Abstract
Purines are metabolic building blocks essential for all living organisms on earth. De novo purine biosynthesis occurs in the brain and appears to play important roles in neural development. Phosphoribosyl formylglycinamidine synthase (FGAMS, also known as PFAS or FGARAT), a core enzyme involved in the de novo synthesis of purines, may play alternative roles in viral pathogenesis. To date, no thorough investigation of the endogenous expression and localization of de novo purine biosynthetic enzymes has been conducted in human neurons or in virally infected cells. In this study, we characterized expression of FGAMS using multiple neuronal models. In differentiated human SH-SY5Y neuroblastoma cells, primary rat hippocampal neurons, and in whole mouse brain sections, FGAMS immunoreactivity was distributed within the neuronal cytoplasm. FGAMS immunolabeling in vitro demonstrated extensive distribution throughout neuronal processes. To investigate potential changes in FGAMS expression and localization following viral infection, we infected cells with the human pathogen herpes simplex virus 1 (HSV-1). In infected fibroblasts, FGAMS immunolabeling shifted from a diffuse cytoplasmic location to a mainly perinuclear localization by 12 hours post infection. In contrast, in infected neurons, FGAMS localization showed no discernable changes in the localization of FGAMS immunoreactivity. There were no changes in total FGAMS protein levels in either cell type. Together, these data provide insight into potential purine biosynthetic mechanisms utilized within neurons during homeostasis as well as viral infection.
Keywords: neuron, de novo purine biosynthesis, purinosome, herpes simplex virus, FGAMS, PFAS, FGARAT
Graphical abstract
We examined expression and localization of the de novo purine biosynthetic enzyme, phophoribosyl formylglycinamidine (FGAMS), in multiple species and neuronal models. We found that endogenous FGAMS is localized throughout neuronal processes and near mitochondria, and is often found in proximity to synapses. These data provide insight into the mechanisms of purine metabolism in neurons.
Introduction
Efficient synthesis of purines is an integral process for the survival of all living organisms and infectious pathogens. Purines are functionally pleiotropic building blocks that are necessary for nucleic acid synthesis, the formation of ATP, and multiple metabolic pathways. Purines are also known to play important roles in cell-to-cell signaling (Abbracchio et al. 2009; An et al. 2008; Stasolla et al. 2003). Inosine monophosphate (IMP) is the first purine synthesized through the de novo pathway and serves as a precursor to the synthesis of the purine nucleosides adenosine monophosphate and guanosine monophosphate. De novo purine biosynthesis begins with 5-phospho-alpha-d-ribose 1-diphosphate (phosphoribosyl pyrophosphate, or PRPP) being converted to IMP via a 10 step process involving six enzymes (Smith et al. 1980). Alternatively, hypoxanthine can be converted to IMP via the salvage pathway (An et al. 2008). Using tumor cell lines transiently transfected with GFP-tagged de novo purine biosynthetic enzymes, recent studies have revealed the formation of distinct puncta following culture in purine-depleted media (An et al. 2010; An et al. 2008; French et al. 2016; Zhao et al. 2013b; Zhao et al. 2015). These data suggest that during periods of high purine demand, a multi-enzyme complex termed the “purinosome” forms, enabling the more efficient generation of purines (An et al. 2008). In further support of a functional role for purinosome formation, fibroblasts derived from patients with mutations in different de novo purine biosynthetic enzymes display distinct changes in purinosome assembly or disassembly (Baresova et al. 2012; Fu et al. 2015).
Neurons are post-mitotic cells with high energy demand (Moreira et al. 2007), making them a unique cell type that requires efficient purine biosynthesis. Most neurons are highly polarized, with numerous projections that extend from the cell body to form connections with neighboring neurons and glial cells. This morphology requires that neurons have sufficient mitochondrial numbers in each cellular compartment to fulfill their high energy requirements (Amadoro et al. 2014). One of the most active compartments in neurons is the synapse, whose high energy demands are evidenced by the presence of many mitochondria and polyribosomes within synaptic compartments (Steward and Levy 1982; Steward and Ribak 1986; Steward 1983; Son et al. 2012). Williamson et al. recently demonstrated the expression of three purine biosynthetic enzymes near mitochondria in rat neurons (Williamson et al. 2017), but their data did not explore the localization of these enzymes relative to synapses.
The rapid generation of purines is not only necessary to maintain intracellular energy stores, but is also required for use in neurotransmission. Adenosine triphosphate (ATP) stored in synaptic vesicles can be released along with other neurotransmitters or serve as a singular neurotransmitter in both the peripheral and central nervous systems (PNS and CNS respectively) (Pankratov et al. 2006; Pankratov et al. 2007) (for review see (Abbracchio et al. 2009)). Purinergic receptors are expressed on both neurons and glia in the PNS and CNS, suggesting that ATP may serve important roles in neuron-neuron, neuron-glia, and glia-glia signaling (Abbracchio et al. 2009; Burnstock 1972; Burnstock 1997; Burnstock 1990; Fields and Burnstock 2006). For example, adenosine has been shown to regulate metabolic processes and also neuronal inhibition and facilitation, depending on the balance of adenosine receptor expression (A1/A2) (Cunha 2001). Dysregulation of purinergic signaling or receptor levels have been linked to the pathophysiology of significant neurological diseases including epilepsy (Boison 2016; Vianna et al. 2002) and Alzheimer’s disease (Parvathenani et al. 2003).
In addition to its active roles in neurotransmission, evidence also suggests that purine nucleotides serve as “danger signals” in the nervous system (Abbracchio et al. 2009). Enhanced ATP release by astrocytes at sites of injury has been shown to trigger local microglial activation and migration to protect healthy tissue (Davalos et al. 2005). In the PNS, sympathetic nerve-derived ATP signaling plays a large role in the regulation of vascular tone (Erlinge and Burnstock 2008), and dysregulation of ATP signaling in sympathetic nerves is thought to underlie the development of cardiovascular disease (Abbracchio et al. 2009; Erlinge and Burnstock 2008). Given the diverse purine requirements of neurons and the pathologies associated with aberrations in purinergic signaling, finely tuned regulation of purine biosynthesis is imperative in the nervous system.
Although few studies have examined changes in purine levels during infection, the high metabolic load of viral infection would be expected to deplete cellular purine resources. Influenza A infection has been shown to induce purine biosynthetic enzyme protein expression in human bronchial airway epithelial cells (Kroeker et al. 2013), but no data is yet available on whether similar alterations occur in neurons. Among the viruses known to infect neurons, herpes simplex virus (HSV-1, also known as human herpesvirus 1, HHV-1) is a widespread pathogen (Looker et al. 2015) that has a known association with mitochondria (Ackermann and Kurtz 1952). HSV-1 infection begins at mucosal or epithelial surfaces, from which the virus traffics into neuronal processes and establishes a lifelong latent reservoir in neurons (Roizman et al. 2013). In non-neuronal cells in vitro, HSV-1 infection results in the destruction of host cell mitochondrial DNA (Saffran et al. 2007; Duguay et al. 2014). ATP concentrations have been shown to increase in cells infected with HSV-1 (Abrantes et al. 2012), and host-derived cellular aspartate fuels de novo pyrimidine, and potentially also purine, nucleotide biosynthesis during HSV-1 infection (Vastag et al. 2011; El-Bacha and Da Poian 2013). One of the core enzymes of the purinosome, phosphoribosyl formylglycinamidine synthase (FGAMS, also known as PFAS or FGARAT) (Deng et al. 2012), has been shown to have a functionally pleiotropic role in the pathogenesis of the distantly related gammaherpesvirus Kaposi’s sarcoma-associated herpesvirus (KSHV or HHV-8) (He et al. 2015; Zhao et al. 2016). These data support the hypothesis that de novo purine biosynthetic enzymes may play important roles in herpesvirus replication and pathogenesis.
