Abstract
Background
Several recent randomized controlled trials (RCTs) in hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (MBC) have demonstrated significant improvements in progression-free survival (PFS); however, few have reported improvement in overall survival (OS). The surrogacy of PFS or time to progression (TTP) for OS has not been formally investigated in HR+, HER2− MBC.
Methods
A systematic literature review of RCTs in HR+, HER2− MBC was conducted to identify studies that reported both median PFS/TTP and OS. The correlation between PFS/TTP and OS was evaluated using Pearson’s product–moment correlation and Spearman’s rank correlation. Subgroup analyses were performed to explore possible reasons for heterogeneity. Errors-in-variables weighted least squares regression (LSR) was used to model incremental OS months as a function of incremental PFS/TTP months. An exploratory analysis investigated the impact of three covariates (chemotherapy vs hormonal/targeted therapy, PFS vs TTP, and first-line therapy vs second-line therapy or greater) on OS prediction. The lower 95% prediction band was used to determine the minimum incremental PFS/TTP months required to predict OS benefit (surrogate threshold effect [STE]).
Results
Forty studies were identified. There was a statistically significant correlation between median PFS/TTP and OS (Pearson =0.741, P=0.000; Spearman =0.650, P=0.000). These results proved consistent for chemotherapy and hormonal/targeted therapy. Univariate LSR analysis yielded an R2 of 0.354 with 1 incremental PFS/TTP month corresponding to 1.13 incremental OS months. Controlling the type of treatment (chemotherapy vs hormonal/targeted therapy), line of therapy (first vs subsequent), and progression measure (PFS vs TTP) led to an improved R2 of 0.569 with 1 PFS/TTP month corresponding to 0.78 OS months. The STE for OS benefit was 5–6 months of incremental PFS/TTP.
Conclusion
We demonstrated a significant association between PFS/TTP and OS, which may justify the use of PFS/TTP as a surrogate for OS benefit in HR+, HER2− MBC.
Keywords: breast cancer, overall survival, progression-free survival, time to progression, correlation analysis, surrogate endpoint
Introduction
Improving overall survival (OS) has long been considered the most important therapeutic goal in advanced breast cancer.1,2 Longer survival profoundly benefits patients and provides a powerful demonstration of the drug’s effectiveness. Measurements of OS, the time from randomization to death, are objective, and their interpretation is straightforward. The preferred efficacy measurement from an economic perspective, OS, is used to calculate the cost per life year or quality-adjusted life year gained. These values are incorporated in the cost-effectiveness or cost-utility analyses that facilitate reimbursement and play an important role in drug access.3
Before mortality is reached and OS data are obtained, there may be opportunities for other factors to interfere. Subsequent therapies distort the relationship between an investigational treatment and OS. For ethical reasons, crossover therapy is provided in many trials: patients in the control arm also receive the investigational treatment, which complicates OS. Demonstrating significant gains in OS, which may amount to only a matter of months, requires large samples and statistical power.4 To assess OS at all, trials must have extended follow-up periods, which raise costs and create long delays in the drug approval process.5
Measurements based on disease behavior during treatment may similarly demonstrate clinical benefit and may be appropriate surrogate endpoints for OS. Progression-free survival (PFS) is defined as the time from randomization to tumor progression or death. The US Food and Drug Administration (FDA) favors PFS as a surrogate endpoint because it accounts for patients who die following tumor progression or following adverse events related to treatment.6 Time to progression (TTP) is a related endpoint defined as the time from randomization to tumor progression explicitly. Both PFS and TTP overcome some of the limitations of OS: they are not affected by subsequent therapies and the followup periods required are shorter. PFS can be assessed in a smaller sample population. However, though progression is typically judged quantitatively, there is a possibility of assessment bias.6 Determining the exact date of progression relies on frequent observation and some amount of interpretation.
PFS is nonetheless considered an acceptable surrogate endpoint for OS in colorectal cancer.7 In colorectal, breast, and other cancers, several therapies have been approved based on improved PFS.8–10 Following investigations focused on wider use of surrogate endpoints in different cancers, it has been proposed that the correlation between PFS/TTP depends on the type of cancer and specific features within cancer types.3
With the presence of numerous confounding factors, randomized clinical trials (RCTs) in advanced breast cancer rarely evaluate OS as a primary endpoint.11 The validation of PFS as a surrogate endpoint for OS in metastatic breast cancer (MBC) is ongoing, though PFS and TTP are sometimes informally accepted in place of OS.9 The process of formal validation of surrogate endpoints in MBC has provoked controversy, and the methodology continues to evolve.12 Additional evidence describing the relationship between PFS or TTP and OS in this disease setting could support the use of a surrogate endpoint. In this study, the correlation between PFS/TTP and OS and, therefore, the suitability of a potential surrogate are assessed in hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) MBC across all lines of therapy.
