Abstract
Background:
Acetazolamide has been investigated for treating sleep apnea in newcomers ascending to high altitude. This study aimed to assess the effect of acetazolamide on sleep apnea at high altitude, determine the optimal therapeutic dose, and compare its effectiveness in healthy trekkers and obstructive sleep apnea (OSA) patients.
Methods:
PubMed, Embase, Scopus, Cochrane Library, and Airiti Library databases were searched up to July 2015 for randomized controlled trials (RCTs) performed above 2500 m in lowlanders and that used acetazolamide as intervention in sleep studies. Studies including participants with medical conditions other than OSA were excluded.
Results:
Eight studies of 190 adults were included. In healthy participants, the pooled mean effect sizes of acetazolamide on Apnea–Hypopnea Index (AHI), percentage of periodic breathing time, and nocturnal oxygenation were 34.66 [95% confidence interval (CI) 25.01–44.30] with low heterogeneity (p = 0.7, I2 = 0%), 38.56% (95% CI 18.92–58.19%) with low heterogeneity (p = 0.24, I2 = 28%), and 4.75% (95% CI 1.35–8.15%) with high heterogeneity (p < 0.01, I2 = 87%), respectively. In OSA patients, the pooled mean effect sizes of acetazolamide on AHI and nocturnal oxygenation were 13.18 (95% CI 9.25–17.1) with low heterogeneity (p = 0.33, I2 = 0%) and 1.85% (95% CI 1.08–2.62%) with low heterogeneity (P = 0.56, I2 = 0%).
Conclusions:
Acetazolamide improves sleep apnea at high altitude by decreasing AHI and percentage of periodic breathing time and increasing nocturnal oxygenation. Acetazolamide is more beneficial in healthy participants than in OSA patients, and a 250 mg daily dose may be as effective as higher daily doses for healthy trekkers.
Keywords: acetazolamide, altitude, sleep apnea syndromes
Introduction
With the increasing popularity of high-altitude traveling, interventions targeting potential associated dysfunctions are of great importance. Sleep-disordered breathing manifesting as frequent arousals, nocturnal hypoxemia, and sleep apnea [Wickramasinghe and Anholm, 1999] is frequently reported in newcomers ascending to high altitudes above 2500 m [Shah et al. 2015]. This pattern of periodic hyperventilation with central apnea or hypopnea is closely related to hypobaric hypoxia and can weaken already exhausted climbers.
Of the two types of sleep apnea, obstructive sleep apnea (OSA) occurs as a result of repeated collapse of the pharyngeal airway due to inadequate motor tone. On the other hand, central sleep apnea (CSA) is characterized by a lack of drive to breathe, which is common in sojourners to high altitude [Eckert et al. 2007; Park et al. 2011]. The severity of sleep apnea can be measured with the Apnea–Hypopnea Index (AHI) that calculates the average episodes of apnea and hypopnea per hour of sleep and is classified as mild (5 to <15), moderate (15 to <30), or severe (⩾30) [Sahin et al. 2014]. A clinical review published in 2015 highlighted that OSA patients may be at higher risk for developing adverse events during their stay at high altitude, because the co-occurrence of central and obstructive apnea might result in sustained hypoxia [Bloch et al. 2015].
Acetazolamide, a carbonic anhydrase inhibitor, is now the standard drug for preventing acute mountain sickness with the dose of 250 mg daily [Low et al. 2012]. Acetazolamide causes metabolic acidosis by increasing bicarbonate secretion from the kidneys, thus increasing ventilation and arterial oxygenation and promoting acclimatization to high altitude [Leaf and Goldfarb, 2007; Shah et al. 2015]. Previous studies showed that acetazolamide was also beneficial for the treatment of sleep apnea at high altitude due to reduced periodic breathing and nocturnal wakefulness [Nicholson et al. 1988; Luks, 2008]. However, the efficacy and optimal dose of acetazolamide for improving sleep apnea at high altitude remain unclear due to the limited number of cases and methodological differences in existing studies [Burgess et al. 2014] and have not been systematically reviewed to date [Luks, 2008; Rolan, 2015], leaving clinicians uncertain on the use of acetazolamide for high-altitude travelers with sleeping disturbances, particularly the more vulnerable OSA patients.
The purpose of this systematic review and meta-analysis of acetazolamide was to assess its effect on sleep apnea improvement at high altitude, to determine its optimal dose, and to compare its efficacy in healthy trekkers and OSA patients.
