Abstract
Purpose
Identification of “clinically significant” disease is crucial for optimal treatment of prostate cancer. Selective detection of prostate cancer with increased microvessel density is possible with contrast-enhanced ultrasound (CEUS). Preliminary studies suggest that pretreatment with a 5α-reductase inhibitor may improve the efficiency of CEUS targeted biopsy. This study was designed to quantify prostate cancer detection with CEUS ± short-term pretreatment with dutasteride.
Methods
In this randomized double blinded placebo controlled trial of oral dutasteride pretreatment, CEUS findings were graded and used to direct targeted biopsy (up to 6 cores/prostate). A blinded twelve core systematic biopsy was performed subsequently on every subject, based on standard medial and lateral sampling of each sextant.
Results
Of 311 subjects who underwent randomization, 272 completed participation. Positive biopsies were obtained in 276/3264 (8.5%) of systematic cores and 203/1237 (16.4%) of targeted cores (odds ratio = 2.1; 95% CI: 1.7–2.6, p<0.001). ROC analysis for detection of all prostate cancers demonstrated an increase in diagnostic accuracy from precontrast imaging to CEUS (Az of 0.60 vs. 0.64, p=0.005). For detection of high grade cancer (Gleason score ≥ 7) ROC analysis demonstrated improved accuracy for precontrast imaging (Az=0.74) and CEUS (Az=0.80), p=0.0005. For detection of high grade cancer with greater than 50% biopsy core involvement, excellent accuracy was demonstrated with precontrast and CEUS, Az =0.83 & 0.90 respectively (p=0.001). Pretreatment with dutasteride had no significant impact on detection of prostate cancer (p=0.97).
Conclusions
CEUS targeted biopsy provides a significant benefit for the detection of high grade / high volume prostate cancer. (clinicaltrials.gov Identifier: NCT00398281)
Introduction
Transrectal ultrasound (TRUS) guided sextant biopsy is a systematic, spatially distributed set of six parasagittal biopsy cores for detection of prostate cancer.[1] Although sextant biopsy was the standard of care for over a decade, the systematic sextant may miss 36% of cancers in glands under 30cm3, and 64% of cancers in larger glands.[2] An optimal systematic biopsy approach may require 12–14 cores for larger glands,[3] or even a “saturation biopsy”.[4] However, this approach to increase cancer detection with more numerous systematic cores must be weighed against the potential for increased detection of clinically insignificant disease.[5]
Targeted biopsy strategies are designed to optimize detection of clinically significant prostate cancer with fewer biopsy cores. Microbubble contrast agents enhance Doppler signal from regions with increased microvessel density and can improve the detection of prostate cancer.[6,7] Hypervascular prostate cancers may be detected with fewer biopsy cores using microbubble contrast-enhanced ultrasound (CEUS) targeted biopsy approach.[8,9] Unfortunately, CEUS is limited by enhancement of benign tissue.[10] Preliminary studies suggest suppression of Doppler flow in benign prostate tissue after therapy with the 5α-reductase inhibitor dutasteride,[11] along with improved detection of prostate cancer by CEUS.[12] The current study was designed to quantify detection of prostate cancer with CEUS techniques combined with short-term pretreatment with dutasteride.
Methods
Patient Population
IRB approval was obtained for this HIPAA compliant, randomized double blinded trial of CEUS with dutasteride pretreatment for detection of prostate cancer. Participants provided written informed consent between November 2006 and June 2011.
Consecutive patients were prospectively enrolled from our urology practice. Inclusion criteria included age>18, and a scheduled prostate biopsy based upon an abnormal digital rectal examination, an elevated prostate specific antigen (PSA≥2.6ng/ml), or a PSA velocity >0.75ng/ml/year. Exclusion criteria included a prostate biopsy within 30 days, participation in a clinical trial involving an investigational agent within 30 days, previous use of a 5-α reductase inhibitor, prior treatment for prostate cancer, or severe concurrent illness.
Patient Preparation
Each subject was randomized to pretreatment with placebo or 0.5mg of oral dutasteride (GlaxoSmithKline; Research Triangle Park, NC) once daily for 2 weeks prior to biopsy. A serum PSA was obtained prior to pretreatment; all PSA samples were processed by the same reference laboratory (Quest Diagnostics). The subject, examining physicians and study coordinators were blinded to the randomization. Patients were asked to refrain from sexual activity for 24 hours prior to biopsy, as this may alter prostate vascularity.[13] Antibiotic prophylaxis was administered with oral ciprofloxacin 500mg one hour prior to biopsy, and continued twice daily for three days.