Methods
Systematic literature review (SLR)
An SLR of RCTs in HR+, HER2− MBC was performed to identify trials that reported both median PFS/TTP and median OS. An electronic literature database search of Embase, MEDLINE, and the Cochrane Library was conducted using the Ovid platform in January 2017. The search strategy for each database is presented in Tables S1–S3. RCTs with Stage IIIb/IV, HR+, HER2− MBC patients (aged ≥18 years) who received hormonal or systemic therapy were selected from the review of titles and abstracts. Among these, only RCTs that reported both incremental months of PFS/TTP and OS were included in this study. Details on the methods of the SLR were reported by Forsythe et al.15
Correlation analysis
For the analysis of the correlation between PFS/TTP and OS, each treatment arm was assumed to provide one observation, meaning that each patient group was treated separately. The relationship between PFS/TTP and OS was assessed using both Pearson’s product–moment correlation and Spearman’s rank correlation. The degree of association between PFS/TTP and OS was evaluated based on Pearson’s and Spearman’s correlation coefficients. If the correlation coefficient ranged from 0.00 to 0.19, 0.20 to 0.39, 0.40 to 0.59, 0.60 to 0.79, and 0.8 to 1, it was considered as a very weak, weak, moderate, strong, and very strong association, respectively.13 Confidence intervals for regression coefficients were calculated using Fisher’s Z-transformation. Specifically, R-coefficients were converted to Z-coefficients, 95% confidence intervals were calculated in terms of Z-statistics, and the resultant values were converted back to R-statistics.
For the initial correlation analysis with median months of PFS/TTP and OS, each treatment arm was assumed to provide one observation. In addition, the correlation between PFS/TTP incremental months and OS incremental months was evaluated as well as the correlation between PFS/TTP hazard ratios (HRs) and OS HRs. In these latter analyses, each study provided one observation. Subgroup analyses, which were defined based on the characteristics of the RCTs, were performed to explore possible reasons for heterogeneity.
Regression methodology using incremental PFS/TTP and OS months
For the multivariate regression analysis, the differences in PFS/TTP (incremental PFS/TTP months) and the differences in OS (incremental OS months) between treatment arms were calculated for each RCT. The pairs of the incremental PFS/TTP months and the incremental OS months from each RCT were used for regression analyses.
Errors-in-variables weighted least squares regression (LSR) was used to model incremental OS months as a function of incremental PFS/TTP months.14 The linear regression model was as follows:
In order to investigate the impact of exploratory factors on the use of PFS/TTP in OS prediction, multivariate linear regression analyses were performed adding three covariates: chemotherapy setting, definition of surrogate, and line of therapy. The multivariate regression model was as follows:
where Chemo is 1 for RCTs that included chemotherapy treatment and Chemo is 0 for RCTs that included hormonal and/or targeted therapy setting. Surrogate is 1 for RCTs that reported TTP, and Surrogate is 0 for RCTs that reported PFS; Line is 1, if RCTs investigated second or higher line of therapy, and Line is 0, if RCTs investigated first line of therapy.
Regression methodology using PFS/TTP and OS HRs
The multivariate regression analysis was conducted using three covariates, which were chemotherapy setting, definition of surrogate, and line of therapy. The pairs of the logarithmic values of PFS/TTP HRs and the log OS HRs from each RCT were used for the regression analyses.
The multivariate regression model was as follows:
where Chemo is 1 for RCTs that included chemotherapy treatment and Chemo is 0 for RCTs that included hormonal and/or targeted therapy setting. Surrogate is 1 for RCTs that reported TTP and Surrogate is 0 for RCTs that reported PFS; Line is 1, if RCTs investigated second or higher line of therapy, and Line is 0, if RCTs investigated first line of therapy.
Surrogate threshold effect (STE)
The concept of the STE described by Burzykowski et al14 can be used to determine threshold values for the estimator effect of the surrogate endpoint. The lower 95% prediction band was used to determine the STE: the minimum incremental PFS/TTP months (or HR value) below which there would be no predicted OS benefit. In the case of HR regression analysis, the STE indicated the minimum value of PFS HR that was associated with the statistically significant OS HR. The analyses were performed using the STATA 14.1 statistics software.
Results
RCTs included in the analysis
A total of 1017 abstracts were identified through the Ovid search (Figure 1). After reviewing the titles and abstracts, 107 abstracts remained for full-text review. During the full-text review, interim reports, updates, sub-group analyses, and meta-analyses and studies not reporting both PFS/TTP and OS months were excluded and a total of 43 unique RCTs were included. Of these, 39 RCTs reported both PFS/TTP and OS in two treatment arms (78 observations). Among the four remaining RCTs, one study reported PFS/TTP and OS in only one treatment arm (one observation). Two RCTs did not report OS and were excluded from this analysis. Finally, one RCT was not included in this analysis because it was terminated early. Therefore, a total of 79 pairs of PFS and OS from 40 RCTs were used for correlation analysis and 39 pairs of incremental PFS/TTP months and incremental OS months from 39 RCTs were used for meta-regression analysis.
Figure 1.
Flow chart of articles included in the analysis.
In 40 RCTs with 79 observations, the total of patient numbers was >20,000. The median patient age ranged from 49 to 66 years. Of the RCTs, 70% (28/40) were Phase III and 30% (12/40) were Phase II. There were more RCTs of chemotherapy than RCTs of hormonal therapy with or without targeted therapy (75 vs 25%). More RCTs reported PFS than TTP (92.5 vs 7.5%).
Correlation between median PFS/TTP and median OS months
There was a statistically significant correlation between median PFS/TTP and OS. Pearson’s correlation coefficient was 0.74 (P=0.000), and Spearman’s correlation coefficient was 0.65 (P=0.000). Both of these values indicate a strong association based on the predefined criteria.
Table 1 presents subgroup analyses of Pearson’s and Spear-man’s correlation coefficients. In general, Pearson’s coefficients indicated strong or very strong associations across almost all subgroups, including patient age, line of therapy, chemotherapy treatment, Eastern Cooperative Oncology Group (ECOG) performance status, HER2−/estrogen receptor positive (ER+)/progesterone receptor positive (PR+) status, number of metastatic sites, and prior chemotherapy (adjuvant or neoadjuvant).
