Abstract
Background
As the WHO verified that China reached the target of 1% prevalence of chronic hepatitis B infection among children targeted by universal hepatitis B immunization of newborns, the country considered new options for hepatitis B prevention and control. We reviewed hepatitis B surveillance in the broader context of viral hepatitis surveillance to propose recommendations to improve the system.
Methods
We described surveillance for viral hepatitis in China with a specific focus on hepatitis B. We assessed critical attributes of the system, including data quality, predictive positive value and usefulness.
Results
While remarkable progress in hepatitis B immunization of infants and children has likely almost eliminated transmission in younger age groups, reported rates of hepatitis B increased steadily in China between 1990 and 2008, probably because of a failure to distinguish acute from chronic infections. Elements that prevented a clearer separation between acute and chronic cases included (1) missed opportunity to report cases accurately among clinicians, (2) low availability and use of tests to detect IgM against the hepatitis B core antigen (IgM anti-HBc) and (3) lack of systems to sort, manage and analyze surveillance data.
Conclusions
To improve hepatitis B surveillance, China may consider (1) training clinicians to diagnose acute cases and to use IgM anti-HBc to confirm them, (2) improving access and use of validated IgM anti-HBc tests and (3) developing data management and analysis techniques that sort out acute from chronic cases.
Keywords: Hepatitis B, Surveillance evaluation, China, Laboratory diagnosis
1. Introduction
In 2010, the 63rd World Health Assembly passed a resolution on viral hepatitis that underlined the role of surveillance [1]. In practice [2], surveillance for viral hepatitis requires (1) a clear, simple system of case definitions, (2) clinicians differentiating acute, recently acquired hepatitis [3] from chronic hepatitis (including flares of disease among chronic patients) [4,5], (3) laboratory diagnosis with standardized testing algorithms and quality assurance systems, (4) collection and appropriate analysis of data and (5) regular feedback for action. In most cases, surveillance for all the specific types of viral hepatitis (A, B, C and E) is integrated because clinical syndromes are identical [2].
The World Health Organization (WHO) region of the Western Pacific (WPR) suffers from a high burden of disease from infection with hepatitis B virus (HBV), with perinatal [6,7] and early horizontal transmission [8,9]. In 2012, the WHO verified that China had reached the hepatitis B surface antigen (HBsAg) 1% prevalence control goal among children under five years of age through large-scale use of hepatitis B vaccine [10]. Hence, China started to consider various options for the next phase of prevention and control of HBV infection, including vaccination of higher risk adults and clinical management of patients. Information from viral hepatitis surveillance would be useful to guide both these potential initiatives.
In China, some laboratory diagnosis for viral hepatitis is available in selected hospitals only and different systems contribute to hepatitis surveillance. However, reports [11] suggested that acute and chronic hepatitis B cases were included in the system in an undifferentiated manner that failed to distinguish acute from chronic hepatitis B cases. This has complicated use of surveillance data to monitor the effectiveness of hepatitis B immunization and identify groups at higher risk for hepatitis B for the purpose of a potential adult immunization programme. In 2011, the Chinese Center for Disease Control and Prevention (China CDC) and the World Health Organization (WHO) decided to jointly review hepatitis B surveillance in China. We aimed at (1) describing the system, (2) identifying its key strengths and weaknesses and (3) proposing interventions for improvement. We mostly used data available from projects already completed in various locations in the country and addressed surveillance for hepatitis B in the broader context of viral hepatitis surveillance as the two are closely related.
2. Methods
As a reference for our review, we defined acute hepatitis B as acute, discrete onset of symptoms with abnormal alanine amino transferases (higher than twice the upper limit of normal) and with presence of IgM antibodies against the core antigen of the hepatitis B virus (anti-HBc IgM). We defined hepatitis A as acute, discrete onset of symptoms with abnormal alanine amino transferases (higher than twice the upper limit of normal) and with the presence of IgM antibodies to hepatitis A virus (HAV).
