Abstract
Background
Greater exposure to urban green spaces has been linked to reduced risks of depression, cardiovascular disease, diabetes and premature death. Alleviation of chronic stress is a hypothesized pathway to improved health. Previous studies linked chronic stress with a biomarker-based composite measure of physiological dysregulation known as allostatic load.
Objective
This study’s objective was to assess the relationship between vegetated land cover near residences and allostatic load.
Methods
This cross-sectional population-based study involved 206 adult residents of the Durham-Chapel Hill, North Carolina metropolitan area. Exposure was quantified using high-resolution metrics of trees and herbaceous vegetation within 500 m of each residence derived from the U.S. Environmental Protection Agency’s EnviroAtlas land cover dataset. Eighteen biomarkers of immune, neuroendocrine, and metabolic functions were measured in serum or saliva samples. Allostatic load was defined as a sum of potentially unhealthy biomarker values dichotomized at 10th or 90th percentile of sample distribution. Regression analysis was conducted using generalized additive models with two-dimensional spline smoothing function of geographic coordinates, weighted measures of vegetated land cover allowing decay of effects with distance, and geographic and demographic covariates.
Results
An inter-quartile range increase in distance-weighted vegetated land cover was associated with 37% (95% Confidence Limits 46%; 27%) reduced allostatic load; significantly reduced adjusted odds of having low level of norepinephrine, dopamine, and dehydroepiandrosterone, and high level of epinephrine, fibrinogen, vascular cell adhesion molecule-1, and interleukin-8 in serum, and α-amylase in saliva; and reduced odds of previously diagnosed depression.
Conclusions
The observed effects of vegetated land cover on allostatic load and individual biomarkers are consistent with prevention of depression, cardiovascular disease and premature mortality.
Keywords: Allostatic load, Green space, Health effect biomarkers, Chronic stress, Depression
1. Introduction
Greater exposure to green and natural environments in urban and suburban settings is associated with various health benefits (WHO 2016). These benefits include improved mental health (Gascon et al. 2015), reduced incidence of type 2 diabetes (Astell-Burt et al. 2014), improved pregnancy outcomes (Dzhambov et al. 2014), reduced cardiovascular disease (Tamosiunas et al. 2014) and reduced mortality (Gascon et al. 2016).
Exposure to the green environment is linked with these health benefits through various interacting pathways, such as chronic stress alleviation, improved social cohesion, enhanced physical activity, and reduced air pollution (Hartig et al. 2014). Relative contributions of these different pathways to specific health outcomes may depend on the type of exposure to the green environment (e.g. urban parks vs. vegetation near residence) and specific population subgroup (WHO 2016). According to the biophilia hypothesis, humans have an innate need to affiliate with the living natural environment (Wilson 1984). As it appears that people are innately predisposed to find non-threatening natural stimuli relaxing, exposure to these stimuli triggers a parasympathetic nervous system response leading to feelings of enhanced well-being and relaxation, and shifting stressed individuals to a more positive emotional state (Ulrich et al. 1991).
Corroborating the biophilia hypothesis, reviews of epidemiological evidence demonstrated that restoration and stress reduction may be the most important pathways to better mental and physical health in individuals living in greener areas (Dadvand et al. 2016; Triguero-Mas et al. 2015). Studies in different countries have linked greater vegetated land cover as well as improved access to geographically defined green spaces to reduced levels of depression, anxiety and psychological distress (Astell-Burt et al. 2013; Beyer et al. 2014; Pope et al. 2015; Reklaitiene et al. 2014; Taylor et al. 2015).
Previous studies explored biological mechanisms underlying mental and physiological benefits of short-term contacts with nature. For example, an experimental study using wearable electroencephalogram (EEG) devices demonstrated the effects of a short walk in a green urban area on brain activity indicative of enhanced relaxation and restoration (Aspinall et al. 2015). Other studies have demonstrated beneficial effects of “forest bathing” and various short-term contacts with nature on biomarkers of cardiovascular, neuroendocrine and immune functions (Haluza et al. 2014). A cross-sectional observational study in the U.K. demonstrated that, among economically deprived sub-populations, residents of greener neighborhoods have healthier diurnal salivary cortisol patterns indicating reduced effects of chronic stress (Roe et al. 2013; Thompson et al. 2012).
Chronic environmental challenges that an individual perceives as stressful are known to have systemic detrimental effects, manifesting in measurable physiological dysregulation. Allostatic load (AL) is a biomarker-based measure of such dysregulation reflecting the physiological consequences of chronically fluctuating neural or endocrine responses resulting from repeated stress (McEwen and Stellar 1993; McEwen 2002; Seeman et al. 1997). Numerous studies have used various AL indices which incorporate biomarkers of neuroendocrine, immune, metabolic and cardiovascular system functioning to demonstrate profound health implications of AL including burnout syndrome, reduced cognitive performance, increased risks of cardiovascular morbidity and death (Juster et al. 2010; Juster et al. 2011; Karlamangla et al. 2014; McEwen 2015; Milot et al. 2014).
AL has been increasingly used in epidemiology and community health research, and linked to adverse social and environmental conditions (Hansen et al. 2014; Jung et al. 2014; Juster et al. 2010; Petrovic et al. 2016; Robinette et al. 2016). While many previously conducted studies of health benefits of short-term exposure to outdoor nature have utilized some AL biomarkers (Haluza et al. 2014), to our knowledge, AL or other composite measures of physiological dysregulation based on multiple biomarkers have not been used to assess effects of long-term exposure to urban green spaces and living natural environments.
The main objective of the present study was to assess an association between vegetated land cover near participants’ residences and AL. Additional objectives were: (i) to explore associations between vegetated land cover and individual biomarkers in order to demonstrate potential mechanisms of beneficial health effects; and (ii) to explore associations between vegetated land cover, and diseases and health conditions.
2. Methods
2.1. Study design
This cross-sectional community-based study used archived serum and saliva samples, and associated questionnaire data from a previously conducted study in the Durham-Chapel Hill, North Carolina metropolitan area, which was designed to identify individual and community level factors affecting general health status and risks of selected chronic infections.
2.2. Human subjects: recruitment and data collection
The study protocol involving the use of human subjects was approved by the Institutional Review Board at the University of North Carolina in Chapel Hill. Participants were recruited through advertisement in local newspapers and internet sites.
Enrollment was limited to individuals of at least 18 years of age. Data collection was conducted at the U.S. Environmental Protection Agency (US EPA) Human Studies Facility in Chapel Hill in May-September 2013. All data were collected during a single visit by a study participant, usually in the morning. The participants signed an informed consent form prior to data collection. Venous blood samples were drawn by registered nurses in BD Vacutainer SST Tubes without an anticoagulant (Becton, Dickinson and Company, Franklin Lakes, NJ). Serum was separated by centrifugation following manufacturer instructions. Oral fluid (hereafter called saliva) samples were collected using Oracol samplers (Malvern Medical Developments, United Kingdom), which consist of a cylindrical sponge with a handle and a container. Sampling involves rubbing the gums with the sponge for one minute or until the sponge becomes saturated with saliva (this sampling method produces variable sample volumes from less than 0.1 mL to 1 mL). Saliva was separated from the sponge by centrifugation in the collection tube at 1,500 g for 5 min. Debris were pelleted by centrifugation at 2,500 g for 5 min. Samples were transferred to microcentrifuge tubes and then further separated from debris by centrifugation at 3,000 g for 3 min and then transferred to a final microcentrifuge tube. Samples were archived immediately after processing at −80°C.
A brief questionnaire included basic demographic information, as well as data on self-assessed health status and medically diagnosed chronic diseases and conditions.
2.3. Land cover data analysis
The residential vegetated land cover data were derived from 1 m resolution classified land cover data published online by the US EPA as part of the EnviroAtlas mapping application and decision toolkit (https://www.epa.gov/enviroatlas). Land cover data for the contiguous United States has 30 m resolution; 1-meter resolution land cover data are available for selected census urban areas including the Durham-Chapel Hill, NC metropolitan area (Pickard et al. 2015). The classified land cover data for Durham - Chapel Hill were developed with remote-sensing methods from U.S. Department of Agriculture 2010 National Agriculture Imagery Program (NAIP) aerial photography and supplemental data including Light Detection and Ranging (LiDAR) data. The land cover data for this area include five categories: (i) Water, (ii) Impervious surface, (iii) Soil & barren, (iv) Trees & forest, and (v) Grass & other herbaceous. US EPA previously conducted error analysis of the land cover data through random sampling of approximately 600 photo-interpreted reference points which demonstrated that the land cover classification for this area had an overall accuracy of 83%. For this study, total vegetated land cover was defined as the proportion of land within the Tree & forest and Grass & other herbaceous categories.
Residential addresses of study participants who lived within the high-resolution land cover data area were geocoded. Only individuals with complete high-resolution land cover data for a 500 m radius around the residence were included in further analysis. The 500 m radius was selected because previous epidemiological studies commonly used 500 m or smaller distance from residence for assessing exposure to greenery (Dadvand et al. 2015; Dadvand et al. 2016; Fuertes et al. 2016; James et al. 2016; Lovasi et al. 2013; Villeneuve et al. 2012). Measures of vegetated land cover were produced for ten concentric 50 m-wide annuli around each residence, from 0–50 m to 450–500 m.
In order to assess the distance-dependence of associations between vegetated land cover and AL, annulus-specific data were combined using seven different weighting schemes (Table 1). The first scheme assigned 100% weight to the first 0 – 50 m annulus and zero weights to the remaining nine annuli to estimate average vegetated land cover within 50 m radius. The next four schemes were based on the assumption that the effect of vegetated land cover decays exponentially with the distance from residence. Weighted exposure measures were calculated as described below using sets of annulus-specific weights derived from cumulative probabilities of four exponential distributions. The cumulative density function of the exponential distribution is
Weight for each annulus was calculated as an interval cumulative density
In this equation, x1 was set equal to the inner radius and x2 to the outer radius of the annulus. The parameter λ varied from 0.02 (a steep decline of weights with distance from residence) to 0.0025 (a slow decline of weights), with 50% reduction at each step. The sixth scheme applied the same weight of 0.1 to all ten annuli. Finally, the seventh scheme used the average vegetated land cover within 500 m from residence. To calculate it from annulus-specific data, weights were set proportional to the area of each annulus: from 1% for the 0–50 m annulus (area 7,854 m2) to 19% for the 450–500 m annulus (area 149,226 m2).
Table 1.
Weights for calculating weighted mean residential vegetated land cover by weighting scheme and 50 m intervals (annuli) from the residence.
| Distance from residence, m | Scheme 1: Average within 50 m radius |
Scheme 2: Exponential decay, distribution with λ = 0.02 |
Scheme 3: Exponential decay, distribution with λ = 0.01 |
Scheme 4: Exponential decay, distribution with λ = 0.005 |
Scheme 5: Exponential decay, distribution with λ = 0.0025* |
Scheme 6: Equal weights for 50 m annuli within 500 m |
Scheme 7: Average within 500 m radius |
|---|---|---|---|---|---|---|---|
| 0–50 | 1.00 | 0.63 | 0.39 | 0.22 | 0.16 | 0.10 | 0.01 |
| 50–100 | 0.00 | 0.23 | 0.24 | 0.17 | 0.15 | 0.10 | 0.03 |
| 100–150 | 0.00 | 0.09 | 0.14 | 0.13 | 0.13 | 0.10 | 0.05 |
| 150–200 | 0.00 | 0.03 | 0.09 | 0.10 | 0.11 | 0.10 | 0.07 |
| 200–250 | 0.00 | 0.01 | 0.05 | 0.08 | 0.10 | 0.10 | 0.09 |
| 250–300 | 0.00 | 0.00 | 0.03 | 0.06 | 0.09 | 0.10 | 0.11 |
| 300–350 | 0.00 | 0.00 | 0.02 | 0.05 | 0.08 | 0.10 | 0.13 |
| 350–400 | 0.00 | 0.00 | 0.01 | 0.04 | 0.07 | 0.10 | 0.15 |
| 400–450 | 0.00 | 0.00 | 0.01 | 0.03 | 0.06 | 0.10 | 0.17 |
| 450–500 | 0.00 | 0.00 | 0.00 | 0.02 | 0.05 | 0.10 | 0.19 |
Weights rescaled so that they add to 1.
To assess the degree of urbanicity, census block-group level data on average numbers of housing units per acre were abstracted from EPA’s Smart Location Database (https://www.epa.gov/smartgrowth/smart-location-mapping#SLD). Housing unit density data were square root transformed for regression analysis.
