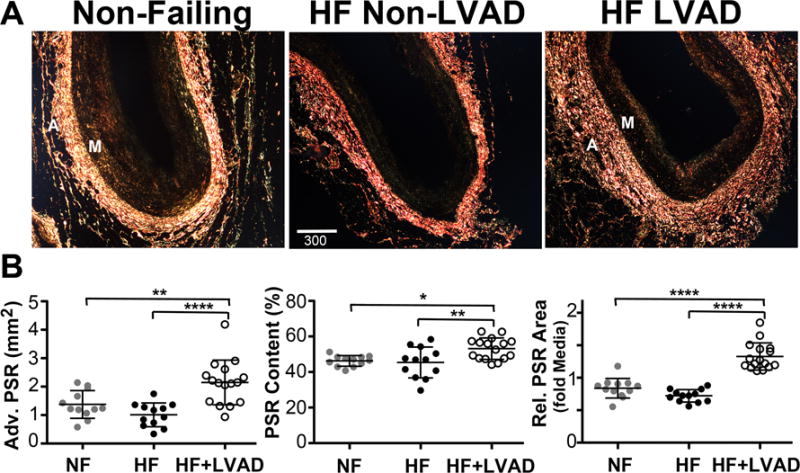
A) Representative polarized darkfield images of PicroSirius Red (PSR) stained coronary artery sections from Non-Failing, HF Non-LVAD, and HF+LVAD individuals demonstrate differences in collagen deposition as measured by PSR birefringence within the adventitia (A). Images are shown at 4x magnification. Scale bar represents 300 μ. B) There was a significant increase in adventitial collagen content among HF+LVAD patients compared to Non-Failing and HF Non-LVAD patients as assessed by the area of PSR birefringence in the adventitia (left panel) and the percent content of PSR birefringence (positive fractional area of PSR signal/adventitia area, middle panel). These differences among groups remained significant when controlling for differences in vessel size by measuring the relative area of PSR birefringence in the adventitia normalized to the vessel media (M) area (right panel).
The changes in the quantity and density of collagen as assessed by PSR staining were also confirmed with SHG microscopy as summarized in Figure 4. Specifically, the percent content of SHG+ collagen in the adventitia, a correlate of collagen density, was higher in coronary arteries from HF+LVAD patients (19.2±3.2%) compared to Non-Failing (13.3±1.9%, P< 0.001) and HF Non-LVAD patients (13.0±3.5%, P<0.0001).
Figure 4. Changes in Coronary Artery Adventitia Fibrillar Collagen as Detected by Second Harmonic Generation Imaging.
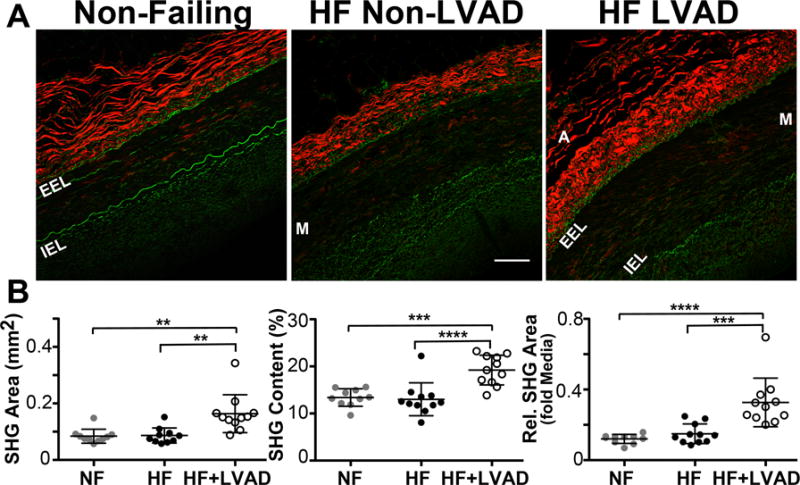
A) Representative second harmonic generation (SHG) images of coronary artery sections from Non-Failing, HF Non-LVAD, and HF+LVAD individuals. The fibrillar collagen within the adventitia (A) produces an autofluorescent SHG signal that is displayed as red in color. The internal elastic lamina (IEL) and external elastic lamina (EEL) surrounding the vessel media (M) is displayed as green in color. Images shown at 20x magnification. Scale bar represents 100 μ. B) The area of SHG+ collagen in the coronary adventitia of HF+LVAD patients is increased relative to Non-Failing and HF Non-LVAD patients (left panel). Furthermore, the percent content of SHG+ collagen in the adventitia, a correlate of collagen density, is higher in the HF+LVAD group compared to Non-Failing and HF Non-LVAD (middle panel). These differences remained significant when controlling for differences in vessel size by measuring the relative area of adventitial SHG+ collagen normalized to the media area (right panel).