Contrast Agent & Ultrasound Technique
The contrast agent, perflutren lipid microsphere (Definity™, Lantheus Medical Imaging; N. Billerica, MA), is a sterile, non-pyrogenic suspension of liposome-encapsulated perfluoropropane microbubbles. The standard-size vial was agitated prior to infusion, diluted into 50ml of saline and infused via a 20 gauge intravenous line at 4ml/min.
TRUS was performed with an Aplio ultrasound unit (Toshiba America Medical Systems, Tustin, CA) using a PVT-661VT endocavitary probe. Subjects were imaged in lithotomy position to minimize perturbation of vascularity measurements.[14] Prior to contrast administration, grayscale ultrasound was performed to measure gland size, and to evaluate for focal echotexture and contour abnormalities. Pre-contrast color and power Doppler imaging were performed to evaluate blood flow. During contrast infusion, continuous harmonic grayscale imaging was performed, followed by color and power Doppler imaging, intermittent grayscale harmonic imaging (with a 2 second interscan delay) and finally flash replenishment imaging coupled with maximum intensity projection imaging (MicroFlow Imaging™ [MFI]). Flash replenishment imaging was first described as a grayscale harmonic technique for perfusion estimation.[15–17] More recently, contrast-enhanced flash replenishment has been combined with maximum intensity projection over 2–4 seconds of low power image frames to demonstrate vascular patterns associated with neovascularity[18] and prostate cancer.[19] For each of the three pre-contrast imaging modes and five post-contrast imaging modes the prostate was imaged in a transverse sweep from base to apex. Findings were rated with a previously described five point subjective rating score (from 1–5; benign to malignant) for each sextant of the prostate.[20,21] These ratings were used to select sites for targeted biopsy and to compare the discrimination of prostate cancer among the various imaging modes (see statistical analysis).
Biopsy Protocol
After the diagnostic examination was complete, local anesthetic was administered by transrectal injection of 2% lidocaine adjacent to the base of the prostate. In order to minimize the chance that CEUS findings might prejudice the selection of locations for systematic core biopsies, two independent, experienced physicians performed the targeted and systematic biopsy procedures. The first physician (EJH) performed the diagnostic CEUS study, administered anesthetic and obtained targeted biopsy cores during contrast infusion which lasted approximately 12 minutes. Although the protocol allowed a maximum of 6 targeted cores, fewer (or no) targeted cores were taken from patients with few (or no) CEUS abnormalities. The second physician (EJT), blinded to the results of the CEUS study, performed a standard 12 core systematic biopsy consisting of a laterally and a medially directed core within each sextant. Biopsy specimens were processed using standard laboratory techniques and read in a blinded fashion by one urologic pathologist (PAM).
Statistical Analysis
Statistical computations were performed with Stata 11.1 (StataCorp; College Station, Tx). Continuous demographic and laboratory variables were compared with a t-test. Subjective ultrasound rating scores were compared with a Wilcoxon rank-sum test, as these scores are not continuous variables and not normally distributed. Comparison of positive biopsy rates in various subgroups was performed with a Pearson Chi-square statistic. Since targeted and systematic biopsy procedures were paired for each subject, the proportions of cancers diagnosed by these techniques were compared with a McNemar’s Chi-square for correlated proportions.
