Abstract
Three new and closely complementary studies have defined the architecture of the circuits underlying the descending control of locomotion, identifying neurons that drive fast motor responses and those that seem to be specialised for exploratory behaviors.
Animals locomote in multiple ways according to their needs and the task at hand, displaying flight or freeze responses to threats, or more exploratory behaviors such as scavenging for food. The initiation, execution and termination of these various locomotor programs involves several brain circuits that converge onto brainstem nuclei, which then relay the appropriate motor commands to effector circuits in the spinal cord. Novel viral and genetic tools have opened up new avenues for dissecting the cellular and anatomical features of the descending locomotor control circuitry. In three recent studies, the groups of Silvia Arber [1], Ole Kiehn [2] and Frederic Bretzner [3] (the last reported in this issue of Current Biology) have identified broadly the neuronal populations in the mesencephalic locomotor region–medulla pathway that control the speed of stepping during locomotion.
Heterogeneity of the Mesencephalic Locomotor Region
It has been 50 years since Orlovsky and colleagues [4,5] first identified an area within the brain, which, upon electrical stimulation, was able to initiate locomotion and modulate its speed. This midbrain area, which encompasses the pedunculopontine nucleus and cuneiform nucleus, was denoted the mesencephalic locomotor region. Subsequent studies in other vertebrates [6,7] have shown that the mesencephalic locomotor region is a highly conserved structure that plays a prominent role in modulating the speed of locomotor movements across many species from lamprey to primates.
While early stimulation experiments identified the gross anatomical location of this key locomotor center, until recently the cellular nature of mesencephalic locomotor region and its downstream targets have remained terra incognita. This is now changing with the advent of new experimental approaches that make it possible to define the contributions of genetically defined neuronal populations to this motor control pathway. The mesencephalic locomotor region comprises at least three neurochemically distinct cell types: excitatory glutamatergic neurons that are intermingled with inhibitory GABAergic interneurons, and cholinergic neurons that are restricted to the pedunculopontine nucleus [7,8].
Roseberry et al. [9] pioneered the dissection of mesencephalic locomotor region heterogeneity, by showing that optogenetic activation of glutamatergic neurons was sufficient to transition the mouse from a stationary state to walking, whereas stimulating glutamatergic and cholinergic mesencephalic locomotor region neurons triggered a transition from walking to running; conversely, the inhibition of glutamatergic neuron activation halted or decelerated locomotion.
In their recent work, Caggiano et al. [2] and Josset et al. [3] further explored the anatomical and molecular heterogeneity of the mesencephalic locomotor region, highlighting the differential role of glutamatergic and cholinergic neurons within the cuneiform and pedunculopontine nuclei. They found that glutamatergic cuneiform nucleus neurons were able to drive the full range of locomotor gaits — walk, trot, bound and gallop — and speed. Glutamatergic pedunculopontine nucleus neurons could only trigger low speed locomotion when stimulated at high intensity, whereas stimulation of cholinergic pedunculopontine nucleus neurons was insufficient to initiate locomotion, as shown by Roseberry et al. [9].
Caggiano et al. [2] went on to characterize the firing properties of glutamatergic neurons during locomotion at different speeds using extracellular recordings, showing that pedunculopontine nucleus neurons are more active at lower speeds, whereas cuneiform nucleus neurons are strongly recruited during fast locomotion. Further behavioral analyses have led the authors to propose a model in which the glutamatergic neurons of the cuneiform nucleus elicit fast locomotion to enable an escape response, whereas glutamatergic neurons of the pedunculopontine nucleus facilitate slow locomotor movements to favor an explorative behavior.
Inputs to the Mesencephalic Locomotor Region
A key step toward understanding how the mesencephalic locomotor region is recruited during behavior has been the identification of presynaptic inputs to excitatory and inhibitory neurons of the region with monosynaptic rabies viral tracings [9]. In the mesencephalic locomotor region, glutamatergic neurons receive strong presynaptic inputs from basal ganglia structures, whereas inhibitory neurons are mainly targeted by the central amygdala, superior colliculus and dorsal raphe [9], a pathway that might be recruited to halt locomotion as part of a fear-freezing response. The basal ganglia-mesencephalic locomotor region connectivity was further explored using optogenetics manipulations to show that mesencephalic locomotor region neurons change their firing pattern in response to direct or indirect striatal pathway activation, and their inhibition is sufficient to reverse striatal-induced initiation or termination of locomotion [9].
