Abstract
Aim
The principal goal of this meta-analysis is to test the hypothesis that circulating total adiponectin or certain fractions may represent a promising biological candidate in modulating the risk of colorectal cancer.
Methods
The processes of paper identification, paper selection and data extraction were accomplished independently by two authors. Effect-size estimates were expressed as weighted mean difference (WMD) and 95% confidence interval (95% CI). A total of 31 papers including 48 qualified studies (7,554 patients with colorectal cancer and 9,798 controls) were meta-analyzed.
Results
Pooling all studies found that circulating total adiponectin was significantly lower in patients with colorectal cancer than in controls (WMD: −0.76 µg/mL, 95% CI: −1.20 to −0.32, p=0.001), with significant heterogeneity (I2: 94.2%) and low publication bias (Egger’s p=0.336). By adiponectin fractions, the difference in high-molecular weight (HMW) adiponectin was comparable between the two groups (WMD: −0.22 µg/mL, 95% CI: −0.70 to 0.25, p=0.350), while non-HMW adiponectin was significantly lower in patients with colorectal cancer than in controls (WMD: −0.27 µg/mL, 95% CI: −0.35 to −0.19, p<0.001), with marginal heterogeneity (I2: 52.3%). Subgroup analysis revealed that effect-size estimates were heterogeneous when grouping studies by cancer subtype, region, study design, matching status, gender and obesity. Further meta-regression analysis indicated that age and gender were significant potential sources of heterogeneity. The results showed the studied subgroups were not subject to publication bias (Egger’s p<0.1).
Conclusion
Our data collectively indicate that low circulating total adiponectin, especially its non-HMW fraction, represents a promising risk factor for colorectal cancer. Further studies are needed to explore underlying mechanisms.
Keywords: colorectal cancer, total adiponectin, high-molecular weight adiponectin, non-high-molecular weight adiponectin, risk factor
Introduction
Adiponectin of molecular mass 28 kDa is a member of the adipocytokines, and it is secreted exclusively by mature adipocytes.1 Biologically speaking, adiponectin possesses a well-defined set of properties, including insulin sensitization, anti-inflammation, anti-atherosclerosis, proapoptosis and antiproliferation.2,3 In circulation, adiponectin exists in forms of trimer, hexamer and high-molecular weight (HMW) complex, and these fractions are proven to have different biological activities.4 For example, HMW adiponectin has a close relationship to insulin sensitivity, while the relevance between non-HMW adiponectin and anti-inflammation is more obvious.5 Mounting evidence from human studies reveal that low total adiponectin in circulation represents a predisposing status for development of colorectal cancer.6–8 By contrast, other researchers failed to confirm this claim,9–11 and some of them even found a higher concentration of circulating total adiponectin in patients with colorectal cancer than in controls.12,13 Several systematic reviews and meta-analyses have summarized published data involving the association between circulating total adiponectin and colorectal cancer risk,14–20 while interpretation of pooled findings in most cases is clouded by the presence of substantial heterogeneity and none considered adiponectin fractions as separate factors. With growing epidemiological data, our understanding on circulating adiponectin and its fractions and colorectal cancer needs replenishment and renewal. Hence, the principal goal of the present study is to test the hypothesis that circulating total adiponectin or certain fractions may represent a promising biological candidate in modulating the risk of colorectal cancer, assessed through a comprehensive meta-analysis. A secondary goal is to explore possible sources of heterogeneity of effect size across studies through both subgroup analysis and meta-regression analysis.
Methods
Guideline
The present study was carried out in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.21
Paper identification
Potentially eligible papers were identified through searching public databases of MEDLINE (PubMed), EMBASE, Web of Knowledge and Scholar (Google). All identified papers from each database were merged together and then duplicates removed. The key items used for paper identification included (“colorectal” or “colon” or “rectal”) in title and (“cancer” or “carcinoma” or “tumor” or “tumour” or “neoplasm” or “adenoma”) in title and (“adiponectin” or “adipocytokine” or “adipokine”) in title/abstract. The process of paper identification was completed independently and in duplicate (Weiqun Lu and Zhiliang Huang) using the same key items mentioned above.
Eligibility criteria
Eligibility criteria consisted of both inclusion criteria and exclusion criteria. Papers were included only when the following conditions were met simultaneously: (i) publication using the English language; (ii) either cross-sectional or nested case–control study design; (iii) involvement of only human beings; and (iv) availability of circulating plasma or serum adiponectin concentration for patients with colorectal or colon or rectal cancer and cancer-free controls. Papers were excluded when any of the following conditions was true: (i) non-original contribution; (ii) narrative or systematic review or meta-analysis; (iii) conference abstract; and (iv) case report or case series.
Paper selection
Two authors (Weiqun Lu and Zhiliang Huang) selected the papers that met the above inclusion criteria through reviewing the title or abstract or the full text, if necessary, of each identified article. When disagreement happened during the selection process, it was resolved by discussion or seeking help from the third author (Haiying Liu).
Data extraction
From each qualified paper, data for analysis including, if available, the first author’s name, publication year, country where the study was conducted, study design, cancer subtype, matching status, source of controls, selection of controls, sample size, assay method for circulating adiponectin, sample type, age, gender, body mass index (BMI), waist–hip ratio (WHR), waist circumference, physical activity, physical inactivity, smoking habit, family history, diabetes mellitus, glucose, insulin, C-reactive protein (CRP) and adiponectin were extracted and entered into databases by two authors (Weiqun Lu and Zhiliang Huang). Extracted data were compared, and disagreement was resolved through consensus and discussion.
Statistical analysis
Data were statistically analyzed by the Stata/SE software version 14.0 (StataCorp LP, College Station, TX, USA). Weighted mean difference (WMD) was adopted to assess the difference in circulating adiponectin concentration between patients and controls in both overall and subgroup analyses, and its 95% confidence interval (95% CI) was estimated accordingly under the random-effects model based on the DerSimonian–Laird method. Heterogeneity was first evaluated with the I2 statistic, and then was explored through both subgroup and meta-regression analyses. The I2 statistic is expressed as a percentage figure, and the higher the figure, the more likely the heterogeneity becomes. It is widely accepted the significance cutoff point of I2 statistic is set at 50%. Publication bias was assessed by the Begg’s funnel plot, filled funnel plot and Egger’s regression asymmetry test. The Egger’s regression asymmetry test records significance if the probability value is <10%.
Results
Qualified studies
A total of 339 papers were identified through searching public databases, and 295 of them were excluded after reading the title and abstract. Further, after full-text reviewing, 13 papers were excluded as they failed to meet our predefined inclusion criteria, leaving 31 eligible papers for meta-analysis in this study.5–13,22–43 Thereof, 16 papers provided data by cancer type, gender or race, and so there were 48 qualified studies.
Study characteristics
Table 1 shows the baseline characteristics of all qualified studies. After removing shared controls, this meta-analysis involved 7,554 patients with colorectal cancer and 9,798 controls. Of 48 qualified studies, only one study focused on HMW adiponectin.39 In addition, eight studies also measured HMW adiponectin, and non-HMW adiponectin was recorded in six studies.
Table 1.