In order to model the detection of prostate cancer with a by-core analysis and to account for the clustering of scores within subjects, a logistic regression model was applied with each subject defined as a cluster. A clustered logistic model was also used to estimate and compare the AZ - area under the receiver operating characteristic (ROC) curve for the detection of prostate cancer by each individual ultrasound imaging mode, as well as for combinations of grayscale and CEUS.[22] In order to determine whether CEUS might have better discriminating ability for higher grade and larger cancers, additional logistic models were created for the detection of tumors with a Gleason score ≥7 and for detection of tumors with a Gleason score ≥7 and >50% core involvement. The AZ for each logistic model was computed based upon the trapezoidal rule; statistical comparisons were performed with a non-parametric technique.[23]
Results
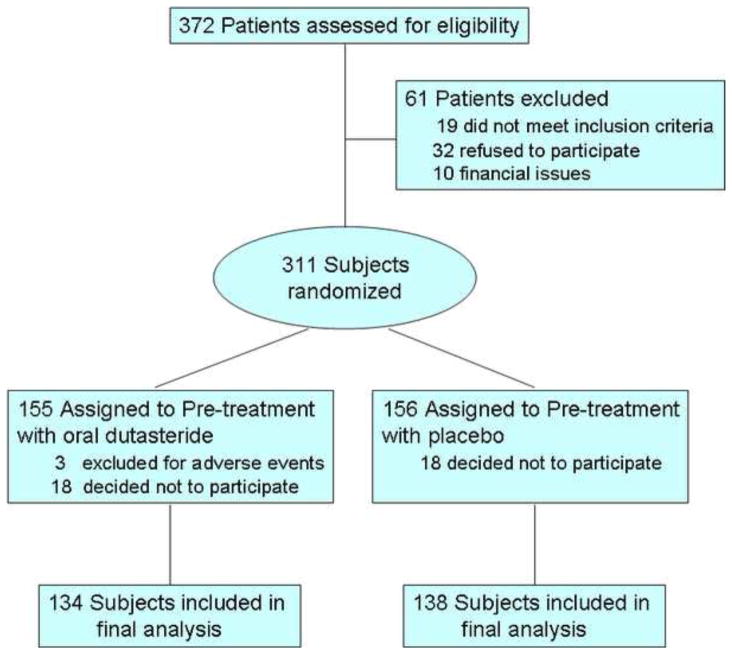
Among 311 eligible subjects, 272 completed participation (Figure 1). The study population included 210 Caucasians, 54 African Americans, 6 Hispanic men and 2 Asians. Mean age was 62 years (range: 36–83), and mean serum PSA was 6.5±7ng/dl. Three patients suffered adverse events during pretreatment and were excluded. Three patients suffered adverse events requiring inpatient admission after the biopsy, but completed the protocol.
Figure 1.
CONSORT flow diagram of study enrollment. Of 372 patients who were assessed for the study, 311 signed informed consent and were randomized to dutasteride versus placebo. Ultimately, 272 patients completed the protocol and received a CEUS with prostate biopsy. No patient was lost to follow-up.
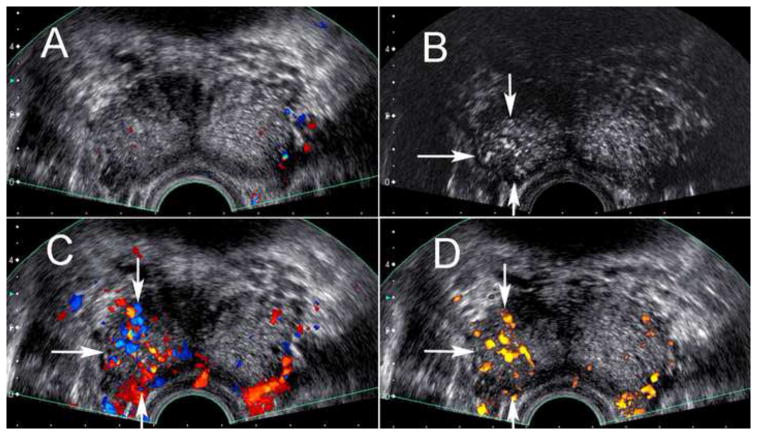
The appearance of prostate cancer on CEUS is demonstrated in Figure 2. A positive biopsy for prostate cancer was obtained in 118/272 study subjects (43%). Mean age among subjects with and without prostate cancer was not significantly different (61.1 vs. 59.6 years; p=0.30). Mean PSA among patients with and without prostate cancer was not significantly different (7.1 vs. 6.4ng/dl;p=0.48).
Figure 2.
Transrectal ultrasound near the base of the prostate in a 62 year old male. Targeted and systematic biopsy cores at the right base demonstrated greater than 50% core involvement with Gleason 8 and 9 prostate cancer. The pre-contrast grayscale and color Doppler image (A) is unremarkable, but the post-contrast harmonic grayscale image (B) as well as the post-contrast color (C) and power (D) Doppler images demonstrate hypervascularity associated with the area of positive targeted cores (arrows).
Dutasteride vs Placebo
Among the 272 study subjects, 134 were randomized to pretreatment with dutasteride and 138 to placebo. There was no significant difference in age (59.5 vs. 61 years, p=0.3) or serum PSA (7.0 vs. 6.4ng/dl, p=0.6) among subjects randomized to dutasteride or placebo. The subjective rating scores for all ultrasound modes were slightly higher among subjects randomized to placebo (Table 1).
Table 1.
