Abstract
Dissimilatory metal-reducing bacteria can mobilize As, but few studies have studied such processes in deeper orange-colored Pleistocene sands containing 1–2 mg kg−1 As that are associated with low-As groundwater in Bangladesh. To address this gap, anaerobic incubations were conducted in replicate over 90 days using natural orange sands initially containing 0.14 mg kg−1 of 1 M phosphate-extractable As (24 hr), >99% as As(V), and 0.8 g kg−1 of 1.2 M HCl-leachable Fe (1 hr at 80°C), 95% as Fe(III). The sediment was resuspended in artificial groundwater, with or without lactate as a labile carbon source, and inoculated with metal-reducing Shewanella sp. ANA-3. Within 23 days, dissolved As concentrations increased to 17 μg L−1 with lactate, 97% as As(III), and 2 μg L−1 without lactate. Phosphate-extractable As concentrations increased 4-fold to 0.6 mg kg−1 in the same incubations, even without the addition of lactate. Dissolved As levels in controls without Shewanella, both with and without lactate, instead remained <1 μg L−1. These observations indicate that metal-reducers such as Shewanella can trigger As release to groundwater by converting sedimentary As to a more mobilizable form without the addition of high levels of labile carbon. Such interactions need to be better understood to determine the vulnerability of low-As aquifers from which drinking water is increasingly drawn in Bangladesh.
Introduction
Shared deep wells, typically more than 150 m deep and installed by the government or nongovernmental organizations, have emerged over the past decade as an important means of reducing the exposure to As of the rural population of Bangladesh (1). There are, however, concerns about the risk of contaminating the deep aquifer with As drawn down from shallow depths, especially if crop irrigation, which accounts for about 90% of all groundwater use in the country, were to tap the deep aquifer (2). The concern is that, in addition to transporting As into the deep aquifer, intruding shallow groundwater could potentially stimulate biogeochemical transformations that release potentially labile As from the sediment to groundwater (3).
A number of microcosm experiments using natural soils and aquifer sands from West Bengal (India), Bangladesh, and Cambodia have demonstrated the release of As following the addition of electron donors in various forms, although typically starting from shallow grey sands containing at least partially reduced amorphous iron minerals (4–7). A subset of these experiments paired with microbial community analysis has shown that metal-reducing bacteria such as Geobacter play a role in releasing As to groundwater either by reducing As-bearing Fe oxides or by reducing sediment-bound As(V) to As(III) (4, 7). Column experiments using ferrihydrite-coated sand loaded with As have separated the role of Fe and As reduction using modified strains of the genus Shewanella in the laboratory (8). The need for substantial reduction of Fe oxides to mobilize As is fairly well established after a decade of research (9–11), but whether reduction of As accompanies its release to solution or is a pre-requisite for mobilization in natural systems remains unclear.
The need for Fe and As reduction is re-examined here on the basis of incubations carried out anaerobically over a period of three months using orange-colored sand from Bangladesh containing primarily Fe(III) and As(V). The choice of starting material reflects the importance of such less-reduced, but still anoxic, aquifers as a source of low-As groundwater (3, 9). Deeper wells tapping such aquifers have reliably supplied numerous villages of Bangladesh with low-As groundwater for over a decade, although a few failures due to increasing As concentrations have also been reported (12). The cause of these failures is presently not known. A shallow break in a PVC pipe could result in high-As groundwater entering a well, but so could potentially a regional intrusion of shallow groundwater into a deeper aquifer. This study was designed to simulate certain aspects of a regional intrusion. The orange sands used in the incubations are representative of deeper aquifers in Bangladesh of likely Pleistocene age. Total As concentrations in the orange sands are close to its crustal abundance of 1–2 mg kg−1 (13) and approximately an order-of-magnitude lower than in shallower grey sands associated with groundwater elevated in As (3, 9).
The orange sands were inoculated with Shewanella sp. strain ANA-3 which is a well-characterized As(V) and Fe(III) reducer that has been used for incubations of synthetic Fe(III)-oxide coated sands containing As (8, 14–16). Although Shewanella species are not major constituents of subsurface environments, there is considerable genomic evidence that the enzymes and genes for As(V) reduction are highly conserved among very diverse bacteria (10), some of which have been detected in aquifer sediments of West Bengal and Cambodia (7, 17–18). Shewanella also uses enzyme pathways for Fe(III) reduction similar to Geobacter which, in contrast, has been shown to be ubiquitous in the subsurface (19). Shewanella sp. strain ANA-3 contains, in addition, the plasmid pBBR1-MCS2 that provides resistance to the antibiotic kanamycin. The intention was to separate the effect of a well-studied organism such as Shewanella from that of the complex natural assemblage of microorganisms present in the sediment by amending the incubations with kanamycin. Although the kanamycin did not fully suppress the activity of the native micro-organisms, the incubations clearly demonstrate that Shewanella amendments can trigger the release of As from orange sands without extensive reduction of Fe oxides driven by a large addition of metabolizable carbon.
