Abstract
Cardiac hypertrophy results from increased mechanical load on the heart and through the action of neuro-humoral mediators. ERK1/2 are known to be activated in response to almost every stress- and agonist-induced hypertrophic stimulus examined to date, suggesting the straightforward hypothesis that these kinases are required for promoting the cardiac growth response. However, recent data from genetically modified mouse models suggest a more complicated picture. For example, inducible expression of dual-specificity phosphatase 6, an ERK1/2-inactivating phosphatase, eliminated ERK1/2 phosphorylation in transgenic mice, but it did not diminish the hypertrophic response to pressure overload. Similarly, Erk1−/− and Erk2+/− mice showed no reduction in stimulus-induced cardiac growth in vivo. However, blockade or deletion of cardiac ERK1/2 did predispose the heart to decompensation and failure after long-term pressure overload. Thus, ERK1/2 signaling is not to be absolutely necessary for mediating cardiac hypertrophy, although it does appear to provide critical protective effects/signals during stress-stimulation.
Keywords: ERK, Cardiac Hypertrophy, Mouse models
Introduction
Cardiac hypertrophy is a leading predictor for the development of serious complications, such as the development of arrhythmias, sudden death and heart failure.1 Current medical treatments for the prevention of pathological hypertrophy rely on pharmacologic blockade of key membrane-bound receptors that respond to neuroendocrine stimuli, such as angiotensin II, endothelin I, and catecholamines. However, even with existing pharmacologic therapies, the incidence of heart failure, with its associated morbidity and mortality, is still on the rise.2 Given these clinical observations, new therapeutic strategies, potentially directed at signaling pathways that transduce these neuro-endocrine signals or stretch mediated signals, could provide additional protection. This review will explore the potential clinical relevance of a therapeutic approach aimed at ERK1/2 signaling branch of the greater mitogen-activated protein kinases (MAPKs). ERK1/2 become activated in cardiac myocytes in response to virtually every type of stress stimulation examined to date,3 and close to 350 manuscripts have been published on ERK1/2 involvement in myocyte hypertrophy. Neuroendocrine effectors act through G protein-coupled receptors, tyrosine kinases, and stretch to induce ERK1/2 activation and, in most cases, this has been associated with induction of the hypertrophic response. Thus, on the surface ERK1/2 would appear to be ideal targets for the development or application of pharmacologic inhibitors as a strategy to antagonize cardiac hypertrophy. However, as will be discussed here the relationship between ERK1/2 signaling and the cardiac hypertrophic response is not entirely straightforward.
MAPK signaling components
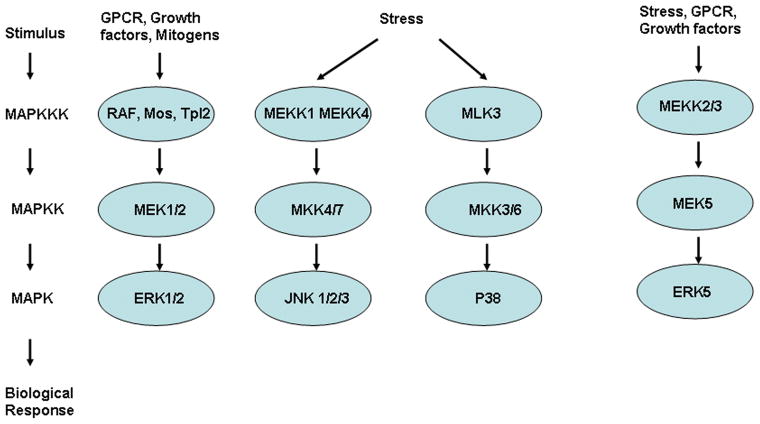
The MAPK signaling cascades transmit extracellular and sometimes intracellular signals into an array of biologic effect that alter or fine-tune cellular processes and gene expression (fig 1). Transmission is classically initiated by activation of small G-proteins, and is followed by sequential activation of successively acting protein kinases. These MAPK cascades are composed of three to five levels of kinases that constitute a phosphorylation-based amplification network. Receiving input from the membrane-associated G-proteins are the MAP3Ks, which in turn activate the MAP2Ks that next activate the MAPKs themselves. The MAPK cascades are generally sub-classified into three main branches, consisting of p38 kinases, c-Jun N-terminal kinases (JNKs), and ERK1/2. 4 However, additional kinases in this cascade include ERK5, which is activated by MEK5, and ERK3/4. The JNKs and p38 kinases generally serve as more specialized transducers of stress or injury responses, hence their sub-classification as stress-activated protein kinases (SAPKs), while ERK1/2 are somewhat more specialized for mitogenic and growth factor stimulation.