FGAMS serves as a marker for the purinosome, and alterations in FGAMS localization have been shown to indicate changes in de novo purine biosynthesis. Formation of purinosomes via the clustering of individual de novo purine biosynthetic enzymes with FGAMS occurs under purine depleted conditions, increasing purine production (An et al. 2008; Zhao et al. 2015). Conversely, activation of 5′ adenosine monophosphate-activated protein kinase during periods of low purine demand causes FGAMS self-clustering, triggering the downregulation of de novo purine biosynthesis (Schmitt et al. 2016). A recent study demonstrated co-localization of FGAMS and two other de novo purine biosynthetic enzymes with mitochondria in primary rat neurons and brain tissue (Williamson et al. 2017). To extend these findings and examine the potential for de novo purine biosynthesis in a model of human neurons, we assessed the localization and expression of FGAMS in differentiated SH-SY5Y neuronal-like cells. We validated these findings in primary rat hippocampal neurons and whole mouse brain sections. To test the effects of viral infection on FGAMS, fibroblasts (MRC5 cells) and differentiated SH-SY5Y neurons were infected with HSV-1. Infection induced strong alterations in FGAMS immunoreactivity in fibroblasts without altering protein levels. However, in neuronal cells, total FGAMS protein levels and localization remained stable following infection. Together, these data demonstrate FGAMS localization in neuronal cytoplasm and projections, and co-localization with mitochondria. These findings were replicable across neuronal models, suggesting conserved localization of de novo purine biosynthesis. Additionally, the re-localization of FGAMS in fibroblasts but not in neurons during HSV-1 infection suggests that viral infection may induce disparate changes in de novo purine biosynthetic pathways in each cell type.
Methods
Animals
Analysis of FGAMS in the brain utilized 3 month old male C57BL/6 mice (average weight = 25.15 grams) purchased from the National Institute on Aging colony at Charles River Laboratories (Wilmington, MA, RRID: SCR_004633). Mice were housed in ventilated, high efficiency particulate air (HEPA)-filtered cages with access to sterile food and water ad libitum at the Pennsylvania State University College of Medicine Hershey Center for Applied Research. Experiments were conducted with approval by the Penn State University Institutional Animal Care and Use Committee. Animals used for the generation of primary neurons were euthanized via CO2 inhalation as per the Animal Care and Use Committee Guidelines for Euthanasia of Rodents. Cultures of hippocampal neurons were prepared from embryonic day 18 (E18) rat brains derived from timed pregnant Sprague-Dawley rats (Charles River Laboratories, RRID: RGD_737891) as described previously (Kaech and Banker 2006; Yao et al. 2015). All animal procedures were approved by the National Institute on Aging’s Animal Care and Use Committee and complied with the National Institutes of Health Guide for Care and Use of Laboratory Animals. No randomization was performed when choosing animals used for this study. Only animals with overt age-related pathology were excluded. The studies detailed below were not pre-registered.
Cell culture, SH-SY5Y neuronal differentiation, and generation of primary rat hippocampal cultures
Human fibroblasts (MRC5 cell line) were obtained from the American Type Culture Collection (ATCC, CCL-171, RRID:CVCL_0440) and maintained in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% (vol/vol) fetal bovine serum (FBS), 2 mM glutamine and 2mM penicillin/streptomycin. SH-SY5Y neuroblastoma cells were obtained from ATCC (CRL-2266, RRID:CVCL_0019) and maintained in EMEM supplemented with 15% (vol/vol) heat-inactivated FBS, 2mM glutamine and 2mM penicillin/streptomycin.
Differentiation of SH-SY5Y neuroblastoma cells was performed as described previously (Christensen et al. 2011; Encinas et al. 2000; Shipley et al. 2016; Shipley et al. 2017). Briefly, cells were grown in EMEM supplemented with 10μM retinoic acid (RA, Sigma) and decreasing concentrations of heat-inactivated FBS for 10 days. Cells were then transferred to MaxGel-coated coverslips (Sigma) or dishes and incubated with media containing Neurobasal (Life Technologies), 20mM KCl (Fisher Scientific), 50ng/mL brain-derived neurotrophic factor (Sigma), 2mM dibutyrl cyclicAMP (Sigma), 1X B-27 (ThermoFisher Scientific), 2mM Glutamax I (Life Technologies), 1X penicillin/streptomycin (Life Technologies), and 10μM RA, for an additional 7 days with media exchanges every 2-3 days.
Cultures of E18 hippocampal neurons were grown on polylysine (1 mg ml−1)-coated glass coverslips and incubated in Neurobasal medium supplemented with 1X B-27. The age of the cultures used for experiments was 2 (young) or 16 (mature) days.
Immunofluorescence of fixed cells
MRC5 cells were grown on glass coverslips while differentiated SH-SY5Y neurons were grown on MaxGel-coated glass coverslips and incubated at 37°C (5% CO2). Cells were then rinsed with 1X phosphate buffered saline (PBS), fixed with 4% paraformaldehyde (PFA) (Alfa Aesar) for 10 minutes at room temperature, washed twice with 1X PBS, and then permeabilized for 10 minutes with 0.1% Triton X-100 in 1X PBS. Cells were blocked with 10% donkey serum (Jackson ImmunoResearch) in 0.1% Triton X-100/PBS for 1 hour at room temperature. Following blocking, cells were incubated with primary antibody (see Table 1 for a complete list of antibodies) overnight at 4°C in a humid chamber. After four-5 minute washes with 0.1% Triton X-100/PBS, cells were incubated with fluorescently labeled species-specific F(ab′)2 fragment secondary antibody (Jackson ImmunoResearch) in a humid chamber for 1.5 hours, at room temperature in the dark. For viral infection experiments, cells were labeled with Hoescht nuclear stain (1:10,000; Thermo Fisher) diluted in 0.1% Triton X-100/PBS with secondary antibody and 10% donkey serum. Cells were washed, then mounted on glass slides (Thermo Fisher Scientific) using ProLong Gold antifade mounting media plus DAPI (Thermo Fisher Scientific) or, for infected cells, ProLong Gold antifade and allowed to dry overnight. For neurons dual-stained for mitochondria and FGAMS, cells were incubated with 500nM MitoTracker Red CMXRos (Thermo Fisher Scientific) in standard neuronal media for 1 hour, and then fixed and processed as described above. Images were acquired using an Olympus FV10i confocal microscope equipped with a 60× water immersion objective. Identical laser settings were applied for comparative pairs. Equivalent background subtraction was performed in ImageJ (RRID:SCR_003070) using the rolling ball method, while brightness and contrast was adjusted in Photoshop (RRID:SCR_014199). These settings were applied equally to all images within a comparison.
Table 1.
Primary antibodies
| Antibody | Protein Target | Manufacturer | RRID | Cat # | Host | Assay | Dilution |
|---|---|---|---|---|---|---|---|
| Actin | Beta actin | Sigma | AB_476743 | A5316 | Mouse | WB | 1:1000 |
| gE | HSV glycoprotein E | Generously provided by Dr. Harvey Friedman | N/A | N/A | Mouse | WB | 1:1000 |
| NeuN | Neuron-specific nuclear protein | Abcam | AB_10711040 | ab104224 | Mouse | IF | 1:500 |
| NSE | Neuron-specific enolase | Enzo Life Sciences | AB_2052014 | BML-NA1501-0100 | Mouse | IF | 1:500 |
| FGAMS/PFAS | Phosphoribosyl formylglycinamidine synthase | Bethyl Laboratories | AB_2620415 | A304-218A | Rabbit | IF, WB | 1:500/1:1000 (primary neurons) IF/1:1000 WB |
| PSD95 | Post-synaptic density 95 | ThermoFisher Scientific | AB_2092361 | MA1-046 | Mouse | IF | 1:500 |
| SMI-31 | Phosphorylated neurofilament heavy chain | Covance | AB_10122491 | SMI-31R-100 | Mouse | IF | 1:500 |
| SV2 | Synaptic vesicle protein 2 | Developmental Studies Hybridoma Bank | AB_2315385 | sv2 | Mouse | IF | 1:250 |
| SYP | Synaptophysin | Sigma | AB_477523 | S5768 | Mouse | IF | 1:1000 |
| Tuj1 | Tubulin β 3 | Covance | AB_2313773 | MMS-435P | Mouse | IF | 1:2000 |
Immunofluorescence labeling of primary rat hippocampal neurons was performed as described previously (Bushlin et al. 2008; Yao et al. 2015). In brief, neurons were fixed in 4% PFA and 4% sucrose for 15 minutes, permeabilized in 0.2% Triton X-100, and blocked in 10% bovine serum albumin. Neurons were then incubated with primary antibody overnight at 4°C (see Table 1). Following washes, neurons were incubated with a species-appropriate Alexa Fluor-tagged secondary antibody (Molecular Probes/ThermoFisher Scientific). Glass coverslips containing labeled neurons were mounted in ProlongGold antifade reagent. Images were acquired using a 63× objective on a Zeiss LSM 880 laser scanning confocal microscope with Airyscan.
