ABSTRACT
We examined the neural correlates underlying response inhibition in early childhood. Five-year-old children completed a Go/No-go task with or without time pressure (Fast vs. Slow condition) while scalp EEG was recorded. On No-go trials where inhibition was required, the left frontal N2 and posterior P3 were enhanced relative to Go trials. Time pressure was detrimental to behavioral performance and modulated the early-occurring P1 component. The topography of ERPs related to response inhibition differed from patterns typically seen in adults, and may indicate a compensatory mechanism to make up for immature inhibition networks in children.
Imagine that you have just stepped out of your house and see a child running after a ball that had gone to the road. Right then, you notice a car coming and you instinctively call out to the child to stop. Will the child be able to comply with your instructions? This scenario is just one example of the adaptive importance of response inhibition, or the process of stopping an action that has been initiated. This ability emerges and undergoes rapid growth in early childhood, particularly between three to six years (Carver, Livesy & Charles, 2001; Garon, Bryson, & Smith, 2008; Wiebe, Sheffield, & Espy, 2012), and continues to mature into early adulthood (Band, van der Molen, Overtoom & Verbaten, 2000). There is a parallel, protracted developmental trajectory of the prefrontal brain regions implicated in higher order cognitive functions (Fuster, 2002). Within the prefrontal cortex, the anterior cingulate, dorsolateral, ventrolateral, inferior frontal and medial prefrontal cortices have been identified as a part of the neural network underlying response inhibition (Casey et al., 1997; Rubia et al., 2001; Tamm, Menon, & Reiss, 2002). The immaturity of these regions in children may underlie the difficulties they face in inhibiting responses. The goal of the present study was to examine response inhibition and its neural correlates in young children, while manipulating task demands expected to affect inhibitory load. Numerous factors have been found to increase inhibitory load—for example, working memory load (Wijeakumar et al., 2015), level of interference (Ciesielski, Harris, & Cofer, 2004), and preceding context (Durston et al., 2002). In this study, we choose to focus on one factor, time pressure (Cragg & Nation, 2008; Jodo & Kayama, 1992; Simpson & Riggs, 2006).
One prominent perspective on response inhibition is the horse race model (Logan & Cowan, 1984), which conceptualizes it as a race between a “go” process, initiating the response, and a “stop” process, inhibiting it. Successful inhibition requires that the stop process be completed in time to interrupt the ongoing go process. Failed inhibitions occur when the stop process is too slow and the go process is completed first. Thus, the timing of these two processes is crucial in determining whether inhibition is successful. Increasing the speed of the go process requires a corresponding increase in the speed of the stop process, and should therefore increase inhibitory load; several previous studies support this suggestion (Cragg & Nation, 2008; Simpson & Riggs, 2006). However, while faster response initiation can be directly observed in response times, faster stopping cannot be directly observed. Consequently, behavioral studies of response inhibition have relied primarily on failed inhibitions to inform us about inhibitory processing. This limitation can be surmounted by neuroimaging methods such as event-related potentials (ERPs), as brain activity is ongoing and can be measured in the absence of observable behavior. Jodo and Kayama (1992) demonstrated the utility of this approach in adults, showing that when the time window allowed for a response was decreased, response initiation was speeded up, and the increased demands were reflected in ERP characteristics.
Two studies (Cragg & Nation, 2008; Simpson & Riggs, 2006) have examined the effects of time pressure on response inhibition in children behaviorally. Both studies used variants of the Go/No-go (GNG) paradigm, commonly used to assess response inhibition in children (e.g., Durston et al., 2002; Wiebe et al., 2012). In a typical GNG task, participants respond to a frequently occurring stimulus type (Go trials), and withhold responding to a less frequently occurring stimulus type (No-go trials). Because the majority of trials require a response, the task induces a prepotent tendency to respond, and consequently No-go trials require inhibitory control. The extent to which individuals are able to inhibit responding on No-go trials serves as a measure of their inhibitory abilities. Cragg and Nation (2008) found that when a shorter time window was allowed for a response, task accuracy decreased in both 5–7-year-old and 9–11-year-old children, suggesting that the response inhibition demands of the task increased with time pressure. Similarly, in a study of 3-year-olds, Simpson and Riggs (2006) found time pressure increased inhibitory demands of the task. However, in their study, this was true only to a certain extent. When the time window was too short for children to respond, Go accuracy plummeted and No-go accuracy no longer correlated with another inhibition measure. Findings from both of these studies suggest that manipulating time pressure should affect response initiation and/or inhibitory demands in children, as it does in adults.
Although numerous studies have documented young children’s behavioral performance on measures of inhibition (Cragg & Nation, 2008; Simpson & Riggs, 2006; Wiebe et al., 2012), only a few studies have examined the neural correlates underlying their performance. Cognitive processes like response inhibition occur on a millisecond time scale, and electroencephalography (EEG) is one of the few neuroimaging methods with the necessary temporal resolution to investigate the neural underpinnings of these processes. Two ERP components, the N2 and P3, have been consistently identified as markers of inhibitory processes: The N2 is a negative peak observed at frontal electrode sites between 200 and 500 ms after stimulus onset (Carter & Van Veen, 2007; Falkenstein, Hoormann, & Hohnsbein, 1999; Jonkman, Sniedt, & Kemner, 2007); the P3 is a positive peak observed at frontocentral electrode sites between 300 and 600 ms after stimulus onset (Bokura, Yamaguchi, & Kobayashi, 2001; Eimer, 1993; Kopp, Mattler, Goertz, & Rist, 1996). In GNG paradigms, the amplitude and latency of these two ERP components differ between No-go trials, where inhibition is required, and Go trials, where it is not (Bokura et al., 2001; Falkenstein et al., 1999; Fallgatter & Strik, 1999).
A few studies with young children have utilized EEG with the GNG task. However, none of these studies have addressed how manipulating time pressure would modulate the neural correlates of response inhibition. In one study that did examine the role of timing, the investigation was limited to how the timing of action decision and response initiation influenced response inhibition in 5- year-old children (Chevalier, Kelsey, Wiebe, & Espy, 2014). In that study, Chevalier and colleagues used a modified GNG task that allowed children to fully or partially inhibit their responses and they found that relative to partial inhibitions, successful inhibitions were marked by an earlier onset of a late negative slow wave thought to represent action decision. Another study examined cross-cultural differences between European- and Chinese-Canadian 5-year-olds. They found no behavioral differences, but Chinese-Canadian children showed a more pronounced N2 component (Lahat, Todd, Mahy, Lau, & Zelazo, 2010). Davis, Bruce, Snyder, and Nelson (2003) found that while adults displayed the enhanced P3 on No-go trials, 6-year-old children did not. Instead, a late positive peak at frontal electrodes distinguished No-go trials from Go trials. Two studies have examined response inhibition in GNG tasks that also required emotion regulation. In a study with both children and adolescents, the N2 and P3 on No-go trials were greater during conditions of negative emotion induction. (Lewis, Lamm, Segalowitz, Stieben & Zelazo, 2006). Adopting a similar paradigm, another study with 5–6 year-olds reported that better response inhibition performance during negative emotional induction was accompanied by greater EEG power in the theta frequency range, though no differences in the N2 amplitude were seen (Farbiash & Berger, 2015). Several other studies have also incorporated EEG with the GNG paradigm to examine brain activity related to error detection and monitoring in 5 to 7-year-old children (Torpey, Hajcak, Kim, Kujawa, & Klein, 2012; Torpey, Hajcak, & Klein, 2009). These studies, however, did not look at the neural correlates associated with response inhibition in early childhood.