Caggiano et al. [2] further examined the connectivity of excitatory neurons within the mesencephalic locomotor region, dissecting the presynaptic input to the anatomically distinct glutamatergic cuneiform nucleus and pedunculopontine nucleus populations. They found that the main inputs to glutamatergic neurons in the pedunculopontine nucleus originate from other midbrain structures, the basal ganglia, and several nuclei in the medulla. By contrast, glutamatergic neurons of the cuneiform nucleus receive little input from basal ganglia structures, but are strongly innervated by midbrain structures such as the periaqueductal grey and inferior colliculus.
Taken together these data reinforce the idea that glutamatergic cuneiform nucleus neurons orchestrate escape responses, whereas the pedunculopontine nucleus pathways favor slow exploratory behaviors and are the primary downstream mediators of basal ganglia-initiated locomotion.
Mesencephalic Locomotor Region Heterogeneity Diversifies the Pattern of Muscle Activation
To better understand how the mesencephalic locomotor region drives movement, Josset et al. [3] took a closer look at muscle activity in resting or freely behaving mice following optogenetic activation of excitatory neurons within the cuneiform and pedunculopontine nuclei. The elicited muscle activity patterns were strongly dependent on whether the mouse was resting or walking (state-dependent), the step cycle phase (spinal gating of descending input) and the duration of photostimulation (temporal dynamics of neuronal recruitment). Stimulating glutamatergic cuneiform and pedunculopontine neurons at rest strongly activated flexor muscles and extensors less so, whereas cholinergic pedunculopontine nucleus neurons preferentially recruited extensor muscles on a slower time scale. Prolonged stimulation of glutamatergic neurons in the cuneiform nucleus also promoted locomotion.
In freely walking mice, short pulses of light stimulation elicited different effects on flexor and extensor muscles when glutamatergic cuneiform versus pedunculopontine neurons were stimulated. Moreover, these effects were modulated during stance and swing, indicating spinal circuits differentially gate descending input during the step cycle. Interestingly, if photostimulation was prolonged — simulating a long-lasting recruitment of these nuclei — the effects on muscle recruitment were dramatically different, prompting a reset in the locomotor rhythm and transition between gaits. Prolonged activation of glutamatergic cuneiform neurons promoted transition to faster gaits and increased the step cycle frequency, by decreasing the durationof extensormuscle burst and anticipating the onset of flexor muscle contraction. Conversely, extended activation of glutamatergic pedunculopontine neurons favored slower gaits and increased the step cycle duration by prolonging extensor muscle contraction and delaying the onset of flexion.
Taken together these results suggest that the type of locomotor behavior initiated depends not only on the neurons being recruited, but also on the context and temporal dynamics of their recruitment. Nonetheless, it remains to be seen how these optogenetic manipulations correlate with the physiological temporal dynamics of mesencephalic locomotor region recruitment during goal-oriented exploratory and escape responses.
Relaying Motor Commands to the Spinal Cord
The extensive characterization of medullary locomotor region projections performed by Caggiano et al. [2] revealed very little input to the motor pools, implying downstream relay centers are needed for the muscle recruitment described by Josset et al. [3]. Shefchyk et al. [10] provided the first evidence localizing key mesencephalic locomotor region downstream effector circuits to the medulla by showing that cooling of the ventral medullary area halts locomotion elicited by electrical stimulation of the mesencephalic locomotor region. Subsequent studies revealed that the mesencephalic locomotor region acts via neurons within the medial reticular formation, the axons of which descend bilaterally through the ventral funiculus to contact interneurons and, to a lesser extent, motor neurons in the spinal cord [11].