Experimental
Sediment Collection and Preservation
A core section from 43.3–43.9 m depth in Dari Satyabandi village (23.785°N, 90.603°E) in Araihazar, Bangladesh, was used in all experiments after manually homogenizing the sand inside an anaerobic chamber containing 95% N2:5% H2 (Coy Lab. Prod. Inc., AALC). Both ends of the PVC liner containing the core were wax-sealed on site immediately after collection in January 2001 (3). The cores were shipped at ambient temperature and stored at 4°C upon their arrival at Lamont-Doherty Earth Observatory’s core repository in May 2001. When the liner was opened inside the anaerobic chamber in July 2005, the sediment was still orange and moist. The presence of water inside the core after four years of storage in a refrigerated and dry environment indicates that the wax seal was intact. Exposure of the core was therefore limited exposed to atmospheric oxygen slowly diffusing through the wax and the PVC liner.
Shewanella Culture
Shewanella sp. strain ANA-3 was previously isolated from an As-treated wooden pier pilling in Woods Hole, Massachusetts (14, 16). The strain was initially grown aerobically and then anaerobically in TME media (20) on an incubator shaker (250 rpm) at 28–30°C for 24 hours and 72 hours respectively. The TME media for aerobic growth was prepared by adding 1.5 g of NH4Cl, 0.6 g of NaH2PO4, 0.1 g of KCl, 0.5 g of yeast extract (50 mL of autoclave sterilized stock solution of 10 g L−1), 20 mmole L−1 Na-lactate (20 ml of autoclaved sterilized stock solution of 1mole L−1) and 10 ml trace metal (modified Wolf’s mineral mix) solution (20) in Milli Q water (18.1 MΩ) to make 1 L of solution. The pH was adjusted to 7 by adding 1 g of Na-HEPES to 1 L of the media. For anaerobic growth of Shewanella, an anaerobic electron acceptor was added to TME media. In this case, 1.56 g of Na2HAsO4.7H2O was added to 500 mL aliquots of initial TME media to reach an As concentration of 10 mmole L−1 (16). The TME media was autoclave sterilized prior to use for aerobic and anaerobic growth. Shewanella sp. ANA-3 strain containing plasmid pBBR1-MCS2 was cultured with 50 μg mL−1 kanamycin (further details provided in Supporting Information).
Incubations
The orange sands were incubated anaerobically for 3 months with five different types of amendments. Three sets of controls were incubated without Shewanella: the first, labeled C0, contained only sediment and artificial groundwater; the second (Clac) was amended with lactate (Fisher, syrup 60% w/w) to a concentration of 20 mmole L−1; the third (Clac+kan) was also amended with the same amount of lactate and with 50 μg mL−1 of the antibiotic kanamycin. Incubations inoculated with Shewanella sp. ANA-3 were all amended with 50 μg mL−1 kanamycin, one with 20 mmole L−1 lactate (Slac+kan) and the other without (Skan).
For all incubations, approximately 5 g of sand was added to sterile 17-mL culture tubes (Hungate 2047-16125) with 10 mL of artificial groundwater previously purged with ultrapure N2 for 2 hours. During preparation of culture tubes, tubes with sediment were also purged with ultrapure N2 and capped immediately after addition of artificial groundwater. Artificial groundwater of composition similar to that of groundwater associated with the Pleistocene aquifer in Dari Satyabandi (3) was prepared by adding salts of Na+, Ca2+, K+, Mg2+, Cl−, HCO3−, PO43−and SO42− to Milli Q water (Table S1) followed by filtration through a disposable sterile system (Corning, 09-761). The initial pH of 7.9 of the artificial groundwater was higher than for groundwater pumped from the orange aquifer in the village (pH 6.5 ± 0.1) (21).
The optical density at 600 nm of 0.05 of the Shewanella cultures indicate that the incubations were inoculated with ~ 5 ×108 cells per 10 mL culture tube (14). The tubes were wrapped in black plastic and set horizontally on an orbital shaker (Cole-Parmer, 51401-15) gently rotating at 50–75 rpm over the entire period. Each set of incubations started with 7 replicates that were sampled sacrificially on day 23 in triplicate, on day 42 in duplicate, and on day 92 in duplicate. The sampling intervals were chosen by monitoring the color change of the sand suspension. After measuring the pH (Orion, model 260A), the supernatant from each sampled tube was filtered in the anaerobic chamber through a 0.23 μm filter.
Sediment Reflectance
Changes in the color of the sediment were recorded over the course of the incubations with a Minolta CM2500D diffuse spectral reflectance spectrophotometer through the round bottom of the glass tubes. The difference in reflectance (ΔR) between wavelengths of 530 nm and 520 nm was used as a measure of conversion of Fe(III) to Fe(II) within the solid phase (22).