Table 1.
Subgroup analysis of correlation between median PFS/TTP and median OS
| Categories | Sub-group | Sample number | Pearson’s coefficient | P-value | Spearman’s coefficient | P-value |
|---|---|---|---|---|---|---|
| Reference case | 79 | 0.741 | 0.000 | 0.650 | 0.000 | |
| Definition of surrogate | PFS | 73 | 0.747 | 0.000 | 0.664 | 0.000 |
| TTP | 6 | 0.532 | 0.278 | 0.086 | 0.862 | |
| Age group (years) | 40–59.9 | 52 | 0.749 | 0.000 | 0.646 | 0.000 |
| ≥60 | 17 | 0.793 | 0.000 | 0.722 | 0.001 | |
| Line of therapy | First line | 43 | 0.747 | 0.000 | 0.659 | 0.000 |
| Second line or more | 26 | 0.673 | 0.000 | 0.534 | 0.002 | |
| Chemotherapy setting | Chemotherapy setting | 59 | 0.741 | 0.000 | 0.681 | 0.000 |
| Others | 20 | 0.790 | 0.000 | 0.646 | 0.021 | |
| ECOG (%) | ECOG 0>50 | 34 | 0.724 | 0.000 | 0.555 | 0.000 |
| ECOG 0<50 | 9 | 0.936 | 0.000 | 0.929 | 0.000 | |
| ECOG 0>60 | 19 | 0.726 | 0.000 | 0.463 | 0.046 | |
| ECOG 0<60 | 24 | 0.780 | 0.000 | 0.739 | 0.000 | |
| HER2− (%) | 100 | 43 | 0.602 | 0.000 | 0.491 | 0.001 |
| <100 | 30 | 0.844 | 0.000 | 0.793 | 0.000 | |
| HR+ (%) | 100 | 14 | 0.827 | 0.000 | 0.899 | 0.000 |
| <100 | 35 | 0.355 | 0.036 | 0.353 | 0.004 | |
| ER+ (%) | >75 | 16 | 0.801 | 0.000 | 0.551 | 0.027 |
| ≤75 | 18 | 0.895 | 0.000 | 0.854 | 0.000 | |
| PR+ (%) | >60 | 12 | 0.765 | 0.004 | 0.413 | 0.183 |
| ≤60 | 14 | 0.922 | 0.000 | 0.820 | 0.000 | |
| Visceral metastases (%) | >50 | 46 | 0.673 | 0.000 | 0.652 | 0.000 |
| ≤50 | 7 | 0.557 | 0.195 | 0.072 | 0.878 | |
| Number of metastatic sites, <3 sitesa (%) | At least 60 | 37 | 0.725 | 0.000 | 0.493 | 0.002 |
| Prior endocrine therapy (%) | At least 50 | 23 | 0.448 | 0.032 | 0.316 | 0.141 |
| <50 | 12 | 0.892 | 0.000 | 0.830 | 0.001 | |
| Prior chemotherapy (adjuvant/neoadjuvant, n) (%) | At least 75 | 11 | 0.666 | 0.025 | 0.360 | 0.277 |
| <75 | 32 | 0.812 | 0.000 | 0.755 | 0.000 |
Note:
The results in the subgroup of <60% are not presented because there were only two observations from one RCT.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ER+, estrogen receptor positive; HER2−, human epidermal growth factor receptor 2 negative; HR+, hormone receptor positive; OS, overall survival; PFS, progression-free survival; PR+, progesterone receptor positive; RCT, randomized controlled trial; TTP, time to progression.
RCTs that reported TTP instead of PFS showed a lower correlation coefficient (r=0.532, P=0.278) than RCTs that reported PFS (r=0.747, P=0.000). However, fewer RCTs reported TTP (n=6) than PFS (n=73).
The number of RCTs in which <50% of patients had visceral metastases was also small (n=7), and their correlation was weaker (r=0.557, P=0.195) than RCTs in which >50% of patients had visceral metastases (r=0.673, P=0.000).
Pearson’s coefficient among the RCTs that had <50% patients with prior endocrine therapy (r=0.892, P=0.000) was higher than among RCTs that had at least 50% patients with prior endocrine therapy (r=0.448, P=0.032).
The RCTs investigating only first-line therapy showed a slightly higher coefficient (r=0.747, P=0.000) than the RCTs investigating second- or higher line therapy (r=0.673, P=0.000), which implies that the correlation between PFS/TTP and OS would be stronger in first-line therapy than in subsequent lines of therapy. There was no substantial difference in Pearson’s coefficients between RCTs investigating PFS/TTP and OS in the chemotherapy setting (r=0.741, P=0.000) and the hormonal or targeted therapy settings (r=0.790, P=0.000).
Pearson’s coefficient among the RCTs that had <75% patients with prior chemotherapy reported as adjuvant or neoadjuvant (r=0.812, P=0.000) was higher than the RCTs that had at least 75% patients with prior adjuvant or neoadjuvant chemotherapy (r=0.666, P=0.025). Pearson’s coefficient among the RCTs that had >50% patients with visceral metastases (r=0.766, P=0.027) was higher than among the RCTS in which 50% of patients or less had visceral metastases (r=0.632, P<0.001).
There was no substantial difference in Pearson’s coefficients between RCTs investigating PFS/TTP and OS in patients with mean age <60 (r=0.749, P=0.000) and ≥60 (r=0.793, P=0.000) years. All sub-groups defined by ECOG performance status showed strong or very strong correlations.
