Abstract
Background:
Endoscopic lung volume reduction coil (LVRC) treatment is an option for selected patients with severe emphysema. In the advanced stages, emphysema leads to respiratory failure: hypoxemia and eventually chronic hypercapnic respiratory failure. It can be hypothesized that LVRC treatment, a procedure targeting hyperinflation and thereby reducing ventilatory workload, may be especially beneficial in patients with chronic hypercapnic respiratory failure. This study was conducted to gain first insights into the effects and the safety of LVRC treatment in patients with emphysema and chronic hypercapnic respiratory failure.
Methods:
A retrospective observational study conducted in the Department of Respiratory Medicine at the University Medical Center Hamburg-Eppendorf, Germany on all patients with chronic hypercapnic respiratory failure in whom bilateral LVRC treatment was performed between 1 April 2012 and 30 September 2015.
Results:
During the study period, bilateral LVRC treatment was performed in 10 patients with chronic hypercapnic respiratory failure. Compared with baseline, bilateral LVRC treatment led to a significant increase in mean forced expiratory volume in one second (FEV1) from 0.5 ± 0.1 l to 0.6 ± 0.2 l (p = 0.004), a decrease in residual volume (RV) from 6.1 ± 0.9 l to 5.6 ± 1.1 l (p = 0.02) and a reduction in partial pressure of carbon dioxide in arterial blood (PaCO2) from 53 ± 5 mmHg to 48 ± 4 mmHg (p = 0.03). One case of hemoptysis requiring readmission to hospital was the only severe adverse event.
Conclusions:
LVRC treatment was safe and effective in patients with nonsevere chronic hypercapnic respiratory failure. It led not only to an improvement in lung function but also to a significant decrease in PaCO2.
Keywords: chronic hypercapnic respiratory failure, emphysema, endoscopic lung volume reduction, endoscopic lung volume reduction coil, interventional pulmonology
Introduction
Emphysema is a debilitating chronic pulmonary disease [Vestbo et al. 2013]. It is characterized by structural changes in lung parenchyma with consecutive reduction of gas exchange surface, loss of elastic recoil and dynamic hyperinflation leading to dyspnea, limited exercise capacity and reduced quality of life. In the advanced stages, it leads to respiratory failure: hypoxemia and eventually chronic hypercapnic respiratory failure (type 2 respiratory failure). Hyperinflation is a major component in the development of chronic hypercapnic respiratory failure as it is associated with an increased ventilatory workload, with key pathophysiological elements being the loss of elastic recoil as well as geometrical changes of diaphragm and thorax compromising the function of the diaphragmatic, intercostal and accessory muscles [Roussos and Koutsoukou, 2003].
The condition is incurable and therapeutic options are limited. In selected patients with severe emphysema, lung volume reduction may be considered [Koegelenberg et al. 2015]. The original procedure, lung volume reduction surgery, has been shown to be beneficial and safe if performed in carefully and properly selected patients [Ginsburg et al. 2016; Meyers et al. 2004; Fishman et al. 2003]. Over the last decade, sophisticated interventional bronchoscopic procedures have been added to the repertoire of lung volume reduction. Procedures developed for bronchoscopic use included one-way valves [Snell et al. 2003], airway bypass stents [Cardoso et al. 2007], biological lung volume reduction [Reilly et al. 2007], bronchoscopic thermal vapor ablation [Snell et al. 2009] and coils [Herth et al. 2010].
Endoscopic lung volume reduction coil (LVRC) treatment was first introduced in 2010 [Herth et al. 2010]. It is a treatment option not only for patients with heterogeneous emphysema but also for patients with homogeneous emphysema [Klooster et al. 2014] and is independent of the presence of collateral ventilation. Beneficial effects of LVRC treatment have been shown regarding pulmonary function, exercise capacity and quality of life [Deslee et al. 2014; Klooster et al. 2014; Shah et al. 2013; Slebos et al. 2012]. LVRC treatment has also been shown to have a good safety profile [Hartman et al. 2015; Shah and Kemp, 2015].
Careful patient selection is mandatory before endoscopic lung volume reduction. In a recently published review, a partial pressure of carbon dioxide in arterial blood (PaCO2) <50 mmHg has been listed among the prerequisites for endoscopic lung volume reduction [Koegelenberg et al. 2015]. However, it can be hypothesized, that LVRC treatment, a procedure explicitly designed to target hyperinflation and thereby reducing ventilatory workload, may be especially beneficial in patients with chronic hypercapnic respiratory failure. We therefore conducted this retrospective analysis on LVRC treatment in patients with chronic hypercapnic respiratory failure. To our knowledge, this is the first analysis of the effectiveness and safety of LVRC treatment in patients with chronic hypercapnic respiratory failure published to date.
Materials and methods
Study design
This was a retrospective observational trial conducted in the Department of Respiratory Medicine at the University Medical Center Hamburg-Eppendorf, Germany. Inclusion criteria were (1) bilateral LVRC treatment and (2) chronic hypercapnic respiratory failure due to emphysema. Chronic hypercapnic respiratory failure was defined as a PaCO2 > 45 mmHg and normal pH at rest while in a clinically stable condition and despite optimal therapy for chronic obstructive pulmonary disease (COPD). The ethics committee of the Hamburg chamber of physicians waived the need for ethics approval and for the need to obtain consent for the collection, analysis, and publication of the retrospectively-obtained and anonymized data for this noninterventional study.