Figure 1.
The MAPK signaling cascades are composed of three to five levels of kinases that constitute a phosphorylation-based amplification network. They are generally sub-classified into three main branches, consisting of p38 kinases, c-Jun N-terminal kinases (JNKs), and ERK1/2. However, additional kinases in this cascade include ERK5, which is activated by MEK5.
Signaling through the ERK1/2 cascade is classically initiated at the cell membrane by activation of the small G protein Ras that then recruits the MAP3K, Raf-1 to the plasma membrane where it is activated.5 Other MAP3Ks components, such as MEKK1, may also be involved in ERK activation under specific conditions.6 These MAP3Ks then phosphorylate and activate the dual-specificity kinases MEK1 and MEK2 (MAP2Ks) that serve as dedicated kinases for ERK1/2 phosphorylation and activation.7 MEK1/2 directly phosphorylate the TEY motif within the activation loops of ERK1 and ERK2 kinases. MEK1/2 are activated by phosphorylation of two serine residues, in a motif typical to all MAP2Ks.8 Other phosphorylation sites also regulate MEK1/2 activity, such as the phosphorylation of ser386 in MEK1 by ERK. MEK1/2 also appear to have a role in nucleo-cytoplasmic shuttling, as well as anchoring of ERK1/2, suggesting yet another level of regulation between these two classes of kinases.9 When translocated to the nucleus, ERK1/2 directly phosphorylate transcription factors such as Elk-1, c-Fos, p53, GATA410, and Ets1/2, which are important regulators of growth and proliferation.
Protein-protein interactions through anchoring domains determine ERK1/2 activity toward specific targets. For example, a region in the C-terminus of ERK1/2 is important for the interaction with MEK1/2 and also many other proteins, and is termed the common docking (CD) domain. The combination of localization, catalytic site interaction, and docking domains contribute to target specificity. Inactivation of ERK1/2 is mediated by dephosphorylation of either or both regulatory tyrosine and threonine residues in the activation loop. This dephosphorylation and inactivation process is mediated by several phosphatases, such as PP2A or a more dedicated class of phosphatases termed the dual specificity phosphatases (DUSPs), formerly known as the MAPK phosphatases.11
ERK1/2 as regulators of cardiac hypertrophy
Despite the wealth of studies on the subject, the precise role of ERK1/2 as necessary mediators of cardiac hypertrophy is unproven.3,12,13 ERK1/2 become activated in cardiac myocytes in response to G protein-coupled receptor agonists (angiotensin II, endothelin-1, and adrenergic receptors), receptor tyrosine kinase agonists (insulin-like growth factor, and fibroblast growth factor receptors), cytokines, reactive oxygen species, and stretch. The vast majority of research conducted to date aimed at establishing causation between ERK1/2 signaling that the hypertrophic response, have been conducted in cultured neonatal rat primary cardiomyocytes. Unfortunately, these previous studies have yielded largely conflicting results, although we will not discuss the issues surrounding them or the limitations inherent to cultured neonatal myocytes further given previous in depth reviews on the topic.14
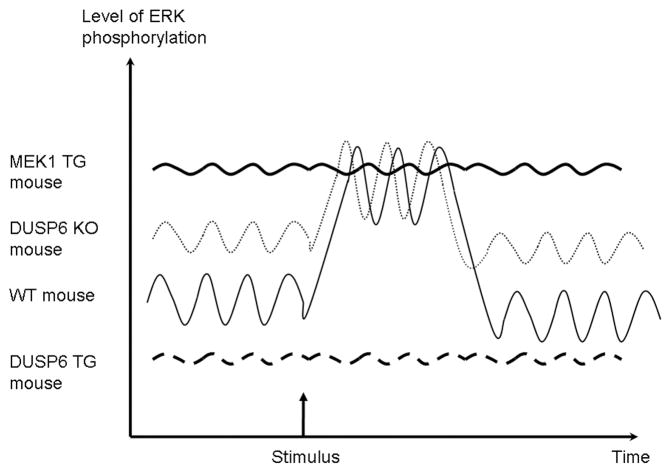
Given the controversies stemming from hypertrophic research conducted in cultured neonatal myocytes, we and others instituted the use of transgenic and gene-targeted mice as a means of re-addressing the causation behind ERK1/2 signaling in regulating cardiac hypertrophy (fig 2).