Protein Isolation
To isolate soluble protein, cell monolayers were scraped into radio immunoprecipitation assay or RIPA buffer (Sigma Aldrich) with protease and phosphatase inhibitors (Thermo Scientific), and then sonicated using a Q500 ultrasonic processor (QSonica). Lysates were then rocked at 4°C for 15 minutes, and centrifuged at 12,500 × g for 15 minutes at 4°C to remove insoluble protein. Total soluble protein isolated in the supernatant was quantified using a bicinchoninic acid assay and a Nanodrop 2000c spectrophotometer (both from Thermo Scientific).
Western Blotting
All protein sample concentrations were diluted to 0.5 μg/μL using supplemented RIPA buffer and Laemmli buffer. A total of 10-15 μg of protein was separated via SDS-PAGE using 7.5% mini-gels (Miniprotean; Bio-Rad), transferred to a nitrocellulose membrane (Amersham GE Healthcare) using a Trans Blot SD semi-dry electrophoresis transfer cell (Bio-Rad), and then blocked using 5% non-fat dry milk prepared in Tris-Buffered saline (1 M, pH 7.4), 154 mM NaCl, and 0.2% Tween 20 (TBS-T) for one hour at room temperature with gentle rocking. Primary antibodies were diluted in respective blocking buffer and then incubated overnight at 4°C with rocking (see Table 1). Membranes were washed in TBS-T, and then incubated in species-appropriate secondary antibody diluted in blocking buffer for 2 hours at room temperature with rocking. Following washing, membranes were visualized using enhanced chemiluminescence substrate or SuperSignal™ West Dura Extended Duration Substrate (Thermo Scientific), imaged on film, and digitized at a resolution of 600 dpi. To ensure equal loading of neuronal lysates, for each immunoblot, an identical gel was run to assess total protein concentration (Supplemental Figure 3). Following size-based separation, gels were fixed in 50% methanol/7% acetic acid and then stained with SYPRO Ruby (ThermoFisher) overnight. Gels were washed in 10% methanol/7% acetic acid, rinsed with water, and then imaged using a Chemidoc MP system (Bio-Rad). Images of SYPRO Ruby stained gels were inverted using PhotoShop (Adobe).
Perfusion fixation and embedding
Whole mouse brains were prepared for immunohistochemical localization as previously described (Mangold et al. 2017a; Mangold et al. 2017b; VanGuilder et al. 2011). Briefly, after acclimation, mice were anesthetized via intraperitoneal administration of a ketamine/xylazine cocktail, and then transcardially perfused with 1X PBS, followed by fresh 4% PFA (pH 7.4). The use of ketamine/xylazine as an anesthetic was chosen in accordance with the American Veterinary Medical Association guidelines and was approved by the Penn State University IACUC. Animals were then decapitated and brains were rapidly hemisected, immersed in 4% PFA, and incubated at 4°C overnight. Brain hemispheres were then washed twice in 1X PBS, and cryoprotected in 30% sucrose. Once processed, hemispheres were embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek), frozen in isopentane on dry ice, and stored at −80°C until cryosectioning.
Immunohistochemical localization of purine enzymes in whole mouse brain
Cryosections immunolabeled for NeuN and FGAMS were processed as previously described (Du et al. 2015). Briefly, 14μM cryosections were blocked in 10% donkey serum and 5% bovine serum albumin diluted in 0.5% Triton X-100/PBS for 1 hour. Sections were then incubated in primary antibody diluted in blocking solution overnight at 4°C (see Table 1 for dilutions). Immunolabeled sections were then washed in PBS, incubated with species-appropriate fluorescence-conjugated F(ab′)2 secondary antibody diluted in blocking solution for 1.5 hours at room temperature in the dark. Nuclear counterstaining was done by incubating sections with 100ng/mL Hoechst for 30 minutes at room temperature. Sections were then rinsed with PBS, mounted with Aqua-Poly/Mount mounting medium (Polysciences, Inc.), and covered with glass coverslips. Images were acquired using a Leica SP2 MP confocal microscope with a 63× oil immersion objective (Leica Microsystems).
Sections immunolabeled for NSE and FGAMS were processed as previously described (Mangold et al. 2017a; Mangold et al. 2017b; VanGuilder et al. 2011). Whole mouse brains were cryosectioned along the sagittal plane at 12μM per slice. Sections were post-fixed in 2% PFA, rinsed in 1XPBS, blocked in 10% donkey serum diluted in 0.1% Triton X-100/PBS for 1 hour at room temperature, and then incubated in primary antibody diluted in blocking solution overnight at 4°C (see Table 1 for dilutions). Sections were washed three times with 0.1% Triton X-100/PBS, and then incubated with species-appropriate fluorescence-conjugated F(ab′)2 secondary antibody diluted in blocking solution and Hoescht nuclear stain (1: 10,000) for 2 hours at room temperature in the dark. Following three washes with 0.1% Triton X-100/PBS, sections were rinsed with 1XPBS, and then covered with glass coverslips using ProLong Gold antifade reagent. Images were acquired using an Olympus Fluoview FV10i confocal microscope equipped with a 60× water immersion objective.
Viral infection
Infections were performed using an HSV-1 strain F modified to express a hybrid viral protein 26 (VP26) capsid protein gene (UL35) that contains monomeric red fluorescent protein (mRFP) coding sequences at the 5′ end of the UL35 open reading frame (Antinone and Smith 2010; Tanaka et al. 2003). Virus (HSV-1 F-GS2822, here referred to as FRed) was grown and titered on monolayers of African green monkey kidney cells (Vero cells; ATCC CCL-81, RRID:CVCL_0059) incubated in Dulbecco’s modified Eagle’s medium or DMEM (HyClone) supplemented with 2% FBS, penicillin-streptomycin, and L-glutamine.
MRC5 cells or differentiated SH-SY5Y neurons (Shipley et al. 2016) were counted and infected at a multiplicity of infection (MOI) of 10 and incubated at 37°C/5% CO2 with viral inoculum diluted in standard media for 1 hour with rocking every 15 minutes. Inoculum was then replaced with standard media and cells were incubated for either 5 or 12 hours. All steps were replicated for mock-infected samples, except that cells were incubated with fresh standard media lacking the viral inoculum. Following infection, cells were then processed for immunofluorescence or Western blotting.
Description of Experimental Replicates
For immunofluorescence assays using differentiated SH-SY5Y neuroblastoma cells and MRC5 fibroblasts, two coverslips from each culture were analyzed with 2-4 fields of view imaged per coverslip. All experiments were repeated using 3-4 independent cultures. Similarly, localization of FGAMS in primary hippocampal neurons was assessed in four independent cultures with two coverslips processed per culture. For immunohistochemical analysis of FGAMS expression in vivo, two animals were used with a total number of 2-4 sections processed per animal, and up to four fields of view analyzed. Analysis of FGAMS protein expression in HSV-1 infected differentiated SH-SY5Y neuroblastoma cells and MRC5 fibroblasts was replicated in three separate cultures with two technical replicates processed per experiment. No blinding or sample calculation was performed for the above experiments.
Results
Localization of endogenous FGAMS in human neuronal cells demonstrates perinuclear localization and distal puncta
Little is known about the localization of endogenously expressed FGAMS, a marker of the purinosome and de novo purine biosynthesis (An et al. 2008; Zhao et al. 2015; Schmitt et al. 2016) in neurons. A recent study demonstrated the expression and localization of FGAMS and two other purine biosynthetic enzymes in primary rat hippocampal neurons (Williamson et al. 2017). As neurons require high levels of energy despite their inability to replicate, these data may provide important insight into the mechanisms of neuronal purine biosynthesis. We therefore investigated the distribution of FGAMS in a model of human neurons, using SH-SY5Y cells differentiated to a neuronal state over approximately 3 weeks of serum withdrawal and neurotrophic support (Christensen et al. 2011; Encinas et al. 2000; Shipley et al. 2017; Shipley et al. 2016). FGAMS expression was assessed in differentiated human SH-SY5Y neurons co-labeled with SMI-31, an antibody that detects phosphorylated neurofilament heavy chain present in neuronal axons. FGAMS labeling in neurons was characterized by dense cytoplasmic staining surrounding the nucleus (indicated by arrowheads), and also by the formation of distinct puncta along neuronal processes (as indicated by arrows) (Figure 1A). It is of note that after differentiation, SH-SY5Y cells often display a small, asymmetric soma with the nucleus taking up the majority of the cell body. The appearance of FGAMS being primarily concentrated to one side of the soma reflects the morphology of differentiated SH-SY5Y neurons rather than a phenotype of physiological relevance.