2.4. Analysis of biomarkers
Biomarkers for this study were selected based on the review of AL indices by Juster et al. (2010) with additions and modifications. The resulting list of 18 biomarkers is presented in Table 2. It includes biomarkers which have been used in previous studies of AL (Cohen et al. 2015; Juster et al. 2010), such as C-reactive protein (CRP), fibrinogen, uric acid, high density and low density lipoproteins (HDL and LDL, respectively), dehydroepiandrosterone (DHEA), catecholamines (epinephrine, norepinephrine and dopamine) and four pro-inflammatory cytokines (interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α); as well as several additional biomarkers reflecting effects of chronic stress including serum amyloid A (SAA), vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and myeloperoxidase (MPO) in serum, and α-amylase in saliva. The study did not include AL biomarkers derived from medical examination, such as systolic and diastolic blood pressure or peak expiratory flow rate, because the parent project did not include such examinations of participants. Therefore, the biomarker-based composite indices which were used in this study differ from AL indices used previously. However, like AL indices employed in previous studies, they are comprised of biomarkers reflecting physiological effects of stress. Moreover, there is no standardized set of AL biomarkers, and new stress and adaptation-related biomarkers can be identified using research on specific health end-points (McEwen 2015).
Table 2.
List of biomarkers and analytical methods.
| Type | Biomarker | Test | Supplier (Cat. #) | Matrix | Biological function | General health implications |
|---|---|---|---|---|---|---|
| Metabolic | α-amylase | Kinetic | Salimetrics (1-1902) | saliva | Major salivary enzyme that hydrolyzes polysaccharides to glucose and maltose. | Biomarker of sympathetic nervous system; elevated levels linked to stress (Nater and Rohleder 2009). |
| Metabolic | Uric acid | Kinetic | Salimetrics (1-3802) | serum | Product of metabolic breakdown of purine nucleotides. | Elevated levels linked to gout, kidney stones and diabetes (Dehghan et al. 2008); low levels may be linked to depression (Jimenez-Fernandez et al. 2015). |
| Metabolic | Low density lipoprotein (LDL) | Enzyme-linked immunosorbent assay (ELISA) | Cell Biolabs (STA-391) | serum | “Bad cholesterol”; lipoprotein synthesized in the liver; transports cholesterol to tissues; can be deposited in the walls of blood vessels and contribute to atherosclerosis. | Elevated serum LDL contributes to the pathogenesis of atherosclerosis (Hansson and Hermansson 2011). |
| Metabolic | High density lipoprotein (HDL) | ELISA | Cell Biolabs (STA-391) | serum | “Good cholesterol”; transports cholesterol from tissues to the liver. | HDL has vascular protective effects; reduces the risk of atherosclerosis (Luscher et al. 2014). High HDL in combination with low SAA is predictive of reduced mortality risk (Zewinger et al. 2015). |
| Metabolic | Serum amyloid A (SAA) | MSD V-plex vascular injury panel | MSD (K15198G-1) | serum | Family of apolipoproteins associated with HDL and secreted during acute phase of inflammation; involved in recruitment of immune cells to inflammatory sites. | High levels linked to inflammatory diseases, diabetes (Marzi et al. 2013), CVD and death (Johnson et al. 2004; Zamani et al. 2013). |
| Immune | C-reactive protein (CRP) | MSD V-plex vascular injury panel | MSD (K15198G- 1) | serum | Protein synthesized in the liver; enhances phagocytosis during acute phase reactions that promote inflammation. | Elevated in chronic inflammatory diseases; risk factor for CVD and death (Barron et al. 2015; Koenig et al. 1999; Zengin et al. 2015). |
| Immune | Vascular cell adhesion molecule 1 (VCAM-1) | MSD V-plex vascular injury panel | MSD (K15198G- 1) | serum | Protein consisting of immunoglobulin domains; expressed in blood vessels upon cytokine stimulation; promotes adhesion of lymphocytes to vascular endothelium. | Elevated levels linked to CVD and risk of death (Zamani et al. 2013). |
| Immune | Intercellular adhesion molecule 1 (ICAM-1) | MSD V-plex vascular injury panel | MSD (K15198G- 1) | serum | Immunoglobulin glycoprotein produced by vascular endothelium and leukocytes; induced by IL-1 and TNF; produces pro-inflammatory effects through cell signaling. | Elevated levels linked with chronic inflammation, diabetes (Gu et al. 2012), stroke and risk of death (Schnabel et al. 2013). |
| Immune | Interleukin (IL)-1β | MSD U-plex pro-inflammatory cytokines panel | MSD (K15053K- 1) | serum | Cytokine protein produced by activated macrophages; mediator of inflammatory responses; involved in cell proliferation, differentiation, and apoptosis. | Central regulator of stress response affecting levels of other stress biomarkers (Goshen and Yirmiya 2009); involved in pathogenesis of atherosclerosis (Hansson and Hermansson 2011). |
| Immune | IL-6 | MSD U-plex pro-inflammatory cytokines panel | MSD (K15053K- 1) | serum | Cytokine produced by macrophages and T-cells; stimulates B cell and T cell differentiation in acute phase response to tissue damage; produces fever. | Drive production of reactant proteins, including CRP; elevated levels linked to major depressive disorder (Haapakoski et al. 2015) and higher risk of death in CVD patients (Zamani et al. 2013). |
| Immune | IL-8 | MSD U-plex pro-inflammatory cytokines panel | MSD (K15053K- 1) | serum | Cytokine produced by macrophages; mediator of pro-inflammatory response; induces chemotaxis and phagocytosis by immune cells at infection sites. | Elevated levels linked with inflammation, obesity and increased risk of death (Cavusoglu et al. 2015). |
| Immune | TNF-α | MSD U-plex pro-inflammatory cytokines panel | MSD (K15053K- 1) | serum | Cytokine produced by macrophages; functions in systemic inflammation by evoking mediators of acute phase reactions as well as in tumor apoptosis. | Produces fever; used pharmacologically; elevated levels linked to autoimmune disorders and major depressive disorder (Haapakoski et al. 2015). |
| Immune | Fibrinogen | ELISA | Abcam (ab108842) | serum | Protein that synthesizes into fibrin in the liver; functions as a blood clotting factor that promotes coagulation. | Elevated levels linked to thrombosis, CVD, and increased risk of death (Barron et al. 2015; Kunutsor et al. 2016). |
| Immune | Myeloperoxidase (MPO) | ELISA | Abcam (ab119605) | serum | Peroxidase enzyme produced by activated neutrophils, monocytes and macrophages. Catalyzes the modification of LDL. | Elevated levels linked to coronary artery disease (Zakynthinos and Pappa 2009). |
| Neuroendocrine | Dehydroepiandrosterone (DHEA) | ELISA | Salimetrics (11202) | serum | Steroid hormone; precursor of sex hormones; suppresses inflammation; improves lipid metabolism; decreases insulin resistance; reduces oxidative brain damage. | Low levels linked to coronary heart disease (Tivesten et al. 2014), immune disorder and increased risk of death (Ohlsson et al. 2015). |
| Neuroendocrine | Epinephrine | ELISA | Eagle Biosciences (AND31-K03) | serum | Catecholamine produced by adrenal glands and brain; increases heart rate and glucose levels, decreases digestive and immune functions during “fight- or-flight” response. | Increased in acute stress and exercise; used to treat cardiac arrest and allergic reactions. Inconsistent reports on associations with burnout and depression; high levels indicative of AL in previous studies (Juster et al. 2010; Juster et al. 2011). |
| Neuroendocrine | Norepinephrine | ELISA | Eagle Biosciences (AND31-K03) | serum | Catecholamine produced by the brain; increases blood pressure, constricts blood vessels, and modulates brain activities during “fight-or-flight” response. | Increases alertness, heart rate and blood pressure; increased in stress (Gu et al. 2016); high level can be predictive of CVD (Yufu et al. 2014); low levels linked with depression (Moret and Briley 2011). |
| Neuroendocrine | Dopamine | ELISA | Eagle Biosciences (AND31-K03) | serum | Catecholamine produced in the brain and adrenal glands; involved in motivation, voluntary movement, and cognition; increases blood pressure and heart rate. | Major role in reward pathways in brain; used to treat cardiac arrest; low levels linked to depression (Dunlop and Nemeroff 2007; Kapur and Mann 1992). |
Commercially available biomarker assay kits (Table 2) were selected based on technical characteristics, laboratory equipment requirements, minimum sample volume and cost of analysis. Two multiplexed assays based on the Meso Scale Discovery (MSD) electrochemiluminescence platform were used to analyze four pro-inflammatory cytokines or four inflammation and vascular injury biomarkers simultaneously.
Samples were analyzed at US EPA laboratories in Chapel Hill, NC and Cincinnati, OH in accordance with manufacturers’ instructions. Most biomarkers were initially analyzed in two matrices (serum and saliva) using a subset of samples (except the salivary enzyme α-amylase, which was tested in saliva only, and lipoproteins, which were tested in serum only). Based on preliminary analysis of distributions of biomarker values and associations between biomarker values in serum and saliva (not shown), further analysis was conducted using one matrix per biomarker: 17 biomarkers were analyzed in serum and one in saliva (Table 2). In each assay, at least 20% of samples were assayed in duplicate. Geometric mean values from duplicate tests were used in data analysis.
Concentrations of analytes were estimated by linear interpolation of standards or by fitting four-parameter logistic dose-response curves to serially diluted standards and solving the resulting equations for individual samples in accordance with manufacturer’s instructions. Biomarker measurements which did not meet pre-set quality control criteria specified by assay manufacturers were excluded from further analysis.
Body mass index (BMI) values were calculated from height and weight data collected at enrollment. Preliminary analysis showed that, as expected, BMI was a strong predictor of many biomarkers of inflammation, vascular injury, metabolic and neuroendocrine functions. At the same time, BMI, as a continuous or categorical variable, was not associated with vegetated land cover in this dataset. Therefore, BMI was included in regression models for AL and individual biomarkers as a covariate to improve precision of parameter estimates.
2.5. Allostatic load indices
In this study, an AL index was defined as a sum of dichotomized biomarker values, which is the most commonly used approach in AL studies (Juster et al. 2010). Data on biomarkers were dichotomized at a low end of the distribution (DHEA, dopamine, and HDL), at a high end of the distribution (IL-1β, IL-6, IL-8, TNF-α, fibrinogen, uric acid, MPO, α-amylase, CRP, LDL, SAA, VCAM-1, and ICAM-1) or at both ends (norepinephrine and epinephrine), depending on which tail of the biomarker distribution is associated with adverse stress-related health effects.
For norepinephrine and epinephrine, both ends of the distribution have been linked with physiological dysregulation and disease. High levels of urinary norepinephrine and epinephrine have been used as indicators of neuroendocrine distress in previous AL studies (Clark et al. 2007; Seeman et al. 1997). While high levels of norepinephrine have been linked with adverse cardiovascular outcomes (Yufu et al. 2014), low levels of norepinephrine are also known to be associated with depression (Moret and Briley 2011). Results of some studies suggest that epinephrine may also be reduced in depression (Ambade et al. 2009).
Due to missing values (mainly lacking saliva samples for α-amylase tests and insufficient serum volume for LDL and HDL tests, which required the largest serum volume of all tests employed in this study), using all 18 biomarkers resulted in a greatly reduced sample size. Therefore, two alternative AL indices were calculated: (i) based on all 18 biomarkers (with two binary variables for epinephrine and norepinephrine each for a total of 20 binary variables); and (ii) based on 15 serological biomarkers, excluding α-amylase, HDL and LDL (17 binary variables in total). The AL indices were count data, which approximately followed Poisson distributions.
Biomarkers were dichotomized using two sets of distribution-based cut-off points: (i) below the 10th percentile or above the 90th percentile of the biomarker distribution; and (ii) below the 25th or above the 75th percentile. The latter quartile-based cut-off points have been used extensively in AL studies (Juster et al. 2010; Seeman et al. 1997). More extreme distribution-based cut-off points (10th and 90th percentiles) were explored because recent research demonstrated that impacts of environmental stressors on some health effect biomarkers are strongest among individuals who were already at risk and had extreme biomarker values at baseline (Bind et al. 2016). AL indices were calculated for both sets of dichotomization cutoff points and both sets of biomarkers (including 15 and 18 biomarkers) for a total of four AL indices.
2.6. Statistical data analysis
Statistical analysis was conducted using SAS version 9.4 (SAS Institute, Cary, NC) software. Regression analyses of associations between vegetated land cover and AL, individual biomarkers and previously diagnosed diseases were conducted using generalized additive models (SAS procedure GAM), which is a common approach in analysis of geographic distributions of health outcomes (Clements et al. 2005). The AL data were analyzed using models for Poisson-distributed outcomes while data on individual biomarkers and diseases were analyzed using models for binomial outcomes.
