Abstract
Background
Quercetin is a natural bioactive flavonoid that is present in a wide variety of vegetables and fruits and exhibits a promising anti-metastasis property in various human cancer cells. However, the effect of quercetin on human HCCLM3 cells is unclear.
Material/Methods
In the current study, a wound-healing assay was performed using quercetin-treated HCCLM3 cells to further explore whether quercetin affects the motility of human HCCLM3 cells. Transwell assay was used to explore the potential effect of quercetin in HCCLM3 cells on cell migration and cell invasion. Western blotting analysis was used to explore the expression of p-Akt1, MMP-2, and MMP-9 in quercetin-treated HCCLM3 cells.
Results
The wound-healing time was delayed in quercetin-treated HCCLM3 cells, and the ability to migrate and invade was inhibited in quercetin-treated human HCCLM3 cells. Moreover, the protein levels of p-Akt1, MMP-2, and MMP-9 were down-regulated in quercetin-treated HCCLM3 cells, as detected by Western blotting.
Conclusions
Our data show that quercetin attenuated cell migration and invasion by suppressing the protein levels of p-Akt1, MMP-2, and MMP-9 in HCCLM3 cells.
MeSH Keywords: Carcinoma, Hepatocellular; Cell Migration Assays; Neoplasm Invasiveness; Quercetin
Background
Hepatocellular carcinoma (HCC) is the fifth most common type of cancer in the world and the second leading cause of cancer-related death [1]. Due to liver dysfunction, the lack of effective treatment options, and high rates of metastasis and recurrence, the survival rate of HCC patients remains low [2]. Therefore, potential molecular mechanisms involved in the progress of metastasis in HCC need to be identified.
MMP-2/MMP-9 are members of the matrix metalloproteinase (MMP) family, and both proteins are implicated in tumor migration, invasion, and metastasis in various cancers, like cervical cancer [3], prostate cancer [4], and gastric cancer [5,6]. Moreover, metastasis is inhibited when MMP-2/MMP-9 are down-regulated in the SK HEP 1 and MHcc97H HCC cell lines [7]. Conversely, induction of MMP-2/MMP-9 facilitates metastasis of HCC cells [8]. The PI3K/Akt signaling transduction pathway was reported to participate in the tumor metastasis of HCC [9–11]. In addition, the PI3K/Akt signaling transduction pathway also plays a critical role in MMP2/MMP-9-mediated tumor cell migration and invasion in various cancers [12–15].
Quercetin is a natural flavonoid from the flavonol subclass that is present in a wide variety of vegetables (e.g., onions), fruits (e.g., apples), and red wine [16]. It functions as a pleiotropic molecule with anti-proliferation, anti-oxidant, anti-inflammatory, and anti-cancer properties [17]. In addition, quercetin was recently shown to have inhibitory effects on cell migration and cell invasion in breast cancer cells [18,19], oral cancer cells [20], glioblastoma cells [21], prostate cancer cells [22], and melanoma cells [23]. Recent evidence demonstrated that quercetin can inhibit cancer cell migration and invasion by reducing the protein level of MMP-2/MMP-9 [20]. Moreover, quercetin also has been reported to regulate the ability to migrate and invade in various cancers by inhibiting Akt phosphorylation through the PI3K/Akt signaling transduction pathway [24,25].
Despite the evidence showing the anti-metastatic potential of quercetin in various tumors, the effect of quercetin on human HCCLM3 cells remains unclear. Therefore, the current study investigated the effects of quercetin on the migration and invasion of human HCCLM3 cells and explored its potential underlying mechanisms.
Material and Methods
Reagents and antibodies
Quercetin was procured from Sigma (St. Louis, MO). Akt1 antibodies (1: 1000 dilution), p-Akt1 (1: 1000 dilution), MMP-2 (1: 500 dilution), and MMP-9 (1: 1000 dilution) were all obtained from Cell Signaling Technology, Beverly, MA. We obtained β-actin (1: 1000 dilution) from Santa Cruz (CA).
Cell culture and treatment
The HCCLM3 cells (human hepatocarcinoma cell line) were received from the China Center for Type Culture Collection (CCTCC, Wuhan, China). The HCCLM3 cells were cultured in RPMI-1640 medium (Invitrogen) with 10% fetal bovine serum (FBS). Cells were incubated at 37°C in an incubator with 5% CO2. Cells were passaged when they reached 70–80% confluence.