Subjective rating scores of patients, stratified by dutasteride vs. placebo
| Imaging Mode | Dutasteride | Placebo | p-value (rank-sum test) |
|---|---|---|---|
| Baseline Grayscale | 1.32 | 1.38 | 0.06 |
| Baseline Color Doppler | 1.27 | 1.39 | 0.0001 |
| Baseline Power Doppler | 1.48 | 1.59 | 0.0095 |
| CE Harmonic Imaging | 1.53 | 1.63 | 0.0048 |
| CE Color Doppler | 1.53 | 1.68 | 0.0016 |
| CE Power Doppler | 1.64 | 1.83 | 0.0000 |
| CE Intermittent Imaging | 1.58 | 1.68 | 0.0071 |
| CE MicroFlow Imaging | 1.76 | 1.91 | 0.0100 |
CE=Contrast Enhanced
There was no significant difference in the proportion of subjects diagnosed with prostate cancer among study participants randomized to placebo (60/138) versus dutasteride (58/134), p=0.97. There was no significant difference in the proportion of positive targeted cores among subjects randomized to placebo (114/659=17.3%) versus dutasteride (88/574=15.3%), p=0.35. There was also no difference in the proportion of positive sextant cores among patients randomized to placebo (150/1656=9.1%) versus dutasteride (126/1608=7.8%), p=0.21. Given these results, the two groups were combined for all subsequent analyses.
Cancer Detection with targeted and systematic biopsy
Prostate cancer was found in 479/4501 cores. Positive biopsies were obtained in 276/3264 (8.5%) of systematic cores and 203/1237 (16.4%) of targeted cores. Based on logistic regression, the odds ratio for a positive core with a targeted versus a systematic biopsy was 2.1 (95% CI:1.7–2.6, p<0.001).
Prostate cancer was detected by targeted biopsy alone in 13 subjects and by systematic biopsy alone in 47 subjects. On a per-patient basis, cancer was more frequently detected by systematic biopsy (n=105) relative to targeted biopsy (n=71; McNemar Chi-square=19.3, p<0.001).
The volume and grade of cancers detected by CEUS targeted biopsy were different from those detected by systematic biopsy alone. Among the 105 subjects detected by systematic biopsy, those with a positive targeted biopsy (n=58) demonstrated a mean of 3.5 positive systematic cores, while subjects undetected by targeted biopsy (n=47) demonstrated a mean of 1.5 positive systematic cores (p<0.001). The mean percentage of systematic core involvement was 32% among subjects with a positive targeted core, compared with 15% among patients who were undetected by a targeted biopsy (p<0.001). Higher grade cancers (Gleason score ≥7) were more common among patients with a positive targeted biopsy (55% versus 17%; p<0.001).
ROC Analysis by Sextant (Table 2)
Table 2.
ROC analysis for detection of prostate cancer.
| Imaging Mode | All sextants with cancer tabulated as true positive | High grade (Gleason score ≥7) sextant tabulated as true positive | High grade (Gleason score ≥7) High volume (>50% core involvement) sextant tabulated as true positive | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AZ | Std Error | AZ 95% CI | AZ | Std Error | AZ 95% CI | AZ | Std Error | AZ 95% CI | |
| Baseline Grayscale | 0.62 | 0.02 | 0.58–0.65 | 0.74 | 0.03 | 0.68–0.81 | 0.83 | 0.04 | 0.77–0.90 |
| Baseline Color Doppler | 0.57 | 0.02 | 0.53–0.60 | 0.68 | 0.03 | 0.61–0.74 | 0.75 | 0.04 | 0.67–0.83 |
| Baseline Power Doppler | 0.57 | 0.02 | 0.53–0.61 | 0.68 | 0.03 | 0.61–0.75 | 0.76 | 0.04 | 0.68–0.83 |
| CE Harmonic Imaging | 0.62 | 0.02 | 0.58–0.64 | 0.76 | 0.03 | 0.70–0.82 | 0.84 | 0.03 | 0.77–0.90 |
| CE Color Doppler | 0.60 | 0.02 | 0.56–0.64 | 0.73 | 0.03 | 0.67–0.79 | 0.79 | 0.04 | 0.71–0.86 |
| CE Power Doppler | 0.60 | 0.02 | 0.55–0.64 | 0.77 | 0.03 | 0.71–0.83 | 0.83 | 0.03 | 0.77–0.90 |
| CE Intermittent Imaging | 0.61 | 0.02 | 0.57–0.65 | 0.76 | 0.03 | 0.70–0.82 | 0.84 | 0.03 | 0.78–0.89 |
| CE MicroFlow Imaging | 0.61 | 0.02 | 0.56–0.65 | 0.76 | 0.03 | 0.70–0.82 | 0.86 | 0.03 | 0.80–0.91 |