As and Fe Concentrations and Speciation
Concentrations of As(III) in the filtered but unacidified supernatant were determined immediately after opening a culture tube by differential pulse cathodic stripping voltammetry (DPCSV) (23). The detection limit of this method is 0.2 μg L−1 (3 nmole L−1). Dissolved Fe(II) was also measured immediately in the filtered unacidified supernatant by colorimetry (detection limit of 30 μg L−1) using ferrozine (24). The concentrations of total As and Fe in the filtered supernatant acidified to 1% Optima HCl were analyzed by high-resolution inductively-coupled plasma spectrometry (HR ICP-MS) (25). The detection limit of the method for As is 0.1 μg L−1 (0.001 μmole L−1).
To analyze the sediment, one 0.5 g aliquot of wet sediment was leached in 10 mL of 1.2 mole L−1 HCl at 80°C for 1 hour, after which the concentration of Fe(II) and Fe(III) in the leachate was measured with ferrozine (22). Another 0.5 g of wet sediment from each tube was leached for 24 hours in 10 mL of 1 M Na2HPO4 (pH 5) and 0.1 M ascorbic acid at room temperature in the anaerobic chamber. This extraction has been shown to release As strongly bound to goethite, presumably by competitive desorption (26). This procedure, previously applied to Bangladesh sediment by Harvey et al. (27), relies on a phosphate concentration that is 10-fold higher than the one used to extract As from aquifer sands in, for instance, Vietnam (28, 29). Subsequent experiments have shown that the addition of ascorbic acid preserves the oxidation state of adsorbed As without causing significant reductive dissolution of Fe oxides (30). The concentration of As(III) in the phosphate extract was also measured by DPCSV (23) and the concentration of total As by HR ICP-MS (25).
Results
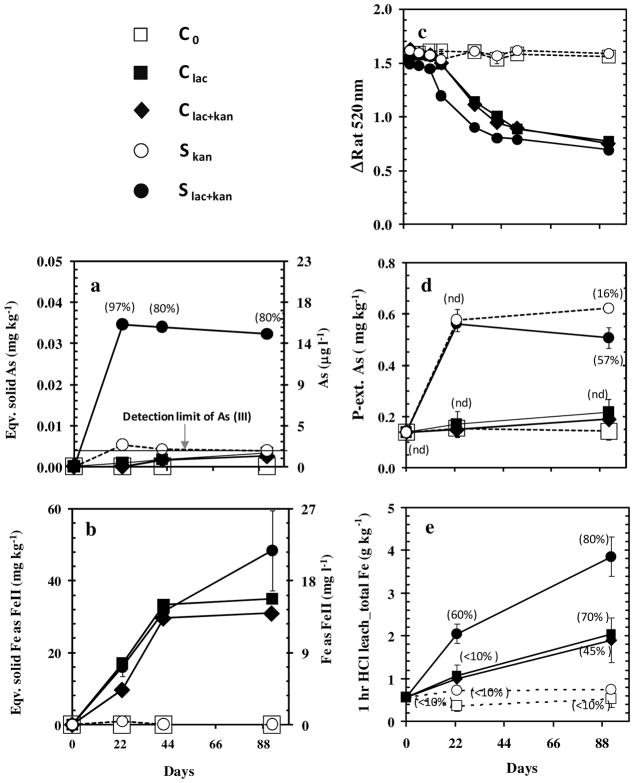
Initial Conditions. Before the incubations, the hot HCl-leachable Fe concentration was 0.8 g kg−1 and the P-extractable As concentration was 0.14 ± 0.03 mg kg−1 (n=3), mostly (>95%) in the form of Fe(III) and As(III), respectively. The Fe(II)/Fe ratio <0.05 in the leachable Fe fraction and the diffuse spectral reflectance are consistent with previous analyses of Pleistocene sediment from the same area (3, 22). The initial difference in reflectance at 530 and 520 nm (ΔR) of 1.5 (Fig. 1c) is higher than previously measured values of ~1.0 for freshly collected orange sands, probably because the reflectance was measured through the bottom of the culture tube instead of through Saran Wrap in direct contact with the sediment. In this study, changes in reflectance therefore provide an effective, albeit qualitative, indication of the redox state of acid-leachable Fe oxides over the course of the experiments.
Figure 1.
Variations in As and Fe concentrations and speciation in the dissolved and the solid phase over the course of incubations of orange sand from Bangladesh in artificial groundwater. The five sets of incubations include a control without lactate (C0), a control with lactate (Clac), a control with lactate and kanamycin (Clac+kan), an amendment with Shewanella in kanamycin without lactate (Skan) and amendment with Shewanella in kanamycin with lactate (Slac+kan). Liquid phase (a) As and (b) Fe measurements are expressed as dissolved concentrations (right) as well as equivalent concentrations in the solid phase (left) by normalizing to sediment weight. Error bars indicate the standard deviation of triplicates on day 22 and duplicates on day 45 and day 90. Some error bars are covered by the symbol. “nd” indicates As(III) was not detected by DPCSV. with a detection limit 2 μg L−1 (0.004 mg kg−1). (c) Changes in sediments color expressed as the difference in reflectance between 530 and 520 nm (ΔR). Also shown are (d) solid phase P-extractable total As, with symbols labeled with the proportion of as As(III) and (e) solid phase 1 hr HCl-leachable Fe, with the proportion of Fe(II).
