Abstract
The dynamic behavior of phytochrome A (phyA) in seedlings of the model plant Arabidopsis was examined by in vivo spectroscopy and by western and northern blotting. Rapid accumulation of phyA was observed, reaching a steady state after 3 d. Both red and far-red light initiated a rapid destruction of the far-red-light-absorbing form of phytochrome (Pfr); the apparent half-life was only 4-fold longer in far-red than in red light. Furthermore, the Pfr-induced destruction of the red-light-absorbing form of phytochrome (Pr) of phyA occurred in darkness with a rate identical to that of Pfr destruction. A 2-fold decrease in mRNA abundance was observed after irradiation, irrespective of the applied light quality. However, reaccumulation occurred rapidly after far-red but slowly after red irradiation, indicating different modes of regulation of phytochrome expression after light-dark transitions depending on the light quality of the preceding irradiation. The wavelength dependency of the destruction rates was distinct from that of mustard, a close relative of Arabidopsis, and was explained on the basis of Pfr-induced Pr destruction and a simple kinetic two-step model. No dark reversion was detectable in the destruction kinetics after a red pulse. From these data we conclude that Arabidopsis phyA differs significantly in several aspects from other dicot phytochromes.
Dependent on their environment, plants can adopt different developmental programs. The light-controlled switch from skoto- to photomorphogenesis is one of the most prominent examples. The red/far-red photoreceptors of the phytochrome family play a key role in sensing light conditions (Casal et al., 1998). These chromoproteins can adopt two major, photoconvertible conformations, Pr and Pfr. The latter is believed to be the physiologically active form.
Five genes encoding phytochromes (PHYA-PHYE) exist in the model plant Arabidopsis (Sharrock and Quail, 1989; Clack et al., 1994). While the chromoproteins phyB to phyE are very stable after irradiation and are present in almost constant amounts throughout the life of a plant (Quail, 1997), phyA is subject to a rapid, light-induced degradation (Clough and Vierstra, 1997). The half-life of light-labile phytochrome differs up to 100-fold (Quail et al., 1973). Immunochemical studies indicated polyubiquitination prior to degradation, suggesting the involvement of the proteasome in the light-dependent destruction of phytochrome (Jabben et al., 1989). Furthermore, rapid formation of sequestered areas of phytochrome (SAPs) was observed in several monocots prior to degradation (Clough and Vierstra, 1997). However, a functional connection between these processes has not yet been demonstrated (Eichenberg et al., 1999). The specific rates of the destruction under continuous irradiation depend highly on the wavelength and fluence rate (Schäfer et al., 1976) and also on the species and developmental state (McArthur and Briggs, 1971). Furthermore, for both monocots and dicots, a Pfr-induced Pr destruction has been described (Clough and Vierstra, 1997).
Dark reversion is the light-independent transition of Pfr into Pr and, therefore, a competing reaction to destruction. In planta investigations measuring mainly phyA have shown that dark reversion occurs in dicots but not in monocots (Briggs and Rice, 1972). Nevertheless, there are reports about dicot phyA without any dark reversion in vivo (Amaranthus caudatus; Kendrick and Hillman, 1971) or in vitro (squash, Vierstra and Quail, 1985).
In addition to the posttranscriptional regulation of receptor abundance, the steady-state level of the phyA mRNA is strongly decreased in light (Colbert et al., 1985; Quail, 1994). However, the other phytochrome genes seem to be expressed constitutively (Quail, 1997; Hirschfeld et al., 1998). In addition to dark reversion and destruction, the extent of regulation of phyA mRNA abundance is diverse, especially between monocots and dicots (Quail, 1994).
As a result of the complex regulation of expression, phyA accumulates predominantly in dark-grown seedlings. The total amount of photoreceptor in green tissues is about 10 times lower than in etiolated seedlings (Clough and Vierstra, 1997). Throughout extensive studies using both mutants and overexpressor lines of Arabidopsis, it became obvious that phyA mediates physiological responses in seedlings to both light pulses and continuous irradiation (Casal et al., 1998).
