Abstract
High-level, acute exposures to individual polycyclic aromatic hydrocarbons (PAHs) and complex PAH mixtures result in cardiac abnormalities in developing fish embryos. Whereas acute PAH exposures can be developmentally lethal, little is known about the later life consequences of early life, lower level PAH exposures in survivors. A population of PAH-adapted Fundulus heteroclitus from the PAH-contaminated Superfund site, Atlantic Wood Industries, Elizabeth River, Portsmouth, Virginia, United States, is highly resistant to acute PAH cardiac teratogenicity. We sought to determine and characterize long-term swimming performance and cardiac histological alterations of a subteratogenic PAH mixture exposure in both reference killifish and PAH-adapted Atlantic Wood killifish embryos. Killifish from a relatively uncontaminated reference site, King’s Creek, Virginia, United States, and Atlantic Wood killifish were treated with dilutions of Elizabeth River sediment extract at 24 h post fertilization (hpf). Two proven subteratogenic dilutions, 0.1 and 1.0% Elizabeth River sediment extract (total PAH 5.04 and 50.4 μg/L, respectively), were used for embryo exposures. Then, at 5-mo post hatching, killifish were subjected to a swim performance test. A separate subset of these individuals was processed for cardiac histological analysis. Unexposed King’s Creek killifish significantly outperformed the unexposed Atlantic Wood killifish in swimming performance as measured by Ucrit (i.e., critical swimming speed). However, King’s Creek killifish exposed to Elizabeth River sediment extract (both 0.1 and 1.0%) showed significant declines in Ucrit. Histological analysis revealed the presence of blood in the pericardium of King’s Creek killifish. Although Atlantic Wood killifish showed baseline performance deficits relative to King’s Creek killifish, their pericardial cavities were nearly free of blood and atrial and ventricular alterations. These findings may explain, in part, the diminished swimming performance of King’s Creek fish.
Keywords: Polycyclic aromatic hydrocarbons (PAHs), Swimming performance, Fundulus heteroclitus (Atlantic killifish), Development, Cardiotoxicity, Subteratogenic
INTRODUCTION
Certain organic environmental contaminants including specific polycyclic aromatic hydrocarbons (PAHs), dioxin-like compounds, 2,3,7,8-tetrachlorodibenzo[p]dioxin (TCDD), 3,3,4,4′,5-pentachlorobiphenyl (PCB-126), and polybrominated diphenyl ethers (PBDEs) disrupt fish embryonic development [1–7]. Developmental exposures to several individual PAHs and to mixtures of petrogenic PAHs lead to anatomical malformations and functional impairments that diminish cardiac output often associated with bradycardia or other forms of arrhythmia [8–10]. Furthermore, exposure to PAHs can result in craniofacial and cardiac malformations and pericardial edema that are similar to effects seen with certain dioxin-like compounds [11,12]. Pericardial alterations are one of the most common abnormalities in fish after developmental exposure to a variety of organic and inorganic compounds [9,10,13]. This, in part, is because of the complex and sensitive movements of cells and tissues during early development [14].
Whereas acute, high-concentration contaminant exposures often cause overt and lethal developmental abnormalities, low-level, environmentally relevant PAH exposures are important causes of early and later life stage behavioral alterations and cardiorespiratory toxicity [5,8,15–17]. Complex organic mixtures, particularly crude oils enriched in PAHs, are associated with later life swimming and cardiac deficiencies [9]. Bradycardia has been found after relatively high-dose exposures to crude oil in herring (Clupea pallasii) [18,19], zebrafish (Danio rerio) [20], sheepshead minnow (Cyprinodon variegatus), and Atlantic killifish (Fundulus heteroclitus) [21]. Environmentally relevant (1–15-μL total PAH concentration) exposures to crude oil from the Deepwater Horizon spill in the Gulf of Mexico perturbed heart development and impacted swimming performance in several species of large pelagic fish [10,22]. Following the Erika oil spill on the west coast of France, Claireaux and Davoodi [23] reported reduced cardiorespiratory performance in adult sole (Solea solea). Similar responses were observed in Pacific herring (C. pallasii) and pink salmon (Oncorhynchus gorbuscha) exposed to low levels of Alaska North Slope crude oil from the Exxon Valdez spill in 1989 [24]. These studies show that exposure causes not only unwanted events in embryos and larvae but also that later life consequences follow early life exposures. The present study describes results of low-level exposures to a pyrogenic PAH mixture (associated with creosote) to an important estuarine fish (Atlantic killifish) and includes an examination of a population that has evolved a resistance to the cardiovascular teratogenesis produced by higher level exposures to unadapted populations.
The Elizabeth River, located in the Tidewater region of southeastern Virginia, United States, was the site of the former Atlantic Wood Industries, a creosote-producing facility [25]. Wide regions of the river sediment have been contaminated with complex mixtures of chemicals consisting primarily of unsubstituted PAHs and several heterocyclic and phenolic PAHs [25–27]. This contamination comes from years of creosote production by Atlantic Wood and other wood-treatment facilities on the Elizabeth River. Sediment PAH concentrations adjacent to the Atlantic Wood site are among the highest PAH sediment concentrations reported [28], ranging from 100 to 500 μg/g in dry sediment [25,29]. The sediment and porewater from the Atlantic Wood site have been chemically characterized and shown to contain an abundance of high molecular-weight PAHs [27]. The US Environmental Protection Agency placed the Atlantic Wood site on their National Priorities List; and remediation was carried out that cleared the former Atlantic Wood site of sediments, covered the site with new soils, and separated it from the Elizabeth River by construction of an impermeable wall barrier [27]. Extracts of sediment porewater characterized by Fang et al. [27] now provide mixtures of water soluble, high molecular-weight PAHs for the present study.
The Atlantic killifish (hereafter referred to as killifish) is a temperate and benthopelagic fish species inhabiting salt marshes and tidal creeks along the Atlantic coast of North America from Newfoundland to Florida [30,31]. It is often the most abundant intertidal fish species in its range and a major component of estuarine food webs [30]. Killifish are a useful environmental and toxicological model as the result of wide distribution, relatively small home range (300–500 m) [32,33], ease of laboratory rearing, high reproductive rate, short development time, and transparent chorion [34]. Killifish residing at the Atlantic Wood site have been chronically exposed to PAHs for decades, and have developed significant resistance to the acute cardiotoxicity and teratogenic effects of Elizabeth River sediment extracts, PAHs, PCB-126, and certain pesticides (chlorpyrifos, permethrin, and carbaryl) [26,35–37]. This population exhibits a degree of resistance compared with populations inhabiting relatively uncontaminated sites in the region including our primary reference site, King’s Creek [38,39]. However, the resistance that Atlantic Wood killifish exhibit has not occurred without fitness costs such as increased sensitivity to hypoxia and reduced survival in captivity [40]. Several other populations of killifish residing in PAH-contaminated regions of the Elizabeth River are similarly resistant to the cardiotoxic effects of PAHs and other environmental contaminants [26].