Increased Adventitia Vasa Vasorum Density in Coronary Arteries with CF-LVAD
In addition, there was an increase in the raw number of VV associated with growth within the coronary adventitia layer. Specifically, the relative density of VV, measured as the number of vessels counted in the entire adventitia area, was 2-fold greater in HF+LVAD coronary arteries (38.7±14.3 vessels/mm2) compared to Non-Failing (19.5±7.4, P<0.0001) and HF Non-LVAD (21.8±10.4, P<0.0001) coronary arteries as seen in Figure 5. Since the density of VV may be higher in larger caliber blood vessels, the mean number of VV was normalized to the media area of the coronary vessel and compared among groups: the relative abundance of VV was higher in HF+LVAD vessels (57.7±25.8 vessels/mm2 media) compared to Non-Failing (19.9±7.2, P<0.0001) and HF Non-LVAD (22.7±8.8, P<0.0001).
Figure 5. Vasa Vasorum Density within the Coronary Artery Adventitia.
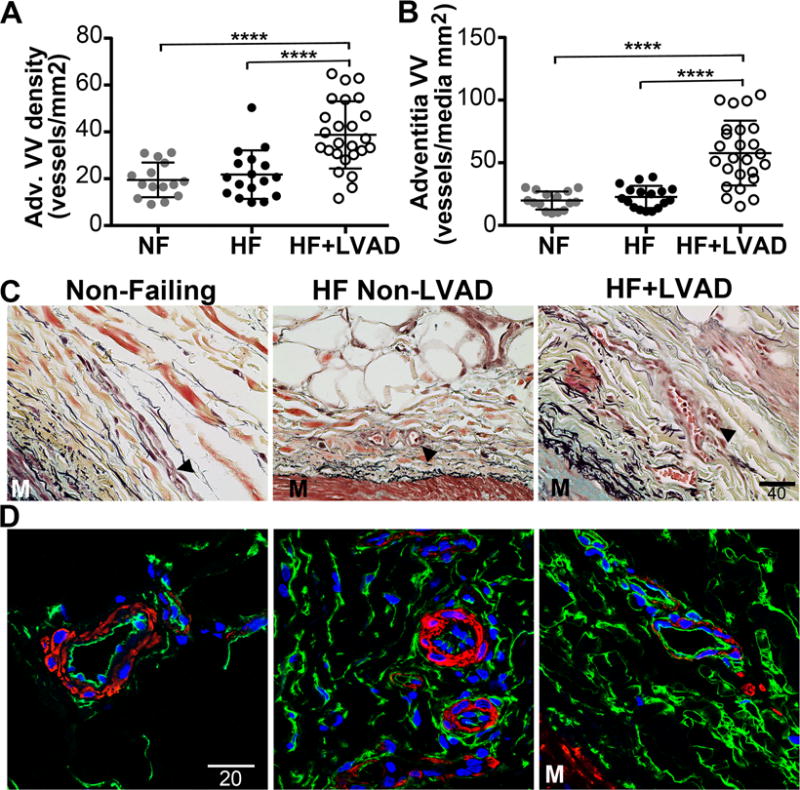
A) Adventitia vasa vasorum (VV) density was assessed by counting the number of microvessels within the surrounding adventitia on Movat’s Pentachrome or CD34 immunohistochemistry stained coronary artery sections. The VV density was significantly higher among the HF+LVAD compared to the Non-Failing and HF Non-LVAD groups. B) The adventitia VV density normalized to the media area (to control for differences in vessel size) remained significantly higher among the HF+LVAD compared to the Non-Failing and HF Non-LVAD groups. C) Representative Movat’s Pentachrome stained sections demonstrating the VV (arrowheads) in the adventitia of coronary arteries. M indicates the outer portion of the media. Images show at 40x magnification. Scale bar represents 40 μ. D) Representative immunofluorescent images of coronary artery adventitia stained with antibodies recognizing CD34 (green) and smooth muscle alpha-actin (red). Images shown at 100x magnification. Scale bar represents 20 μ.
