Abstract
Purpose
Efforts to define the neurovascular bundle (NVB) for prostate radiation have varied. In this series, we sought to determine the reproducibility and reliability of contouring the classical posterolateral NVB on dedicated pelvic magnetic resonance imaging (MRI) scans.
Methods and materials
A total of 120 NVB structures were defined on 10 3-Tesla pelvic MRI scans in patients with prostate cancer but without extraprostatic extension. One pelvic radiologist served as the expert in contouring the right and left NVB for each case. Five radiation oncologists, with varying levels of experience, contoured the right and left NVBs on these same cases. The intraclass correlation coefficient across each rater and the expert, Pearson correlation coefficient between each rater and the expert, and the Dice similarity coefficient (DSC) between each rater and the expert were calculated to evaluate contour agreement and overlap.
Results
The overall intraclass correlation coefficient was 0.89 (95% confidence interval [CI], 0.81-0.95). The Pearson correlation coefficient was 0.95 (95% CI, 0.86-0.98) for rater 1, 0.98 (95% CI, 0.95-0.99) for rater 2, 0.94 (95% CI, 0.86-0.98) for rater 3, 0.98 (95% CI, 0.95-0.99) for rater 4, and 0.84 (95% CI, 0.63-0.93) for rater 5. The mean DSC was 0.72 (standard deviation [SD], 0.07) for rater 1, 0.72 (SD, 0.06) for rater 2, 0.73 (SD, 0.09) for rater 3, 0.74 (SD, 0.09) for rater 4, and 0.68 (SD, 0.13) for rater 5. Overall, across all raters, the average DSC was 0.72 (SD, 0.09).
Conclusions
The classic posterolateral NVB can be accurately and reliably contoured on 3-Tesla pelvic MRI scans by radiation oncologists.
Introduction
Long-term erectile dysfunction (ED) is an important quality of life factor in men receiving treatment for prostate cancer. The cause of ED is related not only to the type of therapy a patient receives but also his medical comorbidities, smoking status, age, weight, and pretreatment erectile function.1–7 Meta-analyses, as well as recently published data comparing active surveillance, surgery, and radiation as therapeutic options for prostate cancer, indicate improved ED with external beam radiation therapy compared with surgery.6–10
Radical prostatectomy causes ED via alteration of the nerves that innervate the corpora cavernosa, which includes the neurovascular bundle (NVB).4,11 External beam radiation therapy induced ED has an unclear cause, with the literature linking radiation dose to the bulb of the penis, the crura, and the NVB to ED,3,12–16 with other series showing no dose effect to these structures causing ED.17–19 The majority of these studies were done in an era before intensity modulated radiation therapy and, in series examining dose to the NVB, were limited to anatomical definitions based on a single cadaver20 or examined dose to the NVB with brachytherapy alone.17,21 As technology evolves and more conformal radiation techniques, including volumetric modulated arc therapy and dose-painting to single lobes of the prostate, are implemented,22 the ability to limit dose to the NVB may be feasible for classically defined NVB in the posterolateral position relative to the prostate.
Given the ability to spare dose to the NVB, correctly identifying and contouring the NVB is important. Previous literature described computed tomography (CT) scan delineation using reference magnetic resonance imaging (MRI) scans.23 Our institution recently reported the dosimetric feasibility of sparing the NVB when in its classical anatomical position using individual CT-MRI registration to define the NVB,24 but no information is currently available on the accuracy and reproducibility of contouring the NVB using this technique. The purpose of this study was to determine the reproducibility and reliability of contouring classically positioned NVB on MRI.
Methods and materials
Selection of patients and MRI scans
Under an institutional review board–approved protocol, the records and images of 16 consecutive patients with prostate cancer undergoing a dedicated pelvic MRI scan with 32-channel surface phased array endorectal coil on a 3-Tesla (T) MRI device (Siemens Medical Solutions, Germany) were reviewed. Patients were excluded if their official pelvic MRI report noted any extraprostatic extension or if extraprostatic extension was noted on rereview of their MRI scan by an expert pelvic radiologist with 15 years’ experience. Periprostatic infiltration, obliterated rectoprostatic angle, irregular contour bulging, and asymmetric NVB were part of the criteria used to determine extraprostatic extension. In total, 10 of these MRI scans met the inclusion criteria; 3 MRI were excluded for gross extraprostatic extension into the NVB, 2 were excluded for obliteration of the rectoprostatic angle/blurring of the NVB and prostate interface, and 1 was excluded because the NVB was not in the classic posterolateral position and instead was found to transverse across the entire posterior lobe of the prostate.