Aqueous As and Fe
The most significant changes in As concentrations occurred within the first 23 days of the incubations (Fig. 1a; Table 1). In the amendments with lactate inoculated with Shewanella sp. strain ANA-3 (Slac+kan), concentrations of dissolved As rose to 17 ± 4 μg L−1 (n=3), which is equivalent to a release of 0.035 mg kg−1 from the sand (Table 1). The increase in dissolved As over the same period was smaller (2.6 ± 0.01 μg L−1; eqv. to 0.005 mg kg−1), but easily detectable by HR ICP-MS for the inoculated incubation without lactate (Skan). No increase above the detection limit of 0.1 μg L−1 dissolved As was observed on day 23 in the supernatant of the three incubations without Shewanella strain (C0, Clac, Clac+kan), two of which were amended with lactate. Aqueous As remained undetectable over three months in the control without amendment (C0), whereas aqueous As in the other two controls amended with lactate (Clac, Clac+kan) gradually increased to 1.7 and 1.3 μg L−1 As (0.003 and 0.002 mg kg−1) (Fig. 1a; Table S2).
Table 1.
Aqueous and post-incubated sediments properties and As, Fe, Mn Concentrations after 23 days
| Liquid phase (solid eqv.) | AGW | Control | SHEWANELLA sp. ANA-3 | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| C0 | Clac | Clac+kan | Skan | Slac+kan | ||
| As_μg l−1(mg kg−1) | nd* | nd* | nd* | nd* | 2.6 ± 0.01 (0.005) | 17.3 ± 3.7 (0.035) |
| As(III)_μg l−1(mg kg−1) | nd* | nd* | nd* | nd* | nd* | 16.1 ± 1.9 (0.032) |
| Fe_mg l−1(mg kg−1) | nd* | nd* | 7.6 ± 0.6 (17) | 4.4 ± 0.4 (10) | 0.4 ± 0.04 (1) | 7.3 ± 1.1 (16) |
| Fe (II)_mg l−1(mg kg−1) | nd* | nd* | 7.6 ± 0.7 (17) | 4.3 ± 0.4 (9) | 0.4 ± 0.06 (1) | 7.2 ± 1.1 (16) |
| Mn_mg l−1(mg kg−1) | nd* | nd* | 7.4 ± 0.9 (17) | 8.1 ± 0.8 (18) | 0.8 ± 0.08 (2) | 7.9 ± 0.2 (18) |
| pH | 7.88 | 8.13 ± 0.02 | 7.41 ± 0.02 | 7.11 ± 0.02 | 7.89 ± 0.02 | 7.13 ± 0.01 |
|
| ||||||
| Solid phase | Pre-incubated | |||||
| Color | Orange | Orange | Orange | Orange | Orange | Grey |
| [Assolid]/[Asliquid] (L/kg) | >320# | >320# | >320# | 218 | 33 | |
| P-extractable As (mg kg−1) | 0.14 ± 0.03 | 0.15 ± 0.01 | 0.17 ± 0.05 | 0.15 ± 0.03 | 0.58 ± 0.04 | 0.56 ± 0.01 |
| P-extractable AsIII (mg kg−1) | nd* | nd* | nd* | nd* | nd* | nd* |
| 24 hrs HCl leachable Fe (g kg−1) | 10.6 ± 1.2 | 12 ± 0.6 | 12 ± 0.8 | 11 ± 0.7 | 11 ± 1.6 | 12 ± 0.3 |
| 1 hr HCl leachable Fe (g kg−1) | 0.79 ± 0.15 | 3.43 ± 0.69 | 5.63 ± 0.63 | 5.11 ± 0.83 | 2.94 ± 0.12 | 5.4 ± 0.04 |
| 1 hr HCl leachable FeII (g kg−1) | 0.04 ± 0.002 | 0.2 ± 0.02 | 0.41 ± 0.01 | 0.33 ± 0.02 | 0.2 ± 0.03 | 3.2 ± 0.02 |
| HCl leachable FeII/Fe ratio | 0.051 | 0.061 | 0.073 | 0.065 | 0.068 | 0.593 |
| HCl leachable Mn (g kg−1) | 0.2 ± 0.05 | 0.2 ± 0.09 | 0.3 ± 0.03 | 0.2 ± 0.04 | 0.2 ± 0.04 | 0.2 ± 0.02 |
: not detectable;
liquid phase As was not detectable.
Limit of detection (LOD) for As and Fe/Mn by HR ICPMS are 0.10 μgl-1 (0.0002 mgkg-1) and 1 μgl-1 (0.002 mgkg-1) respectively. AsIII by DPCSV is 2 μgl-1 (0.004 mgkg-1) and Fe is 0.03 mgl−1 (0.06 mgkg−1) by Ferrozine method.
Most of the As released from the orange sand in the amendments with lactate and Shewanella (Slac+kan) was in the form of As(III) on day 23 and remained in that form for the duration of incubations (Fig. 1a). The proportion of dissolved As(III) in the incubation with Shewanella without lactate (Skan) and all the controls could not be determined because concentrations were below the detection limit < 2 μg L−1 by DPCSV.