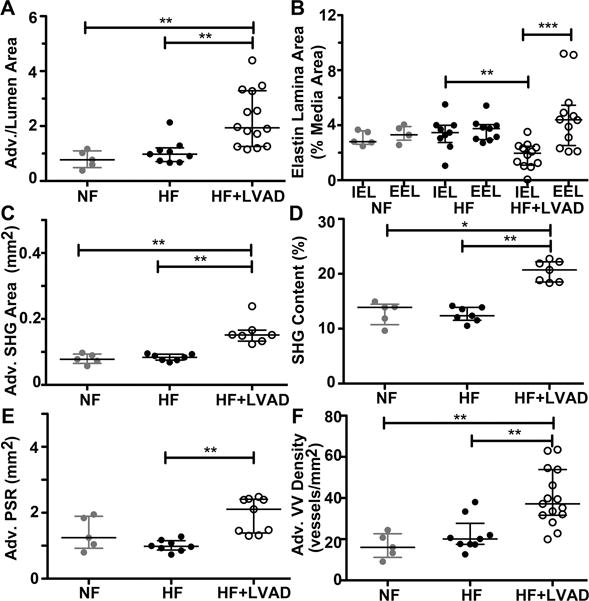
Finally, since systemic factors may influence vascular remodeling in individual patients, all Figures displaying the primary data from each coronary artery sample (n) were analyzed again on a per individual (N) basis and summarized in Figure 6. Statistical analysis by individual confirmed there is attenuation of the IEL and there are statistically significant increases in the adventitia area, area of adventitia fibrosis, and VV density of coronary arteries from HF+LVAD individuals.
Figure 6. Analysis of Adventitia Remodeling and Fibrosis by Individual Patient.
All data-points in panels A-F represent averaged values for different coronary samples from each individual among the Non-failing (NF, gray circles, N=5), Heart Failure Non-LVAD, (HF (black circles, N=9), and HF+LVAD (open circles, N=15) groups. A) Adventitia-to-lumen area ratios were measured on Russel-Movat’s Pentachrome stained coronary artery sections. The coronary arteries of HF+LVAD individuals had increased adventitia areas compared to HF and NF individuals. B) The internal elastin lamina (IEL) and external elastin lamina (EEL) areas were measured as the percent-area relative the area of the media layer. The coronary vessels from HF+LVAD individuals show breakage and attenuation of the IEL compared the EEL of the same vessel and compared to the IEL of coronary arteries from HF individuals. C) The area of second-harmonic generation (SHG) signals from deposited collagen in the coronary adventitia was increased in HF+LVAD individuals compared to NF and HF. D) The density of collagen fibers, as indicated by the percent content of SHG signals in the adventitia, was increased in the coronaries of HF+LVAD individuals compared to the other 2 groups. E) The area of PicroSirius red (PSR) birefringence within the coronary adventitia was increased in HF+LVAD individuals compared to HF individuals that are not on CF-LVAD support. F) The density of adventitia vasa vasorum was significantly higher among coronary arteries of HF+LVAD individuals compared to NF and HF groups. For all figure panels, the error bars indicate the median and interquartile ranges of the data. The non-normal distributed data among the 3 groups was analyzed by Kruskal-Wallis test to determine the overall P value = 0.0002. Dunn’s post-test pairwise comparisons are indicated as *, **, and *** for P values of ≤0.05, ≤0.01, and ≤0.001, respectively.