| Grayscale + | |||||||||
| Baseline Color Doppler | 0.62 | 0.02 | 0.58–0.66 | 0.77 | 0.02 | 0.71–0.84 | 0.87 | 0.03 | 0.82–0.93 |
| Baseline Power Doppler | 0.62 | 0.02 | 0.57–0.66 | 0.77 | 0.02 | 0.71–0.84 | 0.88 | 0.03 | 0.82–0.93 |
| CE Harmonic Imaging | 0.64 | 0.02 | 0.60–0.68 | 0.79 | 0.02 | 0.73–0.86 | 0.88 | 0.03 | 0.82–0.94 |
| CE Color Doppler | 0.64 | 0.02 | 0.60–0.68 | 0.78 | 0.02 | 0.73–0.84 | 0.87 | 0.03 | 0.81–0.93 |
| CE Power Doppler | 0.62 | 0.02 | 0.58–0.67 | 0.80 | 0.02 | 0.75–0.86 | 0.89 | 0.02 | 0.85–0.94 |
| CE Intermittent Imaging | 0.63 | 0.02 | 0.59–0.68 | 0.80 | 0.02 | 0.74–0.86 | 0.89 | 0.03 | 0.83–0.94 |
| CE MicroFlow Imaging | 0.63 | 0.02 | 0.59–0.68 | 0.80 | 0.02 | 0.74–0.86 | 0.90 | 0.03 | 0.86–0.95 |
| All modes in one model (CE=Contrast Enhanced) | 0.64 | 0.02 | 0.60–0.69 | 0.81 | 0.02 | 0.76–0.87 | 0.91 | 0.02 | 0.87–0.95 |
Cancer was demonstrated in 204/1632 sextants by systematic biopsy. The multivariate test for equality demonstrated a significant difference in Az among the eight individual ultrasound modes (p=0.03). However, when pre-contrast color and power Doppler imaging were dropped from the analysis, there was no significant difference in Az among the remaining individual modes (p=0.40), suggesting that the CEUS modes were superior to pre-contrast Doppler imaging. When pre-contrast grayscale imaging was combined with contrast enhanced harmonic imaging into a single predictive model, the Az of 0.64 was increased relative to conventional grayscale imaging alone or harmonic imaging alone (p=0.005).
High grade cancer (Gleason score ≥7) was demonstrated in 70/1632 sextants. When the analysis was repeated with only high grade sextants rated as positive, the test for equality of Az again demonstrated a significant difference among imaging modes (p=0.04). When pre-contrast color and power Doppler imaging were dropped from the analysis, there was no significant difference among the remaining modes (p=0.51), again suggesting that the CEUS modes were superior to pre-contrast Doppler. When pre-contrast grayscale imaging was combined with contrast enhanced continuous harmonic or MFI into a single predictive model, Az increased to 0.79–0.80 and was higher than for either conventional grayscale or harmonic imaging alone (p<0.05). No further increase in Az was obtained from a model that combined all the ultrasound modes (Az=0.81,p=0.6).
High grade, high volume cancer, defined as Gleason 7 or higher with greater than 50% core involvement on systematic biopsy, was demonstrated in 46/1632 sextants. When ROC analysis was repeated with only these cores rated as positive, the test for equality did not demonstrate a significant difference among the individual ultrasound modes (p=0.07). However, when pre-contrast grayscale imaging was combined with any one CEUS technique, Az increased into the range 0.87–0.90 and was significantly higher than those obtained with conventional grayscale imaging alone (p<0.02). A non-significant increase in Az was obtained from a model that combined all the ultrasound modes (Az=0.91,p=0.21). The combination of grayscale imaging with MicroFlow imaging yielded the highest Az (0.91), but was not significantly better than the combination of grayscale and continuous harmonic imaging (p=0.10).
Discussion
Prior studies suggest that CEUS improves prostate cancer detection.[24,25] Our study confirms increased frequency of positive cores among CEUS targeted biopsy relative to systematic biopsy (16.4 versus 8.5%), similar to previous reports.[26] Nonetheless, the combination of grayscale with CEUS imaging provides an ROC area of only 0.64, suggesting that this technique misclassifies many patients. The paired analysis of CEUS targeted versus systematic biopsy confirms that CEUS misses many cancers.