Sub-group analyses using Spearman’s rank correlation showed similar trends to Pearson’s correlation, except regarding visceral metastases. Spearman’s correlation coefficient was higher in RCTs where at least 50% of patients had visceral metastases (r=0.652, P=0.000) than in those where <50% of patients had visceral metastases (r=0.072, P=0.878). Spearman’s correlation coefficient in RCTs that reported TTP represented a very weak association (r=0.086, P=0.862). RCTs in which at least 50% of patients had prior endocrine therapy (r=0.316, P=0.141) and at least 75% had prior adjuvant or neoadjuvant chemotherapy (r=0.360, P=0.277) showed weak associations.
Correlation of incremental PFS/TTP and OS months
Correlation analyses were conducted in 39 studies reporting incremental PFS/TTP and OS months. There was a statistically significant correlation between incremental PFS/TTP and OS months, although the values of coefficients were lower as compared to previous analysis of median PFS/TTP and OS months. Pearson’s correlation coefficient was 0.51 (P=0.0009), indicating a moderate association, while Spearman’s correlation coefficient was 0.36 (P=0.025), indicating a weak association between PFS and OS based on the predefined criteria.
RCTs that reported TTP showed higher correlation coefficients (r=0.891, P=0.301) than RCTs that reported PFS (r=0.484, P=0.003). However, the sample size was much smaller (n=3) than RCTs that reported PFS (n=36).
Correlation of incremental log PFS/TTP HRs and log OS HRs
Correlation analyses were conducted in 32 studies reporting incremental log PFS/TTP HRs and log OS HRs. There was a statistically significant correlation between log PFS/TTP HRs and log OS HRs. Both Pearson’s correlation coefficient at 0.56 (P=0.0010) and Spearman’s correlation coefficient at 0.45 (P=0.0106) indicated a moderate association between PFS and OS based on the predefined criteria.
Results of the regression and STE analyses using incremental PFS/TTP and OS months
Linear weighted regression
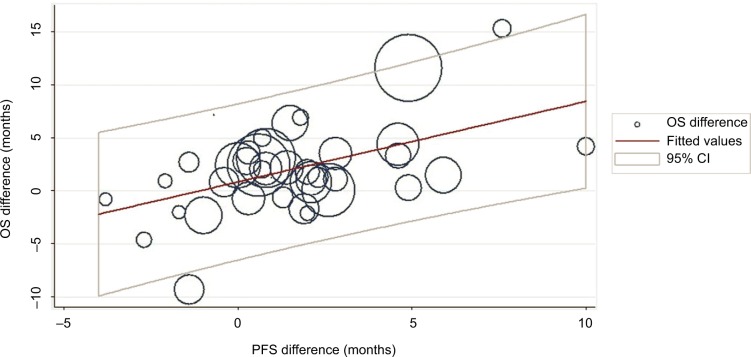
Linear regression analysis was performed with 39 pairs of incremental PFS/TTP months and incremental OS months. Initial LSR analysis with weighted trial size yielded a coefficient of 1.13 (P=0.000) and an R2 of 0.354, which indicates that 1 incremental PFS/TTP month significantly corresponds to 1.13 incremental OS months (Figure 2).
Figure 2.
Regression analysis with weighted trial size.
Note: Point size corresponds to trial size.
Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression-free survival.
The model was: incremental OS months = 0.58 + 1.13 X incremental PFS/TTP months.
Multivariate-weighted regression analysis
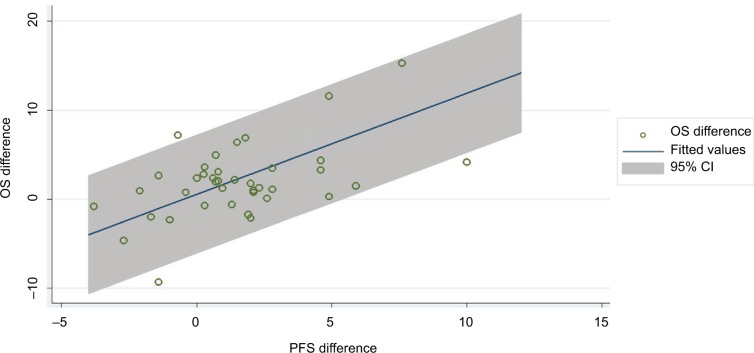
Multivariate regression analysis adding two covariates; chemotherapy treatment (vs hormonal with or without targeted therapy) and definition of surrogate endpoint (TTP vs PFS), improved the R2 to 0.568 (Figure 3). The coefficient of PFS/TTP was 0.76 (P=0.000), indicating that an increase of 1 month in PFS/TTP was significantly associated with an increase of 0.76 months in OS.
Figure 3.
Multivariate regression analysis adding two covariates.
Note: Point size corresponds to trial size.
Abbreviations: CI, confidence interval; OS, overall survival; PFS, progression-free survival.
The model was: incremental OS months =0.65–0.27 X Chemo + 5.80 X Surrogate + 0.76 incremental PFS/TTP months.
The STE for OS benefit was 5–6 months of incremental PFS/TTP; therefore, an incremental PFS/TTP of at least 5–6 months would be required to predict incremental OS.
Upon adding a third covariate, line of therapy, R2 varied little (0.569) and an incremental gain of 1 month of PFS/TTP corresponded to an increase in OS 0.78 months.