More research using ERP to study response inhibition has been conducted in middle childhood. Most of these studies have found ERPs modulated by response inhibition demands were more evident at posterior electrode sites (Brydges, Anderson, Reid, & Fox, 2013; Ciesielski et al., 2004; Durston et al., 2002; Jonkman et al., 2007). Jonkman, Lansbergen, and Stauder (2003) directly compared 9-year-old children and adults, and found that the No-go P3 was maximal at posterior electrode sites in children, but frontally maximal in adults. Furthermore, several studies have found laterality differences in brain activity during response inhibition between children and adults. While response inhibition in adults is typically associated with greater activity in the right hemisphere (Aron, Robbins, & Poldrack, 2004), several studies have found that children display greater activity in the left hemisphere (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Johnstone, Pleffer, Barry, Clarke, & Smith, 2005). In addition to the studies highlighted above, Hoyniak (2017) recently performed a meta-analysis of 65 studies that have utilized the GNG task to assess inhibition in children aged 2 to 12 years old. This meta-analysis found that the N2 was larger on No-go trials than on Go trials, bolstering its position as a neural marker of response inhibition. Furthermore, the N2 decreased in both amplitude and latency with age. However, of these studies, only five involved children in early childhood and none examined how time pressure influenced response inhibition performance or its neural correlates.
In the present study, our main goal was to examine the neural correlates of response inhibition in early childhood, and to do so under varying inhibitory demands. We used the preschool GNG paradigm, because several previous studies have manipulated the inhibitory demands of this task by inducing time pressure (Cragg & Nation, 2008; Jodo & Kayama, 1992; Simpson & Riggs, 2006). In the GNG task, response inhibition is indexed by children’s ability to withhold responding on No-go trials. We chose to focus on 5-year-olds because many studies have found that children at this age are capable of performing well on GNG tasks (Chevalier et al., 2014; Wiebe et al., 2012), and in ERP studies, it is important that children complete sufficient correct trials to generate stable ERP averages. Additionally, this age captures an important phase transition to formal schooling where there is an increased need for children to inhibit inappropriate responses (Blair & Razza, 2007; Lahat et al., 2010). It is, therefore, important for us to gain insights into factors that may influence response inhibition performance in children at this age. Because of the number of trials required in ERP studies, young children’s limited attention span, and to minimize the influence of practice effects and fatigue on children’s performance, we chose to implement the time pressure manipulation in a between-subjects design. Children were randomly assigned to the Fast or Slow condition, and completed the GNG task with shorter or longer time windows in which they could respond. We hypothesized that children in the Fast condition would experience increased inhibitory demands induced by time pressure and that this would be reflected in their performance and their brain activity, particularly the N2 and P3 components.
Method
Participants
The sample included 31 children (15 boys, 16 girls) between 5;0 and 5;11 years (M = 5;8 years, SD = 3 months). Data from eleven additional children were excluded due to poor task performance (n = 6) and/or excessive EEG movement or eye-blink artifact (n = 5). Children were randomly assigned to either the Fast or Slow GNG conditions. There were 15 children (7 boys, 8 girls) between 5;0 and 5;11 years (M = 5;8 years, SD = 3 months) in the Slow condition and 16 children (8 boys, 8 girls) between 5;3 and 5;11 years (M = 5;9 years, SD = 2 months) in the Fast condition. Participants were recruited from a small city in the Midwestern United States through local businesses that served families with young children (e.g., preschools, health offices, pediatricians) and by word of mouth. To be included in the study, children had to be born full-term and have no history of neurological or behavioral disorders. The ethnic composition of the children in the two conditions was similar: the Slow condition included 11 European American, 1 African American, and 3 mixed ethnicity children. The Fast condition included 12 European American, 1 African American, 1 Asian American, and 2 mixed ethnicity children. Parent-reported health insurance status was used as a proxy for socioeconomic status (SES). Most participants (71%; 11 in each condition) were middle or upper-middle SES, with private health insurance. Fewer participants (29%; 4 in the Slow condition, 5 in the Fast condition) were low SES and eligible for public health insurance.
Procedure
The study was carried out at a child development laboratory at a university in the Midwestern United States. Parents accompanied their children to the lab and were briefed about the study before providing informed consent. After EEG net application, children completed two tasks, with the GNG task administered second. The first task was a measure of set-shifting that took approximately 10 minutes. It was unrelated to the present investigation and was the same for all children. Parents remained in the testing room throughout the session.
Response inhibition task
The Preschool GNG task (adapted from Wiebe et al., 2012) was a computerized fishing game (see Figure 1), presented using E-Prime 2.0 Professional (Psychology Software Tools, Sharpsburg, PA). Children were instructed to respond by pressing a single button on a button box whenever a fish appeared (Go trials) and withhold responding when a shark appeared (No-go trials). 1 Each trial began with the onset of a stimulus that remained on the screen for a maximum of 750 ms (Fast) or 1500 ms (Slow), and disappeared when the child responded. On correct Go trials, a net appeared over the fish with a ‘bubbling’ sound to indicate that the fish had been caught. On incorrect No-go trials, a picture of a broken fishing net appeared over the shark with a “buzzer” sound to indicate the shark had broken through the net. The feedback lasted for 1000 ms. No feedback was given on trials when the child did not press the button. Each trial was followed by an inter-stimulus interval of 1000 ms.
Figure 1.
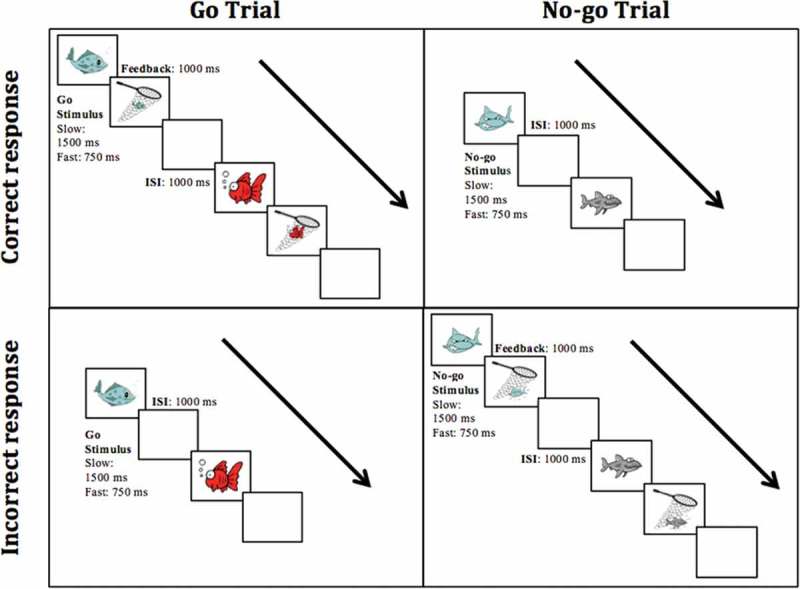
Outline of the Go/No-go task used in the study. Top-left: Correct go trial (Hit), Top-right: Correct No-go trial (Correct miss), Bottom-left: Incorrect Go trial (Miss), Bottom-right: Incorrect No-go trial (False alarm). In the slow condition, stimulus was displayed for up to 1500 ms and in the Fast condition, stimulus was displayed for up to 750 ms.
At the beginning of the task, children were shown a picture of all the stimuli and asked to point out the fish and sharks to ensure they could distinguish between Go and No-go stimuli. Next, children completed a block of 13 practice trials and then proceeded with the test trials. There were a total of 200 (Fast) or 160 (Slow) test trials 2 with 75% Go trials (Fast: 150 trials, Slow: 120 trials) and 25% No-go trials (Fast: 50 trials, Slow: 40 trials). Following the prototypical design of the GNG task (Cragg & Nation, 2008; Durston et al., 2002; Lahat et al., 2010), Go trials were probabilistically dominant to increase children’s bias to respond, thereby maximizing response inhibition requirements on No-go trials. Children in the Fast condition completed a greater number of trials to ensure there was sufficient EEG data on correct trials to permit ERP analyses, as we expected children to make more errors in the speeded condition.
