Abstract
Multiple metabolic and hormonal factors can affect the success of protocols for ovarian superstimulation. In this study, the effect of acute feed restriction and increased LH content in the superstimulatory FSH preparation on numbers of ovulations, fertilization, and embryo quality in lactating dairy cows was evaluated. Two experiments were performed using a Latin square design with treatments arranged as a 2 × 2 factorial: feed restriction (FR; 25% reduction in dry matter intake) compared with ad libitum (AL) feeding, combined with high (H) versus low (L) LH in the last 4 injections of the superstimulatory protocol. As expected, FR decreased circulating insulin concentrations (26.7 vs. 46.0 μU/mL). Two analyses were performed: one that evaluated the complete Latin square in experiment 2 and a second that evaluated only the first periods of experiments 1 and 2. For both analyses, follicle numbers, ovulation rates, and corpora lutea on d 7 were not different. In the first period analysis of experiments 1 and 2, we observed an interaction between feed allowance and amount of LH on fertilization rates, percentage of embryos or oocytes that were quality 1 and 2 embryos, and number of embryos or oocytes that were degenerate. Fertilization rates were greater for the AL-L (89.4%) and FR-H (80.1%) treatments compared with the AL-H (47.9%) and FR-L (59.9%) treatments. Similarly, the proportion of total embryos or oocytes designated as quality 1 and 2 embryos was greater for AL-L (76.7%) and FR-H (73.4%) treatments compared with AL-H (35.6%) and FR-L (47.3%) treatments. In addition, the number of degenerate embryos was decreased for AL-L (1.3) and FR-H (0.4) treatments compared with the AL-H (2.6) and FR-L (2.3) treatments. Thus, cows with either too low (FR-L) or too high (AL-H) insulin and LH stimulation had lesser embryo production after superstimulation because of reduced fertilization rate and increased percentage of degenerate embryos. Therefore, interaction of the gonadotropin content of the superstimulatory preparation with the nutritional program of the donor cow needs to be considered to optimize success of ovarian superstimulatory protocols.
Keywords: superstimulation, feed restriction, luteinizing hormone, dairy cattle
INTRODUCTION
Ovarian superstimulation of lactating dairy cows is a practical tool to increase offspring from cows of high genetic merit. Researchers have acquired valuable biological information on the effects of specific hormonal and metabolic factors on early embryo development by applying treatments to cows that have been superstimulated. Nevertheless, superstimulation results, particularly in lactating dairy cows, vary widely among individual cows (Murphy et al., 1984; Greve et al., 1995) and are somewhat variable among different experiments (Dalton et al., 2000; Sartori et al., 2004, 2010). Protocols that increase embryo yield and quality could make superstimulation more valuable from both a practical and research perspective.
The amount of LH in the FSH preparation of a superstimulatory protocol is one factor that affects the number of ovulations and embryo quality. Results have been surprisingly inconsistent when number of corpora lutea (CL) were evaluated, with some studies reporting an increase (Chupin et al., 1985; Kelly et al., 1997), others reporting no effect (Willmott et al., 1990), and some reporting a detrimental effect of high LH (Chupin et al., 1984; Tribulo et al., 1991). In addition, increasing LH in the FSH preparation has generally been found to decrease embryo quality in most (Chupin et al., 1984; Donaldson and Ward, 1986; Donaldson et al., 1986) but not all (Willmott et al., 1990) studies. Further, intensive studies (Luo et al., 2011) indicate that LH pulses must be present for successful induction of LH receptors on the granulosa cells of the developing future dominant follicle. Induction of LH receptors and other changes in the granulosa cells are expected to be essential for subsequent ovulation of the follicle. Thus, our first objective was to determine the effect of supplemental LH in a superstimulatory protocol on the subsequent number of ovulations and embryo quality.
Numerous metabolic factors can affect reproduction in lactating dairy cows. Changes in feed intake or feed components have been found to alter insulin (Adamiak et al., 2005, 2006), progesterone (P4; Sangsritavong et al., 2002; Vasconcelos et al., 2003), and superstimulatory response (Yaakub et al., 1999a,b). For example, superstimulated beef heifers fed concentrates ad libitum compared with 81% of ad libitum intake had reduced numbers of CL, reduced numbers of recovered embryos or oocytes, and reduced yield of transferable embryos (Yaakub et al., 1999b). In a study with superovulated ewes, overfeeding (2.2 × maintenance) dramatically reduced embryo quality compared with underfeeding (0.5 × maintenance; Lozano et al., 2003). This last study, as well as others in lactating cows (Sangsritavong et al., 2002; Vasconcelos et al., 2003), noted that animals with greater feed intake had reduced circulating P4 concentrations.
Increased circulating P4 concentrations during super-stimulation dramatically increases embryo quality and quantity (Nasser et al., 2011; Rivera et al., 2011). The underlying mechanisms leading to reduced embryo production in the presence of decreased P4 concentrations or increased feed intake have not yet been elucidated. One possibility is that increased LH pulsatility or excess insulin may result in overstimulation of follicles during the superstimulatory protocol, possibly leading to premature resumption of meiosis and ovulation of an oocyte of reduced fertility, as reported in persistent follicle models (Roberson et al., 1989; Revah and Butler, 1996). Regardless of the mechanism, a reduction in DMI could increase circulating P4, as observed by Sangsritavong et al. (2002) and would be expected to reduce circulating insulin. Both of these changes could lead to changes in follicle and oocyte health and subsequent embryo quality. Consistent with this idea, our companion paper (Ferraretto et al., 2014) reports that a 25% reduction in DMI in lactating cows increased P4 concentrations and decreased insulin concentrations. Although an increase in insulin via dietary manipulations in the early postpartum period has potential beneficial effects on resumption of cyclicity and ovulation, higher insulin during the mating period may reduce fertility (Butler et al., 2004, 2006; Garnsworthy et al., 2009a,b). Thus, our second objective was to determine the effect of acute feed restriction on ovarian super-stimulation, fertilization, and embryo quality.
The present study had 2 primary hypotheses. Our first hypothesis was that increasing LH during an ovarian superstimulation protocol would increase the ovulation rate but may reduce embryo quality. Second, we hypothesized that acute feed restriction (25%) during superstimulation would not alter ovulation rate but would increase embryo quality. Of particular importance, we proposed that an important interaction could occur between these 2 treatments with increasing LH in the FSH preparation, potentially leading to increased embryo yield in feed-restricted cows but potentially having a negative effect in cows fed ad libitum.
MATERIALS AND METHODS
Animals and Management
All procedures were approved by the Animal Care and Use Committee of the College of Agriculture and Life Sciences (University of Wisconsin-Madison). This experiment was conducted from April 2011 to June 2011 (experiment 1) and September 2011 to December 2011 (experiment 2) using cows housed in the tiestall barn at the University of Wisconsin dairy herd. During the nontreatment portion of the trial, cows were fed a TMR (Table 1) once daily, formulated to meet or exceed NRC requirements (NRC, 2001) for high-producing lactating dairy cows with ad libitum access to feed and water. Before enrollment in the study, cows had an average daily milk production of 38.7 ± 5.6 kg, were selected from lactations 1 to 6, and had an average DIM of 482 ± 26. Throughout the experiment, cows were milked twice daily at approximately 12-h intervals. All cows received subcutaneous injections of bST (Posilac, 500 mg, Monsanto Co., St. Louis, MO) at 14-d intervals throughout the trial. All procedures, including injections, ultrasonography, blood collection, AI, follicular ablation, and flushing were performed while cows were restrained in tiestalls or in the surgery room of the Dairy Cattle Center of the University of Wisconsin-Madison.
Table 1.
Ingredient and nutrient composition (% of DM unless otherwise noted) of the formulated diet
| Item | TMR |
|---|---|
| Ingredient | |
| Haylage1 | 31.2 |
| Corn silage2 | 16.8 |
| Whole cottonseed | 6.4 |
| Dry ground shelled corn | 24.8 |
| Distillers dried grains | 6.2 |
| Soybean meal, solvent | 4.3 |
| Soy hulls | 3.6 |
| Soybean meal, expeller3 | 3.6 |
| Calcium | 0.91 |
| Megalac4 | 0.80 |
| Sodium bicarbonate | 0.54 |
| Mineral mix5 | 0.30 |
| Urea | 0.22 |
| UW ADE6 | 0.18 |
| Magnesium oxide | 0.13 |
| VIP Rumensin 57 | 0.10 |
| Vitamin E | 0.03 |
| Nutrient | |
| DM (TMR, % of as fed) | 52.0 |
| CP (%) | 17.5 |
| NDF (%) | 31.6 |
| NFC (%) | 39.8 |
| Fat (%) | 5.0 |
| NEL (Mcal/kg) | 1.76 |
| Ca (%) | 0.95 |
| P (%) | 0.37 |
Contained 18.2% CP, 32.5% ADF, and 44.3% NDF.
Contained 8.3% CP, 24.0% ADF, and 38.0% NDF.
Exceller meal (Quality Roasting Inc., Valders, WI).
Megalac (Arm & Hammer, Ewing, NJ).
Contained 88% NaCl, 0.002% Co, 0.2% Cu, 0.012% I, 0.18% Fe, 0.8% Mn, 0.006% Se, and 1.4% Zn.
Vitamin A, 3,300,000 IU/kg; vitamin D, 1,100,000 IU/kg; vitamin E, 11,000 IU/kg.
Premix contained 11 g/kg of Rumensin (Elanco Animal Health, Greenfield, IN).
Experiment 1
Sixteen nonpregnant, cyclic, lactating Holstein dairy cows were used for this experiment. Cows were randomly divided into 2 groups of 8, separated by 1 d, to facilitate ease in cow handling during intensive procedures. Cows underwent ovarian superstimulation, superovulation, and subsequent uterine flush according to the procedures outlined below, in 4 Latin squares, organized in a Williams design to balance first-order carryover effects (Wang et al., 2009). In each period of the Latin square, each cow was exposed to 1 of 4 treatments arranged in a 2 × 2 factorial design. Cows were then resynchronized, ovarian superstimulated, super-ovulated, and uterine flushed, allowing 4 wk between the flushing procedures of each period. The experiment was terminated after period 2; thus, each cow was flushed twice and exposed to 2 of the 4 treatments. In each period, each of the 4 treatments was represented.