Results of the regression and STE analyses using PFS/TTP and OS HRs
Weighted univariate regression
Weighted univariate regression analyses with logarithmic values of 32 pairs of PFS/TTP and OS HRs were conducted. The coefficient of log PFS/TTP HRs was statistically significant at −0.214 (P=0.034); however, the R2 was low (0.171), showing low predictability of OS using PFS.
Weighted multivariate regression
Weighted multivariate regression analysis with logarithmic values of 32 pairs of PFS/TTP and OS HRs was also conducted using three covariates, which were chemotherapy treatment (vs hormonal with or without targeted therapy), definition of surrogate endpoint (TTP vs PFS), and line of therapy. The coefficient of log PFS/TTP HRs was no longer statistically significant at 0.216 (P=0.052); however, there was a slight improvement in the R2 (0.236). Considering the low R2 and nonsignificant coefficient for PFS/TTP, we can conclude that log PFS/TTP HRs were not significant predictors of log OS HRs.
Discussion
The SLR of RCTs in HR+, HER2− MBC identified 40 studies that reported both PFS/TTP and OS. Correlation analysis revealed a statistically significant association between median months of PFS/TTP and OS (Pearson =0.741, P=0.000; Spearman =0.650, P=0.000). This result did not drastically vary with the type of systemic treatment, eg, chemotherapy, hormonal therapy, and targeted therapy. Similarly, demonstrated by Pearson’s coefficient, a strong or very strong association was maintained across almost all subgroups: age, line of therapy, chemotherapy treatment, ECOG performance status, hormone receptor status, visceral metastases, number of metastases, and prior chemotherapy. Therefore, the correlation between PFS/TTP and OS is not highly sensitive to baseline characteristics.
While the correlation between PFS/TTP and OS was fairly consistent, PFS may have greater predictive power in certain subgroups. Trials that included only HR+ patients demonstrated a very strong correlation (r=0.827, P=0.000), and PFS was a very good predictor of OS in this subgroup. The strength of the correlation decreased in trials with <100% HR+ patients (r=0.355, P=0.036). Interestingly, the correlation between PFS/TTP and OS was stronger in RCTs investigating first-line therapies than in those investigating subsequent-line therapies (r=0.747, P=0.000 vs r=0.673, P=0.000), though the result was not statistically significant.
Most of the studies included in this analysis measured PFS (92.5%), and the correlation was stronger in these studies than in those that measured TTP (7.5%). The latter group represents a smaller sample size with less statistical power. Pearson’s correlation in RCTs including <50% of patients with visceral metastases was weaker than RCTs including >50% of patients with visceral metastases, and Spearman’s correlation showed the same trend.
Analyses of incremental PFS/TTP and OS months and log PFS/TTP and OS HRs demonstrated a significantly moderate correlation between incremental PFS/TTP and OS months. The initial univariate LSR analysis with incremental PFS/TTP and OS months yielded an R2 of 0.354 with 1 incremental PFS/TTP month corresponding to 1.13 incremental OS months. Controlling the type of treatment, line of therapy, and progression measure leads to an improved R2 of 0.569 with 1 PFS/TTP month corresponding to 0.78 OS months. The STE for OS benefit was 5–6 months of incremental PFS/TTP, which indicated that only studies demonstrating at least 5–6 months of incremental PFS/TTP improvement should be considered predictive of OS. Weighted multivariate regression analysis with logarithmic values of 32 pairs of PFS/TTP and OS HRs did not support a hypothesis of log PFS/TTP HRs as surrogate for log OS yielding a low R2 without statistical significance.
This analysis addressed the question of PFS as a surrogate marker for OS through the analysis of incremental PFS/OS months, while the HR analyses aimed to address the question of treatment effect. Both incremental analysis and HR analysis have limitations. Incremental PFS can be falsely correlated to OS based on the relationship between the two statistical flags, where PFS is defined as the end of stable disease or mortality. Regression analyses using HRs may be biased as the HRs consider both the treatment effect and the correlation between OS and PFS. These limitations may explain the statistical insignificance of the regression analysis results. Additionally, the sample in the HR analysis was close to 30, which is the minimum for such a regression.
The multivariate regression analysis of incremental PFS/TTP months as a surrogate for OS months demonstrated a high R2 value (the proportion of the variance in the dependent variable that is predictable from the independent variable), and as such, this analysis delivers a convincing argument for PFS as a potential surrogate for OS. Considered together, evidence provided here helps to establish the PFS surrogate as an important tool in HR+, HER2− MBC with applications that will enhance the value of RCT data and promote more concise research practices.
Supplementary materials
Table S1.