2.1. Description of hepatitis B surveillance in the broader context of hepatitis surveillance
We considered two systems that contribute to viral hepatitis surveillance: The National Notifiable Disease Reporting System (NNDRS) and the national immunization programme (NIP), including its sentinel surveillance in 18 counties. We conducted unstructured interviews with staff of all levels of the CDC network, including national, municipal/provincial, prefecture and county. In addition to the Division of Hepatitis, National Immunization Programme (NIP), China CDC, we visited the lower levels of the CDC system in Beijing municipality, Ningxia autonomous region (Western China) and Jiangsu province (Eastern China). We reviewed procedures for collection, transmission, analysis and feedback of data and followed surveillance data from health care facilities to China CDC, through the intermediate levels (i.e., county, prefecture, provincial and China CDC).
In Beijing municipality, Nanjing prefecture in Jiangsu province and Guyuan prefecture in Ningxia autonomous region, we assessed hepatitis laboratory diagnostic capacity through visits to laboratories in county, prefectural and provincial hospitals. The team assessed three components of the laboratory cycle, including pre-analytical logistics, analytical aspects and post analytical procedures.
2.2. Review of hepatitis B surveillance in the broader context of hepatitis surveillance
Our review referred to generic guidelines for the evaluation of surveillance systems [12] focusing on selected attributes critical to hepatitis B surveillance, including (1) data quality, (2) positive predictive value, (3) representativeness and (4) usefulness.
2.2.1. Data quality
To assess the data quality, we described the number of reported hepatitis B cases between 1990 and 2008, compared it with the number of reported hepatitis A cases and assessed how the surveillance data reflected the different epidemiological situations of these two diseases.
2.2.2. Positive predictive value
2.2.2.1. Positive predictive value of clinicians’ diagnosis
A 2008 WHO pilot project that aimed at identifying methods to conduct viral hepatitis surveillance in the Guyuan prefecture of the Ningxia autonomous region provided an opportunity to validate the diagnoses of clinicians using hospital laboratory data. Among patients clinically diagnosed with hepatitis A, acute hepatitis B and chronic hepatitis B, we calculated the proportions that were confirmed by the laboratory (positive predictive value).
2.2.2.2. Use of hospital laboratory tests to document case definitions
We reviewed information available on the frequency of use of diagnostic tests for the diagnosis of hepatitis B in a survey of a sample of hospitals in eight provinces/municipalities of China (reported in details elsewhere) [13].
2.2.2.3. Proportion of chronic hepatitis B cases reported
In the Jiangsu province where this information was available, we calculated the proportion of acute and chronic hepatitis B cases reported in each prefecture. Further, in the 18 sentinel counties where both NNDRS and the sentinel systems were in operation, we compared the capacity of the NNDRS and the sentinel systems to identify and differentiate acute and chronic cases.
2.2.3. Representativeness
To assess representativeness, we examined how the systems that contributed to hepatitis surveillance collected information that reflected the Chinese situation.
2.2.4. Usefulness
To assess usefulness, we reviewed the use of surveillance information to make decisions.
3. Results
3.1. Description of hepatitis B surveillance in the broader context of hepatitis surveillance
In 2011, two different systems contributed to surveillance of viral hepatitis A and B in China: The NNDRS and the NIP, including its sentinel surveillance in 18 counties. Surveillance for hepatitis C was under the responsibility of the National Center for HIV-AIDS at China CDC, did not rely on acute disease reporting and was based mostly on regular serological surveys in selected high-risk groups (e.g., commercial sex workers, injection drug users, men who have sex with men) as is being done for HIV surveillance. Little surveillance information was available for hepatitis E virus (HEV) infection other than ad hoc active surveillance system organized in a specific area of Jiangsu province in the context of a clinical trial of a new vaccine against hepatitis E [14].