Contouring of the NVB
First, an expert pelvic radiologist reviewed each of the 10 3-T prostate pelvic MRI scans and contoured the NVB for each case. Contouring of the NVB was done in 3 dimensions on the T2 turbo spin echo 320 sequences of the 3-T pelvic MRI. This sequence is part of a standard prostate MRI protocol.25,26 On our machine, this sequence specifically had a repetition time of 3000 ms; an echo time of 101 ms; 4 signal averages performed for the sequence; and a flip angle of 150°. This sequence was chosen because the NVBs appear as dark structures against a lighter background of the periprostatic fascia. Our expert radiologist determined that this sequence most reliably allowed for visualization of the NVB, which is consistent with the radiology literature.25,26 This sequence provides high signal-to-noise ratio, high spatial resolution, and significant T2 contrast that allows detailed visualization of the NVB.25 The right and left NVBs were defined separately, giving a total of 20 expert-defined structures across the 10 cases. All contouring was done in Velocity Software (Varian Medical Systems, CA).
Next, 5 radiation oncologists (raters 1-5) with experience ranging from 2 to 20 years were asked to delineate the same right and left NVB structures on the 3-T pelvic MRI scans on the same sequences after being given a brief presentation of a pelvic MRI highlighting what to look for (this MRI was not 1 of the 10 cases). The raters were blinded to the expert contours as well as other rater contours. No feedback was given to each rater after completion of a case. This yielded 100 additional structures from the raters. Figure 1 demonstrates a typical case with left and right NVB contours overlaid.
Figure 1.
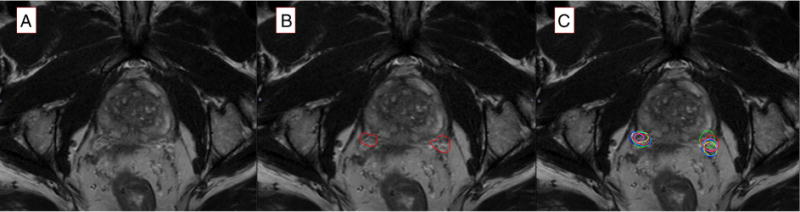
Representative axial slice for a 3-Tesla pelvic magnetic resonance imaging scan without any contours (A), with the expert pelvic radiologist’s neurovascular bundle contours (B), and with all neurovascular bundle contours from each rater and the expert overlaid (C). On these images, the red contours on each side are the expert. Dark blue represents rater 1, yellow represents rater 2, light blue represents rater 3, pink represents rater 4, and lime green represents rater 5.
Statistical analysis
Volumes of all left and right NVBs were determined. Overlapping volumes of each NVB from each rater compared with the expert contour were calculated in Velocity Software. Three separate measures of agreement were then implemented. Intraclass correlation coefficient (ICC) and standard deviation (SD) were determined across the 5 raters and 1 expert over the 120 data points. ICC values >0.75 were considered excellent agreement.27 Next, the pairwise Pearson correlation coefficient (PCC) was estimated for each rater compared with the expert. PCC values range from –1 to 1, with a value of 1 implying a perfectly linear relationship between variables. The Dice similarity coefficient (DSC) was the estimated for each rater volume compared with the expert using the formula , where A is the volume of the expert, B is the volume of the given rater, and A∩B is the intersecting overlap of the 2 volumes. A DSC >0.7 is considered good overlap, with 1 being perfect overlap.28,29 Mean DSCs and SDs were calculated across each sample and each rater. Data were analyzed using R version 3.2.3; ICC was computed using the R package psych.30
Results
Ten 3-T pelvic MRI scans met inclusion criteria. A total of 120 data points were generated: 20 NVBs from the expert and 100 from the raters. The overall ICC for the entire group was 0.89 (95% confidence interval [CI], 0.81-0.95). Table 1 summarizes the individual PCC and CI for each rater. Table 2 summarizes the DSC for each individual contour of each rater across all the cases as well as the mean DSC for each left and right NVB. The mean DSC was 0.72 (SD, 0.07) for rater 1, 0.72 (SD, 0.06) for rater 2, 0.73 (SD, 0.09) for rater 3, 0.74 (SD, 0.09) for rater 4, and 0.68 (SD, 0.13) for rater 5. Overall, across all raters, the average DSC was 0.72 (SD, 0.09). Figure 1 depicts a sample axial slice showing a blank figure (without contours), the same slice with the expert NVB contours, and the same slice with all the contours overlaid. Figure 2 depicts a representative axial slice, sagittal slice, and coronal slice from a patient’s 3-T pelvic MRI scan with the left and right NVB contours from each rater and the expert overlaid. Figure 3 summarizes the patterns of measured volumes for each rater across each sample.