Search strategy for MEDLINE
| 1 | exp Breast Neoplasms/ | 250773 |
| 2 | (breast adj6 cancer$).af. | 246948 |
| 3 | (breast adj6 neoplas$).af. | 252542 |
| 4 | (breast adj6 carcinoma$).af. | 63807 |
| 5 | (breast adj6 tumour$).af. | 7401 |
| 6 | (breast adj6 tumor$).af. | 48416 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 336672 |
| 8 | metasta$.mp. or exp Neoplasm Metastasis/ | 477568 |
| 9 | 7 and 8 | 74656 |
| 10 | (“metastatic breast cancer” or “metastatic breast neoplasms”).af. | 11972 |
| 11 | 9 or 10 | 74661 |
| 12 | ‘hormone receptor positive’.af. | 2300 |
| 13 | ‘hormone receptor-positive’.af. | 2300 |
| 14 | (‘estrogen receptor-positive’ or ‘oestrogen receptor-positive’).af. | 4218 |
| 15 | ‘progesterone receptor-positive’.af. | 732 |
| 16 | ‘hormone sensitive’.af. | 3719 |
| 17 | 12 or 13 or 14 or 15 or 16 | 10513 |
| 18 | 11 and 17 | 1955 |
| 19 | exp randomized controlled trials/ | 111704 |
| 20 | randomized controlled trial.pt. | 448501 |
| 21 | exp random allocation/or exp randomization/ | 89826 |
| 22 | exp placebos/ | 34191 |
| 23 | exp Double-Blind Method/or double-blind$.af. | 180225 |
| 24 | exp Multicenter Study/or multicent$.af. | 279405 |
| 25 | random$.ti,ab,kw,sh. | 1124651 |
| 26 | blind$.ti,ab,kw,sh. | 263750 |
| 27 | placebo$.ti,ab,kw,sh. | 203823 |
| 28 | parallel$.ti,ab,kw,sh. | 266233 |
| 29 | exp clinical trial, phase 3/ | 13116 |
| 30 | exp clinical trial, phase 2/ | 29002 |
| 31 | (‘phase 3’ or ‘phase 2’ or (‘phase III’ or ‘phase II’)).af. | 112861 |
| 32 | 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 | 1752319 |
| 33 | 18 and 32 | 610 |
| 34 | limit 33 to yr=”2006 -Current” | 422 |
| 35 | limit 34 to “review articles” | 74 |
| 36 | 34 not 35 | 348 |
| 37 | limit 36 to humans | 297 |
| 38 | remove duplicates from 37 | 244 |
Table S2.
Search strategy for Embase
| 1 | breast cancer’.af. | 404912 |
| 2 | exp breast tumor/ | 456726 |
| 3 | (breast adj6 tumour*).mp | 10358 |
| 4 | (breast adj6 tumor*).mp | 132238 |
| 5 | (breast adj6 neoplas*).mp | 22949 |
| 6 | (breast adj6 cancer*).mp | 446177 |
| 7 | (breast adj6 carcinoma*).mp | 95202 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 513111 |
| 9 | metastasis/ | 310169 |
| 10 | metasta* | 697570 |
| 11 | 9 or 10 | 697570 |
| 12 | 8 and 11 | 123533 |
| 13 | (‘metastatic breast neoplasms’ or ‘metastatic breast neoplasm’ or ‘metastatic breast cancer’).mp | 19097 |
| 14 | 12 or 13 | 123533 |
| 15 | hormone receptor positive’ OR ‘hormone receptor-positive’ | 3917 |
| 16 | progesterone receptor-positive’ OR ‘progesterone receptor positive’ | 1185 |
| 17 | estrogen receptor-positive’ or ‘oestrogen receptor-positive’ | 7549 |
| 18 | hormone sensitive’ | 4924 |
| 19 | hormone adj3 positive | 5460 |
| 20 | 15 or 16 or 17 or 18 or 19 | 17999 |
| 21 | 14 and 20 | 4258 |
| 22 | exp ‘randomized controlled trial’/ | 481221 |
| 23 | randomization/ | 84943 |
| 24 | random*.ti,ab. | 1181594 |
| 25 | parallel*.ti,ab | 303245 |
| 26 | ((single or double or triple) adj3 (blind* or mask* or dummy)).ti,ab. | 201720 |
| 27 | double-blind’ or ‘double-blinded’ | 219339 |
| 28 | multicenter study’ or multicent* | 277968 |
| 29 | blind*.ti,ab. | 341487 |
| 30 | placebo*.ti,ab | 254042 |
| 31 | (‘phase 3’ OR ‘phase 2’).ti,ab | 40098 |
| 32 | (‘phase iii’ OR ‘phase ii’).ti,ab | 111214 |
| 33 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 2028041 |
| 34 | 21 and 33 | 1173 |
| 35 | limit 34 to human | 1094 |
| 36 | limit 35 to english language | 1075 |
| 37 | limit 36 to yr=”2006 -Current” | 898 |
| 38 | limit 37 to embase | 562 |
| 39 | limit 38 to (article or conference abstract) | 501 |
Table S3.
Search strategy for Cochrane Library
| 1 | exp Breast Neoplasms | 9184 |
| 2 | breast adj6 cancer$ | 21934 |
| 3 | breast adj6 neoplas$ | 10989 |
| 4 | breast adj6 carcinoma$ | 2112 |
| 5 | breast adj6 tumour$ | 573 |
| 6 | breast adj6 tumor$ | 1851 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 23744 |
| 8 | metasta$ | 21265 |
| 9 | Neoplasm Metastasis.sh. | 2226 |
| 10 | 8 or 9 | 21265 |
| 11 | 7 and 10 | 6121 |
| 12 | ‘hormone receptor positive’ | 607 |
| 13 | hormone receptor-positive’ 588 | 607 |
| 14 | ‘estrogen receptor-positive’ or ‘oestrogen receptor-positive’ 654 | 522 |
| 15 | ‘progesterone receptor-positive’ 250 | 109 |
| 16 | hormone sensitive 986 | 210 |
| 17 | 12 or 13 or 14 or 15 or 16 2325 | 1318 |
| 18 | 11 and 17 | 453 |
| 19 | limit 18 to yr=”2006 -Current” | 329 |
| 20 | limit 19 to english language | 284 |
| 21 | limit 20 to humans | 278 |
| 22 | remove duplicates from 21 | 272 |
Acknowledgments
Part of this article was presented at the 2017 European Society for Medical Oncology as a poster presentation with interim findings. The poster’s abstract was published in the Annals of Oncology (2017) 28 (suppl_5): v74–v108. 10.1093/annonc/mdx365. We thank Jaclyn Hearnden for her medical writing contributions and assistance in preparing the article. This study was sponsored by Novartis.