In contrast to As, the incubations of orange sands amended with lactate systematically released more Fe than the incubations without lactate. Concentrations of dissolved Fe(II) in all three lactate amendments (Clac, Clac+kan, Slac+kan) rose above 4 mg L−1 by day 23 (Table 1) and stabilized to ~15 mg/L by day 42 (Fig. 1b; Table S2). The maximum of Fe released to solution by the end of the incubations corresponds to <1 % of the total Fe in the acid-leachable fraction in the original sand. Dissolved Fe(II) remained below the detection limit of 0.03 mg L−1 throughout the incubations in both incubations without lactate (C0, Skan).
Sediment As and Fe
The concentrations of P-extractable As in the sediment rose four-fold to 0.6 mg/kg by day 23 in all six tubes inoculated with Shewanella, with or without lactate (Slac+kan, Skan), and remained constant thereafter (Fig. 1d). In marked contrast, concentrations of P-extractable As remained below 0.23 mg kg−1 throughout the experiment for all the other amendments without Shewanella, including those amended with lactate (C0, Ckan, Clac+kan).
The quantity of As extracted from the solid phase in 1 M phosphate is 20-fold greater than the total released to the supernatant over the same period for the incubations inoculated with Shewanella and lactate (Slac+kan). Whereas As released to the supernatant was primarily in the form of As(III), the P-extractions of the sediment in both sets of inoculated incubations contain no detectable As(III) through day 23 (Skan, Slac+kan) (Fig. 1d). The proportion of As(III) in the P-extractions rose to 20 and 60% in the Skan and Slac+kan amendments by the end of the incubations, respectively.
The orange sediment amended with lactate and Shewanella (Slac+kan) turned grey by day 17, followed by the two incubations with lactate but without Shewanella (Clac, Clac+kan) on day 23. Evidently, the kanamycin did not suppress Fe reduction by the indigenous population of microbes. The remaining amendments without lactate (Skan, C0), including the one with Shewanella, remained orange for the entire period. The timing of the color changes is consistent with the changes in reflectance (Fig. 1c). The proportion of Fe(II)/Fe in the 1 hr HCl-leachable Fe fraction rose to 60% by day 23 in the incubation with lactate and Shewanella (Slac+kan) (Fig. 1e). In all other incubations with lactate or Shewanella, the Fe(II)/Fe ratio remained <10% (Table 1). The leachable Fe(II)/Fe ratio continued to rise as the concentration of total leachable Fe in the incubations amended with lactate (Clac, Clac+kan, Slac+kan) further increased over the course of the incubations (Table S3). In incubations without lactate (C0, Skan), neither the HCl-leachable Fe concentration nor the HCl-leachable Fe(II)/Fe ratio changed over the entire incubation period. The ΔR of ~0.7 for grey sediment at the end of the incubations with lactate was again 0.4 unit higher than observed in the field with freshly collected grey sediment (22). The reflectance of the incubations without lactate did not change over the course of the experiments.
Discussion
Decoupling of As and Fe Release
Incubation of sediment from South and Southeast Asia have to date relied primarily on additions of acetate or lactate to stimulate the reduction of Fe oxides and the transfer of As from the solid to the dissolved phase. A limited number of studies have relied on natural organic matter and have attempted to characterize the role of bacteria by including abiotic controls and characterizing microbial populations (4–5, 7, 31). The incubations presented here differ from previous work because of the nature of the starting material, orange as opposed to grey sands, and because well-characterized bacteria were added with and without lactate. The most surprising result is that the additions of Shewanella alone led to a substantial increase in the concentrations of P-extractable As in the solid phase as well as a release of As to solution, whereas the additions of lactate without Shewanella caused only a marginal increase in the concentrations of P-extractable As in the solid phase. The additions of lactate triggered the anticipated conversion of Fe (III) to Fe(II) within the solid phase as well as reductive dissolution, but without a concomitant release of As in the absence of Shewanella. This extent of decoupling between the release of As and Fe has been reported for model systems but, to our knowledge, rarely for natural sediment.
Underlying Mechanisms
The interpretation of results from this pilot-study is limited by a number of factors. It appears that extended storage of the sediment core had little impact on the speciation of As and Fe, although confirmation by X-ray spectroscopy (6–7) would have been useful. The initial predominance of As(V) in the orange sands is unlikely to be a storage artifact because it has also been reported on the basis of X-ray spectroscopy for freshly collected and preserved orange sands from Bangladesh (32). However, no information is available to determine to what extent the original microbial population was affected by storage and how it evolved in response to the various treatments. Shewanella has not been reported in natural sediment of South and Southeast Asia, although the presence of bacteria with similar properties has been documented (17–19). The pH of the experiments was high compared to natural conditions, even in the incubations amended with lactate (pH 7.1–7.4 at day 23 vs. pH 6.5 in the local groundwater). It is also not known to what extent the sulfate initially contained in the artificial groundwater was reduced and whether this conversion could have resulted in precipitation of As-sulfides and, therefore, could have limited the increase in dissolved As (7). Finally, the amount of kanamycin added was insufficient to inhibit fully microbial activity, particularly that of Fe reducers, in the presence of lactate. This could have been because of resistance of the indigenous population to kanamycin and limits our ability to distinguish biotic and abiotic processes regulating dissolved As levels in the experiments.