Table 1.
The PCC for each rater compared with the expert pelvic radiologist
| Rater | PCC (95% CI) |
|---|---|
| 1 | 0.95 (0.86-0.98) |
| 2 | 0.98 (0.95-0.99) |
| 3 | 0.94 (0.86-0.98) |
| 4 | 0.98 (0.95-0.99) |
| 5 | 0.84 (0.63-0.93) |
PCC, Pearson correlation coefficient.
Table 2.
Dice similarity coefficient of each rater and case compared with the expert pelvic radiologist and the mean values for each left and right neurovascular bundle of all cases
| Case | Rater 1 | Rater 2 | Rater 3 | Rater 4 | Rater 5 | Mean (SD) |
|---|---|---|---|---|---|---|
| 1: left NVB | 0.78 | 0.76 | 0.69 | 0.78 | 0.61 | 0.72 (0.073) |
| 1: right NVB | 0.74 | 0.75 | 0.65 | 0.73 | 0.58 | 0.69 (0.076) |
| 2: left NVB | 0.71 | 0.77 | 0.80 | 0.74 | 0.56 | 0.71 (0.09) |
| 2: right NVB | 0.63 | 0.73 | 0.72 | 0.79 | 0.59 | 0.69 (0.079) |
| 3: left NVB | 0.64 | 0.71 | 0.77 | 0.69 | 0.64 | 0.69 (0.056) |
| 3: right NVB | 0.72 | 0.75 | 0.67 | 0.68 | 0.66 | 0.70 (0.036) |
| 4: left NVB | 0.68 | 0.64 | 0.66 | 0.62 | 0.52 | 0.62 (0.06) |
| 4: right NVB | 0.62 | 0.73 | 0.64 | 0.72 | 0.68 | 0.68 (0.049) |
| 5: left NVB | 0.71 | 0.59 | 0.70 | 0.75 | 0.79 | 0.71 (0.074) |
| 5: right NVB | 0.65 | 0.74 | 0.61 | 0.61 | 0.84 | 0.69 (0.102) |
| 6: left NVB | 0.83 | 0.75 | 0.96 | 0.90 | 0.79 | 0.84 (0.08) |
| 6: right NVB | 0.80 | 0.85 | 0.84 | 0.95 | 0.88 | 0.86 (0.058) |
| 7: left NVB | 0.77 | 0.80 | 0.76 | 0.82 | 0.66 | 0.76 (0.059) |
| 7: right NVB | 0.80 | 0.76 | 0.78 | 0.79 | 0.73 | 0.77 (0.028) |
| 8: left NVB | 0.68 | 0.67 | 0.75 | 0.74 | 0.94 | 0.75 (0.107) |
| 8: right NVB | 0.78 | 0.80 | 0.78 | 0.80 | 0.82 | 0.79 (0.018) |
| 9: left NVB | 0.81 | 0.73 | 0.86 | 0.73 | 0.68 | 0.76 (0.07) |
| 9: right NVB | 0.82 | 0.66 | 0.78 | 0.78 | 0.64 | 0.73 (0.08) |
| 10: left NVB | 0.65 | 0.66 | 0.63 | 0.62 | 0.45 | 0.60 (0.086) |
| 10: right NVB | 0.63 | 0.65 | 0.65 | 0.59 | 0.56 | 0.62 (0.038) |
NVB, neurovascular bundle; SD, standard deviation.