Duration of CF-LVAD Support and Coronary Artery Remodeling
We performed a preliminary exploratory analysis as to whether the duration of CF-LVAD support influenced the extent of coronary artery remodeling. Therefore, data of coronary samples from the quartile of individuals who had the shortest duration of LVAD support (median 40 days, range 27-81 days, N=4 individuals, 6 samples) were compared to data of coronary samples from the upper quartile of individuals with the longest LVAD duration (median 477 days, range 233-661 days, N=4 individuals, 6 samples). The mean areas for adventitia/media ratio, EEL, adventitia collagen, and the densities of VV in the short and long-duration LVAD subgroups were each greater than the Non-Failing and HF Non-LVAD groups. Remarkably, only the VV density had a significant difference in the magnitude of the increase between the short and long-duration of LVAD subgroups (54.0±9.4 vs. 31.6±8.1 vessels/mm2, P<0.01). Although the sample numbers in the subgroups were too small to make definitive conclusions regarding the influence of CF-LVAD duration, these data imply that adventitial remodeling and, in particular, the expansion of VV occurs early after LVAD implantation.
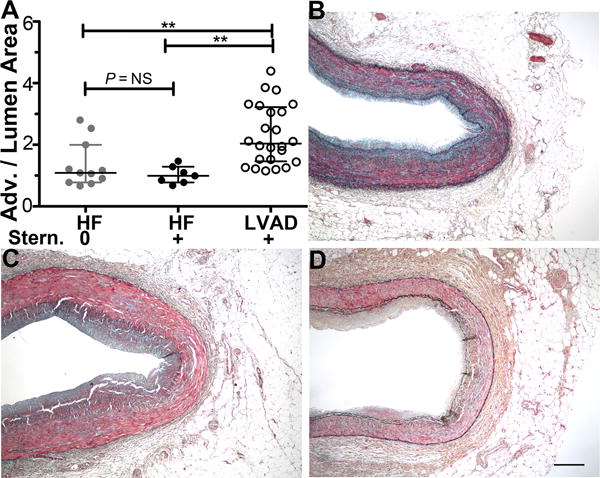
Effect of Prior Sternotomy on Coronary Adventitia Fibrosis
To address the possibility that the structural changes in the HF+LVAD coronaries were related to the sternotomy procedure necessary for CF-LVAD implantation, the surgical history of HF Non-LVAD individuals was reviewed to identify 4 individuals who had a prior sternotomy and 5 individuals who did not have a prior sternotomy. As shown in Figure 7, there were no significant differences in adventitia-to-lumen areas between vessels from HF Non-LVAD individuals with and without prior sternotomy. Each of these HF Non-LVAD subgroups had smaller adventitia areas and fibrosis compared to vessels from HF+LVAD individuals (P≤0.01 for HF no sternotomy vs HF+LVAD and for HF+ sternotomy vs HF+LVAD). Representative images of the adventitia and surrounding margins of periaortic fat show a pattern of adventitia fibrosis adjacent to the media and not extending into the more peripheral periaortic fat.
Figure 7. Sternotomy has Limited Effect on Coronary Adventitia Fibrosis.
A) Coronary Adventitia-to-Lumen areas were similar between coronary samples of the four HF Non-LVAD individuals with a prior sternotomy and the five HF Non-LVAD individuals without a prior sternotomy (Mann-Whitney two-tailed t test, P=0.9048). Both HF Non-LVAD subgroups with and without prior sternotomy had significantly lower coronary adventitia areas compared to coronary arteries of HF+LVAD individuals (Kruskal Wallis test, P=0.0002; post test pairwise comparisons for HF no sternotomy vs. HF+LVAD P≤ 0.01 and HF with sternotomy vs. HF+LVAD P≤0.01). B-D) Representative low power (4x) images of Russel-Movat’s Pentachrome stained coronary arteries from a HF Non-LVAD individual with a prior sternotomy (B), a HF Non-LVAD individual without a prior sternotomy (C), and HF+LVAD individual (D). Note the periaortic fat tissue surrounding the coronary arteries contains limited fibrosis in all groups and the most fibrotic regions of the adventitia are adjacent to the media. Scale bar is 300 microns.