Footnotes
Author contributions
Anna Forsythe and Gabriel Tremblay made substantial contributions to the conception and design, analyzed and interpreted the data, and helped to draft the article. David Chandiwana, Janina Barth, Marroon Thabane, and Johan Baeck contributed to the conception and development of this study and evaluation and interpretation of the data. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
David Chandiwana, Janina Barth, Marroon Thabane, and Johan Baeck are employees of Novartis. The authors report no other conflicts of interest in this work.
References
- 1.Zhuang SH, Xiu L, Elsayed YA. Overall survival: a gold standard in search of a surrogate: the value of progression-free survival and time to progression as end points of drug efficacy. Cancer J. 2009;15(5):395–400. doi: 10.1097/PPO.0b013e3181be231d. [DOI] [PubMed] [Google Scholar]
- 2.Driscoll JJ, Rixe O. Overall survival: still the gold standard: why overall survival remains the definitive end point in cancer clinical trials. Cancer J. 2009;15(5):401–405. doi: 10.1097/PPO.0b013e3181bdc2e0. [DOI] [PubMed] [Google Scholar]
- 3.Davis S, Tappenden P, Cantrell A. A Review of Studies Examining the Relationship between Progression-Free Survival and Overall Survival in Advanced or Metastatic Cancer. Sheffield: School of Health and Related Research, University of Sheffield; 2012. [PubMed] [Google Scholar]
- 4.Di Leo A, Bleiberg H, Buyse M. Overall survival is not a realistic end point for clinical trials of new drugs in advanced solid tumors: a critical assessment based on recently reported phase III trials in colorectal and breast cancer. J Clin Oncol. 2003;21(10):2045–2047. doi: 10.1200/JCO.2003.99.089. [DOI] [PubMed] [Google Scholar]
- 5.Saad ED, Katz A, Buyse M. Overall survival and post-progression survival in advanced breast cancer: a review of recent randomized clinical trials. J Clin Oncol. 2010;28(11):1958–1962. doi: 10.1200/JCO.2009.25.5414. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services Food and Drug Administration Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. [Accessed March 19, 2017]. Available from: https://www.fda.gov/downloads/Drugs/Guidances/ucm071590.pdf.
- 7.Buyse M, Burzykowski T, Carroll K, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;25(33):5218–5224. doi: 10.1200/JCO.2007.11.8836. [DOI] [PubMed] [Google Scholar]
- 8.Sridhara R, Johnson JR, Justice R, Keegan P, Chakravarty A, Pazdur R. Review of oncology and hematology drug product approvals at the US Food and Drug Administration between July 2005 and December 2007. J Natl Cancer Inst. 2010;102(4):230–243. doi: 10.1093/jnci/djp515. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JR, Williams G, Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol. 2003;21(7):1404–1411. doi: 10.1200/JCO.2003.08.072. [DOI] [PubMed] [Google Scholar]
- 10.Cortazar P, Justice R, Johnson J, Sridhara R, Keegan P, Pazdur R. US Food and Drug Administration approval overview in metastatic breast cancer. J Clin Oncol. 2012;30(14):1705–1711. doi: 10.1200/JCO.2011.39.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith I. Goals of treatment for patients with metastatic breast cancer. Semin Oncol. 2006;33(suppl 2):2–5. doi: 10.1053/j.seminoncol.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Saad ED, Buyse M. Statistical controversies in clinical research: end points other than overall survival are vital for regulatory approval of anticancer agents. Ann Oncol. 2016;27(3):373–378. doi: 10.1093/annonc/mdv562. [DOI] [PubMed] [Google Scholar]
- 13.Swinscow TDV, Campbell MJ. Section 11 Correlations and regressions Statistics at Square One. 9th ed. London: BMJ Publishing Group Ltd; 1997. [Accessed March 19, 2017]. webpage on the Internet. Available from: http://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression. [Google Scholar]
- 14.Burzykowski TMG, Buyse M. The Evaluation of Surrogate Endpoints. New York: Springer; 2005. [Google Scholar]
- 15.Forsythe A, Chandiwana D, Barth J, Thabane M, Baeck J, Tremblay G. Is progression-free survival a more relevant endpoint than overall survival in first-line HR+, HER2− metastatic breast cancer? Cancer Manag Res. 2018 doi: 10.2147/CMAR.S162714. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Search strategy for MEDLINE
| 1 | exp Breast Neoplasms/ | 250773 |
| 2 | (breast adj6 cancer$).af. | 246948 |
| 3 | (breast adj6 neoplas$).af. | 252542 |