The purpose of the present study was to determine whether later life swimming performance would be different in offspring of the 2 populations. Killifish were collected from Atlantic Wood and King’s Creek sites, reared in our laboratory, and used to produce fish for the present study. The low-level PAH dilutions used in our study did not induce overt embryonic cardiac abnormalities when screened at 144 h post fertilization (hpf), and embryonically exposed killifish were reared to 5 mo, at which time swimming and histological assessment were performed. King’s Creek killifish exposed to low-level PAHs as embryos showed deficiencies in swimming performance. Unexposed Atlantic Wood killifish exhibited reduced swimming performance relative to that of unexposed King’s Creek fish; however, observed deficiencies in swimming performance were not exacerbated by PAH exposure.
MATERIALS AND METHODS
Adult fish care
Adult killifish were collected with wire mesh minnow traps at the Atlantic Wood Superfund site (36°48′27.2″N, 76°17′38.1″W) and at King’s Creek, a relatively uncontaminated tributary of the Severn River in Virginia (37°18′16.2″N, 76°24′58.9″W) from April 2012 to September 2013. Fish from the latter site comprised the reference population. In the laboratory, adults were maintained in a flow-through system consisting of a series of 30- or 40-L tanks containing 15% artificial seawater (Instant Ocean; Foster and Smith). The system was maintained at 25 to 28 °C on a 14:10-h light:dark cycle. Adults were fed pelleted feed (Aquamax Fingerling Starter 300; PMI Nutritional International) ad libitum.
F1 generation care and dosing
Killifish eggs and sperm from 100 females and 20 males of each laboratory population were used for in vitro fertilization methods approved by the Duke University Institutional Animal Care & Use Committee (A184-13-07). Embryos were collected, cleaned, and maintained according to Clark et al. [26]. At 24 hpf, normally developed screened embryos (Clark et al. [6]) from each population were randomly assigned to individually labeled 20-mL glass scintillation vials (VWR International; Wheaton Worldwide), N = 1 per vial, containing 10-mL dosing solution (Elizabeth River sediment extract diluted in artificial seawater). The treatments were as follows: 0% (control, artificial seawater only), 0.1 or 1.0% Elizabeth River sediment extract (0, 5.04, and 50.4 μg/L total PAHs, respectively). Concentrations for use in experiments were determined following range-finding studies published in Brown et al. [41]. Fifty embryos were initially exposed per treatment group and population before double-blind cardiac screening. Duration of exposure was 144 h, at which time embryos were screened in a double-blinded fashion for cardiac abnormalities using a semi-quantitative scale [6,42]. Only embryos receiving a score of 0 (no abnormalities) were selected for long-term growth and subsequent evaluation (30 embryos were selected to continue forward for long-term rearing). After transfer to absorbent filter paper, embryos were maintained at 27 °C in an incubator until 14 d post fertilization. At this time, artificial seawater was added to the Petri dishes, the absorbent paper was removed, and the dishes were gently rocked in a shaker until hatching. Larvae were separated, maintained in an incubator at 28 °C in 2-L beakers, and fed a diet of brine shrimp with gradual transitioning to a mix of Ziegler’s Adult Zebrafish Complete Diet (Aquatic Habitats) and Cyclopeeze (Argent Chemical Laboratories). After 1 wk, larvae were transferred from beakers to individually labeled 10-gal tanks at a density of 30 individuals per tank. Fish were maintained at 28 °C in one of 2 recirculating AHAB systems (Aquatic Habitats) under a 14:10-h light:dark cycle and reared to age 5 mo. Killifish were considered to be young adults at this stage, based on egg bearing and sexual maturity.
A randomly selected subset of the 5-mo-old individuals was exposed to 1.0% Elizabeth River sediment extract for 24 h immediately before having their swimming capacity tested using a swim tunnel. These repeated-exposure fish were not used in morphological assessments. Adult care and reproductive techniques are noninvasive and have been reviewed and approved by the Duke University Institutional Animal Care & Use Committee (A184-13-07).
Sediment collection and deformity assessment
Sediments were collected from the Atlantic Wood site; a full description of collection procedures and Elizabeth River sediment extract processing can be found in Fang et al. [27]. Developmental cardiac abnormalities were scored blindly by a second researcher at 144 hpf. Chamber deformities were scored as 0, 1, or 2 using scale scoring from Matson et al. [42]. This scale represented, respectively, normal cardiac development (0), atrium and ventricular misalignment (1), and tube heart with no blood circulation (2). Embryos with severe heart abnormalities (score of 2) do not hatch, whereas mild deformities (score of 1) prevent hatching in roughly one-half of affected embryos (data not shown). All killifish embryos used for swim performance and later life studies showed no gross cardiac chamber abnormalities and were scored as 0, using the above methodology.
Swimming performance
To establish the swimming capacity of killifish, critical prolonged swimming speed (Ucrit) was determined using the methodology described by Brett [43]. Briefly, individual fish (both male and female) were introduced randomly into a Beamish-style swim tunnel (4000-mL vol; Loligo Systems) and allowed to acclimate for 45 min (fish that did not show relaxed static swimming at 45 min were given 15 additional min for acclimation). All fish given additional time acclimated to the tunnel conditions. Two identical tunnels were used to accelerate testing, and fish were randomly assigned to either tunnel. Temperature was maintained at 28 °C to match rearing conditions. Once acclimated, the swimming chamber was closed to oxygen flow, and an initial oxygen reading (ppm) was recorded using a dipping probe mini sensor (#OX1125, L100 mm; Loligo Systems). This was repeated after 10 min to establish oxygen consumption. Fish were then given an opportunity to acclimate to the flow of water by swimming for 10 min at one body length per second. During this period, fish oriented to the water current but maintained a stationary position on the bottom of the swim tube with little body movement. Next, fish were swim tested to determine Ucrit. Water velocity was calibrated using a DAQ-M instrument (Loligo Systems) and a swimming interval of 10 min was chosen based on Jones [44], Beamish [45], and Hammer [46]. Velocity increased stepwise by 0.3 body length/s every 10 min, from a starting velocity of 1 body length/s, until the fish became fatigued [47,48]. When fish failed to maintain swim performance and were forced against the chamber screen, water velocity was decreased to 0 to allow the fish to resume swimming. Velocity was then increased to the velocity of failure within 10 s. Final fatigue was established when the fish fell against the back screen 3 consecutive times. Critical swimming was then calculated using the Ucrit formula from Brett [43]. Fish size was less than 5% different for all body metrics; therefore, the measured swimming speeds were not corrected for solid blocking effects [49]. The absolute value of Ucrit was converted to relative swimming speed in body length/s-1 by taking the Ucrit value (cm s-1) and dividing it by the total body length (cm) of each fish [50]. After Ucrit was reached, fish were assessed for a final post Ucrit respiration rate using conditions described earlier. In addition, a randomly selected subset (7 fish per treatment, experimentally replicated n = 14) of the 5-mo-old individuals received an additional 24-h exposure to 1.0% Elizabeth River sediment extract, after which swimming capacity was repeated as already described. By following this procedure, a baseline and post Ucrit oxygen reading were obtained for each fish.