To control for spatial autocorrelation, these regression models included a two dimensional spline smoothing function of geographic coordinates as described previously (Dormann et al. 2007; Webster et al. 2006). Splines were fitted using the “spline2” function in the SAS procedure GAM, which is based on the penalized least squares method. It computes a thin-plate flexible surface approximating spatial variation of the parameter of interest. The default approach was to use the generalized cross validation (GSV) option to automatically select the degrees of freedom which determines the amount of smoothing or flexibility of the thin plate. In several models for individual biomarkers, the number of degrees of freedom had to be constrained to enable model convergence.
All regression models involving vegetated land cover data also included census group block-level average housing unit density (square root transformed to achieve normality) as a covariate. Age was modelled as a continuous variable for biomarkers which are monotonously associated with age, or as a categorical variable for U-shaped or inversely U-shaped associations and included in all regression models for biomarkers and AL. Other covariates were selected using a step-wise selection (bidirectional elimination) procedure for each vegetated land cover weighting scheme and then combined to produce a common set of covariates for all regression models involving a specific outcome variable. In addition to the core covariates listed above, other covariates considered were gender, race, education, smoking status and BMI. Stratified analyses by age, gender, race, obesity status and city of residence were conducted using the same set of covariates as analysis of the entire dataset minus the stratification variable. Regression models for diseases did not include biomarkers as covariates because biomarkers may be intermediate endpoints between exposure to vegetation and prevention of disease. Spatial autocorrelation in model residuals was analyzed using the Moran’s I tests and variogram plots (SAS procedure VARIOGRAM). Only results of models with non-significant Moran’s I test statistics were accepted.
Results of regression analysis were expressed as multiplicative change in the outcome per interquartile range (IQR) increase in proportion of vegetated land cover. In addition, regression analysis of AL was conducted with vegetated land cover modelled as an ordinal variable with categories corresponding to tertiles of vegetated land cover distribution. Associations between biomarkers and diseases were analyzed using general linear models (SAS procedure GENMOD); these models did not include vegetated land cover data.
3. Results
3.1. Descriptive statistics
Complete high resolution land cover data for 500 m radius around the residence was available for 206 individuals residing at 173 unique street addresses. Of these, 148 were addresses with one study participant per address. The remaining 25 street addresses with two to four study participants per address (58 individuals total) were mainly apartment buildings.
The age of study participants ranged from 18 to 85 years (mean 37.4 years, median 33 years); 66.0% of participants were women (Table 3). Women were overrepresented due to their greater willingness to participate in this study. For regression analysis, race and ethnicity data were combined to produce a binary variable defined as non-Hispanic Whites (53.7%) vs. all others.
Table 3.
Demographic, socioeconomic and health characteristics of the study population.
| Variable | Category | Number | Mean or percent |
|---|---|---|---|
| Age (years) | 206 | 37.4 | |
| Gender | Males | 70 | 34.0% |
| Females | 136 | 66.0% | |
| Race | White | 117 | 56.8% |
| African American | 75 | 36.4% | |
| American Indian or Alaska Native | 1 | 0.5% | |
| Asian or Pacific Islander | 4 | 1.9% | |
| Other | 8 | 3.9% | |
| Ethnicity | Hispanic | 13 | 6.3% |
| Education | Did not graduate high school | 13 | 6.3% |
| High school graduate | 32 | 15.5% | |
| Some college, no degree | 40 | 19.4% | |
| Associate degree | 15 | 7.3% | |
| Bachelor’s degree | 60 | 29.1% | |
| Post baccalaureate degree | 46 | 22.3% | |
| Ever lived on a farm | 37 | 18.0% | |
| Water from private well | 24 | 11.7% | |
| Current smoker | 57 | 27.7% | |
| BMI category | Obese | 59 | 28.6% |
| Overweight | 55 | 26.7% | |
| Normal weight | 89 | 43.2% | |
| Underweight | 3 | 1.3% | |
| Self-reported health | Excellent | 49 | 23.8% |
| Very good | 92 | 44.7% | |
| Good | 51 | 24.8% | |
| Fair | 13 | 6.3% | |
| Poor | 0 | 0.0% | |
| Previously diagnosed with: | |||
| Allergy (any) | 107 | 51.9% | |
| Depression | 47 | 22.8% | |
| Asthma | 25 | 12.1% | |
| Arthritis | 20 | 9.7% | |
| IBS | 13 | 6.3% | |
| Diabetes | 9 | 4.4% | |
| Ulcers | 8 | 3.9% | |
| Cancer | 4 | 1.9% | |
| Liver disease | 2 | 1.0% | |
| Heart disease | 1 | 0.5% | |
| Chronic obstructive pulmonary disease (COPD) | 0 | 0.0% | |
| Kidney disease | 0 | 0.0% |
Education data were dichotomized as four-year college degree and above (51.5%) vs. all others. This relatively high level of education reflected the composition of source communities. Approximately half of study participants (51%) lived in Durham (U.S. Census Bureau estimates 258,000 residents in 2015; 47% of adults had a bachelor’s degree or higher education), 29% lived in Chapel Hill (city with a large university campus; 59,000 residents, 74% of adults had at least a bachelor’s degree), 11% lived in Carrboro (town adjacent to Chapel Hill; 21,000 residents, 68% of adults had at least bachelor’s degree) and 9% lived in other towns in the Durham-Chapel Hill metropolitan area. Predominant types of housing in the study population were detached single family homes, townhouses and low-rise apartment buildings.
BMI data indicated that 28.6% of study participants were obese according to the World Health Organization’s definition (BMI ≥ 30.0 kg/m2). The 90th percentile of BMI distribution in this sample corresponded to 36.8 kg/m2. The prevalence of current smoking was 27.7%.
Allergy was the most common health condition reported by 51.9% of participants followed by previously diagnosed depression, asthma, arthritis, IBS and diabetes. These six diseases were analyzed for associations with vegetated land cover. Data on other self-reported diseases were not included in regression analysis due to the small numbers of cases. For regression analysis, self-reported health status was dichotomized as excellent or very good (68.4%) vs. good, fair or poor.
Descriptive statistics for variability in average or weighted vegetated land cover near residences by weighting scheme are presented in Table 4. Variability was the greatest for average vegetated land cover within 50 m radius around residence (weighting scheme 1) ranging from 6.0% to 99.8%, with IQR of 25.8%, and the smallest for average vegetated land cover within the 500 m radius (weighting scheme 7), ranging from 36.9% to 95.2%, IQR 15.0%. Average housing unit density at a census block-group level ranged from 0.08 to 11.2 (median 1.53) housing units per acre or 0.20 to 27.7 (median 3.78) housing units per hectare.
Table 4.
Summary statistics of weighted residential vegetated land cover (%) variability by land cover weighing scheme.
| Vegetated land cover weighting scheme | Min | 25th percentile | Median | 75th percentile | Max | Interquartile range |
|---|---|---|---|---|---|---|
| 1: Average within 50 m radius | 6.0 | 44.7 | 59.9 | 70.6 | 99.8 | 25.9 |
| 2: Exponential decay, distribution with λ = 0.02 | 10.6 | 50.5 | 64.1 | 72.3 | 97.4 | 21.8 |
| 3: Exponential decay, distribution with λ = 0.01 | 16.4 | 53.6 | 63.2 | 74.6 | 94.6 | 21.0 |
| 4: Exponential decay, distribution with λ = 0.005 | 20.9 | 53.0 | 61.1 | 71.4 | 86.1 | 18.3 |
| 5: Exponential decay, distribution with λ = 0.0025* | 27.1 | 59.3 | 67.7 | 78.0 | 94.0 | 18.7 |
| 6: Equal weights for 50 m annuli within 500 m | 31.7 | 61.1 | 68.7 | 78.5 | 94.3 | 17.4 |
| 7: Average within 500 m radius | 36.9 | 63.8 | 72.8 | 78.8 | 95.2 | 15.0 |
Weights were rescaled so that they add up to 1 within the ten 50-m annuli.
Summary statistics on individual biomarkers are presented in Table 5. While the total sample size was 206 individuals, sample sizes for analysis of individual biomarkers ranged from 115 to 204. Reduced sample sizes for some biomarkers were due to insufficient saliva or serum sample volumes which did not allow for all biomarker tests. Pairwise Kendall correlation coefficients τ among dichotomized biomarkers ranged from −0.14 to 0.54, with the strongest correlations between CRP and SAA (τ = 0.54) and between ICAM-1 and VCAM-1 (τ = 0.44).
Table 5.
Summary of individual biomarker values in the study population.
| Parameter | Units | N | 10th percentile | Median | 90th percentile |
|---|---|---|---|---|---|
| Biomarker | |||||
| α-amylase | U/mL | 115 | 28.2 | 65.8 | 160 |
| CRP | ng/mL | 204 | 211 | 1,754 | 11,225 |
| DHEA | ng/mL | 196 | 1.15 | 3.28 | 7.37 |
| Dopamine* | ng/mL | 195 | < LOQ | 0.04 | 0.19 |
| Epinephrine | ng/mL | 200 | 0.04 | 0.11 | 0.22 |
| Fibrinogen | μg/mL | 202 | 1.27 | 1.92 | 2.86 |
| HDL | mg/dL | 162 | 45.4 | 64.9 | 103 |
| ICAM-1 | ng/mL | 204 | 264 | 402 | 672 |
| IL-1β | pg/mL | 203 | < LOQ | < LOQ | 0.71 |
| IL-6 | pg/mL | 203 | < LOQ | 1.6 | 9.86 |
| IL-8 | pg/mL | 202 | 29.6 | 57 | 106 |
| LDL | mg/dL | 162 | 57.1 | 110 | 185 |
| MPO | ng/mL | 202 | 15.9 | 32.7 | 66.3 |
| Norepinephrine | ng/mL | 200 | 0.12 | 0.74 | 1.45 |
| SAA | ng/mL | 204 | 334 | 1042 | 4,451 |
| TNF-α | pg/mL | 203 | < LOQ | 4.75 | 11.5 |
| Uric acid | mg/dL | 198 | 3.41 | 5.09 | 7.62 |
| VCAM-1 | ng/mL | 202 | 361 | 536 | 868 |
Dopamine was dichotomized at the 14th (rather than 10th) or 25th percentile because 14% of results were below the Limit of Quantitation (LOQ).
AL indices involving all 18 biomarkers (AL1 for the 10th and 90th percentile-based dichotomization cut-off points, and AL3 for 25th and 75th percentile cut-offs) were available for a subset of 80 individuals while AL2 and AL4 indices based on 15 serological biomarkers were available for additional 106 individuals for a total of 186 individuals. The 80 individuals with data on all 18 biomarkers did not differ significantly from the other 106 individuals regarding age, gender, education, and prevalence of obesity (not shown). For all four AL indices, the minimum value was zero; the maximum values were seven for AL 1, nine for AL 2 and AL 3, and eleven for AL 4.
3.2. Associations between residential vegetated land cover and individual dichotomized biomarkers
Regression analysis results for biomarkers dichotomized at the 10th or 90th percentile are presented in Table 6 while results for biomarkers dichotomized at the 25th or 75th percentiles are presented in Supplementary Table 1. Effect estimates smaller than one mean that individuals with greater vegetated land cover near their residences were less likely to have a potentially unhealthy value of a certain biomarker. Columns in these two tables correspond to the seven weighting schemes for vegetated land cover described in Table 1. Adjusted odds ratio (aOR) estimates are for an IQR increase in weighted vegetated land cover (IQR data are shown in Table 4). Covariates used in all regression models for each outcome are listed in footnotes for Table 6 and Supplementary Table 1. For each outcome variable, the same set of covariates was used in all regression models with different land cover weighting schemes and dichotomization cut-offs.
Table 6.