Figure 1. FGAMS protein expression occurs in neuronal processes and near mitochondria in differentiated SH-SY5Y human neuroblastoma cells.
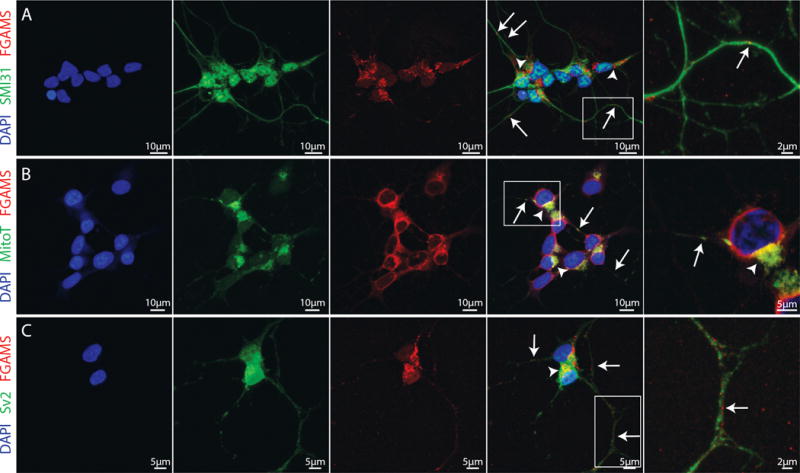
(A) Differentiated SH-SY5Y neurons immunolabeled for the axonal marker SMI31 (green) and the purine biosynthetic enzyme FGAMS (red). FGAMS demonstrated strong localization in the neuronal cytoplasm (arrowheads) with numerous punctate localizations throughout neuronal axons (arrows). (B) Consistent with recent work in primary hippocampal neurons (Williamson et al. 2017), FGAMS (red) co-localized with mitochondria (green), both in neuronal cytoplasm (arrowheads) and along neurites (arrows) in differentiated SH-SY5Y neurons. (C) When neurons were immunolabeled for both FGAMS (red) and Sv2 (synaptic vesicle protein 2 – green), no distinct enrichment of FGAMS at synapses was evident. All cells were counterstained with DAPI nuclear stain (blue). Two coverslips from 3-4 independent cultures were analyzed.
Purinosome formation in non-neuronal cells has been shown to occur at or near mitochondria following purine depletion, as evidenced by the co-localization of GFP-labeled FGAMS puncta and MitoTracker-labeled mitochondria (French et al. 2016). Neuronal cytoplasm and projections contain numerous mitochondria, which provide energy for the high metabolic demands of neuronal communication and the preservation of cell membrane ionic gradients (Chang et al. 2006; Kann and Kovács 2007; Li et al. 2004). To investigate whether endogenous FGAMS co-localizes with mitochondria in neurons, terminally differentiated SH-SY5Y cells were co-stained with MitoTracker Red dye and FGAMS antibody. Co-localization of endogenous FGAMS and mitochondria was evident both in the neuronal cytoplasm (as indicated by arrowheads) and also in neuronal projections (as indicated by arrows) (Figure 1B). Together, these data suggest that FGAMS and mitochondria co-occur not only in neuronal cell bodies, but also along neuronal projections, enabling purine production for neuronal communication and metabolic processes.
Since neurons are known to form synapses along neuronal processes (Wang and Sun 2012), we next asked whether axonally localized FGAMS was found in proximity to potential synapses. Synapses are regions of high energy demand where extensive communication with surrounding cells and active translation of local mRNA transcripts occurs. Following differentiation, SH-SY5Y cells form synapse-like structures localized at sites of contact between neurites. These structures contain synaptic vesicle protein 2 (Sv2), and transport of vesicles is evident along the neurites of differentiated SH-SY5Y neuronal cells (Agholme et al. 2010). We therefore tested for the expression of FGAMS at these synapse-like structures in differentiated SH-SY5Y neuronal cells, as indicated by immunoreactivity near Sv2-labeled puncta. FGAMS did not appear to consistently localize in areas expressing Sv2, suggesting that FGAMS is not enriched in synapses (Figure 1C). However, FGAMS immunoreactivity is frequently found near synapses (see Figure 1C, zoomed in panel). This localization suggests that FGAMS may function to provide purines necessary for local metabolic processes or neurotransmission in neuronal projections.
Cultured primary neurons demonstrate similar FGAMS localization
Differentiated SH-SY5Y neurons provide a facile platform in which to study the physiology of human neuronal-like cells. To extend our findings, we used primary rodent embryonic neurons which are known to form synapses and maintain electrical activity following dissociation (Friedman et al. 2000). A recent publication has demonstrated localization of FGAMS with mitochondria in primary rat hippocampal neurons (Williamson et al. 2017), corroborating our data in differentiated human SH-SY5Y neurons. To determine whether primary neurons also express FGAMS in neuronal projections, we tested for co-localization of FGAMS and Tuj1, which recognizes class III beta tubulin specifically in the microtubules of neurons. In young primary rat hippocampal neurons (2 days in culture), FGAMS demonstrated strong localization both in neuronal cell bodies (arrowheads) and along microtubules within neuronal processes (arrows) (Figure 2A–2B). To test whether FGAMS localized within synapses in this neuronal model, we used mature primary hippocampal neurons (16 days in culture). As observed in differentiated SH-SY5Y neurons, FGAMS did not appear to directly localize within either pre- or post-synaptic compartments, as evidenced by lack of enrichment in regions of pre-synaptic (synaptophysin) and post-synaptic (post-synaptic density 95, PSD-95) staining (Figure 2C–2D). These results corroborate and extend those of the recent publication by Williamson et al. (2017), which examined FGAMS expression in neurons in relation to mitochondria, but did not examine neurofilament or synaptic markers, or present neuronal FGAMS distribution in both young and mature neurons. Taken together, these data demonstrate FGAMS localization along neuronal projections and co-localization with mitochondria in both the cell bodies and neurites of human and rat neurons. However, FGAMS does not appear to be concentrated in synapses.
Figure 2. FGAMS protein is expressed along neuronal projections in primary rat hippocampal neurons.
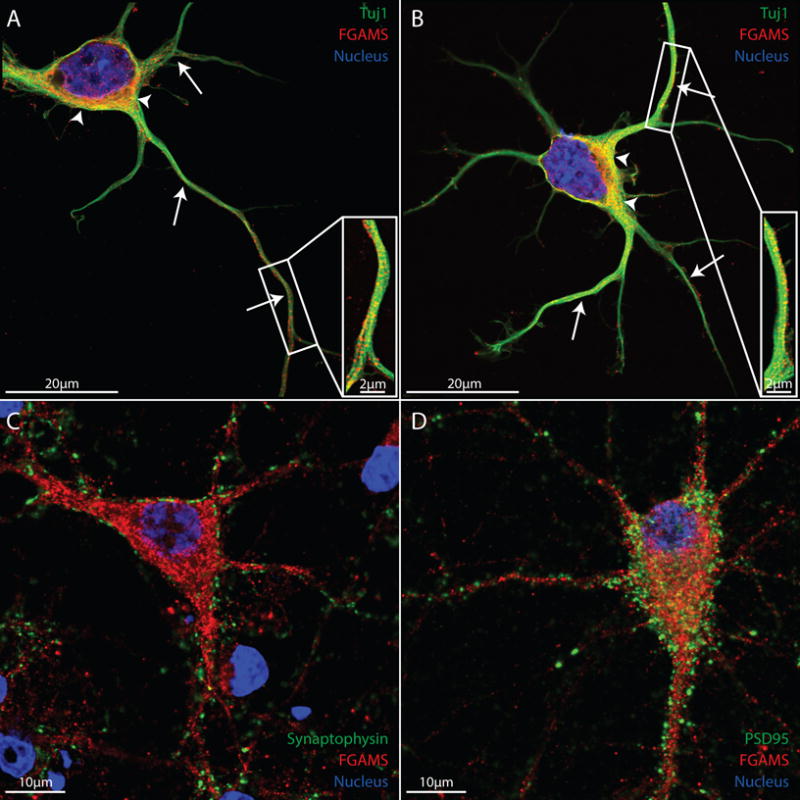
(A-B) Dissociated 2 day old primary hippocampal neurons probed for FGAMS (red) and Tuj1 (β3 tubulin – green) demonstrated similar localization of FGAMS both in neuronal cytoplasm (arrowheads) and in neuronal projections (arrows). (C-D) When co-labeled with the pre- (synaptophysin - green) and post- (PSD-95 – green) synaptic markers, FGAMS demonstrated no distinct enrichment within neuronal synapses in 16 day old mature hippocampal neurons. Two coverslips from 4 different cultures were analyzed.