The baseline characteristics of all qualified studies
| Study | Year | Region | Cancer subtype | Control features | Study design | Matching status | ADI assay (ELISA) | Sample size
|
Sample source | Adiponectin (µg/mL)
|
Age (years)
|
Male gender
|
BMI (kg/m2)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | |||||||||
| Saetang et al25 (CRC) | 2016 | East Asia | CRC | W/o colonic polyps | Retrospective | NA | Thermo Fisher | 82 | 30 | Serum | 17.56 | 19.49 | 62.4 | 59.1 | NA | NA | NA | NA |
| Saetang et al25 (colon) | 2016 | East Asia | Colon | W/o colonic polyps | Retrospective | NA | Thermo Fisher | 52 | 30 | Serum | 15.65 | 19.49 | 62.4 | 59.1 | NA | NA | NA | NA |
| Saetang et al25 (rectum) | 2016 | East Asia | Rectum | W/o colonic polyps | Retrospective | NA | Thermo Fisher | 30 | 30 | Serum | 20.49 | 19.49 | 62.4 | 59.1 | NA | NA | NA | NA |
| Inamura et al29 (HPFS) | 2016 | USA | CRC | W/o CRC | Nested | Yes | ALPCO | 155 | 296 | Plasma | 5.00 | 5.60 | 66.2 | 66.2 | 1.000 | 1.000 | 26.1 | 25.3 |
| Inamura et al29 (NHS) | 2016 | USA | CRC | W/o CRC | Nested | Yes | ALPCO | 152 | 297 | Plasma | 7.90 | 8.20 | 58.5 | 66.2 | 0.000 | 0.000 | 25.2 | 24.7 |
| Zekri et al13 | 2015 | Middle East | CRC | W/o CRC | Retrospective | NA | RayBiotech | 34 | 29 | Serum | 11.28 | 6.59 | 41.3 | 66.2 | 0.588 | 0.552 | NA | NA |
| Demir et al37 | 2015 | Middle East | CRA | W/o CRC | Retrospective | NA | eBioscience | 32 | 30 | Serum | 8.57 | 9.70 | 53.5 | 66.2 | 0.625 | 0.500 | 29.1 | 26.1 |
| Chandler et al11 | 2015 | USA | CRC | W/o CRC | Nested | Yes | ALPCO | 275 | 275 | Plasma | 6.00 | 6.24 | 58.8 | 66.2 | 0.000 | 0.000 | 26.8 | 26.3 |
| Ochs-Balcom et al26 (white) | 2014 | USA | CRA | W/o CRA | Nested | NA | R&D | 217 | 650 | Plasma | 10.58 | 11.64 | 57.4 | 66.2 | 0.521 | 0.369 | 29.1 | 32.3 |
| Ochs-Balcom et al26 (AA) | 2014 | USA | CRA | W/o CRA | Nested | NA | R&D | 175 | 378 | Plasma | 7.76 | 8.02 | 58.2 | 66.2 | 0.406 | 0.241 | 31.7 | 32.3 |
| Aleksandrova et al5 (colon) | 2014 | Europe | Colon | W/o CRC | Nested | Yes | ALPCO | 794 | 794 | Serum | 6.70 | 6.80 | 58.6 | 66.2 | 0.530 | 0.530 | 26.8 | 26.3 |
| Aleksandrova et al5 (rectum) | 2014 | Europe | Rectum | W/o CRC | Nested | Yes | ALPCO | 466 | 466 | Serum | 6.50 | 6.80 | 58.0 | 66.2 | 0.457 | 0.457 | 26.6 | 26.4 |
| Aleksandrova et al40 (men) | 2014 | Europe | Colon | W/o CRC | Nested | Yes | ALPCO | 292 | 292 | Serum | 5.40 | 5.30 | 59.3 | 66.2 | 1.000 | 1.000 | 27.3 | 26.5 |
| Aleksandrova et al40 (women) | 2014 | Europe | Colon | W/o CRC | Nested | Yes | ALPCO | 370 | 370 | Serum | 8.30 | 8.40 | 58.6 | 66.2 | 0.000 | 0.000 | 26.4 | 26.1 |
| Abe Vicente et al43 | 2014 | USA | CRC | Healthy | Retrospective | NA | R&D | 39 | 40 | Serum | 4.60 | 3.60 | 61.0 | 66.2 | 0.590 | 0.650 | 23.9 | 23.9 |
| Song et al24 (men) | 2013 | USA | CRC | W/o CRC | Nested | Yes | Linco | 270 | 519 | Plasma | 4.99 | 5.32 | 65.8 | 66.2 | 1.000 | 1.000 | 26.2 | 25.4 |
| Song et al24 (women) | 2013 | USA | CRC | W/o CRC | Nested | Yes | Linco | 346 | 686 | Plasma | 8.04 | 8.19 | 59.0 | 66.2 | 0.000 | 0.000 | 26.0 | 25.5 |
| Danese et al38 (men) | 2013 | Europe | CRA | W/o CRC | Retrospective | Yes | Mediagnost | 21 | 26 | Plasma | 7.86 | 5.83 | 63.0 | 66.2 | 0.525 | 0.650 | 26.0 | 26.0 |
| Danese et al38 (women) | 2013 | Europe | CRA | W/o CRC | Retrospective | Yes | Mediagnost | 19 | 14 | Plasma | 11.81 | 9.89 | NA | NA | NA | NA | NA | NA |
| Kemik et al8 (colon) | 2012 | Middle East | Colon | Healthy | Retrospective | NA | Linco | 32 | 40 | Serum | 4.80 | 6.50 | 49.5 | 40.4 | 0.563 | 0.550 | 15.9 | 21.5 |
| Kemik et al8 (rectum) | 2012 | Middle East | Rectum | Healthy | Retrospective | NA | Linco | 35 | 40 | Serum | 4.70 | 6.50 | 44.8 | 40.4 | 0.543 | 0.550 | 16.2 | 21.5 |
| Ho et al30 | 2012 | USA | CRC | W/o CRC | Nested | NA | Millipore | 457 | 834 | Plasma | 27.20 | 29.10 | NA | NA | NA | NA | NA | NA |
| Hillenbrand et al31 (men) | 2012 | Europe | CRC | Healthy | Retrospective | NA | Millipore | 42 | 30 | Serum | 14.20 | 11.80 | 65.5 | 46.0 | 1.000 | 1.000 | 27.2 | NA |
| Hillenbrand et al31 (women) | 2012 | Europe | CRC | Healthy | Retrospective | NA | Millipore | 25 | 30 | Serum | 21.90 | 22.50 | 66.0 | 44.0 | 0.000 | 0.000 | 26.4 | NA |
| Gulcelik et al32 | 2012 | Middle East | Colon | W/o cancer | Retrospective | NA | B-Bridge | 27 | 40 | Serum | 9.51 | 13.91 | 52.1 | 52.4 | 0.593 | 0.250 | 26.8 | 26.8 |
| Chen et al39 (E) | 2012 | East Asia | CRC | W/o colonic polyps | Retrospective | NA | Adlitteram | 71 | 102 | Plasma | 10.10 | 13.20 | 62.1 | 58.3 | 1.000 | 1.000 | 23.5 | 22.9 |
| Chen et al39 (A) | 2012 | East Asia | CRC | W/o colonic polyps | Retrospective | NA | Adlitteram | 94 | 102 | Plasma | 9.30 | 13.20 | 61.8 | 58.3 | 1.000 | 1.000 | 22.4 | 22.9 |
| Aleksandrova et al41 (colon) | 2012 | Europe | Colon | W/o CRC | Nested | Yes | ALPCO | 755 | 755 | Serum | 6.71 | 6.84 | 58.6 | 58.6 | 0.542 | 0.542 | 26.9 | 26.3 |
| Aleksandrova et al41 (rectum) | 2012 | Europe | Rectum | W/o CRC | Nested | Yes | ALPCO | 451 | 451 | Serum | 6.38 | 6.79 | 58.0 | 58.0 | 0.452 | 0.452 | 26.5 | 26.4 |
| Al-Harithy and Al-Zahrani42 (men) | 2012 | Middle East | CRC | Healthy | Retrospective | Yes | ALPCO | 31 | 30 | Serum | 5.59 | 6.38 | 55.0 | 49.0 | 1.000 | 1.000 | 24.8 | 29.1 |