Estimated effects with 95% confidence limits of residential vegetated land cover on biomarkers, AL and diseases by land cover weighting scheme: adjusted ORs of potentially unhealthy biomarker values (biomarkers dichotomized at 10th or 90th percentile), adjusted multiplicative changes in mean AL and aORs of diseases per IQR increases in residential vegetated land cover.
| Outcome (set of covariates) | Scheme 1: Average within 50 m radius | Scheme 2: Exponential decay, distribution with λ = 0.02 | Scheme 3: Exponential decay, distribution with λ = 0.01 | Scheme 4: Exponential decay, distribution with λ = 0.005 | Scheme 5: Exponential decay, distribution with λ = 0.0025* | Scheme 6: Equal weights for 50 m annuli within 500 m | Scheme 7: Average within 500 m radius |
|---|---|---|---|---|---|---|---|
| Biomarker a | |||||||
| α-amylase > 90 pctl (1) | 0.46 (0.22, 0.95)* | 0.36 (0.18, 0.74)* | 0.39 (0.19, 0.80)* | 0.36 (0.16, 0.78)* | 0.37 (0.18, 0.79)* | 0.33 (0.15, 0.74)* | 0.42 (0.2, 0.86)* |
| CRP > 90 pctl (2) | 0.48 (0.23, 0.99)* | 0.51 (0.26, 1.00) | 0.50 (0.23, 1.06) | 0.52 (0.23, 1.19) | 0.58 (0.25, 1.32) | 0.66 (0.29, 1.49) | 0.83 (0.40, 1.74) |
| DHEA < 10 pctl (2) | 0.62 (0.31, 1.20) | 0.57 (0.30, 1.07) | 0.49 (0.25, 0.99)* | 0.45 (0.22, 0.95)* | 0.46 (0.22, 0.96)* | 0.48 (0.23, 1.01) | 0.58 (0.29, 1.14) |
| Dopamine < 10 pctl (3) | 0.47 (0.27, 0.83)* | 0.48 (0.29, 0.82)* | 0.46 (0.26, 0.81)* | 0.47 (0.26, 0.84)* | 0.49 (0.28, 0.88)* | 0.53 (0.30, 0.95)* | 0.65 (0.38, 1.10) |
| Epinephrine < 10 pctl (2) | 1.41 (0.73, 2.72) | 1.33 (0.71, 2.47) | 1.28 (0.66, 2.48) | 1.21 (0.61, 2.41) | 1.16 (0.59, 2.29) | 1.10 (0.56, 2.16) | 1.01 (0.55, 1.87) |
| Epinephrine > 90 pctl (2) | 0.47 (0.25, 0.88)* | 0.44 (0.24, 0.79)* | 0.37 (0.19, 0.72)* | 0.34 (0.17, 0.68)* | 0.34 (0.17, 0.67)* | 0.36 (0.18, 0.69)* | 0.43 (0.24, 0.77)* |
| Fibrinogen > 90 pctl (2) | 0.67 (0.36, 1.24) | 0.62 (0.35, 1.12) | 0.56 (0.29, 1.07) | 0.51 (0.25, 1.02) | 0.49 (0.24, 0.98)* | 0.47 (0.24, 0.94)* | 0.50 (0.27, 0.91)* |
| HDL < 10 pctl (4) | 1.12 (0.44, 2.81) | 0.85 (0.35, 2.06) | 0.63 (0.24, 1.63) | 0.46 (0.17, 1.24) | 0.38 (0.14, 1.04) | 0.34 (0.13, 0.90)* | 0.34 (0.14, 0.82)* |
| ICAM-1 > 90 pctl (2) | 0.78 (0.42, 1.48) | 0.74 (0.41, 1.36) | 0.65 (0.34, 1.25) | 0.59 (0.30, 1.16) | 0.57 (0.29, 1.13) | 0.58 (0.30, 1.12) | 0.63 (0.34, 1.14) |
| IL-1β > 90 pctl (1) | 1.25 (0.68, 2.32) | 1.09 (0.62, 1.92) | 0.98 (0.54, 1.78) | 0.86 (0.46, 1.59) | 0.79 (0.43, 1.45) | 0.71 (0.39, 1.30) | 0.63 (0.36, 1.09) |
| IL-6 > 90 pctl (4) | 0.56 (0.29, 1.08) | 0.62 (0.33, 1.15) | 0.63 (0.31, 1.25) | 0.64 (0.31, 1.33) | 0.65 (0.31, 1.37) | 0.67 (0.32, 1.39) | 0.73 (0.38, 1.40) |
| IL-8 > 90 pctl (5) | 0.75 (0.42, 1.35) | 0.67 (0.38, 1.16) | 0.52 (0.28, 0.96)* | 0.40 (0.21, 0.78)* | 0.36 (0.18, 0.70)* | 0.33 (0.17, 0.66)* | 0.36 (0.19, 0.66)* |
| LDL > 90 pctl (4) | 1.64 (0.58, 4.62) | 1.48 (0.54, 4.04) | 1.56 (0.54, 4.57) | 1.73 (0.59, 5.09) | 1.84 (0.64, 5.26) | 1.96 (0.71, 5.42) | 2.04 (0.82, 5.08) |
| MPO > 90 pctl (6) | 1.28 (0.66, 2.46) | 1.12 (0.61, 2.07) | 1.09 (0.56, 2.12) | 1.11 (0.55, 2.22) | 1.12 (0.56, 2.25) | 1.13 (0.56, 2.26) | 1.11 (0.58, 2.12) |
| Norepinephrine<10 pctl (7) | 0.36 (0.18, 0.74)* | 0.42 (0.22, 0.80)* | 0.42 (0.21, 0.82)* | 0.42 (0.21, 0.83)* | 0.42 (0.21, 0.84)* | 0.43 (0.22, 0.85)* | 0.48 (0.26, 0.90)* |
| Norepinephrine>90 pctl (7) | 0.62 (0.33, 1.17) | 0.65 (0.36, 1.19) | 0.61 (0.32, 1.17) | 0.58 (0.30, 1.14) | 0.59 (0.30, 1.14) | 0.61 (0.32, 1.16) | 0.68 (0.38, 1.22) |
| SAA > 90 pctl (2) | 0.52 (0.27, 1.04) | 0.54 (0.28, 1.03) | 0.51 (0.25, 1.05) | 0.51 (0.24, 1.10) | 0.54 (0.25, 1.16) | 0.58 (0.27, 1.24) | 0.70 (0.35, 1.37) |
| TNF-α > 90 pctl (8) | 0.84 (0.47, 1.53) | 0.81 (0.47, 1.42) | 0.79 (0.43, 1.45) | 0.79 (0.42, 1.49) | 0.79 (0.42, 1.49) | 0.79 (0.42, 1.48) | 0.82 (0.46, 1.45) |
| Uric acid > 90 pctl (2) | 1.00 (0.51, 1.98) | 0.91 (0.48, 1.73) | 0.86 (0.43, 1.72) | 0.80 (0.39, 1.65) | 0.76 (0.37, 1.57) | 0.72 (0.35, 1.47) | 0.68 (0.36, 1.30) |
| VCAM-1 > 90 pctl (2) | 0.57 (0.32, 1.04) | 0.56 (0.32, 0.97)* | 0.49 (0.27, 0.91)* | 0.44 (0.23, 0.84)* | 0.42 (0.22, 0.81)* | 0.42 (0.22, 0.78)* | 0.45 (0.25, 0.79)* |
| Allostatic load b | |||||||
| AL 1: 18 biomarkers (2) | 0.78 (0.64, 0.96)* | 0.75 (0.61, 0.92)* | 0.69 (0.55, 0.87)* | 0.62 (0.49, 0.80)* | 0.61 (0.48, 0.78)* | 0.61 (0.49, 0.77)** | 0.64 (0.52, 0.78)** |
| AL 2: 15 biomarkers (2) | 0.71 (0.62, 0.81)** | 0.70 (0.61, 0.79)** | 0.66 (0.57, 0.76)** | 0.63 (0.55, 0.73)** | 0.63 (0.55, 0.73)** | 0.64 (0.55, 0.74)** | 0.69 (0.60, 0.78)** |
| Disease or health condition | |||||||
| Allergy (any) (9) | 0.85 (0.59, 1.24) | 0.91 (0.64, 1.29) | 0.87 (0.59, 1.27) | 0.87 (0.59, 1.31) | 0.94 (0.63, 1.40) | 0.96 (0.65, 1.44) | 1.02 (0.70, 1.47) |
| Asthma (10) | 0.71 (0.41, 1.24) | 0.73 (0.43, 1.21) | 0.70 (0.40, 1.23) | 0.70 (0.39, 1.25) | 0.71 (0.39, 1.28) | 0.74 (0.41, 1.32) | 0.81 (0.48, 1.38) |
| Arthritis (10) | 1.51 (0.76, 3.00) | 1.44 (0.75, 2.77) | 1.49 (0.73, 3.05) | 1.52 (0.71, 3.23) | 1.50 (0.70, 3.20) | 1.46 (0.69, 3.10) | 1.35 (0.68, 2.68) |
| Depression (11) | 0.45 (0.27, 0.75)* | 0.46 (0.29, 0.74)* | 0.44 (0.27, 0.72)* | 0.45 (0.27, 0.75)* | 0.48 (0.29, 0.80)* | 0.53 (0.32, 0.88)* | 0.67 (0.43, 1.06) |
| Diabetes (10) | 0.53 (0.16, 1.77) | 0.54 (0.18, 1.61) | 0.48 (0.15, 1.53) | 0.41 (0.12, 1.39) | 0.39 (0.12, 1.28) | 0.37 (0.11, 1.18) | 0.38 (0.14, 1.08) |
| Irritable Bowel Syndrome | |||||||
| (IBS) (12) | 1.00 (0.49, 2.05) | 0.91 (0.47, 1.75) | 0.83 (0.41, 1.65) | 0.79 (0.38, 1.62) | 0.79 (0.39, 1.63) | 0.82 (0.40, 1.68) | 0.90 (0.46, 1.78) |
| Obesity (3) | 0.97 (0.91, 1.04) | 0.97 (0.91, 1.03) | 0.96 (0.90, 1.03) | 0.96 (0.90, 1.03) | 0.96 (0.90, 1.03) | 0.97 (0.90, 1.04) | 0.98 (0.92, 1.04) |
| Low health status (13) | 0.55 (0.33, 0.92)* | 0.60 (0.36, 0.98)* | 0.63 (0.37, 1.09) | 0.69 (0.40, 1.21) | 0.75 (0.43, 1.31) | 0.93 (0.54, 1.62) | 0.90 (0.58, 1.42) |
Biomarkers dichotomized at 10th percentile (< 10 pctl) or 90th percentile (> 90 pctl)
Allostatic load (AL) indices based on biomarkers dichotomized at 25th or 75th percentile
0.0001 ≤ p < 0.05
p < 0.0001
Sets of covariates:
1. Age, gender, housing density, spline of geographic coordinates
2. Age, gender, race, education, BMI, housing density, spline of geographic coordinates
3. Age, gender, race, education, housing density, spline of geographic coordinates
4. Age (categorical), gender, education, BMI, housing density, spline of geographic coordinates
5. Age, education, BMI, housing density, spline of geographic coordinates
6. Age, race, housing density, spline of geographic coordinates
7. Age, race, education, BMI, housing density, spline of geographic coordinates
8. Age, gender, education, BMI, housing density, spline of geographic coordinates
9. Gender, race, BMI, housing density, spline of geographic coordinates
10. Age, gender, BMI, housing density, spline of geographic coordinates
11. Gender, race, smoking, ethnicity, housing density, spline of geographic coordinates
12. Age, race, BMI, housing density, spline of geographic coordinates
13. Education, smoking, BMI, housing density, spline of geographic coordinates.
General conclusions based on these two sets of dichotomization cut-off points are largely consistent. All statistically significant associations of vegetated land cover with AL and individual biomarkers are in the direction consistent with beneficial health effects. For most biomarkers, however, using quartile-based cut-off points produced smaller effect estimates compared to analysis using 10th and 90th percentile cut-off points.
Results of regression analysis of all biomarkers using the vegetated land cover weighting scheme 5 (exponential decay with λ = 0.0025) are shown in Figure 1 (for 10th and 90th percentile-based cut-offs) and Supplementary Figure 1 (for 25th and 75th percentile-based cutoffs). As in Table 6 and Supplementary Table 1, effect estimates less than one indicate a beneficial effects of vegetated land cover. For example, Figure 1 shows that greater vegetated land cover was associated with reduced adjusted odds of having high (above the 90th percentile) α-amylase in saliva and low (below the 10th percentile) DHEA in serum; both are considered beneficial effects. While Figure 1 shows eight statistically significant associations, only five effect estimates are statistically significant in Supplementary Figure 1.
Fig. 1.