Histological methods
Live adult 5-mo-old killifish (exposed as embryos to various concentrations of Elizabeth River sediment extract) that did not participate in the swimming test(s) were selected and euthanized by placement in an ice bath in preparation for processing for histopathological assessment. Fish were visually inspected for external physical abnormalities (e.g., frayed fins, skin discoloration, lesions, ulcers, parasites, and eye damage/cloudiness), and total length and wet weight were recorded.
Necropsy, fixation, orientation, embedment, and sectioning were done as described in Riley et al. [51]. Step sections (5-μm thickness) were taken beginning at the retina of the left eye and continuing until the midportion of the contralateral eye was reached. In this way parasagittal sections containing all major organs were included. To focus on heart morphology, serial sections of each fish were taken providing a full view of the sinus venosus, atrium, ventricle, and bulbous arteriosus. This approach standardized the orientation and sectioning plane of the 3 heart chambers for imaging and measurements and provided a thorough view of the pericardial cavity.
Slides were stained by hematoxylin and eosin (H&E). Structures were surveyed with emphasis on visualization and orientation of heart and pericardial cavity and then imaged at ×20 magnification on a Nikon Eclipse E600 compound microscope with a Nikon DMX1200 digital camera and NIS-Elements Ver 3.20.01 software (Nikon Instruments). Sections were examined in a double-blinded fashion using a Python script and analyzed using ImageJ Ver 1.48 software (Rasband, National Institutes of Health). Ventricular shape analysis followed methods of Hicken et al. [9]. The color deconvolution plug-in [52,53] was used to generate individual images of single stains from the costained sections using the preset vector for H&E [52]. This separation of the stains allowed for identification of nucleated erythrocytes within the pericardial cavity and digital separation of cells from proteins or other eosinophilic components.
Hematoxylin-stained images were then thresholded to produce a binary image that was measured for area within landmark-based quadrilaterals, allowing for areal quantification of blood in the pericardial cavity (μm2 Figure 1). Two quadrilaterals were used in the pericardial cavity of each image to estimate amount of blood in that space (Figure 1. Quadrilateral A was aligned to the cranial-most margin of the pericardial cavity with a line from the ventral cranial corner of the ventricle to the lowermost portion of the pericardial membrane. From this vertical margin of the box, the quadrilateral ascended within the pericardial cavity to form a corner halfway along the ventral margin of the ventricle. Quadrilateral B started in the corner between the sinus venosus and the atrioventricular margin. From there, the quadrilateral descended within the pericardial cavity along the caudal wall of the ventricle to the apex (Figure 1). The back or left vertical of the box is that part of the pericardial cavity between the ventricle and the septum transversum. The contents of quadrilaterals A and B were added to give the total blood area (μm2) for an individual (Figure 1), and the mean area of the blood was calculated for each treatment group. A total of 10 randomly selected fish per treatment group were sectioned and histologically assessed using this protocol.
Figure 1.
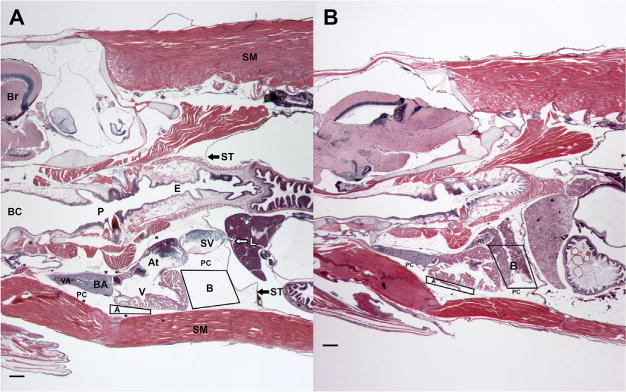
Parasagittal sections of paraffin-embedded, 5-mo-old killifish stained with hematoxylin and eosin (H&E) and oriented with the head to the left. Blood in the pericardial cavity (PC) of each fish was quantified using landmark-based quadrilaterals A and B. Images were separated for H&E staining and the blood within the quadrilaterals was measured for area (A + B = total blood [μm2]). (A) An Atlantic Wood Industries control fish. The dorsal aspect of the individual contains brain (Br) and skeletal muscle (SM). Along the midline, the buccal cavity (BC) is continuous with the pharynx (P) and the esophagus (E). The septum transversum (ST) is denoted by black arrows, with the liver located caudally to ST; associated white arrow indicates hepatic vein. Large segment of skeletal muscle is associated with isthmus and is located ventral to the PC. Cardiovascular structures shown include sinus venosus (SV), atrium (At), ventricle (V), bulbous arteriosus (BA), and ventral aorta (VA), along with the PC. Asterisks mark portions of the parietal pericardium. Note that the PC and associated quadrilaterals are clear of blood. (B) A King’s Creek fish exposed as an embryo to 1.0% Elizabeth River sediment extract. This fish was smaller than the fish in (A), thus the decreased depth. Note that extravasated blood can be seen in both quadrilaterals. Scale bars are 100 μm, ×20, hematoxylin stain.
Statistical analysis
Statistical analyses were performed using JMP Ver 10.1.1 software (SAS Institute). For both swimming and histology experiments, data were analyzed via a 2-way analysis of variance and a Tukey–Kramer post hoc test to determine an overall effect of the Elizabeth River sediment extract treatment. No differences among experimental replicates were observed for any test. Data are represented as mean ± standard error of the mean (SEM). Values are considered significantly different at p < 0.05.
RESULTS
Fish used in swimming experiments were of similar size with no significant differences in length, body mass, and body shape among populations or treatment groups (Table 1). Similar-size matching is imperative for accurate comparison of swimming performance.
Table 1.
Fish body measurementsa
| ERSE treatment (%) | 0.0 | 0.1 | 1.0 |
|---|---|---|---|
| Total body length (cm) | |||
| KC | 3.70 ± 0.03 | 3.67 ± 0.05 | 3.67 ± 0.06 |
| AW | 3.65 ±0.04 | 3.66 ± 0.03 | 3.64 ± 0.05 |
| Total wet weight (g) | |||
| KC | 0.54 ± 0.03 | 0.53± 0.04 | 0.55 ± 0.05 |
| AW | 0.54 ± 0.03 | 0.52 0.02 | 0.52 ± 0.03 |
Total body length and wet weight across treatments for killifish used in all experiments. There were no statistical differences in body metrics between populations or treatments tested. Data represent mean ± the standard error of means.
ERSE = Elizabeth River sediment extract; KC = King’s Creek; AW = Atlantic Wood.
