Abstract
Chronic rhinosinusitis (CRS) is a chronic inflammatory disease of the nose and sinuses that affects up to 12% of the population in Europe and the United States. This complex disease is likely driven by multiple environmental, genetic, and inflammatory mechanisms, and recent studies suggest that B cells may play a critical role in disease pathogenesis. B cells and their antibodies have undisputed roles in health and disease within the airway mucosae. Deficient or inadequate B cell responses can lead to susceptibility to infectious disease in the nose, while excess antibody production, including autoantibodies, can promote damaging inflammation. Thus, patients with B cell defects often have either chronic or recurrent acute infections, and this can be associated with non-polypoid CRS. In contrast, many patients with CRSwNP, which is less likely to be driven by pathogens, have excess production of local immunoglobulins, including autoreactive antibodies. These B cell responses activate complement in many patients and likely contribute to immuno-pathogenic responses. A better understanding of the B cell-associated mechanisms that drive disease in CRS should be a high priority in the quest to understand the pathogenesis of this disease.
Keywords: Antibodies, Antibody deficiency, Autoimmunity, Chronic rhinosinusitis, B cells, Immunodeficiency, Mucosal immunity
Introduction
The sinonasal mucosa is the first epithelial surface encountered by inhaled microbes and allergens.1 B cells and their immunoglobulins play an active role in the surveillance of this mucosal barrier and protection against infectious diseases.2, 3 B cells can directly recognize and respond to pathogens via their surface bound immunoglobulins (also known as the B cell receptor (BCR)),4 and they help other immune effector cells recognize and respond to pathogens through secretion of antibodies that interact with Fc receptors expressed on immune effector cells. In addition, B cells play important roles in the activation of T helper cells, and can produce a variety of cytokines and effector molecules that can contribute to the host response against pathogens. When B cell responses are not properly regulated however, they can lead to disease.
Chronic rhinosinusitis (CRS) is a chronic inflammatory disease of the nose and sinuses that persists for at least 12 weeks and affects up to 12% of the population in Europe and the United States.5 CRS is often divided into two subtypes: CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP), based on clinical findings. CRSwNP patients generally have more severe radiographic disease and a greater propensity for recurrence after sinus surgery, but make up a smaller proportion of all patients with CRS.6 Classically, these two phenotypic forms of CRS were thought to be driven by distinct inflammatory mechanisms, however, recent evidence suggests that both forms of CRS can be characterized by a variety of inflammatory endotypes.7–9 CRS is a complex disease that is likely driven by multiple environmental, genetic, and inflammatory mechanisms, and many recent studies indicate that B cells may play a critical role in the pathogenesis of this disease.10 This review will highlight recent evidence that supports a role for B cells and antibodies in the pathogenesis of CRS.
B Cells and Antibodies in the Upper Airways and Sinuses
B Cell Activation in the Mucosa
B cells are classically thought to recognize their cognate antigen and become activated in secondary lymphoid organs (SLO) via the germinal center reaction. After they leave the secondary lymphoid organ, activated B cells can then traffic to sites of inflammation and participate in inflammatory responses. The details of these classic B cell activation mechanisms have been extensively covered elsewhere, and are beyond the scope of this review.11 Importantly, B cells can also be directly activated at sites of inflammation, including the airways.10 Mucosal associated lymphoid tissues (MALT) have been well characterized, especially Peyer’s patches in the gut and the Waldeyer’s ring (e.g. tonsils and adenoids) in the airways. Rabbits and rats also have constitutive bronchus associated and nasal associated lymphoid tissues (BALT and NALT, respectively), but these tertiary lymphoid organs (TLO) are largely absent in healthy murine and human airways.12 However, the formation of TLO can be induced in the airways by inflammation, and inducible BALT (iBALT) has been shown to be critical for the optimal response to and clearance of pathogens, and even tumors in humans.13, 14 The structures of follicles within TLOs is similar to those found in SLOs, although they tend to be less well organized.10 Because of this lack of organization, and possibly reduction in clonal selection (see below), it has been speculated that TLOs may allow for the activation of autoreactive B and T cell clones, which could contribute to chronic inflammation or development of autoimmunity.15, 16 While TLOs in the airways are associated with protective immune responses, their presence is also associated with chronic inflammatory diseases, including asthma and COPD, as well as autoimmiunity.12