Despite the great importance of phyA in the fate of etiolated Arabidopsis seedlings, up to now the dynamic properties of phyA have not been studied systematically. Based on the complexity of the nonphotochemical properties of phyA, knowledge about the amounts and dynamics of this phytochrome is essential for the interpretation and understanding of its function in light control of seedling development. Conclusions based on data from other species are difficult to extrapolate; therefore, we decided to investigate phyA dynamics (accumulation, dark reversion, and destruction) in Arabidopsis seedlings by in vivo spectroscopy, western blotting, and northern blotting. To avoid any interfering effects mediated by the second abundant phytochrome (phyB), we used the mutant phyB-5 (Reed et al., 1993) throughout this study.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Light Sources
The mutant of Arabidopsis used in this work was phyB-5 (Koornneef et al., 1980; Reed et al., 1993). Seeds were plated on four layers of water-soaked filter papers placed into clear plastic boxes. A 24-h dark treatment at 4°C was followed by induction of germination by white light for 24 h and further incubation of seedlings in the dark at 25°C. Standard red (656 nm), far-red (730 nm), blue (436 nm), or white light fields were used (Heim and Schäfer, 1982). Light of 718 nm, 692 nm, and long-wavelength far-red light (λmax = 750 nm) was applied using light projectors with the appropriate interference filters (Schott, Mainz, Germany) or with RG9 filters. The values of the photoequilibrium (ϕ) as a function of the wavelength were assumed to be close to those given by Mancinelli (1994) for oat phyA. As the value of ϕ in blue light may differ significantly from the theoretical value (Jabben et al., 1982), it was determined in etiolated dark-grown Arabidopsis seedlings. The measurements showed that the applied blue light established a ϕ of 6.2% ± 1.4% in Arabidopsis phyB-5 seedlings (data not shown).
For RNA blotting and immunoblotting, seedlings were harvested under a green safelight and kept at −80°C until use. In vivo spectroscopy was performed with fresh material.
In Vivo Spectroscopy
Accumulation, destruction, reaccumulation, and dark reversion were measured in complete seedlings (cotyledons, hypocotyls, and roots) with a dual-wavelength ratiospectrophotometer at 4°C (Eichenberg et al., 1999). For the measuring beam, interference filters (730 and 800 nm) were used, and for actinic light, interference filters (660 nm) and RG9 filters were used. The measured Δ(ΔA) value represents the different amounts of Pfr after saturating red or far-red light. The total spectroscopically detectable phytochrome (Ptot) was calculated based on the assumed ϕ of phytochrome in red light (Mancinelli, 1994). For each measurement, about 10 mg of seeds were germinated, resulting in about 100 mg fresh weight after 3 d. Δ(ΔA) values were normalized either to the amount of seeds sown (Figs. 1A and 6) or to the fresh weight determined immediately after the measurements (Figs. 2A, 3A, 4, and 5). A control experiment demonstrated the linear relationship between fresh weight and Δ(ΔA) (data not shown). If not indicated otherwise, data are means of at least three parallel measurements, with error bars indicating ses.
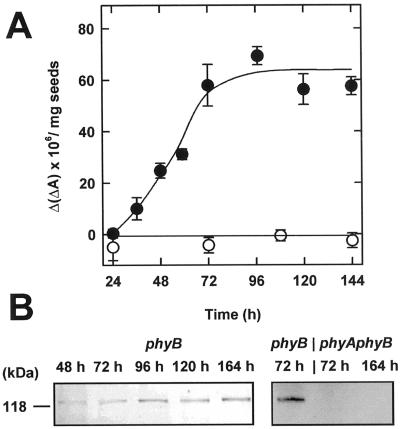
Figure 1.
Accumulation of phyA in Arabidopsis seedlings in the dark. A, From 24 to 144 h after the start of induction of germination by 24 h of white light, Ptot was determined by in vivo spectroscopy in phyB-5 (●) and phyA-201 phyB-5 (○). B, Samples of etiolated seedlings of phyB-5 and phyA-201 phyB-5 were analyzed by immunoblotting of 25 μg of protein and probing with an antiserum against phyA. All subsequent experiments were performed with phyB-5 only.
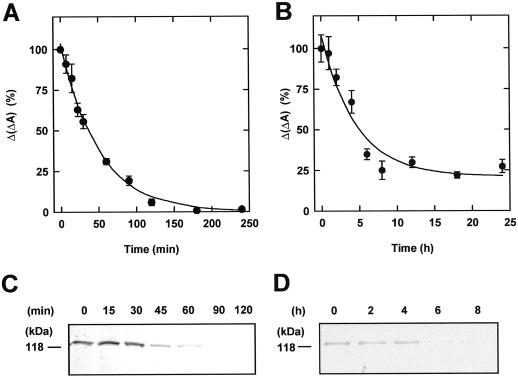
Figure 2.
Destruction of phyA in continuous light. Three-day-old etiolated seedlings were exposed to continuous red light (A and C) or far-red light (B and D). At regular time intervals Ptot was determined by in vivo spectroscopy (A and B) or samples were analyzed by immunoblotting of 25 μg of protein and probing with an antiserum against phyA (C and D).
Protein Extraction and Immunoblotting
Seedlings were extracted with SDS-sample buffer (65 mm Tris-HCl, pH 7.8, 4 m urea, 10 mm DTE, and 5.0% [w/v] bromphenol blue) by sonification (model GM 70 MS 72, Bandelin Sonopuls, Berlin) and heated to 95°C for several minutes. The crude extracts were clarified by centrifugation for 15 min at 20,000g (25°C).