Materials and methods
Following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [Moher et al. 2009], we performed a systematic review with a database search date up to 4 July 2015, using the PubMed, Embase, Scopus, and Cochrane Library databases. The search strategy included the terms Altitude [MeSH] AND acetazolamide [MeSH] AND (sleep apnea syndromes [MeSH] OR sleep apnea OR sleep-disordered breathing OR sleep-related breathing disorders OR periodic breathing during sleep OR nocturnal periodic breathing). We also hand searched related articles on the Airiti Library that consists of two sources, Chinese Electronic Periodical Services and Chinese Journal and Thesis Database (CJTD). The relevant studies were included if they met the following criteria: (1) randomized controlled trial (RCT); (2) lowlanders as trial subjects; (3) trial conducted at high altitudes above 2500 m; (4) acetazolamide as intervention; and (5) sleep study in the trial design. There were no language restrictions, whereas animal studies, reviews, trials with duplicate study populations, or those lacking direct comparisons between acetazolamide and control groups were excluded. Studies focusing on populations with underlying medical conditions other than OSA were also excluded. Two reviewers (HML and CC) independently reviewed titles, abstracts, and full articles for trial inclusion and reached a final consensus through discussion. If necessary, study authors were contacted to retrieve unpublished data. Completed PRISMA checklists are available online in the supplementary material.
Our primary outcome was the effect of acetazolamide on sleep apnea as assessed by either the AHI or percentage of periodic breathing time, and the secondary outcome was nocturnal oxygenation. The bias of included studies were determined by the Cochrane Collaboration’s tool for assessing risk of bias, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias [Higgins and Green 2011].
Analyses were conducted using Review Manager (RevMan) Version 5.3 (Copenhagen, Denmark) and were presented as forest plots in random effects models [The Nordic Cochrane Centre, 2014]. Summary measures were differences in means. Publication bias was assessed by detecting asymmetry in funnel plots if at least 10 studies were included, and sensitivity analysis was assessed for eligibility criteria based on characteristics of the intervention. The healthy trekkers and OSA patients were further divided into two subgroups to compare acetazolamide efficacy. As they measure distinct aspects of sleep apnea, AHI and percentage of periodic breathing time were analyzed separately. If the mean and variance were not reported in a trial, they were estimated from the median, range, and sample size [Hozo et al. 2005]. Heterogeneity was assessed using χ2 test and I2 statistic and was categorized as low (< 30%), moderate (30–60%), or high (> 60%) by I2 values [Higgins and Thompson, 2002].
Results
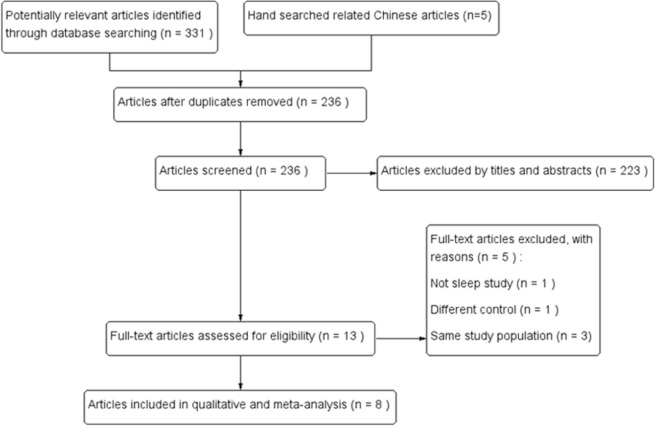
The literature search yielded 331 articles from the PubMed, Embase, Scopus, and Cochrane Library databases and five articles from the CJTD database. After removal of duplicates, the remaining 236 studies were reviewed against the exclusion criteria based on the titles and abstracts; a total of 223 articles were excluded. Of the remaining 13 full-text articles that were reviewed in detail, five did not meet the predetermined inclusion criteria and were excluded. Finally, eight trials including a total of 190 adults were included in the meta-analysis (Figure 1) [Sutton et al. 1979; Hackett et al. 1987; Fischer et al. 2004; Rodway et al. 2011; Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012; Burgess et al. 2014; Caravita et al. 2015].
Figure 1.
Selection flow diagram of the study.
Table 1 summarizes the characteristics of the included eight trials with publication dates ranging from 1979 to 2015. Six trials recruited healthy participants with an age range of 20–56 years [Sutton et al. 1979; Hackett et al. 1987; Fischer et al. 2004; Rodway et al. 2011; Burgess et al. 2014; Caravita et al. 2015]. Two trials included OSA patients with median age of 63 and 64 years, respectively [Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012]. Six trials included both male and female subjects [Sutton et al. 1979; Rodway et al. 2011; Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012; Burgess et al. 2014; Caravita et al. 2015], whereas the other two trials enrolled only male participants [Hackett et al. 1987; Fischer et al. 2004]. Trials were conducted worldwide: two were in North America [Sutton et al. 1979; Hackett et al. 1987], four were in Europe [Fischer et al. 2004; Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012; Caravita et al. 2015], and two were in Nepal [Rodway et al. 2011; Burgess et al. 2014]. The mean final altitude was 4687 m in trials with healthy trekkers and 2590 m in trials with OSA patients.