B Cells in CRS
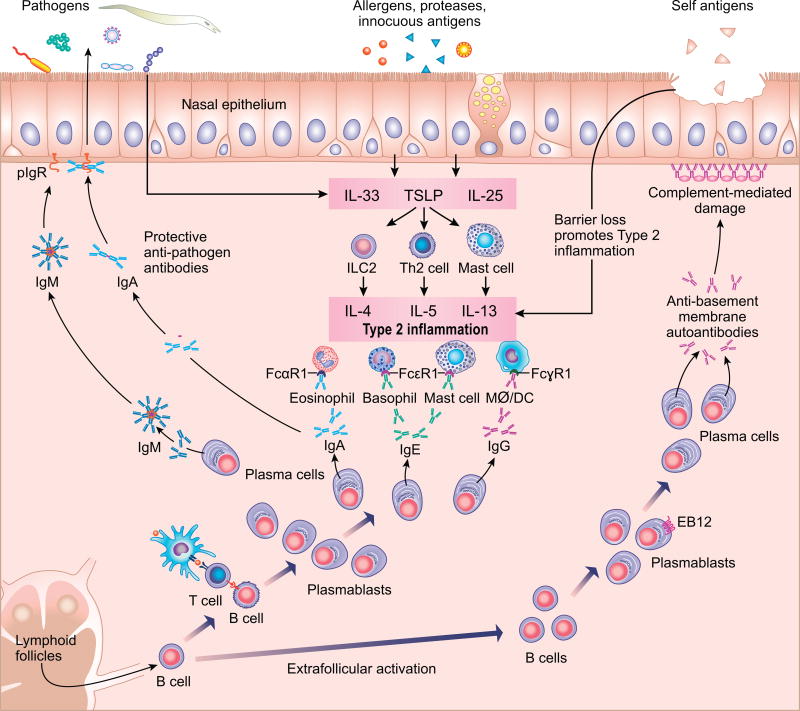
Although B cells and plasma cells had been described in the inflammatory infiltrate in CRS17, the specificity and pathogenic potential of these B cells remained underexplored until recently. Early immunohistology studies demonstrated elevated expression of the B cell marker CD19 and the plasma cell marker CD138 in CRSwNP tissue,18 which were respectively later confirmed to represent elevated numbers of B cells and plasma cells, based on flow cytometry.19 On histopathologic examination, organization of the B cell infiltrates into clusters has been reported in CRS20, 21, but the infrequency of these clusters and the rarity with which they are organized into germinal centers (GC) put their significance in doubt. Our own systematic quantitative histopathologic study of sinus tissues from CRSwNP and control patients found that TLOs were present in nasal polyp tissue, but they were not increased in frequency compared to non-CRS control uncinate tissue when normalized to tissue volume.22 However, a recent report by Lau et. al. found that 37% of tissue from patients with recalcitrant CRSwNP had evidence for the formation of TLOs in their nasal polyps, based on identification of lymphoid clusters with peripheral lymph node addressin (PNAd) and follicular dendritic cells, while none of the tissues from control patients contained such structures.23 Although it is clear that B lineage cells play important roles in CRS pathogenesis, the question of whether TLOs play an important role in the activation of B cells and antibody production in nasal polyps, whether these events occur in extrafollicular locations and whether the appearance of TLOs varies in disease in patients from distinct geographical regions will require more study (Figure 1).
Figure 1.
Overview of the roles of B cells and antibodies in Chronic Rhinosinusitis. The figure is divided into three domains based on the nature of the B cell antigen sources (see top), which corresponds to the organization of the review. Left: summary of the roles of B cells and antibodies in anti-pathogen immunity. Defects in the activation of B cells and production of anti-pathogen antibodies can lead to chronic or recurrent-acute sinus disease. Middle: summary of the effector cells activated in typical type 2 inflammation, as can be found in both CRSwNP and certain endotypes of CRSsNP. Many of the effector cells involved, such as mast cells, basophils and eosinophils, utilize specific antibodies for activation. Right: illustration of the expansion of autoreactive extrafollicular B cell clones, production of autoantibodies, and activation of complement leading to epithelial damage, which may contribute to chronic inflammation.
Recent studies have evaluated the phenotype of B cells within the CRS nasal mucosa to discover mechanisms by which these B cells may be activated. Studies from our group have used B cells from adult tonsil tissue to serve as a control for classically activated B cells in order to compare to the phenotypes of B cells in nasal polyps. We have found that while total CD19+ naïve and germinal center B cells were less frequent in nasal polyps, CD19+CD27+CD38hi plasmablasts were dramatically increased in nasal polyps.22 Interestingly, plasmablasts in nasal polyps also had a higher frequency of expression of Epstein Barr Virus-induced protein 2 (EBI2), compared to plasmablasts from tonsils.22 EBI2 is a chemotactic receptor that guides B cells away from germinal centers to the extrafollicular regions outside the germinal center in secondary lymphoid organs,24 suggesting that some B cells in nasal polyps may be activated via an extrafollicular mechanism. We also found that EBI2 expression by B cells could be upregulated by co-culture of B cells with type 2 innate lymphoid cells (ILC2s). Since ILC2 cells are highly elevated in nasal polyp tissue25, 26, this observation provides a possible alternate novel pathway for local B cell activation in polyp tissue.22 Another recent report found that B cells expressing CD180, an unconventional member of the toll-like receptor family that plays an important role in B cell activation and proliferation, are also elevated in nasal polyps compared to control sinus tissue.27 This suggests that some B cells in nasal polyps may be activated through exposure to PAMPS, such as LPS, that may be abundant in the nasal polyp microenvironment. While most studies of B cells in CRS have focused on CRSwNP, two recent studies have characterized a unique subset of IgD+CD19+CD38+ plasmablasts that are elevated in both CRSwNP and CRSsNP, indicating that B cells may also be important for the pathogenesis of CRSsNP.28, 29 In related studies, IL-21 and T follicular helper-like cells, which are known to secrete IL-21, were found to be elevated in CRSwNP tissue.30, 31 T follicular helper cells are well established to play a key role in the activation of B cells in GCs. Taken together, these studies suggest that activation of mucosal B cells in nasal polyps likely occurs via multiple mechanisms, including canonical GC-like reactions and less regulated extrafollicular responses (Figure 1).