Discussion
The main finding of this study is that the coronary arteries from CF-LVAD patients undergo marked structural remodeling with an expansion of the adventitia, an increase in collagen deposition, and proliferation of the VV within the adventitia compared to age-matched non-LVAD HF patients and non-failing donors. These fibrotic changes were observed in spite of some inhibition of the renin angiotensin aldosterone system with medications in the majority of CF-LVAD patients suggesting that alternate pathways may be contributing to vascular fibrosis. Although the mechanisms for this remodeling within the adventitia layer of coronary arteries are yet to be determined, these findings raise questions about how CF-LVADs induce adventitial remodeling and structural changes that may impact coronary perfusion.
First, continuous flow compared to normal pulsatile blood flow has been reported to impair endothelial cell function via oxidative stress and cytoskeletal and mitochondrial alterations.16 In turn, endothelial dysfunction in the artery lumen impacts the adventitia by increasing hydraulic transport of solutes, microparticles, and oxidation products through the vessel wall.17,18 Hemodynamic conditions, including pulsatility, arterial pressure, and wall permeability, further influence outward mass transport in the artery wall.19 Others have shown endothelial injury or dysfunction is soon followed by the accumulation of inflammatory cells and VV proliferation in the adventitia during early stages of coronary artery disease.10,20,21. Thus, altered mass transport and delivery of vascular mediators to the adventitia is a probable determinant for the expansion of adventitial fibrosis and vasa vasorum in coronary arteries exposed to CF-LVAD.22
Adventitia VV have arterial and venous connections that perfuse the vessel wall and remove waste substances in the adventitia.22,23 The hemodynamics of VV are poorly understood but are likely to be affected by more dense adventitial fibrosis and the loss of the usual diastolic coronary filling with CF-LVADs. Future studies with contrast-enhanced ultrasound might be feasible to measure VV blood flow in a given artery under pulsatile and non-pulsatile flow conditions.24–26 Prior studies have shown that coronary flow reserve is reduced in patients with LVAD support.27 In addition, the extensive adventitia fibrosis in the coronary arteries of HF+LVAD patients can exert vascular compression to further reduce coronary flow reserve, which directly influences myocardial perfusion and could lead to chronic myocardial ischemia during CF-LVAD support.
Another important finding from this study is that the coronary artery elastic lamina is changed in CF-LVAD patients. In normal coronary arteries, the IEL and the EEL that delineate the media borders are similar in thickness, but we observed significant breakdown and attenuation of the IEL with thickening of the EEL in CF-LVAD coronary arteries. The internal and external fibrillar elastin lamina have hydrophobic properties that impede the migration and adhesion of inflammatory cells and support the relative immune privilege of the media layer.28,29 The breakdown of elastin fibers also releases proteolytic products that are chemotactic for inflammatory cells and capable of inducing neovascularization.30,31 These structural changes imply that CF-LVAD coronary arteries are exposed to a pathologic or inflammatory process. Indeed, a greater increase in markers of vascular inflammation has been reported in the aortic endothelium from patients with continuous vs. pulsatile LVADs.32 Similar changes in other systemic vessels may contribute to the pathophysiology of intestinal arteriovenous malformations and inflammatory complications such as allosensitization.
Remodeling within the coronary artery adventitia is a known early indicator of pathology in other vascular disease models.10 We have also previously reported similar adventitial thickening with increased collagen deposition in the aorta after CF-LVAD support.3 The degree to which these observed vascular changes in CF-LVAD coronary arteries occurs in distal coronary arterioles and other vascular beds warrant further investigation. For example, in patients with renal dysfunction undergoing CF-LVAD implantation, there is an initial dramatic improvement in renal function—likely related to the restoration of cardiac output and improvement in hemodynamics with the device.33 However, after one year of chronic CF-LVAD support, renal function deteriorates back to nearly the same level of renal dysfunction as seen prior to CF-LVAD implantation. Animal studies have shown that non-pulsatile flow from CF-LVADs results in marked renal arterial remodeling highlighted by medial smooth muscle hypertrophy and inflammatory cell infiltration of the vessel wall.34,35 Taken together, these findings suggest that vascular remodeling in more distal territories may occur and have clinically significant consequences.