Experiment 2
Sixteen nonpregnant, cyclic, lactating Holstein dairy cows (different from those used in experiment 1) were used for this experiment. Cows were randomly divided into 2 groups of 8, separated by 1 d, to facilitate ease in cow handling during intensive procedures. Cows underwent ovarian superstimulation, superovulation, and subsequent uterine flush according to the procedures outlined below, in 4 Latin squares, again organized in a Williams design to balance first-order carryover effects (Wang et al., 2009). In each period of the Latin square, each cow was exposed to 1 of 4 treatments arranged in a 2 × 2 factorial design. Cows were then resynchronized, ovarian superstimulated, superovulated, and uterine flushed, allowing 4 wk between the flushing procedures of each period. Each cow received all 4 treatments and each treatment was represented in each period of the Latin square.
Treatments: Feed and LH
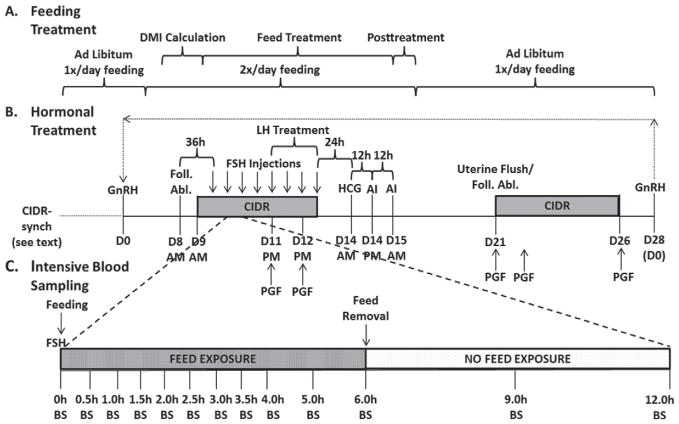
Feed intake and LH quantity in the FSH preparation served as the 2 variables in the 2 × 2 factorial design of treatments. Feed allowance was either ad libitum (AL) or feed restricted (FR; 25% reduction from AL DMI) of the aforementioned TMR. Beginning on d 4 (Figure 1A), the feeding schedule was modified to allow cows to become accustomed to a change in schedule. In the pretreatment interval, all cows were exposed to ad libitum feed for 6 h, and then feed was removed for 6 h. This continued for 2 d. On d 6.5 to d 9.5, daily feed intakes were determined to obtain average DMI for each cow (ample feed was given to ensure proper ad libitum feeding). Weighbacks were recorded each day and an average DMI was calculated. The cows under the AL treatment received 120% of their previous 3-d average DMI, whereas the cows under the FR treatment received 75% of their previous 3-d average DMI. Each ration of feed was equally divided into 2 rations per day to accommodate both 6-h intervals of exposure to feed. The treatment interval began on d 9.5 and continued to d 15.5, at which point all cows were again exposed to ad libitum feeding (still on the modified feeding schedule of exposure to feed for 6 h, and then feed removed for 6 h). Daily feed intakes were monitored; if an AL-treated cow had <10% weighbacks, the feed allowance was increased by approximately 10% the following day. All cows returned to a normal, once per day feeding schedule on d 16.5.
Figure 1.
Feeding protocol (A), synchronization and superstimulation protocol (B), and intensive bleeding protocol (C) from experiments 1 and 2. In both experiments, cows were presynchronized with controlled internal drug release (CIDR)-Synch (see Materials and Methods) with the last GnRH on d 0 (D0). Cows underwent superstimulation as shown while exposed to a 2 × 2 factorial of treatments: with or without feed restriction and with or without supplemental LH. Cows were exposed to either feed restriction (FR; 25% restriction of intake) or ad libitum (AL) feeding during the Feed Treatment period (A). All FSH injections were supplemented with low LH, whereas the last 4 were supplemented with either high (H) or low (L) LH during LH Treatment (B). Cows then received AI with subsequent uterine flushing. Resynchronization was then performed (B) using CIDR-Synch, follicular ablation (Foll. Abl.), and subsequent superstimulation for period 2 (experiment 1), and periods 2, 3, and 4 (experiment 2). During period 2 of experiment 2, intensive blood sampling (BS) occurred following the second, fourth, sixth, and eighth injections (C). HCG = human chorionic gonadotropin.
Luteinizing hormone was modified to either be high (H) or low (L) on the last 4 FSH treatment preparations, thus creating the 4 treatment groups AL-H, ALL, FR-H, and FR-L. Two FSH preparations containing different amounts of FSH and LH were provided by Minitube of America (Verona, WI). Pluset (50% LH, 50% FSH) and Flex H (25% LH, 75% FSH) were used in this trial, and amounts were varied to provide the FSH and LH delivery illustrated in Table 2. All injections coincided with the 6-h interval of time that began with feeding the cows.
Table 2.
Experimental design detailing quantity of FSH and LH in superstimulatory preparation1
| Day/time | Low LH: Flex H only | High LH: Flex H + Pluset | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| FSH (IU) | LH (IU) | Flex H (mL) | Pluset (mL) | FSH (IU) | LH (IU) | Flex H (mL) | Pluset (mL) | |
| Day 9, p.m. | 158 | 53 | 3.00 | — | 158 | 53 | 3.00 | — |
| Day 10, a.m. | 144 | 48 | 2.75 | — | 144 | 48 | 2.75 | — |
| Day 10, p.m. | 118 | 39 | 2.25 | — | 118 | 39 | 2.25 | — |
| Day 11, a.m. | 105 | 35 | 2.00 | — | 105 | 35 | 2.00 | — |
| Day 11, p.m. | 79 | 26 | 1.50 | — | 79 | 79 | — | 2.25 |
| Day 12, a.m. | 53 | 18 | 1.00 | — | 53 | 53 | — | 1.50 |
| Day 12, p.m. | 24 | 8 | 0.45 | — | 24 | 24 | — | 0.70 |
| Day 13, a.m. | 24 | 8 | 0.45 | — | 24 | 24 | — | 0.70 |
| Total | 705 | 235 | 13.40 | — | 705 | 353.5 | 10.00 | 5.15 |
The low LH and high LH treatments were prepared using 2 drugs: Pluset (50% LH, 50% FSH; Minitube of America, Verona, WI) and Flex H (25% LH, 75% FSH; Minitube of America) in different doses. The first 4 injections of the 8-injection protocol were identical; the last 4 injections were varied to accommodate the low LH/high LH treatments.
Synchronization, Superstimulatory Treatments, and Flushing Protocol
Estrous cycles were synchronized using a modified controlled internal drug releasing (CIDR)-synch program (Figure 1B). On d −10, cows received GnRH (100 μg of gonadorelin diacetate tetrahydrate, Fertagyl; Intervet Inc., Millsboro, DE), followed by the insertion of an intravaginal P4-releasing device (Eazi-Breed CIDR; Pfizer Animal Health, New York, NY) the next day (d −9). Prostaglandin F2α (PGF, 25 mg of Lutalyse; Pfizer Animal Health) was administered 6 and 7 d later (d −3 and d −2), with the second injection coinciding with the removal of the CIDR. On d 0, GnRH was again administered (Figure 1B). Throughout the synchronization protocol, ultrasonic evaluation (Ibex, E.I. Medical Imaging, Loveland, CO) was used to evaluate ovarian responses to the hormonal treatments. Ovulation was determined via the presence and subsequent absence of a follicle >9 mm immediately before and 1 d after GnRH administration.
Figure 1B illustrates the protocol described below. To synchronize the start of a new follicular wave before superstimulation, all follicles ≥5 mm were aspirated with an ultrasound-guided needle (Aloka 900, Hitachi Aloka, Wallingford, CT) 8 d after the GnRH injection (d 0) of the synchronization protocol. The superstimulation injections of FSH were initiated 36 h after aspiration in a series of 8 injections, each separated by 12 h. In addition, a used CIDR (5 d of previous use, cleaned and autoclaved) was inserted 24 h after aspiration. Administration of PGF occurred coincident with the fifth and seventh FSH injections. Human chorionic gonadotropin (hCG, 3,300 IU; Chorulon, Intervet Inc.) was administered 24 h after the last FSH injection to ensure ovulation of follicles, with AI occurring both 12 and 24 h following hCG. All cows were inseminated by one experienced technician to 1 of 5 high fertility bulls (Accelerated Genetics, Baraboo, WI) with all semen deposited into the uterine body of the cow.
On d 21 (7 d after hCG), cows were flushed by 1 of 4 experienced technicians, according to procedures similar to those described by Sartori et al. (2003). Briefly, a silicone Foley 2-way catheter (Minitube of America) was inserted through the cervix and into the uterus, where the balloon was inflated in one uterine horn approximately 2 cm cranial to the uterine bifurcation. The uterine horn was flushed into a MiniFlush Filter System (Minitube of America) with approximately 650 mL of medium prepared in our laboratory. The medium was sterile lactated Ringer’s, USP (Abbott Laboratories, Chicago, IL) with polyvinyl alcohol (PVA, 3.0 g/L; Sigma-Aldrich Co., St. Louis, MO) and 10 mL of an antibiotic solution (Cellgro, Mediatech Inc., Manassas, VA) containing penicillin (10,000 units/mL) and streptomycin (10 mg/mL). The balloon was deflated, and a new catheter was inserted in a similar manner to flush the other uterine horn, also with approximately 650 mL of medium. This second catheter was then pulled back to the cervix, where the balloon was inflated caudal to the uterine body, against the cervix. Flush medium flowed into the uterus until the uterus was full. The catheter then remained in the uterus, with the end clamped to prevent loss of flush medium. Cows were then reflushed using a full uterine body flush 30 min later with 650 mL of flush medium. Filters were searched and embryos/oocytes were graded according to quality and stage of development by one experienced technician utilizing the standards set by the International Embryo Transfer Society (grades 1 to 4; IETS; http://www.iets.org/).