SDS-PAGE, protein blotting, and immunodetection were performed as described by Harter et al. (1993). Antiserum raised against phyA of Sinapis alba L. was obtained from B. Thomas (Wellesbourne, Warwick, UK).
RNA Gel Blotting
Total RNA was isolated from seedlings using the guanidinium hydrochloride method (Logemann et al., 1987). Samples of 20 μg were separated on a 1% (w/v) agarose gel containing formaldehyde and blotted onto a positively charged nylon membrane (Boehringer Mannheim, Basel). Preparation of digoxigenin-labeled probes and hybridization were with the DIG labeling kit (Boehringer Mannheim) according to the manufacturer's instructions using Arabidopsis phyA cDNA as the template. Bound probes were visualized by the immunochemiluminescence protocol of Boehringer Mannheim. For detection of phyA mRNAs, x-ray films were exposed for 2 h. To confirm equal loading of the mRNAs, the same blot was hybridized with a probe against the 18S rRNA of Arabidopsis and exposed for 12 min.
RESULTS
Accumulation of Phytochrome in Dark-Grown Seedlings
Total phytochrome was measured spectroscopically in dark-grown Arabidopsis seedlings 24 to 144 h after the start of the 24-h germination-inducing light treatment (Fig. 1A). In the phyA-201 phyB-5 double mutant (Reed et al., 1994), no photoreversible phytochrome could be detected. Therefore, the entire amount of phytochrome observed by in vivo spectroscopy in the phyB-5 mutant was phyA. Similarly, no photoreversible phytochrome (Ptot) was detectable at 24 h in phyB-5. In this case, however, Ptot increased rapidly during the next 2 d, and thereafter phytochrome levels remained almost constant until 144 h. Western-blot analysis confirmed these results (Fig. 1B). The absence of any signal in extracts of seedlings of the phyA-201 phyB-5 double mutant demonstrated that the antiserum is specific. Because constant levels of phytochrome were reached in phyB-5 after 3 d, seedlings of this age were used in all subsequent experiments.
Destruction of Phytochrome in Continuous Light
Ptot was measured after transfer of dark-grown seedlings into red light. Figure 2A demonstrates that Ptot declined with apparent first-order kinetics. This light-induced destruction proceeded with a half-life of about 30 min, leading to a complete loss of spectroscopically detectable phytochrome after 180 min. Western-blot analysis gave similar results: after 15 min only minor changes in phyA were detected, but within 120 min phytochrome was destroyed completely (Fig. 2C).
In a second experiment Ptot was measured during a period of 0 to 24 h after transfer of seedlings into far-red light (Fig. 2B), and a decline with an apparent half-life of 2 h was detected. However, a new steady state of about 25% of the amount in dark-grown seedlings was observed instead of a total loss of phytochrome. Western-blot analysis yielded consistent results (Fig. 2D). Neither bands of higher nor smaller mobility were detected on the blots (Fig. 2, C and D). Furthermore, destruction was analyzed under monochromatic light of 436, 692, and 718 nm. Fitting the kinetics to a first-order time law resulted in apparent half-lives of 70, 40, and 80 min, respectively (data not shown).
In the simple classical phytochrome reaction scheme:
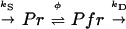 |
1 |
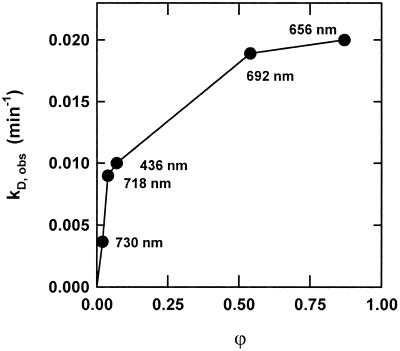
kS is the zero-order rate constant of Pr synthesis and kS is the first-order rate constant of Pfr destruction at a certain ϕ. Based on this scheme, the observed rates of destruction of Ptot under continuous light (kD,obs) should depend linearly on ϕ. However, a strong nonlinear relation between ϕ and kD,obs was observed when kD,obs was plotted versus ϕ (Fig. 3). Therefore, other regulatory mechanisms may be involved (e.g. dark reversion, regulation of synthesis, or Pfr-induced Pr destruction).
Figure 3.
Kinetic analysis of the destruction of phyA in continuous light. Data of Figure 2, A and C, and of similar destruction kinetics at 718, 692, and 436 nm were fitted to a first-order time law. kD,obs was plotted against ϕ.