Despite these limitations, our results show that the additions of Shewanella with lactate and only the additions of Shewanella resulted in a significant conversion of As from a relatively immobile to an exchangeable phase. The extraction method used to quantify this exchangeable phase has previously been shown to effectively release As adsorbed onto ferrihydrite and goethite into solution (30). Because the concentration of phosphate is high, the extraction could potentially over-estimate the pool of mobile arsenic by attacking arsenic that may be incorporated into the mineral lattice. On the other hand, previous work using natural sediments from the Bengal Basin has shown that the phosphate extraction procedure applied to the incubations provides a useful operational definition of a pool of As in the solid phase that is related in fairly systematic way to dissolved concentration of As in groundwater (3, 33).
It is presently unclear which properties of Shewanella led to the conversion to an exchangeable form of As or whether the inoculations provided a source of metabolizable carbon that was utilized, instead, by other resident bacteria that were not inhibited by kanamycin. For comparison with the amount of the lactate added (600 μmol of C per tube), the quantity of reduced carbon in the form of bacterial cells added was on the order of 1 μmol C per tube, assuming a carbon content of the natural bacterial assemblage of 20 fg of C cell−1 (34). The quantity of reduced carbon added in the form of bacteria was clearly insufficient to release as Fe(II) the equivalent of 50 mg kg−1 (Fig. 1b), i.e. 5 μmol Fe per tube, but considerably larger than the maximum of 0.03 mg kg−1, i.e. 0.002 μmol per tube, of As transformed to a more exchangeable form.
It is worth pointing out that the importance of As reduction for As release from natural orange sands remains uncertain in these experiments. On one hand, the incubation with Shewanella and lactate (Slac+kan) showing the highest degree of As reduction in the solid phase at the end of the incubations resulted in the largest release of As concentrations to the dissolved phase and the solution speciation was dominated by As(III) for this incubation (Fig. 1a, b). Taken at face value, this could imply that reduction enhances As release to solution. On the other hand, the marked difference in the extent of reduction of sedimentary As(V) at the end of the experiment between Skan and Slac+kan did not affect to the total increase in P-extractable As. It is not clear which of the two processes, the short-term release of As to solution or the conversion to P-extractable As, is ultimately responsible for increasing groundwater As levels under natural conditions. Another reason the role of As reduction is difficult to ascertain from these experiments is that As can be sequestered in re-mineralized solids, by analogy to the impact of transformations from ferrihydrite to magnetite transformation documented in model systems (35).
Implications for Mitigation
The solid to water ratio in the culture tubes was about 12 times lower than in the sandy aquifer, assuming a typical porosity of 0.3. All other things being equal, the impact of introducing a similar dose of Shewanella in the field could therefore conceivably have increased dissolved As concentrations to 31 μg L−1 without further amendment and 210 μg L−1 with lactate or an equivalent source of metabolizable carbon. The lower concentrations of dissolved As, which still exceeds the WHO guideline for As in drinking water of 10 μg L−1 is probably more realistic because it was observed without any major change in the redox state of Fe on the sediment surface.
Our results suggest that, in the presence of a significant hydraulic head gradient that favors downward transport of groundwater as in Dari Satyabandi (3), shallow groundwater with a different resident population of bacteria and, potentially, higher levels of metabolizable carbon could intrude into the deeper low-As aquifer and trigger the release of As. There is also growing evidence of preferential transport of bacteria in sandy aquifers (36), suggesting that imperfect sealing between the shallow and deep aquifer could be conducive to transferring bacteria and, therefore release of As even from orange sands. As villagers of Bangladesh increasingly rely on deep community wells to meet their needs for safe drinking water, the potential vulnerability of low-As aquifers clearly deserves further study.
Supplementary Material
Acknowledgments
We thank Zhongqi Cheng for helpful discussions and analytical support. The paper benefitted greatly from four anonymous reviews and additional comments by Benjamin Bostick. This study was supported by USEPA-NIEHS/Superfund Research Program grant 2 P42 ES10349. This is LDEO contribution xxxx.
Footnotes
Supporting Information Available
This information is available free of charge via the internet at http://pubs.acs.org/.