Data collection
The electronic endoscopic database (Endobase, version 12.0, Olympus, Tokyo, Japan) was searched for all cases of LVRC treatment specifying a time frame between 1 April 2012 and 30 September 2015. The electronic patient database including the electronic patient record (Soarian Clinicals, version 3.00 SP3, Cerner Health Services, USA) was then used to retrieve patient characteristics, procedural details and data collected during initial assessment and follow-up visits including the results of the assessments of pulmonary function and exercise capacity.
LVRC treatment
In our department, all patients with severe emphysema are thoroughly evaluated considering lung volume reduction surgery, different techniques of endoscopic lung volume reduction and lung transplantation. Criteria to indicate endoscopic lung volume reduction include the presence of emphysema, symptoms despite optimal medical therapy and pulmonary rehabilitation, severe or very severe airflow obstruction as defined by the global initiative for chronic obstructive lung disease (GOLD) definition, hyperinflation with, at the time of this study, a residual volume (RV) >175% of predicted and the absence of excessive sputum or active infection. To select the appropriate technique, the distribution of emphysema, the degree of tissue destruction, the evaluation of interlobar collateral ventilation and comorbidities are taken into account, with homogeneous emphysema, the presence of collateral ventilation in patients with heterogeneous emphysema and tissue destruction <75% being among the criteria in favor of LVRC treatment.
Prior to endoscopic lung volume reduction, patients are routinely screened for signs of right ventricular strain and pulmonary hypertension. Unless pulmonary hypertension has been previously diagnosed or excluded by right heart catheterization, screening includes echocardiography, the measurement of NT-proBNP levels as well as the evaluation of computed tomography scans for the presence of a pulmonary arterial diameter >3 cm, a ratio of the right to left atrial diameter >1, a ratio of the pulmonary arterial to the aortic diameter >1 and reflux of intravenous contrast into the inferior vena cava. In cases where severe pulmonary hypertension, defined as a systolic pulmonary arterial pressure >50 mmHg, has been diagnosed or is suspected, patients are excluded from endoscopic lung volume reduction.
LVRC treatment is performed bilaterally in two sequential procedures unless complications or contraindications arise or the patient opts for unilateral treatment only. The aim is to complete the second procedure 1–3 months after the first procedure. The targeted lobe and the sequence are selected according to the distribution of emphysema and the degree of tissue destruction, with the upper lobes being preferentially treated in patients with homogeneous emphysema [Deslee et al. 2016; Klooster et al. 2014].
Assessment of pulmonary function and exercise capacity
In our department, as a routine standard of care in patients with LVRC treatment, pulmonary function and exercise capacity are assessed in a standardized manner at baseline, between the first and second LVRC treatment and at each outpatient follow-up visit. Pulmonary function tests including spirometry, body plethysmography and carbon monoxide uptake as well as blood gas analyses are performed according to the American Thoracic Society and European Respiratory Society guidelines [Pellegrino et al. 2005; MacIntyre et al. 2005; Wanger et al. 2005; Miller et al. 2005a, 2005b]. Exercise capacity is assessed using the 6-min walk test [Holland et al. 2014; ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories, 2002].
Adverse events
The electronic patient record was systematically reviewed for adverse events. As a routine standard of care, patients after LVRC treatment are questioned about changes in dyspnea and the occurrence of hemoptysis or chest pain on a daily basis during hospitalization and at each outpatient visit. Outpatient visits are routinely scheduled between the first and second treatment approximately 1 month after the first treatment as well as 3 months, 6 months and 12 months after the second treatment. Any complications encountered during the bronchoscopic procedure as well as pneumothoraces, respiratory infections, COPD exacerbations within 4 weeks of the procedure, pleuritic pain associated with the position of coils and hemoptysis occurring at any time during the follow-up period were considered adverse events.
Data analysis
Categorical variables are presented as absolute numbers and percentages. Continuous variables are presented as mean and standard deviation if normally distributed and as median and range if not normally distributed. Comparisons were performed using the Student’s t test for metric data. A two-sided p value <0.05 was considered significant. The software used for statistical analyses was SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Case selection
Between 1 April 2012 and 30 September 2015 a total of 101 LVRC procedures were performed in 62 patients. Among these were 19 patients with chronic hypercapnic respiratory failure. LVRC treatment was performed only unilaterally in nine of these patients. Overall, 10 patients met inclusion criteria and were analyzed in this study. The process of case selection is shown in Figure 1.
Figure 1.
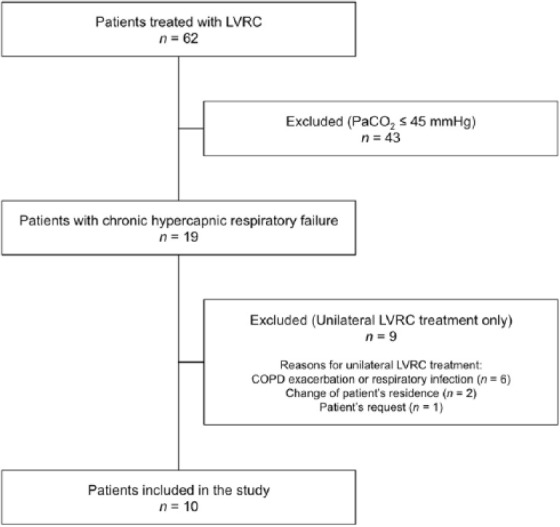
Flow diagram illustrating the process of case selection.