| 4 | (breast adj6 carcinoma$).af. | 63807 |
| 5 | (breast adj6 tumour$).af. | 7401 |
| 6 | (breast adj6 tumor$).af. | 48416 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 336672 |
| 8 | metasta$.mp. or exp Neoplasm Metastasis/ | 477568 |
| 9 | 7 and 8 | 74656 |
| 10 | (“metastatic breast cancer” or “metastatic breast neoplasms”).af. | 11972 |
| 11 | 9 or 10 | 74661 |
| 12 | ‘hormone receptor positive’.af. | 2300 |
| 13 | ‘hormone receptor-positive’.af. | 2300 |
| 14 | (‘estrogen receptor-positive’ or ‘oestrogen receptor-positive’).af. | 4218 |
| 15 | ‘progesterone receptor-positive’.af. | 732 |
| 16 | ‘hormone sensitive’.af. | 3719 |
| 17 | 12 or 13 or 14 or 15 or 16 | 10513 |
| 18 | 11 and 17 | 1955 |
| 19 | exp randomized controlled trials/ | 111704 |
| 20 | randomized controlled trial.pt. | 448501 |
| 21 | exp random allocation/or exp randomization/ | 89826 |
| 22 | exp placebos/ | 34191 |
| 23 | exp Double-Blind Method/or double-blind$.af. | 180225 |
| 24 | exp Multicenter Study/or multicent$.af. | 279405 |
| 25 | random$.ti,ab,kw,sh. | 1124651 |
| 26 | blind$.ti,ab,kw,sh. | 263750 |
| 27 | placebo$.ti,ab,kw,sh. | 203823 |
| 28 | parallel$.ti,ab,kw,sh. | 266233 |
| 29 | exp clinical trial, phase 3/ | 13116 |
| 30 | exp clinical trial, phase 2/ | 29002 |
| 31 | (‘phase 3’ or ‘phase 2’ or (‘phase III’ or ‘phase II’)).af. | 112861 |
| 32 | 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 | 1752319 |
| 33 | 18 and 32 | 610 |
| 34 | limit 33 to yr=”2006 -Current” | 422 |
| 35 | limit 34 to “review articles” | 74 |
| 36 | 34 not 35 | 348 |
| 37 | limit 36 to humans | 297 |
| 38 | remove duplicates from 37 | 244 |
Table S2.
Search strategy for Embase
| 1 | breast cancer’.af. | 404912 |
| 2 | exp breast tumor/ | 456726 |
| 3 | (breast adj6 tumour*).mp | 10358 |
| 4 | (breast adj6 tumor*).mp | 132238 |
| 5 | (breast adj6 neoplas*).mp | 22949 |
| 6 | (breast adj6 cancer*).mp | 446177 |
| 7 | (breast adj6 carcinoma*).mp | 95202 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 513111 |
| 9 | metastasis/ | 310169 |
| 10 | metasta* | 697570 |
| 11 | 9 or 10 | 697570 |
| 12 | 8 and 11 | 123533 |
| 13 | (‘metastatic breast neoplasms’ or ‘metastatic breast neoplasm’ or ‘metastatic breast cancer’).mp | 19097 |
| 14 | 12 or 13 | 123533 |
| 15 | hormone receptor positive’ OR ‘hormone receptor-positive’ | 3917 |
| 16 | progesterone receptor-positive’ OR ‘progesterone receptor positive’ | 1185 |
| 17 | estrogen receptor-positive’ or ‘oestrogen receptor-positive’ | 7549 |
| 18 | hormone sensitive’ | 4924 |
| 19 | hormone adj3 positive | 5460 |
| 20 | 15 or 16 or 17 or 18 or 19 | 17999 |
| 21 | 14 and 20 | 4258 |
| 22 | exp ‘randomized controlled trial’/ | 481221 |
| 23 | randomization/ | 84943 |
| 24 | random*.ti,ab. | 1181594 |
| 25 | parallel*.ti,ab | 303245 |
| 26 | ((single or double or triple) adj3 (blind* or mask* or dummy)).ti,ab. | 201720 |
| 27 | double-blind’ or ‘double-blinded’ | 219339 |
| 28 | multicenter study’ or multicent* | 277968 |
| 29 | blind*.ti,ab. | 341487 |
| 30 | placebo*.ti,ab | 254042 |
| 31 | (‘phase 3’ OR ‘phase 2’).ti,ab | 40098 |
| 32 | (‘phase iii’ OR ‘phase ii’).ti,ab | 111214 |
| 33 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 2028041 |
| 34 | 21 and 33 | 1173 |
| 35 | limit 34 to human | 1094 |
| 36 | limit 35 to english language | 1075 |
| 37 | limit 36 to yr=”2006 -Current” | 898 |
| 38 | limit 37 to embase | 562 |
| 39 | limit 38 to (article or conference abstract) | 501 |
Table S3.
Search strategy for Cochrane Library
| 1 | exp Breast Neoplasms | 9184 |
| 2 | breast adj6 cancer$ | 21934 |
| 3 | breast adj6 neoplas$ | 10989 |
| 4 | breast adj6 carcinoma$ | 2112 |
| 5 | breast adj6 tumour$ | 573 |
| 6 | breast adj6 tumor$ | 1851 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 23744 |
| 8 | metasta$ | 21265 |
| 9 | Neoplasm Metastasis.sh. | 2226 |
| 10 | 8 or 9 | 21265 |
| 11 | 7 and 10 | 6121 |
| 12 | ‘hormone receptor positive’ | 607 |
| 13 | hormone receptor-positive’ 588 | 607 |
| 14 | ‘estrogen receptor-positive’ or ‘oestrogen receptor-positive’ 654 | 522 |
| 15 | ‘progesterone receptor-positive’ 250 | 109 |
| 16 | hormone sensitive 986 | 210 |
| 17 | 12 or 13 or 14 or 15 or 16 2325 | 1318 |
| 18 | 11 and 17 | 453 |
| 19 | limit 18 to yr=”2006 -Current” | 329 |
| 20 | limit 19 to english language | 284 |
| 21 | limit 20 to humans | 278 |
| 22 | remove duplicates from 21 | 272 |