Limitations
We acknowledge a number of limitations to our study. The coronary artery tissue samples were obtained after the heart was removed at transplant. For obvious patient safety reasons, we were unable to obtain CF-LVAD coronary vessels or serial tissues samples before initiation of CV-LVAD support to allow for direct temporal comparisons in the same patient. This was a small single-center study so an institution specific effect cannot be excluded. In addition, the number of available patients and vessel samples that lacked concurrent atherosclerosis and rare number of non-failing donor samples limits the power to discern variations due to different HF etiologies (ischemic, non-ischemic, familial cardiomyopathy), medications, and duration of CF-LVAD support or other LVAD modalities. We also focused on the proximal coronary artery segments for this initial analysis and did not look for differential effects in more distal arterioles. Additional studies would be required to assess whether CF-LVADs alter the progression of existing atherosclerosis as well as effects on the coronary microcirculation.
Conclusions
Among patients supported with CF-LVADs, there are changes in the structure and composition of the coronary vessels compared to age-matched HF patients and non-failing donors. The most notable of these changes is remodeling and expansion of the adventitia with increased fibrosis and VV density. Such fibrotic changes within the coronary arteries may contribute to common CF-LVAD complications such as ventricular arrhythmias and right ventricular dysfunction and may also be an explanation for the low rates of myocardial recovery with CF-LVAD support. As a greater number of patients are supported with CF-LVADs and new pump technology attempts to modulate pulsatility, further research is needed to better understand the mechanisms and long-term sequela of these observed changes in the coronary arteries as well as other vascular beds.
What is New?
Continuous-flow left ventricular assist devices (CF-LVADs) create a unique physiology where blood flow is minimally pulsatile without a clear mechanical systole and diastole.
The influence of this unique physiology on coronary fluid dynamics and coronary arterial structure and composition is unknown.
In this study, coronary artery samples collected from CF-LVAD patients at the time of heart transplantation showed evidence of structural remodeling compared to heart failure patients without CF-LVADs and healthy organ donors.
The most significant remodeling of the coronaries involved adventitial collagen deposition and fibrosis.
What are the Clinical Implications?
Compared to older pulsatile devices, patient outcomes have dramatically improved with modern CF-LVADs. However, minimization of CF-LVAD complications is of paramount importance.
Fibrotic changes within the coronary arteries of CF-LVAD patients may result in chronic myocardial ischemia and contribute to the pathophysiology of complications such as ventricular arrhythmias and right ventricular failure as well as partially explain the low rates of cardiac recovery with CF-LVADs.
Further research is needed to better understand the mechanisms that induce structural remodeling of the coronary arteries and long-term sequelae of the unique flow profiles with CF-LVAD on the coronary arteries as well as other vascular beds. Modulation of LVAD flow characteristics may be a simple therapeutic tool for reducing complications.
Acknowledgments
The authors wish to acknowledge Dr. Peter Buttrick, Dr. Michael Bristow, and the University of Colorado’s Division of Cardiology for ongoing maintenance of the human cardiac tissue biobank. In addition, we thank Radu Moldovan and Greg Glazner of the University of Colorado Advanced Microscopy Core Facility for assistance with confocal microscopy.