Figure 2.
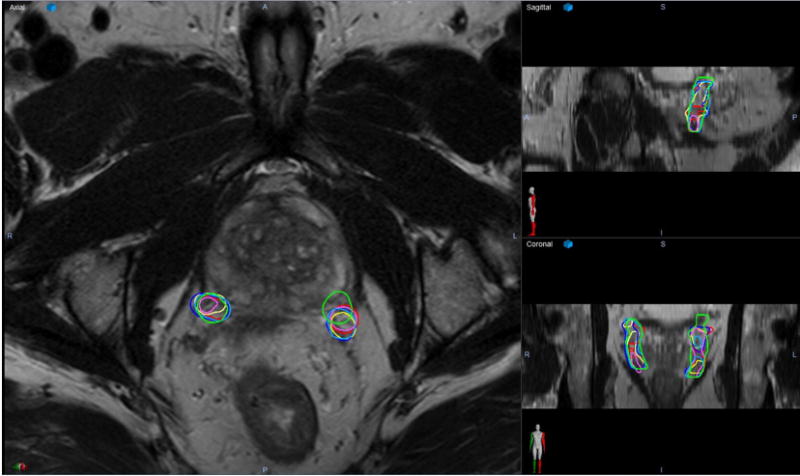
A representative axial (left), sagittal (upper right), and coronal (lower right) slices for a 3-Tesla pelvic magnetic resonance imaging scans with overlaying contours of the left and right neurovascular bundle from each rater and the expert. On this image, the red contours on each side are the expert. Dark blue represents rater 1, yellow represents rater 2, light blue represents rater 3, pink represents rater 4, and lime green represents rater 5.
Figure 3.
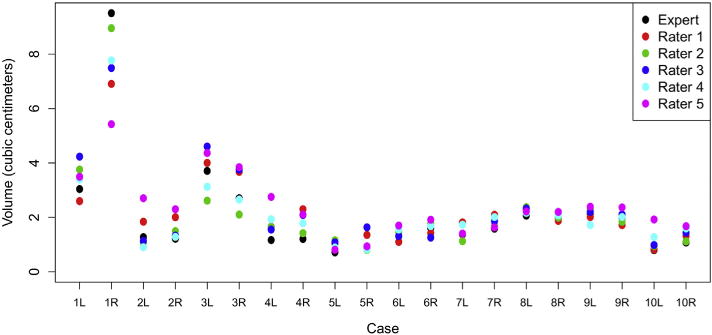
Plotted distribution of the left and right neurovascular bundle volumes (in cubic centimeters) for each rater and the expert across all cases. The volume (in cubic centimeters) for the left (L) and right (R) neurovascular bundle for each case for each rater, 1-5, and the expert was determined. These values were plotted for each case to further detail the spread of the contoured volumes as well as the variation in volume of the neurovascular bundle volume for each case.
Discussion
To our knowledge, this series represents the first attempt to quantify and characterize the accuracy and reproducibility of contouring the NVB when it is in its classical posterolateral position relative to the prostate on dedicated pelvic MRI scans. We demonstrated that there was excellent agreement among the raters and the expert as evidenced by an overall ICC of 0.89 (95% CI, 0.81-0.95). In addition to agreement among the volume of the NVB for each case, we also observed reasonable overlap of each rater’s NVB to the expert contoured NVB as observed that most DSC scores were >0.7. Evaluation of individual volumes for each case showed good overall consistency (Fig 3).