All stimuli measured 10 by 13 cm and were presented at the centre of the screen on a 20-inch DELL desktop monitor. In total, there were 10 different Go stimuli and 3 different No-go stimuli and each stimulus was presented with roughly equal frequency across the task (10–12%). As there were more Go trials (75%) than No-go trials (25%), it was necessary to have a greater variety of Go stimuli to ensure each stimulus was presented at equal frequency across the task.
Electrophysiological recording and data processing
EEG was recorded using a 128-channel Hydrocel Geodesic Sensor Net (Electrical Geodesics Inc., Eugene, OR). First, children’s head circumference was measured to allow selection of the appropriate net, and the vertex was marked to ensure correct net placement. The net was soaked in an electrolyte solution prior to application. Electrode impedances were measured and maintained below 50 kΩ. If needed, additional electrolyte solution was applied to electrodes with high impedances. During task performance, EEG data was recorded continuously at a sampling rate of 250 Hz, with a 0.1–100 Hz bandpass filter, referenced to the vertex.
ERP analysis was conducted with Net Station software (Version 4.3.1, Electrical Geodesics Inc., Eugene, OR). A 0.3–30 Hz bandpass filter was used to filter data offline. EEG data was segmented into 1300 ms epochs beginning 100 ms before stimulus onset and ending 1200 ms post-stimulus. Artifact detection was performed on segmented files and all electrodes where signal fluctuations exceeded 200 µV were marked bad. Segments with more than 12 bad electrodes were rejected. Spline interpolation was used to replace bad electrodes in otherwise acceptable segments. EOG correction was conducted using Gratton’s algorithm (Gratton, Coles, & Donchin, 1983). In addition, the first author visually inspected all segments for EOG or movement artifacts, and corrected segment and channel markups if needed. As recommended by the Net Station data processing manual, following EOG correction, channels were reassessed for artifacts with previously marked information overwritten if necessary and then bad electrodes replaced using spline interpolation. All usable segments were averaged, separately for Go and No-go trial types. EEG data was re-referenced to an average reference using the polar average reference effect correction (Junghöfer, Elbert, Tucker, & Braun, 1999) and then baseline-corrected using the data 100 ms prior to stimulus onset. Incorrect trials and trials with a RT less than 200 ms were excluded from analysis as they do not reflect deliberate behavior (Haith, Hazan, & Goodman, 1988). All ERP data included in the analysis had a minimum of 10 artifact-free trials in each trial type (Go, No-go). In the Slow condition, the number of Go and No-go trials included in data analysis ranged from 27–62 (Total = 120 trials) and 15–37 (Total = 40 trials), respectively. In the Fast condition, the number of Go and No-go trials included ranged from 33–87 (Total = 200 trials) and 14–42 (Total = 50 trials), respectively.
Then, mean amplitude and peak latency measures were calculated for the inhibition-related ERP components, N2 and P3. Because visual inspection of the waveforms suggested that the P1 component at parietal electrodes differed between the Fast and Slow conditions, mean amplitude and peak latency of P1 were also calculated. Mean amplitude was defined as the average amplitude of the waveform within the time window selected for each ERP component. Peak latency was defined as the time taken from the onset of the stimulus to the maximum peak of the ERP component within the time window selected. N2 was examined at the frontal, frontocentral and central electrode sites and was defined as the negative deflection in the time window between 260 and 560 ms. P1 and P3 were examined at parietal electrode sites. P1 was defined as a positive deflection between 60 and 150 ms, and P3 was defined as the positive deflection between 310 and 610 ms. The time windows were determined after considering previous studies with similar age groups (Ciesielski et al., 2004; Davis et al., 2003; Jonkman et al., 2003; Lahat et al., 2010) and visual examination of the grand averaged and individual waveforms. Peak latency and mean amplitude measures were averaged across electrodes within clusters selected to be compatible with the 10–20 electrode placement system. The electrodes included in each cluster are presented in Figure 2. Because visual inspection of waveforms showed that the N2 component was more pronounced laterally than in midline regions, electrode clusters were defined within Left, Midline and Right locations.
Figure 2.
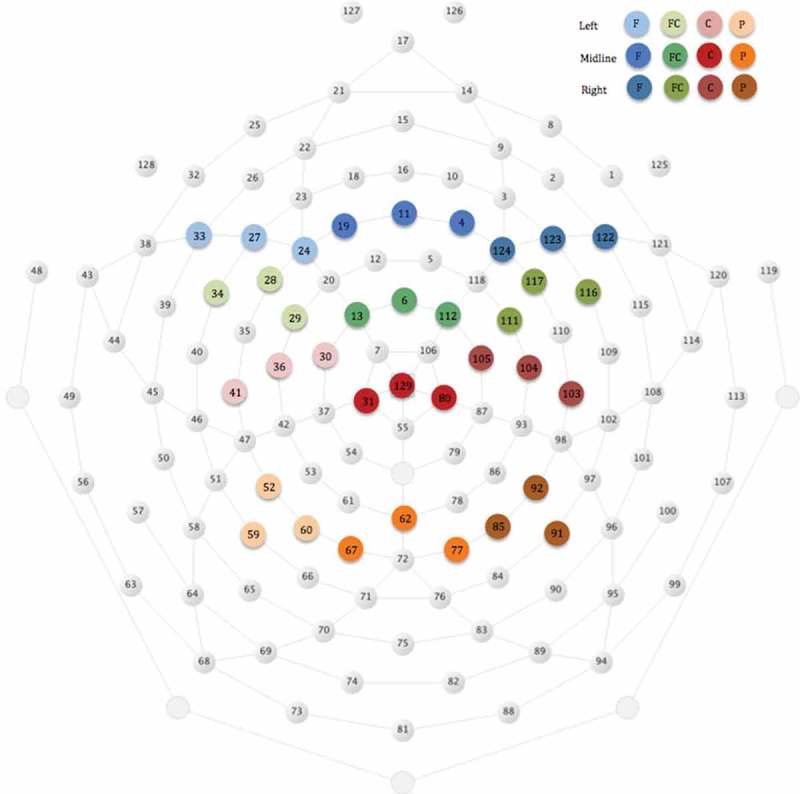
Layout illustrating the electrodes that were included in the ERP analyses at frontal (F), frontocentral (FC), central (C) and parietal (P) regions.
Data analysis
All analyses were conducted using SPSS Version 21.0 (IBM Corp., Armonk, NY). Behavioral and ERP measures were analyzed using general linear models analysis of variance (ANOVA), to account for the unequal sample sizes in the Fast and Slow conditions. Greenhouse-Geisser correction was applied in certain cases where sphericity assumptions were violated. Means are reported as least-squares means, and partial eta-squared (η2) is reported as a measure of effect size for all statistically significant findings.
Analyses of behavioral measures, accuracy and reaction time (RT) were conducted for speed condition (Fast, Slow) and trial type (Go, No-go). Accuracy was calculated as the number of correct trials as a proportion of the total number of Go or No-go trials. RT was measured as the time from stimulus onset to when a button press was recorded.
Analyses of ERP measures, P1, N2 and P3 mean amplitude and latency were conducted for speed condition (Fast, Slow), trial type (Go, No-go), region (Frontal, Frontocentral, Central, and Parietal) and laterality (Left, Midline and Right).
Results
Descriptive statistics for accuracy and response time (RT) are presented in Table 1, broken down by speed condition and trial type. Those for ERP amplitude and latency measures are presented in Table 2, broken down by speed condition, trial type, region, and laterality. Grand-averaged ERP waveforms are shown in Figure 3, separately for each electrode cluster.
Table 1.
