Abstract
We show here that invertase gene expression and the invertase-sucrose (Suc) synthase ratio decrease abruptly in response to low oxygen in maize root tips. In addition to aiding in the conservation of carbon and possibly ATP, this response has the potential to directly affect sugar signaling relative to carbon flux. Experiments were motivated by the potential for a reduced invertase/Suc synthase balance to alter the impact of respiratory and/or membrane carbon flux on sugar signaling. Maize (Zea mays L.) seedlings with 5-cm primary roots were exposed to anoxic (0% [v/v] O2), hypoxic (3% [v/v] O2), and aerobic conditions. Rapid repression of the Ivr1 and Ivr2 maize invertases by low oxygen was evident in root tips within 3 h at both the transcript and activity levels. The speed and extent of this response increased with the degree of oxygen deprivation and differed with genotypes. This decrease in expression also contrasted markedly to that of other genes for respiratory Suc metabolism, such as Suc synthases, which typically increased or remained constant. Although previous work showed that the contrasting effects of sugars on Suc synthase genes were reflected in their regulation by hypoxia and anoxia, the same was not observed for the differentially sugar-responsive invertases. Theoretically advantageous reductions in the invertase/Suc synthase balance thus resulted. However, where this response was extreme (an Oh43 inbred), total sucrolytic capacity dropped below an apparent minimum and root tip viability was reduced. Paradoxically, only Oh43 seedlings showed survival levels >80% (versus <50%) after extreme, long-term stress, suggesting a possible advantage for multiple means of reducing sink activity. Overall, our results demonstrate a rapid change in the regulation and balance of invertases and Suc synthases that could have an immediate impact on limiting the extent of Suc cleavage and reducing the extent of concomitant, hexose-based sugar signaling under low oxygen.
The overall motivation for this work stems from the central importance of Suc metabolism to both respiration and sugar signal transduction (Koch, 1996; Koch et al., 1996; Drew, 1997; Jang et al., 1997; Smeekens and Rook, 1997; Lalonde et al., 1999). In many instances, the essential first step in both processes is Suc cleavage by either invertase or the reversible Suc synthase reaction. Perturbation of these cleavage enzymes by low oxygen can therefore affect not only the entry of carbon into respiration, but also the extent to which this is reflected in sugar sensing pathways.
Under relatively extended periods of low oxygen (approximately 12 h to 7 d), Suc synthase activity typically increases, while that of invertase declines (Bertani et al., 1981; Guglielminetti et al., 1995, 1997; Perata et al., 1997; Ricard et al., 1998). However, changes may also occur during critical periods of earlier adjustment to low oxygen. Recently, time-course analyses of Suc synthase expression have shown that rapid responses can be involved (Zeng et al., 1998). Further, the earliest changes often reverse over time and can differ markedly between hypoxia (3% [v/v] O2) and anoxia (0% [v/v] O2) (Zeng et al., 1998).
We therefore hypothesized that the expression of invertases, which catalyze the only alternative path of sucrolysis, might be subject to similarly rapid regulation, reversals over time, and/or different responses to hypoxia and anoxia. Collectively, these could be fundamental to the tight control of Suc cleavage, and also to metabolic partitioning of its products between different paths under low oxygen. The speed of such a change could directly affect the extent of its contribution to the rapid, protective acclimation that can occur under hypoxia. Short exposures to hypoxia (as little as 3 h) can have striking effects on survival under subsequent anoxia, and can increase the activities of potentially vital enzymes (Andrews et al., 1994; Drew, 1997) such as Suc synthase (Zeng et al., 1998).
Rapid increases in invertase, however, might well be excluded from this protective response. Instead, reduced invertase expression may be specifically important among the many genes down-regulated under anaerobic stress (Sachs et al., 1980). There are at least two ways in which especially rapid repression of invertase genes could be significant to the effectiveness of fast, low-oxygen adjustments.