Swimming performance
The unexposed King’s Creek population significantly outperformed the unexposed Atlantic Wood individuals in swimming performance as measured by terminal velocity (Figure 2) and Ucrit (Figure 3; p < 0.05). However, King’s Creek exposed to Elizabeth River sediment extract (both 0.1 and 1.0%) as embryos showed significant decreases in both terminal velocity and Ucrit (p < 0.05). Thus Atlantic Wood showed baseline performance deficits relative to King’s Creek, but were not affected by Elizabeth River sediment extract exposures.
Figure 2.
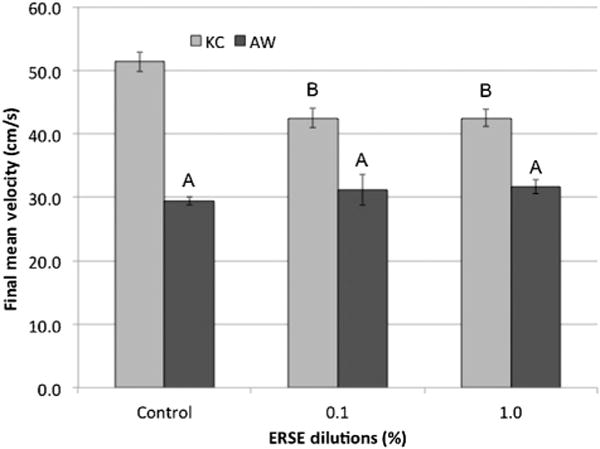
Terminal velocity for King’s Creek (KC) and Atlantic Wood (AW) killifish exposed to dilutions of Elizabeth River sediment extract (ERSE) in a swimming performance assay. Final mean velocity (cm/s) ± standard error of the mean for Elizabeth River sediment extract killifish. King’s Creek (light gray bar) and AW (dark gray bar) killifish were exposed to Elizabeth River sediment extract dilutions at 24 h postfertilization and swim tested at 5 mo. Each bar represents the final mean velocity for 2 experimental replicates with 7 killifish per replicate (total, n = 14). Letters indicate significant difference (p < 0.05) compared with King’s Creek controls and within treatments.
Figure 3.
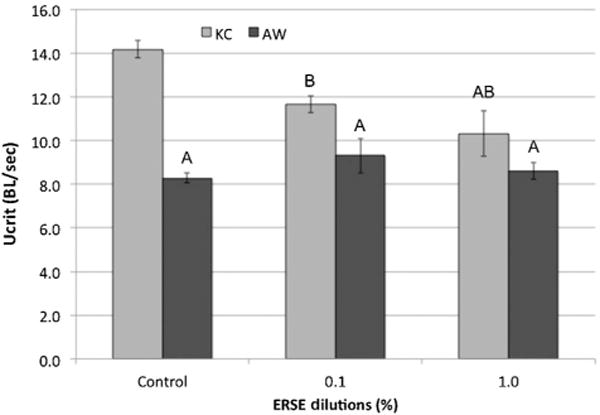
Critical swimming measure (Ucrit) for King’s Creek (KC) and Atlantic Wood (AW) killifish exposed to dilutions of Elizabeth River sediment extract (ERSE) in a swimming performance assay. Final mean Ucrit (body length/s) ± standard error of means for King’s Creek (light gray bar) and AW (dark gray bar) killifish exposed to Elizabeth River sediment extract dilutions at 24 h postfertilization and swim tested at 5 mo. Each bar represents the final mean velocity for 2 experimental replicates with 7 killifish per replicate (total, n = 14). Letters indicate significant difference (p < 0.05) compared with King’s Creek controls and within treatments.
Oxygen consumption at baseline and post Ucrit
Significantly less oxygen was consumed at baseline respiration by unexposed King’s Creek compared with 0.1 and 1.0% Elizabeth River sediment extract exposures (Figure 4; p < 0.05). All Atlantic Wood displayed significantly elevated baseline oxygen consumption relative to unexposed King’s Creek. Post Ucrit oxygen consumption was statistically elevated for all King’s Creek groups relative to King’s Creek baseline measurements (p < 0.05).
Figure 4.
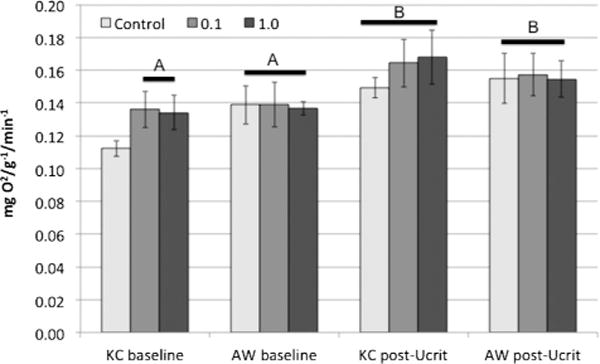
Mean oxygen consumption at baseline and post Ucrit (critical swimming speed) for King’s Creek (KC) and Atlantic Wood (AW) killifish. Mean oxygen consumption (mg O2/g−1/min−1) ± standard error for killifish after acclimation (baseline) and after the Ucrit assay (post Ucrit). Letters represent significant differences in consumed oxygen relative to untreated King’s Creek killifish at baseline. Treatment caused significantly higher oxygen consumption at baseline for King’s Creek and Atlantic Wood killifish (p < 0.05). All exhausted killifish had significantly elevated oxygen consumption levels relative to King’s Creek at baseline, but no significant differences among populations or treatments.
Swimming performance after Elizabeth River sediment extract exposure at 5 mo
Five-mo-old King’s Creek killifish exposed to an additional 24 h of 1.0% Elizabeth River sediment extract exhibited significant declines in terminal velocity when swim tested (Figure 5; p < 0.05). This was true for both King’s Creek killifish exposed or unexposed as embryos. Conversely, exposed Atlantic Wood killifish displayed no significant differences in swimming performance as measured by final velocity (Figure 6), whether exposed or unexposed to 1.0% Elizabeth River sediment extract as embryos.
Figure 5.
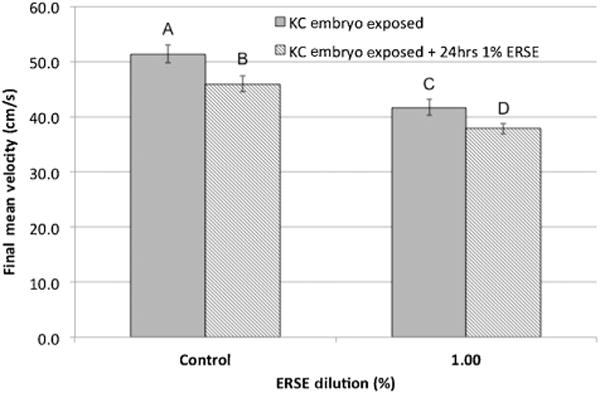
Critical swimming measure for King’s Creek (KC) killifish exposed to 1.0% Elizabeth River sediment extract (ERSE) for an additional 24 h. Final mean velocity (cm/s) ± standard error for King’s Creek killifish after embryo-only Elizabeth River sediment extract exposure (gray bar) and after a single repeat exposure at 5 mo (lined bar). Killifish were swim tested at 5 mo. Each bar represents the final mean velocity for 2 experimental replicates with 7 killifish per replicate (total, n = 14). Letters indicate significant difference (p < 0.05) compared with King’s Creek controls.