Means and standard deviations (in parentheses) for accuracy (proportion correct) and reaction time (milliseconds) by trial type and speed condition.
| Go Trials |
No-go Trials |
|||
|---|---|---|---|---|
| Slow | Fast | Slow | Fast | |
| Accuracy | .98 (.03) | .87 (.07) | .91 (.04) | .88 (.08) |
| Reaction Time | 664 (100.6) | 550 (43.3) | 537 (151.4) | 437 (63.6) |
Notes. Reaction times represent correct go trials and incorrect no-go trials.
Table 2.
Means and standard deviations (in parentheses) for ERP waveform characteristics (amplitude and latency), by trial type and speed condition.
| Component Region Laterality |
Go |
No-go |
||
|---|---|---|---|---|
| Slow | Fast | Slow | Fast | |
| N2 Amplitude (µV) | ||||
| Frontal | ||||
| Midline | −5.07 (4.67) | −5.71 (4.19) | −4.53 (5.57) | −4.91 (5.00) |
| Right | −5.18 (4.94) | −4.78 (2.82) | −4.52 (6.23) | −4.62 (3.39) |
| Left | −5.01 (5.47) | −5.72 (4.49) | −5.74 (7.02) | −7.74 (4.44) |
| Frontocentral | ||||
| Midline | −5.21 (4.99) | −4.81 (3.30) | −4.44 (6.26) | −4.63 (4.58) |
| Right | −4.13 (4.44) | −4.22 (3.33) | −2.85 (7.28) | −3.30 (3.66) |
| Left | −4.28 (4.33) | −4.89 (3.55) | −5.09 (6.34) | −6.93 (4.38) |
| Central | ||||
| Midline | −.97 (5.34) | −1.86 (3.49) | −1.99 (3.32) | −.68 (6.71) |
| Right | −.81 (3.80) | −2.07 (4.10) | −.39 (6.85) | −.31 (3.60) |
| Left | −1.67 (3.72) | −3.43 (3.43) | −1.90 (5.51) | −4.39 (3.49) |
| N2 Latency (ms) | ||||
| Frontal | ||||
| Midline | 400 (67.1) | 384 (71.2) | 427 (63.1) | 429 (51.6) |
| Right | 378 (48.6) | 347 (34.9) | 408 (58.5) | 355 (33.9) |
| Left | 402 (68.9) | 393 (75.8) | 436 (66.7) | 422 (58.2) |
| Frontocentral | ||||
| Midline | 365 (44.1) | 365 (44.9) | 383 (53.2) | 396 (58.9) |
| Right | 361 (34.5) | 348 (36.3) | 378 (51.2) | 351 (40.0) |
| Left | 378 (63.1) | 379 (66.7) | 415 (67.8) | 403 (62.5) |
| Central | ||||
| Midline | 356 (46.7) | 357 (25.6) | 359 (48.6) | 362 (29.6) |
| Right | 363 (37.3) | 347 (30.4) | 370 (61.0) | 346 (31.4) |
| Left | 367 (45.5) | 353 (34.4) | 365 (30.3) | 366 (51.3) |
| P3 Amplitude (µV) Parietal | ||||
| Midline | 11.30 (8.83) | 9.84 (6.16) | 14.12 (7.73) | 11.88 (5.06) |
| Right | 12.71 (9.72) | 9.38 (5.73) | 15.14 (8.46) | 12.96 (3.96) |
| Left | 8.32 (4.41) | 6.27 (5.91) | 11.52 (6.99) | 7.86 (5.78) |
| P3 Latency (ms) Parietal | ||||
| Midline | 344 (45.5) | 382 (84.3) | 372 (75.1) | 380 (65.8) |
| Right | 398 (88.3) | 401 (84.4) | 383 (64.3) | 411 (75.3) |
| Left | 401 (63.6) | 449 (101.9) | 406 (57.4) | 461 (84.6) |
Figure 3.
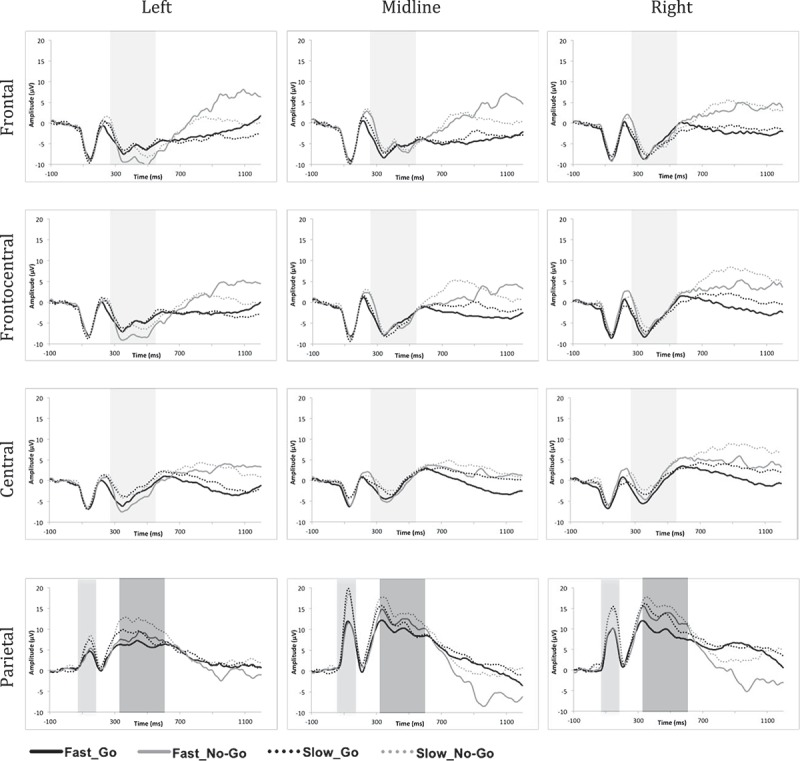
Grand-averaged ERP waveforms separated by region (Frontal, Frontocentral, Central and Parietal) and laterality (Left, Midline and Right). Time windows selected for the analyses of the N2, P1 and P3 are displayed in different shades of gray. N2 (light gray, top three panels) was analyzed between 260–560 ms after stimulus onset at frontal, frontocentral and central electrode sites. P1 (light gray, bottom panel) was analyzed between 60–150 ms and P3 (dark gray, bottom panel) was analyzed between 310–610 ms after stimulus onset at parietal electrode sites.
Behavioral performance
Accuracy and RT were analyzed using speed condition (Fast, Slow) x trial type (Go, No-go) mixed ANOVAs with repeated measures on trial type. For accuracy, there was a main effect of speed condition (F(1,29) = 21.2, p < .001, η2 = .420) and a statistically significant interaction between speed condition and trial type (F(1, 29) = 9.15, p < .01, η2 = .240). Overall, children in the Fast condition responded less accurately (M = .87, SD = .01) than children in the Slow condition (M = .94, SD = .01). Follow-up analysis of the interaction effect (F(1, 29) = 12.08, p < .01, η2 = .294) showed that only in the Slow condition, children had greater accuracy on Go trials (M = .98, SD = .01) than on No-go trials (M = .91, SD = .02). Examined another way, the speed condition effect was significant only for Go trials (F(1, 29) = 32.92, p < .01, η2 = .532): children in the Fast condition performed less accurately (M = .87, SD = .01) than children in the Slow condition (M = .98, SD = .01).
Analyses of RT revealed main effects of speed condition (F(1, 32) = 14.3, p < .005, η2 = .331) and trial type (F(1, 32) = 33.5, p < .001, η2 = .536). Across both trial types, children in the Fast condition responded more quickly (M = 493.9 ms, SD = 19.64) than children in the Slow condition (M = 600.8 ms, SD = 20.28), as expected given the time pressure manipulation. Errors of commission on No-go trials (M = 487.3 ms, SD = 20.61) were characterized by shorter RTs than correct Go trials (M = 607.4 ms, SD = 13.75). This finding, characteristic of inhibitory failures, follows the predictions of the horse race model, indicating the earlier completion of the Go process in No-go trials as the underlying reason for inhibitory failures. The interaction between trial type and speed condition was not significant (p > .05).