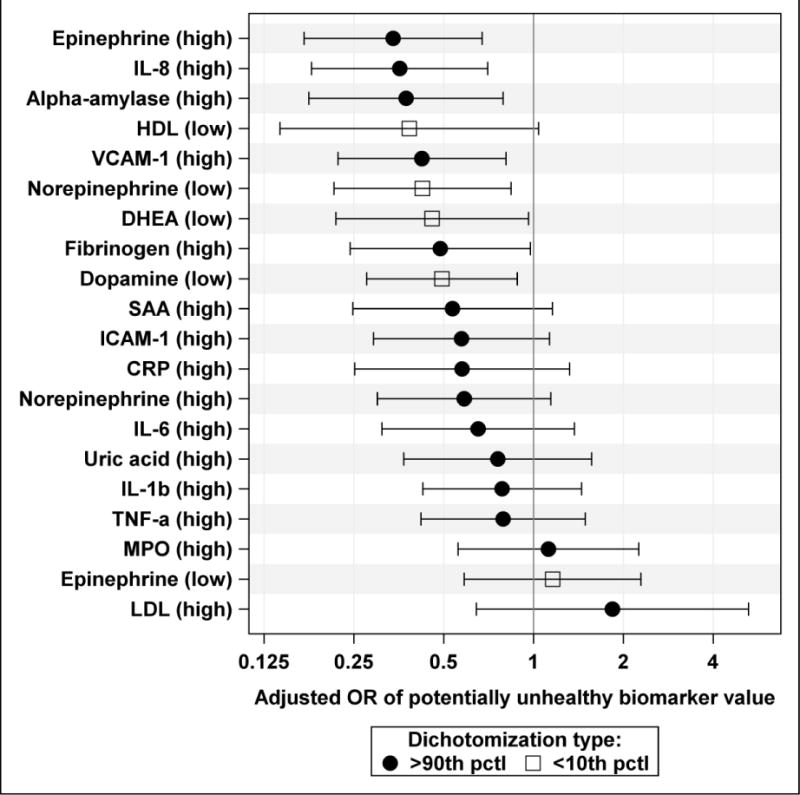
Adjusted ORs of potentially unhealthy biomarker values with 95% confidence limits per IQR increase in residential vegetated land cover (weighting scheme 5 based on exponential decay with λ = 0.0025), plotted on a logarithmic scale. Biomarkers dichotomized at 10th percentile (< 10 pctl) or 90th percentile (> 90 pctl) of their distributions.
Overall, ten biomarkers were statistically significantly (p < 0.05) associated with vegetated land cover in at least one weighting scheme (Table 6). Models using weighting schemes 5 (exponential decay with λ = 0.0025) and 6 (equal weights) produced statistically significant effect estimates for eight biomarkers each. In models using weighting scheme 5, increased vegetated land cover was linked with reduced aOR of having a low (below the 10th percentile) level of serum norepinephrine, dopamine, and DHEA, as well as reduced aOR of having a high (above the 90th percentile) level of α–amylase in saliva, and epinephrine, fibrinogen, IL-8, and VCAM-1 in serum. Models using weighting scheme 6 produced similar results except the effect estimate for DHEA was no longer significant while the effect estimate for HDL became statistically significant. Models using weighting schemes 1 and 2 produced significant associations for five biomarkers each, while models using weighting schemes 3, 4, and 7 produced significant associations for seven biomarkers each. For biomarkers dichotomized at 10th or 90th percentile, the strongest average adjusted effect of an IQR increase in vegetated land on all biomarkers was produced by models using the vegetated land cover weighting scheme 5 (on average, 34.4% reduction in the odds of having a potentially unhealthy biomarker value).
Age was positively and monotonously associated with most biomarkers. As associations between age and LDL, HDL and IL-6 were non-linear, age was entered as a categorical variable in regression models for these biomarkers (see footnotes for Table 6 and Supplementary Table 1). Greater BMI was associated with increased odds of having potentially unhealthy values of many biomarkers. Low educational attainment and minority status were also associated with elevated odds of having potentially unhealthy values of several biomarkers.
3.3. Associations between residential vegetated land cover and allostatic load
All four AL indices were significantly inversely associated with vegetated land cover in all regression models using seven vegetated land cover weighting schemes (Table 6 and Supplementary Table 1). All seven effect estimates for AL2 index based on 15 biomarkers and 10th/90th percentile dichotomization cut-off points were highly significant with p < 0.0001. Figure 2 shows adjusted effect estimates with 95% confidence intervals for four AL indices and seven vegetated land cover weighting schemes. All effect estimates for AL1 and AL2 indices (based on biomarkers dichotomized at 10th or 90th percentiles) were larger than corresponding effect estimates for AL3 and AL4 indices (based on biomarkers dichotomized at 25th or 75th percentiles).
Fig. 2.
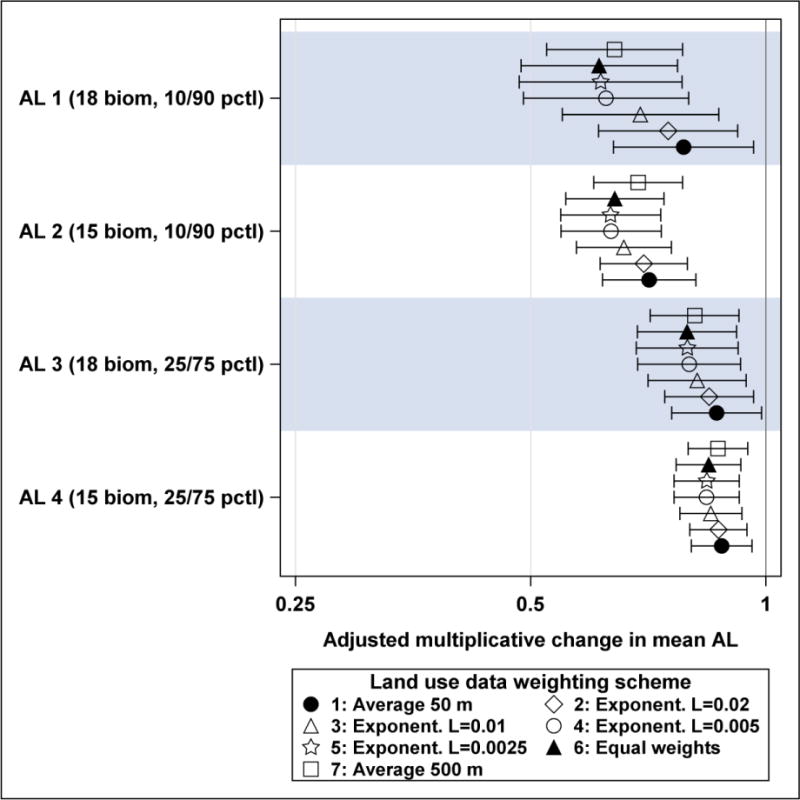
Adjusted multiplicative changes in mean AL indices with 95% confidence limits per IQR increase in residential vegetated land cover plotted on a logarithmic scale. AL1: 18 biomarkers dichotomized at 10th or 90th percentile; AL2: 15 biomarkers dichotomized at 10th or 90th percentile; AL3: 18 biomarkers dichotomized at 25th or 75th percentile; AL4: 15 biomarkers dichotomized at 25th or 75th percentile. Vegetated land cover weighting schemes are: 1 – average within 50 m radius, 2 to 5 – weights from exponential decay functions with lambda (L) parameter from 0.02 to 0.0025, 6 – equal weights of 0.1 assigned to all 50 m annuli; and 7 – average within 500 m.
Results of analysis using an ordinal variable with categories corresponding to tertiles of vegetated land cover distribution for the weighting scheme 5 (exponential decay with λ = 0.0025) show consistent monotonous declines in all four AL indices in the 2nd and 3rd tertiles of vegetated land cover compared to the 1st tertile (Figure 3). Adjusted effect estimates for the 3rd tertile were statistically significant for all four AL indices while adjusted effect estimates for the 2nd tertile were substantially smaller.
Fig. 3.
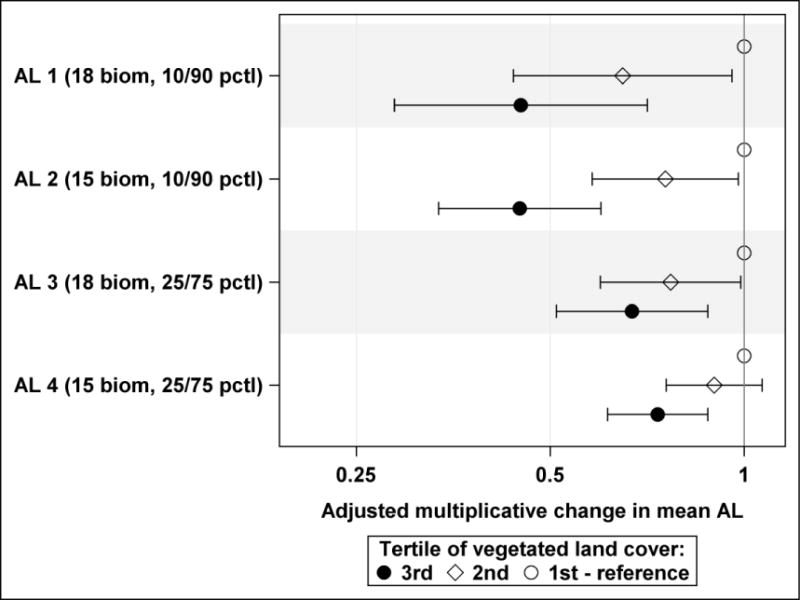
Adjusted multiplicative changes in mean allostatic load (AL) indices with 95% confidence limits by tertiles of residential vegetated land cover (vegetated land cover weighting scheme 5 based on exponential decay with λ = 0.0025), plotted on a logarithmic scale. AL1: 18 biomarkers dichotomized at 10th or 90th percentile; AL2: 15 biomarkers dichotomized at 10th or 90th percentile; AL3: 18 biomarkers dichotomized at 25th or 75th percentile; AL4: 15 biomarkers dichotomized at 25th or 75th percentile.
In analyses stratified by gender, race, city of residence (Durham vs. all others), education and obesity status, associations between vegetated land cover and AL2 index values were consistent and statistically significant (p < 0.05) in all strata; analysis of interaction effects showed that interactions of these factors with vegetated land cover were not significant (Supplementary Table 2).
3.4. Associations between residential vegetated land cover and diseases
There were no significant effects of vegetated land cover on obesity (BMI > 30 kg/m2), allergy, asthma, diabetes, IBS, and self-reported health status (Table 6). While the effect estimates for diabetes were large (from 45% to 61% reduction per IQR increase in green space), confidence intervals were very wide, possibly due to the small sample size (only nine individuals reported having diabetes).
Only previously diagnosed depression was significantly inversely associated with vegetated land cover. The results of regression analysis of vegetated land cover vs. previously diagnosed depression by 50 m annuli demonstrate a decline of beneficial effects of vegetation on the odds of depression with distance from residence (Figure 4). The effect estimates remain rather constant within the first four annuli (0 – 200 m from the residence) and then decline steadily with the smallest effects observed for the 450–500 m annulus.
Fig. 4.
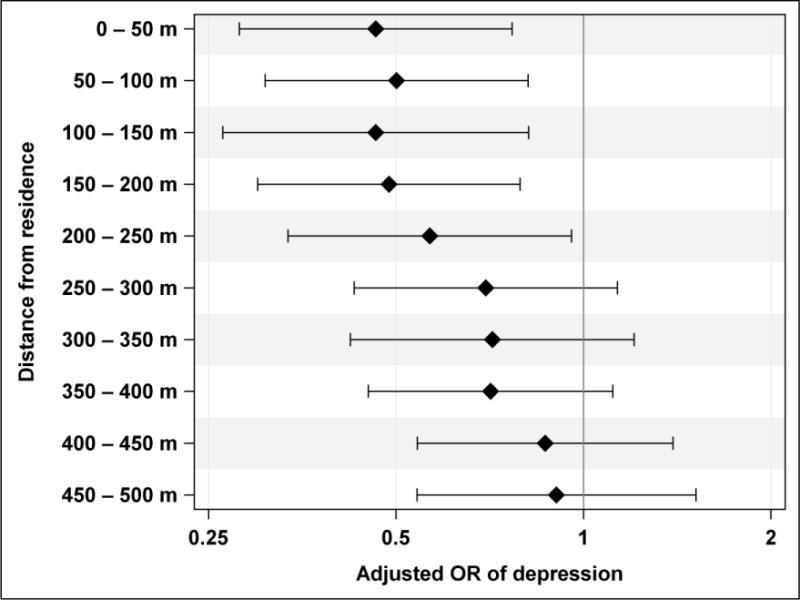
Adjusted ORs of previously diagnosed depression with 95% confidence intervals per IQR increase in residential vegetated land cover by distance from residence intervals (annuli), plotted on a logarithmic scale.
3.5. Association between previously diagnosed depression, and biomarkers and allostatic load
As data were collected on only previously-diagnosed depression, and most individuals with previous depression might not be depressed at the time of data collection, past depression was modelled as a predictor, and biomarkers or AL as outcomes. Individuals who reported previously diagnosed depression had significantly increased odds of having low dopamine compared to controls: aORs were 5.3 (2.0; 14.3) and 2.9 (1.3; 6.4) for dopamine below 10th percentile and below 25th percentile of the sample distribution, respectively, adjusting for age, gender, race, education, smoking, BMI and housing unit density. Associations between previously diagnosed depression and odds of having low norepinephrine were short of statistical significance: aORs were 2.0 (0.7; 6.1) and 1.7 (0.7; 3.9) for norepinephrine below 10th and 25th percentiles respectively. Other biomarkers were not associated with depression (not shown).