FGAMS in the mouse brain demonstrates strong cytoplasmic localization
To assess FGAMS localization and to test whether the observations above were conserved in vivo, we examined FGAMS protein in cryosections of the adult mouse hippocampus and brainstem. To identify neurons in these sections, we counter-stained for the neuronal markers NeuN and neuron-specific enolase (NSE). NeuN is a neuron-specific protein that localizes in neuronal nuclei and in perinuclear regions in mature neurons (Gusel’nikova and Korzhevskiy 2015). NSE is an enolase enzyme that localizes specifically within neuronal cytoplasm. In 3-month-old male mice, we found FGAMS localized within neuronal cell bodies of the CA1 region of the hippocampus (Figure 3A) and also within neuronal cell bodies of the brainstem (Figure 3B). The punctate-like staining of FGAMS seen within neuronal cytoplasm using in vitro model systems (Figures 1 and 2) was also observed in the hippocampal neuronal cell bodies. These data suggest that our in vitro findings reflect the in vivo expression of endogenous neuronal FGAMS.
Figure 3. In vivo expression of FGAMS is evident in neuronal cell bodies.
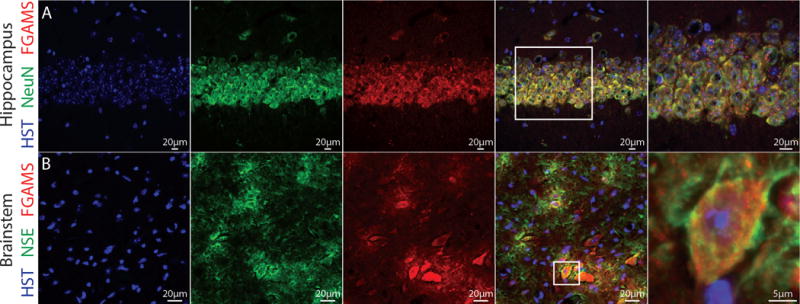
To assess FGAMS expression in neurons in vivo, whole brain sections derived from 3 month old male mice were probed for FGAMS (red) and two neuronal markers (green). (A) Images acquired from the CA1 region of the hippocampus demonstrated co-localization of FGAMS and neuronal nuclei (NeuN) in the cell body layers. (B) In the medulla region of the brainstem, co-localization of neuron-specific enolase (NSE) and FGAMS was observed. All sections were counterstained with Hoescht to mark the nucleus (blue). Sections were acquired from two different animals, and 2-4 sections per animal were processed for analysis.
Infections of fibroblasts and differentiated SH-SY5Y cells with HSV-1 induces enhanced perinuclear localization of FGAMS only in fibroblasts
Prior data on purinosome formation has been accomplished via purine depletion, pharmacological manipulation, or using cells derived from patients with purine biosynthetic enzyme mutations (Pedley and Benkovic 2017). Few studies have examined changes in endogenous purine biosynthetic enzyme expression and localization following viral infection. To assess how HSV-1 infection impacts purine biosynthetic enzyme levels and localization in fibroblasts, MRC5 cells were infected at high multiplicity (MOI =10) with a fluorescently labeled strain of HSV-1 (FRed; mRFP-capsid label; see methods for details). The HSV-1 Fred strain expresses a hybrid VP26 capsid protein gene (UL35) containing mRFP (Antinone and Smith 2010). At 5 hours post infection (hpi), most infected cells did not yet have visible foci of HSV-1 RFP-labeled capsid in the nucleus (Figure 4B). Mock-infected MRC5 cells had a relatively even distribution of FGAMS immunoreactive puncta throughout the entire cell cytoplasm (Figure 4A). In contrast, HSV-1 infected MRC5 cells demonstrated enhanced clustering of FGAMS-immunoreactive puncta, often perinuclear (Figure 4B). After 12 hours of infection, fluorescently-labeled capsids were evident in the nucleus of each infected MRC5 cell (Figure 4D). FGAMS immunoreactivity shifted to a primarily perinuclear phenotype in infected cells, while mock-infected cells maintained an even distribution of FGAMS-immunoreactive puncta (Figure 4C), suggesting that a re-localization of FGAMS in infected fibroblasts may occur during infection by HSV-1.
Figure 4. Infection with HSV-1 caused distinct alterations in FGAMS localization in fibroblasts.
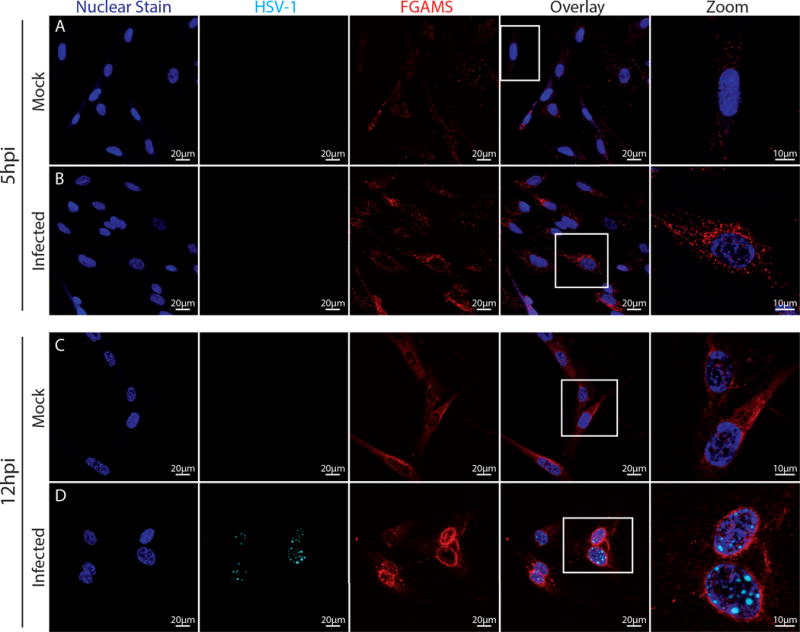
MRC5 cells were infected with HSV-1 Fred for 5 (rows A,B) or 12 hours (rows C,D). Punctate FGAMS staining is more readily apparent in HSV-infected fibroblasts (B,D) than mock-infected fibroblasts (A,C). Fluorescently-labeled HSV-1 capsids were not detectable until 12 hpi, when foci of viral assembly appear in the nuclei of infected cells (D, cyan). At 12 hpi, infected MRC5 cells demonstrated a shift in FGAMS staining from a mainly cytoplasmic distribution to a more pernuclear localization (C). All cells were labeled with Hoescht nuclear stain (blue). Images were acquired at 60× magnification with zoomed panels acquired at 168×. Equal laser power was applied between infected pairs (i.e., mock 5 hpi and infected 5 hpi; mock 12 hpi and infected 12 hpi). Two coverslips from 3-4 independent cultures were analyzed.
HSV-1 infects both skin cells and neurons (Roizman et al. 2013) and has distinct host-virus interactions in these different cell types. Based on the data demonstrating a change in FGAMS localization in HSV-infected fibroblasts, we tested whether neurons undergo any changes in FGAMS immunoreactivity during infection with HSV-1. At 5 hpi, mock-infected and HSV-1-infected neurons demonstrated no distinct differences in FGAMS immunoreactivity or localization (Supplemental Figure 1). At 12 hpi, neuronal detachment from the substrate as a result of infection prevented staining and imaging.
Given the enhanced FGAMS immunoreactivity found in infected MRC5 cells, we sought to determine whether this was due to increased enzyme protein levels or from clustering of pre-existing FGAMS enzyme. FGAMS protein levels were measured by Western blot in both MRC5 and differentiated SH-SY5Y cells infected for either 5 or 12 hours with HSV-1. We found no significant changes in protein levels observed at either 5 or 12 hpi (Supplemental Figure 2). These data suggest that the puncta observed in infected MRC5 cells may be due to alterations in FGAMS clustering and/or localization.