More important than overall detection of prostate cancer is the detection of clinically aggressive prostate cancer. Biochemical screening results in the detection of many insignificant cancers such that “the risk of being diagnosed with prostate cancer is increasingly greater than the risk of dying of it”.[27] The ERSPC, (European Randomized Study of Prostate Cancer screening) found that 1410 men must be screened and 48 cancers treated to prevent prostate cancer death.[28] The authors conclude that “Overdiagnosis and overtreatment are probably the most important adverse effects of prostate-cancer screening.” Our results suggest that while CEUS is not an efficient test for overall detection of prostate cancer, it is a good test for detection of high grade cancer (with an ROC AZ of 0.80), and an excellent test for discrimination of high grade/high volume cancers (AZ=0.90).
The excellent discriminating power of CEUS for high grade/high volume prostate cancer implies that a high proportion of the cancers detected by CEUS targeted biopsy will be aggressive cancers. It stands to reason that the cost-benefit ratio for prostate cancer screening will improve if PSA screening is followed by a limited targeted biopsy based upon CEUS. A second potential clinical application for CEUS is for the decision between definitive therapy and expectant management of patients on active surveillance. Men on active surveillance for prostate cancer undergo periodic repeat biopsy and are referred for definitive therapy when there is a biopsy finding of high grade (Gleason score ≥7) or high volume (core involvement ≥50%) disease. Our results suggest that CEUS can detect the presence of high grade/high volume disease with fewer targeted biopsy cores.
Limitations
The uneven allocation of targeted biopsy cores among subjects is a potential source of bias, since cancer is more likely to be detected among patients subjected to an increased number of biopsy cores. In order to minimize the impact of this biased sampling scheme, ROC analysis was based upon the systematic biopsy protocol, which included an equal number of cores (n=2) from each sextant of every subject. The lack of surgical pathology represents another limitation since prostate cancer may not be detected on needle biopsy. A future study might evaluate CEUS targeted biopsy among patients scheduled for radical prostatectomy. In the present study, however, we evaluated CEUS in a screening population where a minority of men undergo prostatectomy. Although overall sensitivity for detection of prostate cancer cannot be reported since the true number of patients with prostate cancer is not known, the systematic biopsy provides the best possible reference standard in a screening population, allows an unbiased comparison of the relative accuracy of pre-contrast and CEUS findings, and is the clinical standard for prostate cancer treatment decisions.
Conclusion
Recent recommendations by the US Preventive Services Task Force suggest that PSA screening of asymptomatic men may not be beneficial.[29] Prostate cancer screening remains controversial in large part because the risks of prostate biopsy and subsequent therapy may outweigh the benefits of treatment among the many men diagnosed with localized, low grade cancer.[30] The present study demonstrates that a targeted biopsy based upon CEUS selectively detects high grade/high volume cancers, and may be a more appropriate approach to screening for clinically important prostate cancer. Although CEUS does add cost to the biopsy procedure, this additional cost is minimal in comparison to the cost of treating clinically insignificant prostate cancer.
Acknowledgments
Funding/Support: This study was funded by a grant from the National Cancer Institute of the National Institutes of Health - RO1-CA118003. The contrast material for this trial (Definity) – was supplied by Lantheus Medical Imaging, North Billerica, MA. The dutasteride and placebo were supplied by GlaxoSmithKline, Research Triangle Park, NC. The Aplio ultrasound system for this study was supplied by Toshiba America Medical Systems, Tustin, CA.
The authors wish to thank Donna George, RN who cared for all patients during the biopsy procedure, and to thank our study coordinators Colleen Dascenzo, Nancy Pedano, Andrea Frangos, Christine Hubert, Kelly Coggins and Karen Milsip who managed patient recruitment, scheduling and data collection. Thanks are also given to the Urology residents at Thomas Jefferson University who assisted in data collection and biopsy procedures throughout the study, including Drs. Eric Nelson, Paul Gittens, Robert Linden, Mark Pe, Jitesh Patel, James Johannes, Steve Dong, Joseph (JR) Zola, Adeep Thumar, Daniel Sackett, Chandan Kundavaram, Xiaolong (Shawn) Liu and Francisco Gelpi-Hammerschmidt. Lastly, we thank Professor Mario Cleves for his assistance with using the logistic model to perform ROC analysis in STATA.