Previous efforts to define the NVB for radiation planning purposes have varied. Historically, a single cadaver study yielded detailed anatomical localization of the nerves that innervate the corpora cavernosa.20 In a series that used this historical definition to define the NVB, patients that received iodine 125 or palladium 103 prostate brachytherapy were found to have radiation doses to the NVB ranging from 150 to 325 Gy.21 The authors found that 3 patients who had early ED had higher than average radiation doses to the NVB. In a similar study that evaluated patients receiving brachytherapy, there was no dose relationship to the NVB that correlated with ED outcomes.31 Previous consensus reporting defined the NVB using CT-based anatomical regions and not MRI.33 Moreover, a series of 147 patients undergoing prostate brachytherapy who had good potency before therapy found 3-year follow up rates of 23% of ED with 43% of patients using a potency aid.19 The authors did not find any relationship between penile bulb dose or NVB dose and ED outcomes. The delineation of the NVB in this study was an area bounded by the posterolateral prostate, levator ani muscle, and the rectal wall.19 Overall, in a review of the literature at the time, some studies reported NVB dose correlated with ED, whereas others did not.33 It is difficult, however, to draw any clinical conclusions regarding the role of dose to the NVB and ED endpoints given that the NVB was not defined using MRI and instead the anatomical location was based on a single cadaver report or general pelvic muscle relationships. This generality likely does not accurately capture the NVB in all cases because the postprostatectomy surgical literature suggests that only 50% to 60% of patients have classically positioned NVB.34
As the availability and use of pelvic MRI scans become more common, defining the NVB can become more accurate. A previously published report tried to use MRI controls to generate a contouring guide for defining the NVB on pelvic CT.23 In this series, 9 patients, treated with permanent implant brachytherapy with pelvic MRI scans, had the NVB area measured. Their study mandated an endorectal coil MRI but did not specify magnet strength or specific series obtained. They used the NVB measured on MRI and referenced these contours to structures that could plainly be seen on CT (ie, prostate, musculature, bones) to generate volumes that encompassed the NVB.23 No efforts were made to spare the NVB of radiation in this series. In a previous report from our institution, use of individual MRI–CT-based registration was implemented to define the NVB for each patient.24 This series did not report on clinical outcomes but instead found that external beam radiation plans using volumetric modulated arc therapy technology that delivered adequate curative dose to the prostate while limiting the dose to the NVB could be generated. This is the first series to actively try to limit dose to the NVB; previously detailed literature describes dose to the NVB during standard therapy.
Despite the dosimetric ability to limit radiation dose to the NVB when it is in its classic posterolateral position, the delineation of the NVB can vary, even with modern MRI techniques. An abstract by Liss et al reported 40 patients with T2-weighted pelvic MRI scans undergoing definitive therapy for prostate cancer.35 They determined extensive variability of the NVB, ranging from an average of 2.03 cm anteroposteriorly and 1.48 cm laterally. They determined that a 5 mm or 1 cm expansion of the prostate would encompass the NVB in 28% of patients and 55% of patients, respectively.35 As imaging techniques evolve and radiation planning becomes more targeted, including techniques that treat only portions of the prostate,22 accurate delineation and reproducibility of contouring the NVB will be paramount if attempts to limit dose to these structures are explored. Our series partially fills this gap in the current literature because we demonstrate that the NVB, when in its classic posterolateral position in relation to the prostate, can be reliably visualized and accurately contoured by radiation oncologists compared with an expert radiologist on dedicated prostate 3-T MRI sequences.
This study has several limitations. First, we used a single expert to delineate the “ideal” NVB contour for each case. It is possible that several pelvic radiologists may disagree about the exact extent of the NVB, especially superiorly and inferiorly. Our series only examined pelvic MRI scans in which the NVB was in its classic posterolateral position relative to the prostate. As discussed, there is anatomical variability of the NVB between individuals, with surgical literature suggesting that up to 50% of men have anatomical variation, including some men not forming an identifiable bundle.34,36 Our series cannot comment on the ability to reliably contour these anatomical variations, and, as such, these patients should not be considered for NVB-sparing radiation therapy.24 Additionally, our series only examined the NVB for each case. Although we found excellent agreement among the raters to the expert as well as good overlap of NVB volumes, if we had expanded the series to involve delineation of the prostate, bladder, and rectum for example, more variability could be introduced. Our series also used endorectal coil technique on 3-T MRI, the current gold standard. It is possible that using lower magnetic machines or lack of an endorectal coil could introduce a lower signal-to-noise ratio, leading to less structural detail,25 and thereby making delineation of the NVB more difficult. In this context, our results may not be applicable to earlier MRI technology.
In conclusion, our series demonstrates that the NVB, when in its classic posterolateral position to the prostate, can reliably be delineated on 3-T pelvic MRI scans by radiation oncologists. Using an expert pelvic radiologist to initially define the NVB, we found that, among 5 radiation oncologists, the NVB could be contoured accurately, with excellent agreement and acceptable overlap with the expert-defined volumes. Given these results and our previously published series demonstrating the dosimetric feasibility of sparing the NVB during external beam radiation therapy, our institution has begun investigating NVB-sparing radiation therapy in a prospective setting.