Discussion
In this study, we sought to characterize neuronal expression of one of the core enzymes of the purinosome, FGAMS, as well as the effects of HSV-1 infection on FGAMS levels and localization. We examined endogenous FGAMS levels and localization in neuronal cell bodies using multiple models including differentiated human SH-SY5Y neurons, primary rat hippocampal neurons, and whole mouse brain slices. Studying endogenous FGAMS allows for the differentiation of potential purinosome formation from overexpression- or stress-induced protein aggregation (Zhao et al. 2013a; Zhao et al. 2014). In SH-SY5Y neurons and in primary hippocampal neurons, we observed FGAMS as distinct puncta throughout neuronal processes, where it co-localized with neurofilament heavy chain, tubulin, and mitochondria (see also (Williamson et al. 2017)). The co-localization of FGAMS with mitochondria and microtubules is consistent with other recent analyses of non-neuronal cells (An et al. 2010; French et al. 2016), suggesting that the mechanisms of purinosome formation and function in neurons may be conserved across cell types. When we attempted to localize FGAMS within synapses, we were unable to observe consistent enrichment with synaptic markers, although FGAMS immunoreactivity was found close to synapses. After HSV-1 infection of human lung fibroblasts, there was an increase in FGAMS clustering in the cytoplasm and near infected cell nuclei, with no change in FGAMS protein levels. In contrast, HSV-1 infection of differentiated SH-SY5Y induced no detectable changes in FGAMS localization or protein levels.
The de novo purine biosynthetic pathway requires 6 enzymes and 10 enzymatic steps to convert PRPP to IMP, which can then ultimately be converted to ATP or GTP (An et al. 2008). Recent studies have detailed the assembly of the purinosome, which forms during periods of high purine demand and allows for more efficient de novo synthesis of purines (An et al. 2008; Zhao et al. 2015; Pedley and Benkovic 2017). Since the first description of the purinosome almost a decade ago (An et al. 2008), many studies have sought to understand purinosome formation, function, and localization (for review, see (Pedley and Benkovic 2017)). In contrast with previously examined epithelial cell lines, neurons are terminally differentiated cells with high metabolic demand and the need for purines in neuronal communication (Burnstock 1990; Burnstock 1997). A recent study by Williamson et al. (2017) demonstrated localization of three purinosome enzymes near mitochondria in vitro in primary rat neurons and brain slices. These data provided evidence for the existence of a relationship between purine metabolism and mitochondria in neurons.
Unlike previous work, the studies presented here detail the localization of FGAMS across neuronal models and species (e.g., an in vitro model of human neurons, primary rat hippocampal neurons, and in vivo mouse brain). We found that in each model system, the localization pattern of FGAMS was consistent. De novo purine biosynthesis has been shown to occur in the brain (Held and Wells 1969; Howard et al. 1970), although it is under debate whether the de novo or salvage pathway is responsible for neuronal purine maintenance (Fasullo and Endres 2015). Our data suggest that de novo purine biosynthesis may occur at specific localized sites within neurons (i.e., neuronal cytoplasm, axons, and dendrites) and that this phenotype is conserved across species.
Mitochondria and de novo purine biosynthesis are suggested to have a synergistic relationship, and purinosomes and mitochondria are known to co-localize in purine-depleted HeLa cells (French et al. 2016). Observation of FGAMS co-localization with mitochondria in neurons is suggestive that a functional link between mitochondria and de novo purine biosynthesis may be conserved between cell types. A recent study of FGAMS expression in neurons did not test for localization at or near synapses (Williamson et al. 2017). We therefore asked whether FGAMS localized at or near synapses in both differentiated human SH-SY5Y neuronal-like cells and in primary rat hippocampal neurons. In the data presented here, FGAMS did not appear to be enriched within neuronal synapses, although they were at times found in proximity to synapses. This finding is interesting given the high metabolic demand required for processes such as neurotransmitter recycling and packaging at synapses (Shetty et al. 2012), as well as the participation of purines in neurotransmission (Abbracchio et al. 2009; Burnstock 1972; Burnstock 1990; Burnstock 1997; Burnstock 2007). It is possible that de novo purine biosynthesis at synapses may be too time consuming (10 steps, 6 enzymes) vs. the salvage pathway for rapid generation of ATP and purinergic neurotransmission. Alternatively, de novo purine biosynthesis may occur nearby and purinergic neurotransmitters may be actively transported to synapses.
In the studies presented here, we have focused exclusively on examining endogenous FGAMS. In contrast to the recent study by Williamson et al. (2017), we used viral infection as a natural cell stressor to initiate high purine demand. Most viruses, including HSV-1, do not encode the enzymes necessary to form purines for nucleic acid synthesis and viral replication. Viral infection thus results in a re-allocation of purine biosynthetic products from the host cell to the replicating virus (Abrantes et al. 2012; El-Bacha and Da Poian 2013; Vastag et al. 2011). Some antiviral therapeutics target the de novo purine biosynthetic pathways. Specifically, ribavirin and mycophenolic acid are known inhibitors of inosine monophosphate dehydrogenase, a key enzyme involved in the final steps of de novo purine biosynthesis (Debing et al. 2014; Pan et al. 2012; Pawlotsky et al. 2004; Takhampunya et al. 2006; Wang et al. 2016).
During infection with HSV-1, fibroblasts demonstrated enhanced FGAMS clustering in the absence of any change in FGAMS protein levels. As infection progressed, FGAMS clustering became more perinuclear. No changes in FGAMS clustering were observed in infected neurons. These studies raise the question of whether the cell-type-specific differences in FGAMS localization after HSV-1 infection result from higher intrinsic purine biosynthetic activity in neurons, or from other cell-type-specific responses to infection. The alteration in FGAMS localization we observed in fibroblasts may reflect a redistribution of de novo purine biosynthesis to localize this process near infected cell nuclei, where viral DNA replication is rapidly consuming intracellular purine stores. This occurs in the absence of overt changes in FGAMS protein levels, suggesting it is mainly an alteration in localization. Future work will be required to investigate whether other enzymes of the de novo purine biosynthesis pathway demonstrate similar localization to perinuclear regions after infection.
It is of note that purines may participate in the cell-danger response (Naviaux 2014), in crosstalk between nucleotide synthesis and host cell innate immune responses (Wang et al. 2017; Wang et al. 2016), and in autocrine signaling (Xia et al. 2012). Additionally, FGAMS functions in the pathogenesis and immune evasion of gammaherpesviruses, which encode a glutamine amidotransferase with homology to FGAMS (ORF75) (He et al. 2015; Zhao et al. 2016). Given these roles of purines in immune processes and signaling, as well as FGAMS in viral pathogenesis, future studies should fully characterize purinosome formation and the specific roles of FGAMS during viral infection.
Supplementary Material
Acknowledgments
We would like to thank the faculty and staff members of the Huck Microscopy Facility at Penn State University for their support and guidance during all the imaging experiments. We would also like to thank Dr. Anthony Pedley, Dr. Vidhi Pareek, and Dr. James Lata of the Benkovic lab for their valuable insights in experimental planning and data interpretation, as well as members of the Szpara lab. C.A.M. is a postdoctoral fellow of the American Heart Association. This work was supported by the startup funds (M.L.S.) from the Pennsylvania State University, the National Institutes of Health (RO1 GM024129, S.J.B.), the American Heart Association (16POST29920001, C.A.M.), a J. Lloyd Huck Biotechnology mini-grant (C.A.M.) from the Pennsylvania State University, and the Intramural Research Programs of the National Institute of Health/National Institute on Aging (P.Y.). The Sv2 monoclonal antibody used in this study was developed by Dr. Kathleen M. Buckley at Harvard Medical School and was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Abbreviations
- ATP
Adenosine triphosphate
- CNS
central nervous system
- FGAMS
Phosphoribosyl formylglycinamidine synthase, also known as PFAS or FGARAT
- GFP
green fluorescent protein
- hpi
hours post infection
- HSV-1
herpes simplex virus 1, also known as human herpesvirus 1, HHV-1
- IMP
Inosine monophosphate
- MOI
multiplicity of infection
- MRC5
a fibroblast cell line
- NeuN
neuronal nuclear protein
- NSE
neuron-specific enolase
- PNS
peripheral nervous system
- PRPP
5-phospho-alpha-d-ribose 1-diphosphate (phosphoribosyl pyrophosphate)
- PSD-95
Post-synaptic density protein 95
- SH-SY5Y
a neuroblastoma cell line
- SMI-31
antibody to phosphorylated neurofilament heavy chain
- Sv2
synaptic vesicle protein 2
- Tuj1
antibody to class III beta tubulin
- VP26
viral capsid protein 26
Footnotes
DR. MORIAH LOUISE SZPARA (Orcid ID : 0000-0001-9859-1678)
Conflict of interest disclosure
The authors declare no conflicts of interest.