Figure 2.
Working model for ERK activation. Baseline levels of phospho-ERK oscillate at baseline. A stimulus activating the ERK pathway increase the level of activated ERK. In contrast, mice with overexpression of activated MEK1 have sustained increased activation, and mice with overexpression of DUSP6 have very low baseline and post-stimulus levels. Mice with deletion of DUSP6 appear to have increased baseline activation, but unchanged post stimulus levels.
The very first reported application of genetically modified mouse models to this problem revealed a remarkable result; that constitutively ERK1/2 activation in the heart was sufficient to induce cardiac hypertrophy in vivo.12 Indeed, transgenic mice over-expressing an activated MEK1 mutant under the transcriptional control of the cardiac-specific α-myosin heavy chain promoter demonstrated mild concentric hypertrophy characterized by a thicker septum and left ventricular posterior wall.12 Multiple independent lines of activated MEK1 transgenic mice established a uniform profile of increased heart-to-body weight ratio of approximately 25% at 2 and 6 months of age. These mice did not suffer from premature death or show any signs of histopathology, but in fact, were mildly hypercontractile. However, results from our group showed that simple overexpression of ERK2 in the heart with the α-myosin heavy chain promoter did not induce hypertrophy, suggesting that not all overexpression strategies produce a cardiac phenotype in transgenic mice, although crossing ERK2 transgenic mice with MEK1 transgenic mice produced synergistic hypertrophy.14 Consistent with the results obtained in MEK1 transgenic mice, Ras overexpressing transgenic mice also exhibited cardiac hypertrophy, although Ras activates more than MEK-ERK1/2, possibly explaining why these hearts were also cardiomyopathic, while MEK1 mice simply showed stable concentric hypertrophy that is not pathologic.15 Taken together these results suggested the relatively simple hypothesis that activation of MEK1-ERK1/2 in vivo is sufficient to induce the cardiac hypertrophic response.
One important caveat to the results obtained in MEK1 transgenic mice is that this model can be criticized as unphysiologic, since ERK1/2 activation is constitutive, a situation that never occurs in vivo. In other words, MEK1-ERK1/2 signaling in vivo are always cyclic with waves of activation and inactivation in response to select stimuli. The MEK1 transgene produces constitutive ERK1/2 activation in the heart, bringing into question the simple interpretation that MEK1-ERK1/2 signaling is truly capable of inducing hypertrophy in vivo under real physiologic conditions. Precedence for this concern arose last year with the description of Dusp6−/− mice.
The dual specificity phosphatase 6 (DUSP6) is a highly specific regulator of ERK1/2 dephosphorylation, but not other MAPK family member.11 Mice with targeted disruption of Dusp6 showed an increase in basal ERK1/2 phosphorylation in the heart, spleen, kidney, brain, and fibroblasts, but no change in ERK5, p38, or c-Jun N-terminal kinases activation.16 Interestingly, mice lacking Dusp6 had larger hearts, but this was not due to hypertrophy, rather it was due to hypercellularity of myocytes. When subjected to 8 weeks of surgically-induced pressure overload on the heart, an intense hypertrophic stimulus, Wt and Dusp6−/− mice produced a similar robust hypertrophic response, although analysis of myocyte cross-sectional surface areas revealed less cellular hypertrophy in Dusp6−/− mice. Thus, increased ERK1/2 basal signaling associated with the Dusp6 gene deletion reduced the growth of individual myocytes during long term pressure overload, an effect that was likely associated with the increased cellularity in the heart. These results are very intriguing as they suggest that a “physiologic” increase in ERK1/2 activity in the heart may not induce the hypertrophic response. Indeed, the results in Dusp6−/− mice suggest that MEK1-induced hypertrophy may simply result from the unphysiologic state of constitutive ERK1/2 activation produced by this transgene.
Novel ERK phosphorylation sites
More recently a novel autophosphorylation site in ERK1/2 on Thr188 was identified.17 Thr188 autophosphorylation requires the activation and assembly of the entire Raf1-MEK1-ERK1/2 kinase cascade, phosphorylation of the TEY motif, dimerization of ERK1/2 and binding to G protein βγ subunits released from activated Gq. Thr188 phosphorylation appears to facilitate ERK1/2 activity towards nuclear targets that may be important for cardiac hypertrophy induction. Specifically, the authors overexpressed wildtype ERK2 or Thr188 mutants of ERK2 in the mouse heart. In agreement with previous data, no hypertrophy was observed, when a wildtype or mutated ERK2 were overexpressed in the heart. As expected, following pressure overload, both wildtype ERK2 and mutated ERK2 overexpressing mice exhibited hypertrophy. However, mice with overexpression of a phophomimetic mutated ERK2 (T188D), displayed more cardiac hypertrophy than mice overexpressing wildtype ERK2. This study raises the notion that a specific mode of ERK activation may be accompanied by a unique auto-phosphorylation to promote hypertrophy.