First, the most immediate possible conservation of the Suc, carbon, and ATP supplies would be of great advantage under low oxygen, and would be aided by an invertase shutdown. Vital Suc supplies can drop below threshold levels for respiration within the first 2 h of anaerobic stress of maize root tips (Bouny and Saglio, 1996; Drew, 1997), and phloem delivery of Suc is also markedly impaired under low oxygen (Saglio, 1985). In addition, carbohydrate supplies, and Suc in particular (Bouny and Saglio, 1996; Ricard et al., 1998), have been shown to markedly enhance the survival of tissues under anoxic stress. The most rapid reduction in invertase action would seemingly have the greatest advantage under these conditions, as long as the total capacity for Suc use did not drop below that needed for maintenance or post-stress recovery. Invertase reductions may also conserve ATP, with one source of savings being a decrease in the “futile” cycling of Suc through hydrolysis and resynthesis. This process is estimated to consume a significant portion of the ATP budget in aerobic root tips (Dieuaide-Nouhani et al., 1995). Invertase has also been suggested to increase ATP costs relative to Suc cleavage by Suc synthase, since invertase generates 2-fold more hexoses, each requiring ATP for phosphorylation (Huber and Akazawa, 1986; Xu et al., 1989; Guglielminetti et al., 1995; Perata et al., 1997). This comparative ATP savings, however, has been questioned due to the ultimate cost of regenerating PPi and its apparently limited supply under low oxygen (Drew, 1997).
Second, early reductions in invertase activity could have a greater effect than those of Suc synthase in limiting the influence of Suc cleavage products on sugar signal transduction (Koch, 1996; Koch et al., 1996; Zeng et al., 1998). Signals of sugar availability can otherwise enhance the expression of specific genes favoring carbohydrate allocation to biosynthetic processes (Koch, 1996). This could have a deleterious effect on carbon use under conditions in which these resources are essential for respiration (Drew, 1997; Hochachka et al., 1996; Perata et al., 1997; Vartapetian and Jackson, 1997). The extent of such signals can vary depending on the path of Suc cleavage and, as noted above, 2-fold more non-phosphorylated hexoses (Glc + Fru) are produced by invertase than by Suc synthase (Fru + UDP-Glc). Since these hexoses are substrates of the sugar signal transduction paths (with both hexokinase-based and membrane-linked sensors), invertase action can potentially exert a greater influence on responsive genes than the action of Suc synthase. Suc cleavage via invertase could thus have a broad yet detrimental effect on the expression of other genes under low-oxygen stress.
Beyond the speed of these responses, we were also interested in possible overlap between sugar and oxygen signaling mechanisms. Invertase genes are themselves sugar responsive (Roitsch et al., 1995; Stitt and Sonnewald, 1995; Xu et al., 1996), and also have the capacity to affect other sugar-modulated genes (Herbers et al., 1996; Koch, 1996; Koch et al., 1996). In addition, they share a differential sugar-responsiveness with Suc synthase genes, there being sugar-induced and sugar-repressed forms of each (Xu et al., 1996). Furthermore, the Suc synthase genes (Sus1 and Sh1) respond differentially to hypoxia and anoxia (Zeng et al., 1998) in parallel with their respective enhancement by conditions of carbohydrate abundance or depletion (Koch et al., 1992). Since the invertase genes Ivr1 and Ivr2 share this differential modulation by sugars (Xu et al., 1996), it seemed likely that their responses to hypoxia and anoxia might also be similar to those of the Suc synthases. This seemed especially likely if there were a consistent overlap between sugar and oxygen signaling mechanisms. This possibility was suggested by the potentially common role of hexokinases in both processes.
Finally, during the course of this investigation, analyses of long-term survival and genotype comparisons were conducted at the whole-seedling level to appraise the potential physiological significance of the observed changes. Also, as noted by Vartepetian and Jackson (1997) and Drew (1997), flood tolerance can differ markedly between the organ- and whole-plant levels, with flood-tolerant species often showing surprisingly sensitive roots (Vartepetian and Jackson, 1997). Escape mechanisms for these organs include aerenchyma formation and passive dormancy. Data presented here are also compatible with the possibility that the sacrifice of non-essential root sinks may be advantageous to ultimate long-term survival under extreme conditions.
The present study shows a rapid repression of maize invertases under low oxygen, and sharp changes in the invertase/Suc synthase balance. Advantages at the organ level would include prompt contributions to control of Suc use, possible ATP savings, and reduced signal transduction to genes for non-respiratory carbon utilization. In addition, analyses at the whole-seedling level indicated that there may be advantages for multiple mechanisms of reducing sink demands for carbon use under flooding stress.