3.1.1. The National Notifiable Disease Reporting System (NNDRS)
In 1959, viral hepatitis became a nationally notifiable disease in China. In 1990, the system differentiated the types of hepatitis (i.e., A, B, C, D, E). In 2008, THE Ministry of Health of China issued a document describing the clinical and laboratory characteristics of viral hepatitis that was used as a guidance document to diagnose cases. However, the document could have outlined better inclusion and exclusion criteria for public health surveillance case definitions. Health care workers reported cases electronically from each hospital. Information that could be collected included the type of hepatitis, demographic and epidemiological characteristics and the clinical characteristics of hepatitis B cases (‘acute’, ‘chronic’ or ‘unclassified’). However, the form did not include laboratory results that could have helped verifying the diagnosis. Each level of the CDC analyzed data for the levels that reported to it and the central China CDC produced regular data analysis reports.
3.1.2. The NIP information system, including sentinel viral hepatitis surveillance in 18 counties
Local CDCs investigated cases of acute hepatitis B to collect information on exposures and on immunization status. CDC staff entered this information into the immunization health information system, a system designed to manage all information related to the NIP including coverage. In 2011, temporary limitations in the bandwidth of the computer network prevented the intended real time data analysis. In addition, between 2006 and 2010, the Hepatitis Division of the NIP maintained a sentinel viral hepatitis surveillance system in 18 counties. In this enhanced system, China CDC used laboratory tests conducted at the local CDC and confirmed at provincial CDC to classify viral hepatitis by virus type, eliminate duplicate reports, and collected epidemiological information regarding exposures among acute cases.
3.2. Evaluation of hepatitis B surveillance in the broader context of hepatitis surveillance
3.2.1. Data quality
Between 1990 and 2008, the number of cases of hepatitis B reported through NNDRS increased nationwide, even though there had been substantial progress in increasing immunization coverage (Fig. 1); information available at China CDC did not allow the differentiation of chronic from acute hepatitis B cases completely. In contrast, the number of hepatitis A cases decreased steadily.
Fig. 1.
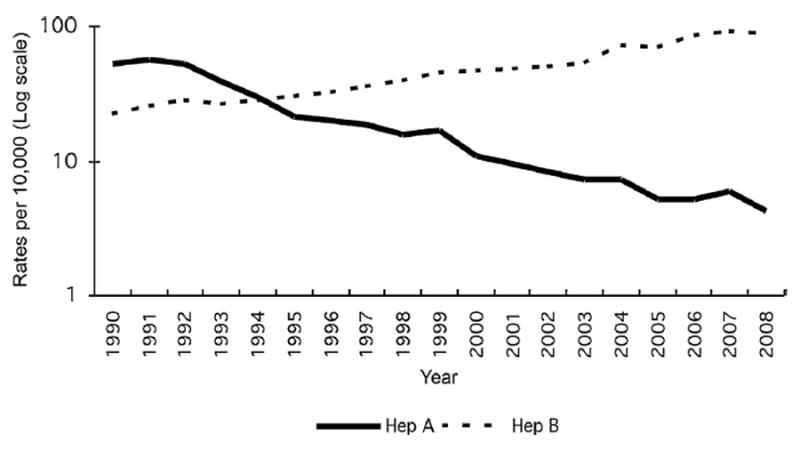
Reported rates of hepatitis A and B, National Notifiable Diseases Reporting System (NNDRS), China, 1990–2008 (logarithmic scale).
3.2.2. Positive predictive value
3.2.2.1. Positive predictive value of clinicians’ diagnosis
In Guyuan, comparison of the diagnoses made by clinicians with the results of laboratory diagnosis at hospital laboratories pointed to different levels of reliability in the clinicians’ capacity to diagnose viral hepatitis (Table 1). Among viral hepatitis cases for whom laboratory data were available, 444 out of 484 cases clinically diagnosed as hepatitis A had laboratory-confirmed hepatitis A (positive predictive value: 92%). In contrast, only 35 out 107 cases clinically diagnosed as acute hepatitis B had laboratory-confirmed acute hepatitis B (positive predictive value: 33%). Among 892 cases clinically diagnosed as chronic hepatitis B, 792 had laboratory-confirmed chronic hepatitis B (positive predictive value: 89%).
Table 1.