Glossary
- ROC curve
Receiver Operating Characteristic Curve
- Az
area under the ROC curve
- CEUS
contrast enhanced ultrasound
- TRUS
transrectal ultrasound
- PSA
prostate specific antigen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. Journal of Urology. 1989;142:71. doi: 10.1016/s0022-5347(17)38664-0. [DOI] [PubMed] [Google Scholar]
- 2.Naughton CK, Smith DS, Humphrey PA, Catalona WJ, Keetch DW. Clinical and pathologic tumor characteristics of prostate cancer as a function of the number of biopsy cores: a retrospective study. Urology. 1998;52:808–813. doi: 10.1016/s0090-4295(98)00344-6. [DOI] [PubMed] [Google Scholar]
- 3.Mariappan P, Chong WL, Sundram M, Mohamed SR. Increasing prostate biopsy cores based on volume vs the sextant biopsy: a prospective randomized controlled clinical study on cancer detection rates and morbidity. BJU International. 2004;94(3):307–10. doi: 10.1111/j.1464-410X.2004.04928.x. [DOI] [PubMed] [Google Scholar]
- 4.Stewart CS, Leibovich BC, Weaver AL, Lieber MM. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. Journal of Urology. 2001;166(1):86–92. [PubMed] [Google Scholar]
- 5.Zaytoun OM, Moussa AS, Gao T, fareed K, Jones JS. Office Based Transrectal Saturation Biopsy Improves Prostate Cancer Detection Compared to Extended Biopsy in the Repeat Biopsy Population. J Urol. 2011 Sep;186(3):850–4. doi: 10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 6.Bogers HA, Sedelaar JP, Beerlage HP, de la Rosette JJ, Debruyne FM, Wijkstra H, Aarnink RG. Contrast-enhanced three-dimensional power Doppler angiography of the human prostate: correlation with biopsy outcome. Urology. 1999;54(1):97–104. doi: 10.1016/s0090-4295(99)00040-0. [DOI] [PubMed] [Google Scholar]
- 7.Roy C, Buy X, Lang H, Saussine Jacqmin D. Contrast enhanced color Doppler endorectal sonography of the prostate: efficiency for detecting peripheral zone tumors and role for biopsy procedure. The Journal of Urology. 2003;170:69–72. doi: 10.1097/01.ju.0000072342.01573.8d. [DOI] [PubMed] [Google Scholar]
- 8.Frauscher F, Klauser A, Halpern EJ, Horninger W, Bartsch G. Detection of Prostate Cancer with a Microbubble Ultrasound Contrast Agent. The Lancet. 2001;357:1849–1850. doi: 10.1016/s0140-6736(00)04970-9. [DOI] [PubMed] [Google Scholar]
- 9.Frauscher F, Klauser A, Volgger H, Halpern EJ, Pallwein L, Steiner H, Schuster A, Horninger W, Rogatsch H, Bartsch G. Comparison of Contrast-enhanced Color Doppler Targeted Biopsy to Conventional Systematic Biopsy: Impact on Prostate Cancer Detection. The Journal of Urology. 2002;167:1648–1652. [PubMed] [Google Scholar]
- 10.Halpern EJ, McCue PA, Aksnes AK, Hagen EK, Frauscher F, Gomella LG. Contrast Enhanced Sonography of the Prostate with Sonazoid: Comparison with Prostatectomy Specimens in Twelve Patients. Radiology. 2002;222:361–366. doi: 10.1148/radiol.2222010582. [DOI] [PubMed] [Google Scholar]
- 11.Ives EP, Gomella LG, Halpern EJ. Effect of dutasteride therapy on Doppler Evaluation of the Prostate: Preliminary Results. Radiology. 2005;237:197–201. doi: 10.1148/radiol.2371041543. [DOI] [PubMed] [Google Scholar]
- 12.Mitterberger M, Pinggera G, Horninger W, Strasser H, Halpern E, Pallwein L, Gradl J, Bartsch G, Frauscher F. Dutasteride prior to contrast-enhanced colour Doppler ultrasound prostate biopsy increases prostate cancer detection. Eur Urol. 2008 Jan;53(1):112–7. doi: 10.1016/j.eururo.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Keener TS, Winter TC, Berger R, Krieger JN, Nodell C, Rothman I, Nghiem HV. Prostate vascular flow: The effect of ejaculation as revealed on transrectal power Doppler sonography. American Journal of Roentgenology. 2000;175:1169–1172. doi: 10.2214/ajr.175.4.1751169. [DOI] [PubMed] [Google Scholar]