ERP amplitude and latency
P1 amplitude and latency were analyzed using speed condition (Fast, Slow) x trial type (Go, No-go) x laterality (Left, Midline, Right) mixed ANOVAs with repeated measures on trial type and laterality. For P1 amplitude, there were main effects of speed condition (F(1, 29) = 4.26, p < .05, η2 = .128) and laterality (F(2, 58) = 42.23, p < .001, η2 = .593). P1 amplitude was greater for children in the Slow condition (M = 7.3 µV, SD = .86) than children in the Fast condition (M = 4.9 µV, SD = .84). It was also greater at the midline electrodes (M = 8.5 µV, SD = .73) than at the right (M = 6.8 µV, SD = .81) or left (M = 3.0 µV, SD = .53) electrodes and greater at the right (M = 6.8 µV, SD = .81) than left (M = 3.0 µV, SD = .53) electrodes. No other main effects or interactions were significant.
For P1 latency, there was a main effect of laterality (F(2, 58) = 42.23, p < .001, η2 = .593). P1 latency was earlier in the left electrodes (M = 118.8 ms, SD = 3.9) than at the right electrodes (M = 128.6 ms, SD = 1.8). P1 latency at midline electrodes (M = 125.7 ms, SD = 1.8) did not differ from right or left electrodes. No other main effects or interactions were significant.
N2 amplitude and latency were analyzed using speed condition (Fast, Slow) x trial type (Go, No-go) x region (Frontal, Frontocentral, Central) x laterality (Left, Midline, Right) mixed ANOVAs with repeated measures on trial type, region, and laterality. For amplitude, there was a main effect of region (F(2, 58) = 34.5, p < .001, η2 = .544), an interaction between trial type and laterality (F(2, 58) = 5.3., p < .01, η2 = .154) and a three-way interaction between trial type, laterality and region (F(4, 116) = 4.2., p < .01, η2 = .127). N2 amplitude was significantly greater at frontal electrodes (M = −5.3 µV, SD = .68) than at frontocentral (M = −4.6 µV, SD = .63) or central electrodes (M = −1.6 µV, SD = .65) and it was significantly greater at frontocentral (M = −4.6 µV, SD = .63) than at central electrodes (M = −1.6 µV, SD = .65). At left electrodes only (F(1, 29) = 6.7., p < .05, η2 = .188), N2 amplitude was greater on No-go trials (M = −5.3 µV, SD = .88) than Go trials (M = −4.2 µV, SD = .68), whereas at midline and right electrodes, amplitude did not differ by trial type (p > .05). Finally, follow-up tests of the three-way interaction between trial type, laterality and region showed that the increased No-go amplitude at left electrodes was only significant at frontal (F(1, 29) = 5.9., p < .05, η2 = .169) (Go: M = −5.4 µV, SD = .90; No-go: M = −6.7 µV, SD = 1.0) and frontocentral regions (F(1, 29) = 7.7., p < .01, η2 = .211) (Go: M = −4.6 µV, SD = .71; No-go: M = −6.0 µV, SD = .97). N2 amplitude in left electrodes at the central region did not differ between Go and No-go trials (p > .05). There were no significant effects involving speed condition, and no other main effects or interactions were significant.
For N2 latency, there were main effects of trial type (F(1, 29) = 15.0, p < .01, η2 = .341), region (F(1.4, 33.4) = 23.8, p < .001, η2 = .451) and laterality (F(1.5, 44.2) = 7.4, p < .01, η2 = .203). N2 peaked later on No-go trials (M = 387.4 ms, SD = 5.6) than on Go trials (M = 369.0 ms, SD = 5.1) and at frontal (M = 398.4 ms, SD = 7.2) than at frontocentral (M = 376.8 ms, SD = 6.2) or central (M = 359.3 ms, SD = 3.4) regions. N2 latency was also later at left (M = 389.9 ms, SD = 7.6) and midline (M = 382.0 ms, SD = 6.2) electrodes than at right (M = 362.7 ms, SD = 5.2) electrodes. These main effects were qualified by interactions between trial type and region (F(2, 58) = 6.8, p < .01, η2 = .189) and between laterality and region (F(3, 71.8) = 4.8, p < .01, η2 = .141). The N2 peaked later on No-go trials than Go trials in the frontal (F(1, 29) = 17.2., p < .01, η2 = .372) (Go: (M = 383.8 ms, SD = 8.2); No-go: (M = 412.9 ms, SD = 7.9)) and frontocentral electrodes (F(1, 29) = 10.0., p < .01, η2 = .256) (Go: (M = 366 ms, SD = 6.4); No-go: (M = 387.7 ms, SD = 7.7)), but not at central electrodes (p > .05). Furthermore, N2 peaked later at left and midline leads relative to right leads in the frontal (F(2, 28) = 8.9., p < .01, η2 = .389) (left: (M = 413.1 ms, SD = 10.1); midline: (M = 409.9 ms, SD = 10.4); right: (M = 372.1 ms, SD = 6.7)) and frontocentral regions (F(2, 28) = 4.3., p < .05, η2 = .234) (left: (M = 377.3 ms, SD = 7.7); midline: (M = 393.7, SD = 10.4); right: (M = 359.6 ms, SD = 5.7)). In the central region, N2 latency did not significantly differ between left, right and midline leads (p > .05). Again, there were no significant effects involving speed condition, and no other main effects or interactions were significant.
P3 amplitude and latency were analyzed using speed condition (Fast, Slow) x trial type (Go, No-go) x laterality (Left, Midline, Right) mixed ANOVAs with repeated measures on trial type and laterality. Analyses of P3 amplitude revealed main effects of trial type (F(1, 29) = 13.0, p < .01, η2 = .310) and laterality (F(1.6, 45.8) = 7.0, p < .01, η2 = .195). P3 amplitude was greater on No-go trials (M = 12.3 µV, SD = .92) than Go trials (M = 9.6 µV, SD = 1.1). Topographically, P3 amplitude was greater in midline (M = 11.8 µV, SD = 1.2) and right electrodes (M = 12.6 µV, SD = 1.2) than in left electrodes (M = 8.5 µV, SD = 1.0).
For P3 latency, there was a main effect of laterality (F(2,58) = 12.1, p < .01, η2 = .195). The P3 latency was significantly earlier in the midline electrodes (M = 369.6 ms, SD = 11.2) than in the right (M = 398.6 ms, SD = 13.1) or the left electrodes (M = 429.4 ms, SD = 12.8) and significantly earlier in the right electrodes (M = 398.6 ms, SD = 13.1) than in the left electrodes (M = 429.4 ms, SD = 12.8). There were no significant effects involving speed condition for both P3 amplitude and latency. No other main effects or interactions were significant.
Discussion
We sought to examine the neural correlates of successful response inhibition in early childhood, and how response inhibition is modulated under conditions of time pressure. Children completed a GNG task where they had to inhibit responding on the less frequent No-go trials. The time pressure manipulation affected children’s task performance: children in the Fast condition responded faster but less accurately than children in the Slow condition. In addition, children in the Slow condition displayed greater accuracy on Go trials than on No-go trials, whereas children in the Fast condition performed equivalently on Go and No-go trials. The time pressure manipulation also affected early ERP activity, as the P1 was greater for children in the Slow condition than the Fast condition. However, ERP markers of response inhibition did not differ between the Fast and Slow conditions. Across both conditions, relative to Go trials, No-go trials elicited a left-lateralized enhanced N2 and an enhanced P3 at midline electrode sites.