Laboratory diagnosis among patients clinically diagnosed with hepatitis A, acute hepatitis B, chronic hepatitis B and other acute hepatitis, March 2008–December 2009, Guyuan prefecture sentinel sites, Ningxia autonomous region, China (Bold values denote positive predictive values).a
| Clinical diagnosis | Diagnosis on the basis of laboratory test results
|
Not tested | Grand total | ||||
|---|---|---|---|---|---|---|---|
| Acute Ab | Acute Bc | Chronic Bd | NonA–NonBe | Total tested | |||
| Hepatitis A | 444 (92%)f | 6 (1%) | 30 (6%) | 4 (1%) | 484 | 40 | 524 |
| Acute hepatitis B | 9 (8%) | 35 (33%)g | 58 (54%) | 5 (5%) | 107 | 14 | 121 |
| Chronic hepatitis B | 13 (1%) | 75 (8%) | 792 (89%)h | 12 (1%) | 892 | 57 | 949 |
| Un-specified/other acute hepatitis | 12 (21%) | 2 (4%) | 20 (36%) | 22 (39%) | 56 | 5 | 61 |
| Total | 478 | 118 | 900 | 43 | 1539 | 116 | 1655 |
Excludes one case with markers of acute hepatitis A and B.
Positive for IgM antibodies to hepatitis A virus (HAV).
Positive for IgM antibodies to hepatitis B virus core antigen (anti-HBc IgM).
Positive for hepatitis B virus surface antigen (HBsAg), with no IgM antibodies against hepatitis viruses.
No serological markers of hepatitis A or B.
444 out of 484 cases clinically diagnosed as hepatitis A had laboratory-confirmed hepatitis A (positive predictive value: 92%).
35 out 107 cases clinically diagnosed as acute hepatitis B had laboratory-confirmed acute hepatitis B (positive predictive value: 33%).
792 out of 892 cases clinically diagnosed as chronic hepatitis B had laboratory-confirmed chronic hepatitis B (positive predictive value: 89%).
3.2.2.2. Use of hospital laboratory tests to document case definitions
The frequency of the diagnostic tests used by hospitals among 95 hospitals investigated in eight provinces/municipalities (Fig. 2) indicated that HBsAg – a marker of current acute or chronic infection – was the most commonly used laboratory test (n = 94, 99%). Antibody against the hepatitis B surface antigen (anti-HBs), a marker of natural or vaccine-induced immunity, ranked second (n = 93, 98%). The hepatitis B “e” antigen (HBeAg) and the antibody against the hepatitis B “e” antigen (anti-HBe), indirect markers of viral replication level mostly used to make treatment decisions in the absence of HBV DNA tests, ranked third (n = 92, 97% and n = 92, 97%), respectively. IgM antibody to the hepatitis B core antigen (anti-HBc IgM), the diagnostic test that best differentiates acute from chronic hepatitis B, ranked fifth and was used only in 28 hospitals (30%).
Fig. 2.
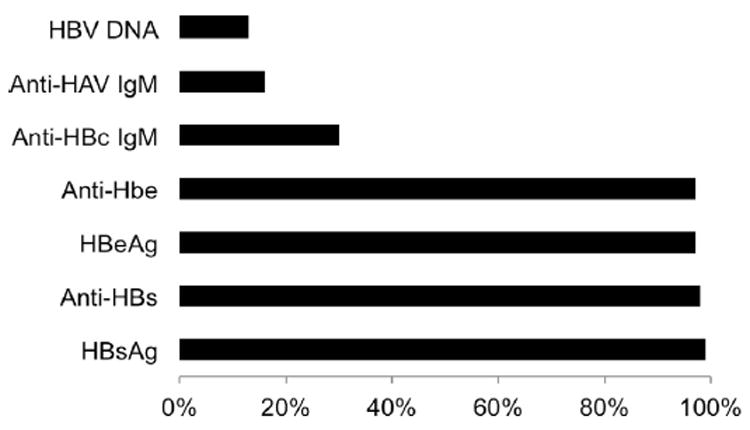
Proportion of hospitals investigated that used selected laboratory tests for the diagnosis of hepatitis B, eight provinces/municipalities, China, 2006 (n = 95).