Figure 6.
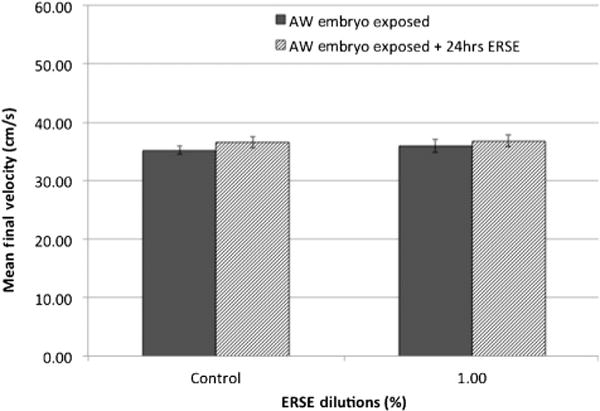
Critical swimming measure for Atlantic Wood (AW) killifish exposed to 1.0% Elizabeth River sediment extract (ERSE) for an additional 24 h. Mean final velocity (cm/s) ± standard error for Atlantic Wood killifish after embryo-only Elizabeth River sediment extract exposure (dark gray bar) and after a single repeat exposure at 5 mo (lined bar). Killifish were swim tested at 5 mo. Each bar represents the mean final velocity for 2 experimental replicates with 7 killifish per replicate (total, n = 14). No significant differences were observed following exposures.
Histology
Histological analysis emphasized all aspects of the heart and pericardium. These efforts were greatly aided by descriptions of various fish species [54]. The parietal pericardium (Figure 1) extended from the septum transversum along the lateral, dorsal, and ventral walls of the pericardial cavity. The parasagittally oriented section planes were particularly advantageous in determining specific elements and their relationships to other structures (Figure 1). During this investigation, we discovered blood in the pericardial cavity of several individuals (Figure 1B). Assessment of ventricular rounding showed no change in either Atlantic Wood or King’s Creek groups in unexposed and exposed individuals. However, blood was found in the pericardial cavity of Elizabeth River sediment extract-exposed King’s Creek killifish as measured by area of thresholded blood (Figure 1). The 1.0%-treated King’s Creek killifish had significantly more blood in the pericardial cavity (p < 0.05). Atlantic Wood killifish showed little blood in the pericardial cavity across all treatment groups (Figure 7).
Figure 7.
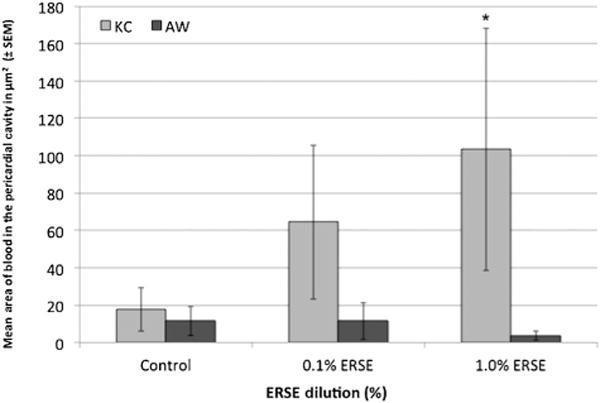
Areal analysis of blood in the pericardial cavity of 5-mo-old killifish. Area (μm2) of blood found in the pericardial cavities. Blood was quantified by drawing 2 quadrilaterals in the pericardial cavity and using ImageJ Ver 1.48 software (Rasband, National Institutes of Health). Bars represent the means of 2 combined experimental replicates of killifish for a total of 10 killifish per bar (n = 10) ± standard error of means. King’s Creek (KC) killifish exposed to 1.0% Elizabeth River sediment extract (ERSE) had significantly more blood (*p < 0.05). AW = Atlantic Wood; SEM = standard error of the mean.
DISCUSSION
The teratogenic impacts of PAH exposures at relatively high concentrations, both as pure compounds and mixtures, on fish embryos have been well established. Embryonic fish develop cardiac failure, jaw deformities, and are unlikely to survive past the yolk-sac stage (i.e., fail to reach free swimming and feeding larval forms) [55–58]. Such exposures are relevant in certain contexts: crude oil spills, and contamination by industries such as wood-treatment facilities utilizing creosote. Nevertheless, perhaps of broader environmental relevance are lower level PAH exposures that do not cause mortality or produce visible defects in offspring (Figure 2). Previous research has indicated that such exposures, including crude oil, can cause subtle yet permanent changes in cardiovascular development that persist into later life stages affecting physiology and behavior of adults [9,16,17,24,58,59]. The present study builds on our understanding of the morphological and physiological consequences of early-life embryonic exposure, using a key sentinel species for North American Atlantic coastal estuaries. The present study also includes the added dimension of comparisons between offspring of wild-caught fish from an uncontaminated site (King’s Creek) with those from a highly PAH-contaminated site (Atlantic Wood). The latter has been the subject of numerous studies examining heritable adaption to PAHs (reviewed by Di Giulio and Clark [39]). The present study focused on swimming performance, oxygen consumption, and cardiac histology providing structural and functional correlates.
Swimming performance is a key fitness process for fish, given its direct relationship to predator–prey interactions, migration capacity, and the selection of favorable environmental conditions. Furthermore, swimming performance is important during all post hatch life stages [60], and survivorship depends on the ability to optimize physiological performance in response to environmental stresses. Our study demonstrated that embryonic exposure to a complex PAH mixture (Elizabeth River sediment extract) altered the capacity of adult King’s Creek killifish to maintain critical swimming levels, thereby identifying an important persistent effect of low-level exposure during embryonic development. Moreover, a marked deficit in swimming performance of the PAH-adapted Atlantic Wood killifish population relative to unexposed King’s Creek fish was determined. These data support previous experiments that indicated fitness costs associated with PAH adaptation in the Atlantic Wood population [40] that are consistent with evolutionary theory [61,62]. Nonetheless, in contrast to King’s Creek killifish, Elizabeth River sediment extract exposures had no impact on swimming performance in Atlantic Wood killifish. This is consistent with earlier investigations detailing the resistance of Atlantic Wood offspring to teratogenic effects of higher level exposures to PAHs and other contaminants [39]. Our findings were further supported by our experiment involving an additional exposure at age 5 mo to 1.0% Elizabeth River sediment extract, mirroring those of embryonic exposures.
