Abstract
Human exposure to consumer and personal care products chemicals such as phenols, including parabens and other antimicrobial agents, can be assessed through biomonitoring by quantifying urinary concentrations of the parent chemical or its metabolites, often after hydrolysis of phase II conjugates. Developing suitable analytical methods for the concurrent quantification of multiple exposure biomarkers is challenging because optimal conditions for the hydrolysis of such conjugates (e.g., O-glucuronides, N-glucuronides, sulfates) may differ depending on the biomarker. We evaluated the effectiveness of seven commercial hydrolytic enzymes to simultaneously hydrolyze N-glucuronides (using the antibacterial triclocarban as example compound) and other conjugates (using select phenols and parabens as examples) by using on-line solid phase extraction–high performance liquid chromatography-isotope dilution-tandem mass spectrometry. Incubation (30 min, 55 °C) with a genetically engineered β-glucuronidase (IMCS, ≥ 15 units/μL urine) hydrolyzed N-glucuronide triclocarban, but did not fully hydrolyze the conjugates of phenols and parabens. By contrast, incubation (4 h, 37 °C) with solid β-glucuronidase (Helix pomatia, Type H-1, ≥ 30 units/μL urine) or liquid β-glucuronidase/arylsulfatase (Helix pomatia, 30 units/μL urine [i.e., 30 μL/100 μL urine]) in the presence of 100 μL methanol for 100 μL urine completely hydrolyzed N-glucuronide triclocarban and the conjugates of several phenols and parabens, without cleaving the ester bond of the parabens to form p-hydroxybenzoic acid. These results highlight the relevance of method validation procedures that include optimizing the hydrolysis of phase II urinary conjugates (e.g., enzyme type and amount used, reaction time, temperature) to quantify accurately and concurrently multiple exposure biomarkers for biomonitoring purposes.
Keywords: Human biomonitoring, Solid phase extraction-LC-MS, β-Glucuronidase enzyme hydrolysis, Environmental exposure, Triclocarban, Parabens, Personal care products
1. Introduction
Exposure to environmental chemicals (e.g., preservatives, antimicrobial agents, UV filters) may occur through use of consumer and personal care products. Some of these xenobiotics are suspect endocrine disruptors (Calafat, 2012), and concerns exist about their potential adverse health effects in humans. Biomonitoring—the measurement of the chemicals or their metabolites in biological matrices—is a useful tool to estimate human exposure to these chemicals (Calafat et al., 2006).
Upon exposure, xenobiotics may undergo phase II biotransformation, a common detoxification pathway to facilitate urinary elimination (Tephly, 1990; Zhou and Miners, 2014). During phase II biotransformations, xenobiotics undergo reactions such as glucuronidation, sulfation, methylation, acetylation, or glutathione and amino acid conjugation to form hydrophilic conjugates (Kaivosaari et al., 2011; Kroemer and Klotz, 1992; Tephly and Burchell, 1990). For example, environmental phenolic compounds present in personal care products such as benzophenone-3 (ultraviolet filter), parabens (preservatives), and triclosan (antimicrobial), often form O-glucuronide and sulfate conjugates (Ye et al., 2011). Other personal care product chemicals such as the antimicrobial triclocarban (3,4,4′-trichlorocarbanilide) can form N-glucuronide conjugates (Howes and Black, 1976; Hawes, 1998; Schebb et al., 2012).
Human exposure can be assessed from the urinary concentrations of xenobiotics or their metabolites; these biomarker concentrations are often obtained after enzymatic hydrolysis of phase II conjugates (e.g., glucuronides, sulfates) and reported as total (free [unconjugated] plus conjugated) concentrations. Thus, optimizing the hydrolysis of urinary conjugates is critical for accurate quantification of the total concentration of these chemicals for exposure assessment. Biomonitoring is an expensive effort, and high-throughput multi-analyte methods that concurrently quantify multiple biomarkers are most cost effective. However, optimal hydrolysis conditions for deconjugation of some conjugates (e.g., O-glucuronides) may not be appropriate for others (e.g., N-glucuronides) when both O–and N-glucuronides of various biomarkers are included in the same analytical method. For instance, β-glucuronidases preferentially hydrolyze O-glucuronides over N-glucuronides (Zenser et al., 1999; Babu et al., 1996; Kowalczyk et al., 2000), and N-glucuronides may be labile under acidic conditions while O-glucuronides are labile under basic conditions. Furthermore, some enzymes also possess nonspecific lipase activity capable of hydrolyzing ester bonds such as those present in phthalates and parabens (Abbas et al., 2010; Blount et al., 2000a).
We investigated seven commercially available enzymes used before for biomonitoring applications (Morris et al., 2014; Frederiksen et al., 2013; Kato et al., 2005; Moos et al., 2014; Schmidt et al., 2013) to assess their effectiveness on the enzymatic deconjugation of urinary N-glucuronides (using triclocarban as example compound) and of other conjugates (using select phenols and parabens) for the concurrent quantification of these bio-markers by using a multi-analyte mass spectrometry-based analytical method.