B Cell Function in CRS
In addition to expanding understanding of the phenotypes of activated B cells present in CRS tissues, it is also important to determine the role these cells may play in disease pathogenesis. A recent study has evaluated the antibody secretion potential of B cells from CRSwNP tissue using ELISPOT and ex vivo cultures and found that nasal polyp-derived B cells more frequently and abundantly secrete IgG, IgA, and IgE compared to tonsil B cells.22 These findings are supported by evidence of accumulation of antibodies of every isotype, except IgD, in nasal polyp tissue.32–34 Although the highest total levels of antibodies are usually found in CRSwNP, tissue IgD levels were highest in a subpopulation of CRSsNP patients.35 There is also accumulating evidence that B cells are activated locally within nasal polyps to secrete antibodies (Figure 1). Elevated expression levels of germline transcripts for IgG, IgA and IgE have been reported in nasal polyp tissues.22, 32 Germline transcripts are expressed very briefly during class switch recombination and serve as markers of cells actively undergoing this process.36 In addition, expression of activation-induced cytidine deaminase (AID) and the recombination activating genes (RAG) proteins, both of which are required for generation of antibody diversity and class switch recombination, are elevated in nasal polyps.22, 32 While the antigen specificity of the antibodies in nasal polyps remains largely unknown, there is evidence that some of the antibodies are autoreactive37, 38 (see below), and some of them, especially among the IgE antibodies, are specific for enterotoxins from Staphylococcus aureus.39, 40 Interestingly, the presence of either local or systemic IgE to S. aureus and its enterotoxins may serve as a biomarker for more severe disease.9, 41 Moreover, nasal polyp-localized polyclonal IgE appears to be functional, as it induces histamine release from tissue extracts exposed to antigens.42 Another potential mechanism for local activation of B cells in CRSwNP is the overexpression of B cell activating factor of the TNF family (BAFF), which plays a critical role in B cell activation and differentiation to plasma cells.1, 43 Likewise, the type 2 cytokines IL-5 and IL-13 are overexpressed in nasal polyp tissue,44 and each are capable of activating B cells or promoting class switching.45, 46 Overall, it is clear that activated B cells accumulate in the sinus tissues of patients with CRS, and many of these B cells produce large amounts of antibodies.
B Cell Immunodeficiencies: What Happens When Normal B Cell Responses Are Lost?
Antibody Defects Associated with Sinus and/or Airway Symptoms
Overproduction of antibodies can lead to inflammation and disease through the activation of complement and/or innate effector immune cells that express Fc receptors. However, decreased antibody production can also lead to disease due to inadequate protective humoral immune responses against microbes (Figure 1 - left side). Interestingly, antibody deficiencies are the most common immunodeficiencies in patients with rhinosinusitis. Diseases associated with antibody deficiencies have very heterogeneous clinical presentations and their exact pathogenesis is not known. The three best-described antibody immunodeficiencies are selective IgA (sIgA) deficiency, specific antibody deficiency (SAD), and common variable immunodeficiency (CVID). Antibody deficiencies are categorized as mild or severe based on their pathogenesis and levels of antibody production and function. CVID is the most common symptomatic immunodeficiency in adults and is characterized by low systemic levels of IgG and IgA and/or IgM antibodies. IgG levels in these patients are typically less than two standard deviations below the mean, adjusted for age. Antibody function in patients with CVID is also impaired, as supported by a poor response to both polysaccharide and protein based vaccines47. Selective IgA deficiency is characterized by serum IgA levels less than 7mg/dL, with normal levels of both IgG and IgM antibodies. Antibody responses to polysaccharide vaccines may or may not be normal in patients with sIgA deficiency.48 Specific antibody deficiency is characterized by normal or low-normal levels of quantitative immunoglobulins but a poor response to polysaccharide antigens49. In general, disorders with a significant decrease in the quantity of IgG antibodies, like CVID, tend to cause more severe immunodeficiency, while IgA deficiency and specific antibody deficiencies tend to be milder.
Individuals with symptomatic antibody deficiencies often present with sinopulmonary infections, and thus it is important for healthcare professionals who treat patients with rhinosinusitis to be aware of these immunodeficiencies. Among patients with difficult to treat CRS, a meta-analysis estimated that the prevalence of pooled IgG, IgA, and IgM deficiency was 23%, while 8–34% of patients had specific antibody deficiency50. More recently, in a well characterized cohort of patients with CRS who were screened for an immunodeficiency, 5% were found to have CVID, while 24% were noted to have a specific antibody deficiency, which is much higher than the estimated rate of 1 in 100,000 to 1 in 10,000 in the general population47, 51, 52. Interestingly, patients with CRSsNP were more likely to have CVID and specific antibody deficiency compared to patients with CRSwNP. We can speculate that this may explain some of the differences seen in activated B cells and elevated antibody levels in CRSwNP compared to CRSsNP tissues.
Mechanisms of B Cell Immunodeficiencies
B cell dysfunction underlies the pathogenesis of antibody deficiencies, although defects in other innate and adaptive immune cells, such as T cells and NK cells, have also been described53. Characteristic B cell defects in patients with antibody deficiencies involve reduced B cell survival, defective isotype switching and/or decreased numbers of switched memory B cells54,55. More rarely, peripheral B cells numbers may be low56. Defects in the polymeric immunoglobulin receptor, which transports IgA and IgM across epithelium, can also lead to insufficient levels of these protective antibodies on the mucosal surface.57
Genetic defects have been linked to about 10% of CVID and IgA deficiency cases. Mutations in the inducible costimulator (ICOS) gene were among the first defects described in patients with CVID58. ICOS is important in B cell activation and generation of memory B cells and plasma cells. Accordingly, mutations in ICOS result in decreased production of antibodies and reduced numbers of memory and switched memory B cells59.
Additional defects reported in patients with CVID include mutations of the proteins in the B cell co-receptor complex (CD21−CD19−CD81)60, 61,62. CD19 functions with CD21 and CD81 to enhance signaling from the B cell receptor. Defects in these receptors are associated with hypogammaglobulinemia, decreased memory B cells, and impaired specific antibody responses60,63. Further B cell dysfunction in patients with CVID results from mutations in the receptors for BAFF, BAFF receptor (BAFF-R) and TACI (transmembrane activator and calcium modulator and cyclophilin ligand interactor)64–68. BAFF, and the related cytokine APRIL (a proliferation-inducing ligand), are important for B cell survival, proliferation, and T cell-independent class switch recombination69,70. Defects in these receptors lead to hypogammaglobulinemia and associated clinical manifestations of antibody deficiencies. TACI mutations have also been reported in sIgA deficiency65, 66. These mutations in sIgA deficiency eventually lead to defects in the differentiation of IgA+ B cells to IgA-secreting plasma cells, which results in reduced IgA production71.