3.2.2.3. Proportion of chronic hepatitis B cases reported
In Jiangsu province, prefectures reported hepatitis B cases as ‘acute’, ‘chronic’ or ‘unclassified’ (Fig. 3). The number of reported hepatitis B cases varied substantially between prefectures – from 211 to 1888. In prefectures that reported less than 1000 cases, the proportion of acute cases was 39%. In contrast, in prefectures that reported 1000 cases or more, the proportion of acute cases was 18%. The 18 viral hepatitis sentinel counties allowed a direct comparison of the NNDRS and sentinel surveillance systems (Table 2). In these 18 viral hepatitis sentinel counties, the sentinel system reported fewer cases of hepatitis B (acute and chronic) than the NNDRS as they were able to eliminate some duplicate reports. When the sentinel system excluded chronic cases to only consider acute hepatitis B cases, the number of cases was further reduced by a factor of 10.
Fig. 3.
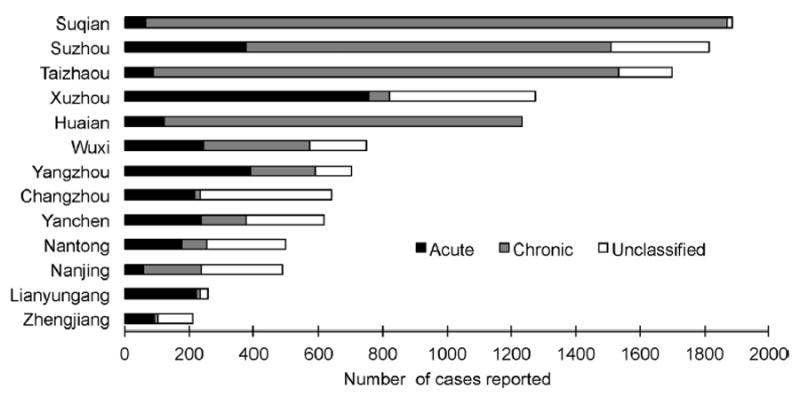
Number of acute, chronic and unclassified hepatitis B cases reported in the National Notifiable Diseases Reporting System (NNDRS), by prefecture, Jiangsu province, China, 2010.
Table 2.
Comparison of the number of hepatitis B cases reported through National Notifiable Diseases Reporting System (NNDRS) and the sentinel system, 18 sentinel counties, China, 2006–2010.
| Year | Hepatitis B cases reported in NNDRS | Total hepatitis B cases reported through the sentinel system | Acute hepatitis B cases reported through the sentinel system | Rate of acute hepatitis B (1/100,000)a,b |
|---|---|---|---|---|
| 2006 | 9454 | 2858 | 404 | 6 |
| 2007 | 9620 | 2816 | 366 | 4 |
| 2008 | 10,590 | 1841 | 227 | 8 |
| 2009 | 5907 | 2708 | 260 | 7 |
| 2010 | 5443 | 2893 | 211 | 4 |
Acute hepatitis cases divided by the population of the county.
Data from the sentinel system.
3.2.3. Representativeness
NNDRS functioned in China nationwide. In addition, the 18 viral hepatitis sentinel counties were spread across nine provinces (three in Eastern China, three in Central China and three in Western China).
3.2.4. Usefulness
Discussions with China CDC staff indicated that the surveillance for hepatitis B had little usefulness in its current status. The absence of an adequate laboratory-based case definition and the low use of anti-HBc IgM led to inaccurate case classification. Inclusion of chronic cases among acute cases led to two major problems. First, the rates of acute hepatitis B generated by this system were grossly inaccurate and increasing despite substantial immunization coverage. Second, the system could not identify real cases of acute hepatitis B (including possible clusters or outbreaks). Identification of acute hepatitis B cases could have pointed to weaknesses of the immunization programme among vaccinated cohorts of children or to risk factors for HBV infection among older age group in the vaccination era. The sentinel system in operation in 18 counties could have represented a better opportunity to review the situation in terms of acute cases of hepatitis B only. However, this system suffered from an incomplete collection of information for selected exposure and from a lack of routine analysis and feedback.