2. Experimental section
2.1. Analytical materials and standards
We obtained HPLC-grade methanol (MeOH) from Fisher Scientific (Pittsburgh, PA, USA), analytical-grade formic acid (>98%) from EM Science (Gibbstown, NJ, USA), and 75 mM potassium phosphate (K2HPO4) buffer, pH 6.8 from Tedia (Fairfield, OH, USA). We used water (18.0 MΩ/cm resistance) from an ultrapure water system (AQUA Solutions, Jasper, GA, USA). We purchased ammonium acetate (>98%), 4-methylumbelliferyl glucuronide, 4-methylumbelliferyl sulfate, triclocarban, triclosan, bisphenol A (BPA), bisphenol F (BPF), bisphenol S (BPS), 2,4-dichlorophenol, 2,5-dichlorophenol, and methyl-, ethyl-, propyl-, and butyl parabens from Sigma-Aldrich Laboratories, Inc. (St. Louis, MO, USA); benzophenone-3 was provided by EMD Chemicals Inc. (Hawthorne, NY, USA). We obtained 13C4-4-methylumbelliferone, 13C12-BPA, 13C6-2,4-dichlorophenol, 13C6-2,5-dichlorophenol, and D2-triclo-carban from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA); 13C12-triclosan from Wellington laboratories Inc. (Ontario, Canada); D3,13C-benzophenone-3 from Los Alamos National Laboratory (Los Alamos, NM, USA); D4-methyl paraben from CDN Isotopes (Quebec, Canada); and 13C12-BPS, D4-ethyl, D4-propyl, and D4-butyl parabens from CanSyn Chem Corp. (Toronto, Canada).
For the present study, we evaluated seven commercially available enzymes, used before for other similar biomonitoring applications (Morris et al., 2014; Frederiksen et al., 2013; Kato et al., 2005; Moos et al., 2014; Schmidt et al., 2013); their sources were:
H-1: β-Glucuronidase from Helix Pomatia, Type H-1 (Sigma Aldrich, Item #G0751, β-glucuronidase activity ≥ 300,000 units/g solid; sulfatase activity 10,000 units/g solid).
SH-1: Sulfatase from Helix pomatia, Type H-1 (Sigma Aldrich, Item #S9626, sulfatase activity ≥ 10,000 units/g solid, β-glucuronidase activity ≥300,000 units/g solid).
K-12: BGALA-RO β-Glucuronidase from E. coli-K12 liquid enzyme (Roche Biomedical through Sigma Aldrich, Item #03707601001, β-glucuronidase activity ≈140 units/mg protein).
BL-21: β-Glucuronidase from E. coli-BL21 (Sigma Aldrich, Item #G8420, β-Glucuronidase activity ≥20,000,000 units/g protein).
HP-2: β-Glucuronidase from Helix Pomatia, Type HP-2 (Sigma Aldrich, Item #G7017, β-glucuronidase activity ≥ 100,000 units/mL; sulfatase activity 7500 units/mL).
IMCS: Recombinant β-Glucuronidase from snail (Integrated Micro-Chromatography Systems Columbia, Item #04–E1F, β-glucuronidase activity >50,000 units/mL).
ALS: BGALA-RO β-Glucuronidase/Arylsulfatase liquid enzyme from Helix pomatia (Roche Life Science through Sigma Aldrich, Item #10127698001, β-glucuronidase activity ≈100,000 units/mL; sulfatase activity ≈47,500 units/mL).
For the experiments, unless specified, we evaluated the testing conditions (e.g., buffer, pH) recommended by the manufacturer of the enzyme. We used archived urine samples (stored at −70 °C) collected in Atlanta, GA, USA between 2010 and 2016 from convenience samplings of male and female adults. No personal information from the subjects was available. The Centers for Disease Control and Prevention’s (CDC) Human Subjects Institutional Review Board reviewed and approved the study protocol. A waiver of informed consent was requested under 45 CFR 46.116(d).
2.2. Preparation of standard stock solutions and quality control (QC) materials
Stock solutions and QC materials were prepared as described before (Zhou et al., 2012, 2014; Ye et al., 2006). Initial stock solutions of analytical standards and stable isotope-labeled internal standards of BPA, BPS, BPF, methyl-, ethyl-, butyl-, and propyl parabens, 2,4-dichlorophenol, 2,5-dichlorophenol, benzophenone-3, triclosan, and triclocarban were prepared in MeOH. Ten working standard spiking solutions that contained all twelve analytes were generated by serial dilutions of the initial stock with MeOH. Final concentrations of the ten working standards were such that a 100-μL spike covered a concentration range from 0.1 to 100 ng/mL (three bisphenols, 2,4-dichlorophenol), 200 ng/mL (triclocarban), or 1000 ng/mL (parabens, 2,5-dichlorophenol, benzophenone-3, triclosan). The stable isotope-labeled internal standard (IS) working solution was prepared by diluting the internal standard stock solution using MeOH, so that 50-μL spike would result in a 25 ng/mL or 100 ng/mL concentration of internal standards. The IS working solution also contained 4-methylumbelliferyl sulfate, 4-methylumbelliferyl glucuronide, and 13C4-4-methylumbelliferone, compounds used as deconjugation standards, as described in detail before (CDC, 2017; 2016; Ye et al., 2005a; Zhou et al., 2014). QC materials were prepared from pooled urine. The QC low (QCL) and the QC high (QCH) pools were enriched with different levels of the target analytes. All standard stock solutions, spiking solutions, and QC materials were dispensed into 2 mL glass vials and stored at −70 °C until analysis.