B cell maturation to antibody secreting plasma cells, antibody isotype switching, and development of switched memory B cells are dependent on downstream activation of the canonical and non-canonical NF-kB pathways through the B cell receptor complex, BAFF-R, and TACI. Mutations in the NF-kB2 (non-canonical) and NF-kB1 (canonical) pathways have been reported in patients with CVID. NF-kB1 mutations have also been described in patients with IgA deficiency72. Interestingly, BAFF-R, TACI, and NF-kB mutations are noted in relatives of CVID patients who don’t have the abnormal clinical features typical of antibody deficiencies68 73. It has been speculated that the disease manifestations may appear later in life in these individuals, or that there may be incomplete penetrance of these mutations.
Similar to CVID and sIgA deficiency, specific antibody deficiency has been associated with a low percentage of CD19+CD27+IgDneg switched memory B cells compared to controls74, 75. Alachkar et al. have also reported that the reduced frequency of switched memory B cells was associated with a significantly higher prevalence of bronchiectasis and autoimmunity in patients with humoral immunodeficiency, including CVID and specific antibody deficiency.74
Overall, antibody deficiency should be considered in patients presenting with persistent CRS. Underlying genetic mutations have been identified in about 10% of patients with primary antibody immunodeficiencies, as mentioned above. In general, many of the known genetic mutations in primary antibody deficiencies affect B cell survival, isotype switching, B cell signaling or transport of immunoglobulins to the mucosal surface (e.g. the polymeric immunoglobulin receptor (PIgR)). Although available evidence suggests that increased frequency and intensity of sinonasal infections results from these defects in B cell responses, how these antibody deficiencies are pathogenic remains unclear in the majority of patients with CRS, and further studies are necessary to elucidate the underlying mechanisms responsible for this common, and often disabling disease.
Autoimmunity: What Happens When B Cell Responses Are Directed Towards Self?
B cell manifestations of airway disease
In mice, mucosal B cell responses vary depending on whether the B cells are derived from B1 B cells that undergo maturation outside the bone marrow, or B2 B cells that undergo maturation within the bone marrow.76 B1 B cells are found at high density at mucosal sites, such as the pleural and peritoneal cavities, and can be directly activated without T cells. B2 B cells are concentrated in germinal centers of lymph nodes but require T cell help for activation. B1 B cells innately express immunoglobulin sequences that bind microbial motifs but are also autoreactive and polyreactive. B1 B cells are also long-lived and non-circulating. In contrast, due to their history of germinal center generation, the affinity of mature B2 B cell antibodies is higher and B2 cell derived antibodies are less likely to manifest autoreactivity due to clonal selection during development in the bone marrow. Although the question of whether humans also have distinct B1 and B2 lineage B cells remains controversial77, 78, the canonical characteristics of mucosal B cells in mice may have significant value in interpretation of the nature of B cells isolated from human sinonasal mucosa.
In healthy nasal mucosa, B cells and their derived plasma cells are relatively rare,34 but dysregulation and expansion of B cell responses in the nasal mucosa play a pathogenic role in allergic and autoimmune disorders of the upper airway. In allergic rhinitis, uptake, processing and presentation of allergens by dendritic cells to T cells results in Th2 priming and ultimately switching of B cells toward production of systemic and local aeroallergen-specific IgE. Mast cells and basophils primed with aeroallergen-specific IgE degranulate when they encounter their cognate aeroallergen and mediate classic allergic symptoms of rhinorrhea and congestion.79 In contrast, when hypersensitivity becomes targeted against self-antigens, B cells can mediate highly destructive inflammation in the upper airway. For example, anti-neutrophil cytoplasmic antibodies (ANCA) are the primary cause of a spectrum of autoimmune necrotizing vasculitic diseases, including granulomatous polyangitis (GPA- formerly known as Wegener’s granulomatosis) and eosinophilic GPA (formerly known as Churg-Strauss syndrome), that have prominent sinonasal and pulmonary manifestations80–82. While less commonly recognized, other systemic autoimmune conditions, particularly rheumatoid arthritis, can be associated with the development of interstitial lung disease and increased pulmonary ectopic lymphoid tissue where high-affinity autoreactive B cell clones are generated.80, 83 Another airway entity in which B cells play a prominent pathogenic role is "IgG4-related disease" characterized by a lymphoplasmacytic infiltrate enriched in IgG4+ plasma cells as well as CD20+ B cells and T cells that affect multiple organ sites.84 While salivary gland involvement is the most common upper airway manifestation, IgG4 related disease can cause symptoms of CRS and these cells can directly infiltrate sinonasal mucosa, causing fibrolytic lesions in the sinuses.85
Evidence for activation of auto-reactive B cells in CRS
While the overall specificity of the antibodies found in CRS has not been systematically characterized, elevated mucosal levels of autoreactive IgG and IgA to nuclear antigens, including double stranded DNA (dsDNA), and basement membrane components have been reported.37, 38, 86 Interestingly, the elevated levels of anti-dsDNA IgG were further correlated with local IgE and eosinophilic cationic protein (ECP) levels, and associated with more severe phenotypes of CRSwNP, such as those with aspirin exacerbated respiratory disease or recurrent nasal polyposis. To delineate the potential role of complement activation in pathogenicity of the local antibody response, a subsequent study evaluated levels of activated neo-epitopes (C1rs-inh, C4d and C5b-9) of the classical, or antibody-mediated, complement pathways and found evidence for strong activation of complement in nasal polyp tissue compared to control tissue.87 Importantly, the complement activation neoepitope C4d is known to bind at the site of activation, and the complement activation appeared to be highly localized to the basement membrane of the nasal polyp epithelium (Figure 1). Whether this localization is due to activation of complement at the basement membrane by specific anti-basement membrane autoantibodies or specific antibodies against a pathogen localized at the same site remains to be established, but the presence of autoantibodies against basement membrane components would favor the former.