COPD, chronic obstructive pulmonary disease; LVRC, endoscopic lung volume reduction coil; PaCO2, partial pressure of carbon dioxide in arterial blood.
Characteristics of patients at baseline
Of the 10 patients included in the study, 70% were female and 30% were male. Mean age was 64 ± 9 years. All patients were on optimal pharmacological therapy for their pulmonary disease. Additionally, all patients were on long-term oxygen therapy due to chronic hypoxemia and 70% were on intermittent noninvasive ventilation. Emphysema was homogeneous in 80% and heterogeneous in 20% of cases. All patients had very severe airflow obstruction as defined by the GOLD criteria. At baseline, mean forced expired volume in one second (FEV1) was 0.5 ± 0.1 l equal to 17 ± 5% of predicted. Mean RV was 6.1 ± 0.9 l equal to 280 ± 48% of predicted. Mean forced vital capacity (FVC) was 1.5 ± 0.5 l equal to 42 ± 10% of predicted. Mean PaCO2 was 53 ± 5 mmHg. Mean 6-min walking distance was 203 ± 97 m. Characteristics of patients at baseline are summarized in Table 1.
Table 1.
Characteristics of patients at baseline.
| Characteristic | Values |
|---|---|
| Number of patients | 10 |
| Age, years | 64 ± 9 |
| Sex | |
| Female | 7 (70.0%) |
| Male | 3 (30.0%) |
| Distribution of emphysema | |
| Homogeneous | 8 (80.0%) |
| Heterogeneous | 2 (20.0%) |
| Treatment for lung disease | |
| β2-agonist | 10 (100.0%) |
| Anticholinergic | 10 (100.0%) |
| Inhaled corticosteroid | 8 (80.0%) |
| Systemic corticosteroid | 1 (10.0%) |
| Theophylline | 4 (40.0%) |
| Roflumilast | 2 (20.0%) |
| Acetylcysteine | 2 (20.0%) |
| Long-term oxygen therapy | 10 (100.0%) |
| Intermittent noninvasive ventilation | 7 (70.0%) |
| Blood gas analysis | |
| PaCO2 (mmHg) | 53 ± 5 |
| Base excess (mmol/l) | 7.2 ± 2.7 |
| HCO3- (mmol/l) | 32.9 ± 2.8 |
| pH | 7.42 ± 0.04 |
| Pulmonary function | |
| FEV1/FVC (%) | 32 ± 5 |
| FEV1 (l) | 0.5 ± 0.1 |
| FEV1 (% of predicted) | 17 ± 5 |
| FVC (l) | 1.5 ± 0.5 |
| FVC (% of predicted) | 42 ± 10 |
| TLC (l) | 7.6 ± 1.1 |
| TLC (% of predicted) | 130 ± 14 |
| RV (l) | 6.1 ± 0.9 |
| RV (% of predicted) | 280 ± 48 |
| RV/TLC (%) | 81 ± 5 |
| Rawtot (kPa•s/l) | 1.8 ± 0.6 |
| sRawtot (kPa•s) | 11.6 ± 4.6 |
| TLCO (ml/min/kPa) | 0.7 ± 0.3 |
| TLCO (% of predicted) | 8.6 ± 3.6 |
| 6-min walk test (m) | 203 ± 97 |
Values are given as mean and standard deviation or as absolute numbers and percentages.
FEV1, forced expired volume in one second; FVC, forced vital capacity; PaCO2, partial pressure of carbon dioxide in arterial blood; Rawtot, total airway resistance; RV, residual volume; sRawtot, specific total airway resistance; TLC, total lung capacity; TLCO, transfer factor of the lung for carbon monoxide.
Characteristics of the LVRC procedure
As defined in the inclusion criteria, LVRC treatment was performed bilaterally in two sequential procedures in all patients. The median interval between the two procedures was 84 days. In 90% of procedures one of the upper lobes was treated. A median number of 10 coils (range, 8–10) were placed per procedure. The mean length of hospital stay after the procedure was 6.8 ± 1.4 days. Characteristics of the LVRC procedure are shown in Table 2.
Table 2.
Characteristics of the LVRC procedures.
| First treatment | Second treatment | |
|---|---|---|
| Number of procedures | 10 | 10 |
| Treated lobe | ||
| Right upper lobe | 6 (60%) | 3 (30%) |
| Left upper lobe | 3 (30%) | 6 (60%) |
| Right lower lobe | 1 (10%) | 1 (10%) |
| Coils | ||
| Total number of coils | 10 (range, 8–10) | 10 (range, 9–10) |
| Coil size 100 mm | 8 (range, 4–10) | 6 (range, 1–10) |
| Coil size 125 mm | 8 (range, 2–5) | 4 (range, 2– 9) |
| Coil size 150 mm | 1 (range, 1–3) | 2 (range, 1– 2) |
Values are given as absolute numbers and percentages or as median and range.