The enhanced N2 observed on No-go trials as compared to Go trials is consistent with the literature, and typically thought to reflect neural activity underlying response inhibition. Alongside amplitude effects, we found parallel differences in N2 latency, with longer N2 latencies for No-go trials and at left frontal electrodes. However, in adults N2 differences are typically observed at midline (Bekker, Kenemans, & Verbaten, 2005; Jonkman et al., 2007) or right (Bokura et al., 2001) electrode sites, whereas in our study the effect was left lateralized. Interestingly, in a previous study with 7- to 16-year-olds, both younger participants and participants who performed poorly exhibited greater left lateralization of the No-go N2 (Lamm, Zelazo, & Lewis, 2006). This suggests that there may be reorganization of networks underlying response inhibition, with the shift from the left-lateralized to midline and right-lateralized activity indicating a more mature neural network. Another alternative is that the left lateralized N2 indicates that children employ a different strategy to perform the task. There is evidence to indicate that children frequently employ verbal strategies to perform cognitive tasks that are inherently nonverbal (Berk, 1992; Winsler & Naglieri, 2003), which would presumably lead to greater reliance on the left hemisphere. For example, in the present study children may have used verbal labeling or self-talk as a strategy to withhold a button press on No-go trials. In a neuroimaging study, 9- to 12-year-old children performing a cognitive control task displayed greater activity in the left hemisphere, and verbal ability was correlated with performance (Bunge et al., 2002). Future research should examine how children’s use of different strategies on cognitive control tasks influences their performance as well as the neural resources recruited to perform them.
We also found an enhanced No-go P3; that is, the P3 was more pronounced on No-go trials than on Go trials (Bokura et al., 2001), although there were no P3 latency differences by trial type. However, as with the N2, the topography of the enhanced No-go P3 observed in our study differed markedly from studies of adults. In our study of early childhood, the No-go P3 was observed at posterior midline electrode sites, whereas in adults, the enhanced No-go P3 is typically seen at frontal midline electrode sites, a phenomenon referred to as the “No-go anteriorization” (Fallgatter & Strik, 1999). At posterior electrode sites, adults show a more pronounced Go P3 than No-go P3 reflecting attention to targets (Bruin, Wijers, & Van Staveren, 2001). A posterior P3 is also seen in adults in the oddball task indicating the processing of infrequent targets (Friedman, Cycowicz, & Gaeta, 2001; Gaeta, Friedman, & Hunt, 2003). One might question whether the P3 effect in the present study is an oddball effect; however, we believe it is unlikely to be a result of infrequent target probability, as individual Go and No-go stimuli were presented with equal frequency. We argue that the difference in the topography of the No-go P3 found in our study could indicate children’s reliance on additional posterior brain regions to support response inhibition. Similar findings have been observed in middle childhood with regard to the N2 (Jonkman et al., 2007). Using source localization methods, Jonkman and colleagues found that the neural activity underlying response inhibition in adults was adequately explained by frontal sources, but in children, contributions from additional posterior sources were required. Supporting evidence also comes from studies utilizing brain imaging techniques that have shown that as children develop, the neural networks controlling inhibitory processes shift from a more posterior, distributed pattern to a more frontal, localized one (Bunge et al., 2002; Casey, Thomas, Davidson, Kunz, & Franzen, 2002; Durston et al., 2006).
Considered together, our ERP findings suggest that the neural correlates underlying response inhibition in early childhood differ in important ways from those seen in adulthood. Consistent with the adult literature, both the N2 and the P3 were more pronounced on No-go trials than on Go trials in 5-year old children. However, the topography of these No-go effects differed markedly, suggesting that the brain regions supporting response inhibition in early childhood differ from those in adults.
It is sometimes argued that the enhanced N2 and P3 amplitudes seen on No-go trials are a result of motor related neural activity rather than a reflection of inhibition. This argument is based on the grounds that unlike Go trials, No-go trials do not require a motor response and that this disparity could explain the amplitude differences observed in both ERP components. However, given that motor preparation is typically associated with a negative-going response (Shibasaki, Barrett, Halliday, & Halliday, 1980), we should have observed an increased N2 amplitude on Go trials. Our findings, in contrast, show increased N2 amplitude on No-go trials making its association with inhibitory processes a more tenable explanation. Similarly, attributing the No-go P3 to motor related activity can be ruled out based on the findings of studies where an enhanced No-go P3 was found despite eliminating motor demands from the task (Smith, Johnstone, & Barry, 2008). However, it should be noted that these studies have typically looked at adults, so future studies should examine motoric contributions to No-go ERP effects in children, particularly given topographic differences between the No-go P3 in children and adults.
We experimentally manipulated time pressure by giving children in the Fast condition a shorter time window in which they could make a response. We expected this manipulation to increase the prepotency of responding, leading to greater inhibitory demands on No-go trials. However, examination of our accuracy findings showed that despite their faster response times, children in the Fast condition did not appear to display prepotent responding, in that their No-go performance was equivalent to children in the Slow condition. Rather, differences between the conditions emerged only on Go trials, where children in the Fast condition made more errors. One possible explanation for the differences in accuracy between the Fast and Slow conditions is that the time pressure manipulation resulted in differences in attentional engagement on the task. This suggestion is consistent with the finding that the parietal P1 component was more pronounced in the Slow condition than in the Fast condition. Greater P1 amplitude is typically associated with heightened selective attention or higher levels of vigilance (Key, Dove, & Maguire, 2005). Hence, it is possible that the lower Go accuracy observed in the Fast condition was due to the time pressure manipulation interfering with children’s ability to recruit or sustain attentional resources. However, another possibility is that this difference is due to children’s inability to respond quickly enough. To test this possibility, we examined the Go reaction time distribution of children in the Fast condition to evaluate whether they were truncated. All children responded between 400–500 ms on a majority of Go trials and, with one exception, RTs greater than 650 ms comprised less than 20% of each child’s RTs distribution. This suggests that children successfully adjusted their speed of responding to the time pressure manipulation, and the behavioral differences between the conditions were not simply a result of the children in the Fast condition having insufficient time to respond. Behavioral differences could also indicate that children in the Fast condition adopted a cautious strategy, placing a higher priority on not catching the sharks on No-go trials, at the expense of missing more fish on Go trials.
One might ask whether the P1 difference between the Fast and Slow condition affected the other ERP findings. Notably, this difference involved only the speed condition factor, with no hint of a main effect or interaction involving trial type. The converse was true for the N2 and P3, which differed between Go and No-go trials but were not modulated by the speed condition manipulation. Therefore, although there were indications that early attentional processes were affected by the speed condition manipulation, it seems unlikely that these differences contributed to later inhibitory processes.
An important limitation of this study is its between-subject design. This was necessary to prevent training effects and exposure to the task. Furthermore, the short attention span of young children would have made it hard for them to participate in both versions of the task, given the high number of trials required in each condition for ERP studies. Children were randomly assigned to the two conditions to minimize the possible confounding effects of unrelated third variables; however, sampling error may have resulted in differences in the makeup up of the children in the two conditions that may have contributed to the findings. Unfortunately, measures of IQ and processing speed were not administered and information on parental education levels was not collected to allow us to assess and control for possible confounding differences.
In order to make the task appropriate for young children, it was designed with fish as the Go stimuli and sharks as the No-go stimuli. However, because stimuli were drawn from two distinct categories, any differences between the stimulus sets could have contributed to observed differences between Go and No-go trials (for example, salience). A supplementary analysis (see Note 1) indicated that behavioral findings from our study remained unchanged after taking into consideration differences in stimulus salience. Furthermore, as the Go stimuli were more colorful, differences in salience should have resulted in a more pronounced N2 for the Go trials, whereas our findings showed the reversed pattern, a more pronounced N2 for No-go trials.