Clinical implications of B cell pathogenesis in airway disease
As illustrated in this review, B cell responses in the upper airway require careful calibration to balance between insufficient responses, which permit recurrent and/or chronic infections (Figure 1 – left side), and unregulated responses, which promote chronic inflammation and/or autoimmunity (Figure 1 – right side). From a clinical perspective, these B cell responses have immediate diagnostic and therapeutic utility, as demonstrated by the clinically beneficial effects of intravenous gamma globulin and rituximab on sinusitis symptoms seen in CVID and GPA, respectively.82, 88 Targeting B cell inflammation in the more common varieties of CRS, where disease is confined to the upper airways without systemic manifestations, is a focus of current research efforts to identify B cell- and/or antibody-based biomarkers of disease severity and validating the utility of such markers for identifying patients with poor treatment outcomes. Furthermore, the increasing availability of safe targeted B cell therapies may open new avenues for treating patients with B cell-associated inflammation of the paranasal sinuses, although careful selection of the appropriate medication and clinical trials will be needed to demonstrate their efficacy in patients with CRS.
Summary/Conclusions
B cells and their antibody products play undisputed roles in health and disease within the mucosae of the nose and sinuses. Deficient or inadequate B cell responses can lead to susceptibility to infectious disease in the nose. Thus, patients with defects in B cell activation, expansion, survival, antibody secretion etc. often have either chronic or recurrent acute infections, and this can be associated with non-polypoid CRS. In contrast, many patients with CRSwNP, which is less likely to be driven by pathogens, have excess expression of local immunoglobulins, including autoreactive antibodies. These B cell responses activate complement in many patients and likely contribute to immuno-pathogenic responses in the sinonasal mucosae. Finally, CRSwNP in general, whether associated with autoantibodies or not, is characterized by frank expansion of B cells and high levels of local production of IgE and other isotypes of antibodies that can participate in type 2 inflammation. Understanding the molecular and cellular mechanisms of CRS that may be mediated by either inadequate B cell responses or inappropriate autoimmune B cell responses should be a high priority in the quest to understand the pathogenesis of the various forms of CRS.
What do we know?
Activated B cells and antibodies of most isotypes are locally elevated in CRS tissues.
B cells are activated locally to produce antibodies in CRSwNP.
Antibodies specific for self-proteins and pathogens are elevated in CRSwNP tissue.
Decreased antibody production is associated with recurrent acute rhinosinusitis or recalcitrant CRS.
What is still unknown?
What roles do B cells play in the pathogenesis of CRS?
What is the specificity of the local antibody repertoire?
What are the mechanisms that drive the local activation of B cells and antibody production in CRS?
Does antibody-mediated activation of complement play a role in CRS pathogenesis?
Are B cell-targeted therapies effective for treating CRS?
Acknowledgments
The authors would like to thank Ms. Jacqueline Schaffer for her outstanding artwork in Figure 1.
Funding: NIH Grants: R56 AI121239, U19 AI106683, R01 AI072570, and the Ernest S. Bazley Trust
Abbreviations
- AID
Activation induced cytidine deaminase
- ANCA
Anti-neutrophil cytoplasmic antibodies
- APRIL
A proliferation-inducing ligand
- BAFF
B cell activating factor of the TNF family
- BAFFR
BAFF receptor
- BALT
Bronchus-associated lymphoid tissue
- BCR
B cell receptor
- CRS
Chronic rhinosinusitis
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- CVID
Common variable immunodeficiency
- EBI2
Epstien Barr virus-induced protein 2
- ECP
Eosinophil cationic protein
- GC
Germinal center
- iBALT
Inducible bronchus-associated lymphoid tissue
- ICOS
Inducible costimulator
- ILC2
Group 2 innate lymphoid cells
- MALT
Mucosal-associated lymphoid tissue
- NALT
Nasal-associated lymphoid tissue
- PNAd
Peripheral lymph node addressin
- PIgR
Polymeric immunoglobulin receptor
- RAG
Recombination activating genes
- SAD
Specific antibody deficiency
- SLO
Secondary lymphoid organ
- TACI
Transmembrane activator and calcium modulator and cyclophilin ligand interactor
- TLO
Tertiary lymphoid organ
Glossary Word
- Anti-neutrophil cytoplasmic antibodies
Auto-antibodies specific for different enzymes contained within primary granules of neutrophils and macrophages. C-ANCA and p-ANCA descriptions of antibodies refers to the pattern seen on immunofluorescences studies (cytoplasmic and perinuclear respectively). The C-ANCA pattern is usually caused by antibodies to serine proteinase 3 (PR-3) and is found in granulomatosis with polyangiitis (GPA). The P-ANCA pattern is typically caused by antibodies to myeloperoxidase (MPO).
- CD180
Also known as RP105. A cell surface molecule that associated with MD-1 to form a complex that functions as a toll-like receptor that recognizes lipopolysaccharide (LPS) from gram negative bacteria.
- Follicular dendritic cells
Cells that reside primarily in the light zone of germinal centers and display antigen. Together with follicular helper T cells, they help promote the survival of B cells with the highest affinity receptors.