Immediately after the flushing procedure, cows were resynchronized (following period 1 for experiment 1, and periods 1, 2, and 3 for experiment 2) to be flushed again (Figure 1B). On the same day as uterine flushing, cows underwent follicular ablation to remove any remaining follicles and begin a new follicular wave. A CIDR was inserted, and PGF was administered (2 injections, d 21 and d 22). After 5 d (d 26), the CIDR was removed, and PGF was again administered. Then, GnRH was administered 2 d later (d 28), which also coincided with d 0 to restart the protocol as the following period. If cows did not ovulate to the GnRH injection, 2 CIDR inserts were inserted during the superstimulatory protocol to mimic P4 production from the CL. Specifically, if the cow did not ovulate to the GnRH (d 0), a CIDR was inserted on d 6, and an additional CIDR was inserted on d 8. One CIDR was removed after each PGF injection of the superstimulatory protocol.
Blood Sampling and Hormonal Analyses
Blood samples were collected via puncture of the jugular vein into evacuated tubes (Vacutainer, Becton Dickinson, Franklin Lakes, NJ) every 6 h beginning on d 8.5 before feeding and continuing every 6 h (immediately before any treatments) through d 16, as well as a blood sample immediately before uterine flushing. Clotted blood for serum was immediately stored at 4°C and centrifuged (3,000 × g, 20 min) after 24 h had elapsed. Whole blood for plasma was immediately placed on ice and centrifuged (3,000 × g, 20 min) within 10 min. Both serum and plasma samples were stored at −20°C until assayed.
As illustrated in Figure 1C, blood samples were taken into evacuated tubes (Vacutainer, Becton Dickinson) during experiment 2 in period 2 following the second, fourth, sixth, and eighth FSH treatments using a jugular catheter that was installed 2 d before sample time.
Plasma samples were used for both NEFA and glucose assays from 1 period of experiment 2. The NEFA Color Assay (Wako Pure Chemical Industries Ltd., Osaka, Japan) was utilized for NEFA determination and had a sensitivity calculated by the manufacturer of 0.0014 mEq/L, an interassay CV of 2.6%, and an intraassay CV of 3.9%. Glucose was colorimetrically assayed via a glucose oxidase-peroxidase-chromogen assay (Karkalas, 1985) with an interassay CV of 6.0% and an intraassay CV of 5.5%.
Serum samples were used for P4, estradiol (E2), and insulin analyses from 1 period of experiment 2. Progesterone was analyzed via ELISA (Rasmussen et al., 1996), with an average extraction efficiency of 67.5%, average sensitivity of 0.047 ng/mL, interassay CV of 16.4%, and an intraassay CV of 3.8%. Serum insulin samples were assayed via a Porcine Insulin RIA (Millipore Corp., Billerica, MA) with a sensitivity of 1.611 μU/mL, an interassay CV of 6.7%, and an intraassay CV of 6.4%. Analysis of E2 was completed by an Estradiol Double-Antibody RIA (Siemens Healthcare Diagnostics, Los Angeles, CA) and had a sensitivity of 0.0535 pg/mL (calculated as 2 times the standard deviation of maximum binding), an interassay CV of 4.3%, and an intraassay CV of 3.4%.
Embryo Evaluation and Analyses
All embryo analysis occurred on a per flush basis (as reported in Tables 2 to 5). All ultrasonography was evaluated by carefully recording an ultrasound video of the ovaries and subsequently evaluating the ovaries slowly to try to accurately count all follicles and CL present. Follicles >9 mm were counted via ultrasonography immediately before the hCG injection, and again at 60 h after the hCG injection. Ovulation rates are calculated via disappearance of a follicle >9 mm at 60 h. Ultrasonography was again performed at 1 d before uterine flushing to determine CL. The total number of embryos or oocytes recovered during each flush (both fertilized and unfertilized) was determined, as well as the recovery rate, defined as the total number of embryos or oocytes recovered as a proportion of total CL number. The numerical count variables for quality 1 embryos, quality 1 and 2 embryos, and degenerate embryos refer to an actual count of the number of those embryos or oocytes on a per flush basis, whereas the percentage variables refer to the numerical count as a proportion of fertilized oocytes or total embryos or oocytes, averaged on a per flush basis. Least squares means (LSM) are reported for all variables.
Table 5.
Embryo quality results (LSM ± SEM) from experiments 1 and 2 analyzed by 2 methods: experiment 2 only and the first periods from experiments 1 and 21
| Item | Interactions: Feed × LH content | P-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| AL-H | AL-L | FR-H | FR-L | Feed | LH | Feed × LH | |
| Experiment 2 (no.) | 12 | 10 | 12 | 10 | |||
| Quality 1 embryos (no.) | 2.1AB ± 1.1 | 2.9A ± 1.1 | 1.6B ± 1.1 | 2.5AB ± 1.1 | 0.49 | 0.17 | 0.83 |
| (% of fertilized) | 17.3B ± 8.4 | 36.4A ± 9.1 | 15.3B ± 8.4 | 26.1AB ± 9.1 | 0.45 | 0.07 | 0.61 |
| Quality 1 and 2 embryos (no.) | 4.5 ± 1.7 | 5.2 ± 1.8 | 2.9 ± 1.7 | 3.8 ± 1.8 | 0.26 | 0.56 | 0.95 |
| (% of fertilized) | 45.5 ± 15.4 | 60.0 ± 16.2 | 49.8 ± 15.4 | 43.8 ± 16.2 | 0.59 | 0.71 | 0.37 |
| (% of total) | 28.9 ± 13.7 | 46.8 ± 14.4 | 42.3 ± 13.7 | 32.7 ± 14.4 | 0.99 | 0.88 | 0.20 |
| Degenerate (no.) | 3.1A ± 0.9 | 1.4B ± 0.9 | 2.3AB ± 0.9 | 3.7A ± 0.9 | 0.43 | 0.65 | 0.05 |
| (% of fertilized) | 52.1 ± 15.4 | 38.6 ± 16.2 | 46.1 ± 15.4 | 56.1 ± 16.3 | 0.72 | 0.83 | 0.27 |
| First periods (no.) | 7 | 7 | 7 | 8 | |||
| Quality 1 embryos (no.) | 2.4AB ± 1.2 | 4.9A ± 1.2 | 1.3B ± 1.2 | 4.1A ± 1.2 | 0.45 | 0.04 | 0.88 |
| (% of fertilized) | 20.7b ± 11.8 | 55.5a ± 11.8 | 41.3ab ± 11.8 | 41.6ab ± 10.9 | 0.77 | 0.14 | 0.15 |
| Quality 1 and 2 embryos (no.) | 5.5AB ± 1.9 | 7.6A ± 1.9 | 2.5B ± 1.9 | 5.9AB ± 1.8 | 0.23 | 0.16 | 0.76 |
| (% of fertilized) | 59.5B ± 12.0 | 76.7AB ± 12.0 | 88.3A ± 12.0 | 70.3AB ± 11.1 | 0.35 | 0.97 | 0.15 |
| (% of total) | 35.6b,B ± 11.6 | 76.7a,A ± 11.6 | 73.4a,A ± 11.6 | 47.3ab,B ± 10.8 | 0.72 | 0.52 | <0.01 |
| Degenerate (no.) | 2.6a,A ± 0.7 | 1.3ab,AB ± 0.7 | 0.4b,B ± 0.7 | 2.3ab,A ± 0.6 | 0.38 | 0.73 | 0.03 |
| (% of fertilized) | 37.8A ± 12.0 | 22.8AB ± 12.0 | 9.1B ± 12.0 | 29.8AB ± 11.1 | 0.37 | 0.81 | 0.14 |
Within response variable, means with different superscripts differ (P < 0.05)
Within response variable, means with different superscripts tended to differ (P < 0.15).
Superstimulated, lactating Holstein dairy cows were fed either ad libitum (AL) or feed restricted (FR) and exposed to either low LH (L) or high LH (H) to create 4 treatments: AL-H, AL-L, FR-H, and FR-L.
Statistical Analyses
Because of failure to meet assumptions of normality, E2, glucose, NEFA, and intensive insulin data at each time point were analyzed in a nonparametric fashion using the RANK procedure of SAS, which analyzes the ranks of the data rather than the original data values (version 9.3, SAS Institute Inc., Cary, NC). The E2, glucose, and NEFA data were then analyzed via the MIXED procedure of SAS, with time and treatment as fixed variables. Progesterone was analyzed via the MIXED procedure of SAS, with treatment and time as fixed variables. Main effects of LH and feeding treatment, as well as the interaction between treatments were included in the model statement. The intensive insulin samples generated from experiment 2, period 2 were also analyzed via the MIXED procedure of SAS using an area under the curve analysis (trapezoid method), including time, treatment, and day as fixed variables. Main effects of LH and feeding treatments and interactions between treatments were included in the statistical model. Finally, after a log-transformation, 6-h insulin samples met all statistical assumptions and were analyzed via the MIXED procedure of SAS, with time and treatment as fixed variables, and main effects of LH and feeding treatments and interactions between treatments included in the model.
Milk production and feed intake were analyzed via the MIXED procedure of SAS, with period as a random variable and treatment and day as fixed variables.
This experiment produced data from a total of 94 superstimulations and 93 embryo flushes performed in 2 different experiments. To properly evaluate the results, several different approaches were used during the statistical analyses of the superstimulatory response, uterine flush, and embryo quality results. The first approach was the analysis of the full Latin square (experiment 2), using the MIXED procedure of SAS, with cow and period as random variables, and main effects of LH and feeding treatment and interactions between treatments as fixed effects included in the model. Although this analysis of only experiment 2 was the most straightforward analysis from a statistical perspective, it did not use data from all cows. A second approach was to analyze data from both experiments 1 and 2, as if both experiments used a full Latin square design. The complete results from this analysis can be obtained from the MS thesis of R. W. Bender (Bender, 2012).
In addition, a third analysis was performed using only the results from the first period of the experiments. We performed this analysis because the first period had much better embryo yield, fertilization rates, and embryo quality than the other periods in both experiments. Thus, the first periods from experiments 1 and 2 were analyzed as a complete randomized design via the MIXED procedure of SAS, with treatments, experiment number, and interactions between treatments as fixed effects (and no random effects) in the model statement.