The oxygen consumption results generally followed those for swimming performance, which is perhaps not surprising considering how closely the 2 variables are linked [63]. King’s Creek killifish exposed as embryos to either concentration of Elizabeth River sediment extract had significantly elevated rates of oxygen consumption at baseline (before the swimming tests). A similar treatment effect was noted after the swim tests (i.e., at exhaustion) but was not statistically significant. Oxygen consumption rates were elevated for all groups after the swim tests relative to their respective controls. Baseline oxygen consumption rates by control Atlantic Wood fish were significantly greater than control King’s Creek fish, but were unchanged by embryonic Elizabeth River sediment extract exposures. Again, these suggest a fitness cost for the Atlantic Wood fish, but no impact by laboratory PAH exposure.
Fish with naturally rounded ventricles are slower swimmers [64]. The adult killifish of the present study showed no changes in cardiac shape. However, we observed blood in the pericardial cavity of King’s Creek fish corresponding with increasing Elizabeth River sediment extract exposure. It is important to note that this could be an artifact of fixation practices; however, the absence of blood in the pericardial cavity of control fish indicates that this is unlikely. To our knowledge, this is the first report of this condition in the context of a chemical exposure. This observation contrasted strongly with the pericardial cavities of Atlantic Wood killifish, which showed little to no blood in the pericardial cavity. Although early-life stage (embryo) exposures caused concentration-dependent alterations, including pericardial edema, individuals free of such findings were allowed to grow out for 5 mo before use in swimming performance studies and morphological analysis. This implies that injury to the heart and/or surrounding visceral pericardium (epicardium) could have been responsible for such extravasation of blood leading to hemopericardium.
Polycyclic aromatic hydrocarbon mixture exposures are capable of causing cardiac structural deformities. They can cause cardiac dysfunction that deleteriously affects later morphogenetic steps such as looping of the atrial and ventricular chambers into their normal side-by-side positioning [4,57]. While these morphological defects are often lethal, milder transient cardiac irregularities caused by low-level PAH exposures may lead to thinner cardiac chambers/walls and possibly to extravasated blood, which in turn could lead to decreased performance and poor cardiac output. To make this association, we consulted the review and discussion by Satchell [65] of pressures in the pericardial cavities of various fish. When Farrell et al. [66] perfused the trout heart in situ under conditions of an intact versus an opened pericardium, the latter condition reduced cardiac output by 44%. We are extending our investigations to detailed analyses of the visceral and parietal pericardium, sinus venosus, atrium, and ventricle. These evaluations may provide detailed information regarding sites of leakage/hemorrhage into the pericardial cavity. Certainly, reduction of cardiac output by extravasation of blood into the pericardial cavity would be likely to negatively affect swimming performance. Developmental exposure to low levels (i.e., subteratogenic) of a complex PAH mixture resulted in persistent deficiency in swimming, cardiovascular performance, and in morphological alterations of the pericardium. Taken together, survivorship in predator–prey scenarios may be at risk. Overall, our findings corroborate and may also inform human health studies of effects related to early-life exposures to PAHs.
CONCLUSIONS
Low-level embryonic exposure to a complex PAH mixture derived from creosote-contaminated sediments (Elizabeth River sediment extract) is capable of altering later life swimming performance in adult killifish. In addition, acute point exposure to PAH contamination can also cause swimming performance deficits. Despite showing baseline swimming performance deficits, the Atlantic Wood killifish displayed no observable adverse effects following Elizabeth River sediment extract exposure. The resistance to Elizabeth River sediment extract exposure shown in our study illustrates the heritable effects of anthropogenic contamination within a select population of killifish. The present study has extended our understanding of fitness costs associated with PAH exposures and the PAH adaptation in the Atlantic Wood killifish population. Because killifish are an integral part of Atlantic estuarine ecosystems, subtle inputs of PAH contamination could result in ecosystem-wide consequences that warrant further examination.
Footnotes
Data Availability—Data, associated metadata, and calculation tools are available from the corresponding author (daniel.r.browndu@gmail.com).
References
- 1.Cantrell SM, Lutz LH, Tillitt DE, Hannink M. Embryotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): The embryonic vasculature is a physiological target for TCDD-induced DNA damage and apoptotic cell death in medaka (Orizias latipes) Toxicol Appl Pharm. 1996;141:23–34. doi: 10.1006/taap.1996.0256. [DOI] [PubMed] [Google Scholar]
- 2.Barron M, Carls MG, Heintz R, Rice SD. Evaluation of fish early life-stage toxicity models of chronic embryonic exposures to complex polycyclic aromatic hydrocarbon mixtures. Toxicol Sci. 2004;78:60–67. doi: 10.1093/toxsci/kfh051. [DOI] [PubMed] [Google Scholar]
- 3.Carney SA, Prasch AL, Heideman W, Peterson RE. Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res, A Clin Molec Teratol. 2006;76:7–18. doi: 10.1002/bdra.20216. [DOI] [PubMed] [Google Scholar]
- 4.Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharm. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Usenko CY, Robinson EM, Usenko S, Brooks BW, Bruce ED. Pbde developmental effects on embryonic zebrafish. Environ Toxicol Chem. 2011;30:1865–1872. doi: 10.1002/etc.570. [DOI] [PubMed] [Google Scholar]
- 6.Clark BW, Matson CW, Jung D, Di Giulio RT. AHR2 mediates cardiac teratogenesis of polycyclic aromatic hydrocarbons and PCB-126 in Atlantic killifish (Fundulus heteroclitus) Aquat Toxicol. 2010;99:232–240. doi: 10.1016/j.aquatox.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macaulay LJ, Bailey JM, Levin ED, Stapleton HM. Persisting effects of a PBDE metabolite, 6-OH-BDE-47, on larval and juvenile zebrafish swimming behavior. Neurotoxicol Teratol. 2015;52:119–126. doi: 10.1016/j.ntt.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Incardona JP, Carls MG, Day HL, Sloan CA, Bolton JL, Collier TK, Scholz NL. Cardiac arrhythmia is the primary response of embryonic Pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environ Sci Technol. 2009;43:201–207. doi: 10.1021/es802270t. [DOI] [PubMed] [Google Scholar]