- Germinal center reaction
Please see reference 11 for details. Briefly, lymph nodes have T cell and B cell-rich zones. B cell zones form lymphoid follicles in the cortex. Primary follicles do not have germinal centers. Secondary follicles contain germinal centers. Activated helper T cells migrate to the edge of a primary follicle to meet and activate B cells. B cells then migrate into the follicle and proliferate. Isotype switching and affinity maturation occur in the germinal center. B cells then differentiate into plasma cells and memory B cells.
- IL-21
The signature cytokine of follicular helper T cells. IL-21 is required for germinal center development. IL-21 also induces B cell apoptosis that is only averted if the B cell is rescued by high affinity receptors for antigen.
- Incomplete penetrance
In complete penetrance, everyone with a pathogenic genotype expresses the disease. In incomplete penetrance, only some will express the disease, indicating other genetic or environmental factors must be present. Penetrance represents the probability of disease when a pathogenic genotype is present. One of the more common clinical examples of penetrance variability is in women with breast cancer susceptibility mutations of the BRCA genes: mutations along with other factors, such as family history, ethnicity, etc determine probability of disease.
- Recombination activating genes (RAG)
Lymphoid specific proteins important in lymphocyte antigen receptor gene rearrangement. Rag-1 and Rag-2 create double stranded DNA breaks in immunoglobulin and TCR genes to allow for V(D)J recombination, a process that randomly generates V(D)J exons that will code for the variable regions of antigen receptor proteins. V(D)J recombination allows for the development of a diverse array of antigen receptors on B and T cells.
- Secondary Lymphoid Organs (SLOs)
Lymphoid tissue where lymphocyte responses to foreign antigens are triggered and enhanced. SLOs include lymph nodes, the spleen, the cutaneous immune system, and the mucosal immune system. Lymph nodes promote the initiation of adaptive immune responses to antigens. Red pulp areas of the spleen contain macrophages that assist with removing aging and/or damaged blood cells, immune complexes, and opsonized microbes. The white pulp is contains high numbers of lymphocytes and promotes adaptive responses to blood borne antigens.
- Switched memory B cells
Memory B cells are derived from germinal center reactions. They are capable of prolonged survival due to expression of the antiapoptotic protein Bcl-2. CD40 on B cells binding to CD40L on T cells is critical for memory B cell formation. Mutations in CD40 or CD40L results in hyper-IgM syndrome. Memory B cells are identified by surface markers CD19 and CD27. Switched memory B cells refers to B cells that have undergone antibody class switching and are often designated as CD19+CD27+IgD−IgM−.
- Type 2 Innate Lymphoid Cells (ILC2s)
Many articles, as well as a recent review in JACI, have explained how innate lymphoid cells (ILCs) are emerging as key contributors to the pathogenesis of inflammatory disease. Group 2 ILCs produce type 2 cytokines, such as IL-4, IL-5, IL-9, and IL-13, upon stimulation with epithelium-derived cytokines, such as IL-33, IL-25, and TSLP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–92. 92 e1–2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandtzaeg P, Farstad IN, Johansen FE, Morton HC, Norderhaug IN, Yamanaka T. The B-cell system of human mucosae and exocrine glands. Immunol Rev. 1999;171:45–87. doi: 10.1111/j.1600-065X.1999.tb01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–93. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–41. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 5.Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol. 2017;12:331–57. doi: 10.1146/annurev-pathol-052016-100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70:995–1003. doi: 10.1111/all.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan BK, Klingler AI, Poposki JA, Stevens WW, Peters AT, Suh LA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2017;139:699–703 e7. doi: 10.1016/j.jaci.2016.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derycke L, Eyerich S, Van Crombruggen K, Perez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014;9:e97581. doi: 10.1371/journal.pone.0097581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–56 e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 10.Kato A, Hulse KE, Tan BK, Schleimer RP. B-lymphocyte lineage cells and the respiratory system. J Allergy Clin Immunol. 2013;131:933–57. doi: 10.1016/j.jaci.2013.02.023. quiz 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–48. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foo SY, Phipps S. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol. 2010;3:537–44. doi: 10.1038/mi.2010.52. [DOI] [PubMed] [Google Scholar]
- 13.Waffarn EE, Baumgarth N. Protective B cell responses to flu--no fluke! J Immunol. 2011;186:3823–9. doi: 10.4049/jimmunol.1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germain C, Gnjatic S, Dieu-Nosjean MC. Tertiary Lymphoid Structure-Associated B Cells are Key Players in Anti-Tumor Immunity. Front Immunol. 2015;6:67. doi: 10.3389/fimmu.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 16.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. doi: 10.1016/j.smim.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoop AE, van der Heijden HA, Biewenga J, van der Baan S. Lymphocytes and nonlymphoid cells in human nasal polyps. J Allergy Clin Immunol. 1991;87:470–5. doi: 10.1016/0091-6749(91)90004-8. [DOI] [PubMed] [Google Scholar]
- 18.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 19.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gevaert P, Holtappels G, Johansson SGO, Cuvelier C, van Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–9. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 21.Patadia M, Dixon J, Conley D, Chandra R, Peters A, Suh LA, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2009 doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman S, Kasjanski R, Poposki J, Hernandez D, Chen JN, Norton JE, et al. Chronic airway inflammation provides a unique environment for B cell activation and antibody production. Clin Exp Allergy. 2017;47:457–66. doi: 10.1111/cea.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau A, Lester S, Moraitis S, Ou J, Psaltis AJ, McColl S, et al. Tertiary lymphoid organs in recalcitrant chronic rhinosinusitis. J Allergy Clin Immunol. 2017;139:1371–3 e6. doi: 10.1016/j.jaci.2016.08.052. [DOI] [PubMed] [Google Scholar]