2.3. Preparation of samples and standards for extraction and quantitation
Urine, QCs, and standards were thawed at room temperature, vortex mixed, and aliquoted (100 μL) into a 1.5 mL conical silanized glass autosampler vial. Then, 50 μL of internal standard was added to the sample vial. For a subset of experiments discussed in section 3.2, we also spiked the urine with 100 μL of methanol before incubation. Enzyme volume and incubation procedures varied depending on the experiment and enzyme used (see Results section). After incubation, we added 0.1 M formic acid to the samples such that the final volume in the vial was 1 mL. Reagent blanks were prepared using the same above procedure but with deionized water instead of urine. All sample vials were vortex mixed and centrifuged at 3000 RPM for 15 min before analysis by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry (SPE-HPLC-MS/MS) following the protocol used for the biomonitoring of environmental phenols and parabens for the 2013–2014 National Health and Nutrition Examination Survey (NHANES) (CDC, 2016).
3. Results and discussion
The original environmental phenols and parabens bio-monitoring method developed and performed in our laboratory used solid β-glucuronidase/sulfatase from Helix Pomatia type H-1 to hydrolyze both O-glucuronide and sulfate conjugates of multiple phenols and parabens biomarkers (Ye et al., 2005a; Zhou et al., 2014). In the original method, complete hydrolysis was fully validated for each target analyte through time course experiments with varying amounts of enzyme, different pHs, incubation temperatures, and incubation times (CDC, 2017; 2016; Ye et al., 2005a; Zhou et al., 2014). For routine application of the method, we also monitored the extent of the enzymatic hydrolysis of 4-methylumbelliferyl sulfate and 4-methylumbelliferyl glucuronide, commercially available conjugate standards, as an additional quality control measure to ensure the enzyme functions properly during sample preparation (CDC, 2017; 2016; Ye et al., 2005a; Zhou et al., 2014). When we decided to incorporate triclocarban, we evaluated the effectiveness of Helix Pomatia type H-1 and other enzymes to hydrolyze N-glucuronide triclocarban, as well as the O-glucuronides and sulfates of the phenols and parabens included in the original method. For all conditions described below, we analyzed multiple samples, but because of volume limitations, for each experimental setting, we only analyzed each sample once.
3.1. Enzymatic hydrolysis of urinary conjugates of triclocarban, parabens, and phenols
3.1.1. Solid β-glucuronidase (H1) or sulfatase (SH-1) from Helix Pomatia (type H-1)
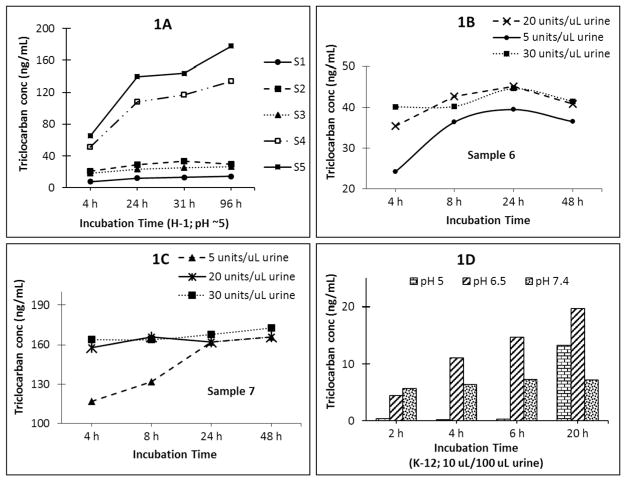
We have successfully used Helix Pomatia H-1 (5 units/μL urine, pH 5, 37 °C, 4 h) for the complete hydrolysis of the urinary conjugates of up to fourteen phenols including bisphenol A, select parabens, benzophenone-3, and triclosan (Zhou et al., 2014). To evaluate whether Helix Pomatia H-1 can also hydrolyze N-glucuronide triclocarban, we aliquoted 100 μL of five urine samples (S1–S5), 50 μL isotope-labeled internal standard solution, and 50 μL enzyme solution prepared in 1 M ammonium acetate buffer (pH 5) providing 5 units enzyme/μL urine, and incubated at 37 °C for 4, 8, 24, 31, or 96 h. At the end of each time point, hydrolysis was quenched by adding 800 μL of 0.1% formic acid, and the urine was analyzed by on-line SPE-HPLC-MS/MS as previously described (Ye et al., 2005a; Zhou et al., 2014). Complete hydrolysis within 4 h of incubation of the urinary conjugates of bisphenol A, parabens, benzophenone-3, triclosan, and other phenols confirmed previous findings (Ye et al., 2005a; Zhou et al., 2014). However, for some samples, total triclocarban concentrations increased with increasing incubation time (Fig. 1A). For example, for sample S5, triclocarban concentration increased from ~70 ng/mL after 4 h incubation to >180 ng/mL after 96 h; we observed a similar pattern for sample S4. On the other hand, for other samples having relatively low triclocarban concentrations, the concentrations of triclocarban initially increased but did not appreciably change after 31 h incubation (Fig. 1A). These observations showed that 5 units H-1 enzyme/μL urine, used before for the hydrolysis of environmental phenols and parabens (Ye et al., 2005a; Zhou et al., 2014), did not completely deconjugate N-glucuronide triclocarban in samples with relatively high triclocarban concentration even after relatively long incubation times (>96 h). For samples with relatively low triclocarban concentrations, 5 units H-1 enzyme/μL urine completely hydrolyzed N-glucuronide triclocarban, but only after at least 31 h, too long of an incubation time for large-scale bio-monitoring studies which favor high throughput analytical methods. The results from this experiment also supported β-glucuronidase preferential hydrolysis of O-glucuronides over N-glucuronides, as reported before (Babu et al., 1996; Hawes, 1998; Zenser et al., 1999).