References
- 1.Ahmed MF, Ahuja S, Alauddin M, Hug SJ, Lloyd JR, Pfaff A, Pichler T, Saltikov C, Stute M, van Geen A. Ensuring safe drinking water in Bangladesh. Science. 2006;314:1687–1688. doi: 10.1126/science.1133146. [DOI] [PubMed] [Google Scholar]
- 2.Michael HA, Voss CI. Evaluation of the sustainability of deep groundwater as an arsenic-safe resource in the Bengal Basin. Proc Natl Acad Sci USA. 2009;105(25):8531–8536. doi: 10.1073/pnas.0710477105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng Y, van Geen A, Stute M, Dhar R, Mo Z, Cheng Z, Horneman A, Gavrieli I, Simpson HJ, Versteeg R. Geochemical and hydrogeological contrasts between shallow and deeper aquifers in two villages of Araihazar, Bangladesh: Implications for deeper aquifers as drinking water sources. Geochim Cosmochim Acta. 2005;69:5203. [Google Scholar]
- 4.Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Loyd LJR. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature. 2004;430:68–71. doi: 10.1038/nature02638. [DOI] [PubMed] [Google Scholar]
- 5.van Geen A, Rose J, Thoral S, Garnier JM, Zheng Y, Bottero JY. Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions. Part II: Evidence from sediment incubations. Geochim Cosmochim Acta. 2004;68:3475–3486. [Google Scholar]
- 6.Gault AG, Islam FS, Polya DA, Charnock JM, Boothman C, Chatterjee D, Loyd LJR. Microcosm depth profiles of arsenic release in a shallow aquifer, West Bengal. Miner Mag. 2005;69:855–863. [Google Scholar]
- 7.Hery M, Dongen BEV, Gill F, Mondal D, Vaughan DJ, Pancost RD, Polya DA, Lloyd JR. Arsenic release and attenuation in low organic carbon aquifer sediments from West Bengal. Geobiology. 2010;8:155–168. doi: 10.1111/j.1472-4669.2010.00233.x. [DOI] [PubMed] [Google Scholar]
- 8.Tufano KJ, Reyes C, Saltikov CW, Fendorf S. Reductive processes controlling arsenic retention: revealing the relative importance of iron arsenic reduction. Environ Sci Technol. 2008;42:8283–8289. doi: 10.1021/es801059s. [DOI] [PubMed] [Google Scholar]
- 9.BGS/DPHE. Final Report. British Geological Survey, Dept. of Public Health Engineering; Keyworth, UK: 2001. Arsenic Contamination of Groundwater in Bangladesh. [Google Scholar]
- 10.Oremland R, Stolz J. Arsenic, microbes and contaminated aquifers. Trends Microbiol. 2005;13(2):45–49. doi: 10.1016/j.tim.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Fendorf S, Michael H, van Geen A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science. 2010;328:1123–1127. doi: 10.1126/science.1172974. [DOI] [PubMed] [Google Scholar]
- 12.van Geen A, Cheng Z, Jia Q, Seddique AA, Rahman MW, Rahman MM, Ahmed KM. Monitoring 51 deep community wells in Araihazar, Bangladesh, for up to 5 years: Implications for arsenic mitigation. Environ Sci Health. 2007;42:1729–1740. doi: 10.1080/10934520701564236. [DOI] [PubMed] [Google Scholar]
- 13.Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chemical Review. 1989;89:713–764. [Google Scholar]
- 14.Saltikov CW, Newman DK. Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci USA. 2003;100:10983–10988. doi: 10.1073/pnas.1834303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saltikov CW, Cifuentes A, Venkateswaran K, Newman DK. The ars detoxification system is advantageous but not required for As(V) respiration by the genetically tractable Shewanella sp. strain ANA-3. Appl Environ Microbiol. 2003;69:2800–2809. doi: 10.1128/AEM.69.5.2800-2809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saltikov CW, Wildman RA, Jr, Newman DK. Expression Dynamics of Arsenic Respiration and Detoxification in Shewanella sp. Strain ANA-3. Bacteriology. 2005;187:7390–7396. doi: 10.1128/JB.187.21.7390-7396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lear G, Polya DA, Song B, Gault AG, Lloyd JR. Molecular analysis of arsenate-reducing bacteria within Cambodian sediments following amendment with acetate. Appl Environ Microbiol. 2007;73:1041–1048. doi: 10.1128/AEM.01654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hery M, Gault A, Rowland HAL, Lear G, Polya DA, Lloyd JR. Molecular and cultivation-dependent analysis of metal-reducing bacteria implicated in arsenic mobilisation in south-east asian aquifers. Appl Geochem. 2008;23(11):3215–3233. [Google Scholar]
- 19.Shi L, Squier TC, Zachara JM, Fredrickson JK. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol Microbiol. 2007;65(1):2–20. doi: 10.1111/j.1365-2958.2007.05783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostka J, Nealson KH. Isolation, cultivation and characterization of iron- and manganese-reducing bacteria. In: Burlage RS, Atlas R, Stahl D, Greesy G, Sayler G, editors. Techniques in Microbial Ecology. Oxford University Press; New York, Oxford: 1998. pp. 58–78. [Google Scholar]