- 9.Hicken CE, Linbo TL, Baldwin DH, Willis ML, Myers MS, Holland L, Larsen M, Stekoll MS, Rice SD, Collier TK, Scholz NL, Incardona JP. Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proc Natl Acad Sci U S A. 2011;108:7086–7090. doi: 10.1073/pnas.1019031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Incardona JP, Gardner LD, Linbo TL, Brown TL, Esbaugh AJ, Mager EM, Stieglitz JD, French BL, Labenia JS, Laetz CA, Tagal M, Sloan CA, Elizar A, Benetti DD, Grosell M, Block BA, Scholz NL. Deepwater Horizon crude oil impacts the developing hearts of large predatory pelagic fish. Proc Natl Acad Sci U S A. 2014;111:E1510–E1518. doi: 10.1073/pnas.1320950111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- 12.Billiard SM, Meyer JN, Wassenberg DM, Hodson PV, Di Giulio RT. Nonadditive effects of PAHs on early vertebrate development: Mechanisms and implications for risk assessment. Toxicol Sci. 2008;105:5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Incardona JP, Day HL, Collier TK, Scholz NL. Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol Appl Pharm. 2006;217:308–321. doi: 10.1016/j.taap.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Brown DR, Samsa LA, Qian L, Liu J. Advances in the study of heart development and disease using zebrafish. J Cardiovasc Dev Dis. 2016;3:13. doi: 10.3390/jcdd3020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DR, Bailey JM, Oliveri AN, Levin ED, Di Giulio RT. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicol Teratol. 2016;53:55–63. doi: 10.1016/j.ntt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vignet C, Devier MH, Le Menach K, Lyphout L, Potier J, Cachot J, Budzinski H, Begout ML, Cousin X. Long-term disruption of growth, reproduction, and behavior after embryonic exposure of zebrafish to PAH-spiked sediment. Environ Sci Pollut Res. 2014a;21:13877–13887. doi: 10.1007/s11356-014-2585-5. [DOI] [PubMed] [Google Scholar]
- 17.Vignet C, Le Menach K, Lyphout L, Guionnet T, Fr ere L, Leguay D, Budzinski H, Cousin X, Begout ML. Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in zebrafish—Part II: Behavior. Environ Sci Pollut Res. 2014b;21:13818–13832. doi: 10.1007/s11356-014-2762-6. [DOI] [PubMed] [Google Scholar]
- 18.Vines CA, Robbins T, Griffin FJ, Cherr GN. The effects of diffusible creosote-derived compounds on development in Pacific herring (Clupea pallasi) Aquat Toxicol. 2000;51:225–239. doi: 10.1016/s0166-445x(00)00107-7. [DOI] [PubMed] [Google Scholar]
- 19.Middaugh DP, Shelton ME, McKenney CL, Cherr GN, Chapman PJ, Courtney LA. Preliminary observations on responses of embryonic and larval Pacific herring, Clupea pallasai, to neutral fraction biodegradation products of weathered Alaska North Slope oil. Arch Environ Contam Toxicol. 1998;34:188–196. doi: 10.1007/s002449900303. [DOI] [PubMed] [Google Scholar]
- 20.Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio) Toxicol Appl Pharm. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JW, Dixit DB, Ward GS, Foster RS. Effects of petroleum hydrocarbons on the rate of heart beat and hatching success of estuarine fish embryos. In: Vernberg FJ, Calabrese A, Thurberg FP, Vernberg WB, editors. Physiological Responses of Marine Biota to Pollutants. Academic; New York, NY, USA: 1977. pp. 241–258. [Google Scholar]
- 22.Mager EM, Esbaugh AJ, Stieglitz JD, Hoenig R, Bodinier C, Incardona JP, Scholz NL, Benetti DD, Grosell M. Acute embryonic or juvenile exposure to Deepwater Horizon crude oil impairs the swimming performance of mahi-mahi (Coryphaena hippurus) Environ Sci Technol. 2014;48:7053–7061. doi: 10.1021/es501628k. [DOI] [PubMed] [Google Scholar]
- 23.Claireaux G, Davoodi F. Effect of exposure to petroleum hydrocarbons upon cardio-respiratory function in the common sole (Solea solea) Aquat Toxicol. 2010;98:113–119. doi: 10.1016/j.aquatox.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Incardona JP, Carls MG, Holland L, Linbo TL, Baldwin DH, Myers MS, Peck KA, Tagal M, Rice SD, Scholz NL. Very low embryonic crude oil exposures cause lasting cardiac defects in salmon and herring. Sci Rep. 2015;5:13499. doi: 10.1038/srep13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker SE, Dickhut RM, Chisholm-Brause C. Polycyclic aromatic hydrocarbons in a highly industrialized urban estuary: Inventories and trends. Environ Toxicol Chem. 2004;23:2655–2664. doi: 10.1897/03-628. [DOI] [PubMed] [Google Scholar]
- 26.Clark BW, Cooper EM, Stapleton HM, Di Giulio RT. Compound- and mixture-specific differences in resistance to polycyclic aromatic hydrocarbons and PCB-126 among Fundulus heteroclitus subpopulations throughout the Elizabeth River Estuary (Virginia, USA) Environ Sci Technol. 2013;47:10556–10566. doi: 10.1021/es401604b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang ML, Getzinger GJ, Cooper EM, Clark BW, Garner LVT, Di Giulio RT, Ferguson PL, Stapleton HM. Effect-directed analysis of Elizabeth River porewater: Developmental toxicity in zebrafish (Danio rerio) Environ Toxicol Chem. 2014;33:2767–2774. doi: 10.1002/etc.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padma TV, Hale RC, Roberts MH. Toxicity of water-soluble fractions derived from whole creosote and creosote-contaminated sediments. Environ Toxicol Chem. 1998;17:1606–1610. [Google Scholar]
- 29.Mulvey M, Newman M, Vogelbein WK, Unger MA. Genetic structure of Fundulus heteroclitus from PAH-contaminated and neighboring sites in the Elizabeth and York Rivers. Aquat Toxicol. 2003;61:195–209. doi: 10.1016/s0166-445x(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 30.Kneib RT. The role of Fundulus-heteroclitus in salt-marsh trophic dynamics. Am Zool. 1986;26:259–269. [Google Scholar]
- 31.Teo SLH, Able KW. Habitat use and movement of the mummichog (Fundulus heteroclitus) in a restored salt marsh. Estuaries. 2003;26:720–730. [Google Scholar]
- 32.Lotrich VA. Summer home range and movements of Fundulus-heteroclitus (Pisces-Cyprinodontidae) in a tidal creek. Ecology. 1975;56:191–198. [Google Scholar]
- 33.Skinner MA, Courtenay SC, Parker WR, Curry RA. Site fidelity of mummichogs (Fundulus heteroclitus) in an Atlantic Canadian estuary. Water Qual Res J Can. 2005;40:288–298. [Google Scholar]