LVRC, endoscopic lung volume reduction coil.
Adverse events
There were no adverse events during bronchoscopy or related to general anesthesia used for the procedure. Mild self-limiting hemoptysis occurred in the first days after 15 LVRC procedures (75%), of which 8 were first procedures and 7 were second procedures. In one case, hemoptysis required readmission to hospital and bronchial artery embolization to stop the bleeding and was therefore classified as a severe adverse event. Exacerbations of COPD within 4 weeks of LVRC treatment occurred after six procedures (30.0%). Of these, three were first procedures and three were second procedures in different patients. Apart from the one case of hemoptysis that was classified as a severe adverse event, all other adverse events resolved spontaneously or with routine medical care. There were no deaths.
Outcome
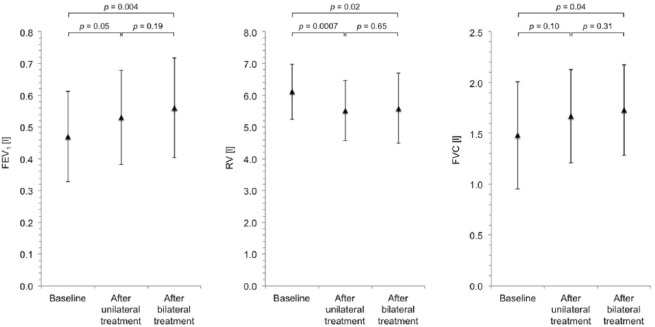
Compared with baseline, after bilateral LVRC treatment, there was a significant increase in mean FEV1 of 19.1% from 0.5 ± 0.1 l to 0.6 ± 0.2 l (p = 0.004), a significant decrease in mean RV of 8.5% from 6.1 ± 0.9 l to 5.6 ± 1.1 l (p = 0.02) and a significant increase in mean FVC of 16.9% from 1.5 ± 0.5 l to 1.7 ± 0.4 l (p = 0.04). Changes in lung function are shown in Figure 2. Mean 6-min walk distance increased by 20.1% from 203 ± 97 m to 244 ± 104 m (p = 0.22). An illustration of the changes in 6-min walk distance is provided in Figure 3. Mean PaCO2 decreased significantly by 8.6% from 53 ± 5 mmHg to 48 ± 4 mmHg (p = 0.03). Changes in PaCO2 are shown in Figure 4. Of the three patients in whom LVRC treatment was performed as a bridge to lung transplantation, two patients underwent lung transplantation during the follow-up period.
Figure 2.
Changes in lung function. Lung function at baseline, after unilateral LVRC treatment and after bilateral LVRC treatment.
FEV1, forced expired volume in one second; FVC, forced vital capacity; LVRC, endoscopic lung volume reduction coil; RV, residual volume.
Figure 3.
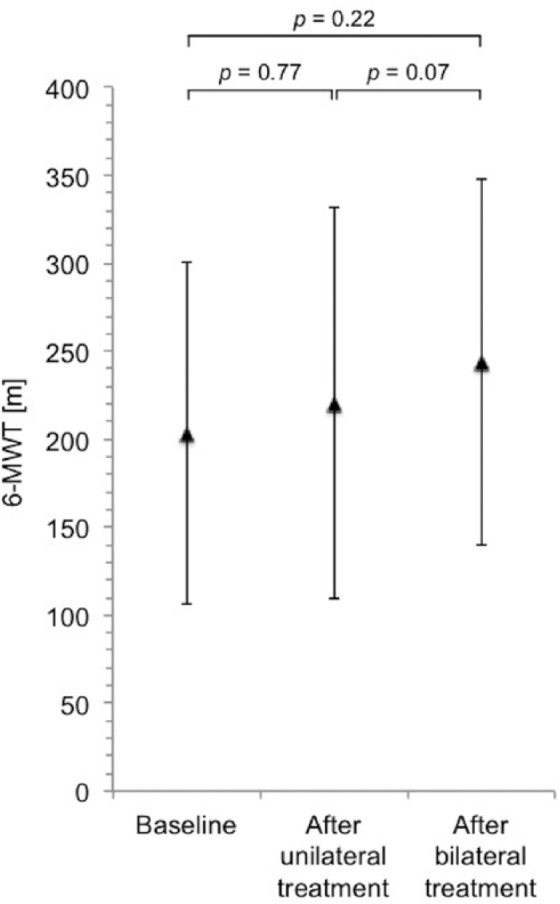
Changes in exercise capacity. Showing 6-min walk distance at baseline, after unilateral LVRC treatment and after bilateral LVRC treatment.
LVRC, endoscopic lung volume reduction coil; 6-MWT, 6-minute walk test.
Figure 4.