Previously diagnosed depression was also significantly associated with a greater AL2 index values (15 biomarkers dichotomized at 10th and 90th percentile), while associations with other AL indices were short of being significant. Adjusted multiplicative effects of previously diagnosed depression on mean AL were: 1.4 (1.0; 2.1) for AL1; 1.3 (1.0; 1.7) for AL2; 1.1 (0.8; 1.4) for AL3; and 1.0 (0.9; 1.2) for AL4. Consistent with the pattern observed for associations between vegetated land cover and biomarkers and AL, the effects of depression were greater for AL1 and AL2 indices based on biomarkers dichotomized at 10th or 90th percentiles.
4. Discussion
4.1. Main findings
To our knowledge, this is the first epidemiological study to apply a composite biomarker-based measure of physiological dysregulation known as AL to assess sub-clinical health effects of residential vegetated land cover. Biomarkers of immune, neuroendocrine, and metabolic functions, and AL indices based on these biomarkers were analyzed in this study in combination with 1 m resolution land cover data from US EPA’s mapping and analysis tool, the EnviroAtlas. The results demonstrated highly significant associations between greater vegetated land cover and reduced AL in adults.
Greater residential vegetated land cover was also significantly associated (p < 0.05) with reduced adjusted odds of potentially unhealthy levels of several individual biomarkers. In generalized additive regression models using biomarkers dichotomized at 10th or 90th percentiles, and vegetated land cover weighting scheme based on exponential decay with distance from residence (exponential distribution with λ = 0.0025), greater vegetated land cover was significantly associated with reduced adjusted odds of having high levels of epinephrine, IL-8, fibrinogen and VCAM-1 in serum, and α-amylase in saliva, and low levels of norepinephrine, DHEA, and dopamine in serum. All of these associations were in the direction consistent with reduced physiological dysregulation and improved health.
The inverse associations between vegetated land cover and AL, as well as beneficial effects on individual biomarkers, are consistent with previously observed associations between greater exposure to urban green spaces and reduced mortality (Gascon et al. 2016; James et al. 2016). Previous studies have demonstrated that AL is associated with increased risk of mortality (Borrell et al. 2010; Cohen et al. 2014; Hwang et al. 2014; Milot et al. 2014). Low levels of the sex hormone precursor DHEA have been linked to elevated risk of mortality in the general population (Ohlsson et al. 2010; Ohlsson et al. 2015); high levels of fibrinogen have also been shown to be predictive of increased mortality (Barron et al. 2015). Finally, depression, which was inversely associated with vegetated land cover in this study, has also been linked to mortality risk elevated by 40 – 70%, according to different estimates (Chesney et al. 2014; Laursen et al. 2016; Walker et al. 2015).
The results of this study also corroborate results of previous research demonstrating stress-alleviating and mental health-promoting effects of residential vegetated land cover and urban green spaces (Alcock et al. 2014; Beyer et al. 2014; Nieuwenhuijsen et al. 2017), including reduced risk of depression (Reklaitiene et al. 2014) and reduced consumption of antidepressants (Taylor et al. 2015). In previous studies, chronic stress has been linked with greater AL (McEwen 2002) as well as reduced levels of DHEA (Maninger et al. 2009). Low norepinephrine is known to be associated with depression (Moret and Briley 2011); low dopamine also has a role in the pathophysiology of depression (Dunlop and Nemeroff 2007; Kapur and Mann 1992). Salivary α-amylase is a biomarker of the sympathetic nervous system reflecting stress-mediated effects (Nater and Rohleder 2009).
Similarly, the observed effects of vegetated land cover on biomarkers are consistent with previously reported protective effects of urban green spaces on type 2 diabetes (Astell-Burt et al. 2014), CVD (Tamosiunas et al. 2014), and mortality due to CVD (Gascon et al. 2016). Previous research has demonstrated that type 2 diabetes and its progression are associated with elevated fibrinogen (Barazzoni et al. 2000), CRP (Evrin et al. 2005), and VCAM-1 (Tousoulis et al. 2013), the latter also being linked to vascular impairment in diabetes patients. High levels of vascular injury biomarkers including VCAM-1 are also associated with increased risk of coronary artery disease and other CVDs (Demerath et al. 2001; Evrin et al. 2005; Johnson et al. 2004; Koenig et al. 1999; Zakynthinos and Pappa 2009). Elevated fibrinogen is a known predictor of CVD (Kaptoge et al. 2012; Kunutsor et al. 2016). Low levels of DHEA are predictive of coronary heart disease (Tivesten et al. 2014) while elevated IL-8 is predictive of all-cause mortality in patients with acute coronary syndrome (Cavusoglu et al. 2015).
The results of this study are consistent with alleviation of chronic stress leading to prevention of systemic physiological dysregulation and affecting a broad set of biomarkers of metabolic, immune and neuroendocrine functions. The similar findings produced using AL indices based on a full set and a subset of biomarkers are also consistent with previous research demonstrating that AL, as a measure of physiological dysregulation, is robust to changes in the composition of component biomarkers (Cohen et al. 2015).
Most biomarkers applied in this study have been used in previous research on AL. Among several new biomarkers of chronic stress, which have not been used in previous AL research, three biomarkers, VCAM-1, fibrinogen, and α-amylase, were significantly associated with vegetated land cover in the direction suggesting potential health benefits.
4.2. Potential pathway to health
The results of analysis using various distance-dependent weighting schemes to assess exposure to vegetation suggest that its effects on AL and individual biomarkers tend to decline quickly with distance from residence. The rapid decline of the effects of vegetation with distance from residence and the lack of association between vegetated land cover and obesity suggest that psychological relaxation and stress alleviation were dominant pathways to improved health in this population. Viewing vegetation, spending time outdoors near home, gardening and similar activities could be behavioral mediators of the observed effects.
Exposure to diverse microorganisms in soil might also be an important pathway leading to improved mental health. Recent studies demonstrated that exposure to pathogens and commensal microorganisms in soil can improve immunoregulation and lead to improved mental health and resilience to stress (Lowry et al. 2016; Reber et al. 2016). These recent findings are consistent with research demonstrating health benefits of gardening (Clatworthy et al. 2013; Van Den Berg and Custers 2010). It has also been shown that urban vegetation influences the composition of airborne bacteria (Mhuireach et al. 2016) suggesting another potential pathway leading to the health benefits observed in the present study. Reduced levels of traffic-related air pollutants could be another pathway contributing to the observed beneficial health effects. Previous research has demonstrated that trees and other vegetation along streets can serve as a barrier reducing exposure to air pollutants (Brantley et al. 2014; Tong et al. 2016). It has also been shown that exposure to common air pollutants in urban air is associated with increased levels of biomarkers of inflammation and endothelial function (Bind et al. 2012; Hajat et al. 2015), which were measured in the present study.
4.3. Approaches to estimating allostatic load
This study used the most common approach to estimating AL based on the count of biomarkers that fall within a range of potentially unhealthy values. Other approaches to estimating AL are based on analysis of continuous biomarker data. A basic alternative approach includes using z-scores of continuous biomarker values representing their deviations from the sample means (Juster et al. 2010). A more complex approach is based on a multi-dimensional generalization of the concept of statistical distance in standard deviations from the mean known as the Mahalanobis distance. This measure shows how unlikely is a given combination of biomarker values taking in account covariance among biomarkers (Cohen et al. 2013). The approaches based on continuous biomarkers do not take into account the direction of deviation from the center assuming that any deviation is indicative of physiological distress.
Many previous studies of AL used 25th or 75th percentiles of biomarker distribution as cut-off points for dichotomizing individual biomarkers (Juster et al. 2010; Seeman et al. 1997). In this study, the observed effects of vegetation near residence on AL indices based on biomarkers dichotomized at 10th or 90th percentile were of larger magnitude compared to AL indices based on biomarkers dichotomized at 25th or 75th percentile cut-offs. Similar patterns were observed for a majority of individual biomarkers. Recent research demonstrated that exposure to environmental factors may have stronger effects on the values of certain biomarkers in individuals who already had elevated levels of these biomarkers (Bind et al. 2016). Conversely, exposure to health-promoting environmental factors, such as vegetation near residences, can have stronger beneficial effects in vulnerable individuals influencing the tail of biomarker distribution associated with health risks. This may explain the pattern of associations in the present study with stronger effects on biomarkers dichotomized at more extreme percentiles. Although confidence intervals for the 10th/90th percentile dichotomized biomarkers and corresponding AL indices were wider due to greater random effects, using 10th/90th percentiles or, perhaps, even more extreme cut-off points for dichotomization may be warranted, especially in larger studies.
4.4. Approaches to modelling residential vegetated land cover
In this study, the application of 1 m resolution EnviroAtlas data based on aerial photography enabled a very precise characterization of vegetated land cover within narrow distance intervals from residences. This is a substantial improvement over many previously conducted studies of urban vegetation and health, which relied on lower resolution land cover or Normalized Difference Vegetation Index (NDVI) data (WHO 2016).
Distributed lag models have been used in environmental research to analyze temporal lagged effect, such as lagged associations between episodes of exposure to pathogens and outbreaks of infections (Egorov et al. 2003; Naumova and Macneill 2008). This approach has also been applied in analysis of spatial associations between built environment and health (Baek et al. 2016). The use of high resolution land cover data in the present study enabled detailed analysis of effects with distance from the residence using 50 m wide annuli. The results demonstrated that the effects of vegetation decline gradually with distance from the residence within the 500 m radius.
In most cases, regression models using distance-weighted estimates of vegetated land cover produced stronger and more significant associations with AL and individual biomarkers, and better measures of model fit compared to models using average vegetated land cover within 500 m of residence. The former models assume that the importance of each square meter of area covered with vegetation declines with distance from the residence. For example, the exponential decay weighting scheme 4 (weights from exponential distribution with λ = 0.005) assigned a 22.1% weight to 0 – 50 m distance from the residence and a 2.3% weight to the 450 – 500 m annulus. As the area of the latter annulus is 19 times larger, this model assumes that 1 m2 of vegetated land cover within the first 50 m has an approximately 180 times greater contribution to health than a similar 1 m2 of vegetation within the 450 – 500 m annulus. In contrast, estimates of the average vegetated land cover within 500 m of residence are based on the assumption that each square meter of vegetation within 500 m from residence is equally important. In other words, the 450 – 500 m annulus with the largest area contributes 19 times more to the mean than the smallest 0 – 50 m annulus.
4.5. Limitations of the study
This cross-sectional study employed a brief questionnaire which did not include questions on lifestyle and activities potentially affecting exposure to and contact with vegetation near residences. As with any cross-sectional observational study, the observed associations could be affected, one way or another, by unmeasured confounding factors, which are correlated with vegetated land cover and linked with improved health. Further research involving more detailed behavioral and socioeconomic data is needed to confirm the results of this study and to provide information on behavioral factors mediating effects of residential vegetated land cover on health.
This exploratory study involved adults only. Therefore, the findings are not applicable to children. Approximately two-thirds of study participants were women reflecting the nonrandom sampling procedure, which was favored by women. Reflecting the source population in the Durham-Chapel Hill, NC metropolitan area, the study participants were better educated on average than the general adult US population. However, stratified analysis demonstrated consistent beneficial effects of vegetated land cover in men and women, and in individuals with and without bachelor’s degree suggesting that the findings are generalizable to the general adult US population living in similar geographic conditions.
While this study demonstrated several statistically significant (p < 0.05) associations between vegetated land cover and individual biomarkers, some of the observed significant effects could be due to type 1 errors (false positive findings due to random sampling effects). However, all significant associations were in the direction indicative of beneficial health effects; this suggests that these findings are consistent with the underlying patterns in the population.
Previous research demonstrated that improved access to urban green spaces is associated with improved diurnal salivary cortisol patterns, and reduced hypocortisolemia (Roe et al. 2013; Thompson et al. 2012). Due to logistical reasons, this study did not include the salivary cortisol biomarker. Data collection involved only one saliva sample from each participant, which precluded analysis of diurnal patterns. While most samples were collected in the morning, exact time intervals since waking up were not recorded, which precluded analysis of morning peak levels. These limitations made analysis of salivary cortisol data uninformative. Instead, the study employed salivary α-amylase, which has been used extensively in research on health effects of chronic stress as a potential alternative to cortisol (Cozma et al. 2017).
Urine sampling and physical examinations were not conducted. Therefore, urinary biomarkers, such as urinary epinephrine and norepinephrine, as well as blood pressure and pulse rate measures which have been applied in previous AL studies (Juster et al. 2010) were not available in this study.