| Al-Harithy and Al-Zahrani42 (women) | 2012 | Middle East | CRC | Healthy | Retrospective | Yes | ALPCO | 29 | 30 | Serum | 4.47 | 8.89 | 53.5 | 52.0 | 0.000 | 0.000 | 26.8 | 28.8 |
| Gialamas et al34 | 2011 | Europe | CRC | Healthy | Retrospective | Yes | RI | 104 | 208 | Serum | 9.45 | 10.38 | 69.8 | 69.1 | 0.625 | 0.625 | NA | NA |
| Chronis et al10 (NOAA) | 2011 | Europe | CRA | W/o CRA | Retrospective | NA | Linco | 22 | 138 | Serum | 10.90 | 10.20 | 65.1 | 63.5 | 0.545 | 0.587 | 26.4 | 27.4 |
| Chronis et al10 (AA) | 2011 | Europe | CRA | W/o CRA | Retrospective | NA | Linco | 46 | 138 | Serum | 11.20 | 10.20 | 66.2 | 63.5 | 0.565 | 0.587 | 27.1 | 27.4 |
| Catalan et al7 | 2011 | Europe | Colon | Healthy | Retrospective | NA | R&D | 11 | 18 | Plasma | 4.07 | 8.09 | 66.0 | 44.0 | NA | NA | 26.9 | 29.3 |
| Al Khaldi et al12 | 2011 | Middle East | Colon | Healthy | Retrospective | Yes | Linco | 58 | 68 | Plasma | 8.60 | 4.10 | 53.0 | 60.0 | 0.500 | 0.500 | 27.9 | 26.0 |
| Yamaji et al22 (men) | 2010 | East Asia | CRA | W/o CRA | Nested | NA | NA | 523 | 480 | Plasma | 3.98 | 4.37 | NA | NA | 1.000 | 1.000 | NA | NA |
| Yamaji et al22 (women) | 2010 | East Asia | CRA | W/o CRA | Nested | NA | NA | 255 | 255 | Plasma | 6.81 | 7.36 | NA | NA | 0.000 | 0.000 | NA | NA |
| Nakajima et al27 | 2010 | East Asia | CRA | W/o CRC | Retrospective | Yes | Otsuka | 115 | 115 | Serum | 8.90 | 8.90 | 63.7 | 63.5 | 0.600 | 0.600 | 22.9 | 23.1 |
| Kemik et al28 | 2010 | Middle East | Colon | Healthy | Retrospective | NA | Linco | 126 | 36 | Serum | 4.30 | 6.50 | 43.5 | 40.4 | 0.421 | 0.444 | NA | NA |
| Gonullu et al33 | 2010 | Middle East | CRC | Healthy | Retrospective | NA | Bio-Source | 36 | 37 | Serum | 5.50 | 6.20 | 56.6 | 51.0 | 0.500 | 0.541 | 27.2 | 27.0 |
| Erarslan et al36 (CRA) | 2009 | Middle East | CRA | W/o colonic polyps | Retrospective | NA | RayBiotech | 31 | 50 | Serum | 7.40 | 9.20 | 63.0 | 59.0 | 0.548 | 0.540 | 26.0 | 29.2 |
| Erarslan et al36 (CRC) | 2009 | Middle East | CRC | W/o colonic polyps | Retrospective | NA | RayBiotech | 23 | 50 | Serum | 7.10 | 9.20 | 57.0 | 59.0 | 0.609 | 0.540 | 24.6 | 29.2 |
| Stocks et al9 (men) | 2008 | Europe | CRC | W/o cancer | Nested | Yes | R&D | 125 | 245 | Plasma | 7.00 | 6.60 | 59.8 | NA | 1.000 | 1.000 | NA | NA |
| Stocks et al9 (women) | 2008 | Europe | CRC | W/o cancer | Nested | Yes | R&D | 181 | 350 | Plasma | 11.40 | 11.40 | 59.7 | NA | 0.000 | 0.000 | NA | NA |
| Ferroni et al35 | 2007 | Europe | CRC | W/o CRC | Retrospective | NA | BioVendor | 60 | 35 | Serum | 8.30 | 13.10 | 64.0 | 63.0 | 0.517 | 0.514 | NA | NA |
| Wei et al23 | 2005 | USA | CRC | W/o CRC | Nested | Yes | Linco | 179 | 177 | Plasma | 7.40 | 7.80 | 66.6 | 66.5 | 1.000 | 1.000 | 25.9 | 25.4 |
| Otake et al6 | 2005 | East Asia | CRA | W/o colonic polyps | Retrospective | NA | Otsuka | 51 | 52 | Plasma | 7.00 | 10.60 | 59.0 | 58.0 | 0.686 | 0.654 | 23.6 | 22.8 |
| Study | Year | WHR
|
WC (cm)
|
DM
|
Physical activity (h/week)
|
Physical inactivity
|
Smoking
|
Family history
|
Glucose (mmol/L)
|
Insulin (µ U/mL)
|
CRP (mg/L)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | Patients | Controls | ||
| Saetang et al25 (CRC) | 2016 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Saetang et al25 (colon) | 2016 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Saetang et al25 (rectum) | 2016 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Inamura et al29 (HPFS) | 2016 | NA | NA | NA | NA | NA | NA | 32.1 | 30.4 | NA | NA | 0.610 | 0.550 | 0.790 | 0.860 | NA | NA | NA | NA | NA | NA |
| Inamura et al29 (NHS) | 2016 | NA | NA | NA | NA | NA | NA | 15.2 | 14.8 | NA | NA | 0.560 | 0.510 | 0.840 | 0.850 | NA | NA | NA | NA | NA | NA |
| Zekri et al13 | 2015 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Demir et al37 | 2015 | NA | NA | 102.8 | 95.0 | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | 5.48 | 5.47 | 12.05 | 6.93 | NA | NA |
| Chandler et al11 | 2015 | NA | NA | NA | NA | NA | NA | 15.4 | 16.5 | NA | NA | 0.098 | 0.113 | 0.120 | 0.098 | NA | NA | NA | NA | NA | NA |
| Ochs-Balcom et al26 (white) | 2014 | 0.92 | 0.90 | 99.6 | 95.1 | 0.078 | 0.086 | NA | NA | NA | NA | 0.553 | 0.489 | 0.267 | 0.246 | 4.79 | 4.73 | 7.70 | 6.20 | 3.00 | 2.30 |
| Ochs-Balcom et al26 (AA) | 2014 | 0.97 | 0.93 | 105.0 | 104.1 | 0.252 | 0.226 | NA | NA | NA | NA | 0.703 | 0.608 | 0.166 | 0.238 | 5.55 | 5.22 | 15.30 | 9.90 | 2.40 | 2.30 |
| Aleksandrova et al5 (colon) | 2014 | NA | NA | 90.7 | 88.5 | NA | NA | NA | NA | 0.152 | 0.116 | 0.237 | 0.215 | NA | NA | NA | NA | NA | NA | NA | NA |
| Aleksandrova et al5 (rectum) | 2014 | NA | NA | 90.4 | 89.7 | NA | NA | NA | NA | 0.146 | 0.133 | 0.281 | 0.288 | NA | NA | NA | NA | NA | NA | NA | NA |
| Aleksandrova et al40 (men) | 2014 | 0.96 | 0.94 | 97.8 | 94.8 | NA | NA | NA | NA | 0.178 | 0.147 | 0.281 | 0.253 | NA | NA | NA | NA | NA | NA | 2.80 | 1.90 |
| Aleksandrova et al40 (women) | 2014 | 0.81 | 0.81 | 84.3 | 82.6 | NA | NA | NA | NA | 0.127 | 0.097 | 0.200 | 0.170 | NA | NA | NA | NA | NA | NA | 3.30 | 2.70 |
| Abe Vicente et al43 | 2014 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Song et al24 (men) | 2013 | 0.96 | 0.94 | 98.0 | 95.3 | NA | NA | 31.9 | 31.0 | NA | NA | 0.050 | 0.049 | 0.196 | 0.139 | NA | NA | NA | NA | 1.34 | 1.13 |