- 24.Gatto D, Brink R. B cell localization: regulation by EBI2 and its oxysterol ligand. Trends Immunol. 2013;34:336–41. doi: 10.1016/j.it.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Poposki JA, Klingler AI, Tan BK, Soroosh P, Banie H, Lewis G, et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis. 2017;5:233–43. doi: 10.1002/iid3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miljkovic D, Ou J, Kirana C, Hulse KE, Hauben E, Psaltis A, et al. Discordant frequencies of tissue-resident and circulating CD180-negative B cells in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017 doi: 10.1002/alr.21924. [DOI] [PubMed] [Google Scholar]
- 28.Min JY, Nayak JV, Hulse KE, Stevens WW, Raju PA, Huang JH, et al. Evidence for altered levels of IgD in the nasal airway mucosa of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokoya M, Ramakrishnan VR, Frank DN, Rahkola J, Getz A, Kingdom TT, et al. Expression of immunoglobulin D is increased in chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2017;119:317–23 e1. doi: 10.1016/j.anai.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YN, Song J, Wang H, Wang H, Zeng M, Zhai GT, et al. Nasal IL-4CXCR5CD4 T follicular helper cell counts correlate with local IgE production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Calus L, Derycke L, Dullaers M, Van Zele T, De Ruyck N, Perez-Novo C, et al. IL-21 Is Increased in Nasal Polyposis and after Stimulation with Staphylococcus aureus Enterotoxin B. Int Arch Allergy Immunol. 2017 doi: 10.1159/000481435. [DOI] [PubMed] [Google Scholar]
- 32.Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68:55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- 33.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840–7. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 34.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–83. 83 e1–7. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min JYKR, Hulse KE, Chandra R, Conley D, Suh L, Carter R, Norton J, Huang JJ, Kato A, Schleimer RP, Tan BT. Evidence For Immunoglobulin D In Patients With Chronic Rhinosinusitis. J Allergy Clin Immunol. 2014;133:AB236. [Google Scholar]
- 36.Cameron L, Hamid Q, Wright E, Nakamura Y, Christodoulopoulos P, Muro S, et al. Local synthesis of epsilon germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure. J Allergy Clin Immunol. 2000;106:46–52. doi: 10.1067/mai.2000.107398. [DOI] [PubMed] [Google Scholar]
- 37.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206 e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeffe JS, Seshadri S, Hamill KJ, Huang JH, Carter R, Suh L, et al. A Role for Anti-BP180 Autoantibodies in Chronic Rhinosinusitis. Laryngoscope. 2013;123:2104–11. doi: 10.1002/lary.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126:962–8. 8 e1–6. doi: 10.1016/j.jaci.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Chen JB, James LK, Davies AM, Wu YB, Rimmer J, Lund VJ, et al. Antibodies and superantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2017;139:1195–204 e11. doi: 10.1016/j.jaci.2016.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Schryver E, Devuyst L, Derycke L, Dullaers M, Van Zele T, Bachert C, et al. Local immunoglobulin e in the nasal mucosa: clinical implications. Allergy Asthma Immunol Res. 2015;7:321–31. doi: 10.4168/aair.2015.7.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang N, Holtappels G, Gevaert P, Patou J, Dhaliwal B, Gould H, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011;66:141–8. doi: 10.1111/j.1398-9995.2010.02448.x. [DOI] [PubMed] [Google Scholar]
- 43.Chan TD, Gardam S, Gatto D, Turner VM, Silke J, Brink R. In vivo control of B-cell survival and antigen-specific B-cell responses. Immunol Rev. 2010;237:90–103. doi: 10.1111/j.1600-065X.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- 44.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am J Respir Crit Care Med. 2015;192:682–94. doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon BG, Takaki S, Miyake K, Takatsu K. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol. 2004;172:6020–9. doi: 10.4049/jimmunol.172.10.6020. [DOI] [PubMed] [Google Scholar]
- 46.Cocks BG, de Waal Malefyt R, Galizzi JP, de Vries JE, Aversa G. IL-13 induces proliferation and differentiation of human B cells activated by the CD40 ligand. Int Immunol. 1993;5:657–63. doi: 10.1093/intimm/5.6.657. [DOI] [PubMed] [Google Scholar]
- 47.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4:38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J Clin Immunol. 2001;21:303–9. doi: 10.1023/a:1012241117984. [DOI] [PubMed] [Google Scholar]
- 49.Orange JS, Ballow M, Stiehm ER, Ballas ZK, Chinen J, De La Morena M, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2012;130:S1–24. doi: 10.1016/j.jaci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Schwitzguebel AJ, Jandus P, Lacroix JS, Seebach JD, Harr T. Immunoglobulin deficiency in patients with chronic rhinosinusitis: Systematic review of the literature and meta-analysis. J Allergy Clin Immunol. 2015;136:1523–31. doi: 10.1016/j.jaci.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 51.Kashani S, Carr TF, Grammer LC, Schleimer RP, Hulse KE, Kato A, et al. Clinical characteristics of adults with chronic rhinosinusitis and specific antibody deficiency. J Allergy Clin Immunol Pract. 2015;3:236–42. doi: 10.1016/j.jaip.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keswani A, Dunn NM, Manzur A, Kashani S, Bossuyt X, Grammer LC, et al. The Clinical Significance of Specific Antibody Deficiency (SAD) Severity in Chronic Rhinosinusitis (CRS) J Allergy Clin Immunol Pract. 2017;5:1105–11. doi: 10.1016/j.jaip.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giovannetti A, Pierdominici M, Mazzetta F, Marziali M, Renzi C, Mileo AM, et al. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–43. doi: 10.4049/jimmunol.178.6.3932. [DOI] [PubMed] [Google Scholar]