Destruction and Dark Reversion of Phytochrome after a Red-Light Pulse
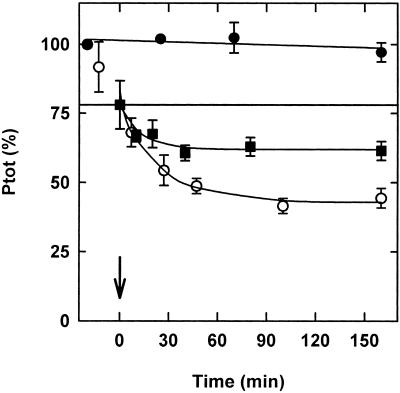
To analyze the reaction of phyA after a light pulse, dark-grown seedlings were irradiated with red light for 5 min, transferred back to the dark, and the levels of Ptot and Pfr were measured (Fig. 4). Total phytochrome levels decreased with an apparent half-life of 20 min. The Pfr trace indicates that almost 30% of total phytochrome remained in its Pfr form. In contrast, a complete loss of phytochrome was observed in continuous red light. No increase of Pr suggesting dark reversion could be observed. Furthermore, no changes in the amount of Ptot were detected during the 5-min red-light pulse (data not shown).
Figure 4.
Destruction and dark reversion of phyA after a red-light pulse. Following a red-light pulse of 5 min and retransfer into darkness (23°C), Ptot (●) and Pfr (□) were determined in 3-d-old etiolated seedlings by in vivo spectroscopy. Pr (▪) was calculated as difference between Ptot and Pfr. Data were fitted to a first-order time law (lines). Destruction (decrease of Ptot) proceeded with a half-life of 20 min. The decrease in Pfr (destruction plus dark reversion) paralleled the decrease of Ptot exactly. There was no dark reversion (increase in Pr) detectable. Data points represent the means of six parallels for Pfr-Pr pairs and means of eight parallels for Ptot values. Error bars indicate ses.
Levels of Phytochrome mRNA after Irradiation
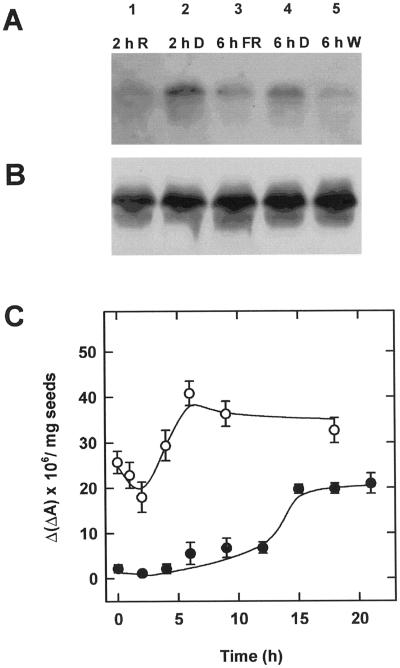
In conjunction with light-induced destruction, the abundance of the phyA mRNA determines steady-state amounts of the photoreceptor. In continuous red and far-red light, Ptot reaches a new steady state after about 2 and 6 h, respectively (Fig. 2). Therefore, phyA transcript levels after these irradiations were analyzed by northern blotting (Fig. 5A). Total RNA was extracted from 48-h-old dark-grown seedlings after 2 h in red light (lane 1) or after 2 h in the dark (lane 2). Alternatively, RNA was extracted from seedlings of the same age after 6 h in far-red light (lane 3), after 6 h in the dark (lane 4), or after 6 h in white light (lane 5). RNA blotting and hybridization revealed a moderate decrease of phyA transcript levels. Quantification showed that the reduction was about 2-fold after red and far-red light, and about 4-fold after white light (data not shown). Thus, the results in Figure 5A do not indicate a strong difference in transcriptional regulation by red or far-red light. Reprobing the blot with 18S rRNA demonstrated equal loading and transfer of RNA (Fig. 5B).
Figure 5.
Levels of phyA mRNA after irradiation and subsequent reaccumulation of phyA. After 2 h of red light (lane 1), 2 h of darkness (lane 2), 6 h of far-red-light (lane 3), 6 h of darkness (lane 4), or 6 h of white light (lane 5), 3-d-old etiolated seedlings were harvested and total RNA was extracted. Blots were hybridized with a probe against Arabidopsis phyA (A), and reprobed with an Arabidopsis 18 S rRNA as a loading control (B). Following 2 h of red light (●) or 6 h of far-red light (○) and retransfer into darkness, Ptot was determined by in vivo spectroscopy in 3-d-old etiolated seedlings (C).
Reaccumulation of Phytochrome after Continuous Red or Far-Red Light
After treatment for either 2 h in red light or 6 h in far-red light, seedlings were transferred into the dark and the Ptot was measured spectroscopically (Fig. 5C). Following far-red irradiation, Ptot first decreased further and then started to increase after a lag period of only about 2 h. This transient decrease of Ptot in the dark suggests the occurrence of Pr destruction. Thereafter, a reaccumulation was observed that leveled off after 6 h at about 60% of the amount of Ptot in dark-grown seedlings. In seedlings irradiated with red light, Ptot also started to reaccumulate after 2 to 4 h. Nonetheless, only after 15 h was a new steady state of about 30% of the amount of Ptot in dark-grown seedlings reached.