MATERIALS AND METHODS
Plant Material
Maize (Zea mays L.) seeds of hybrid NK508, inbred W22, and inbred Oh43 were surface-sterilized for 20 min in 0.525% (v/v) sodium hypochlorite, and rinsed with water for 20 min. Germination progressed in darkness at 18°C on moistened filter paper (3 MM, Whatman, Clifton, NJ) in 27 × 39-cm glass pans. Each pan was sealed and supplied with a continuous, humidified air flow at 1 L min−1 for the duration of the 5- to 7-d germination period. Terminal 1-cm tips were excised from primary roots at selected time points during subsequent experimental treatments. For each sample, about 90 root tips (approximately 0.65 g) were pooled and divided into two groups, one for the assay of enzyme activity and one for the analysis of mRNA levels. Each of these was weighed, frozen in liquid N2, and stored at −80°C.
Hypoxic and Anaerobic Treatments
Experimental treatments were initiated when primary roots had reached approximately 5 cm (about 5 d for NK508, 7 d for W22, and 7 d for OH43). A positive pressure and fully humidified gas flow were maintained at 1 L min−1 for anoxic (N2 only), hypoxic (3% [v/v] O2 in N2), and aerobic (ambient air) treatments. The influence of these low-oxygen treatments may have been delayed somewhat for the inner tissues by residual internal oxygen. Root tip samples were harvested after 3, 6, 12, and 24 h of treatment. Intact controls were monitored for the post-stress seedling regrowth that occurred to varying degrees.
Enzyme Extraction and Assay
Frozen samples were ground to a fine powder in liquid N2, and enzymes were extracted in ice-cold medium containing 200 mm HEPES buffer (pH 7.5), 1 mm DTT, 5 mm MgCl, 1 mm EGTA, 20 mm sodium ascorbate, 1 mm PMSF, and 10% (w/v) polyvinylpolypyrrolidone. The buffer/tissue ratio was 10:1, and the extract was centrifuged at 14,000g for 10 min. The supernatant was dialyzed using a 25,000 Mr cutoff at 4°C for 24 h against extraction buffer diluted 1:40. The buffer was changed several times during dialysis. Later dialysis of the enzyme extract at a 50,000 Mr cutoff indicated the presence of an inhibitory fraction with relatively high Mr, but comparison between lines and treatments indicated a lack of a significant, differential effect in the present experiments.
Invertase was assayed in a total volume of 500 μL of reaction medium containing 10 mm sodium acetate buffer (pH 4.5), 20 mm Suc, and enzyme extract. Reactions conducted at 30°C for 15 min were terminated by adding 500 μL of potassium phosphate buffer (pH 7.5) and boiling for 1 min. Glc was quantified by Somogyi's and arsenomolebdate reagents and measured as A660. Insoluble invertase activity was approximately one-tenth that of soluble activity in root tips (Xu et al., 1996). Protein was quantified by the Bradford method using a BSA standard.
Suc synthase was assayed in the synthetic direction as described previously (Zeng et al., 1998). Twenty microliters of enzyme extract was assayed in 70 μL of reaction buffer at 30°C for 30 min, and this reaction was terminated by adding 30% (w/v) KOH and boiling. This was incubated with 1 mL of 0.14% (w/v) anthrone in H2SO4, and the product was quantified by measuring A620.
RNA Extraction and Analysis
After grinding frozen samples to a fine powder in liquid N2, RNA was extracted according to the method of McCarty (1986), and quantified by A260. For RNA separation and analysis, 10 μg of total RNA were electrophoresed along each lane of a 1% (w/v) agarose gel containing formaldehyde, transferred to a nylon membrane, and hybridized with maize cDNA probes as in Koch et al. (1992) using Ivr1 and Ivr2 invertases (Xu et al., 1996), Sus1 and Sh1 Suc synthases (from L.C. Hannah, University of Florida), and Adh1 (from R. Ferl, University of Florida). The labeled mRNA was visualized using x-ray film at −80°C, and its relative abundance was quantified using a phosphor imager (Molecular Dynamics, Sunnyvale, CA).