Sources of Funding: Dr. Ambardekar is supported by a Scientist Development Grant from the American Heart Association and by the Boettcher Foundation’s Webb-Waring Biomedical Research Program. Studies were supported in part by NIH Grant Number 1R01 HL123616 (M.W.-E.). REDCap provided by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082. Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advanced Light Microscopy Core supported in part by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.McIlvennan CK, Magid KH, Ambardekar AV, Thompson JS, Matlock DD, Allen LA. Clinical outcomes after continuous-flow left ventricular assist device: a systematic review. Circ Heart Fail. 2014;7:1003–1013. doi: 10.1161/CIRCHEARTFAILURE.114.001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambardekar AV, Hunter KS, Babu AN, Tuder RM, Dodson RB, Lindenfeld J. Changes in Aortic Wall Structure, Composition, and Stiffness With Continuous-Flow Left Ventricular Assist Devices: A Pilot Study. Circ Heart Fail. 2015;8:944–952. doi: 10.1161/CIRCHEARTFAILURE.114.001955. [DOI] [PubMed] [Google Scholar]
- 4.Patel AC, Dodson RB, Cornwell WK, Hunter KS, Cleveland JC, Brieke A, Lindenfeld J, Ambardekar AV. Dynamic Changes in Aortic Vascular Stiffness in Patients Bridged to Transplant With Continuous-Flow Left Ventricular Assist Devices. JACC Heart Fail. 2017;5:449–459. doi: 10.1016/j.jchf.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 6.Ovchinnikova O, Robertson A-KL, Wågsäter D, Folco EJ, Hyry M, Myllyharju J, Eriksson P, Libby P, Hansson GK. T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of Apoe(−/−) mice. The American Journal of Pathology. 2009;174:693–700. doi: 10.2353/ajpath.2009.080561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc. 2012;7:654–669. doi: 10.1038/nprot.2012.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drifka CR, Loeffler AG, Mathewson K, Mehta G, Keikhosravi A, Liu Y, Lemancik S, Ricke WA, Weber SM, Kao WJ, Eliceiri KW. Comparison of Picrosirius Red Staining With Second Harmonic Generation Imaging for the Quantification of Clinically Relevant Collagen Fiber Features in Histopathology Samples. Journal of Histochemistry & Cytochemistry. 2016;64:519–529. doi: 10.1369/0022155416659249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barger AC, Beeuwkes R, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 11.Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, Biedermann BC. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–2850. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 12.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- 13.Psaltis PJ, Harbuzariu A, Delacroix S, Witt TA, Holroyd EW, Spoon DB, Hoffman SJ, Pan S, Kleppe LS, Mueske CS, Gulati R, Sandhu GS, Simari RD. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation. 2012;125:592–603. doi: 10.1161/CIRCULATIONAHA.111.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proceedings of the National Academy of Sciences. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacilli A, Pasquinelli G. Vascular wall resident progenitor cells: a review. Exp Cell Res. 2009;315:901–914. doi: 10.1016/j.yexcr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Patibandla PK, Rajasekaran NS, Shelar SB, Giridharan GA, Litovsky SH, Sethu P. Evaluation of the effect of diminished pulsatility as seen in continuous flow ventricular assist devices on arterial endothelial cell phenotype and function. J Heart Lung Transplant. 2016;35:930–932. doi: 10.1016/j.healun.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Gössl M, Zamir M, Ritman EL. Vasa vasorum growth in the coronary arteries of newborn pigs. Anat Embryol. 2004;208:351–357. doi: 10.1007/s00429-004-0400-7. [DOI] [PubMed] [Google Scholar]
- 18.Michel J-B, Thaunat O, Houard X, Meilhac O, Caligiuri G, Nicoletti A. Topological determinants and consequences of adventitial responses to arterial wall injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:1259–1268. doi: 10.1161/ATVBAHA.106.137851. [DOI] [PubMed] [Google Scholar]