Red-Light-Induced Pr Destruction
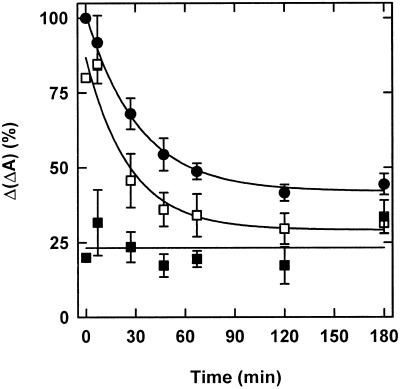
Dark-grown seedlings were irradiated for 5 min with red light and transferred into darkness. After 15 min, the dark period was interrupted by a long-wavelength far-red-light pulse of 5 min, and Ptot was monitored over the following 3 h (Fig. 6). The curve of Ptot destruction after a 5-min red-light pulse is redrawn from Figure 4. Immediately after the far-red-light pulse, 80% of the starting total phytochrome content was present. During the course of the next 3 h, Ptot declined in the dark to 60%. Because of the reverting far-red pulse, the amount of phytochrome present in the Pfr form during this period was too low to account for the decay of Ptot levels. Therefore, the observed decrease must have been the result of Pr destruction. The reverting far-red-light pulse caused a decrease in the fraction of Ptot, which was destroyed after the first 20 min following the red-light pulse, from 35% to 17%. The apparent half-life of destruction was significantly shorter after the far-red-light pulse (10 min) than after the red-light pulse alone (20 min). A far-red-light pulse that was not preceded by a red-light pulse did not lead to any signs of phyA destruction.
Figure 6.
Pfr-induced Pr destruction. Following a red-light pulse of 5 min and retransfer into darkness, Ptot was determined in 3-d-old etiolated seedlings by in vivo spectroscopy (○, replot of data from Fig. 3). Alternatively, 15 min after the red-light pulse, a far-red-light pulse of 5 min was applied. The time course of Ptot in subsequent darkness was measured (▪). Similarly, the time course of Ptot after the far-red-light pulse alone was measured (●). The ordinate was shifted by 20 min to have the end of the initial red light pulse at −20 min for both curves and the end of the reverting far-red-light pulse at 0 min. Data points after 0 min were fitted to a first-order time law (lines). Destruction of Pfr (○) and Pr (▪) proceeded with half-lives of 20 and 10 min, respectively. The amplitude of Pr destruction (17%) was one-half of the amplitude of Pfr destruction (36%) after 20 min.
DISCUSSION
The complex, light-controlled regulation of phytochromes has been the focus of research for many years. Light-induced destruction and dark reversion of phytochrome have been extensively analyzed in mustard, pea, oat, and other species, resulting in kinetics of great variability between different species and developmental stages. The majority of results seem to apply to phyA-like phytochromes, while data obtained by analyzing light-grown tissue probably represent the properties of a mixture of phyA-like and phyB-like phytochromes. Recent investigations of the phytochrome system have been based on the physiology of mutants and transgenic lines of Arabidopsis. Therefore, we wanted to give a framework of data describing the kinetic behavior of endogenous phyA in seedlings of Arabidopsis. The availability of mutants allowed us to distinguish between the two most abundant phytochromes phyA and phyB and to unequivocally observe only phyA. However, the use of the phyB-5 mutant could alter the results if levels of phyA were in part controlled by the action of phyB.
During seedling development in the dark, Ptot increased almost linearly until a constant level was reached within 3 d (Fig. 1A). This pattern resembles that of GUS activity in the phyA-promoter-GUS lines (Somers and Quail, 1995). Transcripts of phyA can be detected after 2 d (Fig. 6) and after 7 d (Sharrock and Quail, 1989). This indicates that the constant level of spectroscopically detectable phyA is the result of a steady state of synthesis and phyA degradation in darkness. The half-life of Pr turnover can be estimated to be 35 h, a value comparable to that observed in squash (Quail et al., 1973).
Analysis of light-induced destruction by in vivo spectroscopy and by western blotting demonstrated that the decrease in the photoreversible photoreceptor was caused by a loss of phyA protein (Fig. 2). Thus, destruction involved bona fide proteolysis of phyA, as has already been shown for grass seedlings (Pratt et al., 1974). While destruction follows zero-order kinetics in monocots in both red and far-red light (Schäfer et al., 1976), first-order kinetics were observed in Arabidopsis. In continuous red light, Ptot disappeared with a half-life of 30 min and after 180 min, photoreversible phytochrome was undetectable. In continuous far-red light, Ptot declined with a half-life of 2 h, and a steady-state level of 25% was observed (Fig. 2). Thus, neither steady-state levels nor rates of destruction reflect the photoequilibria, i.e. the abundance of light-labile Pfr. One of the reasons for this discrepancy could be a differential regulation of Pr synthesis in red and far-red light. Nevertheless, measurements of phyA mRNA levels did not indicate a strong differential regulation of Pr synthesis in red and far-red-light (Fig. 5A). In addition, similar results were obtained with 7-d-old Arabidopsis seedlings (Sharrock and Quail, 1989), implying that the regulation of phyA transcripts does not differ between these two developmental stages.