Acknowledgments
Portions of this work were accepted for an oral presentation at the 2017 ASTRO Annual Meeting in San Diego, California.
Footnotes
Conflicts of interest: None.
References
- 1.Incrocci L. Sexual function after external-beam radiotherapy for prostate cancer: what do we know? Crit Rev Oncol Hematol. 2006;57:165–173. doi: 10.1016/j.critrevonc.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Eid JF. Elucidating the etiology of erectile dysfunction after definitive therapy for prostatic cancer. Int J Radiat Oncol Biol Phys. 1998;40:129–133. doi: 10.1016/s0360-3016(97)00554-3. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Henderson RH, Indelicato DJ, Keole SR, Mendenhall NP. Erectile dysfunction after radiotherapy for prostate cancer. Am J Clin Oncol. 2009;32:443–447. doi: 10.1097/COC.0b013e318173a563. [DOI] [PubMed] [Google Scholar]
- 4.Walsh PC, Donker PJ. Impotence following radical prostatectomy: Insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 5.Pardo Y, Guedea F, Aguilo F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 2010;28:4687–4696. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- 6.Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA. 2017;317:1141–1150. doi: 10.1001/jama.2017.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017;317:1126–1140. doi: 10.1001/jama.2017.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:1063–1068. doi: 10.1016/s0360-3016(02)03030-4. [DOI] [PubMed] [Google Scholar]
- 9.Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306:1205–1214. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh PC. Anatomic radical prostatectomy: Evolution of the surgical technique. J Urol. 1998;160:2418–2424. doi: 10.1097/00005392-199812020-00010. [DOI] [PubMed] [Google Scholar]
- 12.Roach M, III, Nam J, Gagliardi G, El Naqa I, Deasy JO, Marks LB. Radiation dose-volume effects and the penile bulb. Int J Radiat Oncol Biol Phys. 2010;76:S130–S134. doi: 10.1016/j.ijrobp.2009.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Wielen GJ, Mulhall JP, Incrocci L. Erectile dysfunction after radiotherapy for prostate cancer and radiation dose to the penile structures: A critical review. Radiother Oncol. 2007;84:107–113. doi: 10.1016/j.radonc.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Roach M, Winter K, Michalski JM, et al. Penile bulb dose and impotence after three-dimensional conformal radiotherapy for prostate cancer on RTOG 9406: Findings from a prospective, multi-institutional, phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys. 2004;60:1351–1356. doi: 10.1016/j.ijrobp.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Wernicke AG, Valicenti R, Dieva K, Houser C, Pequignot E. Radiation dose delivered to the proximal penis as a predictor of the risk of erectile dysfunction after three-dimensional conformal radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60:1357–1363. doi: 10.1016/j.ijrobp.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Kao J, Turian J, Meyers A, et al. Sparing of the penile bulb and proximal penile structures with intensity-modulated radiation therapy for prostate cancer. Br J Radiol. 2004;77:129–136. doi: 10.1259/bjr/37893924. [DOI] [PubMed] [Google Scholar]
- 17.Kiteley RA, Lee WR, deGuzman AF, Mirzaei M, McCullough DL. Radiation dose to the neurovascular bundles or penile bulb does not predict erectile dysfunction after prostate brachytherapy. Brachytherapy. 2002;1:90–94. doi: 10.1016/s1538-4721(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 18.Brown MW, Brooks JP, Albert PS, Poggi MM. An analysis of erectile function after intensity modulated radiation therapy for localized prostate carcinoma. Prostate Cancer Prostatic Dis. 2007;10:189–193. doi: 10.1038/sj.pcan.4500938. [DOI] [PubMed] [Google Scholar]
- 19.Solan AN, Cesaretti JA, Stone NN, Stock RG. There is no correlation between erectile dysfunction and dose to penile bulb and neurovascular bundles following real-time low-dose-rate prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2009;73:1468–1474. doi: 10.1016/j.ijrobp.2008.06.1946. [DOI] [PubMed] [Google Scholar]