For all data, a difference between the levels of a response variable was considered significant when P < 0.05, whereas differences between P > 0.05 and P < 0.15 were considered a statistical tendency. Data presented in the tables are LSM estimates and standard error of the mean (±SEM).
RESULTS
Experiment 1 was intentionally terminated after 2 periods because of an expected reduction in fertility and embryo quality as the experiment approached the summer months. In experiment 2, 1 cow was superstimulated but not flushed because of difficulty in passing the catheter through the cervix, and 1 cow died at the beginning of period 3, and thus was not superstimulated and flushed in periods 3 and 4.
Milk Production and Feed Intake
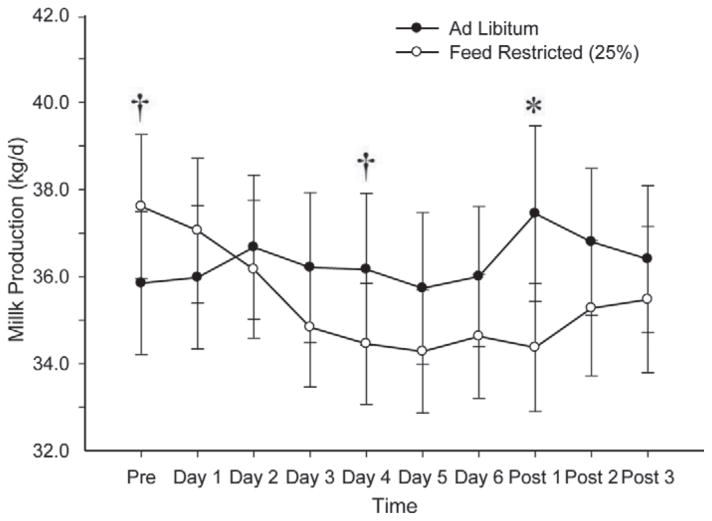
Milk production (Figure 2) tended (P = 0.14) to be greater for the FR cows before initiation of feed treatments. On d 4 of the treatment period, milk production tended (P = 0.11) to increase in AL compared with FR cows. Milk production was also greater (P < 0.05) for AL cows on d 1 after treatment.
Figure 2.
Milk production in experiment 2 before treatment (Pre), during feed treatment (d 1 – d 6), and after all cows returned to ad libitum feed (Post 1 – Post 3). We observed no effect of feed (P = 0.33), LH (P = 0.64), or time (P = 0.11), but we did detect an interaction of feed × time (P = 0.0097), as shown. †P < 0.15; *P < 0.05.
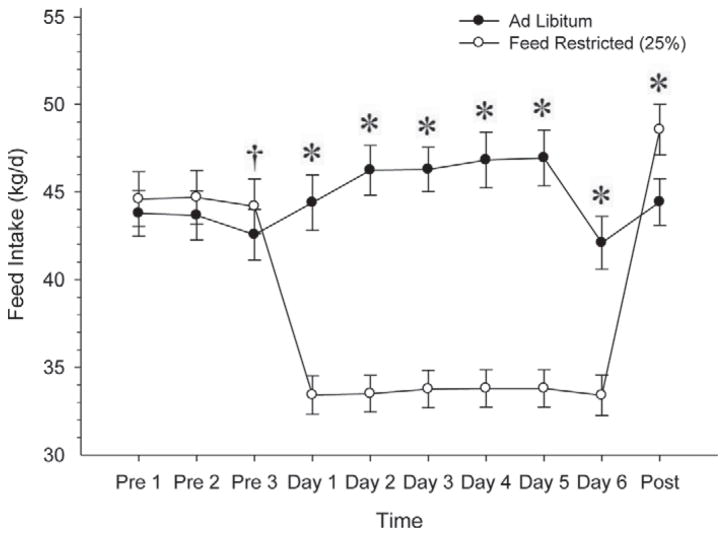
Feed intake (Figure 3), measured on an ad libitum basis, tended (P < 0.15) to be greater for the FR cows on the day immediately preceding initiation of treatment, but was not different 2 and 3 d before treatment. On d 1 through d 6 of treatment, feed intake was greater (P < 0.05) for the AL group compared with the FR group. After treatment, on the day immediately after all cows returned to ad libitum feeding, the FR group had a greater (P < 0.05) feed intake than the AL group.
Figure 3.
Feed intake (ad libitum basis) before treatment (Pre 1 – Pre 3), during feed treatment (Day 1 – Day 6), and after all cows returned to ad libitum feed (Post). We observed no effect of LH (P = 0.89) but we did detect an effect of feed (P < 0.0001), time (P < 0.0001), and an interaction of feed × time (P < 0.0001). †P < 0.15; *P < 0.05.
Metabolic and Reproductive Hormones
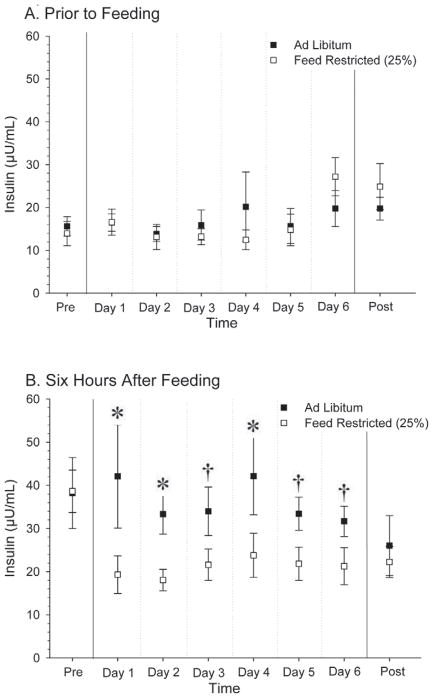
Circulating insulin concentrations (Figure 4) were similar before initiation of feed treatment, as seen in the pretreatment period (Pre-Trt) of Figure 4A and 4B. Insulin concentrations were greater (P < 0.05) after exposure to feed for 6 h in the AL treatment compared with the FR treatment on d 1, 2, and 4, and showed a tendency (P < 0.15) to be greater on d 3, 5, and 6 (Figure 4B). During the treatment protocol, no differences were observed in circulating insulin levels before feeding (Figure 4A). After all cows returned to ad libitum feeding (Post-Trt), similar insulin concentrations were observed in all cows.
Figure 4.
Circulating serum insulin concentrations after 6 h without feed (A) and after exposure to feed ad libitum or 25% feed restriction for 6 h (B). For (A), we observed no differences (P > 0.15), based on ANOVA. For (B), we observed no effect of LH (P = 0.73) or feed (P = 0.43), but we did detect an effect of time (P < 0.0001) and an interaction of feed × time (P < 0.0001). †P < 0.15; *P < 0.05.
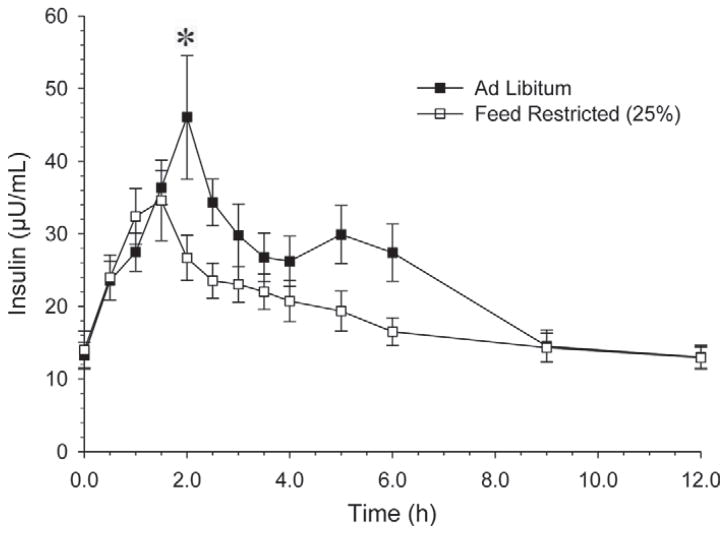
More frequent evaluations of insulin concentrations (Figure 5) were performed during experiment 2, period 2. Overall, insulin concentrations were greater (P < 0.05) in the AL than the FR group at 2.0 h after feeding. Comparisons at individual times did not differ, although insulin concentrations were consistently greater for cows in the AL treatment than in the FR treatment from 1.5 to 6.0 h after feeding. Overall, there was a time × treatment interaction (P < 0.01). In addition, insulin was similar (P = 0.37) in the 0- to 1.5-h interval, and greater in the AL treatment compared with the FR treatment in both the 1.5- to 6.0-h interval (P < 0.01) and the 0- to 6.0-h interval (P < 0.05).
Figure 5.
Circulating serum insulin concentrations following feeding (0 h) with removal of feed at 6.0 h. The data presented are the average after d 4 and 5 of feed treatment. We observed no effect of feed (P < 0.38) but we did detect an effect of time (P < 0.0001) and an interaction of feed × time (P = 0.0023). *P < 0.05; Feed × Time: P < 0.01.
Plasma glucose concentrations were similar (P > 0.05) throughout the treatments for all data points both before (AL = 70.9 mg/dL; FR = 71.3 mg/dL) and after (AL = 71.4 mg/dL; FR = 68.8 mg/dL) exposure to feed. In addition, both feed × time and feed × LH interactions were detected for plasma glucose concentrations (P = 0.04 and P = 0.04, respectively).