Table 1.
Characteristics of RCTs (n = 8) of acetazolamide effects on sleep apnea at high altitude.
| Study | Participants | Intervention | Outcomes |
Risk of bias assessment
a
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3b | 4 | 5 | 6 | ||||
|
Sutton
et al. [1979]
Study design: randomized controlled, data analyzer blinded, crossover |
9 healthy trekkers M/F 5/4 Age range 22–36 years |
ACZ 250 mg/8 h (oral), total 5 doses Final altitude: 5360 m in North America |
Oxygen saturation Breathing frequency |
U | U | A | A | A | U |
|
Hackett
et al. [1987]
Study design: randomized controlled, double-blind, crossover |
4 healthy trekkers Male Age range 26–35 years |
ACZ 250 mg/8 h (oral), total 3 doses Final altitude: 4400 m in North America |
Hypoxic ventilatory response Sleep study |
U | U | A | U | A | U |
|
Fischer
et al. [2004]
Study design: randomized controlled, double-blind |
20 healthy trekkers Male ACZ/control 10/10 BMI <25 |
ACZ 250 mg twice daily (oral), for 4 days Final altitude: 3454 m in Switzerland |
Polysomnography Acute mountain sickness Pulse rate, Arterial blood gases Oxyhemoglobin saturation |
U | U | A | A | A | U |
|
Rodway
et al. [2011]
Study design: randomized Controlled |
8 healthy trekkers M/F 12/3c ACZ/control 4/4 Age range 25–55 years |
ACZ 125 mg/day (oral), for 1 night Final altitude: 5300 m in Nepal |
Heart rate Respiratory rate Oxygen saturation Tidal volume, Minute volume AHI |
A | U | A | U | A | U |
|
Nussbaumer-Ochsner et al. [2012]
Study design: randomized controlled, double-blind, crossover |
45 OSA patients M/F 42/3 Median age 64 years |
ACZ 250 mg twice daily (oral), for 3 nights Final altitude: 2590 m in Switzerland |
Polysomnography Vigilance, Symptoms Oxygen saturation AHI |
U | A | A | A | A | A |
|
Latshang
et al. [2012]
Study design: randomized controlled, double-blind, crossover |
51 OSA patients M/F 48/3 Median age 63 years |
ACZ 250/500 mg (morning /evening, oral), for 3 nights Final altitude: 2590 m in Switzerland |
Oxygen saturation AHI Sleep structure Vigilance Symptoms Adverse effects Exercise performance |
U | A | A | A | A | A |
|
Burgess
et al. [2014],
2014 Study design: randomized controlled, single-blind, crossover |
12 healthy trekkers M/F 8/4 Mean age 30 years |
ACZ 10 mg/kg (intravenous), for 1 night Final altitude: 5050 m in Nepal |
Arterial blood gas Cerebral blood flow Ventilatory response Sleep study |
U | U | A | U | A | U |
|
Caravita
et al. [2015]
Study design: randomized controlled, double-blind |
41 healthy trekkers ACZ 20 (M/F 10/10) Control 21 (M/F
11/10) Mean age 36 years |
ACZ 250 mg/day (oral), for 2 days Final altitude: 4559 m in Italy |
Resting ventilation Chemoreflex Cardiorespiratory sleep study |
U | U | A | U | A | A |
M, male; F, female; ACZ, acetazolamide; AHI, Apnea–Hypopnea Index; BMI, body mass index; OSA, obstructive sleep apnea; U, unclear; A, adequate.
Risk of bias assessment determined by the Cochrane Collaboration’s tool for assessing risk of bias, including: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting.
Though six studies that included healthy participants did not provide adequate information to permit the assessment of performance bias or were not double-blinded, the outcomes were judged not likely to be influenced by lack of blinding because sleep apnea and nocturnal oxygenation were unlikely to have a placebo effect.
The total 15 participants in the study that were assigned to 4 different treatment groups, of which ACZ and control group were included in this meta-analysis.