While this study showed that residential vegetated land cover was inversely associated with previously diagnosed depression, the relatively small sample size did not support analysis of potential associations with less prevalent chronic diseases such as diabetes and cardiovascular disease. The lack of significant effects of vegetated land cover on allergy and asthma in this study does not necessarily suggest that beneficial or detrimental effects do not exist in certain sub-populations. Previous birth cohort studies demonstrated significant detrimental as well as significant beneficial effects of residential greenness on allergic rhinitis in different areas and countries (Fuertes et al. 2014; Fuertes et al. 2016). While greater exposure to pollen in greener areas can have detrimental effects in allergic individuals, it has also been shown that reduced contacts with natural environments in childhood may adversely affect the human microbiome leading to increased risk of allergic sensitization (Hanski et al. 2012). Further studies involving children are needed to provide information on the potential links between urban green spaces and allergy.
This project employed an exposure metric based on total vegetated land cover including trees and grass. While the observed effect of total vegetated land cover on AL was very strong, further research is needed to identify the types of vegetation or landscape features that have the strongest beneficial health effects and to characterize main pathways to health. Finally, data on the duration of residence at present address, and on previous residences of study participants, were not collected. Thus, long-term exposure to residential vegetation could not be characterized precisely for some study participants. Further research on green space and health effect biomarkers should include analysis of residential history data.
5. Conclusions
The results of this study demonstrate beneficial effects of residential vegetated land cover on AL and individual biomarkers, and are consistent with previously observed health benefits of exposure to urban vegetation and urban green spaces, including reduced levels of chronic stress, improved mental health, reduced risk of type 2 diabetes, CVD and premature mortality. Application of health effect biomarkers can help to elucidate biological mechanisms of health benefits of urban vegetation. It also enables in-depth analysis of common sub-clinical beneficial health effects in epidemiological studies involving extensive data collection on a relatively small number of participants.
Supplementary Material
Highlights.
Greater vegetated land cover near residence is linked with reduced allostatic load.
Greater vegetated land cover is linked with healthier levels of stress biomarkers.
Greater vegetated land cover is linked with reduced odds of previous depression.
Psychological relaxation and stress relief are likely pathways to improved health.
Acknowledgments
The authors are thankful to internal US EPA reviewers, Elizabeth Hilborn and Joachim Pleil, and to three anonymous reviewers for their helpful comments.
Funding sources
This articles presents results of intramural research at U.S. Environmental Protection Agency (US EPA). Jennifer Styles was funded by the US EPA-UNC-CH Cooperative Training Agreement CR-83591401-0. Anthony Wilson was funded by the EPA Cooperative Agreement X3-83555301 with the Association of Schools and Programs of Public Health.
Abbreviations
- aOR
adjusted odds ratio
- AL
allostatic load
- BMI
body mass index
- CL
confidence Limits
- CRP
C-reactive protein
- DHEA
dehydroepiandrosterone
- HDL
high density lipoprotein
- IBS
irritable bowel syndrome
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- IQR
interquartile range
- LDL
low density lipoprotein
- pctl
percentile
- SAA
serum amyloid A
- TNF
tumor necrosis factor
- US EPA
United States Environmental Protection Agency
- VCAM-1
vascular cell adhesion protein 1
Footnotes
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views or policies of US EPA. Mention of trade names, products, or services does not convey, and should not be interpreted as conveying official US EPA approval, endorsement or recommendation.
Human subjects
The observational study described in this article involved human subjects. It has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The study protocol involving the use of human subjects was approved by the Institutional Review Board at the University of North Carolina in Chapel Hill. Informed consent was obtained from all study participants prior to data collection.
References
- Alcock I, White MP, Wheeler BW, Fleming LE, Depledge MH. Longitudinal effects on mental health of moving to greener and less green urban areas. Environmental science & technology. 2014;48:1247–1255. doi: 10.1021/es403688w. [DOI] [PubMed] [Google Scholar]
- Ambade V, Arora M, Singh P, Somani B, Basannar D. Adrenaline, noradrenaline and dopamine level estimation in depression: Does it help? Medical Journal Armed Forces India. 2009;65:216–220. doi: 10.1016/S0377-1237(09)80006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall P, Mavros P, Coyne R, Roe J. The urban brain: Analysing outdoor physical activity with mobile eeg. British journal of sports medicine. 2015;49:272–276. doi: 10.1136/bjsports-2012-091877. [DOI] [PubMed] [Google Scholar]
- Astell-Burt T, Feng X, Kolt GS. Mental health benefits of neighbourhood green space are stronger among physically active adults in middle-to-older age: Evidence from 260,061 Australians. Preventive medicine. 2013;57:601–606. doi: 10.1016/j.ypmed.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Astell-Burt T, Feng X, Kolt GS. Is neighborhood green space associated with a lower risk of type 2 diabetes? Evidence from 267,072 Australians. Diabetes care. 2014;37:197–201. doi: 10.2337/dc13-1325. [DOI] [PubMed] [Google Scholar]
- Baek J, Sanchez BN, Berrocal VJ, Sanchez-Vaznaugh EV. Distributed lag models: Examining associations between the built environment and health. Epidemiology. 2016;27:116–124. doi: 10.1097/EDE.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzoni R, Zanetti M, Davanzo G, Kiwanuka E, Carraro P, Tiengo A, et al. Increased fibrinogen production in type 2 diabetic patients without detectable vascular complications: Correlation with plasma glucagon concentrations. The Journal of clinical endocrinology and metabolism. 2000;85:3121–3125. doi: 10.1210/jcem.85.9.6779. [DOI] [PubMed] [Google Scholar]
- Barron E, Lara J, White M, Mathers JC. Blood-borne biomarkers of mortality risk: Systematic review of cohort studies. PloS one. 2015;10:e0127550. doi: 10.1371/journal.pone.0127550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer KM, Kaltenbach A, Szabo A, Bogar S, Nieto FJ, Malecki KM. Exposure to neighborhood green space and mental health: Evidence from the survey of the health of Wisconsin. International journal of environmental research and public health. 2014;11:3453–3472. doi: 10.3390/ijerph110303453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, Baccarelli A, Zanobetti A, Tarantini L, Suh H, Vokonas P, et al. Air pollution and markers of coagulation, inflammation, and endothelial function: Associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012;23:332–340. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, Peters A, Koutrakis P, Coull B, Vokonas P, Schwartz J. Quantile regression analysis of the distributional effects of air pollution on blood pressure, heart rate variability, blood lipids, and biomarkers of inflammation in elderly american men: The normative aging study. Environmental health perspectives. 2016;124:1189–1198. doi: 10.1289/ehp.1510044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Dallo FJ, Nguyen N. Racial/ethnic disparities in all-cause mortality in U.S. adults: The effect of allostatic load. Public health reports. 2010;125:810–816. doi: 10.1177/003335491012500608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley HL, Hagler GS, Deshmukh PJ, Baldauf RW. Field assessment of the effects of roadside vegetation on near-road black carbon and particulate matter. The Science of the total environment. 2014;468–469:120–129. doi: 10.1016/j.scitotenv.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Cavusoglu E, Marmur JD, Yanamadala S, Chopra V, Hegde S, Nazli A, et al. Elevated baseline plasma il-8 levels are an independent predictor of long-term all-cause mortality in patients with acute coronary syndrome. Atherosclerosis. 2015;242:589–594. doi: 10.1016/j.atherosclerosis.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: A meta-review. World psychiatry : official journal of the World Psychiatric Association. 2014;13:153–160. doi: 10.1002/wps.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Bond MJ, Hecker JR. Environmental stress, psychological stress and allostatic load. Psychology, health & medicine. 2007;12:18–30. doi: 10.1080/13548500500429338. [DOI] [PubMed] [Google Scholar]
- Clatworthy J, Hinds J, Camic PM. Gardening as a mental health intervention: A review. Mental Health Review Journal. 2013;18:214–225. [Google Scholar]
- Clements MS, Armstrong BK, Moolgavkar SH. Lung cancer rate predictions using generalized additive models. Biostatistics. 2005;6:576–589. doi: 10.1093/biostatistics/kxi028. [DOI] [PubMed] [Google Scholar]
- Cohen AA, Milot E, Yong J, Seplaki CL, Fülöp T, Bandeen-Roche K, et al. A novel statistical approach shows evidence for multi-system physiological dysregulation during aging. Mechanisms of ageing and development. 2013;134:110–117. doi: 10.1016/j.mad.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Milot E, Li Q, Legault V, Fried LP, Ferrucci L. Cross-population validation of statistical distance as a measure of physiological dysregulation during aging. Experimental gerontology. 2014;57:203–210. doi: 10.1016/j.exger.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Li Q, Milot E, Leroux M, Faucher S, Morissette-Thomas V, et al. Statistical distance as a measure of physiological dysregulation is largely robust to variation in its biomarker composition. PloS one. 2015;10:e0122541. doi: 10.1371/journal.pone.0122541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozma S, Dima-Cozma LC, Ghiciuc CM, Pasquali V, Saponaro A, Patacchioli FR. Salivary cortisol and alpha-amylase: Subclinical indicators of stress as cardiometabolic risk. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2017;50:e5577. doi: 10.1590/1414-431X20165577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Nieuwenhuijsen MJ, Esnaola M, Forns J, Basagana X, Alvarez-Pedrerol M, et al. Green spaces and cognitive development in primary schoolchildren. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7937–7942. doi: 10.1073/pnas.1503402112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadvand P, Bartoll X, Basagana X, Dalmau-Bueno A, Martinez D, Ambros A, et al. Green spaces and general health: Roles of mental health status, social support, and physical activity. Environment international. 2016;91:161–167. doi: 10.1016/j.envint.2016.02.029. [DOI] [PubMed] [Google Scholar]
- Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes care. 2008;31:361–362. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- Demerath E, Towne B, Blangero J, Siervogel RM. The relationship of soluble icam-1, vcam-1, p-selectin and e-selectin to cardiovascular disease risk factors in healthy men and women. Annals of human biology. 2001;28:664–678. doi: 10.1080/03014460110048530. [DOI] [PubMed] [Google Scholar]
- Dormann C, McPherson J, Araújo M, Bivand R, Bolliger J, Carl G, et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography. 2007;30:609–628. [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of general psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Dzhambov AM, Dimitrova DD, Dimitrakova ED. Association between residential greenness and birth weight: Systematic review and meta-analysis. Urban Forestry & Urban Greening. 2014;13:621–629. [Google Scholar]
- Egorov AI, Naumova EN, Tereschenko AA, Kislitsin VA, Ford TE. Daily variations in effluent water turbidity and diarrhoeal illness in a russian city. International journal of environmental health research. 2003;13:81–94. doi: 10.1080/0960312021000071567. [DOI] [PubMed] [Google Scholar]
- Evrin PE, Nilsson SE, Oberg T, Malmberg B. Serum c-reactive protein in elderly men and women: Association with mortality, morbidity and various biochemical values. Scandinavian journal of clinical and laboratory investigation. 2005;65:23–31. doi: 10.1080/00365510510013505. [DOI] [PubMed] [Google Scholar]
- Fuertes E, Markevych I, von Berg A, Bauer CP, Berdel D, Koletzko S, et al. Greenness and allergies: Evidence of differential associations in two areas in germany. Journal of epidemiology and community health. 2014;68:787–790. doi: 10.1136/jech-2014-203903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes E, Markevych I, Bowatte G, Gruzieva O, Gehring U, Becker A, et al. Residential greenness is differentially associated with childhood allergic rhinitis and aeroallergen sensitization in seven birth cohorts. Allergy. 2016;71:1461–1471. doi: 10.1111/all.12915. [DOI] [PubMed] [Google Scholar]
- Gascon M, Triguero-Mas M, Martinez D, Dadvand P, Forns J, Plasencia A, et al. Mental health benefits of long-term exposure to residential green and blue spaces: A systematic review. International journal of environmental research and public health. 2015;12:4354–4379. doi: 10.3390/ijerph120404354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Triguero-Mas M, Martinez D, Dadvand P, Rojas-Rueda D, Plasencia A, et al. Residential green spaces and mortality: A systematic review. Environment international. 2016;86:60–67. doi: 10.1016/j.envint.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (il-1): A central regulator of stress responses. Frontiers in neuroendocrinology. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Gu HF, Ma J, Gu KT, Brismar K. Association of intercellular adhesion molecule 1 (icam1) with diabetes and diabetic nephropathy. Frontiers in endocrinology. 2012;3:179. doi: 10.3389/fendo.2012.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Wang W, Wang F, Huang JH. Neuromodulator and emotion biomarker for stress induced mental disorders. Neural plasticity. 2016;2016:2609128. doi: 10.1155/2016/2609128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and c-reactive protein in patients with major depressive disorder. Brain, behavior, and immunity. 2015;49:206–215. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, et al. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: A repeat-measures analysis in the multi-ethnic study of atherosclerosis (mesa) Epidemiology. 2015;26:310–320. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluza D, Schonbauer R, Cervinka R. Green perspectives for public health: A narrative review on the physiological effects of experiencing outdoor nature. International journal of environmental research and public health. 2014;11:5445–5461. doi: 10.3390/ijerph110505445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AM, Lund R, Bruunsgaard H, Rod NH, Garde AH, Molbo D, et al. Social gradient in allostatic load among danish men and women in late midlife. J Aging Health. 2014;26:72–87. doi: 10.1177/0898264313508187. [DOI] [PubMed] [Google Scholar]