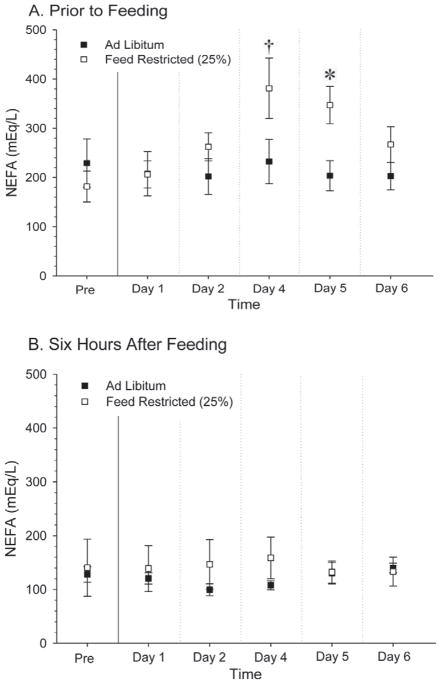
Plasma NEFA concentrations before feeding (Figure 6A) did not differ (P > 0.05) before initiation of treatment or during the first 2 d of treatment. Concentrations of NEFA were greater in the FR treatment compared with the AL treatment at d 4 (P < 0.15) and 5 (P < 0.05) after initiation of feed restriction at the time just before feeding; however, NEFA concentrations did not differ on any day when measurements were performed at 6 h after feeding (Figure 6B).
Figure 6.
Circulating plasma NEFA concentrations before feeding (A) and after exposure to feed ad libitum or 25% feed restriction for 6 h (B). We observed no effect of LH (P = 0.16) or feed (P = 0.41) but we did detect an effect of time (P < 0.0001) and an interaction of feed by time (P = 0.0018). †P < 0.15; *P < 0.05.
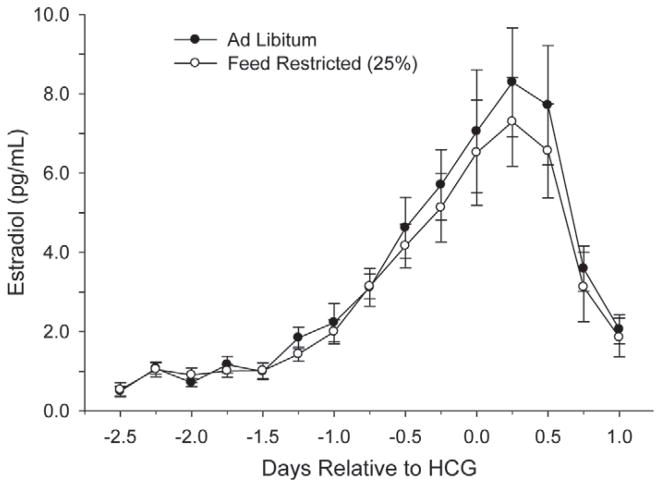
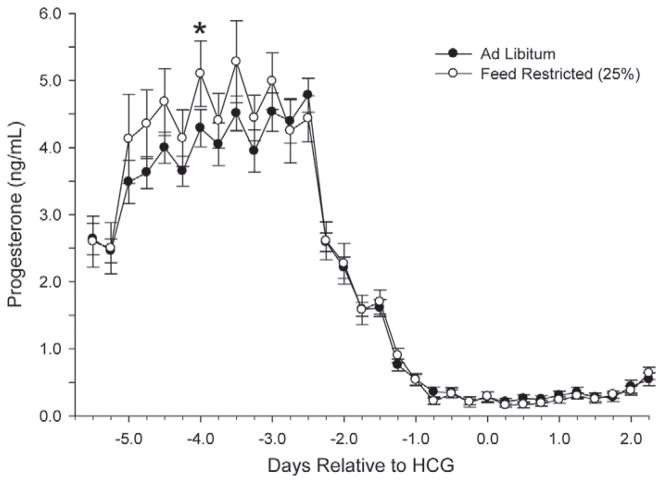
Figures 7 and 8 show the circulating concentrations of serum E2 and P4, respectively. Despite FR, no differences (P > 0.05) were observed throughout the treatment interval for either main effects or interactions for E2 or P4. Average P4 concentrations were also similar at the time of embryo flushing, and averaged 18.2 ± 3.9 ng/mL.
Figure 7.
Circulating serum estradiol concentrations every 6 h throughout the superstimulatory protocol. HCG = human chorionic gonadotropin. No effects and interactions were significant (P > 0.15) except time (P < 0.0001).
Figure 8.
Circulating serum progesterone concentrations every 6 h throughout the superstimulatory protocol. HCG = human chorionic gonadotropin. No effects and interactions were significant (P > 0.15) except time (P < 0.0001); *P < 0.05.
Superstimulatory Response, Fertilization, and Embryo Quality
Our analysis of the superstimulatory response, fertilization, and embryo data was performed in several ways. First, a complete analysis of experiment 2 (full Latin square) was completed, which included data from 63 uterine flushes. Next, analysis of all flushes from both experiments was done in a manner similar to the first analysis in an effort to get a full, cumulative analysis. Because of similarities between results from the experiment 2 analysis and the combined analysis, only the results from the experiment 2 analysis are presented. Finally, the first period of both experiments had greater fertilization rates (67.5 vs. 42.5%, respectively) and a reduced percentage of degenerate embryos of fertilized oocytes (24.8 vs. 53.1%, respectively). Therefore, a third analysis was completed that included only results from the first periods of experiments 1 and 2.
Table 3 details the superstimulatory response from experiments 1 and 2. The number of follicles >9 mm immediately before the hCG injection did not differ among treatments in the experiment 2 analysis. The first period analysis also did not differ for number of follicles >9 mm for main effects; however, we observed an interaction indicating a tendency (P = 0.19) for the AL-H treatment to have increased follicle numbers compared with the FR-H treatment. The ovulation rate at 60 h did not differ among treatments in the experiment 2 analysis; however, the analysis of first periods indicated a feed effect, with FR treatments having a greater (P = 0.06) ovulation rate (88.9 ± 4.9) compared with the AL treatments (75.4 ± 4.9) at 60 h. In addition, an interaction was detected: the AL-H, FR-H, and FR-L treatments had greater (P < 0.05) ovulation rates than the AL-L treatment. The numbers of CL did not differ at the time of flushing for any of the analyses, either as main effects or interactions.
Table 3.
Stimulatory response (LSM ± SEM) from experiments 1 and 2 analyzed by 2 methods: experiment 2 only and the first periods from experiments 1 and 21
| Item | Interactions: Feed × LH content | P-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| AL-H | AL-L | FR-H | FR-L | Feed | LH | Feed × LH | |
| Experiment 2 (no.) | 16 | 15 | 15 | 16 | |||
| Follicles >9 mm (no.) | 18.8 ± 2.1 | 16.6 ± 2.1 | 15.8 ± 2.1 | 16.6 ± 2.1 | 0.34 | 0.68 | 0.38 |
| Ovulation rate – 60 h (%) | 81.4 ± 5.3 | 77.1 ± 5.5 | 74.8 ± 5.5 | 76.9 ± 5.3 | 0.83 | 0.74 | 0.39 |
| CL number (no.) | 18.3 ± 2.8 | 15.5 ± 2.8 | 17.9 ± 2.8 | 14.9 ± 2.8 | 0.78 | 0.10 | 0.96 |
| First period (no.) | 8 | 8 | 8 | 8 | |||
| Follicles >9 mm (no.) | 22.0A ± 3.6 | 17.9AB ± 3.6 | 13.6B ± 3.6 | 19.1AB ± 3.6 | 0.33 | 0.85 | 0.19 |
| Ovulation rate – 60 h (%) | 87.3a ± 6.9 | 63.5b ± 6.9 | 85.5a ± 6.9 | 92.3a ± 6.9 | 0.06 | 0.23 | 0.04 |
| CL number (no.) | 22.1 ± 3.9 | 16.6 ± 3.9 | 17.0 ± 3.9 | 19.4 ± 3.9 | 0.76 | 0.69 | 0.32 |
Within response variable, means with different superscripts differ (P < 0.05)
Within response variable, means with different superscripts tended to differ (P < 0.15).
Superstimulated, lactating Holstein dairy cows were fed either ad libitum (AL) or feed restricted (FR) and exposed to either low LH (L) or high LH (H) to create 4 treatments: AL-H, AL-L, FR-H, and FR-L.
The uterine flush results (Table 4) using the experiment 2 analysis showed that the numbers of embryos or oocytes recovered, recovery rate, number of fertilized and unfertilized oocytes, and fertilization rate did not differ between treatments or show any interactions. The first period analysis did show differences: although main effects were similar across treatments, an interaction resulted in the FR-L and AL-H treatments having a greater (P < 0.05) number of embryos or oocytes recovered compared with the FR-H treatment. The recovery rate (%) tended (P = 0.13) to be greater in the low LH (63.9 ± 7.8) treatment compared with the high LH (46.7 ± 7.8) treatment in the first period analysis. Also in the first period analysis, an interaction indicated that a greater (P = 0.03) recovery rate was observed in the FR-L treatment compared with the FR-H treatment. Further, in the first period analysis, although no main effects were observed for fertilized oocytes, unfertilized oocytes, or fertilization rate, significant interactions were detected. We observed a tendency (P = 0.15) for an increase in fertilized oocytes recovered from the FR-L treatment compared with the FR-H treatment. In addition, a greater (P < 0.05) number of unfertilized oocytes was recovered from the AL-H treatment compared with the AL-L and FR-H treatments, a greater (P < 0.01) number was recovered from the FR-L compared with the AL-L treatment, and a tendency (P < 0.15) was detected for an increase in the FR-L treatment compared with the FR-H treatment. This resulted in a greater (P < 0.05) fertilization rate for the AL-L and FR-H treatments compared with the AL-H and FR-L treatments in the first period analysis.
Table 4.