More recently, the Arber lab [12] has used retrograde rabies viral tracings to identify the brainstem nuclei that directly target specific motor pools or components of the central pattern generator network. Building on this knowledge [10,11], Capelli et al. [1] have now identified the magnocellular and the gigantocellular nuclei as the structures most likely to mediate locomotor behaviors induced by the mesencephalic locomotor region. As with the mesencephalic locomotor region, these nuclei are also heterogeneous with respect to their neurotransmitter phenotype. By combining mouse genetics, viral tools and optogenetics, Capelli et al. [1] went on to show that activating glutamatergic neurons of the lateral paragigantocellular nucleus alone is sufficient to initiate locomotion. Conversely, optogenetic activation of inhibitory neurons within the magnocellular and gigantocellular nuclei halted ongoing locomotion, producing either spasms or atonia depending on the targeted nucleus [1].
Taking advantage once more of their ability to trace presynaptic connections, Capelli et al. [1] identified the anatomical inputs from the mesencephalic locomotor region to glutamatergic neurons of the lateral paragigantocellular nucleus. They found that glutamatergic neurons of the lateral paragigantocellular nucleus receive input from both cuneiform and pedunculopontine nuclei, with a stronger bias from cuneiform nucleus. This latter finding is very interesting in light of the finding of Caggiano et al. [2] that cuneiform nucleus neurons have fewer and more restricted projections than pedunculopontine nucleus neurons. In an elegant experiment, Capelli et al. [1] went on to demonstrate that the ablation of glutamatergic neurons of the lateral paragigantocellular nucleus dampens the speed of mesencephalic locomotor region-induced locomotion.
Together, these three new studies [1–3] point to the presence of a dedicated glutamatergic cuneiform nucleus–lateral paragigantocellular nucleus circuit that can be used to elicit an escape response (Figure 1). Interestingly there appears to be a marked divergence in downstream targets of inhibitory versus excitatory neurons of the lateral paragigantocellular nucleus. Although both subpopulations target ventral spinal laminae where the locomotor central pattern generator resides, while medullary glycinergic neurons directly contact motor neurons, medullary glutamatergic synapses are concentrated in the ventral central grey matter and for the most part avoid motor neuron cell bodies [1].
Figure 1. Anatomical and functional characterization of a bimodal circuit for speed.
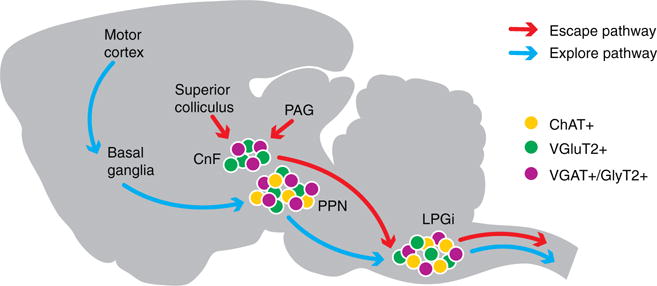
The mesencephalic motor region comprises two areas: the pedunculopontine nucleus (PPN) and cuneiform nucleus (CnF). The pedunculopontine nucleus has at least three neurochemically distinct populations: glutamatergic (purple), cholinergic (yellow) and inhibitory neurons (green). The cuneiform nucleus is a mix of glutamatergic and inhibitory interneurons. The lateral paragigantocellular nucleus (LPGi) resides in the medulla and is as heterogeneous as the pedunculopontine nucleus. The midbrain areas, like the superior colliculus and periaqueductal grey, initiate fast escape responses, which are relayed to the effector circuits in the spinal cord via the cuneiform nucleus–lateral paragigantocellular nucleus pathway (red arrows). Conversely, motor cortex and basal ganglia promote a series of goal-directed movements that are translated into slow-paced exploratory motor behavior via the pedunculopontine nucleus-induced activation of probably lateral paragigantocellular nucleus and other medullary and spinal nuclei (blue arrows).
Outlook
The findings of Caggiano et al. [2], Josset et al. [3] and Roseberry et al. [9] point to a bimodal organization for the mesencephalic locomotor region, with the cuneiform nucleus transforming escape responses initiated in the midbrain into fast running motor programs, and the pedunculopontine nucleus promoting slower explorative behaviors as selected by the basal ganglia. This raises the question as to whether these two locomotor modes are generated by selectively driving the cuneiform nucleus or pedunculopontine nucleus as a whole, or whether subpopulations within these two broad anatomical structures, and/or defined temporal patterns of activity, provide for a more refined contextualization of locomotion. Indications of further specialization in these structures comes from: first, the presence of mesencephalic locomotor region neurons with ascending projections to the basal forebrain that change the gain of visual cortex responses without affecting locomotion [13]; second, the differential effects of the cuneiform and pedunculopontine nuclei on flexor versus extensor muscle activation [3]; and third, the increased number of postsynaptic targets contacted by pedunculopontine nucleus neurons when compared to the cuneiform nucleus neuronal ensemble [2]. This latter finding suggests that the greater variety of behavioral contexts that are associated with slow-paced locomotion might be mediated by different spatial and temporal patterns of pedunculopontine nucleus neuron recruitment.