4. Discussion
China has a well-developed infrastructure for viral hepatitis surveillance. First, patients suffering from acute or chronic hepatitis can easily access health care. Second, health care workers have a good level of awareness with respect to viral hepatitis [15]. Third, laboratory testing for HBsAg is easily accessible. Fourth, national guidelines are available for the clinical diagnosis of hepatitis B. Fifth, specialized physicians understand acute and chronic hepatitis B. Sixth, selected health care facilities used electronic systems to manage patients’ information. Seventh, the national system managed individual clinical and hospital laboratory information using a uniform, internet-based and Geographic Information System [GIS] enabled system. This infrastructure functioned well for hepatitis A. Hepatitis A surveillance is technically easier: There are no chronic cases and the positive predictive value of clinical diagnosis was high. As a result, analysis of surveillance data allowed characterizing hepatitis A by time, place and person [16]. This analysis documented the decrease in reported rates that followed improvements in environmental conditions and increasing use of hepatitis A vaccine (Integrated in the Expanded Programme on Immunization in 2008). In addition to its usefulness for hepatitis A, surveillance for acute hepatitis in China occasionally captured clusters of nosocomial hepatitis C, even though hepatitis C surveillance was under the responsibility of the National Center for HIV-AIDS at China CDC. Despite this infrastructure that functioned well for hepatitis A surveillance, anecdotal information gathered during our field visits pointed to a number of issues that limited the capacity of the system to provide useful information to direct hepatitis B prevention and control at the levels of data collection, laboratory testing and data management.
Case identification and reporting by clinicians suffered from a few weaknesses. First, among primary care clinicians, the concepts of “acute hepatitis” did not necessarily correspond with the concept of “recently acquired new infection”, particularly as understood from a surveillance perspective in terms of the need to identify possible risk factors for infection that may have been present during the incubation period. Clinicians’ capacity to diagnose acute hepatitis varied and some clinicians practicing in primary care were not aware of the national diagnosis guidelines. In fact, in some primary care facilities “Acute hepatitis” referred more to the intensity of symptoms and was used for flares among chronic patients. Second, clinicians used laboratory tests according to the clinical needs, and not for surveillance purposes. Our interactions in the field suggested that they did not always understand how to use laboratory tests for surveillance purposes and how to interpret them. Third, IgM anti-HBc tests were not used sufficiently, either because the test was not available in the hospital or because the patient could not pay for it. Fourth, the national guidance did not clearly differentiate the information provided to assist clinicians in diagnosis and management of viral hepatitis and the criteria to be used for reporting cases for surveillance. Fifth, most clinicians did not use the option of “selecting out” chronic hepatitis on report forms. This item was often filled with incorrect or unspecified information, leading to excessive reporting of chronic cases, including for patients who returned regularly for long-term care. The 18 counties sentinel surveillance system captured a smaller number of hepatitis B cases than NNDRS because local CDC staff de-duplicated the records by excluding patients who repeatedly presented themselves for care of a known chronic disease.
Most provincial CDCs had laboratories that could function as reference laboratories locally as they had good buildings, well-designed space, senior and technical personnel, adequate instruments, internal quality control programmes, national accreditation and External Quality Assurance (EQA) programmes. In some provinces, hospital laboratory information systems integrated with the hospital information system allowed laboratory results to be accessed in real time. However, testing algorithms allowing for an optimal decision-tree approach in test ordering were not available. Instead, laboratories tested based on clinician requests, using extensive panels that often included unnecessary tests. This expensive approach might also have contributed to misclassification of cases: Our informal interactions with clinicians suggested that occasionally, the laboratory provided more information than the clinicians could interpret (e.g., HBe antigen in an acute case may have been interpreted as an indicator of chronicity). Finally, available laboratory results were not used for verification of the case classification in the surveillance system. Instead, surveillance relied mainly on the classification of the clinicians’ who had initiated the mandatory report.