- Hanski I, von Hertzen L, Fyhrquist N, Koskinen K, Torppa K, Laatikainen T, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8334–8339. doi: 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, Hermansson A. The immune system in atherosclerosis. Nature immunology. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- Hartig T, Mitchell R, De Vries S, Frumkin H. Nature and health. Annual Review of Public Health. 2014;35:207–228. doi: 10.1146/annurev-publhealth-032013-182443. [DOI] [PubMed] [Google Scholar]
- Hwang AC, Peng LN, Wen YW, Tsai YW, Chang LC, Chiou ST, et al. Predicting all-cause and cause-specific mortality by static and dynamic measurements of allostatic load: A 10-year population-based cohort study in taiwan. Journal of the American Medical Directors Association. 2014;15:490–496. doi: 10.1016/j.jamda.2014.02.001. [DOI] [PubMed] [Google Scholar]
- James P, Hart JE, Banay RF, Laden F. Exposure to greenness and mortality in a nationwide prospective cohort study of women. Environ Health Perspect. 2016;124:1344–1352. doi: 10.1289/ehp.1510363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Fernandez S, Gurpegui M, Diaz-Atienza F, Perez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: Results from a meta-analysis. The Journal of clinical psychiatry. 2015;76:1658–1667. doi: 10.4088/JCP.14r09179. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, et al. Serum amyloid a as a predictor of coronary artery disease and cardiovascular outcome in women: The national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation (wise) Circulation. 2004;109:726–732. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- Jung CC, Liang HH, Lee HL, Hsu NY, Su HJ. Allostatic load model associated with indoor environmental quality and sick building syndrome among office workers. PloS one. 2014;9:e95791. doi: 10.1371/journal.pone.0095791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and biobehavioral reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Juster RP, Sindi S, Marin MF, Perna A, Hashemi A, Pruessner JC, et al. A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology. 2011;36:797–805. doi: 10.1016/j.psyneuen.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. The New England journal of medicine. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Mann JJ. Role of the dopaminergic system in depression. Biological psychiatry. 1992;32:1–17. doi: 10.1016/0006-3223(92)90137-o. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Lachman ME, Tun PA, Koretz BK, Seeman TE. Biological correlates of adult cognition: Midlife in the united states (midus) Neurobiology of aging. 2014;35:387–394. doi: 10.1016/j.neurobiolaging.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, et al. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: Results from the monica (monitoring trends and determinants in cardiovascular disease) augsburg cohort study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- Kunutsor SK, Kurl S, Zaccardi F, Laukkanen JA. Baseline and long-term fibrinogen levels and risk of sudden cardiac death: A new prospective study and meta-analysis. Atherosclerosis. 2016;245:171–180. doi: 10.1016/j.atherosclerosis.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Laursen TM, Musliner KL, Benros ME, Vestergaard M, Munk-Olsen T. Mortality and life expectancy in persons with severe unipolar depression. Journal of affective disorders. 2016;193:203–207. doi: 10.1016/j.jad.2015.12.067. [DOI] [PubMed] [Google Scholar]
- Lovasi GS, O’Neil-Dunne JP, Lu JW, Sheehan D, Perzanowski MS, Macfaden SW, et al. Urban tree canopy and asthma, wheeze, rhinitis, and allergic sensitization to tree pollen in a new york city birth cohort. Environmental health perspectives. 2013;121:494–500. doi: 10.1289/ehp.1205513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Smith DG, Siebler PH, Schmidt D, Stamper CE, Hassell JE, Jr, et al. The microbiota, immunoregulation, and mental health: Implications for public health. Current environmental health reports. 2016;3:270–286. doi: 10.1007/s40572-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher TF, Landmesser U, von Eckardstein A, Fogelman AM. High-density lipoprotein: Vascular protective effects, dysfunction, and potential as therapeutic target. Circulation research. 2014;114:171–182. doi: 10.1161/CIRCRESAHA.114.300935. [DOI] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (dhea) and dhea sulfate (dheas) Frontiers in neuroendocrinology. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi C, Huth C, Herder C, Baumert J, Thorand B, Rathmann W, et al. Acute-phase serum amyloid a protein and its implication in the development of type 2 diabetes in the kora s4/f4 study. Diabetes care. 2013;36:1321–1326. doi: 10.2337/dc12-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of internal medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiology of aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism: clinical and experimental. 2015;64:S2–S10. doi: 10.1016/j.metabol.2014.10.029. [DOI] [PubMed] [Google Scholar]
- Mhuireach G, Johnson BR, Altrichter AE, Ladau J, Meadow JF, Pollard KS, et al. Urban greenness influences airborne bacterial community composition. The Science of the total environment. 2016;571:680–687. doi: 10.1016/j.scitotenv.2016.07.037. [DOI] [PubMed] [Google Scholar]
- Milot E, Morissette-Thomas V, Li Q, Fried LP, Ferrucci L, Cohen AA. Trajectories of physiological dysregulation predicts mortality and health outcomes in a consistent manner across three populations. Mech Ageing Dev. 2014;141–142:56–63. doi: 10.1016/j.mad.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatric disease and treatment. 2011;7:9–13. doi: 10.2147/NDT.S19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Naumova EN, Macneill IB. Time-distributed effect of exposure and infectious outbreaks. Environmetrics. 2008;20:235–248. doi: 10.1002/env.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Khreis H, Triguero-Mas M, Gascon M, Dadvand P. Fifty shades of green: Pathway to healthy urban living. Epidemiology. 2017;28:63–71. doi: 10.1097/EDE.0000000000000549. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Labrie F, Barrett-Connor E, Karlsson MK, Ljunggren O, Vandenput L, et al. Low serum levels of dehydroepiandrosterone sulfate predict all-cause and cardiovascular mortality in elderly swedish men. The Journal of clinical endocrinology and metabolism. 2010;95:4406–4414. doi: 10.1210/jc.2010-0760. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Vandenput L, Tivesten A. Dhea and mortality: What is the nature of the association? The Journal of steroid biochemistry and molecular biology. 2015;145:248–253. doi: 10.1016/j.jsbmb.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Petrovic D, Pivin E, Ponte B, Dhayat N, Pruijm M, Ehret G, et al. Sociodemographic, behavioral and genetic determinants of allostatic load in a swiss population-based study. Psychoneuroendocrinology. 2016;67:76–85. doi: 10.1016/j.psyneuen.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Pickard BR, Daniel J, Mehaffey M, Jackson LE, Neale A. Enviroatlas: A new geospatial tool to foster ecosystem services science and resource management. Ecosystem Services. 2015;14:45–55. [Google Scholar]
- Pope D, Tisdall R, Middleton J, Verma A, van Ameijden E, Birt C, et al. Quality of and access to green space in relation to psychological distress: Results from a population-based cross-sectional study as part of the euro-urhis 2 project. European journal of public health. 2015 doi: 10.1093/eurpub/ckx217. [DOI] [PubMed] [Google Scholar]
- Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, et al. Immunization with a heat-killed preparation of the environmental bacterium mycobacterium vaccae promotes stress resilience in mice. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E3130–3139. doi: 10.1073/pnas.1600324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reklaitiene R, Grazuleviciene R, Dedele A, Virviciute D, Vensloviene J, Tamosiunas A, et al. The relationship of green space, depressive symptoms and perceived general health in urban population. Scandinavian journal of public health. 2014;42:669–676. doi: 10.1177/1403494814544494. [DOI] [PubMed] [Google Scholar]
- Robinette JW, Charles ST, Almeida DM, Gruenewald TL. Neighborhood features and physiological risk: An examination of allostatic load. Health & place. 2016;41:110–118. doi: 10.1016/j.healthplace.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe JJ, Thompson CW, Aspinall PA, Brewer MJ, Duff EI, Miller D, et al. Green space and stress: Evidence from cortisol measures in deprived urban communities. International journal of environmental research and public health. 2013;10:4086–4103. doi: 10.3390/ijerph10094086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, et al. Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1728–1733. doi: 10.1161/ATVBAHA.112.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation–allostatic load and its health consequences. Macarthur studies of successful aging. Archives of internal medicine. 1997;157:2259–2268. [PubMed] [Google Scholar]
- Tamosiunas A, Grazuleviciene R, Luksiene D, Dedele A, Reklaitiene R, Baceviciene M, et al. Accessibility and use of urban green spaces, and cardiovascular health: Findings from a kaunas cohort study. Environmental health : a global access science source. 2014;13:20. doi: 10.1186/1476-069X-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MS, Wheeler BW, White MP, Economou T, Osborne NJ. Research note: Urban street tree density and antidepressant prescription rates—a cross-sectional study in london, uk. Landscape and Urban Planning. 2015;136:174–179. [Google Scholar]
- Thompson CW, Roe J, Aspinall P, Mitchell R, Clow A, Miller D. More green space is linked to less stress in deprived communities: Evidence from salivary cortisol patterns. Landscape and Urban Planning. 2012;105:221–229. [Google Scholar]
- Tivesten A, Vandenput L, Carlzon D, Nilsson M, Karlsson MK, Ljunggren O, et al. Dehydroepiandrosterone and its sulfate predict the 5-year risk of coronary heart disease events in elderly men. Journal of the American College of Cardiology. 2014;64:1801–1810. doi: 10.1016/j.jacc.2014.05.076. [DOI] [PubMed] [Google Scholar]
- Tong Z, Baldauf RW, Isakov V, Deshmukh P, Zhang KM. Roadside vegetation barrier designs to mitigate near-road air pollution impacts. The Science of the total environment. 2016;541:920–927. doi: 10.1016/j.scitotenv.2015.09.067. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Papageorgiou N, Androulakis E, Siasos G, Latsios G, Tentolouris K, et al. Diabetes mellitus-associated vascular impairment: Novel circulating biomarkers and therapeutic approaches. Journal of the American College of Cardiology. 2013;62:667–676. doi: 10.1016/j.jacc.2013.03.089. [DOI] [PubMed] [Google Scholar]
- Triguero-Mas M, Dadvand P, Cirach M, Martinez D, Medina A, Mompart A, et al. Natural outdoor environments and mental and physical health: Relationships and mechanisms. Environment international. 2015;77:35–41. doi: 10.1016/j.envint.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Ulrich RS, Simons RF, Losito BD, Fiorito E, Miles MA, Zelson M. Stress recovery during exposure to natural and urban environments. Journal of environmental psychology. 1991;11:201–230. [Google Scholar]
- Van Den Berg AE, Custers MH. Gardening promotes neuroendocrine and affective restoration from stress. Journal of Health Psychology. 2010 doi: 10.1177/1359105310365577. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Jerrett M, Su JG, Burnett RT, Chen H, Wheeler AJ, et al. A cohort study relating urban green space with mortality in ontario, canada. Environmental research. 2012;115:51–58. doi: 10.1016/j.envres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA psychiatry. 2015;72:334–341. doi: 10.1001/jamapsychiatry.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster T, Vieira V, Weinberg J, Aschengrau A. Method for mapping population-based case-control studies: An application using generalized additive models. International journal of health geographics. 2006;5:26. doi: 10.1186/1476-072X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Urban green spaces and health - a review of evidence. Copenhagen: World Health Organization; 2016. [Google Scholar]
- Wilson EO. Biophilia. Harvard University Press; 1984. [Google Scholar]
- Yufu K, Okada N, Ebata Y, Murozono Y, Shinohara T, Nakagawa M, et al. Plasma norepinephrine is an independent predictor of adverse cerebral and cardiovascular events in type 2 diabetic patients without structural heart disease. Journal of cardiology. 2014;64:225–230. doi: 10.1016/j.jjcc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. Journal of cardiology. 2009;53:317–333. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Zamani P, Schwartz GG, Olsson AG, Rifai N, Bao W, Libby P, et al. Inflammatory biomarkers, death, and recurrent nonfatal coronary events after an acute coronary syndrome in the miracl study. Journal of the American Heart Association. 2013;2:e003103. doi: 10.1161/JAHA.112.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin E, Bickel C, Schnabel RB, Zeller T, Lackner KJ, Rupprecht HJ, et al. Risk factors of coronary artery disease in secondary prevention–results from the atherogene–study. PloS one. 2015;10:e0131434. doi: 10.1371/journal.pone.0131434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zewinger S, Drechsler C, Kleber ME, Dressel A, Riffel J, Triem S, et al. Serum amyloid a: High-density lipoproteins interaction and cardiovascular risk. European heart journal. 2015;36:3007–3016. doi: 10.1093/eurheartj/ehv352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