References
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Abrantes JL, Alves CM, Costa J, Almeida FCL, Sola-Penna M, Fontes CFL, Souza TML. Herpes simplex type 1 activates glycolysis through engagement of the enzyme 6-phosphofructo-1-kinase (PFK-1) Biochim Biophys Acta BBA - Mol Basis Dis. 2012;1822:1198–1206. doi: 10.1016/j.bbadis.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Ackermann WW, Kurtz H. The relation of herpes virus to host cell mitochondria. J Exp Med. 1952;96:151–157. doi: 10.1084/jem.96.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agholme L, Lindström T, Kågedal K, Marcusson J, Hallbeck M. An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimers Dis JAD. 2010;20:1069–1082. doi: 10.3233/JAD-2010-091363. [DOI] [PubMed] [Google Scholar]
- Amadoro G, Corsetti V, Florenzano F, Atlante A, Bobba A, Nicolin V, Nori SL, Calissano P. Morphological and bioenergetic demands underlying the mitophagy in post-mitotic neurons: the pink–parkin pathway. Front Aging Neurosci. 2014;6 doi: 10.3389/fnagi.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Deng Y, Tomsho JW, Kyoung M, Benkovic SJ. Microtubule-assisted mechanism for functional metabolic macromolecular complex formation. Proc Natl Acad Sci. 2010;107:12872–12876. doi: 10.1073/pnas.1008451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Kumar R, Sheets ED, Benkovic SJ. Reversible Compartmentalization of de Novo Purine Biosynthetic Complexes in Living Cells. Science. 2008;320:103–106. doi: 10.1126/science.1152241. [DOI] [PubMed] [Google Scholar]
- Antinone SE, Smith GA. Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis. J Virol. 2010;84:1504–12. doi: 10.1128/JVI.02029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baresova V, Skopova V, Sikora J, Patterson D, Sovova J, Zikanova M, Kmoch S. Mutations of ATIC and ADSL affect purinosome assembly in cultured skin fibroblasts from patients with AICA-ribosiduria and ADSL deficiency. Hum Mol Genet. 2012;21:1534–1543. doi: 10.1093/hmg/ddr591. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine and Epilepsy: From Therapeutic Rationale to New Therapeutic Strategies. The Neuroscientist. 2016 doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. Noradrenaline and ATP as cotransmitters in sympathetic nerves. Neurochem Int. 1990;17:357–368. doi: 10.1016/0197-0186(90)90158-p. [DOI] [PubMed] [Google Scholar]
- Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Bushlin I, Petralia RS, Wu F, Harel A, Mughal MR, Mattson MP, Yao PJ. Clathrin Assembly Protein AP180 and CALM Differentially Control Axogenesis and Dendrite Outgrowth in Embryonic Hippocampal Neurons. J Neurosci. 2008;28:10257–10271. doi: 10.1523/JNEUROSCI.2471-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DTW, Honick AS, Reynolds IJ. Mitochondrial Trafficking to Synapses in Cultured Primary Cortical Neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Steain M, Slobedman B, Abendroth A. Differentiated neuroblastoma cells provide a highly efficient model for studies of productive varicella-zoster virus infection of neuronal cells. J Virol. 2011;85:8436–42. doi: 10.1128/JVI.00515-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim J V, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Debing Y, Emerson SU, Wang Y, Pan Q, Balzarini J, Dallmeier K, Neyts J. Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob Agents Chemother. 2014;58:267–273. doi: 10.1128/AAC.01795-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Gam J, French JB, Zhao H, An S, Benkovic SJ. Mapping protein-protein proximity in the purinosome. J Biol Chem. 2012;287:36201–36207. doi: 10.1074/jbc.M112.407056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Otalora L, Martin AA, Moiseyev G, Vanlandingham P, Wang Q, Farjo R, Yeganeh A, Quiambao A, Farjo KM. Transgenic Mice Overexpressing Serum Retinol-Binding Protein Develop Progressive Retinal Degeneration through a Retinoid-Independent Mechanism. Mol Cell Biol. 2015;35:2771–2789. doi: 10.1128/MCB.00181-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguay BA, Saffran HA, Ponomarev A, Duley SA, Eaton HE, Smiley JR. Elimination of Mitochondrial DNA Is Not Required for Herpes Simplex Virus 1 Replication. J Virol. 2014;88:2967. doi: 10.1128/JVI.03129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bacha T, Da Poian AT. Virus-induced changes in mitochondrial bioenergetics as potential targets for therapy. Int J Biochem Cell Biol. 2013;45:41–46. doi: 10.1016/j.biocel.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Ceña V, Gallego C, Comella JX. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75:991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasullo M, Endres L. Nucleotide Salvage Deficiencies, DNA Damage and Neurodegeneration. Int J Mol Sci. 2015;16:9431. doi: 10.3390/ijms16059431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JB, Jones SA, Deng H, Pedley AM, Kim D, Chan CY, Hu H, et al. Spatial colocalization and functional link of purinosomes with mitochondria. Science. 2016;351:733–737. doi: 10.1126/science.aac6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of New Individual Excitatory Synapses: Time Course and Temporal Order of Synaptic Molecule Recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Fu R, Sutcliffe D, Zhao H, Huang X, Schretlen DJ, Benkovic S, Jinnah HA. Clinical severity in Lesch-Nyhan disease: the role of residual enzyme and compensatory pathways. Mol Genet Metab. 2015;114:55–61. doi: 10.1016/j.ymgme.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusel’nikova VV, Korzhevskiy DE. NeuN As a Neuronal Nuclear Antigen and Neuron Differentiation Marker. Acta Naturae. 2015;7:42. [PMC free article] [PubMed] [Google Scholar]
- He S, Zhao J, Song S, He X, Minassian A, Zhou Y, Zhang J, et al. Viral Pseudo-Enzymes Activate RIG-I via Deamidation to Evade Cytokine Production. Mol Cell. 2015;58:134–146. doi: 10.1016/j.molcel.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held I, Wells W. Observations on Purine Metabolism in Rat Brain. J Neurochem. 1969;16:529–536. doi: 10.1111/j.1471-4159.1969.tb06851.x. [DOI] [PubMed] [Google Scholar]
- Howard WJ, Kerson LA, Appel SH. Synthesis de novo of purines in slices of rat brain and liver1. J Neurochem. 1970;17:121–123. doi: 10.1111/j.1471-4159.1970.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol - Cell Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- Kroeker AL, Ezzati P, Coombs KM, Halayko AJ. Influenza A Infection of Primary Human Airway Epithelial Cells Up-Regulates Proteins Related to Purine Metabolism and Ubiquitin-Related Signaling. J Proteome Res. 2013;12:3139–3151. doi: 10.1021/pr400464p. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K-I, Hayashi Y, Sheng M. The Importance of Dendritic Mitochondria in the Morphogenesis and Plasticity of Spines and Synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Looker KJ, Magaret AS, Turner KME, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PloS One. 2015;10:e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold CA, Masser DR, Stanford DR, Bixler GV, Pisupati A, Giles CB, Wren JD, Ford MM, Sonntag WE, Freeman WM. CNS-wide Sexually Dimorphic Induction of the Major Histocompatibility Complex 1 Pathway With Aging. J Gerontol A Biol Sci Med Sci. 2017a;72:16–29. doi: 10.1093/gerona/glv232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold CA, Wronowski B, Du M, Masser DR, Hadad N, Bixler GV, Brucklacher RM, Ford MM, Sonntag WE, Freeman WM. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation. 2017b;14:141. doi: 10.1186/s12974-017-0920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PI, Nunomura A, Honda K, Aliev G, Casadesus G, Zhu X, Smith MA, Perry G. Chapter 12 - The Key Role of Oxidative Stress in Alzheimer’s Disease A2 - QURESHI, G. ALI. In: PARVEZ SH, editor. Oxidative Stress Neurodegener Disord. Elsevier Science B.V.; Amsterdam: 2007. pp. 267–281. [Google Scholar]