All analyzed trials compared the efficacy of acetazolamide on the improvement of sleep apnea at high altitude, as measured by nocturnal oxygenation [Sutton et al. 1979; Hackett et al. 1987; Fischer et al. 2004; Rodway et al. 2011; Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012; Burgess et al. 2014; Caravita et al. 2015], AHI [Fischer et al. 2004; Rodway et al. 2011; Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012; Burgess et al. 2014; Caravita et al. 2015], or percentage of periodic breathing time [Sutton et al. 1979; Hackett et al. 1987]. The dose of acetazolamide as intervention varied between different trials. One trial used 125 mg per day orally [Rodway et al. 2011], whereas another trial tested 250 mg per day orally [Caravita et al. 2015]. Four studies included 250 mg/dose acetazolamide at different dosing schedules: two trials evaluated twice-daily oral administration [Fischer et al. 2004; Nussbaumer-Ochsner et al. 2012], whereas the other two evaluated oral dosing every 8 h [Sutton et al. 1979; Hackett et al. 1987]. One trial assessed 750 mg per day oral acetazolamide [Latshang et al. 2012], and only one trial used 10 mg/kg administered intravenously 30 min before further testing [Burgess et al. 2014].
The risk of bias assessment of the included RCTs is also presented in Table 1. Random sequence generation was assessed as an unclear risk of bias in seven of the eight trials [Sutton et al. 1979; Hackett et al. 1987; Fischer et al. 2004; Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012; Burgess et al. 2014; Caravita et al. 2015] one study stated that the subjects were assigned using a computer-based random procedure and thus were judged as having a low risk of bias [Rodway et al. 2011]. Six of the eight trials were assessed as an unclear risk of bias for allocation concealment [Sutton et al. 1979; Hackett et al. 1987; Fischer et al. 2004; Rodway et al. 2011; Burgess et al. 2014; Caravita et al. 2015], whereas two trials were assessed as adequate [Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012]. Because the majority of included trials provided insufficient information, the overall selection bias remained unclear.
Blinding of participants and personnel was judged as adequate in eight studies. Though six studies that included healthy participants did not provide adequate information to permit the assessment of performance bias or were not double-blinded [Sutton et al. 1979; Hackett et al. 1987; Fischer et al. 2004; Rodway et al. 2011; Burgess et al. 2014; Caravita et al. 2015], the outcomes were judged not likely to be influenced by lack of blinding because sleep apnea and nocturnal oxygenation were unlikely to have a placebo effect. Blinding of the outcome assessment yielded inconsistent results among trials. Four studies provided insufficient information to make a judgment [Hackett et al. 1987; Rodway et al. 2011; Burgess et al. 2014; Caravita et al. 2015], and the remaining four trials clearly stated the procedure of blinding of outcomes and had a lower risk of detection bias [Sutton et al. 1979; Fischer et al. 2004; Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012].
All included trials were judged as having a low risk of attrition bias. Five trials did not have missing outcome data [Sutton et al. 1979; Hackett et al. 1987; Rodway et al. 2011; Latshang et al. 2012; Burgess et al. 2014]. The other three trials discussed withdrawals; the missing outcome data was either balanced in numbers across intervention groups [Nussbaumer-Ochsner et al. 2012] or only consisted of a small portion of participants [Fischer et al. 2004; Caravita et al. 2015]. Three trials were judged as having a low risk of reporting bias as the study protocols were available and all predetermined study outcomes of this meta-analysis were reported [Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012; Caravita et al. 2015]. The other five trials provided insufficient information to judge on selective reporting [Sutton et al. 1979; Hackett et al. 1987; Fischer et al. 2004; Rodway et al. 2011; Burgess et al. 2014]. The ascent profiles, adaptation of altitude, route of administration and dose of acetazolamide were not consistent among studies.