Fig. 1.
Fig. 1A, Total triclocarban concentration (ng/mL) in five urine samples (S1–S5) with increasing incubation time (h) with 5 units H-1 enzyme/μL urine. Fig. 1B and C, Total triclocarban concentrations with increasing amounts of H-1 enzyme for Sample 6 (1B) and 7 (1C). Hydrolysis was complete within 4 h of incubation at 37 °C when using 30 units enzyme/μL urine. Fig. 1D, Total triclocarban concentrations after incubation with K-12 enzyme (10 μL/100 μL urine) prepared in ammonium acetate at different pHs.
We then tested the efficiency of N-glucuronide triclocarban hydrolysis with variable amounts of H-1 enzyme (equivalent to 5, 20, 30, or 36 units/μL urine) added to 100 μL of urine (S6 and S7), and incubated at 37 °C for 4, 8, 24, or 48 h. Hydrolysis was complete within 4 h of incubation when using 30 units enzyme/μL urine (Fig. 1B and C), 6 times more than the amount used previously for the quantification of environmental phenols (Ye et al., 2005a; Zhou et al., 2014). Similar results were observed with 36 units enzyme/μL urine (data not shown). Under these conditions, concentrations of the six phenols and most parabens, except methyl paraben, compared well with those obtained using the original phenol method (Ye et al., 2006; Zhou et al., 2014) (data not shown). However, we observed a decrease (10–30%) in urinary methyl paraben concentrations with increasing amount of enzyme.
The above experiments using sulfatase SH-1 from Helix pomatia (Type H-1), containing comparable glucuronidase activity as H-1, produced similar results, including decreasing urinary methyl paraben concentrations with increasing enzyme amounts (data not shown).
In summary, reproducible and complete deconjugation of N-glucuronide triclocarban was possible only when the H-1 or SH-1 enzyme amount was ≥30 units/μL urine with 4 h of incubation at 37°C; under those conditions, conjugates of all other biomarkers could also be completely hydrolyzed, but concentrations of methyl paraben were underestimated.
3.1.2. β-Glucuronidase from Escherichia coli K12 (K-12)
K12 is a liquid enzyme used in biomonitoring applications, including the quantification of phthalate metabolites (Silva et al., 2008, 2013). We tested this enzyme and monitored total triclocarban concentrations at different incubation times and pH buffers. First, 100 μL of one urine sample (S7 with ~180 ng/mL triclocarban as suggested using solid β-glucuronidase (H1) or sulfatase (SH-1) from Helix Pomatia (Type H-1), a concentration above the 95th percentile in NHANES 2013–2014) (CDC, 2017) was treated with 10 μL of K-12 enzyme and 90 μL of 1 M ammonium acetate buffer at pH 5, 6.5, or 7.4 (Fig. 1D). The maximum triclocarban concentration, obtained after incubation for 20 h at pH 6.5, was almost 10 times lower (~20 ng/mL) than expected (~180 ng/mL). Interestingly, even with 20 times more K-12 enzyme amount and 10 times longer incubation time than those used for the hydrolysis of phthalate metabolite conjugates (Silva et al., 2008, 2013), we observed incomplete hydrolysis of N-glucuronide triclocarban. Though increasing the enzyme amount could have potentially increased the total concentration of triclocarban, we performed no further experiments with K-12 because of its rather poor efficiency for triclocarban hydrolysis, and concomitant estimated high cost of this approach (compared to others evaluated for this study) for routine analysis. More importantly, K-12 lacks sulfatase activity (Blount et al., 2000b), and use of this enzyme could potentially underestimate concentrations of other biomarkers also present in urine as sulfate conjugates (Ye et al., 2005b, 2006; Abbas et al., 2010).
3.1.3. Mixture of solid β-glucuronidases (H-1) with liquid β-glucuronidases from E. Coli-B-21 (BL-21)
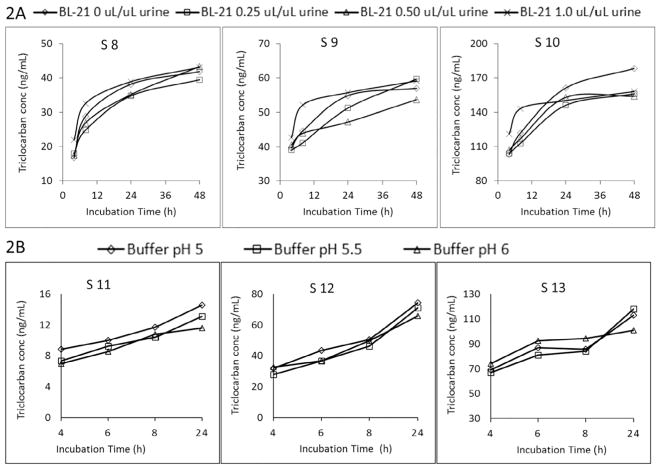
We evaluated these mixtures to minimize some of the problems associated with the use of large amounts of enzyme (e.g., extra protein precipitate from the enzyme occasionally plugging the autosampler injection lines) while also providing both sulfatase (H-1) and β-glucuronidase (H-1, BL-21) activities. The enzyme mixture solution was prepared in ammonium acetate buffer at pH 6. We incubated for 4, 8, 24, or 48 h at 37 °C three urine samples with urinary triclocarban concentrations around 40, 60, and 180 ng/mL (obtained using 30 units H-1/μL urine as described in 3.1.1), with 5 units H-1/μL urine and variable amounts (0, 0.25, 0.50, 1.0 μL) of BL-21 enzyme solution/μL urine (Fig. 2A). Even when using the highest amount of BL-21 (1.0 μL/μL urine), N-glucuronide triclocarban did not fully hydrolyze after 48 h of incubation (Fig. 2A).