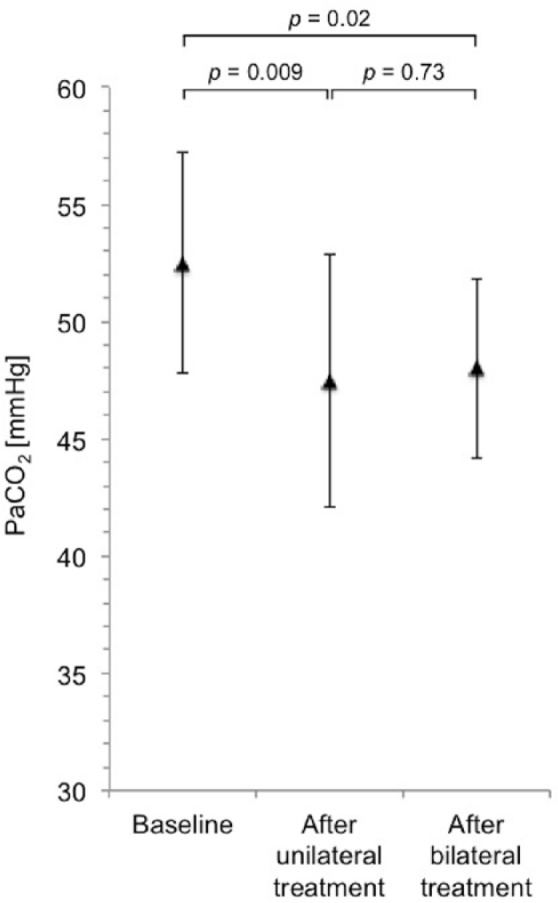
Changes in PaCO2. PaCO2 at baseline, after unilateral LVRC treatment and after bilateral LVRC treatment.
LVRC, endoscopic lung volume reduction coil; PaCO2, partial pressure of carbon dioxide in arterial blood.
Discussion
In this retrospective observational study, we analyzed 10 patients with nonsevere chronic hypercapnic respiratory failure defined as a PaCO2 between 45–65 mmHg due to emphysema in whom bilateral endoscopic LVRC treatment was performed. LVRC treatment was found to be effective and safe in this group of patients. After LVRC treatment, we observed not only an improvement in lung function but also a reduction of hypercapnia.
LVRC treatment has been shown to improve lung function, exercise capacity and quality of life in patients with emphysema [Deslee et al. 2014; Klooster et al. 2014; Shah et al. 2013; Slebos et al. 2012]. The recently published REVOLENS trial is the largest randomized trial on LVRC treatment published to date including 100 patients. In the 50 patients randomized to receive LVRC treatment, 6 months after the procedure, the authors found improvements in lung function with an increase in FEV1 of 9%, a decrease in RV of 9% and an increase in FVC of 15% as well as an improvement in 6-minute walk distance of 9% and improved quality of life [Deslee et al. 2016]. In our study, after LVRC treatment in patients with chronic hypercapnic respiratory failure, we observed similar ameliorations of lung function with an increase in mean FEV1 of 19.1%, a decrease in mean RV of 8.5% and an increase in mean FVC of 16.9%.
Chronic hypercapnic respiratory failure puts the patient at an increased periprocedural risk of death especially should complications arise. Indeed, hypercapnia has been reported to increase mortality associated with lung volume reduction surgery [National Emphysema Treatment Trial Research Group, 2001]. LVRC treatment has been shown to have a good safety profile [Hartman et al. 2015; Shah and Kemp, 2015]. The REVOLENS trial reported 17 severe adverse events within 1 month of the procedure (4 chronic obstructive pulmonary disease exacerbations, 3 pneumothoraces, 1 case of hemoptysis, 1 case of thoracic pain, 5 cases of pneumonia, 1 cardiovascular event and 2 others) as well as 1 death which was however due to peritonitis [Deslee et al. 2016]. LVRC in patients with chronic hypercapnic respiratory failure in our study was shown to have an acceptable safety profile with only one serious adverse event, being hemoptysis, requiring readmission to hospital and bronchial artery embolization to stop the bleeding.
Chronic hypercapnic respiratory failure has been shown to be associated with poor prognosis. A prospective study by Yang and colleagues including 275 patients with COPD showed a median survival of 5.0 years in patients with hypercapnia compared with 6.5 years in patients with normocapnia (p = 0.016) [Yang et al. 2015]. Therapeutic measures providing ventilatory support and thereby reducing hypercapnia have been shown to be beneficial in patients with chronic hypercapnic respiratory failure [Schönhofer, 2015]. In particular, the use of intermittent noninvasive positive-pressure ventilation has been thoroughly investigated. In a randomized controlled trial, Köhnlein and colleagues observed that the addition of long-term noninvasive pressure ventilation to standard treatment improved survival in patients with chronic hypercapnic respiratory failure when noninvasive ventilation was targeted to reduce hypercapnia [Köhnlein et al. 2014]. Budweiser and colleagues observed a significant weight gain in cachectic patients with COPD and chronic hypercapnic respiratory failure receiving noninvasive ventilation [Budweiser et al. 2006]. The question of whether or not an improvement of prognosis or nutritional status may also be achieved by other measures leading to the reduction of hypercapnia by decreasing ventilatory workload like lung volume reduction remains to be investigated. However, the concept appears promising in theory.