Uterine flush results (LSM ± SEM) from experiments 1 and 2 analyzed by 2 methods: experiment 2 only and the first periods from experiments 1 and 21
| Item | Interactions: Feed × LH content | P-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| AL-H | AL-L | FR-H | FR-L | Feed | LH | Feed × LH | |
| Experiment 2 (no.) | 16 | 15 | 14 | 16 | |||
| Embryos/oocytes recovered (no.) | 11.6 ± 2.3 | 8.3 ± 2.4 | 9.6 ± 2.4 | 10.1 ± 2.3 | 0.95 | 0.36 | 0.21 |
| Recovery rate (%) | 59.0 ± 8.9 | 53.6 ± 9.6 | 61.7 ± 9.2 | 53.6 ± 8.9 | 0.88 | 0.46 | 0.88 |
| Fertilized oocytes (no.) | 6.2 ± 1.7 | 4.9 ± 1.8 | 4.6 ± 1.8 | 5.0 ± 1.7 | 0.51 | 0.67 | 0.46 |
| Unfertilized oocytes (no.) | 5.4 ± 1.4 | 3.1 ± 1.5 | 4.7 ± 1.5 | 5.1 ± 1.4 | 0.95 | 0.20 | 0.24 |
| Fertilization rate (%) | 42.5 ± 12.1 | 55.8 ± 12.6 | 56.8 ± 12.4 | 47.7 ± 12.7 | 0.76 | 0.79 | 0.19 |
| First period (no.) | 8 | 8 | 7 | 8 | |||
| Embryos/oocytes recovered (no.) | 14.5a ± 2.9 | 9.1ab ± 2.9 | 4.7b ± 3.2 | 13.4a ± 2.9 | 0.36 | 0.59 | 0.03 |
| Recovery rate (%) | 57.8ab ± 10.6 | 55.6ab ± 11.5 | 35.6b ± 11.5 | 72.1a ± 10.6 | 0.80 | 0.13 | 0.09 |
| Fertilized oocytes (no.) | 7.1AB ± 2.3 | 7.8AB ± 2.3 | 3.1B ± 2.5 | 8.1A ± 2.3 | 0.44 | 0.24 | 0.35 |
| Unfertilized oocytes (no.) | 7.4a,A ± 1.5 | 0.8b,B ± 1.5 | 1.6b,AB ± 1.6 | 5.3a,A ± 1.5 | 0.81 | 0.22 | <0.01 |
| Fertilization rate (%) | 47.9b ± 10.0 | 89.4a ± 10.8 | 80.1a ± 10.8 | 59.9b ± 10.0 | 0.90 | 0.32 | <0.01 |
Within response variable, means with different superscripts differ (P < 0.05)
Within response variable, means with different superscripts tended to differ (P < 0.15).
Superstimulated, lactating Holstein dairy cows were fed either ad libitum (AL) or feed restricted (FR) and exposed to either low LH (L) or high LH (H) to create 4 treatments: AL-H, AL-L, FR-H, and FR-L.
In the experiment 2 analysis (Table 5), the number of quality 1 embryos tended (P = 0.14) to be greater for the AL-L treatment compared with the FR-H treatment. As a proportion of fertilized oocytes, the AL-L treatment tended (P = 0.15) to increase quality 1 embryos compared with the AL-H and FR-H treatments. Also in the experiment 2 analysis, when considering the number of quality 1 and 2 embryos and the percentage of the fertilized oocytes and total embryos or oocytes that were quality 1 and 2 embryos, the results did not differ between main effects or interactions. When only the first period was analyzed, the number of quality 1 embryos tended (P < 0.15) to be greater for the AL-L and FR-L treatments compared with the FR-H treatment. In terms of the proportion of fertilized oocytes designated as quality 1 embryos, the AL-L treatment was greater (P < 0.05) than the AL-H treatment, with a tendency (P = 0.14) to be increased in the low LH treatments compared with the high LH treatments. The number of quality 1 and 2 embryos continued the trend observed for quality 1 embryos; the AL-L treatment tended (P = 0.08) to be greater than the FR-H treatment. However, as a percentage of the number of fertilized oocytes designated as quality 1 and 2, the FR-H treatment tended (P = 0.10) to be greater than the AL-H treatment. Finally, when quality 1 and 2 embryos were analyzed as a proportion of total embryos or oocytes recovered, both AL-L and FR-H were greater (P < 0.05) than AL-H and tended (P < 0.15) to be greater than the FR-L treatments.
In the experiment 2 analysis of degenerate embryos (Table 5), a tendency (P < 0.15) for greater numbers of degenerate embryos was observed in FR-L and AL-H treatments compared with the AL-L treatment. No differences were observed in the percentage of fertilized oocytes designated as degenerate. Similarly, in the first period analysis, the number of degenerate embryos in the AL-H treatment was greater (P = 0.03), whereas the FR-L treatment tended (P = 0.06) to be greater than the FR-H treatment. When analyzed as the percentage of fertilized oocytes that were graded as degenerate, the AL-H treatment tended (P = 0.10) to be greater than the FR-H treatment.
DISCUSSION
This study evaluated the interaction of 2 factors that may affect superstimulation, fertilization, and subsequent embryo quality: alteration of metabolic state by acute feed restriction and alteration of gonadotropin stimulation by altering the LH content of the FSH preparation. Both feed restriction (Freret et al., 2006; Walsh et al., 2012a,b) and increasing LH (Ginther et al., 2012) produce major changes in follicular development and reproductive success. We chose to use a Latin square experimental design to potentially increase the statistical power by utilizing a smaller number of cows but exposing them to all experimental groups. However, the unexpected period effect observed in both experiments 1 and 2 potentially negated some of the positive effects of the Latin square design. In our experiment, feed restriction had a substantial effect on circulating insulin concentrations without changing plasma glucose concentrations. Large changes were not observed in numbers of large follicles on the final day of superstimulation, in the percentage of these follicles that ovulated, or in the number of CL on the day of flushing. In addition, fertilization of oocytes was not altered by treatments, although we observed an intriguing tendency for an interaction of these treatments on fertilization, as discussed below. Probably the most consistent and biologically interesting result from this study was found in the embryo results. Although we observed no treatment effects on embryo yield, we did detect an interaction between feed restriction and amount of LH during the superstimulation protocol on the number of embryos or oocytes that were degenerate and on the percentage of total embryos or oocytes that were quality 1 and 2 embryos. It appears that combining ad libitum feeding and high LH reduced embryo quality and increased the number of degenerate embryos. Conversely, feed-restricted cows with low LH in the superstimulation preparation also had reduced percentage of quality 1 and 2 embryos (of total embryos/oocytes) and increased degenerate embryos. Thus, an interaction existed between these 2 treatments on embryo quality that is consistent with the idea that optimizing ovarian superstimulatory success requires consideration of both the hormonal and metabolic state of the superstimulated cow.
Few superstimulation studies have reported super-stimulatory response in terms of the number of follicles of ovulatory size immediately before ovulation that subsequently ovulated. Generally, researchers have reported ovulation in terms of CL number at the time of uterine flushing. Previous studies that compared differing FSH:LH ratios on CL number after superstimulation reported surprisingly variable results, with 3 studies (Chupin et al., 1984; Murphy et al., 1984; Tribulo et al., 1991) showing increased CL in cows receiving the FSH preparation with the lowest proportion of LH in the FSH preparation, whereas Kelly et al. (1997) reported the opposite effect and Herrler et al. (1991) reported that the treatment with a medium amount of LH provided the best superstimulatory response. In addition, Chupin et al. (1985) reported conflicting results; superstimulation utilizing a high LH preparation yielded the most CL in beef cattle (6.8 vs. 13.5), whereas the low LH preparation yielded the most CL in dairy cows (12.3 vs. 5.1). Another study (Willmott et al., 1990) reported no effect of LH concentration on CL number.
In our study, treatment had no effect on ovulation rate, although analysis of only the first period of both experiments indicated a tendency for a decrease in ovulation rate in cows with AL feeding, primarily due to a substantial decrease in ovulation rate of cows in the AL-L treatment (63.5%) compared with cows in the other 3 treatments, which all had an ovulation rate exceeding 85%. In this treatment, it is possible that there was inadequate LH during the superstimulation protocol to adequately develop ovulatory capacity in all of the superstimulated follicles. Luo et al. (2011) reported that LH is required for induction of LH receptors in granulosa cells, a likely a requirement for ovulation. Nevertheless, the minimal effect of alteration in feeding or LH content on number of superstimulated follicles may have been due to use of lower doses of LH that were only targeted to the final 2 d of the superstimulation protocol. All previous evaluations of LH content in the superstimulation preparation have increased LH amounts during all FSH treatments.
Our study reported an average fertilization rate of 51.8% per flush across all treatments and periods, which is similar to other studies in lactating dairy cattle (Hawk and Tanabe, 1986; Chebel et al., 2008). However, it was puzzling why the first periods of experiments 1 and 2 had substantially greater fertilization rates compared with later periods (67.5 vs. 42.5%, respectively). We were unable to find reports of this type of period effect in previous studies that have sequentially superstimulated cows. This could be due to the resynchronization protocol that we used after the first period. We speculate that the use of a CIDR after flushing the embryos may have resulted in inadequate cleaning of the uterus and some subclinical uterine inflammation or infection that could reduce fertilization in some cows. Obviously, this idea remains to be tested but it may be an important consideration for commercial farms that repetitively superstimulate cattle of high genetic merit. In our study, the treatments AL-L and FR-H had the greatest fertilization rates, which were significantly greater than those achieved by the AL-H and FR-L treatments in the first period analysis. The other analyses also found similar numerical trends that were not significant. This potential interaction of treatments could be interesting, with the group with the greatest stimulation of insulin and LH (AL-H) and the group with the least stimulation (FR-L) having reduced fertilization rates. In a previous study, we reported a lower fertilization rate in heifers superstimulated with greater amounts of FSH compared with lesser FSH (Souza et al., 2007), consistent with the idea that overstimulation with excessive insulin, LH, or FSH may decrease fertilization rate in superstimulation protocols. We were unable to find previous studies showing an adverse effect of inadequate LH or feed restriction on fertilization rates during superstimulation.