Fig. 2.
Fig. 2A, Total triclocarban concentrations for urine samples S8–S10 after incubating with enzyme mixture solution (BL-21 + 5 units H-1/μL urine) at 37 °C and different amounts of BL-21 (0, 0.25, 0.50, 1.0 μL per μL urine). Fig. 2B, Total triclocarban concentrations for urine samples S11–S13 after incubating with enzyme mixture solution (1.0 μL BL-21/μL urine + 5 units H-1/μL urine) prepared with buffers at different pH values.
Further, to evaluate the effect of pH of the ammonium acetate buffer, we used 5 units H-1/μL urine and 1.0 μL BL-21/μL urine of enzyme mixture at pH 5, 5.5 or 6, and then incubated for 4, 6, 8, or 24 h (Fig. 2B). Total triclocarban concentration increased with increasing incubation time, but did not vary with the pH of the buffer (Fig. 2B).
3.1.4. Liquid β-glucuronidase from Helix Pomatia (type HP-2)
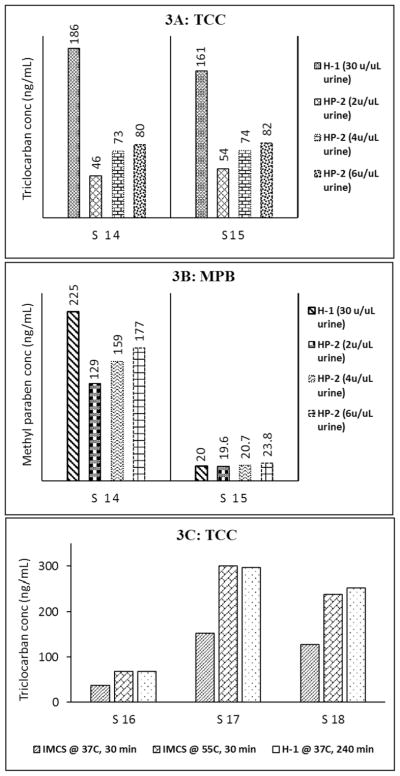
We tested a liquid β-glucuronidase HP-2, previously used for the simultaneous determination of parabens, environmental phenols, and triclocarban in urine (300 μL) treated with 6 μL of HP-2 (2 units/μL urine, 3.5 h incubation at 37 °C) at pH 5.0 with 1 M ammonium acetate (Moos et al., 2014). Fig. 3A and B shows the total concentrations of triclocarban and methyl paraben, respectively, in two example urine samples after 4 h of incubation at 37 °C with 2, 4, or 6 units HP-2/μL urine; for comparison, the figures also include the concentrations obtained after incubation at 37 °C for 4 h with 30 units H-1/μL urine. Even using 6 units HP-2/μL urine, triclocarban concentrations were approximately half of the concentrations after treatment with H-1 (Fig. 3A), thus suggesting incomplete hydrolysis of N-conjugated triclocarban. Regarding methyl paraben, when concentrations were relatively low (S15 in Fig. 3B), we observed similar hydrolysis efficiency for H-1 (30 units/μL urine) and HP-2 (regardless of the volume used); however, for relatively high concentrations (S14 in Fig. 3B), H-1 seemed to be more efficient than HP-2. Furthermore, we observed that concentrations of some target phenols (e.g., methyl paraben, ethyl paraben, triclosan) in a water blank, after incubation with HP-2, were more than 10 times higher than the background concentrations of the same analytes in a water blank after treatment with H-1 enzyme (data not shown). These results raised concerns that the limits of the detection of these analytes would likely increase by using the HP-2 enzyme.
Fig. 3.
Fig. 3A and B: Total concentrations of triclocarban (Fig. 3A) and methyl paraben (Fig. 3B) in urine samples (S14 and S15) after incubating with H-1 (30 units/μL urine) or HP-2 (2, 4, or 6 units/μL urine) for 4 h in 37 °C. Fig. 3C, Total concentrations of triclocarban after incubation urine S16, S17, S18 with IMCS enzyme (15 units/μL urine, 37°C or 55 °C for 30 min) or H-1 enzyme (30 units/μL urine, 37 °C for 4 h).