Concerning lung volume reduction surgery, there has been much debate on the effects and risks of the procedure in patients with chronic hypercapnic respiratory failure and on whether or not the procedure should be performed in patients with hypercapnia at all. However, the data available suggest that lung volume reduction surgery is capable of improving gas exchange, lung function and quality of life while exhibiting an acceptable safety profile in patients with chronic hypercapnic respiratory failure. The national emphysema treatment trial included patients with a PaCO2 of up to 60 mmHg [Fishman et al. 2003]. Although hypercapnia was found to increase the mortality rate associated with lung volume reduction surgery, it did not clearly identify patients for whom surgery posed a substantially higher risk than medical treatment [National Emphysema Treatment Trial Research Group, 2001]. Other studies on lung volume reduction surgery [Argenziano et al. 1996; Albert et al. 1998; Wisser et al. 1998; O’Brien et al. 1999; Shade et al. 1999; Tsunezuka et al. 2000; Mitsui et al. 2001] analyzing patients with hypercapnic respiratory failure showed improvements of hypercapnia, pulmonary function, exercise capacity, dyspnea and quality of life. The studies are summarized in Table 3. Shade and colleagues found the degree of decrease in PaCO2 after lung volume reduction surgery in patients with moderate hypercapnia to be dependent on the baseline level of PaCO2 with the greatest reduction in PaCO2 seen in patients with higher baseline levels of PaCO2 [Shade et al. 1999].
Table 3.
Studies on lung volume reduction surgery in patients with hypercapnic respiratory failure.
| Study | Number of patients with HRF | Severity of HRF (PaCO2) | Outcome |
|---|---|---|---|
| Argenziano et al. [1996] | 9 | >55 mmHg | Improvement in pulmonary function. Improvement in exercise capacity. Improvement in dyspnea. |
| Albert et al. [1998] | 12 | >45 mmHg | Decrease in PaCO2 from 53 ± 6 mmHg to 47 ± 5 mmHg. |
| Wisser et al. [1998] | 22 | ⩾45 mmHg | Decrease in PaCO2 from 51.7 ± 1.7 mmHg to 41.3 ± 1.7 mmHg. |
| O’Brien et al. [1999] | 15 | >45 mmHg | Decrease in PaCO2 from 59 ± 7 mmHg to 50 ± 9
mmHg. Improvement in pulmonary function. Improvement in exercise capacity. Improvement in quality of life. |
| Shade et al. [1999] | 33 | Decrease in PaCO2 from 44 ± 7 mmHg to 42 ± 5
mmHg. Improvement in pulmonary function. |
|
| Tsunezuka et al. [2000] | 3 | >50 mmHg | Decrease in PaCO2 from 52 ± 1 mmHg to 48 ± 2 mmHg. |
| Mitsui et al. [2001] | 6 | ⩾60 mmHg | Decrease in PaCO2 from 70.4 ± 9.4 mmHg to 46.9 ± 3.4
mmHg. Improvement in pulmonary function. |
HRF, hypercapnic respiratory failure; PaCO2, partial pressure of carbon dioxide in arterial blood.
At present, the evidence for bronchoscopic lung volume reduction in patients with respiratory failure is limited to anecdotal data about the use of occlusive devices in patients with bullous emphysema and acute ventilatory failure requiring mechanical ventilation. Tsujino and colleagues described the use of endobronchial silicone spigots in a patient with acute respiratory failure and bullous emphysema whose respiratory condition improved after endoscopic lung volume reduction [Tsujino et al. 2009]. Sexton and colleagues, Votruba and colleagues and Bierach and colleagues each reported cases of a patient with acute respiratory failure and bullous emphysema who could be liberated from mechanical ventilation after endoscopic lung volume reduction using endobronchial valves [Sexton et al. 2010; Votruba et al. 2011; Bierach et al. 2013].
To our knowledge, to date there is no study on the effects of endoscopic lung volume reduction in patients with chronic hypercapnic respiratory failure. In this analysis on LVRC treatment in patients with chronic hypercapnic respiratory failure, mean PaCO2 was seen to decrease significantly from 53 ± 5 mmHg to 48 ± 4 mmHg (p = 0.03) after LVRC treatment.
In this study, only patients were included in whom bilateral LVRC treatment was performed. While LVRC treatment has traditionally been a primarily bilateral approach and data for bilateral lung volume reduction surgery is also available, for one-way valves, data predominantly rely on unilateral procedures. Recently, a study on sequential bilateral bronchoscopic lung volume reduction with one-way valves has been published showing improvements in respiratory function in patients with bilateral heterogeneous emphysema [Fiorelli et al. 2016]. The question of whether endoscopic lung volume reduction should be primarily planned as a unilateral or as a bilateral approach and what the criteria should be to continue with contralateral treatment in cases where a primarily unilateral approach was chosen cannot be definitively answered to date. An individualized approach being tailored to the characteristics of patient and emphysema, taking into consideration the changes in physiological parameters resulting after the first procedure and possibly involving hybrid procedures incorporating different techniques of lung volume reduction might be expected.
The study has some methodological limitations. The interpretation of the results is limited by potential biases introduced by the retrospective study design and the small number of patients. However, the outcome and safety profile of LVRC treatment in this selected and highly vulnerable subgroup of patients with emphysema were comparable with the overall results of previously published studies on LVRC treatment. LVRC treatment was additionally shown to be capable of reducing ventilatory workload leading to the improvement of hypercapnia. Thus, patients with chronic hypercapnic respiratory failure should probably not be excluded from further evaluation for LVRC treatment solely for the presence of hypercapnia. Prospective studies including more patients are now needed to further assess the value of LVRC treatment in patients with chronic hypercapnic respiratory failure.