The embryo quality results we observed followed a trend similar to the fertilization results. In all analyses, the greatest number of quality 1 and quality 1 and 2 embryos was observed in cows from the AL-L group, representing a high amount of insulin stimulation but little LH stimulation. Although the FR-H group had a low total number of quality 1 and quality 1 and 2 embryos produced in both analyses, the proportion of fertilized (quality 1 and quality 1 and 2 embryos) and proportion of total embryos/oocytes (quality 1 and 2 embryos) indicated that both the AL-L and FR-H treatments achieve greater superovulatory success than the FR-L and AL-H treatments. In addition, degenerate embryos were numerically and proportionally the least for cows in both the AL-L and FR-H treatments across all analyses. Other published studies have shown similar but inconsistent results. Donaldson and Ward (1986) reported that in superstimulated beef and dairy cows, the middle LH treatment performed similarly to the low LH (5.8 vs. 5.7 high-quality embryos, respectively) but both exceeded (P < 0.006) the high LH treatment (3.3 high-quality embryos). In another study (Donaldson and Ward, 1987), 108 beef and dairy cows were superstimulated and it was observed that the middle level of LH (6.0 quality embryos) showed superior results (P = 0.014) compared with the low and high levels of LH (2.4 vs. 1.9 quality embryos, respectively). An additional study (Donaldson et al., 1986) reported that the low LH treatment yielded significantly (P = 0.001) more embryos than the high LH treatment (6.3 vs. 2.9). Herrler et al. (1991) also reported that the middle LH preparation achieved the most high-quality embryos, although significance was not achieved. Thus, it appears that varying levels of LH during follicular development could affect embryo quality. Our data support the concept that different LH treatments may be necessary in cows that are in different physiological conditions.
The effects of long-term restrictions of nutrient and energy intake in early lactation are well documented: a delay in the resumption of cyclicity and a reduction in follicle competence and oocyte quality, as reviewed by Santos et al. (2008). However, feed restriction over an acute period of time during follicle growth may have a beneficial effect on subsequent oocyte and embryo quality, as indicated by previous literature in dairy heifers (Freret et al., 2006), beef heifers (Nolan et al., 1998), and sheep (Papadopoulos et al., 2001). It is possible that this short-term effect is mediated by a reduction in circulating insulin levels in feed-restricted animals. Our companion study (Ferraretto et al., 2014) also found that short-term 25% feed restriction reduced circulating insulin concentrations, in agreement with the present study. Previous studies, however, have reported variable results on the effects of feed restriction and insulin concentrations on reproduction. In beef and dairy heifers fed at maintenance or twice maintenance (Adamiak et al., 2005), insulin concentrations differed, as expected. Heifers exposed to the 2× maintenance diet, however, had impaired oocyte quality, although this was only observed in heifers of moderately fat body condition, with a beneficial effect of the 2 × maintenance diet observed in heifers of low body condition. Conversely, in a study from the same laboratory (Adamiak et al., 2006), insulin increases, achieved via feeding highly digestible starch, reduced blastocyst yields in low, but not moderate, body condition heifers. Other studies (Yaakub et al., 1999b) have reported that embryo yield and quality is decreased in superstimulated beef cattle fed high levels of concentrates or rapidly fermentable starch, although insulin was not measured in that study. Finally, in superstimulated ewes fed at either 0.5 or 1.5 × maintenance, the high energy diet (1.5 × maintenance) was found to decrease both the superstimulatory response and subsequent embryo quality, although insulin was also not measured in that study (Lozano et al., 2003). This literature indicates that insulin may be a factor influencing superstimulatory success and embryo quality; however, in the present study, we did not observe any main effect of feed treatment on superovulatory success or embryo quality. Rather, interactions with the LH treatment indicate that an ideal level of stimulation may be necessary for optimum embryo quality, thus the feed-restricted cows (low insulin) exposed to high levels of LH and the ad libitum fed cows (high insulin) exposed to low levels of LH achieved superior embryo quality.
The present study showed no effect of feed restriction on circulating concentrations of P4 or E2, in contrast to the companion study (Ferraretto et al., 2014) that reported increased P4 during feed restriction of pregnant cows. At least 2 factors primarily affect the metabolism of P4: liver blood flow and changes in the hepatic enzymes that metabolize P4. Feed restriction in lactating dairy cows would be expected to reduce liver blood flow and thereby reduce P4 metabolism and increase circulating concentrations of P4 (Sangsritavong et al., 2002; Vasconcelos et al., 2003). Conversely, greater circulating insulin concentrations, achieved through high starch diets, can decrease expression of catabolic liver enzymes P4502C and 3A, enzymes known to metabolize P4 in the liver (Lemley et al., 2008, 2010a,b). Thus, nutritional alterations could have 2 competing actions on P4 metabolism by increasing liver blood flow and also reducing liver P4 metabolism enzymes. In the present study, we did not detect a change in circulating P4 although insulin was clearly increased.
CONCLUSIONS
Our results are consistent with the idea that an ideal range of LH exposure exists during a superstimulatory protocol that may vary according to the metabolic state of the cow. Our research supports the hypothesis that an ideal protocol is one in which some LH stimulation is necessary to acquire LH receptors and achieve ovulation, but too much LH stimulation exerts a negative effect on fertilization rate and subsequent embryo quality, possibly through an overstimulated, aged oocyte. Achieving an optimal amount of LH stimulation during a superstimulatory protocol could be achieved through feed restriction of the cow combined with supplementation with greater doses of LH, or alternatively having a cow with AL intake that receives less supplemental LH. Although the exact physiological mechanisms have yet to be elucidated, further research should delve into specific nuclear and cytoplasmic changes that occur in the cumulus, granulosa, and oocyte exposed to varying levels of LH and various metabolic environments to validate this hypothesis. Thus, we propose that optimization of ovarian superstimulatory protocols may entail consideration of the LH content of the superstimulatory preparation along with consideration of the nutritional program provided to the donor cow. It will be important in future experiments to consider the quality of the embryos produced in different metabolic and hormonal environments in relation to pregnancies produced after embryo transfer and not just by using morphological evaluation of the embryo.
Acknowledgments
The authors thank the numerous undergraduate and graduate students who contributed to this project, including Giovanni Baez Sandoval, Luiz Ferraretto, Brian Schnell, Cara Biely, Sarah Maerske, and Megan Nelson (all of the Department of Dairy Science, University of Wisconsin-Madison). In addition, thanks to Accelerated Genetics (Baraboo, WI) for donation of semen and Minitube of America (Verona, WI) for the donation of the FSH products. Thank you to Mike Maroney, Kay Nelson, and Theresa Hirsch (all part of the animal care staff of University of Wisconsin-Madison) for veterinary assistance throughout the research trial. Finally, the authors acknowledge The Wisconsin Experiment Station Hatch Fund #WIS01240 to M.C.W. and USDA National Institute of Food & Agriculture #2010-85122-20612 to M.C.W., R.D.S., and P.M.F. for the funding for this experiment.
References
- Adamiak SJ, Mackie K, Watt RG, Webb R, Sinclair KD. Impact of nutrition on oocyte quality: Cumulative effects of body composition and diet leading to hyperinsulinemia in cattle. Biol Reprod. 2005;73:918–926. doi: 10.1095/biolreprod.105.041483. [DOI] [PubMed] [Google Scholar]
- Adamiak SJ, Powell K, Rooke JA, Webb R, Sinclair KD. Body composition, dietary carbohydrates and fatty acids determine post-fertilisation development of bovine oocytes in vitro. Reproduction. 2006;131:247–258. doi: 10.1530/rep.1.00871. [DOI] [PubMed] [Google Scholar]
- Bender RW. MS Thesis. University of Wisconsin-Madison; Madison: 2012. Methods to improve superovulatory success using feed restriction and targeted use of increasing LH content of FSH preparations. [Google Scholar]
- Butler ST, Pelton SH, Butler WR. Insulin increases 17 β-estradiol production by the dominant follicle of the first postpartum follicle wave in dairy cows. Reproduction. 2004;127:537–545. doi: 10.1530/rep.1.00079. [DOI] [PubMed] [Google Scholar]
- Butler ST, Pelton SH, Butler WR. Energy balance, metabolic status, and the first postpartum ovarian follicle wave in cows administered propylene glycol. J Dairy Sci. 2006;89:2938–2951. doi: 10.3168/jds.S0022-0302(06)72566-8. [DOI] [PubMed] [Google Scholar]
- Chebel RC, Demetrio DGB, Metzger J. Factors affecting success of embryo collection and transfer in large dairy herds. Theriogenology. 2008;69:98–106. doi: 10.1016/j.theriogenology.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Chupin D, Combarnous Y, Procureur R. Antagonistic effect of LH on FSH-induced superovulation in cattle. Theriogenology. 1984;21:229. (Abstr.) [Google Scholar]
- Chupin D, Combarnous Y, Procureur R. Different effect of LH on FSH-induced superovulation in 2 breeds of cattle. Theriogenology. 1985;23:184. (Abstr.) [Google Scholar]
- Dalton JC, Nadir S, Bame JH, Noftsinger M, Saacke RG. The effect of time of artificial insemination on fertilization status and embryo quality in superovulated cows. J Anim Sci. 2000;78:2081–2085. doi: 10.2527/2000.7882081x. [DOI] [PubMed] [Google Scholar]