- 21.Dhar RK, Zheng Y, Stute M, van Geen A, Cheng Z, Shanewaz M, Shamsudduha M, Hoque MA, Rahman W, Ahmed KM. Temporal variability of groundwater chemistry in shallow and deep aquifers of Araihazar, Bangladesh. J Cont Hydrol. 2008;99(1–4):97–111. doi: 10.1016/j.jconhyd.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horneman A, van Geen A, Kent D, Mathe PE, Zheng Y, Dhar RK, O'Connell S, Hoque MA, Aziz Z, Shamsudduha M, Seddique A, Ahmed KM. Decoupling of arsenic and iron release to Bangladesh groundwater under reducing conditions. Part I: Evidence from sediment profiles. Geochim Cosmochim Acta. 2004;68:3459–3473. [Google Scholar]
- 23.He Y, Zheng Y, Ramnaraine M, Locke DC. Differential pulse cathodic stripping voltammetric speciation of trace level inorganic arsenic compounds in natural water samples. Anal Chim Acta. 2004;511:55–61. [Google Scholar]
- 24.Viollier E, Inglett PW, Hunter K, Roychoudhury AN, Cappellen P. The ferrozine method revisited: Fe(II)/Fe(III) determination in natural waters. Appl Geochem. 2000;15:785–790. [Google Scholar]
- 25.Cheng Z, Zheng Y, Mortlock R, van Geen A. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379:512–518. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 26.Keon NE, Swartz CH, Brabander DJ, Ha C. Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ Sci Technol. 2001;35:2778–2784. doi: 10.1021/es001511o. [DOI] [PubMed] [Google Scholar]
- 27.Harvey CF, Swartz CH, Badruzzaman ABM, Keon-Blute N, Yu W, Ali MA, Jay J, Beckie R, Niedan V, Brabander D, Oates PM, Ashfaque KN, Islam S, Hemond HF, Ahmed MF. Arsenic mobility and groundwater extraction in Bangladesh. Science. 2002;298(5598):1602–1606. doi: 10.1126/science.1076978. [DOI] [PubMed] [Google Scholar]
- 28.Berg M, Stengel C, Trang PKT, Viet PH, Sampson ML, Leng M, Samreth S, Fredericks D. Magnitude of arsenic pollution in the Mekong and Red River Deltas- Cambodia and Vietnam. Sci Tot Environ. 2007;372:413–425. doi: 10.1016/j.scitotenv.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Postma D, Larsen F, Hue NTM, Duc MT, Viet PH, Nhan PQ, Jessen S. Arsenic in groundwater of the Red River floodplain, Vietnam: controlling geochemical processes and reactive transport modeling. Geochim Cosmochim Act. 2007;71:5054–5071. [Google Scholar]
- 30.Jung HB, Zheng Y. Enhanced recovery of arsenite sorbed onto synthetic oxides by L-ascorbic acid addition to phosphate solution: calibrating a sequential leaching method for the speciation analysis of arsenic in natural samples. Water Research. 2006;40:2168–2180. doi: 10.1016/j.watres.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 31.Rowland HAL, Pederick RL, Polya DA, Pancost RD, Van Dongen BE, Gault AG, Vaughan DJ, Bryant C, Anderson B, Lloyd JR. The control of organic matter on microbially mediated iron reduction and arsenic release in shallow alluvial aquifers, Cambodia. Geobiology. 2007;5:281–292. [Google Scholar]
- 32.Foster AL, Breit GN, Welch AH, Whitney JW, Yount JC, Islam MS, Alam MM, Islam MK, Islam MN. In-situ identification of arsenic species in soil and aquifer sediment from Ramrail, Brahmanbaria, Bangladesh. Abstract H21D-01. Am. Geophys. Union Fall Annual Meeting; San Francisco. 15–19 Dec. 2000; Washington, DC: AGU; [Google Scholar]
- 33.van Geen A, Zheng Y, Goodbred JrS, Horneman A, Aziz Z, Cheng Z, Stute M, Mailloux B, Weinman B, Hoque MA, Seddique AA, Hossain MS, Chowdhury SH, Ahmed KM. Flushing history as a hydrogeological control on the regional distribution of arsenic in shallow groundwater of the Bengal Basin. Environ Sci Technol. 2008;42:2283–2288. doi: 10.1021/es702316k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukuda R, Ogawa H, Nagata T, Koike I. Direct determination of carbon and nitrogen contents of natural bacterial assemblages in marine environments. App Environ Microbiol. 1998;64(9):3352–3358. doi: 10.1128/aem.64.9.3352-3358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coker VS, Gault AG, Pearce CI, Van Der Laan G, Telling ND, Charnock JM, Polya DA, Lloyd JR. XAS and XMCD evidence for species-dependent partitioning of arsenic during microbial reduction of ferrihydrite to magnetite. Environ Sci Technol. 2006;40:7745–7750. doi: 10.1021/es060990+. [DOI] [PubMed] [Google Scholar]
- 36.van Geen A, Ahmed KM, Akita Y, Alam MJ, Culligan PJ, Feighery J, Ferguson A, Emch M, Escamilla V, Knappett P, Layton AC, Mailloux BJ, McKay LD, Mey JL, Serre ML, Streatfield PK, Wu J, Yunus M. Fecal contamination of shallow tubewells in Bangladesh inversely related to arsenic. Environ Sci Technol. 2011 doi: 10.1021/es103192b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.