Conclusion
LVRC treatment could be performed safely in patients with nonsevere chronic hypercapnic respiratory failure. It led not only to an improvement in lung function but also to an improvement of hypercapnia. Thus, patients with chronic hypercapnic respiratory failure should probably not be excluded from further evaluation for LVRC treatment solely for the presence of hypercapnia.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MS and HK declare that they serve as advisors for PneumRx and have received honoraria for talks and workshops. None of the authors has any financial interest in the company.
Contributor Information
Marcel Simon, Department of Respiratory Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Lars Harbaum, Department of Respiratory Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Tim Oqueka, Department of Respiratory Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Stefan Kluge, Department of Intensive Care Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Hans Klose, Department of Respiratory Medicine, University Medical Center Hamburg-Eppendorf, Martinistrasse 52, 20246 Hamburg, Germany.
References
- Albert R., Benditt J., Hildebrandt J., Wood D., Hlastala M. (1998) Lung volume reduction surgery has variable effects on blood gases in patients with emphysema. Am J Respir Crit Care Med 158: 71–76. [DOI] [PubMed] [Google Scholar]
- Argenziano M., Moazami N., Thomashow B., Jellen P., Gorenstein L., Rose E., et al. (1996) Extended indications for lung volume reduction surgery in advanced emphysema. Ann Thorac Surg 62: 1588–1597. [DOI] [PubMed] [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111–117. [DOI] [PubMed] [Google Scholar]
- Bierach J., Maloney J., Ferguson J. (2013) Endobronchial valve placement for a giant bulla in a patient with hypercapnic respiratory failure. Ann Am Thorac Soc 10: 521–524. [DOI] [PubMed] [Google Scholar]
- Budweiser S., Heinemann F., Meyer K., Wild P., Pfeifer M. (2006) Weight gain in cachectic COPD patients receiving noninvasive positive-pressure ventilation. Respir Care 51: 126–132. [PubMed] [Google Scholar]
- Cardoso P., Snell G., Hopkins P., Sybrecht G., Stamatis G., Ng A., et al. (2007) Clinical application of airway bypass with paclitaxel-eluting stents: early results. J Thorac Cardiovasc Surg 134: 974–981. [DOI] [PubMed] [Google Scholar]
- Deslee G., Klooster K., Hetzel M., Stanzel F., Kessler R., Marquette C., et al. (2014) Lung volume reduction coil treatment for patients with severe emphysema: a European multicentre trial. Thorax 69: 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslee G., Mal H., Dutau H., Bourdin A., Vergnon J., Pison C., et al. (2016) Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. J Am Med Assoc 315: 175–184. [DOI] [PubMed] [Google Scholar]
- Fiorelli A., D’Andrilli A., Anile M., Diso D., Poggi C., Polverino M., et al. (2016) Sequential bilateral bronchoscopic lung volume reduction with one-way valves for heterogeneous emphysema. Ann Thorac Surg 102: 287–294. [DOI] [PubMed] [Google Scholar]
- Fishman A., Martinez F., Naunheim K., Piantadosi S., Wise R., Ries A., et al. (2003) A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Eng J Med 348: 2059–2073. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Thomashow B., Bulman W., Jellen P., Whippo B., Chiuzan C., et al. (2016) The safety, efficacy, and durability of lung-volume reduction surgery: a 10-year experience. J Thorac Cardiovasc Surg 151: 717–724.e1. [DOI] [PubMed] [Google Scholar]
- Hartman J., Klooster K., Gortzak K., Hacken Ten N., Slebos D. (2015) Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 20: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herth F., Eberhard R., Gompelmann D., Slebos D., Ernst A. (2010) Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis 4: 225–231. [DOI] [PubMed] [Google Scholar]
- Holland A., Spruit M., Troosters T., Puhan M., Pepin V., Saey D., et al. (2014) An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 44: 1428–1446. [DOI] [PubMed] [Google Scholar]
- Klooster K., Hacken Ten N., Franz I., Kerstjens H., van Rikxoort E., Slebos D. (2014) Lung volume reduction coil treatment in chronic obstructive pulmonary disease patients with homogeneous emphysema: a prospective feasibility trial. Respiration 88: 116–125. [DOI] [PubMed] [Google Scholar]
- Koegelenberg C., Slebos D., Shah P., Theron J., Dheda K., Allwood B., et al. (2015) Time for the global rollout of endoscopic lung volume reduction. Respiration 90: 430–440. [DOI] [PubMed] [Google Scholar]
- Köhnlein T., Windisch W., Köhler D., Drabik A., Geiseler J., Hartl S., et al. (2014) Non-invasive positive-pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med 2: 698–705. [DOI] [PubMed] [Google Scholar]
- MacIntyre N., Crapo R., Viegi G., Johnson D., van der Grinten C., Brusasco V., et al. (2005) Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 26: 720–735. [DOI] [PubMed] [Google Scholar]