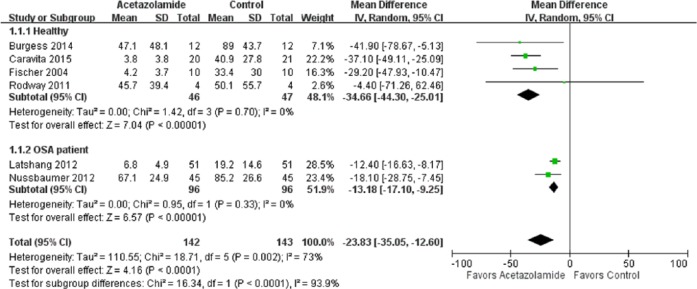
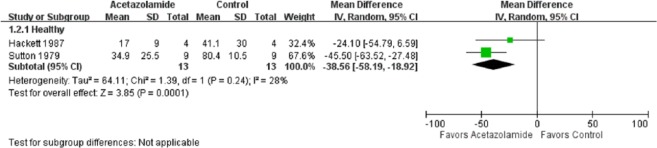
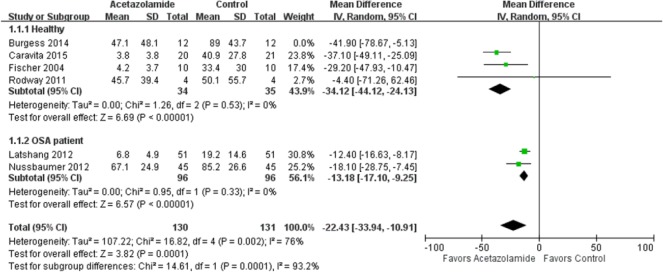
In the meta-analysis of the effects of acetazolamide on AHI, the point and pooled estimates of four trials [Fischer et al. 2004; Rodway et al. 2011; Burgess et al. 2014; Caravita et al. 2015] that included 93 healthy cases favored acetazolamide intervention, with low heterogeneity (p = 0.7, I2 = 0%) (Figure 2). Of these trials, only one was not significant at the 5% significance level [Rodway et al. 2011]. In addition, the point and pooled estimates of two trials [Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012] that included 192 OSA cases indicated a beneficial effect of acetazolamide on AHI, with low heterogeneity (p = 0.33, I2 = 0%), while the effect size seemed to be smaller than that of healthy participants. In other words, acetazolamide is more beneficial in healthy trekkers than in patients with OSA. The overall treatment effect of acetazolamide on AHI in healthy and OSA participants (n = 285) was a mean difference of 23.83 [95% confidence interval (CI) 12.60–35.05] with high heterogeneity (p = 0.002, I2 = 73%). The pooled estimates of two trials [Sutton et al. 1979; Hackett et al. 1987] with 26 healthy cases favored the acetazolamide intervention effect on the percentage of periodic breathing time, with low heterogeneity (p = 0.24, I2 = 28%) (Figure 3).
Figure 2.
Forest plot of the effects of acetazolamide on Apnea Hypopnea Index.
CI, confidence interval; df, degrees of freedom; IV, ; OSA, obstructive sleep apnea; SD, standard deviation.
Figure 3.
Forest plot of the effects of acetazolamide on periodic breathing time (%).
CI, confidence interval; df, degrees of freedom; IV, inverse variance; SD, standard deviation.
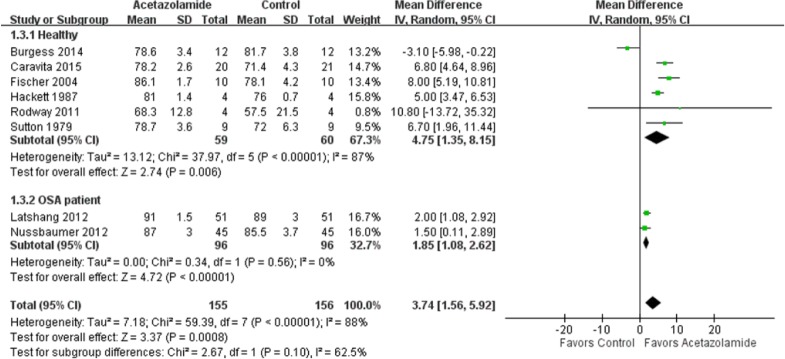
The combined effect of acetazolamide on nocturnal oxygenation in six trials [Sutton et al. 1979; Hackett et al. 1987; Fischer et al. 2004; Rodway et al. 2011; Burgess et al. 2014; Caravita et al. 2015] that included 119 healthy cases was a mean difference of 4.75% (95% CI 1.35–8.15%), with high heterogeneity (p < 0.001, I2 = 87%) (Figure 4). Of these trials, one did not reach the 5% significance level [Rodway et al. 2011], and one did not favor the intervention with acetazolamide [Burgess et al. 2014]. The pooled effect of acetazolamide on nocturnal oxygenation in two trials [Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012] with 192 OSA cases was a mean difference of 1.85% (95% CI 1.08–2.62%), with low heterogeneity (p = 0.56, I2 = 0%). The overall treatment effect of acetazolamide on nocturnal oxygenation in a total of 311 healthy and OSA cases was a mean difference of 3.74% (95% CI 1.56–5.92%), with high heterogeneity (p < 0.001, I2 = 88%).
Figure 4.
Forest plot of the effects of acetazolamide on nocturnal oxygenation.
CI, confidence interval; df, degrees of freedom; IV, ; OSA, obstructive sleep apnea; SD, standard deviation.