To address the question of whether there is a similar capacity of phyA synthesis after light-induced destruction of the photoreceptor, the reaccumulation of phyA was measured after both red- and far-red-light treatments. In both cases an increase started after only a lag period of approximately 2 h, and supplied about 30% of Ptot of the dark control. Only the kinetics of resynthesis depended on the light quality, as synthesis led to a new steady state after about 6 h in far-red-light-irradiated seedlings, compared with 15 h in red-light-irradiated seedlings. mRNA resynthesis in parsley is much slower after a red-light pulse than after a far-red-light pulse (Poppe et al., 1994), suggesting that differential mRNA reappearance in darkness could cause different reaccumulation kinetics of phyA. In fact, Ptot reaccumulation after 2 h of red light followed by 5 min of far-red light occurred with the same kinetics as reaccumulation after 6 h of far-red light (data not shown). Alternatively, the light regulation of phyA resynthesis could be the result of light regulation of the translation rather than transcription.
Differences in destruction in red and far-red light could be due to dark reversion, which is discussed as a process opposing both light-induced destruction of phyA and the activation of signal transduction chains by Pfr. Regarding the assumption that all dicot phytochromes display dark reversion except those in the family Centrospermae (Kendrick and Hillman, 1971), it is surprising that dark reversion could not be detected in Arabidopsis phyB-5 seedlings (Fig. 4). Recently, the functional relevance of phytochrome dark reversion was emphasized (Elich and Chory, 1997). That study demonstrated that the loss-of-function mutation E812K in Arabidopsis phyB (phyB-101) caused a strongly enhanced dark reversion. Based on the failure to detect dark reversion in Arabidopsis phyB-5 seedlings, it will be very interesting to determine whether some of the known missense phyA alleles behave differently in this respect, in a manner similar to phyB-101.
While dark reversion was not observed, Pfr-induced Pr destruction remains an alternative possibility to explain the destruction kinetics. Figure 6 demonstrates that red-light-initiated destruction of Ptot continued even after a reverting far-red-light pulse, which is similar to data reported for oat (Stone and Pratt, 1979; Jabben et al., 1989). Previous studies have suggested that Pfr-induced Pr destruction is composed of two competing parallel reactions, destruction and escape. Both start from cycled Pr and are controlled by two first-order rate constants (Speth et al., 1987). Such a treatment predicts that the ratio of the degraded fraction of Ptot after the far-red-light pulse (17%) to the degraded fraction after the red-light pulse (36%) is determined by the ratio of the two rate constants, and that the apparent rate of Ptot destruction after the far-red-light pulse is accelerated. After an appropriate reanalysis of the data shown in Figure 6, a half-life of 14 min for escape and 18 min for destruction was calculated. The latter value is in perfect agreement with the half-life of Pfr obtained independently (20 min, time points >20 min from Fig. 4). Thus, Pfr destruction and Pfr-induced Pr destruction proceed with similar rates. The kinetics of destruction of Ptot under continuous irradiation should be affected by Pfr-induced Pr destruction.
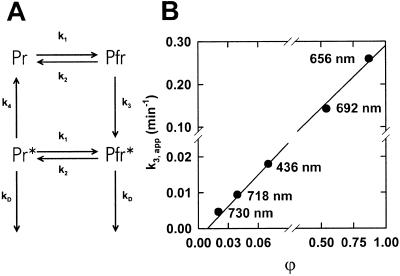
Analysis of Ptot levels in continuous light revealed a nonlinear relationship between kD,obs and ϕ (Fig. 3). While in other dicots the expected linearity could be found (e.g. A. caudatus, Kendrick and Frankland, 1968; Mirabilis jalapa, Kendrick and Hillman, 1971), a saturation-like dependency was observed in oat (Schäfer et al., 1976), which is similar to our results. To account for the nonlinear relationship of kD,obs to ϕ and the observed Pfr-induced Pr destruction, a simple two-step model of phytochrome destruction was employed (Fig. 7A, modified after Brockmann et al. [1987] and Vierstra [1994]): After the formation of Pfr, a modification is required for the start of destruction—only the modified Pfr (Pfr*) is degraded. Pfr*, being still photoconvertible, yields modified Pr (Pr*) subject to Pfr-induced Pr destruction and to an escape reaction forming Pr. The de novo synthesis of phytochrome, which was low at 3 d (Fig. 1), and dark reversion, which was not detectable (Fig. 4), were neglected. The formalization of this model contains the light reactions (rate constants k1 and k2), the first-order formation of Pfr* (rate constant k3), the first-order escape of Pr* to Pr (rate constant k4), and the first-order destruction of Pr* and Pfr* (rate constant kD).