Survival Tests
For seedling survival tests, anaerobic treatments were initiated as above at 5 to 7 d after germination, when primary roots had reached approximately 5 cm. Entire seedlings were exposed to a N2 gas flow of 0.1 L min−1 for 7 d. The first 1 to 2 h may have involved varying degrees of internal hypoxia during tissue equilibration with exogenous N2. Seedling regrowth was tested by replacing the N2 flow with ambient air (21% [v/v] O2) for an additional 6 d. Viability was appraised by the appearance of new adventitious roots on seedlings retaining a green, growing shoot.
For root tip survival analyses, anaerobic treatments were conducted similarly but were limited to 12 and 24 h. The viability of any given tip could be accurately predicted by their resumption of growth after the return of ambient airflow. Elongation was measured by marking a point 1 cm behind each root tip with particulate charcoal immediately before the return of ambient airflow. No additional elongation occurred for any of the root tips during the low-O2 treatments, and the extent of subsequent aerobic growth was relatively uniform for those that remained viable.
RESULTS
Invertase Activity Dropped Rapidly with the Degree of Oxygen Deprivation and Genotype
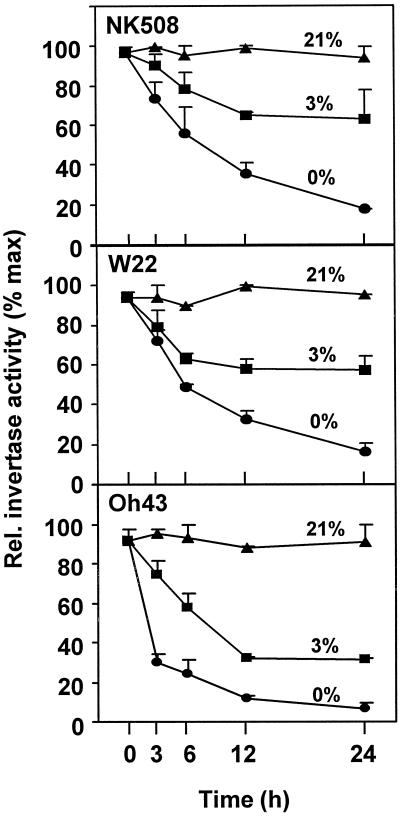
Figure 1 shows sharp decreases in soluble invertase activity from primary root tips during the first hours of seedling anoxia in all three maize lines tested. Mean activity values dropped to between 20% to 60% of aerobic levels during the first 6 h. The most rapid and pronounced reductions were evident in root tips from the Oh43 inbred (previously identified as a low-invertase line [Duke et al., 1991]), in which activity dropped within the first 3 h of anoxia to approximately 30% of the original values. Invertase activity from root tips of the NK508 hybrid and the W22 inbred stabilized under anoxia approximately 20% after 24 h (not shown), whereas that of the Oh43 inbred dropped below detectable levels. Under hypoxia (3% [v/v] O2), similar decreases in activity took place over a 12-h period, and soluble invertase activity stabilized at approximately 60% of aerobic values for both the NK508 hybrid and the W22 inbred. Activity in the Oh43 inbred dropped to a constant level of approximately 30% under hypoxia.
Figure 1.
Time course of the effects of low oxygen on invertase activity from root tips of intact maize seedlings under 0% (v/v) O2 (anoxic), 3% (v/v) O2 (hypoxic), or 21% (v/v) O2 (aerobic) conditions for a hybrid (NK508) and two inbreds (W22 and Oh43). Soluble invertase activities are presented and insoluble activity was approximately 10% of these values (not shown). Treatments were initiated after 5 to 7 d of germination, when roots had reached approximately 5 cm. One-centimeter tips of primary roots (approximately 90 total, for approximately 0.6 g) were excised at each time point. Data are means ± se of three separate experiments, and values are plotted as percentage of maximum activity (determined as μm Glc g−1 fresh weight h−1). At 21% (v/v) O2, soluble invertase activities for NK508, W22, and Oh43 were 282 (±8), 351 (±7), and 213 (±4) g−1 fresh weight h−1, respectively. Results were similar if expressed per unit of protein.