- Meyers B., Yusen R., Guthrie T., Patterson G., Lefrak S., Davis G., et al. (2004) Results of lung volume reduction surgery in patients meeting a national emphysema treatment trial high-risk criterion. J Thorac Cardiovasc Surg 127: 829–835. [DOI] [PubMed] [Google Scholar]
- Miller M., Crapo R., Hankinson J., Brusasco V., Burgos F., Casaburi R., et al. (2005a) General considerations for lung function testing. Eur Respir J 26: 153–161. [DOI] [PubMed] [Google Scholar]
- Miller M., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., et al. (2005b) Standardisation of spirometry. Eur Respir J 26: 319–338. [DOI] [PubMed] [Google Scholar]
- Mitsui K., Kurokawa Y., Kaiwa Y., Ando K., Kurosawa H., Hida W., et al. (2001) Thoracoscopic lung volume reduction surgery for pulmonary emphysema patients with severe hypercapnia. Jpn J Thorac Cardiovasc Surg 49: 481–488. [DOI] [PubMed] [Google Scholar]
- National Emphysema Treatment Trial Research Group. (2001) Patients at high risk of death after lung-volume-reduction surgery. N Eng J Med 345: 1075–1083. [DOI] [PubMed] [Google Scholar]
- O’Brien G., Furukawa S., Kuzma A., Cordova F., Criner G. (1999) Improvements in lung function, exercise, and quality of life in hypercapnic COPD patients after lung volume reduction surgery. Chest 115: 75–84. [DOI] [PubMed] [Google Scholar]
- Pellegrino R., Viegi G., Brusasco V., Crapo R., Burgos F., Casaburi R., et al. (2005) Interpretative strategies for lung function tests. Eur Respir J 26: 948–968. [DOI] [PubMed] [Google Scholar]
- Reilly J., Washko G., Pinto-Plata V., Velez E., Kenney L., Berger R., et al. (2007) Biological lung volume reduction: a new bronchoscopic therapy for advanced emphysema. Chest 131: 1108–1113. [DOI] [PubMed] [Google Scholar]
- Roussos C., Koutsoukou A. (2003) Respiratory Failure. Eur Respir J Suppl 47: 3s–14s. [DOI] [PubMed] [Google Scholar]
- Schönhofer B. (2015) Noninvasive ventilation in patients with persistent hypercapnia. Med Klin Intensivmed Notfmed 110: 182–187. [DOI] [PubMed] [Google Scholar]
- Sexton P., Garrett J., Rankin N., Anderson G. (2010) Endoscopic lung volume reduction effectively treats acute respiratory failure secondary to bullous emphysema. Respirology 15: 1141–1145. [DOI] [PubMed] [Google Scholar]
- Shade D., Cordova F., Lando Y., Travaline J., Furukawa S., Kuzma A., et al. (1999) Relationship between resting hypercapnia and physiologic parameters before and after lung volume reduction surgery in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159(5 Pt 1): 1405–1411. [DOI] [PubMed] [Google Scholar]
- Shah P., Kemp S. (2015) Springing forward to medium-term results for endobronchial coils for emphysema. Respirology 20: 176–178. [DOI] [PubMed] [Google Scholar]
- Shah P., Zoumot Z., Singh S., Bicknell S., Ross E., Quiring J., et al. (2013) Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 1: 233–240. [DOI] [PubMed] [Google Scholar]
- Slebos D., Klooster K., Ernst A., Herth F., Kerstjens H. (2012) Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest 142: 574–582. [DOI] [PubMed] [Google Scholar]
- Snell G., Holsworth L., Borrill Z., Thomson K., Kalff V., Smith J., et al. (2003) The potential for bronchoscopic lung volume reduction using bronchial prostheses: a pilot study. Chest 124: 1073–1080. [DOI] [PubMed] [Google Scholar]
- Snell G., Hopkins P., Westall G., Holsworth L., Carle A., Williams T. (2009) A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg 88: 1993–1998. [DOI] [PubMed] [Google Scholar]
- Tsujino K., Sasada S., Kodama M., Ishihara H., Kawase I. (2009) Severe bullous emphysema and hypercapnia successfully treated by bronchoscopic lung volume reduction. Respirology 14: 907–909. [DOI] [PubMed] [Google Scholar]
- Tsunezuka Y., Sato H., Tsubota M., Seki M. (2000) Significance of percutaneous cardiopulmonary bypass support for volume reduction surgery with severe hypercapnia. Artif Organs 24: 70–73. [DOI] [PubMed] [Google Scholar]
- Vestbo J., Hurd S., Agustí A., Jones P., Vogelmeier C., Anzueto A., et al. (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187: 347–365. [DOI] [PubMed] [Google Scholar]
- Votruba J., Collins J., Herth F. (2011) Successful treatment of ventilator dependent emphysema with Chartis treatment planning and endobronchial valves. Int J Surg Case Rep 2: 285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger J., Clausen J., Coates A., Pedersen O., Brusasco V., Burgos F., et al. (2005) Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522. [DOI] [PubMed] [Google Scholar]
- Wisser W., Klepetko W., Senbaklavaci O., Wanke T., Gruber E., Tschernko E., et al. (1998) Chronic hypercapnia should not exclude patients from lung volume reduction surgery. Eur J Cardiothoracic Surg 14: 107–112. [DOI] [PubMed] [Google Scholar]
- Yang H., Xiang P., Zhang E., Guo W., Shi Y., Zhang S., et al. (2015) Is hypercapnia associated with poor prognosis in chronic obstructive pulmonary disease? A long-term follow-up cohort study. BMJ Open 5: e008909. [DOI] [PMC free article] [PubMed] [Google Scholar]