Wound-healing assay
HCCLM3 cells were cultured in 6-well plates at 5×105, and the cells were scratched using a 200-μL pipette tip when they grew to 90% confluence. Then, the cells were washed twice using PBS, and the RPMI-1640 medium was completely changed.
Then, we added the different concentrations of quercetin into the wounded cells and incubated them for 36 h. Photos of the wound were taken at 0 and 36 h, and 7 randomly fields were used to assess cell migration ability by using microscopy.
Transwell migration assay
The anti-migration effect of quercetin in HCCLM3 cells was detected by using Transwell assays. In the upper chamber, 4×104 cells were cultured in 600 μL of RPMI-1640 medium with different concentrations of quercetin. In the lower chamber, 800 μL of RPMI-1640 medium with 10% FBS was added. After incubation for 36 h at 37°C, the upper chamber was cleaned by using a cotton swab to clean out the cells that were not migrated. The migrated cells were routinely processed by fixing in 4% formalin for 10 min, then stained in 0.1% crystal violet for 15 min, and 5 randomly selected fields were captured using microscopy at 100× magnification.
Matrigel invasion assay
The upper chamber was coated with an 80-μL mixture of Matrigel/RPMI1640 medium (1: 5), and 4×104 cells were cultured in 600 μL of RPMI-1640 medium with different concentrations of quercetin. In the lower chamber, 800 μL of RPMI-1640 medium with 10% FBS was added. After incubation for 36 h at 37°C, the upper chamber was cleaned by using a cotton swab to clean out the cells that were not migrated. The migrated cells were routinely processed by fixing in 4% formalin for 10 min, then staining in 0.1% crystal violet for 15 min, and 5 randomly selected fields were captured by using microscopy at 100× magnification.
Western blotting
The HCCLM3 cells were treated with different concentrations of quercetin (0 μmol and 40 μmol). Then, the cell lysate was collected and the protein lysates were quantified. The quantified protein lysates were electrophoresed in 10% SDS-PAGE (SDS-polyacrylamide gel) and then was transferred to the PVDF (polyvinylidene difluoride) membranes. After transferring, the PVDF membranes were incubated with the primary antibodies at 4°C overnight. Then, after washing twice with TBST buffer, the PVDF membranes were incubated with secondary antibody for 1 h. Finally, the proteins were visualization with enhanced chemiluminescence method by using the Western blot image-forming system (Tanon 5200, China). All of the experiments were repeated 3 times.
Statistical analysis
Statistical differences from the respective controls for each experimental test condition were compared by ANOVA (one-way analysis of variance) or the t test. The p<0.01 or p<0.05 represented a significant difference. Values are expressed as the mean ±SD.
Results
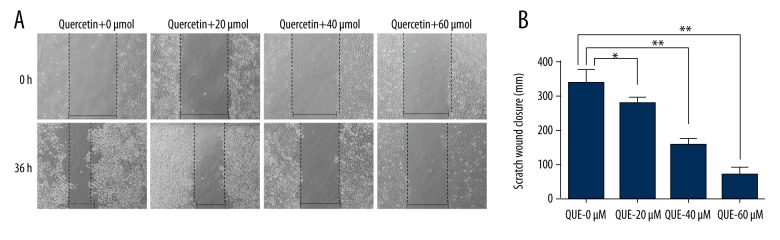
Quercetin delayed the wound-healing time in human HCCLM3 cells
A wound-healing assay was performed using quercetin-treated HCCLM3 cells to further explore whether quercetin affects the motility of human HCCLM3 cells. As shown in Figure 1, quercetin-treated HCCLM3 cells (20 μmol, 40 μmol, and 60 μmol) migrated more slowly than cells in the control group (0 μmol) at 36 h. Thus, quercetin delayed the wound-healing time in human HCCLM3 cells and inhibited HCCLM3 cell motility in a dose-dependent manner.
Figure 1.
Quercetin delayed the wound-healing time in human HCCLM3 cells. (A) The inhibitory effects of quercetin on HCCLM3 cells were determined using a wound closure assay. The width of the scratch was measured in the control and quercetin groups, and the quantitative data are shown (B). The values are presented as means ±S.D. All experiments were performed in triplicate (* p<0.05, ** p<0.01).