- Donaldson LE, Ward DN. Effects of luteinizing-hormone on embryo production in superovulated cows. Vet Rec. 1986;119:625–626. [PubMed] [Google Scholar]
- Donaldson LE, Ward DN. LH effects on superovulation and fertilization rates. Theriogenology. 1987;27:225. (Abstr.) [Google Scholar]
- Donaldson LE, Ward DN, Glenn SD. Use of porcine follicle-stimulating-hormone after chromatographic purification in superovulation of cattle. Theriogenology. 1986;25:747–757. [Google Scholar]
- Ferraretto LF, Gencoglu H, Hackbart KS, Nascimento AB, Dalla Costa F, Bender RW, Guenther JN, Shaver RD, Wiltbank MC. Effect of feed restriction on reproductive and metabolic hormones in dairy cattle. J Dairy Sci. 2014;97:754–763. doi: 10.3168/jds.2013-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freret S, Grimard B, Ponter AA, Joly C, Ponsart C, Humblot P. Reduction of body-weight gain enhances in vitro embryo production in overfed superovulated dairy heifers. Reproduction. 2006;131:783–794. doi: 10.1530/rep.1.00689. [DOI] [PubMed] [Google Scholar]
- Garnsworthy PC, Fouladi-Nashtaa AA, Mann GE, Sinclair KD, Webb R. Effect of dietary-induced changes in plasma insulin concentrations during the early postpartum period on pregnancy rate in dairy cows. Reproduction. 2009a;137:759–768. doi: 10.1530/REP-08-0488. [DOI] [PubMed] [Google Scholar]
- Garnsworthy PC, Gong JG, Armstrong DG, Mann GE, Sinclair KD, Webb R. Effect of site of starch digestion on metabolic hormones and ovarian function in dairy cows. Livest Sci. 2009b;125:161–168. [Google Scholar]
- Ginther OJ, Khan FA, Hannan MA, Rodriguez MB, Pugliesi G, Beg MA. Role of LH in luteolysis and growth of the ovulatory follicle and estradiol regulation of LH secretion in heifers. Theriogenology. 2012;77:1442–1452. doi: 10.1016/j.theriogenology.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Greve T, Callesen H, Hyttel P, Hoier R, Assey R. The effects of exogenous gonadotropins on oocyte and embryo quality in cattle. Theriogenology. 1995;43:41–50. [Google Scholar]
- Hawk HW, Tanabe TY. Effect of unilateral cornual insemination upon fertilization rate in superovulating and single-ovulating cattle. J Anim Sci. 1986;63:551–560. doi: 10.2527/jas1986.632551x. [DOI] [PubMed] [Google Scholar]
- Herrler A, Elsaesser F, Parvizi N, Niemann H. Superovulation of dairy-cows with purified FSH supplemented with defined amounts of LH. Theriogenology. 1991;35:633–643. doi: 10.1016/0093-691x(91)90459-q. [DOI] [PubMed] [Google Scholar]
- Karkalas J. An improved enzymatic method for the determination of native and modified starch. J Sci Food Agric. 1985;36:1019–1027. [Google Scholar]
- Kelly P, Duffy P, Roche JF, Boland MP. Superovulation in cattle: Effect of FSH type and method of administration on follicular growth, ovulatory response and endocrine patterns. Anim Reprod Sci. 1997;46:1–14. doi: 10.1016/s0378-4320(96)01589-8. [DOI] [PubMed] [Google Scholar]
- Lemley CO, Butler ST, Butler WR, Wilson ME. Short communication: Insulin alters hepatic progesterone catabolic enzymes cytochrome P4502C and 3A in dairy cows. J Dairy Sci. 2008;91:641–645. doi: 10.3168/jds.2007-0636. [DOI] [PubMed] [Google Scholar]
- Lemley CO, Vonnahme KA, Tager LR, Krause KM, Wilson ME. Diet-induced alterations in hepatic progesterone (P4) catabolic enzyme activity and P4 clearance rate in lactating dairy cows. J Endocrinol. 2010a;205:233–241. doi: 10.1677/JOE-10-0042. [DOI] [PubMed] [Google Scholar]
- Lemley CO, Wilmoth TA, Tager LR, Krause KM, Wilson ME. Effect of a high cornstarch diet on hepatic cytochrome P450 2C and 3A activity and progesterone half-life in dairy cows. J Dairy Sci. 2010b;93:1012–1021. doi: 10.3168/jds.2009-2539. [DOI] [PubMed] [Google Scholar]
- Lozano JM, Lonergan P, Boland MP, O’Callaghan D. Influence of nutrition on the effectiveness of superovulation programmes in ewes: Effect on oocyte quality and post-fertilization development. Reproduction. 2003;125:543–553. [PubMed] [Google Scholar]
- Luo W, Gumen A, Haughian JM, Wiltbank MC. The role of luteinizing hormone in regulating gene expression during selection of a dominant follicle in cattle. Biol Reprod. 2011;84:369–378. doi: 10.1095/biolreprod.110.085274. [DOI] [PubMed] [Google Scholar]
- Murphy BD, Mapletoft RJ, Manns J, Humphrey WD. Variability in gonadotropin preparations as a factor in the superovulatory response. Theriogenology. 1984;21:117–125. [Google Scholar]
- Nasser LF, Sa MF, Reis EL, Rezende CR, Mapletoft RJ, Bo GA, Baruselli PS. Exogenous progesterone enhances ova and embryo quality following superstimulation of the first follicular wave in Nelore (Bos indicus) donors. Theriogenology. 2011;76:320–327. doi: 10.1016/j.theriogenology.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Nolan R, O’Callaghan D, Duby RT, Lonergan P, Boland MP. The influence of short-term nutrient changes on follicle growth and embryo production following superovulation in beef heifers. Theriogenology. 1998;50:1263–1274. doi: 10.1016/s0093-691x(98)00225-8. [DOI] [PubMed] [Google Scholar]
- NRC. Nutrient Requirements of Dairy Cattle. 7. Natl. Acad. Sci; Washington, DC: 2001. [Google Scholar]
- Papadopoulos S, Lonergan P, Gath V, Quinn KM, Evans ACO, O’Callaghan D, Boland MP. Effect of diet quantity and urea supplementation on oocyte and embryo quality in sheep. Theriogenology. 2001;55:1059–1069. doi: 10.1016/s0093-691x(01)00466-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen FE, Wiltbank MC, Christensen JO, Grummer RR. Effects of fenprostalene and estradiol-17β benzoate on parturition and retained placenta in dairy cows and heifers. J Dairy Sci. 1996;79:227–234. doi: 10.3168/jds.S0022-0302(96)76355-5. [DOI] [PubMed] [Google Scholar]
- Revah I, Butler WR. Prolonged dominance of follicles and reduced viability of bovine oocytes. J Reprod Fertil. 1996;106:39–47. doi: 10.1530/jrf.0.1060039. [DOI] [PubMed] [Google Scholar]
- Rivera FA, Mendonca LGD, Lopes G, Santos JEP, Perez RV, Amstalden M, Correa-Calderon A, Chebel RC. Reduced progesterone concentration during growth of the first follicular wave affects embryo quality but has no effect on embryo survival post transfer in lactating dairy cows. Reproduction. 2011;141:333–342. doi: 10.1530/REP-10-0375. [DOI] [PubMed] [Google Scholar]
- Roberson MS, Wolfe MW, Stumpf TT, Kittok RJ, Kinder JE. Luteinizing-hormone secretion and corpusluteum function in cows receiving 2 levels of progesterone. Biol Reprod. 1989;41:997–1003. doi: 10.1095/biolreprod41.6.997. [DOI] [PubMed] [Google Scholar]
- Sangsritavong S, Combs DK, Sartori R, Armentano LE, Wiltbank MC. High feed intake increases liver blood flow and metabolism of progesterone and estradiol-17β in dairy cattle. J Dairy Sci. 2002;85:2831–2842. doi: 10.3168/jds.S0022-0302(02)74370-1. [DOI] [PubMed] [Google Scholar]
- Santos JEP, Curi RLA, Sartori R. Nutritional management of the donor cow. Theriogenology. 2008;69:88–97. doi: 10.1016/j.theriogenology.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Sartori R, Bastos MR, Wiltbank MC. Factors affecting fertilisation and early embryo quality in single- and superovulated dairy cattle. Reprod Fertil Dev. 2010;22:151–158. doi: 10.1071/RD09221. [DOI] [PubMed] [Google Scholar]
- Sartori R, Souza AH, Guenther JN, Caraviello DZ, Geiger LN, Schenk JL, Wiltbank MC. Fertilization rate and embryo quality in superovulated Holstein heifers artificially inseminated with x-sorted or unsorted sperm. Anim Reprod. 2004;1:86–90. [Google Scholar]
- Sartori R, Suarez-Fernandez CA, Monson RL, Guenther JN, Rosa GJM, Wiltbank MC. Improvement in recovery of embryos/ova using a shallow uterine horn flushing technique in superovulated Holstein heifers. Theriogenology. 2003;60:1319–1330. doi: 10.1016/s0093-691x(03)00147-x. [DOI] [PubMed] [Google Scholar]
- Souza AH, Sartori R, Guenther JN, Caraviello D, Monson R, Wiltbank MC. Effect of semen source and dose of FSH on superovulatory response and embryo production in Holstein heifers. Anim Reprod. 2007;4:70–76. [Google Scholar]
- Tribulo H, Bo GA, Jofre F, Carcedo J, Alonso A, Mapletoft RJ. The effect of LH concentration in a porcine pituitary extract and season on superovulatory response in Bos indicus heifers. Theriogenology. 1991;35:286. (Abstr.) [Google Scholar]
- Vasconcelos JLM, Sangsritavong S, Tsai SJ, Wiltbank MC. Acute reduction in serum progesterone concentrations after feed intake in dairy cows. Theriogenology. 2003;60:795–807. doi: 10.1016/s0093-691x(03)00102-x. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Matthews D, Browne JA, Forde N, Crowe MA, Mihm M, Diskin M, Evans ACO. Acute dietary restriction in heifers alters expression of genes regulating exposure and response to gonadotrophins and IGF in dominant follicles. Anim Reprod Sci. 2012a;133:43–51. doi: 10.1016/j.anireprosci.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Mehta JP, McGettigan PA, Browne JA, Forde N, Alibrahim RM, Mulligan FJ, Loftus B, Crowe MA, Matthews D, Diskin M, Mihm M, Evans ACO. Effect of the metabolic environment at key stages of follicle development in cattle: Focus on steroid biosynthesis. Physiol Genomics. 2012b;44:504–517. doi: 10.1152/physiolgenomics.00178.2011. [DOI] [PubMed] [Google Scholar]
- Wang B, Wang X, Gong L. The construction of a Williams design and randomization in cross-over clinical trials using SAS. J Stat Softw. 2009;29:1–10. [Google Scholar]
- Willmott N, Saunders J, Bo GA, Palasz A, Pierson RA, Mapletoft RJ. The effect of FSH/LH ratio in pituitary extracts on superovulatory response in the cow. Theriogenology. 1990;34:33–37. [Google Scholar]
- Yaakub H, O’Callaghan D, Boland MP. Effect of roughage type and concentrate supplementation on follicle numbers and in vitro fertilisation and development of oocytes recovered from beef heifers. Anim Reprod Sci. 1999a;55:1–12. doi: 10.1016/s0378-4320(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Yaakub H, O’Callaghan D, Boland MP. Effect of type and quantity of concentrates on superovulation and embryo yield in beef heifers. Theriogenology. 1999b;51:1259–1266. doi: 10.1016/S0093-691X(99)00070-9. [DOI] [PubMed] [Google Scholar]