Evidence that ERK1/2 are not required for hypertrophy in vivo
Since ERK1/2 are activated by a large variety of pro-hypertophic stimuli it may appear as an ideal target for small molecules aimed at reducing cardiac hypertrophy. However, loss-of-function studies in transgenic and gene-targeted mice suggest that ERK1/2 signaling may not be required to mediate cardiac growth in vivo, although it is important for cardioprotection. Overexpression of the phosphatase DUSP6 in the mouse heart using an inducible system resulted in a nearly complete inactivation of all cardiac ERK1/2 at baseline and after multiple stimuli (pressure overload and neuro-endocrine agonist infusion), with no effect on other MAPK effectors.13 However, complete inhibition of ERK1/2 associated with DUSP6 overexpression did not reduce the ability of the heart to hypertrophy following pressure overload stimulation, neuroendocrine agonist infusion, or physiologic exercise stimulation. Although chronic inhibition of ERK1/2 was without a pathologic effect in mice as they aged past 1 year of life (based on echocardiography and histology), a profile of decompensation after long-term pressure overload stimulation was observed. Specifically, medium- and high- DUSP6 overexpressing mice actually showed greater cardiac hypertrophy after 14 weeks of pressure overload, which was associated with greater dilation and myopathy. Echocardiographic data and the presence of pulmonary congestion indicated more advanced heart failure in these mice. However, at the cellular level, myocyte hypertrophy was equally increased across each of the groups. Thus, prevention of ERK1/2 activation in vivo did not prevent hypertrophy, but it did sensitize the heart to failure.
While the data obtained in DUSP6 overexpressing transgenic mice were definitive, further analysis in gene-deleted mice is still warranted to conclusively address the issue in its entirety. Such an approach would require the generation of Erk1/2 double null mice, although Erk2 nulls are embryonic lethal, and it is exceedingly difficult to even generate Erk1−/− Erk2+/− mice (3 of 4 alleles deleted). Erk1−/− mice and Erk2+/− mice were analyzed, and in agreement with the DUSP6 overexpressing mice, failed to show a significant reduction in cardiac hypertrophy after pressure overload induced by transverse aortic constriction (TAC). 13 In addition, Erk1−/− mice and Erk2+/− mice also showed no reduction in exercise-induced cardiac hypertrophy after swimming compared with controls. These results suggest that either ERK1/2 are not critical for mediating cardiac hypertrophy to pathophysiologic stress or that the remaining ERK1/2 alleles in each gene-targeted mouse models (Erk2 in Erk1−/− mice and ERK1 and half of ERK2 in Erk2 +/− mice) was sufficient to mediate the signaling events required to drive the hypertrophic response. Future studies with Erk2-loxP targeted mice, which will permit complete deletion of all ERK1/2 from the heart (when crossed with Erk1−/− and the appropriate cardiac Cre-expressing transgenic line), should provide the final data set in the ongoing saga as to the requirement of ERK1/2 in mediating cardiac hypertrophy in vivo.
ERK1/2 targets in the heart and higher level regulation
A potential explanation for the discrepancies and contradictions in the literature concerning the role of ERK1/2 in hypertrophy may result from their pleotrophy in regulation and the manner in which the direct downstream effectors. For example, the ability of ERK1/2 to potentially regulate the hypertrophic response may depend on the duration and strength of any given signal, interaction with various scaffold proteins, subcellular localization, and even cross-talk with other intracellular signaling pathways. A classic model for these kinds of differential activities was demonstrated in PC12 cells. Stimulation of these cells with epidermal growth factors (EGF) causes proliferation, while stimulation with nerve growth factor (NGF) leads to differentiation. It was shown that EGF stimulation causes a strong but transient activation of the ERK cascade, while NGF causes an equally strong but sustained activation;18 and both effects were shown to be dependent on the activity of ERKs. Thus, two stimuli can achieve opposing effects through ERK activation. This also likely explains why MEK1 transgenic mice show robust cardiac hypertrophy given the constitutive nature of ERK1/2 activation in the heart, while Dusp6−/− mice do not.