Figure 7.
Model of phyA destruction in continuous light. A, Schematic representation of the employed two-step model of destruction (for details, see text). B, Data of destruction kinetics (Fig. 3) were fitted according to the model in Figure 7A. The apparent rate constant k3,app was plotted against ϕ; kD was 2.53 × 10−2 min−1.
According to this model, Ptot destruction is composed of two consecutive first-order reactions. Only the apparent k of the first step (k3,app) is expected to depend on ϕ, because subsequently both Pr* and Pfr* are degraded with similar rates. All destruction data were reanalyzed assuming a variable k3,app and an invariable kD. The obtained k3,app values were plotted against ϕ (Fig. 7B). This analysis of the experimental data resulted in a linear relationship between rate constants and ϕ, demonstrating that the employed model (Fig. 7A) is a satisfactory description of the observed destruction kinetics. Consequently, in far-red-light (low Pfr) the kD,obs is determined by the apparent slow modification (k3,app), while in red light (high Pfr) the degradation step (kD) becomes rate limiting.
The molecular nature of the proposed modification remains elusive. Biochemical data have indicated that phyA is ubiquitinated prior to degradation, probably without impairing the photoreversibility (Shanklin et al., 1989). Because polyubiquitination is a commonly observed prerequisite for regulated proteolysis, it is tempting to speculate that the modification is a ligation of ubiquitin moieties to phyA. Data analysis based on the proposed model suggests that a large fraction of Ptot transiently accumulates in the modified state (up to 95% after 20 min in red light, calculations not shown). However, the absence of any signals of higher molecular mass in the western blots argues against polyubiquitination as the rate-limiting degradation signal at this stage. Due to their short half-life, ubiquitinated forms of phyA may have escaped detection in the western blot, requiring overdevelopment of the blots. On the other hand, formation of SAPs may provide an initial degradation signal by means of intracellular localization. Nevertheless, there have thus far been no reports about SAPs in Arabidopsis. Two further striking findings are the high steady-state level of Ptot in far-red-light (25%) and the significant fraction of stable Pfr after a red-light pulse (30%) that undergoes neither destruction nor dark reversion. Experiments addressing both phenomena are in progress.
To summarize, phyA in Arabidopsis is characterized by several properties that have so far been primarily observed in monocots. Light regulation of the synthesis of phyA and Pr destruction could not be observed in mustard, a close dicot relative of Arabidopsis. However, dark reversion occurs. In contrast, there is both a pronounced regulation of the synthesis of phyA and Pr destruction, but no dark reversion in oat and other monocots. Therefore, Arabidopsis, which displays a moderate regulation of the synthesis of phyA, no dark reversion, and Pr destruction, differs significantly not only from monocots but also from other dicots.
ACKNOWLEDGMENTS
We thank B. Thomas for the antiserum against phyA, Prof. Peter H. Quail for providing the Arabidopsis phyA cDNA, and Prof. Gunther Neuhaus for providing the 18S rRNA probe. Furthermore, we thank students Annette Martin and Sabine Unger for excellent technical assistance.
Footnotes
This work was supported by the Deutsch Forschungsgemeinschaft (fellowship to L.H. and grant to E.S.) and by Evangelisches Studienwerk Villigst (fellowship to K.E.).