3.1.5. Liquid recombinant β-glucuronidase from snail (IMCS)
ICMS has been successfully used for rapid (<30 min) hydrolysis of glucuronide conjugates of some drugs (Morris et al., 2014). The enzyme is odorless, has water-like consistency and thus is easy to handle. We added 30 μL of ICMS enzyme (15 units/μL urine), 40 μL of IMCS buffer (pH 6.8), and 100 μL internal standard solution to three urine samples (100 μL), and incubated for 30 min at 37 °C or 55 °C. We also processed the same urine samples with H-1 enzyme (30 units/μL urine, pH 5.5, 37 °C) instead of ICMS. After incubation, we added 730 μL of 0.1 M formic acid to quench the enzymatic process and the samples were analyzed by on-line SPE-HPLC-MS/MS. Total triclocarban concentrations ranged from about 50 to 300 ng/mL (Fig. 3C). Compared to H-1 treatment, IMCS fully hydrolyzed triclocarban conjugates in a shorter time (30 min vs 4 h) but only at 55 °C; hydrolysis was incomplete at 37 °C. Our results suggest that IMCS enzyme may be a good alternative to HP-1 for complete hydrolysis of N-glucuronide triclocarban for bio-monitoring purposes because of the short incubation time, high potency, cost effectiveness, and handling convenience. However, total urinary concentrations of several phenols (e.g., 2,5-dichlorophenol, benzophenone-3) and parabens were underestimated when using 15 units ICMS/μL urine, 30 min incubation, 55 °C (data not shown). These results may relate to the fact that ICMS does not provide sulfatase activity, and, therefore, could not hydrolyze the sulfate conjugates of these chemicals (Ye et al., 2005b, 2006; Abbas et al., 2010).
3.1.6. Liquid β-glucuronidase/Arylsulfatase from Helix Pomatia (ALS)
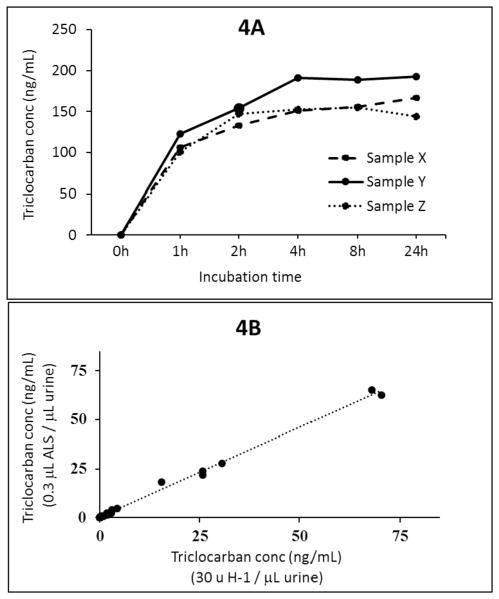
We compared triclocarban concentrations obtained after 4 h of incubation at 37 °C with either 30 units H-1/μL urine or ALS using 10, 20, 30, or 40 μL/100 μL urine (i.e., 10, 20, 30, 40 units/μL urine). Compared with the concentrations obtained after treatment with H-1, which ranged between approximately 1 to 150 ng/mL we observed no noticeable difference (within 10% deviation in measured concentrations) in concentrations when using 30 or 40 units ALS/μL urine, but for samples with triclocarban concentration >30 ng/mL, concentrations were lower (>25% deviation in measured concentration) after treatment with 10 or 20 units ALS/μL urine. Fig. 4A shows the total concentrations of triclocarban after 1, 2, 4, 8, or 24 h of incubation at 37 °C with 30 units ALS/μL urine for samples S19, S20, and S21. Triclocarban was completely deconjugated within 4 h of incubation and so did all phenols and parabens evaluated in the same biomonitoring method (data not shown). Measured analyte concentrations in 40 urine samples using ALS or H-1, both at 30 units/μL urine, correlated very well (correlation coefficient R2 > 0.98) for all analytes (except methyl paraben) (Fig. 4B). We did not observe chemical interferences when using ALS enzyme as did during HP-2 enzyme hydrolysis. These results suggest that at least 30 units ALS/μL urine was needed for the complete deconjugation of N-glucuronide triclocarban within 4 h. Under these same conditions (≥30 units ALS/μL urine, 4 h at 37 °C), concentrations of the six phenols and most parabens, except methyl paraben, compared well with those obtained using the original phenol method (Ye et al., 2005a; Zhou et al., 2014), thus suggesting complete deconjugation for these analytes by ALS.
Fig. 4.
Fig. 4A: Total concentrations of triclocarban after 1, 2, 4, 8, or 24 h of incubation at 37 °C using 30 units of ALS enzyme/μL urine for samples S19, S20, and S21. Fig. 4B: Correlation plot for total triclocarban measured in 40 urine samples after incubation with H-1 (30 units/μL urine) or ALS (0.3 μL/μL urine [i.e., 30 units/μL urine] for 4 h at 37°C).
3.2. Hydrolysis of methyl paraben conjugates
Out of the seven enzymes tested, three (≥30 units H-1, SH-1, or ALS/μL urine) completely hydrolyzed the conjugates of triclocarban, of all six other phenols, and of most parabens (except methyl paraben) within 4 h of incubation at 37 °C. However, compared to our original method (5 units Helix Pomatia H-1/μL urine, pH 5, 37 °C, 4 h) (Ye et al., 2005a; Zhou et al., 2014), under those experimental conditions using considerably large amounts of enzyme, we observed a ~20–50% decrease in concentrations of methyl paraben. We speculate that the incomplete recovery of methyl paraben under the aforementioned enzymatic digestion conditions may relate to the alkyl/aryl sulfatase (lipase)-mediated hydrolysis of the alkyl-ester bond of methyl paraben to p-hydroxybenzoic acid and methanol (Abbas et al., 2010). For example, several enzymes with similar lipase activity also hydrolyzed phthalate diesters to the corresponding monoesters during the enzymatic deconjugation reaction (Blount et al., 2000b). Interestingly, with the addition of 100 μL of methanol before incubation, we obtained total urinary methyl paraben concentrations comparable to those obtained using our original method (Ye et al., 2005a; Zhou et al., 2014). Furthermore, the addition of methanol did not seem to compromise the enzyme efficacy because all of the other analytes included in the method also deconjugated completely. We speculate that the addition of methanol before incubation prevented the possible hydrolysis of the ester bond of methyl paraben under our enzymatic conditions and facilitated the quantitative recovery of methyl paraben even in the presence of considerable amounts of enzyme without compromising the hydrolysis of the conjugates of triclocarban, and other parabens and phenols included in this method.