Data management in the various levels of the CDC could also be improved so that the date sources available for hepatitis surveillance could be best integrated to generate useful information. First, there was no reliable method in place to de-duplicate records collected by various data-systems (e.g., NNDRS, NIP, electronic medical records, laboratory database). These data systems tended to run in parallel rather than in a coordinated, integrated way. Second, systems lacked functions or automations that would have allowed clinical and laboratory data reconciliation, or reminders for missing/unclear data. As a result, too many unclassified reports persisted even when laboratory data could have been available locally. The heterogeneity of the proportion of chronic cases in the Jiangsu surveillance database and the results from the 18 county sentinel system suggest that in some areas, methods had already been identified to sort out chronic and acute cases. Third, chronic cases captured by the system were not used to guide potential national policies on management and there were no mechanisms to link them with other possible sources of information regarding outcome (e.g., cirrhosis, death). Finally, no analysis focused on cases among persons < 16 year of age. In 2008, these cases among children born in the hepatitis B vaccination era, whether chronic or acute, should have become rare events. Thus, surveillance in that age group could document the impact of immunization and use persisting cases to generate useful information to improve immunization activities.
Our surveillance review had several limitations. First, we did not use a pre-defined protocol for a formal systematic evaluation; instead, we collated experiences from data reported at the national level, various pilot projects and field visits. Second, none of the eighteen China CDC sentinel sites were visited, preventing us from describing activities there. These sentinel counties already implement some of the interventions that could improve the system (e.g., exclusion of patients coming repeatedly for care, collection of information on exposures). As a result, our review may fail to mention innovative solutions that have already been tested or used in the field. Third, we did not conduct a full assessment of the viral hepatitis laboratory diagnosis quality management system and did not examine EQA for viral hepatitis markers in those eighteen sentinel sites.
In conclusion, the Chinese health system offers a solid foundation for viral hepatitis surveillance if some challenges in case reporting, laboratory diagnosis and data management could be addressed. First, continuous medical education of providers could improve knowledge and practices with respect to ordering of laboratory tests, interpretation of viral hepatitis markers and use of precise criteria for case reporting. ‘Just in time’ tools (e.g., pamphlets, job aids) might be sufficient given the good baseline knowledge. Also, a review of the national viral hepatitis diagnostic guidelines may help to optimize laboratory diagnosis and differentiate clinical and surveillance issues. These diagnostic guidelines could include simple messages for better clinical management on one side and better, simpler case classification to improve surveillance on the other side (i.e., case definitions formulated as reporting criteria). Second, evidence-based viral hepatitis diagnostic and interpretation algorithms could be proposed to support accurate case classification criteria. This would allow a better use of the results of the laboratory investigations for surveillance purposes. With respect to IgM anti-HBc, there is need to determine validated positivity thresholds that can differentiate recent infections from flares among chronic cases [4,5] in the Chinese context where chronic infections are highly prevalent. Affordable, nationally produced IgM anti-HBc tests of documented performance must be available. Third, CDC at its various levels, including NNDRS could consider healthcare system wide use of laboratory data for case classification. This could be partially automated through a data management system that would link clinical and laboratory data at health facilities. Such systems could include notifications when data are needed to classify cases, error messages when clinical and laboratory data are incompatible or mechanisms to de-duplicate cases on the basis of demographic information. Overall, these small technical changes using the existing rich data sources could allow China to refine its viral hepatitis surveillance system so that acute and chronic infections can be separated. Surveillance data for acute cases could be analyzed to guide primary prevention. Selective identification of non-acute HBsAg-positive cases could create added value at a time when scaled-up management of chronic viral hepatitis B will emerge as a public health issue in China. Improved surveillance could create linkages with care and treatment and use newly collected information on outcomes (e.g., cirrhosis, hepatocellular carcinoma, liver transplantation registration, deaths). In 2012, WHO began to work with China CDC to test innovative surveillance approaches in pilot, community trials. Lessons from these experiences could lead to a larger network of improved sentinel surveillance sites. This would change the way China triangulates information from viral hepatitis surveillance, HIV programmes and integrated prevention of mother to child transmission of HIV, hepatitis B and syphilis to frame policies for prevention and control of HBV infection. Such an initiative could create a model for other countries, in Asia and worldwide.