LITERATURE CITED
- Briggs W, Rice HV. Phytochrome: chemical and physical properties and mechanism of action. Annu Rev Plant Physiol. 1972;23:293–334. [Google Scholar]
- Brockmann J, Rieble S, Kazarinova-Fukshansky N, Seyfried M, Schäfer E. Phytochrome behaves as a dimer in vivo. Plant Cell Environ. 1987;10:105–111. [Google Scholar]
- Casal JJ, Sánchez RA, Botto JF. Modes of action of phytochromes. J Exp Bot. 1998;49:127–138. [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Clough RC, Vierstra RD. Phytochrome degradation. Plant Cell Environ. 1997;20:713–721. [Google Scholar]
- Colbert JT, Hershey HP, Quail PH. Phytochrome regulation of phytochrome mRNA abundance. Plant Mol Biol. 1985;5:91–101. doi: 10.1007/BF00020091. [DOI] [PubMed] [Google Scholar]
- Eichenberg K, Kunkel T, Kretsch T, Speth V, Schäfer E. In vivo charcterization of chimeric phytochromes in yeast. J Biol Chem. 1999;274:354–359. doi: 10.1074/jbc.274.1.354. [DOI] [PubMed] [Google Scholar]
- Elich TD, Chory J. Biochemical characterization of Arabidopsis wild-type and mutant phytochrome B holoproteins. Plant Cell. 1997;9:2271–2280. doi: 10.1105/tpc.9.12.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter K, Talke-Messerer C, Barz W, Schäfer E. Light-dependent and sucrose-dependent gene expression in photomixotrophic cell suspension cultures and protoplasts of rape (Brassica napus L.) Plant J. 1993;4:507–516. [Google Scholar]
- Heim B, Schäfer E. Light-controlled inhibition of hypocotyl growth in Sinapis alba seedlings: fluence rate dependence of hourly light pulses and continuous irradiation. Planta. 1982;154:150–155. doi: 10.1007/BF00387909. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Tepperman JM, Clack T, Quail PH, Sharrock RA. Coordination of phytochrome levels in phyB mutants of Arabidopsis as revealed by apoprotein-specific monoclonal antibodies. Genetics. 1998;149:523–535. doi: 10.1093/genetics/149.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabben M, Beggs C, Schäfer E. Dependence of Pfr/Ptot-ratios on light quality and light quantity. Photochem Photobiol. 1982;35:709–712. [Google Scholar]
- Jabben M, Shanklin J, Vierstra RD. Red light-induced accumulation of ubiquitin-phytochrome conjugates in both monocots and dicots. Plant Physiol. 1989;90:380–384. doi: 10.1104/pp.90.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick RE, Frankland B. Kinetics of phytochrome decay in Amaranthus seedlings. Planta. 1968;82:317–320. doi: 10.1007/BF00386434. [DOI] [PubMed] [Google Scholar]
- Kendrick RE, Hillman WS. Absence of phytochrome dark reversion in seedlings of the Centrospermae. Am J Bot. 1971;58:424–428. [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Mancinelli AL. The physiology of phytochrome action. In: Kendrick RE, Kronenberg HHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 211–270. [Google Scholar]
- McArthur JA, Briggs WR. In vivo phytochrome reversion in immature tissue of the Alaska pea seedling. Plant Physiol. 1971;48:46–49. doi: 10.1104/pp.48.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Ehmann B, Frohnmeyer H, Furuya M, Schäfer E. Regulation of phytochrome A mRNA abundance in parsley seedlings and cell-suspension cultures. Plant Mol Biol. 1994;26:481–486. doi: 10.1007/BF00039558. [DOI] [PubMed] [Google Scholar]
- Pratt LH, Kidd GH, Coleman RA. An immunochemical characterization of the phytochrome destruction reaction. Biochim Biophys Acta. 1974;365:93–107. doi: 10.1016/0005-2795(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Quail PH. Phytochrome genes and their expression. In: Kendrick RE, Kronenberg HHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 71–104. [Google Scholar]
- Quail PH. An emerging molecular map of the phytochromes. Plant Cell Environ. 1997;20:657–665. [Google Scholar]
- Quail PH, Schäfer E, Marmé D. Turnover of phytochrome in pumpkin cotyledons. Plant Physiol. 1973;52:128–131. doi: 10.1104/pp.52.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red-far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer E, Lassig T-U, Schopfer P. Photocontrol of phytochrome destruction and binding in dicotyledonous vs monocotyledonous seedlings: the influence of wavelength and irradiance. Photochem Photobiol. 1976;24:567–572. doi: 10.1111/j.1751-1097.1975.tb06736.x. [DOI] [PubMed] [Google Scholar]
- Shanklin J, Jabben M, Vierstra RD. Partial purification and peptide mapping of ubiquitin phytochrome conjugates from oat. Biochemistry. 1989;28:6028–6034. [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana structure evolution and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Somers DE, Quail PH. Temporal and spatial expression patterns of PHYA and PHYB genes in Arabidopsis. Plant J. 1995;7:413–427. doi: 10.1046/j.1365-313x.1995.7030413.x. [DOI] [PubMed] [Google Scholar]
- Speth V, Otto V, Schäfer E. Intracellular localization of phytochrome and ubiquitin in red light-irradiated oat coleoptiles by electron microscopy. Planta. 1987;171:332–338. doi: 10.1007/BF00398678. [DOI] [PubMed] [Google Scholar]
- Stone HJ, Pratt LH. Characterization of the destruction of phytochrome in the red absorbing form. Plant Physiol. 1979;63:680–682. doi: 10.1104/pp.63.4.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD. Phytochrome degradation. In: Kendrick RE, Kronenberg HHM, editors. Photomorphogenesis in Plants. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 141–160. [Google Scholar]
- Vierstra RD, Quail PH. Spectral characterization and proteolytic mapping of native 120-kilodalton phytochrome from Cucurbita pepo L. Plant Physiol. 1985;77:990–998. doi: 10.1104/pp.77.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]