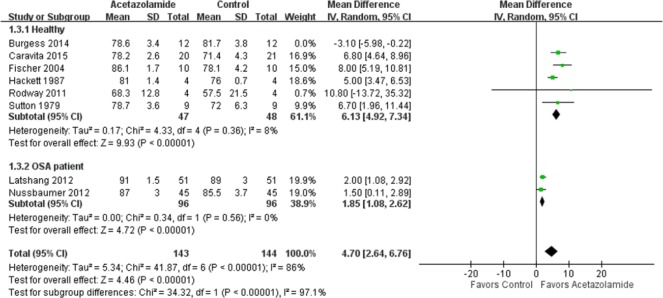
The intervention effect of random and fixed models yielded mild differences in both subtotal and overall effect of acetazolamide on AHI, the percentage of periodic breathing time, and nocturnal oxygenation. Sensitivity analysis of the effect of acetazolamide on AHI showed a similar outcome when only those studies with oral acetazolamide administration were included (Figure 5) [Fischer et al. 2004; Rodway et al. 2011; Latshang et al. 2012; Nussbaumer-Ochsner et al. 2012; Caravita et al. 2015]. Sensitivity analysis was not necessary for the percentage of periodic breathing time as both studies that included periodic breathing time administered acetazolamide orally [Sutton et al. 1979; Hackett et al. 1987]. However, in healthy participants, the effect of oral acetazolamide on nocturnal oxygenation was a mean difference of 6.13% (95% CI 4.92–7.34%) with low heterogeneity (p = 0.36, I2 = 8%) by sensitivity analysis (Figure 6), whereas the original mean difference was only 4.75% (95% CI 1.35–8.15%) with high heterogeneity (p < 0.001, I2 = 87%).
Figure 5.
Sensitivity analysis of the effects of acetazolamide on Apnea Hypopnea Index.
CI, confidence interval; df, degrees of freedom; IV, ; OSA, obstructive sleep apnea; SD, standard deviation.
Figure 6.
Sensitivity analysis of the effects of acetazolamide on nocturnal oxygenation.
CI, confidence interval; df, degrees of freedom; IV, ; OSA, obstructive sleep apnea; SD, standard deviation.
Discussion
The results of this systematic review and meta-analysis including 311 cases from eight RCTs indicate that acetazolamide is effective in improving sleep apnea at high altitudes by decreasing the AHI and percentage of periodic breathing time and increasing nocturnal oxygenation. In addition, acetazolamide is more beneficial in healthy participants than in OSA patients. Though risk of bias for all outcomes across studies did not result in a high risk of bias, many of them were judged as unclear risk of bias due to insufficient information provided. We are unable to determine the most effective dose of acetazolamide due to the variety of doses used among the included studies. However, in this review, a 250 mg daily dose may be as effective as, if not more, higher or more frequent daily doses for healthy trekkers [Caravita et al. 2015].
Our findings support two recently published literature reviews, which concluded that acetazolamide was beneficial for OSA patients during their altitude sojourn by improving oxygenation and reducing sleep apnea [Bloch et al. 2015; Rolan, 2015]. The results of this meta-analysis are in agreement with a literature review from 2008 showing that acetazolamide improved sleep in healthy individuals at high altitudes [Luks, 2008]. Our study is the first systematic review and meta-analysis evaluating the efficacy of acetazolamide in improving sleep apnea at high altitudes, which is a major difference from previous reviews. Furthermore, the participants in our study consist of not only healthy trekkers but also OSA patients, whereas previous studies mostly examined one or the other.
The findings of this study demonstrate that healthy participants are more susceptible to the beneficial effect of acetazolamide than OSA patients. One possible explanation for this outcome is that CSA occurs predominantly when ascending to high altitude and serves as a target of acetazolamide [Javaheri, 2006; Burgess et al. 2014]. OSA patients, however, continue to suffer from the coincident repeated pharyngeal airway collapse. In other words, the effect of acetazolamide on CSA is there, but not on OSA. Note that of the two studies that included OSA patients, one mentioned the application of a background continuous positive airway pressure device [Latshang et al. 2012], whereas the other discontinued it at altitude [Nussbaumer-Ochsner et al. 2012]. The combination of both acetazolamide and continuous positive airway pressure device is believed to be the best practice for OSA patients to improve sleep at altitude [Rolan, 2015]. One other possibility is the difference in the mean final altitude between the two participant groups: 4687 m in healthy participants and 2590 m in OSA patients. As oxygen levels are much lower at higher altitude, the severity of sleep apnea may increase and require longer times for acclimatization, thus enhancing the effect of acetazolamide [Pagel et al. 2011].