| Song et al24 (women) | 2013 | 0.79 | 0.78 | 81.0 | 79.5 | NA | NA | 16.6 | 16.9 | NA | NA | 0.145 | 0.123 | 0.139 | 0.120 | NA | NA | NA | NA | 1.52 | 1.67 |
| Danese et al38 (men) | 2013 | NA | NA | 97.5 | 95.9 | NA | NA | NA | NA | NA | 0.294 | NA | NA | NA | NA | 5.53 | 5.38 | NA | NA | 1.90 | 1.80 |
| Danese et al38 (women) | 2013 | NA | NA | 94.2 | 81.1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Kemik et al8 (colon) | 2012 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 9.10 | 3.50 |
| Kemik et al8 (rectum) | 2012 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 9.10 | 3.50 |
| Ho et al30 | 2012 | NA | NA | NA | NA | 0.000 | 0.000 | NA | NA | 0.296 | 0.294 | 0.540 | 0.458 | 0.182 | 0.155 | NA | NA | 6.50 | 5.30 | NA | NA |
| Hillenbrand et al31 (men) | 2012 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Hillenbrand et al31 (women) | 2012 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Gulcelik et al32 | 2012 | NA | NA | NA | NA | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chen et al39 (E) | 2012 | 0.89 | 0.86 | NA | NA | 0.000 | 0.000 | NA | NA | 0.690 | 0.725 | 0.380 | 0.304 | NA | NA | NA | NA | NA | NA | NA | NA |
| Chen et al39 (A) | 2012 | 0.85 | 0.86 | NA | NA | 0.000 | 0.000 | NA | NA | 0.777 | 0.725 | 0.415 | 0.304 | NA | NA | NA | NA | NA | NA | NA | NA |
| Aleksandrova et al41 (colon) | 2012 | 0.88 | 0.87 | 90.4 | 88.1 | NA | NA | NA | NA | 0.144 | 0.114 | 0.567 | 0.538 | NA | NA | NA | NA | NA | NA | 3.10 | 2.30 |
| Aleksandrova et al41 (rectum) | 2012 | 0.89 | 0.88 | 90.4 | 89.9 | NA | NA | NA | NA | 0.151 | 0.131 | 0.609 | 0.603 | NA | NA | NA | NA | NA | NA | 2.40 | 2.30 |
| Al-Harithy and Al-Zahrani42 (men) | 2012 | 1.23 | 0.95 | 72.6 | 75.7 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Al-Harithy and Al-Zahrani42 (women) | 2012 | 0.95 | 1.01 | 61.0 | 84.6 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Gialamas et al34 | 2011 | 1.01 | 0.94 | NA | NA | 0.279 | 0.173 | NA | NA | 0.279 | 0.178 | 0.490 | 0.519 | NA | NA | NA | NA | NA | NA | NA | NA |
| Chronis et al10 (NOAA) | 2011 | NA | NA | 99.3 | 99.9 | 0.000 | 0.000 | NA | NA | NA | NA | 0.318 | 0.304 | NA | NA | 5.34 | 5.58 | 5.80 | 5.90 | NA | NA |
| Chronis et al10 (AA) | 2011 | NA | NA | 99.2 | 99.9 | 0.000 | 0.000 | NA | NA | NA | NA | 0.261 | 0.304 | NA | NA | 5.70 | 5.58 | 7.10 | 5.90 | NA | NA |
| Catalan et al7 | 2011 | NA | NA | NA | NA | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | 6.06 | 5.50 | 3.80 | 12.00 | 11.96 | 4.31 |
| Al Khaldi et al12 | 2011 | NA | NA | 106.0 | 102.5 | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | 7.30 | 5.80 | 10.40 | 11.00 | NA | NA |
| Yamaji et al22 (men) | 2010 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.138 | 0.135 | NA | NA | NA | NA | NA | NA | NA | NA |
| Yamaji et al22 (women) | 2010 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0.216 | 0.102 | NA | NA | NA | NA | NA | NA | NA | NA |
| Nakajima et al27 | 2010 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Kemik et al28 | 2010 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 9.80 | 3.50 |
| Gonullu et al33 | 2010 | 0.92 | 0.93 | NA | NA | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | 5.33 | 5.15 | 10.50 | 6.10 | NA | NA |
| Erarslan et al36 (CRA) | 2009 | NA | NA | 94.0 | 102.0 | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | 5.34 | 5.24 | 8.60 | 12.90 | NA | NA |
| Erarslan et al36 (CRC) | 2009 | NA | NA | 84.0 | 102.0 | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | 5.12 | 5.24 | 9.70 | 12.90 | NA | NA |
| Stocks et al9 (men) | 2008 | NA | NA | NA | NA | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Stocks et al9 (women) | 2008 | NA | NA | NA | NA | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Ferroni et al35 | 2007 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Wei et al23 | 2005 | 0.95 | 0.94 | 97.0 | 95.0 | NA | NA | 28.6 | 28.9 | NA | NA | 0.051 | 0.054 | 0.207 | 0.126 | NA | NA | NA | NA | NA | NA |
| Otake et al6 | 2005 | NA | NA | NA | NA | 0.000 | 0.000 | NA | NA | NA | NA | NA | NA | NA | NA | 5.83 | 5.11 | 8.50 | 6.10 | NA | NA |
Abbreviations: ADI, adiponectin; ELISA, enzyme-linked immunosorbent assay; CRC, colorectal cancer; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; CRA, colorectal adenoma; AA, advanced adenoma; NOAA, without advanced adenoma; W/o, without; BMI, body mass index; WHR, waist–hip ratio; WC, waist circumference; DM, diabetes mellitus; CRP, C-reactive protein; NA, not available.
Overall analysis
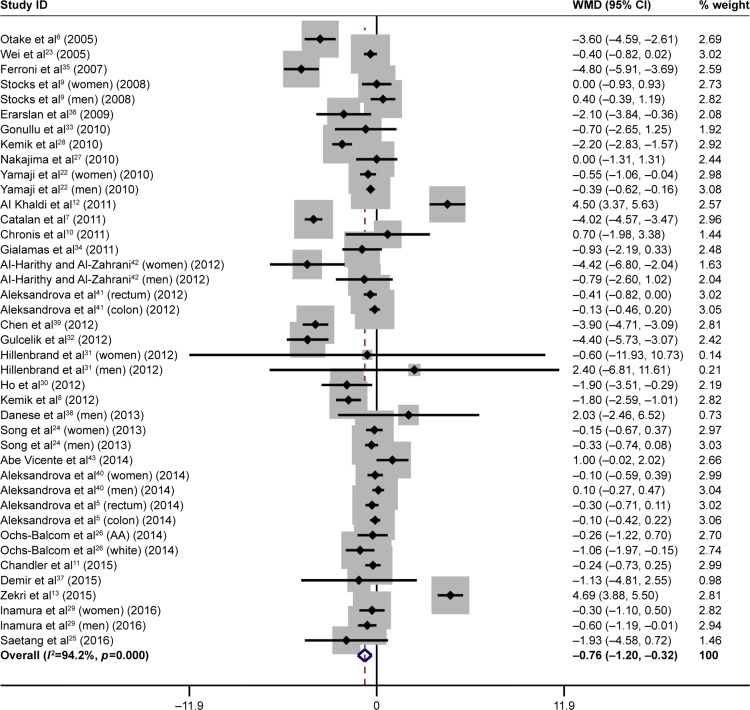
Overall analysis was based on 41 qualified studies, and the funnel plot illustrating the difference in total adiponectin concentrations between patients with colorectal cancer and controls is presented in Figure 1. Pooling effect size of all studies found that total adiponectin was significantly lower in patients with colorectal cancer than in controls (WMD: −0.76 µg/mL, 95% CI: −1.20 to −0.32, p=0.001). However, this significant finding was clouded by strong evidence of statistical heterogeneity (I2: 94.2%, p<0.001).
Figure 1.
Forest plot for overall difference in total adiponectin between patients with colorectal cancer and controls.
Note: Weights are from random-effects analysis.