Capelli et al. [1] also highlight the potential for heterogeneity in the lateral paragigantocellular nucleus by describing a ventral area within the glutamatergic lateral paragigantocellular nucleus that shows high levels of cFos activation during movement and coincidentally receives more input from the mesencephalic locomotor region. Thus, future quests probing the molecular heterogeneity of the mesencephalic locomotor region and medulla nuclei might unravel more dedicated pathways recruited in a context-dependent manner. Finally, another more restricted glutamatergic subpopulation (Chx10+) in the medulla has been described as necessary to halt locomotion [14], and understanding if and how this subpopulation is recruited by the mesencephalic locomotor region–lateral paragigantocellular nucleus pathway and whether it acts in conjunction with the pedunculopontine nucleus to promote a stop and explore behavior would shed further light on the logic of descending motor control.
References
- 1.Capelli P, Pivetta C, Soledad Esposito M, Arber S. Locomotor speed control circuits in the caudal brainstem. Nature. 2017;552:373–377. doi: 10.1038/nature24064. [DOI] [PubMed] [Google Scholar]
- 2.Caggiano V, Leiras R, Goñi-Erro H, Masini D, Bellardita C, Bouvier J, Caldeira V, Fisone G, Kiehn O. Midbrain circuits that set locomotor speed and gait selection. Nature. 2018;553:455–460. doi: 10.1038/nature25448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josset N, Roussel M, Lemieux M, Lafrance-Zougba D, Rastqar A, Bretzner F. Distinct contributions of mesencephalic locomotor region nuclei to locomotor control in the freely behaving mouse. Curr Biol. 2018;28:884–901. doi: 10.1016/j.cub.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Shik ML, Severin FV, Orlovsky GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- 5.Shik ML, Orlovsky GN, Severin FV. Organization of locomotor synergism. Biofizika. 1966;11:879–886. [PubMed] [Google Scholar]
- 6.Orlovsky GN, Deliagina T, Grillner S. Neuronal Control of Locomotion: From Mollusc to Man. Oxford University Press; 1999. [Google Scholar]
- 7.Ryczko D, Dubuc R. The multifunctional mesencephalic locomotor region. Curr Pharm Des. 2013;19:4448–4470. doi: 10.2174/1381612811319240011. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat. 2011;5:22. doi: 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, Kreitzer AC. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell. 2016;164:526–537. doi: 10.1016/j.cell.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shefchyk SJ, Jell RM, Jordan LM. Reversible cooling of the brainstem reveals areas required for mesencephalic locomotor region evoked treadmill locomotion. Exp Brain Res. 1984;56:257–262. doi: 10.1007/BF00236281. [DOI] [PubMed] [Google Scholar]
- 11.Noga BR, Kriellaars DJ, Brownstone RM, Jordan LM. Mechanism for activation of locomotor centers in the spinal cord by stimulation of the mesencephalic locomotor region. J Neurophysiol. 2003;90:1464–1478. doi: 10.1152/jn.00034.2003. [DOI] [PubMed] [Google Scholar]
- 12.Pivetta C, Esposito MS, Sigrist M, Arber S. Motor-circuit communication matrix from spinal cord to brainstem neurons revealed by developmental origin. Cell. 2014;156:537–548. doi: 10.1016/j.cell.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Lee AM, Hoy JL, Bonci A, Wilbrecht L, Stryker MP, Niell CM. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron. 2014;83:455–466. doi: 10.1016/j.neuron.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, Kiehn O. Descending Command Neurons in the Brainstem that Halt Locomotion. Cell. 2015;163:1191–1203. doi: 10.1016/j.cell.2015.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]