- Naviaux RK. Metabolic features of the cell danger response. Mitochondrion. 2014;16:7–17. doi: 10.1016/j.mito.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Pan Q, de Ruiter PE, Metselaar HJ, Kwekkeboom J, de Jonge J, Tilanus HW, Janssen HLA, van der Laan LJW. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C Virus infection in vitro and in vivo. Hepatol Baltim Md. 2012;55:1673–1683. doi: 10.1002/hep.25562. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflüg Arch Eur J Physiol. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A, North RA. Quantal release of ATP in mouse cortex. J Gen Physiol. 2007;129:257–265. doi: 10.1085/jgp.200609693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 Mediates Superoxide Production in Primary Microglia and Is Up-regulated in a Transgenic Mouse Model of Alzheimer’s Disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- Pawlotsky J-M, Dahari H, Neumann AU, Hezode C, Germanidis G, Lonjon I, Castera L, Dhumeaux D. Antiviral action of ribavirin in chronic hepatitis C. Gastroenterology. 2004;126:703–714. doi: 10.1053/j.gastro.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Pedley AM, Benkovic SJ. A New View into the Regulation of Purine Metabolism: The Purinosome. Trends Biochem Sci. 2017;42:141–154. doi: 10.1016/j.tibs.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Knipe DM, Whitley R. Fields Virol. Vol. 2. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. Herpes Simplex Viruses; pp. 1823–1897. [Google Scholar]
- Saffran HA, Pare JM, Corcoran JA, Weller SK, Smiley JR. Herpes simplex virus eliminates host mitochondrial DNA. EMBO Rep. 2007;8:188. doi: 10.1038/sj.embor.7400878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt DL, Cheng Y, Park J, An S. Sequestration-Mediated Downregulation of de Novo Purine Biosynthesis by AMPK. ACS Chem Biol. 2016;11:1917–1924. doi: 10.1021/acschembio.6b00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty PK, Galeffi F, Turner DA. Cellular Links between Neuronal Activity and Energy Homeostasis. Front Pharmacol. 2012;3 doi: 10.3389/fphar.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MM, Mangold CA, Kuny CV, Szpara ML. Differentiated SH-SY5Y human cells provide a reductionist model of HSV-1 neurotropism. J Virol. 2017:JVI.00958–17. doi: 10.1128/JVI.00958-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MM, Mangold CA, Szpara ML. Differentiation of the SH-SY5Y human neuroblastoma cell line. J Vis Exp. 2016;108 doi: 10.3791/53193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GK, Mueller WT, Wasserman GF, Taylor WD, Benkovic SJ. Characterization of the enzyme complex involving the folate-requiring enzymes of de novo purine biosynthesis. Biochemistry (Mosc) 1980;19:4313–4321. doi: 10.1021/bi00559a026. [DOI] [PubMed] [Google Scholar]
- Son JH, Shim JH, Kim K-H, Ha J-Y, Han JY. Neuronal autophagy and neurodegenerative diseases. Exp Mol Med. 2012;44:89–98. doi: 10.3858/emm.2012.44.2.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasolla C, Katahira R, Thorpe TA, Ashihara H. Purine and pyrimidine nucleotide metabolism in higher plants. J Plant Physiol. 2003;160:1271–1295. doi: 10.1078/0176-1617-01169. [DOI] [PubMed] [Google Scholar]
- Steward O. Polyribosomes at the base of dendritic spines of central nervous system neurons–their possible role in synapse construction and modification. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):745–759. doi: 10.1101/sqb.1983.048.01.077. [DOI] [PubMed] [Google Scholar]
- Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci Off J Soc Neurosci. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Ribak CE. Polyribosomes associated with synaptic specializations on axon initial segments: localization of protein-synthetic machinery at inhibitory synapses. J Neurosci Off J Soc Neurosci. 1986;6:3079–3085. doi: 10.1523/JNEUROSCI.06-10-03079.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takhampunya R, Ubol S, Houng H-S, Cameron CE, Padmanabhan R. Inhibition of dengue virus replication by mycophenolic acid and ribavirin. J Gen Virol. 2006;87:1947–1952. doi: 10.1099/vir.0.81655-0. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kagawa H, Yamanashi Y, Sata T, Kawaguchi Y. Construction of an Excisable Bacterial Artificial Chromosome Containing a Full-Length Infectious Clone of Herpes Simplex Virus Type 1: Viruses Reconstituted from the Clone Exhibit Wild-Type Properties In Vitro and In Vivo. J Virol. 2003;77:1382–1391. doi: 10.1128/JVI.77.2.1382-1391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, Warrington JP, Sonntag WE, Freeman WM. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J Neuroinflammation. 2011;8:138. doi: 10.1186/1742-2094-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastag L, Koyuncu E, Grady SL, Shenk TE, Rabinowitz JD. Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism. PLOS Pathog. 2011;7:e1002124. doi: 10.1371/journal.ppat.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna EPM, Ferreira AT, Naffah-Mazzacoratti MG, Sanabria ERG, Funke M, Cavalheiro EA, Fernandes MJS. Evidence that ATP participates in the pathophysiology of pilocarpine-induced temporal lobe epilepsy: fluorimetric, immunohistochemical, and Western blot studies. Epilepsia. 2002;43(Suppl 5):227–229. doi: 10.1046/j.1528-1157.43.s.5.26.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Xu L, Su J, Peppelenbosch MP, Pan Q. Transcriptional Regulation of Antiviral Interferon-Stimulated Genes. Trends Microbiol. 2017;25:573–584. doi: 10.1016/j.tim.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sun QQ. Characterization of axo‐axonic synapses in the piriform cortex of Mus musculus. J Comp Neurol. 2012;520:832–847. doi: 10.1002/cne.22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang W, Xu L, Zhou X, Shokrollahi E, Felczak K, van der Laan LJW, et al. Crosstalk between Nucleotide Synthesis Pathways with Cellular Immunity in Constraining Hepatitis E Virus Replication. Antimicrob Agents Chemother. 2016 doi: 10.1128/AAC.02700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J, Petralia RS, Wang YX, Mattson MP, Yao PJ. Purine Biosynthesis Enzymes in Hippocampal Neurons. NeuroMolecular Med. 2017 doi: 10.1007/s12017-017-8466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH. Neurons respond directly to mechanical deformation with pannexin‐mediated ATP release and autostimulation of P2X7 receptors. J Physiol. 2012;590:2285–2304. doi: 10.1113/jphysiol.2012.227983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PJ, Petralia RS, Ott C, Wang Y-X, Lippincott-Schwartz J, Mattson MP. Dendrosomatic Sonic Hedgehog Signaling in Hippocampal Neurons Regulates Axon Elongation. J Neurosci. 2015;35:16126–16141. doi: 10.1523/JNEUROSCI.1360-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Tsechansky M, Ellington AD, Marcotte EM. Revisiting and revising the purinosome. Mol Biosyst. 2014;10:369. doi: 10.1039/c3mb70397e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Tsechansky M, Swaminathan J, Cook L, Ellington AD, Marcotte EM. Transiently Transfected Purine Biosynthetic Enzymes Form Stress Bodies. PLoS ONE. 2013a;8:e56203. doi: 10.1371/journal.pone.0056203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chiaro CR, Zhang L, Smith PB, Chan CY, Pedley AM, Pugh RJ, French JB, Patterson AD, Benkovic SJ. Quantitative Analysis of Purine Nucleotides Indicates That Purinosomes Increase de Novo Purine Biosynthesis. J Biol Chem. 2015;290:6705–6713. doi: 10.1074/jbc.M114.628701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, French JB, Fang Y, Benkovic SJ. The purinosome, a multi-protein complex involved in the de novo biosynthesis of purines in humans. Chem Commun Camb Engl. 2013b;49:4444–4452. doi: 10.1039/c3cc41437j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li J, Xu S, Feng P. Emerging roles of regulated protein deamidation in innate immune signaling. J Virol. 2016:JVI.01980–15. doi: 10.1128/JVI.01980-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