Abbreviations: WMD, weighted mean difference; CI, confidence interval.
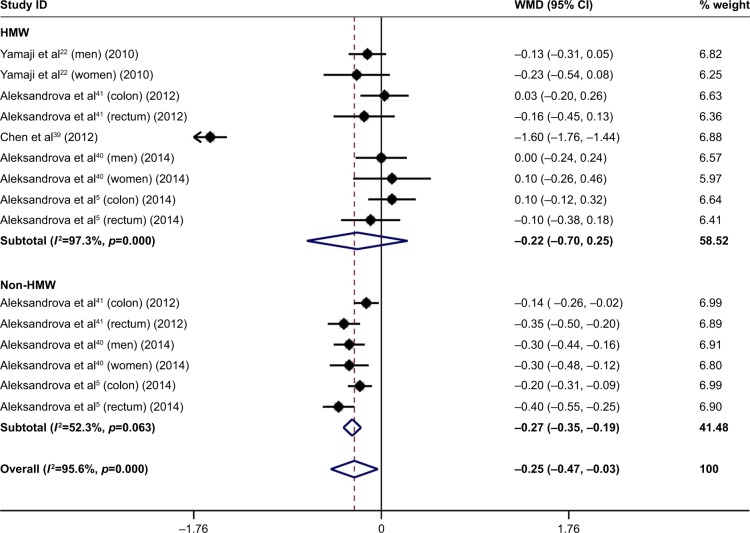
Figure 2 shows another funnel plot quantifying the differences in adiponectin fractions – HMW adiponectin and non-HMW adiponectin – between patients with colorectal cancer and controls. The difference in HMW adiponectin was comparable between the two groups (WMD: −0.22 µg/mL, 95% CI: −0.70 to 0.25, p=0.350), with significant heterogeneity (I2: 97.3%, p<0.001). By contrast, non-HMW adiponectin was significantly lower in patients with colorectal cancer than in controls (WMD: −0.27 µg/mL, 95% CI: −0.35 to −0.19, p<0.001), with only marginal heterogeneity (I2: 52.3%, p=0.063).
Figure 2.
Forest plot for differences in two adiponectin fractions (HMW and non-HMW) between patients with colorectal cancer and controls.
Note: Weights are from random-effects analysis.
Abbreviations: HMW, high molecular weight; WMD, weighted mean difference; CI, confidence interval.
Subgroup analysis
Table 2 summarizes the differences in total adiponectin between patients and controls by grouping studies according to cancer subtype, region, study design, matching status, sample source, gender, BMI, WHR and diabetes mellitus, respectively.
Table 2.
Subgroup analysis of the difference in circulating adiponectin between patients with colorectal cancer and controls
| Characteristics | Subgroups | Studies (number) | WMD | 95% CI | pD | I2 | pH | pE |
|---|---|---|---|---|---|---|---|---|
| Cancer subtype | CRC | 20 | −0.736 | −1.519 to 0.047 | 0.065 | 94.0% | <0.001 | 0.514 |
| CRA | 10 | −0.892 | −1.573 to −0.211 | 0.010 | 80.1% | <0.001 | 0.473 | |
| Colon | 10 | −1.073 | −2.095 to −0.050 | 0.040 | 97.3% | <0.001 | 0.403 | |
| Rectum | 4 | −0.681 | −1.358 to −0.005 | 0.048 | 75.4% | 0.007 | 0.778 | |
| Region | East Asia | 6 | −1.707 | −2.979 to −0.436 | 0.008 | 95.1% | <0.001 | 0.213 |
| America | 10 | −0.343 | −0.599 to −0.087 | 0.009 | 36.7% | 0.115 | 0.683 | |
| Europe | 15 | −0.749 | −1.451 to −0.047 | 0.037 | 94.3% | <0.001 | 0.541 | |
| Middle East | 10 | −0.787 | −3.015 to 1.442 | 0.489 | 97.1% | <0.001 | 0.787 | |
| Study design | Retrospective | 22 | −1.179 | −2.536 to 0.177 | 0.088 | 96.2% | <0.001 | 0.489 |
| Nested | 19 | −0.266 | −0.380 to −0.152 | <0.001 | 12.4% | 0.303 | 0.409 | |
| Matching status | NA | 21 | −1.472 | −2.452 to −0.491 | 0.003 | 96.1% | <0.001 | 0.411 |
| Yes | 20 | −0.099 | −0.389 to 0.191 | 0.504 | 79.0% | <0.001 | 0.693 | |
| Sample source | Plasma | 18 | −0.714 | −1.358 to −0.070 | 0.030 | 95.1% | <0.001 | 0.783 |
| Serum | 23 | −0.803 | −1.430 to −0.176 | 0.012 | 93.3% | <0.001 | 0.303 | |
| Sample size (median) | ≤230 | 21 | −1.189 | −2.609 to 0.231 | 0.101 | 96.4% | <0.001 | 0.511 |
| >230 | 20 | −0.271 | −0.385 to −0.157 | <0.001 | 12.1% | 0.304 | 0.262 | |
| Gender | Men | 9 | −0.671 | −1.255 to −0.086 | 0.025 | 90.4% | <0.001 | 0.536 |
| Women | 8 | −0.329 | −0.693 to 0.035 | 0.077 | 49.6% | <0.001 | 0.206 | |
| BMI | <25 kg/m2 | 7 | −1.627 | −3.092 to −0.163 | 0.029 | 92.1% | <0.001 | 0.434 |
| ≥25 kg/m2 | 24 | −0.527 | −1.016 to −0.039 | 0.034 | 92.4% | <0.001 | 0.611 | |
| WHR | <0.9 m | 5 | −0.878 | −1.798 to 0.043 | 0.062 | 94.7% | <0.001 | 0.112 |
| ≥0.9 m | 9 | −0.522 | −0.939 to −0.106 | 0.014 | 60.6% | 0.009 | 0.496 | |
| DM | Without | 12 | −1.385 | −3.003 to 0.233 | 0.093 | 96.1% | <0.001 | 0.349 |
| TNM stage | I–II | 9 | −1.280 | −2.115 to −0.446 | 0.003 | 90.1% | <0.001 | 0.103 |
| III–IV | 9 | −1.196 | −2.033 to −0.358 | 0.005 | 92.6% | <0.001 | 0.219 |
Notes: pD, the p-value for WMD; I2, inconsistency index; pH, the p-value for heterogeneity; pE, the p-value for Egger’s regression asymmetry test.
Abbreviations: WMD, weighted mean difference; CI, confidence interval; CRC, colorectal cancer; CRA, colorectal adenoma; BMI, body mass index; WHR, waist–hip ratio; DM, diabetes mellitus; TNM, tumor node metastasis; NA, not available.
By cancer subtype, significant difference in total adiponectin was observed in patients with colorectal adenoma (CRA), colon cancer and rectum cancer, respectively, relative to controls (WMD: −0.892, −1.073 and −0.681 µg/mL, p=0.010), with significant heterogeneity (I2: 80.1%, 97.3% and 75.4%).
By geographic region, besides Middle East, total adiponectin was significantly lower in patients than in controls from America, Europe and especially East Asia (WMD: −1.707 µg/mL, p=0.008), and only in America, there was no evidence of heterogeneity (I2: 36.7%).
By study design, significant difference was observed only in nested case–control studies (WMD: −0.266 µg/mL, p<0.001), without heterogeneity (I2: 12.4%).
By matching status, studies with matched patients and controls failed to identify any significance (WMD: −0.099 µg/mL, p=0.504) (I2: 79.0%), while in studies with unclear matching status, there was significant difference in total adiponectin between patients and controls (I2: 96.1%).
By sample source, total adiponectin was significantly and comparably reduced in studies collecting plasma and serum samples for adiponectin measurement (WMD: −0.714 and −0.803 µg/mL, p=0.030 and 0.012, I2: 95.1% and 93.3%, respectively).