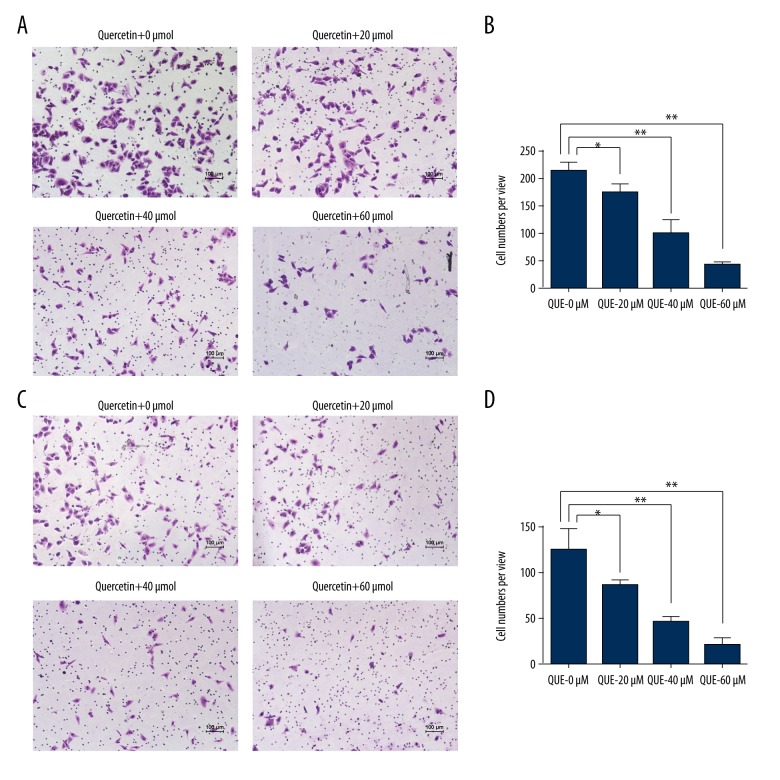
Quercetin inhibited the cell migration and invasion in human HCCLM3
Furthermore, a Transwell assay was used to explore the effect of quercetin on the migration and invasion of HCCLM3 cells. As shown in Figure 2A and 2B, the number of migrated cells decreased from 219.73±14.01 (0 μmol) to 173.32±14.84 (20 μmol), 100.31±25.03 (40 μmol), and 44.33±5.03 (60 μmol) after 36 h of quercetin treatment. Thus, quercetin inhibited the migration of human HCCLM3 cells in vitro. Moreover, the quercetin treatment also suppressed the ability of HCCLM3 cells to invade through a Matrigel basement membrane. As shown in Figure 2C and 2D, the number of invaded cells decreased from 123.75±24.11 (0 μmol) to 85.33±5.86 (20 μmol), 45.01±6.03 (40 μmol), and 20.65±8.51 (60 μmol) after 36 h of quercetin treatment. Thus, quercetin suppressed the invasion of human HCCLM3 cells in vitro. Based on these results, quercetin attenuated the migration and invasion of HCCLM3 cells in vitro.
Figure 2.
Quercetin inhibited the migration and invasion of human HCCLM3 cells. (A) The inhibitory effects of quercetin on the migration of HCCLM3 cells were determined using a Transwell assay, and the quantitative data are shown (B). (C) The inhibitory effects of quercetin on the invasion of HCCLM3 cells were determined using a Transwell assay, and the quantitative data are shown (D). The values are presented as means ±S.D. All experiments were performed in triplicate (* p<0.05, ** p<0.01).