Acknowledgments
We are grateful to Alicia Barrasa, Erika Duffel, Michael Edelstein, Dale Hu, Lance Rodewald and Nicole Seguy for critical comments on the manuscript. This work benefited from funding support from the United States Centers for Disease Control and Prevention, from the Zeshan Foundation (Hong Kong) and from the WHO.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention (USCDC).
Conflicts of interest
The authors declare that they have no conflict if interest.
References
- 1.World Health Organization. Viral hepatitis. [05.12.12];Sixty-third world health assembly WHA63.18. 2010 May; Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA63-REC1/WHA63REC1-P2-en.pdf.
- 2.Centers for Disease Control and Prevention. Guidelines for viral hepatitis surveillance and case management. Atlanta, GA: 2005. [02.12.12]. Available at: www.cdc.gov/hepatitis/pdfs/2005guidlinessurv-casemngmt.pdf. [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Establishment of a viral hepatitis surveillance system – Pakistan, 2009–2011. Morb Mortal Wkly Rep. 2011;60:1385–90. [PubMed] [Google Scholar]
- 4.Tassopoulos NC, Papatheodoridis GV, Kalantzakis Y, Tzala E, Delladetsima JK, Koutelou MG, et al. Differential diagnosis of acute HBsAg positive hepatitis using IgM anti-HBc by a rapid, fully automated microparticle enzyme immunoassay. J Hepatol. 1997 Jan;26(1):14–9. doi: 10.1016/s0168-8278(97)80003-7. [DOI] [PubMed] [Google Scholar]
- 5.Huang YW, Lin CL, Chen PJ, Lai MY, Kao JH, Chen DS. Higher cut-off index value of immunoglobulin M antibody to hepatitis B core antigen in Taiwanese patients with hepatitis B. J Gastroenterol Hepatol. 2006 May;21(5):859–62. doi: 10.1111/j.1440-1746.2006.04280.x. [DOI] [PubMed] [Google Scholar]
- 6.Rani M, Yang B, Nesbit R. Hepatitis B control by 2012 in the WHO Western Pacific Region: rationale and implications. Bull World Health Organ. 2009;87:707–13. doi: 10.2471/BLT.08.059220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–4. doi: 10.1056/NEJM197504102921503. [DOI] [PubMed] [Google Scholar]
- 8.Beasley RP, Hwang LY, Lin CC, Leu ML, Stevens CE, Szmuness W, et al. Incidence of hepatitis B virus infections in preschool children in Taiwan. J Infect Dis. 1982;146:198–204. doi: 10.1093/infdis/146.2.198. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Progress towards meeting the 2012 hepatitis B control milestone: WHO Western Pacific Region, 2011. Wkly Epidemiol Rec. 2011;86:180–8. [PubMed] [Google Scholar]
- 10.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992–2005 in China. J Infect Dis. 2009;200:39–47. doi: 10.1086/599332. [DOI] [PubMed] [Google Scholar]
- 11.Cui F, Lu Y, Wang F, et al. Analysis of proportion of reported hepatitis B cases on pilot surveillance in China. Chin J Epidemiol. 2007;28:872–4. [PubMed] [Google Scholar]
- 12.German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50(RR-13):1–35. [PubMed] [Google Scholar]
-
13.Lu Y, Cui F, Wang X, Gong X, Dong H, Hu Y, et al. Investigation of the capacity to confirm the diagnosis of hepatitis B with laboratory tests in health care facilities at all levels in eight provinces and municipalities of China. Chin J Epidemiol. 2006;9:802.

- 14.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 15.Chao J, Chang ET, So SK. Hepatitis B and liver cancer knowledge and practices among healthcare and public health professionals in China: a cross-sectional study. BMC Public Health. 2010 Feb;10:98. doi: 10.1186/1471-2458-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui F, Hadler SC, Zheng H, Wang F, Zhenhua W, Yuansheng H, et al. Hepatitis A surveillance and vaccine use in China from 1990 through 2007. J Epidemiol. 2009;19:189–95. doi: 10.2188/jea.JE20080087. [DOI] [PMC free article] [PubMed] [Google Scholar]