Oral acetazolamide can increase bicarbonate secretion from the kidneys and induce metabolic acidosis, while intravenous acetazolamide works predominantly on altering cerebral blood flow and does not cause significant metabolic acidosis [Burgess et al. 2014]. This may explain the high heterogeneity in the overall treatment effect of acetazolamide on nocturnal oxygenation but not on AHI, as sensitivity analysis showed a similar outcome when only those studies with oral acetazolamide administration were included.
The relatively smaller oral dose of 250 mg per day may be as effective as higher or more frequent daily doses in improving sleep apnea for healthy trekkers [Caravita et al. 2015], which is in line with the lowest effective acetazolamide dose for acute mountain sickness [Low et al. 2012], implying an additional protective role for acetazolamide during mountaineering. The side effects of acetazolamide are minor and include change in taste; however, as a diuretic, its use can exacerbate dehydration and is a concern [Kupper et al. 2008]. Therefore, trekkers should hydrate well, especially when taking acetazolamide.
AHI measures sleep apnea and is categorized as mild (5 to < 15), moderate (15 to < 30), or severe (> 30) [Sahin et al. 2014]. Our results show that AHI reduction of approximately 35 points (95% CI 25–44) in healthy participants and 13 (95% CI 9–17) in OSA patients, both with low heterogeneity (I2 = 0%), suggesting meaningful clinical significance. When taken orally, acetazolamide improves nocturnal oxygenation by approximately 6% (95% CI 5–7%) in healthy participants with low heterogeneity (I2 = 8%), which is much higher than 1.2% reported by a trekking company [Eigenberger et al. 2014].
Our study has several limitations. First, not all included trials reported outcomes as mean values with standard deviation. In order to improve the inclusiveness of all relevant trials for this meta-analysis, we estimated the mean and variance from the median, range, and sample size from a distribution-free formula [Hozo et al. 2005]. Second, two studies used the percentage of periodic breathing time instead of AHI to measure sleep apnea; thus, the data could not be pooled [Sutton et al. 1979; Hackett et al. 1987]. Though another two studies did not perform standard polysomnography [Rodway et al. 2011; Caravita et al. 2015], their devices for detecting AHI had been supported by previous research. Third, gender distribution was balanced in only two of the eight trials [Sutton et al. 1979; Caravita et al. 2015], and two studies included only males [Hackett et al. 1987; Fischer et al. 2004]. One of the included trials concluded that males suffered from more severe periodic breathing at high altitude, which was alleviated by acetazolamide in both sexes [Caravita et al. 2015]. Finally, the total length of journey and duration of acetazolamide intervention varied across trials, and this leads to differences in altitude adaptation, making the pooled results of studies performed in the first few nights at altitude with studies performed after five [Burgess et al. 2014] or ten [Sutton et al. 1979] days at altitude not physiologically sound. Thus, further studies including participants with similar characteristics and comparable parameters are needed, and the confirmation of dose-dependent efficacy of acetazolamide on sleep apnea at high altitude requires larger clinical trials.
Conclusion
This systematic review and meta-analysis has provided evidence that acetazolamide is effective in improving sleep apnea at high altitude by decreasing AHI and percentage of periodic breathing time and increasing nocturnal oxygenation. During overnight travel at altitude, it is more beneficial in healthy participants than in OSA patients, and a 250 mg daily dose may be as effective as higher or more frequent daily doses for healthy trekkers.
Supplementary Material
Footnotes
Author contributions: Liu HM contributed to study concept and design, data analysis and interpretation, and writing of the report. Chiang IJ, Liou CM and Chen C contributed substantially to study concept, data analysis and interpretation. Kuo KN contributed to data interpretation, revision of the manuscript and provided important intellectual enrichment.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Hsin-Ming Liu, Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University, Taipei, Taiwan.
I-Jen Chiang, Graduate Institute of Data Science, Taipei Medical University, Taipei, Taiwan.
Ken N. Kuo, Cochrane Taiwan, Taipei Medical University and Department of Orthopedic Surgery, National Taiwan University Hospital and Children Hospital, Taipei, Taiwan
Cher-Ming Liou, Department of Anesthesiology, Chung Shan Medical University Hospital and Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
Chiehfeng Chen, Department of Public Health, School of Medicine, College of Medicine, Cochrane Taiwan, and Division of Plastic Surgery, Department of Surgery, Wan Fang Hospital, Taipei Medical University, No.250 Wuxing Street, Taipei, 110, Taiwan.