- 20.Lepor H, Gregerman M, Crosby R, Mostofi FK, Walsh PC. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol. 1985;133:207–212. doi: 10.1016/s0022-5347(17)48885-9. [DOI] [PubMed] [Google Scholar]
- 21.DiBiase SJ, Wallner K, Tralins K, Sutlief S. Brachytherapy radiation doses to the neurovascular bundles. Int J Radiat Oncol Biol Phys. 2000;46:1301–1307. doi: 10.1016/s0360-3016(99)00551-9. [DOI] [PubMed] [Google Scholar]
- 22.Amini A, Westerly DC, Waxweiler TV, Ryan N, Raben D. Dose painting to treat single-lobe prostate cancer with hypofractionated high-dose radiation using targeted external beam radiation: Is it feasible? Med Dosim. 2015;40:256–261. doi: 10.1016/j.meddos.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Wright JL, Newhouse JH, Laguna JL, Vecchio D, Ennis RD. Localization of neurovascular bundles on pelvic CT and evaluation of radiation dose to structures putatively involved in erectile dysfunction after prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2004;59:426–435. doi: 10.1016/j.ijrobp.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Cassidy RJ, Yang X, Liu T, Thomas M, Nour SG, Jani AB. Neurovascular bundle-sparing radiotherapy for prostate cancer using MRI-CT registration: A dosimetric feasibility study. Med Dosim. 2016;41:339–343. doi: 10.1016/j.meddos.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Hegde JV, Mulkern RV, Panych LP, et al. Multiparametric MRI of prostate cancer: An update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging. 2013;37:1035–1054. doi: 10.1002/jmri.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naiki T, Okamura T, Nagata D, et al. Preoperative prediction of neurovascular bundle involvement of localized prostate cancer by combined T2 and diffusion-weighted imaging of magnetic resonance imaging, number of positive biopsy cores, and Gleason score. Asian Pac J Cancer Prev. 2011;12:909–913. [PubMed] [Google Scholar]
- 27.Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]
- 29.Zijdenbos AP, Dawant BM, Margolin RA, Palmer AC. Morphometric analysis of white matter lesions in MR images: method and validation. IEEE Trans Med Imaging. 1994;13:716–724. doi: 10.1109/42.363096. [DOI] [PubMed] [Google Scholar]
- 30.Revelle W. Procedures for Psychological, Psychometric, and Personality Research. Northwestern University; 2016. [Google Scholar]
- 31.Merrick GS, Butler WM, Dorsey AT, Lief JH, Donzella JG. A comparison of radiation dose to the neurovascular bundles in men with and without prostate brachytherapy-induced erectile dysfunction. Int J Radiat Oncol Biol Phys. 2000;48:1069–1074. doi: 10.1016/s0360-3016(00)00746-x. [DOI] [PubMed] [Google Scholar]
- 32.Crook JM, Potters L, Stock RG, Zelefsky MJ. Critical organ dosimetry in permanent seed prostate brachytherapy: Defining the organs at risk. Brachytherapy. 2005;4:186–194. doi: 10.1016/j.brachy.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Akbal C, Tinay I, Simsek F, Turkeri LN. Erectile dysfunction following radiotherapy and brachytherapy for prostate cancer: Pathophysiology, prevention and treatment. Int Urol Nephrol. 2008;40:355–363. doi: 10.1007/s11255-007-9247-1. [DOI] [PubMed] [Google Scholar]
- 34.Kiyoshima K, Yokomizo A, Yoshida T, et al. Anatomical features of periprostatic tissue and its surroundings: A histological analysis of 79 radical retropubic prostatectomy specimens. Jpn J Clin Oncol. 2004;34:463–468. doi: 10.1093/jjco/hyh078. [DOI] [PubMed] [Google Scholar]
- 35.Liss AZJ, Evans C, et al. Anatomic variability of the neurovascular elements defined by MRI. Brachytherapy. 2014;13(suppl 1):S42–43. [Google Scholar]
- 36.Hong H, Koch MO, Foster RS, et al. Anatomic distribution of periprostatic adipose tissue: A mapping study of 100 radical prostatectomy specimens. Cancer. 2003;97:1639–1643. doi: 10.1002/cncr.11231. [DOI] [PubMed] [Google Scholar]