- 54.Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(−)IgD(−)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–51. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 55.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 56.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 57.Polosukhin VV, Cates JM, Lawson WE, Zaynagetdinov R, Milstone AP, Massion PP, et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:317–27. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–8. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 59.Warnatz K, Bossaller L, Salzer U, Skrabl-Baumgartner A, Schwinger W, van der Burg M, et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 2006;107:3045–52. doi: 10.1182/blood-2005-07-2955. [DOI] [PubMed] [Google Scholar]
- 60.van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354:1901–12. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 61.Kanegane H, Agematsu K, Futatani T, Sira MM, Suga K, Sekiguchi T, et al. Novel mutations in a Japanese patient with CD19 deficiency. Genes Immun. 2007;8:663–70. doi: 10.1038/sj.gene.6364431. [DOI] [PubMed] [Google Scholar]
- 62.van Zelm MC, Smet J, Adams B, Mascart F, Schandene L, Janssen F, et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest. 2010;120:1265–74. doi: 10.1172/JCI39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thiel J, Kimmig L, Salzer U, Grudzien M, Lebrecht D, Hagena T, et al. Genetic CD21 deficiency is associated with hypogammaglobulinemia. J Allergy Clin Immunol. 2012;129:801–10 e6. doi: 10.1016/j.jaci.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 64.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 65.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–34. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 66.Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci U S A. 2009;106:13945–50. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pieper K, Rizzi M, Speletas M, Smulski CR, Sic H, Kraus H, et al. A common single nucleotide polymorphism impairs B-cell activating factor receptor's multimerization, contributing to common variable immunodeficiency. J Allergy Clin Immunol. 2014;133:1222–5. doi: 10.1016/j.jaci.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 68.Losi CG, Silini A, Fiorini C, Soresina A, Meini A, Ferrari S, et al. Mutational analysis of human BAFF receptor TNFRSF13C (BAFF-R) in patients with common variable immunodeficiency. J Clin Immunol. 2005;25:496–502. doi: 10.1007/s10875-005-5637-2. [DOI] [PubMed] [Google Scholar]
- 69.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–52. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 71.Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–6. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schipp C, Nabhani S, Bienemann K, Simanovsky N, Kfir-Erenfeld S, Assayag-Asherie N, et al. Specific antibody deficiency and autoinflammatory disease extend the clinical and immunological spectrum of heterozygous NFKB1 loss-of-function mutations in humans. Haematologica. 2016;101:e392–e6. doi: 10.3324/haematol.2016.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fliegauf M, Bryant VL, Frede N, Slade C, Woon ST, Lehnert K, et al. Haploinsufficiency of the NF-kappaB1 Subunit p50 in Common Variable Immunodeficiency. Am J Hum Genet. 2015;97:389–403. doi: 10.1016/j.ajhg.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alachkar H, Taubenheim N, Haeney MR, Durandy A, Arkwright PD. Memory switched B cell percentage and not serum immunoglobulin concentration is associated with clinical complications in children and adults with specific antibody deficiency and common variable immunodeficiency. Clin Immunol. 2006;120:310–8. doi: 10.1016/j.clim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Aghamohammadi A, Abolhassani H, Biglari M, Abolmaali S, Moazzami K, Tabatabaeiyan M, et al. Analysis of switched memory B cells in patients with IgA deficiency. Int Arch Allergy Immunol. 2011;156:462–8. doi: 10.1159/000323903. [DOI] [PubMed] [Google Scholar]
- 76.Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol. 2015;15:149–59. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- 77.Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H. Human B-1 cells take the stage. Annals of the New York Academy of Sciences. 2013;1285:97–114. doi: 10.1111/nyas.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tangye SG. To B1 or not to B1: that really is still the question! Blood. 2013;121:5109–10. doi: 10.1182/blood-2013-05-500074. [DOI] [PubMed] [Google Scholar]
- 79.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 80.Crestani B. The respiratory system in connective tissue disorders. Allergy. 2005;60:715–34. doi: 10.1111/j.1398-9995.2005.00761.x. [DOI] [PubMed] [Google Scholar]
- 81.Drolet JP, Frangie H, Guay J, Hajoui O, Hamid Q, Mazer BD. B lymphocytes in inflammatory airway diseases. Clin Exp Allergy. 2010;40:841–9. doi: 10.1111/j.1365-2222.2010.03512.x. [DOI] [PubMed] [Google Scholar]
- 82.Jennette JC, Falk RJ. B cell-mediated pathogenesis of ANCA-mediated vasculitis. Semin Immunopathol. 2014;36:327–38. doi: 10.1007/s00281-014-0431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116:3183–94. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74:1171–7. doi: 10.1136/annrheumdis-2014-206605. [DOI] [PubMed] [Google Scholar]
- 85.Moteki H, Yasuo M, Hamano H, Uehara T, Usami S. IgG4-related chronic rhinosinusitis: a new clinical entity of nasal disease. Acta Otolaryngol. 2011;131:518–26. doi: 10.3109/00016489.2010.533699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Schryver E, Calus L, Bonte H, Natalie de R, Gould H, Donovan E, et al. The quest for autoreactive antibodies in nasal polyps. J Allergy Clin Immunol. 2016;138:893–5 e5. doi: 10.1016/j.jaci.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Roey GA, Vanison CC, Wu J, Huang JH, Suh LA, Carter RG, et al. Classical complement pathway activation in the nasal tissue of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walsh JE, Gurrola JG, Graham SM, Mott SL, Ballas ZK. Immunoglobulin replacement therapy reduces chronic rhinosinusitis in patients with antibody deficiency. International Forum of Allergy & Rhinology. 2017;7:30–6. doi: 10.1002/alr.21839. [DOI] [PubMed] [Google Scholar]