References
- Bloch K., Latshang T., Ulrich S. (2015) Patients with obstructive sleep apnea at altitude. High Alt Med Biol 16: 110–116. [DOI] [PubMed] [Google Scholar]
- Burgess K., Lucas S., Shepherd K., Dawson A., Swart M., Thomas K., et al. (2014) Influence of cerebral blood flow on central sleep apnea at high altitude. Sleep 37: 1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravita S., Faini A., Lombardi C., Valentini M., Gregorini F., Rossi J., et al. (2015) Sex and acetazolamide effects on chemoreflex and periodic breathing during sleep at altitude. Chest, 147: 120–131. [DOI] [PubMed] [Google Scholar]
- Eckert D., Jordan A., Merchia P., Malhotra A. (2007) Central sleep apnea: pathophysiology and treatment. Chest 131: 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenberger P., Faino A., Maltzahn J., Lisk C., Frank E., Frank A., et al. (2014) A retrospective study of acute mountain sickness on Mt. Kilimanjaro using trekking company data. Aviat Space Environ Med 85: 1125–1129. [DOI] [PubMed] [Google Scholar]
- Fischer R., Lang S., Leitl M., Thiere M., Steiner U., Huber R. (2004) Theophylline and acetazolamide reduce sleep-disordered breathing at high altitude. Eur Respir J 23: 47–52. [DOI] [PubMed] [Google Scholar]
- Hackett P., Roach R., Harrison G. (1987) Respiratory stimulants and sleep periodic breathing at high altitude: almitrine versus acetazolamide. Am Rev Respir Dis 135: 896–906. [DOI] [PubMed] [Google Scholar]
- Higgins J., Green S. (eds) (2011) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration. [Google Scholar]
- Higgins J., Thompson S. (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- Hozo S., Djulbegovic B., Hozo I. (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S. (2006) Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med 173: 234–237. [DOI] [PubMed] [Google Scholar]
- Kupper T., Schoffl V., Netzer N. (2008) Cheyne stokes breathing at high altitude: a helpful response or a troublemaker? Sleep Breath 12: 123–127. [DOI] [PubMed] [Google Scholar]
- Latshang T., Nussbaumer-Ochsner Y., Henn R., Ulrich S., Lo Cascio C., Ledergerber B., et al. (2012) Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA 308: 2390–2398. [DOI] [PubMed] [Google Scholar]
- Leaf D., Goldfarb D. (2007) Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol (1985) 102: 1313–1322. [DOI] [PubMed] [Google Scholar]
- Low E., Avery A., Gupta V., Schedlbauer A., Grocott M. (2012) Identifying the lowest effective dose of acetazolamide for the prophylaxis of acute mountain sickness: systematic review and meta-analysis. BMJ 345: e6779. doi: 10.1136/bmj.e6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks A. (2008) Which medications are safe and effective for improving sleep at high altitude? High Alt Med Biol 9: 195–198. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A., Smith P., Stone B., Bradwell A., Coote J. (1988) Altitude insomnia: studies during an expedition to the Himalayas. Sleep 11: 354–361. [DOI] [PubMed] [Google Scholar]
- Nussbaumer-Ochsner Y., Latshang T., Ulrich S., Kohler M., Thurnheer R., Bloch K. (2012) Patients with obstructive sleep apnea syndrome benefit from acetazolamide during an altitude sojourn: a randomized, placebo-controlled, double-blind trial. Chest 141: 131–138. [DOI] [PubMed] [Google Scholar]
- Pagel J., Kwiatkowski C., Parnes B. (2011) The effects of altitude associated central apnea on the diagnosis and treatment of obstructive sleep apnea: comparative data from three different altitude locations in the mountain west. J Clin Sleep Med 7: 610–615A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Ramar K., Olson E. (2011) Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc 86: 549–554; quiz 554–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodway G., Edsell M., Wong B., Windsor J. Caudwell Xtreme Everest Research, G. (2011) Improving sleep at altitude: a comparison of therapies. Wilderness Environ Med 22: 316–320. [DOI] [PubMed] [Google Scholar]
- Rolan T. (2015) Neurology and altitude illness. Neurol Clin Pract 5: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M., Bilgen C., Tasbakan M., Midilli R., Basoglu O. (2014) A clinical prediction formula for apnea-hypopnea index. Int J Otolaryngol 2014: 438376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N., Hussain S., Cooke M., O’Hara J., Mellor A. (2015) Wilderness medicine at high altitude: recent developments in the field. Open Access J Sports Med 6: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton J., Houston C., Mansell A., Mcfadden M., Hackett P., Rigg J., et al. (1979) Effect of acetazolamide on hypoxemia during sleep at high altitude. N Engl J Med 301: 1329–1331. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre (2014) Review Manager (RevMan) [Computer program]. Version 5.3. ed. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration Copenhagen. [Google Scholar]
- Wickramasinghe H., Anholm J. (1999) Sleep and breathing at high altitude. Sleep Breath 3: 89–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.