4. Conclusions
Extent of enzymatic hydrolysis of conjugates depends on the analyte, the source of the enzyme, enzyme activity, incubation time, pH of the buffer used for enzyme preparation, and amount of enzyme used. Our data demonstrate that optimal enzymatic conditions to hydrolyze conjugates of phenols and parabens may not be optimal for N-glucuronide triclocarban. Of the conditions tested, conjugates of triclocarban and 10 phenols and parabens completely hydrolyzed after incubation at 37 °C for 4 h with ≥ 30 units/μL urine of β-glucuronidase (H-1) or sulfatase (SH-1) from Helix pomatia (both in powder form), or β-glucuronidase/arylsulfatase from Helix pomatia (ALS, liquid) in the presence of 100 μL methanol per 100 μL urine. Addition of methanol likely quenched the unwanted enzyme lipase activity that could cleave the alkyl-ester bond of some biomarkers (e.g., methyl paraben) without compromising the glucuronidase/sulfatase activity. In summary, the data presented here stress the importance of optimizing the hydrolysis of urinary conjugates (e.g., enzyme type and amount used, buffer pH, reaction time, temperature) to quantify accurately and concurrently multiple exposure biomarkers for biomonitoring purposes.
HIGHLIGHTS.
Finding one optimal condition for complete hydrolysis of multiple biomarkers was challenging.
Enzyme type and amount used, reaction time, temperature critically affected hydrolysis extent.
Only two/seven enzymes evaluated completely hydrolyzed O– and N-glucuronides simultaneously.
≥30 units/μL urine of solid β-glucuronidase (Helix pomatia, H-1) resulted in complete hydrolysis.
≥30 μL/100 μL urine of liquid β-glucuronidase/arylsulfatase (Helix pomatia) resulted in complete hydrolysis.
Acknowledgments
This research was supported in part by an appointment (Tolar Powell) to the Research Participation Program at the Centers for Disease Control and Prevention, National Center for Environmental Health, Division of Laboratory Sciences, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC. The authors thank Josh Kramer for technical assistance.
Footnotes
Disclaimer
The findings and conclusions in this presentation are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References
- Abbas S, Greige-Gerges H, Karam N, Piet MH, Netter P, Magdalou J. Metabolism of parabens (4-hydroxybenzoic acid esters) by hepatic esterases and UDP-glucuronosyltransferases in man. Drug Metabol Pharmacokinet. 2010;25(6):568–577. doi: 10.2133/dmpk.dmpk-10-rg-013. [DOI] [PubMed] [Google Scholar]
- Babu SR, Lakshmi VM, Huang GP, Zenser TV, Davis BB. Glucuronide conjugates of 4-aminobiphenyl and its N-hydroxy metabolites. pH stability and synthesis by human and dog liver. Biochem Pharmacol. 1996;51(12):1679–1685. doi: 10.1016/0006-2952(96)00165-7. [DOI] [PubMed] [Google Scholar]
- Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000;72(17):4127–4134. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000;108(10):979–982. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM. The U.S. national health and nutrition examination Survey and human exposure to environmental chemicals. Int J Hyg Environ Health. 2012;215(2):99–101. doi: 10.1016/j.ijheh.2011.08.014. https://doi.org/10.1016/j.ijheh.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Silva MJ, Kuklenyik Z, Needham LL. Human exposure assessment to environmental chemicals using biomonitoring. Int J Androl. 2006;29(1):166–171. doi: 10.1111/j.1365-2605.2005.00570.x. https://doi.org/10.1111/j.1365-2605.2005.00570.x discussion 181-165. [DOI] [PubMed] [Google Scholar]
- CDC. Laboratory Procedure Manual. 2016 https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/EPHPP_H_MET.pdf.
- CDC. National Report on Human Exposure to Environmental Chemicals. 2017 https://www.cdc.gov/exposurereport.