The subcellular localization and interactions with scaffolds and other proteins are another major determinant of the target specificity of ERK1/2, although very little is understood of the pro-hypertrophic targets and scaffolds for ERK1/2 in the heart. Studies in non-myocytes have shown that MEK1-ERK1/2 signaling can regulate the transcription of polymerase II–associated genes by direct phosphorylation of cardiac-expressed transcription factors. In nonmyocytes, ERK1/2 directly phosphorylate the transcriptional effectors Elk-1, Ets1, Sap1a, c-Myc, and STAT factors. In cardiac myocytes ERK1/2 activation following phenylephrine stimulation was reported to be associated with Elk-1 phosphorylation. 19 Perhaps more importantly, ERK1/2 signaling has been associated with phosphorylation and activation of the cardiac-enriched pro-hypertrophic transcription factor GATA4.10 Study of the novel autophosphorylation (Thr188) site in ERK2 suggested a role in regulating nuclear ERK1/2 targets.17 Collectively, it is attractive to propose that ERK1/2 activation within the nuclear compartment may play a more directed role in controlling the cardiac hypertrophic response. However, additional experimentation with subcellular restricted mutants of ERK1/2 will be needed to more carefully address this putative model.
An additional element to consider is that MEK1 and MEK2, as well as ERK1 and ERK2, may not be completely overlapping in function. The extensive sequence similarity between MEK1 and MEK2, and separately ERK1 and ERK2, and their identical substrate recognition, led to the initial hypothesis that these isoforms are functionally redundant.20 However, it is now clear that some differences exist. For example, while the kinase domains are essentially identical between MEK1 and MEK2, the N-termini of these two kinases only share 40% identity. Indeed, differential functions of MEK1 and MEK2 were demonstrated using Mek1 and Mek2 knockout mice. Deletion of MEK1 caused embryonic lethality at 10.5 days while Mek2−/− mice are viable and fertile, with no observed morphological alternation.21 Likewise, Erk1 and Erk2 genes are also very similar in sequence and co-expressed in essentially all cells and tissues in about the same relative amounts. While both proteins appear to share similar activation kinetics, cellular localization and substrates, Erk1 deficient mice are viable and have normal cardiac function,13 while Erk2 deficient mice die early in development, showing that Erk1 cannot compensate for Erk2 in the embryo. Moreover, some studies have suggested that differences between ERK1 and ERK2 are more than quantitative.22 Despite all these considerations, the potential differential functions between ERK1 and ERK2 in the heart are unknown.
ERK1/2 are anti-apoptotic in the heart
Data from several mouse models suggests that ERK signaling plays a protective, anti-apoptotic role in the heart. For example, MEK1 transgenic mice were profoundly protected from ischemia-reperfusion injury to the heart, showing reduced DNA laddering and less area of myocardial damage.12 Consistent with these observations, loss of function experiments in Erk2 heterozygote gene-targeted mice showed enhanced myocardial injury following ischemia-reperfusion.23 Similarly, inhibition of ERK1/2 with DUSP6 overexpression in a model of long-term pressure overload predisposed the myocardium to decompensation associated with greater TUNEL rates.13 The mechanisms whereby ERK1/2 might directly protect cardiac myocytes from cell death have yet to be definitively identified, although some associations with the Bcl-2 family have been shown.10,24 In our hands, DUSP6 transgenic mice showed increased Bcl-2 and Bcl-xl protein levels after TAC. However, this up-regulation of Bcl-2 and Bcl-xl in DUSP6 transgenic hearts may be a compensatory response due to the loss of other survival signals that are more proximal to ERK1/2. It is likely that many other downstream targets of ERK1/2 mediate cardioprotection following long-term pressure overload and/or ischemia-reperfusion injury.