By sample size, grouping studies by median total sample size showed that the reduction of total adiponectin was obvious in studies with total sample size >230 (WMD: −0.271 µg/mL, p<0.001), and the likelihood of heterogeneity was low (I2: 12.1%).
By gender, total adiponectin was reduced significantly in male patients relative to male controls (WMD: −0.671 µg/mL, p=0.025) (I2: 90.4%), and there was no observable significance in females.
By obesity, studies were grouped by mean or median BMI at 25 kg/m2, and significant reduction in total adiponectin was noted in both groups, especially in studies with BMI <25 kg/m2 (WMD: −1.627 µg/mL, p=0.029) (I2: 92.1%). In addition, when studies were grouped by mean or median WHR at 0.9 m, there was significant reduction in studies with WHR ≥0.9 m (WMD: −0.522 µg/mL, p=0.014).
After restricting to studies involving subjects free of diabetes mellitus, the reduction in total adiponectin was not statistically significant, and there was significant heterogeneity.
By tumor node metastasis stage, the reduction in magnitude of total adiponectin was slightly stronger in patients with stage I–II colorectal cancer (WMD: −1.280 µg/mL, p=0.003) than in patients with stage III–IV cancer (WMD: −1.196 µg/mL, p=0.005), and significance was detected in both subgroups with significant heterogeneity but a low probability of publication bias.
Meta-regression analysis
Table 3 lists the results of meta-regression analysis by incorporating all characteristics to assess whether they can explain the heterogeneity between total adiponectin and colorectal cancer. Age and smoking in both patients and controls were significant potential sources of heterogeneity, and BMI, waist circumference and CRP can account for heterogeneity only in patients (p<0.05).
Table 3.
The meta-regression analysis of all characteristics in both patients with colorectal cancer and controls
| Characteristics | Studies (number) | Patients
|
Controls
|
||
|---|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | ||
| Age | 36 | −0.21 | 0.012 | 0.18 | 0.019 |
| Gender | 38 | −8.45 | 0.076 | 8.37 | 0.073 |
| BMI | 29 | 0.41 | 0.039 | −0.35 | 0.091 |
| WHR | 14 | 5.04 | 0.387 | −10.47 | 0.282 |
| WC | 19 | 0.14 | 0.008 | −0.09 | 0.177 |
| DM | 15 | −3.71 | 0.913 | 8.86 | 0.840 |
| Physical activity | 6 | −0.05 | 0.785 | 0.04 | 0.842 |
| Physical inactivity | 9 | 1.46 | 0.836 | −7.74 | 0.315 |
| Smoking | 20 | −10.59 | 0.032 | 10.59 | 0.043 |
| Family history | 9 | −0.66 | 0.842 | 0.34 | 0.909 |
| Glucose | 10 | 0.93 | 0.640 | 2.37 | 0.609 |
| Insulin | 10 | 0.28 | 0.316 | 0.03 | 0.930 |
| CRP | 12 | −0.37 | 0.015 | 0.20 | 0.698 |
Abbreviations: BMI, body mass index; WHR, waist–hip ratio; WC, waist circumference; DM, diabetes mellitus; CRP, C-reactive protein.
Publication bias
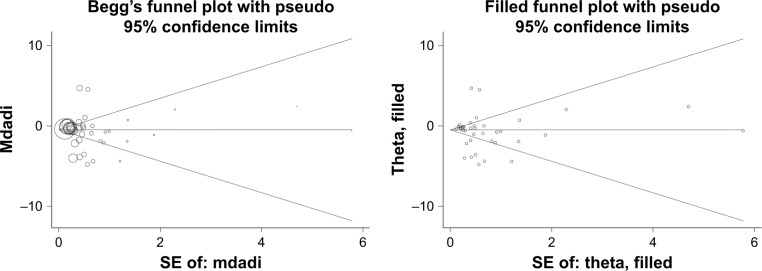
Overall comparison of total adiponectin between patients with colorectal cancer and controls was not subject to publication bias, which was illustrated by both Begg’s and filled funnel plots in Figure 3, as well as by the Egger’s regression asymmetry test (p=0.336). For comparisons in subgroup analysis, there was consistently low likelihood of publication bias across all subgroups (Egger’s test, p<0.1), as presented in Table 2.
Figure 3.
Begg’s and filled funnel plots for difference in circulating total adiponectin between patients with colorectal cancer and controls.
Abbreviations: mdadi, mean value of total adiponectin; SE, standard error.
Discussion
The most noteworthy finding of this present study is that in line with the conclusions of most previous studies,14–20 our data indicated that low total adiponectin in circulation was a significant risk factor for colorectal cancer. Moreover, for the first time, we pooled data according to adiponectin fractions and found that only non-HMW adiponectin differed significantly between patients with colorectal cancer and controls. We further identified age, gender, smoking, obesity, study design, matching status and sample size as potential sources of heterogeneity. To our knowledge, this is thus far the largest meta-analysis dealing with the relationship of circulating adiponectin and its fractions with colorectal cancer.
The connection between obesity and colorectal cancer is well established,44,45 and a great deal of supporting evidence from clinical and epidemiological data has suggested that this connection is probably mediated by abnormal adiponectin in circulation.46 Adiponectin is the most abundant hormone secreted by adipose tissue, and its anti-inflammatory and insulin-sensitizing properties may, at least in part, expound on the etiologic link between obesity and colorectal cancer.47,48 Moreover, accumulating evidence has highlighted a contributory role of adiponectin in anti-carcinogenesis.49,50 In vitro studies have showed that adiponectin can promote endothelial apoptosis, repress the maturation and proliferation of colorectal cancer cells and control colony formation, likely through the activation of AMPK-mTOR signaling pathway.39,51,52 Based on the above evidence, it is more reasonable to presume that circulating adiponectin may be involved in the pathophysiological process of colorectal cancer.
Consistent with the findings of major published studies,14–20 we confirmed in this present study that low total adiponectin in circulation was a significant risk factor for colorectal cancer. As with most previous meta-analyses, heterogeneity is a serious issue that limits interpretation of effect estimates and requires careful exploration.53 As a secondary goal of this study, we employed both subgroup analysis and meta-regression analysis to seek possible causes of heterogeneity between studies. In particular, the reduction in total adiponectin was more obvious for CRA and colon cancer, and in studies enrolling subjects of male gender, from East Asia and with normal weight. In addition, the association of total adiponectin with colorectal cancer was markedly significant in studies with a nested case–control design and a large sample size, relative to studies with a retrospective case–control design and a small sample size, indicating the robustness of our observation. Colorectal cancer is a highly heterogeneous disease to which environmental exposure, germ-line susceptibility determinants and accumulated genetic and epigenetic changes contribute interactively.54 For example, cigarette smoking and alcohol drinking, which are more prevalent in men than in women, are established risk factors for the development and progression of colorectal gender-specific difference in adiponectin observed in the present study. Nevertheless, even though we have made great endeavors to seek causes of heterogeneity, there is still strong evidence of heterogeneity in some subgroups, indicating that residual confounding from other sources of heterogeneity in colorectal cancer risk is likely. We agree that further explorations are necessary, and especially analysis of individual participant data could yield further insights.
However, a growing body of research has reported diverse biological activities of different adiponectin fractions, mainly focusing on HMW (insulin sensitivity) and non-HMW (inflammation response) forms.5,24 In this context, the sufficient number of eligible studies in this meta-analysis enables us to investigate different adiponectin fractions, and our findings revealed that low non-HMW adiponectin in circulation was a significant risk factor for colorectal cancer and there was no observable significant difference for HMW adiponectin. As previously discussed, non-HMW adiponectin plays a major role in the inflammatory process.24 Systemic inflammation is a key manifestation of cancer progression and metastasis in many types of cancer including colorectal cancer.57 We thus develop a further presumption that non-HMW adiponectin may be involved in colorectal carcinogenesis by regulating inflammatory responses. Addressing this presumption is beyond the scope of this meta-analysis, and further experimental studies are required.