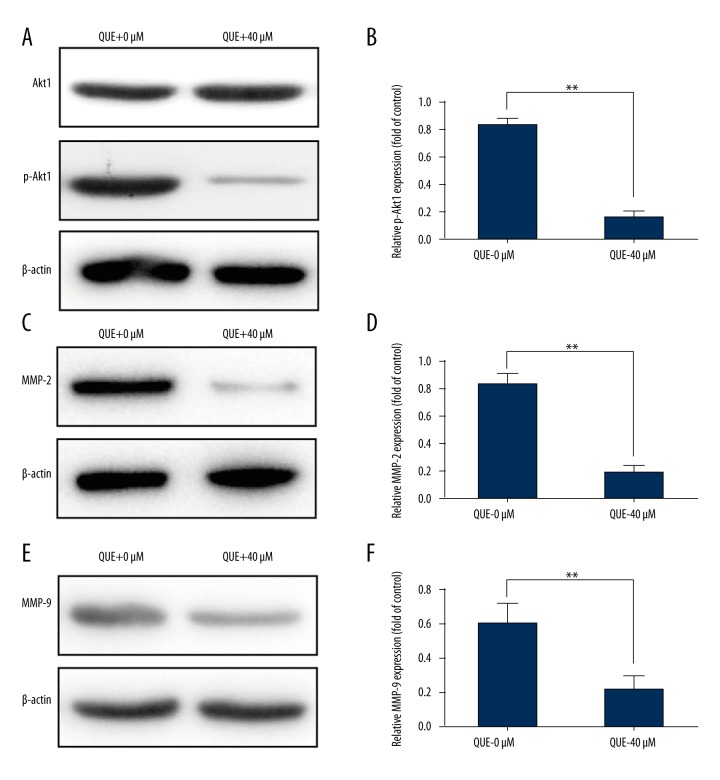
Quercetin down-regulated the protein levels of p-Akt1, MMP-2, and MMP-9 in human HCCLM3
According to previous reports, the PI3K/Akt1 signaling pathway is positively correlated with tumor metastasis [26,27], and quercetin was also reported to inhibit p-Akt1 expression in various cancers [12,28]. Accordingly, Western blotting was used to explore whether the PI3K/Akt1 signaling transduction pathway was necessary for the anti-migration and anti-invasion effects of quercetin on HCCLM3 cells. As shown in Figure 3A and 3B, the administration of 40 μmol quercetin significantly reduced p-Akt1 levels compared with the 0 μmol group (p<0.05).
Figure 3.
Quercetin markedly decreased p-Akt1, MMP-2, and MMP-9 expression in human HCCLM3 cells. (A) Akt1 and p-Akt1 levels were detected by Western blotting, and the quantitative data are shown (B). (C) MMP-2 expression was detected by Western blotting, and the quantitative data are shown (D). (E) MMP-9 expression was detected by Western blotting, and the quantitative data are shown (F). The values are presented as means ±S.D. All experiments were performed in triplicate (* p<0.05, ** p<0.01).
MMP-2/MMP-9 are regulated by the PI3K/Akt1 signaling transduction pathway [29,30], and both MMPs have been implicated in the invasion of hepatocellular carcinoma [31,32]. Therefore, the protein level of MMP-2/MMP-9 was examined in control cells and quercetin-treated cells. After 36-h quercetin treatment, the quercetin-treated cells (40 μmol) expressed a much lower level of the MMP-2/MMP-9 proteins than did control cells (0 μmol). Based on these results, quercetin inhibited the protein levels of p-Akt1, MMP-2, and MMP-9 in the HCCLM3 cell line.
Discussion
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related death. Although the therapeutic approaches have improved, the 5-year survival rate of HCC patients after surgical resection remains low due to the high rates of metastasis and recurrence [2]. Quercetin is a natural bioactive flavonoid, widely present in vegetables and fruits [16], and has exhibited promising anti-metastasis properties in various human cancer cells, both in vitro or/and in vivo, including breast cancer [18,19], oral cancer cells [20], glioblastoma [21], prostate cancer [22] and melanoma [23]. Evidences from human head and neck squamous cell carcinoma shows that quercetin dose-dependently delayed wound-healing time in both HSC-3 and FaDu cells [33]. However, the effect of quercetin on cell migration and invasion has been less clearly defined. In the present study, quercetin delayed the wound-healing time in HCCLM3 cells (Figure 1, p<0.05), indicating the inhibitory effects of quercetin on HCCLM3 cell motility. Moreover, the results from the Transwell assay also suggest that quercetin inhibits cell migration and invasion in HCCLM3 cells (Figure 2, p<0.05).
A previous study indicated that quercetin had remarkable effects in suppressing cell migration in renal cell carcinoma cells by altering AKT-mTOR-ERK1/2 signaling pathways [28]. However, whether the expression of p-Akt1 was altering by treating with quercetin in HCCLM3 cell has been unclear. Accordingly, we assessed the expression of p-Akt1 by Western blotting in HCCLM3 cell after treating with quercetin for 36 h. Similar to the effect of quercetin in renal cell carcinoma cells, the expression of p-Akt1 was significantly decreased in HCCLM3 cell (Figure 3A, p<0.05), suggesting that quercetin inhibits p-Akt1 expression in HCCLM3 cells.