Conclusions
In summary many lines of evidence from cultured systems, transgenic, and gene-targeted mice, show that the ERK pathway plays an important role in the heart. Activation of this system is capable of inducing cardiac hypertrophy in cultured neonatal myocytes (not discussed) and in the hearts of MEK1 transgenic mice. However, interpretation of causation becomes more complicated when other factors are considered. For example, mild enhancement of baseline ERK1/2 activity in Dusp6 null mice did not induce cardiac hypertrophy in vivo. These data suggest that there are likely many modes of ERK activation in the heart that depend on timing and duration of signaling, the intensity of the signal, intracellular location of ERK1/2, and the activation of co-regulatory pathways. Despite these issues regarding the ability of ERK1/2 to contribute to the hypertrophic response when activated, loss-of-function data strongly suggest that ERK1/2 are not necessary for hypertrophy. It is probable that other critical regulatory pathways are fully capable of compensating for the loss of ERK1/2 in effectively “bypassing” their functions during stress or agonist stimulation. Thus, this pathway is likely a poor choice for pharmacologic intervention in humans with hypertrophic disease. Moreover, inhibition of this pathway might even be detrimental to heart in select disease states, as several lines of evidence show that ERK1/2 are protective during injury and failure. Future studies aimed at identifying downstream targets of ERK1/2 in the heart as mediators of cardiac protection will clearly be important. It will also be essential to further dissect exactly how ERK1/2 are regulated in the cardiac myocyte by select signals, so that we can generate a hierarchy of biologic effects (signal intensity, signal duration, subcellular localization, etc) that ultimately determine if ERK1/2 can modify the hypertrophic response or merely affect other cellular processes.
Acknowledgments
This work was supported in part by a fellowship from the Human Frontier Science Program to I.K.
References
- 1.HO KK, PINSKY JL, KANNEL WB, LEVY D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 2.KLEIN L, et al. Pharmacologic therapy for patients with chronic heart failure and reduced systolic function: review of trials and practical considerations. Am J Cardiol. 2003;91:18F–40F. doi: 10.1016/s0002-9149(02)03336-2. [DOI] [PubMed] [Google Scholar]
- 3.BUENO OF, MOLKENTIN JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–81. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 4.GARRINGTON TP, JOHNSON GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–8. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 5.WELLBROCK C, KARASARIDES M, MARAIS R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–85. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 6.LANGE-CARTER CA, PLEIMAN CM, GARDNER AM, et al. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993;260:315–9. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 7.SHAUL YD, SEGER R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–26. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8.ALESSI DR, et al. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. Embo J. 1994;13:1610–9. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ADACHI M, FUKUDA M, NISHIDA E. Nuclear export of MAP kinase (ERK) involves a MAP kinase kinase (MEK)-dependent active transport mechanism. J Cell Biol. 2000;148:849–56. doi: 10.1083/jcb.148.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LIANG Q, et al. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol Cell Biol. 2001;21:7460–9. doi: 10.1128/MCB.21.21.7460-7469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FJELD CC, RICE AE, KIM Y, et al. Mechanistic basis for catalytic activation of mitogen-activated protein kinase phosphatase 3 by extracellular signal-regulated kinase. J Biol Chem. 2000;275:6749–57. doi: 10.1074/jbc.275.10.6749. [DOI] [PubMed] [Google Scholar]
- 12.BUENO OF, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. Embo J. 2000;19:6341–50. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PURCELL NH, et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–9. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MOLKENTIN JD, ROBBINS J. With great power comes great responsibility: using mouse genetics to study cardiac hypertrophy and failure. J Mol Cell Cardiol. 2009;46:130–6. doi: 10.1016/j.yjmcc.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HUNTER JJ, TANAKA N, ROCKMAN HA, et al. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270:23173–8. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 16.MAILLET M, et al. DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem. 2008;283:31246–55. doi: 10.1074/jbc.M806085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LORENZ K, SCHMITT JP, SCHMITTECKERT EM, LOHSE MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15:75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 18.MARSHALL CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–85. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 19.BABU GJ, LALLI MJ, SUSSMAN MA, et al. Phosphorylation of elk-1 by MEK/ERK pathway is necessary for c-fos gene activation during cardiac myocyte hypertrophy. J Mol Cell Cardiol. 2000;32:1447–57. doi: 10.1006/jmcc.2000.1185. [DOI] [PubMed] [Google Scholar]
- 20.ZHENG CF, GUAN KL. Properties of MEKs, the kinases that phosphorylate and activate the extracellular signal-regulated kinases. J Biol Chem. 1993;268:23933–9. [PubMed] [Google Scholar]
- 21.BELANGER LF, et al. Mek2 is dispensable for mouse growth and development. Mol Cell Biol. 2003;23:4778–87. doi: 10.1128/MCB.23.14.4778-4787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FISCHER AM, KATAYAMA CD, PAGES G, et al. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–43. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 23.LIPS DJ, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–41. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 24.ARIES A, PARADIS P, LEFEBVRE C, et al. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci U S A. 2004;101:6975–80. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]