Finally, the present study needs to be interpreted cautiously, bearing in mind the following limitations. First, we only retrieved the literature for the papers published in English language, and selection bias cannot be ruled out. Second, all involved studies in this meta-analysis were observational in nature, either cross-sectional or nested, which hindered further causality inference. Third, as discussed above, sources of heterogeneity for a majority of comparisons were not fully accounted for, and additional considerations were necessary. For example, low circulating adiponectin was found to be associated with KRAS-mutant colorectal cancer risk but not with KRAS-wild-type cancer risk.29 Fourth, only data on circulating plasma/serum adiponectin were summa-rized, and it is expected that tissue adiponectin concentration in tumor environment may be more relevant to the evaluation of colorectal cancer risk than its circulating concentration. However, data on tissue adiponectin are rarely reported. Fifth, circulating adiponectin concentration was affected by medical treatment or drug intervention, which cannot be taken into account because of lack of data.
In conclusion, through a comprehensive meta-analysis of 7,554 patients with colorectal cancer and 9,798 controls, our data indicate that low total adiponectin, especially its non-HMW fraction, represents a promising risk factor for colorectal cancer. Although there is still residual confounding unaccounted for, we believe that this study can aid in better understanding cancer heterogeneity, highlighting the importance of anti-inflammation therapies to prevent or delay the occurrence of colorectal cancer and thereby providing new insight in its physiology. In addition, further studies are needed to explore underlying mechanisms.
Footnotes
Author contributions
WL and ZH searched the literature and identified potential papers, extracted the data, performed statistical analysis and drafted the manuscript. NL checked the data and results. HL designed the study and polished the language of the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Heiker JT, Kosel D, Beck-Sickinger AG. Molecular mechanisms of signal transduction via adiponectin and adiponectin receptors. Biol Chem. 2010;391(9):1005–1018. doi: 10.1515/BC.2010.104. [DOI] [PubMed] [Google Scholar]
- 2.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(6) doi: 10.3390/ijms18061321. pii E1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148(3):293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 4.Liu M, Liu F. Regulation of adiponectin multimerization, signaling and function. Best Pract Res Clin Endocrinol Metab. 2014;28(1):25–31. doi: 10.1016/j.beem.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleksandrova K, Jenab M, Bueno-de-Mesquita HB, et al. Biomarker patterns of inflammatory and metabolic pathways are associated with risk of colorectal cancer: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Eur J Epidemiol. 2014;29(4):261–275. doi: 10.1007/s10654-014-9901-8. [DOI] [PubMed] [Google Scholar]
- 6.Otake S, Takeda H, Suzuki Y, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res. 2005;11(10):3642–3646. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 7.Catalan V, Gomez-Ambrosi J, Rodriguez A, et al. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. J Nutr Biochem. 2011;22(7):634–641. doi: 10.1016/j.jnutbio.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Kemik O, Kemik AS, Begenik H, et al. The relationship among acute-phase response proteins, cytokines, and hormones in various gastrointestinal cancer types patients with cachectic. Hum Exp Toxicol. 2012;31(2):117–125. doi: 10.1177/0960327111417271. [DOI] [PubMed] [Google Scholar]
- 9.Stocks T, Lukanova A, Johansson M, et al. Components of the metabolic syndrome and colorectal cancer risk; a prospective study. Int J Obes (Lond) 2008;32(2):304–314. doi: 10.1038/sj.ijo.0803713. [DOI] [PubMed] [Google Scholar]
- 10.Chronis A, Thomopoulos K, Sapountzis A, et al. Adiposity factors are not related to the presence of colorectal adenomas. Clin Exp Gastroenterol. 2011;4:257–261. doi: 10.2147/CEG.S25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandler PD, Buring JE, Manson JE, et al. Association between plasma adiponectin levels and colorectal cancer risk in women. Cancer Causes Control. 2015;26(7):1047–1052. doi: 10.1007/s10552-015-0590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Khaldi RM, Al Mulla F, Al Awadhi S, Kapila K, Mojiminiyi OA. Associations of single nucleotide polymorphisms in the adiponectin gene with adiponectin levels and cardio-metabolic risk factors in patients with cancer. Dis Markers. 2011;30(4):197–212. doi: 10.3233/DMA-2011-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zekri AR, Bakr YM, Ezzat MM, Zakaria MS, Elbaz TM. Circulating levels of adipocytokines as potential biomarkers for early detection of colorectal carcinoma in Egyptian patients. Asian Pac J Cancer Prev. 2015;16(16):6923–6928. doi: 10.7314/apjcp.2015.16.16.6923. [DOI] [PubMed] [Google Scholar]
- 14.Pei Y, Xu Y, Niu W. Causal relevance of circulating adiponectin with cancer: a meta-analysis implementing Mendelian randomization. Tumour Biol. 2015;36(2):585–594. doi: 10.1007/s13277-014-2654-x. [DOI] [PubMed] [Google Scholar]
- 15.Joshi RK, Lee SA. Obesity related adipokines and colorectal cancer: a review and meta-analysis. Asian Pac J Cancer Prev. 2014;15(1):397–405. doi: 10.7314/apjcp.2014.15.1.397. [DOI] [PubMed] [Google Scholar]
- 16.Joshi RK, Kim WJ, Lee SA. Association between obesity-related adipokines and colorectal cancer: a case-control study and meta-analysis. World J Gastroenterol. 2014;20(24):7941–7949. doi: 10.3748/wjg.v20.i24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrier S, Jarde T. Adiponectin, an anti-carcinogenic hormone? A systematic review on breast, colorectal, liver and prostate cancer. Curr Med Chem. 2012;19(32):5501–5512. doi: 10.2174/092986712803833137. [DOI] [PubMed] [Google Scholar]
- 18.Izadi V, Farabad E, Azadbakht L. Serum adiponectin level and different kinds of cancer: a review of recent evidence. ISRN Oncol. 2012;2012:982769. doi: 10.5402/2012/982769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An W, Bai Y, Deng SX, et al. Adiponectin levels in patients with colorectal cancer and adenoma: a meta-analysis. Eur J Cancer Prev. 2012;21(2):126–133. doi: 10.1097/CEJ.0b013e32834c9b55. [DOI] [PubMed] [Google Scholar]
- 20.Xu XT, Xu Q, Tong JL, et al. Meta-analysis: circulating adiponectin levels and risk of colorectal cancer and adenoma. J Dig Dis. 2011;12(4):234–244. doi: 10.1111/j.1751-2980.2011.00504.x. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaji T, Iwasaki M, Sasazuki S, Tsugane S. Interaction between adiponectin and leptin influences the risk of colorectal adenoma. Cancer Res. 2010;70(13):5430–5437. doi: 10.1158/0008-5472.CAN-10-0178. [DOI] [PubMed] [Google Scholar]
- 23.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97(22):1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 24.Song M, Zhang X, Wu K, et al. Plasma adiponectin and soluble leptin receptor and risk of colorectal cancer: a prospective study. Cancer Prev Res (Phila) 2013;6(9):875–885. doi: 10.1158/1940-6207.CAPR-13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saetang N, Boonpipattanapong T, Palanusont A, Maneechay W, Sangkhathat S. Alteration of leptin and adiponectin in multistep colorectal tumorigenesis. Asian Pac J Cancer Prev. 2016;17(4):2119–2123. doi: 10.7314/apjcp.2016.17.4.2119. [DOI] [PubMed] [Google Scholar]