- 14.Halpern EJ, Frauscher F, Forsberg F, Strup SE, Nazarian LN, O'Kane P, Gomella LG. High-frequency Doppler US of the prostate: effect of patient position. Radiology. 2002;222:634–9. doi: 10.1148/radiol.2223010946. [DOI] [PubMed] [Google Scholar]
- 15.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998 Feb 10;97(5):473–83. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 16.Kamiyama N, Moriyasu F, Mine Y, Goto Y. Analysis of flash echo from contrast agent for designing optimal ultrasound diagnostic systems. Ultrasound Med Biol. 1999 Mar;25(3):411–20. doi: 10.1016/s0301-5629(98)00182-3. [DOI] [PubMed] [Google Scholar]
- 17.Shi WT, Forsberg F, Liu JB, Rawool NM, Goldberg BB. Blood flow estimation with harmonic Flash Echo Imaging. Ultrason Imaging. 2001 Jul;23(3):161–70. doi: 10.1177/016173460102300303. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto K, Moriyasu F, Kamiyama N, Metoki R, Yamada M, Imai Y, Iijima H. Analysis of morphological vascular changes of hepatocellular carcinoma by microflow imaging using contrast-enhanced sonography. Hepatol Res. 2008;38(8):790–9. doi: 10.1111/j.1872-034X.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 19.Linden RA, Trabulsi EJ, Forsberg F, Gittens PR, Gomella LG, Halpern EJ. Contrast enhanced ultrasound flash replenishment method for directed prostate biopsies. J Urol. 2007;178(6):2354–8. doi: 10.1016/j.juro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Halpern EJ, Ramey JR, Strup SE, Frauscher F, McCue P, Gomella LG. Detection of Prostate Cance with Contrast Enhanced Sonography Using Intermittent Harmonic Imaging. Cancer. 2005;104(11):2372–2383. doi: 10.1002/cncr.21440. [DOI] [PubMed] [Google Scholar]
- 21.Mitterberger M, Aigner F, Pinggera GM, Steiner E, Rehder P, Ulmer H, Halpern EJ, Horninger W, Frauscher F. Contrast-enhanced colour Doppler-targeted prostate biopsy: correlation of a subjective blood-flow rating scale with the histopathological outcome of the biopsy. BJU Int. 2010;106(9):1315–8. doi: 10.1111/j.1464-410X.2010.09335.x. [DOI] [PubMed] [Google Scholar]
- 22.Cleves MA. Comparing areas under receiver operating characteristic curves from two or more probit or logit models. The Stata Journal. 2002;2(3):301–313. [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 24.Mitterberger M, Pinggera GM, Horninger W, Bartsch G, Strasser H, Schäfer G, Brunner A, Halpern EJ, Gradl J, Pallwein L, Frauscher F. A prospective randomized trial comparing contrast-enhanced targeted versus systematic ultrasound guided biopsies: impact on prostate cancer detection. Prostate. 2007 Oct 1;67(14):1537–42. doi: 10.1002/pros.20639. [DOI] [PubMed] [Google Scholar]
- 25.Mitterberger M, Horninger W, Aigner F, Pinggera GM, Rehder P, Steiner E, Wiunig C, Reissigl A, Frauscher Contrast-enhanced colour Doppler-targeted vs a 10-core systematic repeat biopsy strategy in patients with previous high-grade prostatic intraepithelial neoplasia. BJU Int. 2010 Jun;105(12):1660–2. doi: 10.1111/j.1464-410X.2009.08963.x. [DOI] [PubMed] [Google Scholar]
- 26.Mitterberger MJ, Aigner F, Horninger W, Ulmer H, Cavuto S, Halpern EJ, Frauscher F. Comparative efficiency of contrast-enhanced colour Doppler ultrasound targeted versus systematic biopsy for prostate cancer detection. Eur Radiol. 2010 Dec;20(12):2791–6. doi: 10.1007/s00330-010-1860-1. [DOI] [PubMed] [Google Scholar]
- 27.Penson DF, Rossignol M, Sartor AO, Scardino PT, Abenhaim LL. Prostate cancer: epidemiology and health-related quality of life. Urology. 2008;72(6 Suppl):S3–11. doi: 10.1016/j.urology.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg P. USPSTF to Downgrade PSA Screening from “I” to “D” – As In “Don’t Do It”. Cancer Letter. 2011;37(37):1. [Google Scholar]
- 30.Gomella LG, Liu XS, Trabulsi EJ, Kelly WK, Myers R, Showalter T, Dicker A, Wender R. Screening for prostate cancer: the current evidence of guidelines controversy. Can J Urol. 2011;18(5):5875–5883. [PubMed] [Google Scholar]