Another limitation of this study is our inability to examine the error-related negativity (ERN)—the neural correlate associated with error detection and monitoring. While several other studies utilizing similar task designs have examined ERN (Torpey et al., 2012, 2009), we were unable to do so in this study because the children made few errors. However, analyzing ERN could potentially give us additional insights into the development of response inhibition abilities in early childhood and future studies should undertake such an investigation.
We ventured to understand the neural correlates underlying response inhibition in early childhood, a period critical for the development of the neural networks underlying higher order cognitive processes like response inhibition. Few studies have examined the neural correlates underlying response inhibition in early childhood. We found both similarities and differences in the pattern of brain activity underlying response inhibition in early childhood compared with that seen in adults. Response inhibition was associated with an enhanced left-lateralized frontal N2 and a midline posterior P3, differing topographically from patterns in adults. These differences may suggest that the immaturity of inhibitory brain networks may result in children’s recruitment of additional, different brain regions to perform response inhibition tasks.
Notes
To make the rules of the task easy for children to understand, two different categories of stimuli (fish, sharks) were used on Go and No-go trials. However, this may have introduced differences between the Go and No-go trials, for example in stimulus salience. To investigate whether the fish and shark stimuli differed in salience, we administered a target detection task to an adult sample (n = 6). This study identified two stimuli as outliers: one fish was .53 standard deviations above the mean in salience and one shark was .52 standard deviations below the mean. We re-analyzed the behavioral data using repeated measures ANOVA excluding these two stimuli. For accuracy, there was a main effect of speed condition (F(1,29) = 12.33, p < .01, η² = .298) and an interaction between speed condition and trial type (F(1, 29) = 15.22, p < .01, η² = .344). For RT, there were main effects of speed condition (F(1, 29) = 68.0, p < .001, η² = .701) and trial type (F(1, 29) = 1381.48, p < .001, η² = .979). As the pattern of findings did not differ from those including the complete stimulus set, the latter are reported in the Results section.
Because we expected children in the Fast condition to make more errors, we had them complete a greater number of trials. To test whether this difference in procedure affected study findings, we analyzed the behavioral data using repeated measures ANOVA excluding the last block of trials from children in the Fast condition so that both conditions contributed an equal number of trials. For accuracy, there was a main effect of speed condition (F(1,29) = 17.25, p < .001, η² = .373) and an interaction between speed condition and trial type (F(1, 29) = 13.53, p < .01, η² = .318). For RT, there were main effects of speed condition (F(1, 29) = 17.94, p < .001, η² = .382) and trial type (F(1, 29) = 33.59, p < .001, η² = .537). As the pattern of findings did not differ from those including the complete stimulus set, the latter are reported in the Results section.
References
- Aron A. R., Robbins T. W., & Poldrack R. A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8(4), 170–177. doi: 10.1016/j.tics.2004.02.010 [DOI] [PubMed] [Google Scholar]
- Band G. P., Van Der Molen M. W., Overtoom C. C., & Verbaten M. N. (2000). The ability to activate and inhibit speeded responses: Separate developmental trends. Journal of Experimental Child Psychology, 75(4), 263–290. doi: 10.1006/jecp.1999.2538 [DOI] [PubMed] [Google Scholar]
- Bekker E. M., Kenemans J. L., & Verbaten M. N. (2005). Source analysis of the N2 in a cued Go/NoGo task. Cognitive Brain Research, 22(2), 221–231. doi: 10.1016/j.cogbrainres.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Berk L. E. (1992). Children’s private speech: An overview of theory and the status of research In Diaz R. M., & Berk L. E. (Eds.), Private speech: From social interaction to self-regulation (pp. 17–53). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Blair C., & Razza R. P. (2007). Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development, 78(2), 647–663. doi: 10.1111/desc.12330 [DOI] [PubMed] [Google Scholar]
- Bokura H., Yamaguchi S., & Kobayashi S. (2001). Electrophysiological correlates for response inhibition in a Go/NoGo task. Clinical Neurophysiology, 112(12), 2224–2232. doi: 10.1016/S1388-2457(01)00691-5 [DOI] [PubMed] [Google Scholar]
- Bruin K. J., Wijers A. A., & Van Staveren A. S. J. (2001). Response priming in a go/nogo task: Do we have to explain the go/nogo N2 effect in terms of response activation instead of inhibition? Clinical Neurophysiology, 112(9), 1660–1671. doi: 10.1016/S1388-2457(01)00601-0 [DOI] [PubMed] [Google Scholar]
- Brydges C. R., Anderson M., Reid C. L., & Fox A. M. (2013). Maturation of cognitive control: Delineating response inhibition and interference suppression. PLoS One, 8(7), e69826. doi: 10.1371/journal.pone.0069826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S. A., Dudukovic N. M., Thomason M. E., Vaidya C. J., & Gabrieli J. D. (2002). Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron, 33(2), 301–311. doi: 10.1016/S0896-6273(01)00583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. S., & Van Veen V. (2007). Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective and Behavioral Neuroscience, 7(4), 367–379. doi: 10.3758/CABN.7.4.367 [DOI] [PubMed] [Google Scholar]
- Carver A. C., Livesey D. J., & Charles M. (2001). Age related changes in inhibitory control as measured by stop signal task performance. International Journal of Neuroscience, 107(1–2), 43–61. doi: 10.3109/00207450109149756 [DOI] [PubMed] [Google Scholar]
- Casey B. J., Thomas K. M., Davidson M. C., Kunz K., & Franzen P. L. (2002). Dissociating striatal and hippocampal function developmentally with a stimulus–response compatibility task. The Journal of Neuroscience, 22(19). Retrieved from www.jneurosci.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. J., Trainor R. J., Orendi J. L., Schubert A. B., Nystrom L. E., Giedd J. N., … Forman S. D. (1997). A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience, 9(6), 835–847. doi: 10.1162/jocn.1997.9.6.835 [DOI] [PubMed] [Google Scholar]
- Chevalier N., Kelsey K. M., Wiebe S. A., & Espy K. A. (2014). The temporal dynamic of response inhibition in early childhood: An ERP study of partial and successful inhibition. Developmental Neuropsychology, 39(8), 585–599. doi: 10.1080/87565641.2014.973497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski K. T., Harris R. J., & Cofer L. F. (2004). Posterior brain ERP patterns related to the go/no‐go task in children. Psychophysiology, 41(6), 882–892. doi: 10.1111/j.1469-8986.2004.00250.x [DOI] [PubMed] [Google Scholar]
- Clark J. M. (1996). Contributions of inhibitory mechanisms to unified theory in neuroscience and psychology. Brain and Cognition, 30, 127–152. doi: 10.1006/brcg.1996.0008 [DOI] [PubMed] [Google Scholar]
- Cragg L., & Nation K. (2008). Go or no‐go? Developmental improvements in the efficiency of response inhibition in mid‐childhood. Developmental Science, 11(6), 819–827. doi: 10.1111/j.1467-7687.2008.00730.x [DOI] [PubMed] [Google Scholar]
- Davis E. P., Bruce J., Snyder K., & Nelson C. A. (2003). The X-trials: Neural correlates of an inhibitory control task in children and adults. Journal of Cognitive Neuroscience, 15(3), 432–443. doi: 10.1162/089892903321593144 [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M. C., Tottenham N., Galvan A., Spicer J., Fossella J. A., & Casey B. J. (2006). A shift from diffuse to focal cortical activity with development. Developmental Science, 9(1), 1–8. doi: 10.1111/j.1467-7687.2005.00454.x [DOI] [PubMed] [Google Scholar]