Moreover, MMP-2 and MMP-9 have been found to be involved in tumor migration, invasion, and metastasis in various cancers, and a previous study indicated that these inhibitory effects of quercetin on cell migration are due to suppressing the expression of MMP2 or/and MMP9 through altering the PI3K/Akt signaling pathway. Previous studies indicated that quercetin significantly inhibited cell migration and invasion in breast cancer cells [19] and glioma cells [24] by down-regulating the expression of p-Akt and MMP9. In the present study, as shown in Figure 3B and 3C, the expression of MMP2 and MMP9 was significantly decreased in quercetin-treated HCCLM3 cells (p<0.05). These results suggest that these inhibitory effects of quercetin on cell migration and invasion in HCCLM3 cell are due to its inhibitory effects on MMP2 and MMP9 through altering the PI3K/Akt signaling pathway.
In summary, our study provides a novel mechanistic insight that quercetin inhibits cell migration and invasion in HCCLM3 cell in vitro, and that these anti-migration and anti-invasion effects of quercetin are due to its capacity to down-regulate the expression of MMP-2 and MMP-9 by altering the PI3K/Akt signaling pathway in HCCLM3 cells. Therefore, these data support the possibility of quercetin has value as an efficient anti-cancer cell migration and invasion agent to improve hepatocellular carcinoma treatment.
Conclusions
Our study provides evidence that quercetin inhibits cell migration and invasion in HCCLM3 cells in vitro by down-regulating the expression of MMP-2 and MMP-9 through the PI3K/Akt signaling pathway. Therefore, quercetin may be a novel agent for treatment of hepatocellular carcinoma.
Footnotes
Source of support: This work was supported by a grant to Prof. Yanmei Zhu from the Science and Technology Plan Project of Qinghai Province (No.2014-ZJ-755 and 2016-ZJ-790), and the Project Supported By Young Grant Of Qinghai University (No. 2014-QYY-3)
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. Cancer J Clin. 2012;62:394–99. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 3.Song C, Zhu S, Wu C, Kang J. Histone deacetylase (HDAC) 10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J Biol Chem. 2013;288:28021–33. doi: 10.1074/jbc.M113.498758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiorentini C, Bodei S, Bedussi F, et al. GPNMB/OA protein increases the invasiveness of human metastatic prostate cancer cell lines DU145 and PC3 through MMP-2 and MMP-9 activity. Exp Cell Res. 2014;323:100–11. doi: 10.1016/j.yexcr.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Yang GL, Tao HR, Wang HW, et al. Ara-C increases gastric cancer cell invasion by upregulating CD-147-MMP-2/MMP9 via the ERK signaling pathway. Oncol Rep. 2015;33:2045–51. doi: 10.3892/or.2015.3748. [DOI] [PubMed] [Google Scholar]
- 6.Choi BD, Jeong SJ, Wang G, et al. Secretory leukocyte protease inhibitor is associated with MMP-2 and MMP-9 to promote migration and invasion in SNU638 gastric cancer cells. Int J Mol Med. 2011;28:527–34. doi: 10.3892/ijmm.2011.726. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Wen X, Liu B, et al. Diosmetin inhibits the metastasis of hepatocellular carcinoma cells by downregulating the expression levels of MMP-2 and MMP-9. Mol Med Rep. 2016;13:2401–8. doi: 10.3892/mmr.2016.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Yang Z, Song W, et al. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int J Oncol. 2013;43:793–802. doi: 10.3892/ijo.2013.1992. [DOI] [PubMed] [Google Scholar]
- 9.Liu CJ, Yang JH, Huang FZ, et al. Glutathione-s-transferase A 4 (GSTA4) suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting AKT pathway. Am J Translat Res. 2017;9:301–15. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Qu L, Deng B, et al. STYK1 promotes epithelial-mesenchymal transition and tumor metastasis in human hepatocellular carcinoma through MEK/ERK and PI3K/AKT signaling. Sci Rep. 2016;6:33205. doi: 10.1038/srep33205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei X, Xu JF, Chang RM, Fang F, et al. JARID2 promotes invasion and metastasis of hepatocellular carcinoma by facilitating epithelial-mesenchymal transition through PTEN/AKT signaling. Oncotarget. 2016;7:40266–84. doi: 10.18632/oncotarget.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi H, Wu Y, Wang Y, et al. Liquiritigenin potentiates the inhibitory effects of cisplatin on invasion and metastasis via downregulation MMP-2/9 and PI3 K/AKT signaling pathway in B16F10 melanoma cells and mice model. Nutr Cancer. 2015;67:761–70. doi: 10.1080/01635581.2015.1037962. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Yue Y, Qin J, et al. Plumbagin suppresses the migration and invasion of glioma cells via downregulation of MMP-2/9 expression and inaction of PI3K/Akt signaling pathway in vitro. J Pharmacol Sci. 2017;134:59–67. doi: 10.1016/j.jphs.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Su Y, Wan D, Song W. Dryofragin inhibits the migration and invasion of human osteosarcoma U2OS cells by suppressing MMP-2/9 and elevating TIMP-1/2 through PI3K/AKT and p38 MAPK signaling pathways. Anticancer Drugs. 2016;27:660–68. doi: 10.1097/CAD.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Zheng J, Zhang Y, et al. Fucoxanthin activates apoptosis via inhibition of PI3K/Akt/mTOR pathway and suppresses invasion and migration by restriction of p38-MMP-2/9 pathway in human glioblastoma cells. Neurochem Res. 2016;41:2728–51. doi: 10.1007/s11064-016-1989-7. [DOI] [PubMed] [Google Scholar]
- 16.Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, et al. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-kappaB inhibition. Eur J Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Ross JA, Kasum CM. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 18.Balakrishnan S, Bhat FA, Raja Singh P, et al. Gold nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016;49:678–97. doi: 10.1111/cpr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Zhang J, Wang Y, et al. Synergistic inhibition of migration and invasion of breast cancer cells by dual docetaxel/quercetin-loaded nanoparticles via Akt/MMP-9 pathway. Int J Pharm. 2017;523:300–9. doi: 10.1016/j.ijpharm.2017.03.040. [DOI] [PubMed] [Google Scholar]
- 20.Lai WW, Hsu SC, Chueh FS, et al. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-kappaB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013;33:1941–50. [PubMed] [Google Scholar]
- 21.Michaud-Levesque J, Bousquet-Gagnon N, Beliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012;318:925–35. doi: 10.1016/j.yexcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Baruah MM, Khandwekar AP, Sharma N. Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumour Biol. 2016;37:14025–34. doi: 10.1007/s13277-016-5277-6. [DOI] [PubMed] [Google Scholar]
- 23.Cao HH, Cheng CY, Su T, et al. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer. 2015;14:103. doi: 10.1186/s12943-015-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan HC, Jiang Q, Yu Y, et al. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochem Int. 2015;80:60–71. doi: 10.1016/j.neuint.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Bhat FA, Sharmila G, Balakrishnan S, et al. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014;25:1132–39. doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Liu Z, Zhang Y, et al. miR-24 represses metastasis of human osteosarcoma cells by targeting Ack1 via AKT/MMPs pathway. Biochem Biophys Res Commun. 2017;486:211–17. doi: 10.1016/j.bbrc.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 27.Tan X, Chen S, Wu J, et al. PI3K/AKT-mediated upregulation of WDR5 promotes colorectal cancer metastasis by directly targeting ZNF407. Cell Death Dis. 2017;8:e2686. doi: 10.1038/cddis.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng FD, Li Y, Tian X, et al. Synergistic effects of snail and quercetin on renal cell carcinoma Caki-2 by altering AKT/mTOR/ERK1/2 signaling pathways. Int J Clin Exp Pathol. 2015;8:6157–68. [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, Guan Z, Chen J, et al. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int J Oncol. 2015;47:690–700. doi: 10.3892/ijo.2015.3041. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Xiao W, Sun J, et al. MiRNA-181c inhibits EGFR-signaling-dependent MMP9 activation via suppressing Akt phosphorylation in glioblastoma. Tumour Biol. 2014;35:8653–58. doi: 10.1007/s13277-014-2131-6. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Yu W, Huang T, et al. RUNX2 promotes hepatocellular carcinoma cell migration and invasion by upregulating MMP9 expression. Oncol Rep. 2016;36:2777–84. doi: 10.3892/or.2016.5101. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Yang P, Yang J, et al. GIT1 is a novel prognostic biomarker and facilitates tumor progression via activating ERK/MMP9 signaling in hepatocellular carcinoma. Onco Targets Ther. 2015;8:3731–42. doi: 10.2147/OTT.S96715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan CY, Lien CH, Lee MF, Huang CY. Quercetin suppresses cellular migration and invasion in human head and neck squamous cell carcinoma (HNSCC) BioMedicine. 2016;6:15. doi: 10.7603/s40681-016-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]