- Frederiksen H, Aksglaede L, Sorensen K, Nielsen O, Main KM, Skakkebaek NE, Juul A, Andersson AM. Bisphenol A and other phenols in urine from Danish children and adolescents analyzed by isotope diluted TurboFlow-LC-MS/ MS. Int J Hyg Environ Health. 2013;216(6):710–720. doi: 10.1016/j.ijheh.2013.01.007. https://doi.org/10.1016/j.ijheh.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Hawes EM. N+-glucuronidation, a common pathway in human metabolism of drugs with a tertiary amine group. Drug Metabol Dispos Biol Fate Chem. 1998;26(9):830–837. [PubMed] [Google Scholar]
- Howes D, Black JG. Percutaneous absorption of triclocarban in rat and man. Toxicology. 1976;6(1):67–76. doi: 10.1016/0300-483x(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Kaivosaari S, Finel M, Koskinen M. N-glucuronidation of drugs and other xenobiotics by human and animal UDP-glucuronosyltransferases. Xenobiotica. 2011;41(8):652–669. doi: 10.3109/00498254.2011.563327. https://doi.org/10.3109/00498254.2011.563327. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77(9):2985–2991. doi: 10.1021/ac0481248. https://doi.org/10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Kowalczyk I, Hawes EM, McKay G. Stability and enzymatic hydrolysis of quaternary ammonium-linked glucuronide metabolites of drugs with an aliphatic tertiary amine-implications for analysis. J Pharmaceut Biomed Anal. 2000;22(5):803–811. doi: 10.1016/s0731-7085(00)00244-2. [DOI] [PubMed] [Google Scholar]
- Kroemer HK, Klotz U. Glucuronidation of drugs. A re-evaluation of the pharmacological significance of the conjugates and modulating factors. Clin Pharmacokinet. 1992;23(4):292–310. doi: 10.2165/00003088-199223040-00005. https://doi.org/10.2165/00003088-199223040-00005. [DOI] [PubMed] [Google Scholar]
- Moos RK, Angerer J, Wittsiepe J, Wilhelm M, Brüning T, Koch HM. Rapid determination of nine parabens and seven other environmental phenols in urine samples of German children and adults. Int J Hyg Environ Health. 2014;217(8):845–853. doi: 10.1016/j.ijheh.2014.06.003. https://doi.org/10.1016/j.ijheh.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Morris AA, Chester SA, Strickland EC, McIntire GL. Rapid enzymatic hydrolysis using a novel recombinant beta-glucuronidase in benzodiazepine urinalysis. J Anal Toxicol. 2014;38(8):610–614. doi: 10.1093/jat/bku083. https://doi.org/10.1093/jat/bku083. [DOI] [PubMed] [Google Scholar]
- Schebb NH, Franze B, Maul R, Ranganathan A, Hammock BD. In vitro glucuronidation of the antibacterial triclocarban and its oxidative metabolites. Drug Metabol Dispos Biol Fate Chem. 2012;40(1):25–31. doi: 10.1124/dmd.111.042283. https://doi.org/10.1124/dmd.111.042283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L, Muller J, Goen T. Simultaneous monitoring of seven phenolic metabolites of endocrine disrupting compounds (EDC) in human urine using gas chromatography with tandem mass spectrometry. Anal Bioanal Chem. 2013;405(6):2019–2029. doi: 10.1007/s00216-012-6618-y. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Preau JL, Jr, Needham LL, Calafat AM. Cross validation and ruggedness testing of analytical methods used for the quantification of urinary phthalate metabolites. J Chromatogr B Anal Technol Biomed Life Sci. 2008;873(2):180–186. doi: 10.1016/j.jchromb.2008.08.017. https://doi.org/10.1016/j.jchromb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Jia T, Samandar E, Preau JL, Jr, Calafat AM. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000–2012) Environ Res. 2013;126:159–163. doi: 10.1016/j.envres.2013.05.007. https://doi.org/10.1016/j.envres.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly TR. Isolation and purification of UDP-glucuronosyltransferases. Chem Res Toxicol. 1990;3(6):509–516. doi: 10.1021/tx00018a004. [DOI] [PubMed] [Google Scholar]
- Tephly TR, Burchell B. UDP-glucuronosyltransferases: a family of detoxifying enzymes. Trends Pharmacol Sci. 1990;11(7):276–279. doi: 10.1016/0165-6147(90)90008-v. [DOI] [PubMed] [Google Scholar]
- Ye XY, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- Ye XY, Kuklenyik Z, Needham LL, Calafat AM. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2005;383(4):638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. Parabens as urinary biomarkers of exposure in humans. Environ Health Perspect. 2006;114(12):1843–1846. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Zhou X, Furr J, Ahn KC, Hammock BD, Gray EL, Calafat AM. Biomarkers of exposure to triclocarban in urine and serum. Toxicology. 2011;286(1–3):69–74. doi: 10.1016/j.tox.2011.05.008. https://doi.org/10.1016/j.tox.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser TV, Lakshmi VM, Davis BB. Human and Escherichia coli beta-glucuronidase hydrolysis of glucuronide conjugates of benzidine and 4-aminobiphenyl, and their hydroxy metabolites. Drug Metabol Dispos Biol Fate Chem. 1999;27(9):1064–1067. [PubMed] [Google Scholar]
- Zhou J, Miners JO. Enzyme kinetics of uridine diphosphate glucuronosyl-transferases (UGTs) Meth Mol Biol. 2014;1113:203–228. doi: 10.1007/978-1-62703-758-7_11. https://doi.org/10.1007/978-1-62703-758-7_11. [DOI] [PubMed] [Google Scholar]
- Zhou X, Ye X, Calafat AM. Automated on-line column-switching HPLC-MS/ MS method for the quantification of triclocarban and its oxidative metabolites in human urine and serum. J Chromatogr B Anal Technol Biomed Life Sci. 2012;881–882:27–33. doi: 10.1016/j.jchromb.2011.11.024. https://doi.org/10.1016/j.jchromb.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Anal Technol Biomed Life Sci. 2014;944:152–156. doi: 10.1016/j.jchromb.2013.11.009. https://doi.org/10.1016/j.jchromb.2013.11.009. [DOI] [PubMed] [Google Scholar]