- Durston S., Thomas K. M., Yang Y., Uluğ A. M., Zimmerman R. D., & Casey B. J. (2002). A neural basis for the development of inhibitory control. Developmental Science, 5(4), F9–F16. doi: 10.1111/1467-7687.00235 [DOI] [Google Scholar]
- Eimer M. (1993). Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biological Psychology, 35(2), 123–138. doi: 10.1016/0301-0511(93)90009-W [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., & Hohnsbein J. (1999). ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica, 101(2–3), 267–291. doi: 10.1016/S0001-6918(99)00008-6 [DOI] [PubMed] [Google Scholar]
- Fallgatter A. J., & Strik W. K. (1999). The NoGo-anteriorization as a neurophysiological standard-index for cognitive response control. International Journal of Psychophysiology, 32(3), 233–238. doi: 10.1016/S0167-8760(99)00018-5 [DOI] [PubMed] [Google Scholar]
- Farbiash T., & Berger A. (2015). Brain and behavioral inhibitory control of kindergartners facing negative emotions. Developmental Science, 1–16. doi: 10.1111/desc.12330 [DOI] [PubMed] [Google Scholar]
- Friedman D., Cycowicz Y. M., & Gaeta H. (2001). The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and Biobehavioral Reviews, 25(4), 355–373. doi: 10.1016/S0149-7634(01)00019-7 [DOI] [PubMed] [Google Scholar]
- Fuster J. M. (2002). Frontal lobe and cognitive development. Journal of Neurocytology, 31(3–5), 373–385. doi: 10.1023/A:1024190429920 [DOI] [PubMed] [Google Scholar]
- Gaeta H., Friedman D., & Hunt G. (2003). Stimulus characteristics and task category dissociate the anterior and posterior aspects of the novelty P3. Psychophysiology, 40(2), 198–208. doi: 10.1111/1469-8986.00022 [DOI] [PubMed] [Google Scholar]
- Garon N., Bryson S. E., & Smith I. M. (2008). Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin, 134(1), 31–60. doi: 10.1037/0033-2909.134.1.31 [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M. G., & Donchin E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. doi: 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Haith M. M., Hazan C., & Goodman G. S. (1988). Expectation and anticipation of dynamic visual events by 3.5-month-old babies. Child Development, 59(2), 467–479. doi: 10.2307/1130325 [DOI] [PubMed] [Google Scholar]
- Hoyniak C. (2017). Changes in the NoGo N2 event-related potential component across childhood: A systematic review and meta-analysis. Developmental Neuropsychology, 42(1), 1–24. doi: 10.1080/87565641.2016.1247162 [DOI] [PubMed] [Google Scholar]
- Jodo E., & Kayama Y. (1992). Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalography and Clinical Neurophysiology, 82(6), 477–482. doi: 10.1016/0013-4694(92)90054-L [DOI] [PubMed] [Google Scholar]
- Johnstone S. J., Pleffer C. B., Barry R. J., Clarke A. R., & Smith J. L. (2005). Development of inhibitory processing during the go/nogo task. Journal of Psychophysiology, 19(1), 11–23. doi: 10.1027/0269-8803.19.1.11 [DOI] [Google Scholar]
- Jonkman L. M., Lansbergen M., & Stauder J. E. A. (2003). Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology, 40, 752–761. doi: 10.1111/1469-8986.00075 [DOI] [PubMed] [Google Scholar]
- Jonkman L. M., Sniedt F. L. F., & Kemner C. (2007). Source localization of the Nogo-N2: A developmental study. Clinical Neurophysiology, 118(5), 1069–1077. doi: 10.1016/j.clinph.2007.01.017 [DOI] [PubMed] [Google Scholar]
- Junghöfer M., Elbert T., Tucker D. M., & Braun C. (1999). The polar average reference effect: A bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology, 110(6), 1149–1155. doi: 10.1016/S1388-2457(99)00044-9 [DOI] [PubMed] [Google Scholar]
- Key A. P. F., Dove G. O., & Maguire M. J. (2005). Linking brainwaves to the brain: An ERP primer. Developmental Neuropsychology, 27(2), 183–215. doi: 10.1207/s15326942dn2702_1 [DOI] [PubMed] [Google Scholar]
- Kopp B., Mattler U., Goertz R., & Rist F. (1996). N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalography and Clinical Neurophysiology, 99(1), 19–27. doi: 10.1016/0921-884X(96)95617-9 [DOI] [PubMed] [Google Scholar]
- Lahat A., Todd R., Mahy C. E., Lau K., & Zelazo P. D. (2010). Neurophysiological correlates of executive function: A comparison of European-Canadian and Chinese-Canadian 5-year-olds. Frontiers in Human Neuroscience, 3, 72. doi: 10.3389/neuro.09.072.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Zelazo P. D., & Lewis M. D. (2006). Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia, 44(11), 2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013 [DOI] [PubMed] [Google Scholar]
- Lewis M. D., Lamm C., Segalowitz S. J., Stieben J., & Zelazo P. D. (2006). Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience, 18(3), 430–443. doi: 10.1162/jocn.2006.18.3.430 [DOI] [PubMed] [Google Scholar]
- Logan G. D., & Cowan W. B. (1984). On the ability to inhibit thought and action: A theory of an act of control. Psychological Review, 91(3), 295–327. doi: 10.1037/0033-295X.91.3.295 [DOI] [PubMed] [Google Scholar]
- Rubia K., Russell T., Overmeyer S., Brammer M. J., Bullmore E. T., Sharma T., … Taylor E. (2001). Mapping motor inhibition: Conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage, 13(2), 250–261. doi: 10.1006/nimg.2000.0685 [DOI] [PubMed] [Google Scholar]
- Shibasaki H., Barrett G., Halliday E., & Halliday A. M. (1980). Components of the movement-related cortical potential and their scalp topography. Electroencephalography and Clinical Neurophysiology, 49(3–4), 213–226. doi: 10.1016/0013-4694(80)90216-3 [DOI] [PubMed] [Google Scholar]
- Simpson A., & Riggs K. J. (2006). Conditions under which children experience inhibitory difficulty with a “button-press” go/no-go task. Journal of Experimental Child Psychology, 94(1), 18–26. doi: 10.1016/j.jecp.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Smith J. L., Johnstone S. J., & Barry R. J. (2008). Movement-related potentials in the Go/NoGo task: The P3 reflects both cognitive and motor inhibition. Clinical Neurophysiology, 119(3), 704–714. doi: 10.1016/j.clinph.2007.11.042 [DOI] [PubMed] [Google Scholar]
- Tamm L., Menon V., & Reiss A. L. (2002). Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry, 41(10), 1231–1238. doi: 10.1097/01.CHI.0000020272.43550.5E [DOI] [PubMed] [Google Scholar]
- Torpey D. C., Hajcak G., Kim J., Kujawa A., & Klein D. N. (2012). Electrocortical and behavioral measures of response monitoring in young children during a Go/No-Go task. Developmental Psychobiology, 54(2), 139–150. doi: 10.1002/dev.20590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey D. C., Hajcak G., & Klein D. N. (2009). An examination of error-related brain activity and its modulation by error value in young children. Developmental Neuropsychology, 34(6), 749–761. doi: 10.1080/87565640903265103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S. A., Sheffield T. D., & Espy K. A. (2012). Separating the fish from the sharks: A longitudinal study of preschool response inhibition. Child Development, 83(4), 1245–1261. doi: 10.1111/j.1467-8624.2012.01765.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeakumar S., Magnotta V. A., Buss A. T., Ambrose J. P., Wifall T. A., Hazeltine E., & Spencer J. P. (2015). Response control networks are selectively modulated by attention to rare events and memory load regardless of the need for inhibition. NeuroImage, 120, 331–344. doi: 10.1016/j.neuroimage.2015.07.026 [DOI] [PubMed] [Google Scholar]
- Winsler A., & Naglieri J. (2003). Overt and covert verbal problem‐solving strategies: Developmental trends in use, awareness, and relations with task performance in children aged 5 to 17. Child Development, 74(3), 659–678. doi: 10.1111/1467-8624.00561 [DOI] [PubMed] [Google Scholar]