- 26.Ochs-Balcom HM, Cannioto R, Nie J, et al. Adipokines do not mediate the association of obesity and colorectal adenoma. J Cancer Epidemiol. 2014;2014:371254. doi: 10.1155/2014/371254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima TE, Yamada Y, Hamano T, et al. Adipocytokines as new promising markers of colorectal tumors: adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci. 2010;101(5):1286–1291. doi: 10.1111/j.1349-7006.2010.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemik O, Sumer A, Kemik AS, et al. The relationship among acute-phase response proteins, cytokines and hormones in cachectic patients with colon cancer. World J Surg Oncol. 2010;8:85. doi: 10.1186/1477-7819-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inamura K, Song M, Jung S, et al. Prediagnosis plasma adiponectin in relation to colorectal cancer risk according to KRAS mutation status. J Natl Cancer Inst. 2016;108(4) doi: 10.1093/jnci/djv363. pii djv363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho GY, Wang T, Gunter MJ, et al. Adipokines linking obesity with colorectal cancer risk in postmenopausal women. Cancer Res. 2012;72(12):3029–3037. doi: 10.1158/0008-5472.CAN-11-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillenbrand A, Fassler J, Huber N, et al. Changed adipocytokine concentrations in colorectal tumor patients and morbidly obese patients compared to healthy controls. BMC Cancer. 2012;12:545. doi: 10.1186/1471-2407-12-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulcelik MA, Colakoglu K, Dincer H, Dogan L, Yenidogan E, Gulcelik NE. Associations between adiponectin and two different cancers: breast and colon. Asian Pac J Cancer Prev. 2012;13(1):395–398. doi: 10.7314/apjcp.2012.13.1.395. [DOI] [PubMed] [Google Scholar]
- 33.Gonullu G, Kahraman H, Bedir A, Bektas A, Yucel I. Association between adiponectin, resistin, insulin resistance, and colorectal tumors. Int J Colorectal Dis. 2010;25(2):205–212. doi: 10.1007/s00384-009-0828-6. [DOI] [PubMed] [Google Scholar]
- 34.Gialamas SP, Petridou ET, Tseleni-Balafouta S, et al. Serum adiponectin levels and tissue expression of adiponectin receptors are associated with risk, stage, and grade of colorectal cancer. Metabolism. 2011;60(11):1530–1538. doi: 10.1016/j.metabol.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Ferroni P, Palmirotta R, Spila A, et al. Prognostic significance of adiponectin levels in non-metastatic colorectal cancer. Anticancer Res. 2007;27(1B):483–489. [PubMed] [Google Scholar]
- 36.Erarslan E, Turkay C, Koktener A, Koca C, Uz B, Bavbek N. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci. 2009;54(4):862–868. doi: 10.1007/s10620-008-0440-6. [DOI] [PubMed] [Google Scholar]
- 37.Demir N, Ahishali E, Dolapcioglu C, et al. The relationship between serum adiponectin and resistin levels, insulin resistance and colorectal adenomas. Turk J Gastroenterol. 2015;26(1):20–24. doi: 10.5152/tjg.2015.3626. [DOI] [PubMed] [Google Scholar]
- 38.Danese E, Minicozzi AM, Montagnana M, De Manzoni G, Lippi G, Guidi GC. Lack of an association between circulating adiponectin levels and risk of colorectal adenoma. Clin Lab. 2013;59(1–2):211–214. doi: 10.7754/clin.lab.2012.120717. [DOI] [PubMed] [Google Scholar]
- 39.Chen MW, Ye S, Zhao LL, et al. Association of plasma total and high-molecular-weight adiponectin with risk of colorectal cancer: an observational study in Chinese male. Med Oncol. 2012;29(5):3129–3135. doi: 10.1007/s12032-012-0280-2. [DOI] [PubMed] [Google Scholar]
- 40.Aleksandrova K, Drogan D, Boeing H, et al. Adiposity, mediating biomarkers and risk of colon cancer in the European prospective investigation into cancer and nutrition study. Int J Cancer. 2014;134(3):612–621. doi: 10.1002/ijc.28368. [DOI] [PubMed] [Google Scholar]
- 41.Aleksandrova K, Boeing H, Jenab M, et al. Total and high-molecular weight adiponectin and risk of colorectal cancer: the European Prospective Investigation into Cancer and Nutrition Study. Carcinogenesis. 2012;33(6):1211–1218. doi: 10.1093/carcin/bgs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Harithy RN, Al-Zahrani MH. The adiponectin gene, ADIPOQ, and genetic susceptibility to colon cancer. Oncol Lett. 2012;3(1):176–180. doi: 10.3892/ol.2011.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abe Vicente M, Donizetti Silva T, Barao K, Vitor Felipe A, Oyama Missae L, Manoukian Forones N. The influence of nutritional status and disease on adiponectin and TNF-alpha; levels in colorectal cancer patients. Nutr Hosp. 2014;30(1):140–146. doi: 10.3305/nh.2014.30.1.7132. [DOI] [PubMed] [Google Scholar]
- 44.Billy M, Sholihah H, Andanni K, Anggraeni MI, Siregar SM, Mirtha LT. Obesity as predictor of mortality of colorectal cancer: an evidence-based case report. Acta Med Indones. 2016;48(3):242–246. [PubMed] [Google Scholar]
- 45.Kasi PM, Zafar SY, Grothey A. Is obesity an advantage in patients with colorectal cancer? Expert Rev Gastroenterol Hepatol. 2015;9(11):1339–1342. doi: 10.1586/17474124.2015.1089170. [DOI] [PubMed] [Google Scholar]
- 46.La Cava A. Adiponectin: a relevant player in obesity-related colorectal cancer? Gut. 2013;62(4):483–484. doi: 10.1136/gutjnl-2012-303034. [DOI] [PubMed] [Google Scholar]
- 47.Otani K, Ishihara S, Yamaguchi H, et al. Adiponectin and colorectal cancer. Surg Today. 2017;47(2):151–158. doi: 10.1007/s00595-016-1334-4. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto H, Maeda K, Arima H, et al. Perioperative adiponectin measurement is useful for prediction of postoperative infection in patients with colorectal cancer. Ann Surg Oncol. 2016;23(Suppl 4):540–545. doi: 10.1245/s10434-016-5386-x. [DOI] [PubMed] [Google Scholar]
- 49.Tae CH, Kim SE, Jung SA, et al. Involvement of adiponectin in early stage of colorectal carcinogenesis. BMC Cancer. 2014;14:811. doi: 10.1186/1471-2407-14-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapoor S. Adiponectin and its role in gastrointestinal carcinogenesis. Am J Gastroenterol. 2009;104(1):243–244. doi: 10.1038/ajg.2008.27. author reply 244–245. [DOI] [PubMed] [Google Scholar]
- 51.Sugiyama M, Takahashi H, Hosono K, et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int J Oncol. 2009;34(2):339–344. [PubMed] [Google Scholar]
- 52.Kim AY, Lee YS, Kim KH, et al. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol Endocrinol. 2010;24(7):1441–1452. doi: 10.1210/me.2009-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petitti DB. Approaches to heterogeneity in meta-analysis. Stat Med. 2001;20(23):3625–3633. doi: 10.1002/sim.1091. [DOI] [PubMed] [Google Scholar]
- 54.Takayama T, Miyanishi K, Hayashi T, Sato Y, Niitsu Y. Colorectal cancer: genetics of development and metastasis. J Gastroenterol. 2006;41(3):185–192. doi: 10.1007/s00535-006-1801-6. [DOI] [PubMed] [Google Scholar]
- 55.Cho S, Shin A, Park SK, Shin HR, Chang SH, Yoo KY. Alcohol drinking, cigarette smoking and risk of colorectal cancer in the Korean multi-center cancer cohort. J Cancer Prev. 2015;20(2):147–152. doi: 10.15430/JCP.2015.20.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hashibe M, Hunt J, Wei M, Buys S, Gren L, Lee YC. Tobacco, alcohol, body mass index, physical activity, and the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Head Neck. 2013;35(7):914–922. doi: 10.1002/hed.23052. [DOI] [PubMed] [Google Scholar]
- 57.Rossi S, Basso M, Strippoli A, et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. 2017;16(4):264–274. doi: 10.1016/j.clcc.2017.03.015. [DOI] [PubMed] [Google Scholar]