- 34.Burnett KG, Bain LJ, Baldwin WS, Callard GV, Cohen S, Di Giulio RT, Evans DH, Gomez-Chiarri M, Hahn ME, Hoover CA, Karchner SI, Katoh F, Maclatchy DL, Marshall WS, Meyer JN, Nacci DE, Oleksiak MF, Rees BB, Singer TD, Stegeman JJ, Towle DW, Van Veld PA, Vogelbein WK, Whitehead A, Winn RN, Crawford DL. Fundulus as the premier teleost model in environmental biology: Opportunities for new insights using genomics. Comp Biochem Phys D. 2007;2:257–286. doi: 10.1016/j.cbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer J, Di Giulio R. Patterns of heritability of decreased EROD activity and resistance to PCB 126-induced teratogenesis in laboratory-reared offspring of killifish (Fundulus heteroclitus) from a creosote-contaminated site in the Elizabeth River, VA, USA. Mar Environ Res. 2002;54:621–626. doi: 10.1016/s0141-1136(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 36.Ownby D, Newman M, Mulvey M, Vogelbein WK, Unger MA, Arzayus F. Fish (Fundulus heteroclitus) populations with different exposure histories differ in tolerance of creosote-contaminated sediments. Environ Toxicol Chem. 2002;21:1897–1902. [PubMed] [Google Scholar]
- 37.Clark BW, Di Giulio RT. Fundulus heteroclitus adapted to PAHs are cross-resistant to multiple insecticides. Ecotoxicology. 2012;21:465–474. doi: 10.1007/s10646-011-0807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wills LP, Matson CW, Landon CD, Di Giulio RT. Characterization of the recalcitrant CYP1 phenotype found in Atlantic killifish (Fundulus heteroclitus) inhabiting a Superfund site on the Elizabeth River, VA. Aquat Toxicol. 2010;99:33–41. doi: 10.1016/j.aquatox.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Giulio RT, Clark BW. The Elizabeth River story: A case study in evolutionary toxicology. J Toxicol Environ Health B. 2015;18:259–298. doi: 10.1080/15320383.2015.1074841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer JN, Di Giuliuo RT. Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol Appl. 2003;13:490–503. [Google Scholar]
- 41.Brown DR, Clark BW, Garner LV, Di Giulio RT. Zebrafish cardiotoxicity: The effects of CYP1A inhibition and AHR2 knockdown following exposure to weak aryl hydrocarbon receptor agonists. Environ Sci Pollut Res. 2015;22:8329–8338. doi: 10.1007/s11356-014-3969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matson CW, Clark BW, Jenny MJ, Fleming CR, Hahn ME, Di Giulio RT. Development of the morpholino gene knockdown technique in Fundulus heteroclitus: A tool for studying molecular mechanisms in an established environmental model. Aquat Toxicol. 2008;87:289–295. doi: 10.1016/j.aquatox.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brett JR. The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can. 1964;30:1779–1809. or 21:1183–1226. [Google Scholar]
- 44.Jones DR. The effect of hypoxia and anaemia on the swimming performance of rainbow trout (Salmo gairdneri) J Exp Biol. 1971;5:541–551. doi: 10.1242/jeb.55.2.541. [DOI] [PubMed] [Google Scholar]
- 45.Beamish FWH. Swimming capacity. Fish Physiology. 1978;7:101–187. [Google Scholar]
- 46.Hammer C. Fatigue and exercise tests with fish. Comp Biochem Phys. 1995;112:1–20. [Google Scholar]
- 47.Jain KE, Hamilton JC, Farrell AP. Use of a ramp velocity test to measure critical swimming speed in rainbow trout, Oncorhynchus mykiss. Comp Biochem Phys A. 1997;117:441–444. [Google Scholar]
- 48.MacNutt MJ, Hinch SG, Farrell AP, Topp S. The effect of temperature and acclimation period on repeat swimming performance in cutthroat trout. J Fish Biol. 2004;65:342–353. [Google Scholar]
- 49.Bell WH, Terhune LB. Technical Report No. 15. Fisheries Research Board of Canada, Biological Station; Nanaimo, BC: 1970. Water tunnel design for fisheries research. [Google Scholar]
- 50.Kolok Interindividual variation in the prolonged locomotor performance of ectothermic vertebrates: A comparison of fish and herpetofaunal methodologies and a brief review of the recent fish literature. Can J Fish Aquat Sci. 1999;56:700–710. [Google Scholar]
- 51.Riley AK, Chernick M, Brown DR, Hinton DE, Di Giulio RT. Hepatic responses of juvenile Fundulus heteroclitus from pollution-adapted and nonadapted populations exposed to Elizabeth River sediment extract. Toxicol Pathol. 2016;44:738–748. doi: 10.1177/0192623316636717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landini G. Proceedings, Second ImageJ User and Developer Conference. Luxembourg: 2008. Nov 6–7, Advanced shape analysis with ImageJ; pp. 116–121. 2008. [cited 2016 August 11]. Available from: http://www.mecourse.com/landinig/software/software.html. [Google Scholar]
- 53.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol. 2001;23:291–299. [PubMed] [Google Scholar]
- 54.Satchell GH. Circulation in Fishes. Cambridge University; New York, NY, USA: 1971. [Google Scholar]
- 55.Carls MG, Rice SD, Hose JE. Sensitivity of fish embryos to weathered crude oil: Part I. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval Pacific herring (Clupea pallasi) Environ Toxicol Chem. 1999;18:481–493. [Google Scholar]
- 56.Wassenberg DM, Di Giulio RT. Teratogenesis in Fundulus heteroclitus embryos exposed to a creosote-contaminated sediment extract and CYP1A inhibitors. Mar Environ Res. 2004;58:163–168. doi: 10.1016/j.marenvres.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Incardona JP, Carls MG, Teraoka H, Sloan CA, Collier TK, Scholz NL. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ Health Perspect. 2005;113:1755–1762. doi: 10.1289/ehp.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi X, He C, Zuo Z, Li R, Chen D, Chen R, Wang C. Pyrene exposure influences the craniofacial cartilage development of Sebastiscus marmoratus embryos. Mar Environ Res. 2012;77:30–34. doi: 10.1016/j.marenvres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Short JW, Heintz RA. Identification of Exxon Valdez oil in sediments and tissues from Prince William Sound and the northwestern Gulf of Alaska based on a PAH weathering model. Environ Sci Technol. 1997;31:2375–2384. [Google Scholar]
- 60.Fuiman LA, Rose KA, Cowan JH, Smith EP. Survival skills required for predator evasion by fish larvae and their relation to laboratory measures of performance. Anim Behav. 2006;71:1389–1399. [Google Scholar]
- 61.Coustau C, Chevillon C, French-Constant R. Resistance to xenobiotics and parasites: Can we count the cost? Trends Ecol Evol. 2000;15:378–383. doi: 10.1016/s0169-5347(00)01929-7. [DOI] [PubMed] [Google Scholar]
- 62.Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: Contemporary evolution and the dynamics of persistence. Funct Ecol. 2007;21:444–454. [Google Scholar]
- 63.Plaut I, Malcolm SG. Swimming metabolism of wild-type and cloned zebrafish (Brachydanio rerio) J Exp Biol. 1994;194:209–223. doi: 10.1242/jeb.194.1.209. [DOI] [PubMed] [Google Scholar]
- 64.Claireaux G, McKenzie DJ, Genge AG, Chatelier A, Aubin J, Farrell AP. Linking swimming performance, cardiac pumping ability and cardiac anatomy in rainbow trout. J Exp Biol. 2005;208:1775–1784. doi: 10.1242/jeb.01587. [DOI] [PubMed] [Google Scholar]
- 65.Satchell GH. Physiology and Form of Fish Circulation. Cambridge University; New York, NY, USA: 1991. [Google Scholar]
- 66.Farrell AP, Johansen JA, Graham MS. The role of the pericardium in cardiac performance of the trout (Salmo gairdneri) Physiol Zool. 1988;61:213–221. [Google Scholar]