- 19.Alberding JP, Baldwin AL, Barton JK, Wiley E. Effects of pulsation frequency and endothelial integrity on enhanced arterial transmural filtration produced by pulsatile pressure. Am J Physiol Heart Circ Physiol. 2005;289:H931–7. doi: 10.1152/ajpheart.00775.2004. [DOI] [PubMed] [Google Scholar]
- 20.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–1556. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi B-J, Matsuo Y, Aoki T, Kwon T-G, Prasad A, Gulati R, Lennon RJ, Lerman LO, Lerman A. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:2473–2477. doi: 10.1161/ATVBAHA.114.304445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–658. doi: 10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gössl M, Beighley PE, Malyar NM, Ritman EL. Role of vasa vasorum in transendothelial solute transport in the coronary vessel wall: a study with cryostatic micro-CT. Am J Physiol Heart Circ Physiol. 2004;287:H2346–51. doi: 10.1152/ajpheart.00066.2004. [DOI] [PubMed] [Google Scholar]
- 24.Lee SC, Carr CL, Davidson BP, Ellegala D, Xie A, Ammi A, Belcik T, Lindner JR. Temporal characterization of the functional density of the vasa vasorum by contrast-enhanced ultrasonography maximum intensity projection imaging. JACC Cardiovasc Imaging. 2010;3:1265–1272. doi: 10.1016/j.jcmg.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staub D, Partovi S, Schinkel AFL, Coll B, Uthoff H, Aschwanden M, Jaeger KA, Feinstein SB. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology. 2011;258:618–626. doi: 10.1148/radiol.10101008. [DOI] [PubMed] [Google Scholar]
- 26.Moritz R, Eaker DR, Anderson JL, Kline TL, Jorgensen SM, Lerman A, Ritman EL. IVUS detection of vasa vasorum blood flow distribution in coronary artery vessel wall. JACC Cardiovasc Imaging. 2012;5:935–940. doi: 10.1016/j.jcmg.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tansley P, Yacoub M, Rimoldi O, Birks E, Hardy J, Hipkin M, Bowles C, Kindler H, Dutka D, Camici PG. Effect of left ventricular assist device combination therapy on myocardial blood flow in patients with end-stage dilated cardiomyopathy. Journal of Heart and Lung Transplantation. 2004;23:1283–1289. doi: 10.1016/j.healun.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Tellides G, Pober JS. Inflammatory and Immune Responses in the Arterial Media. Circulation Research. 2015;116:312–322. doi: 10.1161/CIRCRESAHA.116.301312. [DOI] [PubMed] [Google Scholar]
- 29.Dal Canto AJ, Swanson PE, O’Guin AK, Speck SH, Virgin HW. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J Clin Invest. 2001;107:R15–22. doi: 10.1172/JCI11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nackman GB, Karkowski FJ, Halpern VJ, Gaetz HP, Tilson MD. Elastin degradation products induce adventitial angiogenesis in the Anidjar/Dobrin rat aneurysm model. Surgery. 1997;122:39–44. doi: 10.1016/s0039-6060(97)90262-2. [DOI] [PubMed] [Google Scholar]
- 32.Lee M, Akashi H, Kato TS, Takayama H, Wu C, Xu K, Collado E, Weber MP, Kennel PJ, Brunjes DL, Ji R, Naka Y, George I, Mancini D, Farr M, Schulze PC. Vascular inflammation and abnormal aortic histomorphometry in patients after pulsatile- and continuous-flow left ventricular assist device placement. Journal of Heart and Lung Transplantation. 2016;35:1085–1091. doi: 10.1016/j.healun.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WWH, Parikh CR, Testani JM. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. 2014;7:68–75. doi: 10.1161/CIRCHEARTFAILURE.113.000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ootaki C, Yamashita M, Ootaki Y, Kamohara K, Weber S, Klatte RS, Smith WA, Massiello AL, Emancipator SN, Golding LAR, Fukamachi K. Reduced Pulsatility Induces Periarteritis in Kidney: Role of the Local Renin–Angiotensin System. The Journal of Thoracic and Cardiovascular Surgery. 2008;136:150–158. doi: 10.1016/j.jtcvs.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kihara S, Litwak KN, Nichols L, Litwak P, Kameneva MV, Wu Z, Kormos RL, Griffith BP. Smooth muscle cell hypertrophy of renal cortex arteries with chronic continuous flow left ventricular assist. Ann Thorac Surg. 2003;75:178–83. doi: 10.1016/s0003-4975(02)04087-0. discussion 183. [DOI] [PubMed] [Google Scholar]