Anoxic Repression of Invertases Involved Sharp Changes in Transcript Abundance
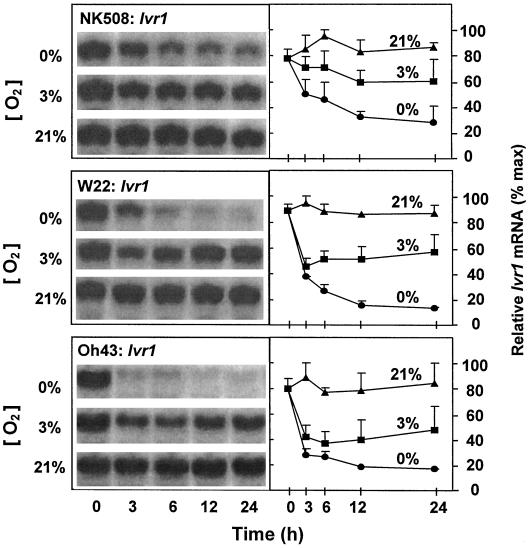
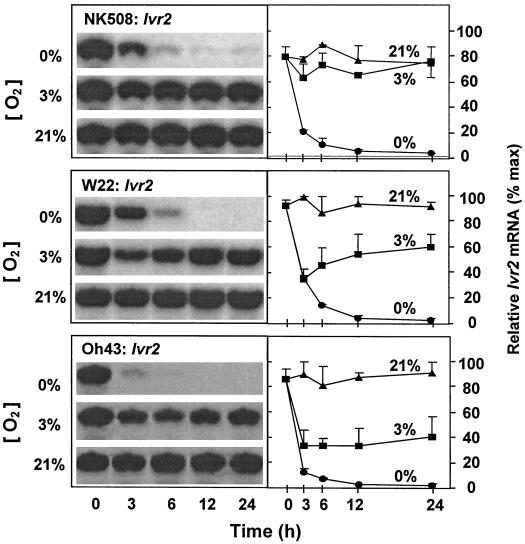
Levels of Ivr1 and Ivr2 mRNA were examined to determine whether transcript levels could have changed rapidly enough to contribute to the decreased enzyme activity in each of the lines tested, and also to compare responses of the two genes. Figures 2 and 3 show that in each instance, Ivr1 and Ivr2 mRNA levels were markedly reduced in root tips during the first 3 h of anoxia. Similar drops were also evident under hypoxia for the inbred lines. In addition, there was an unexpected similarity in these responses despite differences in sugar regulation of the genes involved.
Figure 2.
Time course of changes in Ivr1 mRNA levels from root tips of intact maize seedlings under 0% (v/v) O2 (anoxic), 3% (v/v) O2 (hypoxic), or 21% (v/v) O2 (aerobic) conditions for a hybrid (NK508) and two inbreds (W22 and Oh43). Treatments and samples were as noted for Figure 1, and were from the same experiments. RNA gel blots were visualized by autoradiography, and the abundance of 32P-mRNA was quantified with a phosphor imager (Molecular Dynamics). Ten micrograms of total RNA was loaded in each lane and uniformity was verified by visualization of rRNA bands (not shown) and by the constancy of Ivr1 mRNA levels under 21% (v/v) O2 (bottom panels in each figure). For each experiment, data from the three oxygen treatments were compared in adjacent lanes on the same blot. Data represent the means ± se of three separate experiments. Identical blots were probed with Ivr2 in Figure 3.
Figure 3.
Time course of changes in Ivr2 mRNA levels from root tips of intact maize seedlings under 0% (v/v) O2 (anoxic), 3% (v/v) O2 (hypoxic), or 21% (v/v) O2 (aerobic) conditions for a hybrid (NK508) and two inbreds (W22, and Oh43). Blots were identical to those probed with Ivr1 in Figure 2, except that mRNA was hybridized with a cDNA for Ivr2. Visualization and quantification were also as in Figure 2. Data represent the means ± se of three separate experiments.
Differences between the Ivr1 and Ivr2 responses were also observed, however. The extent to which Ivr1 mRNA levels dropped under low oxygen (Fig. 2) was less than that observed for Ivr2 (Fig. 3), and occurred primarily in the first 3 h. After 24 h of anoxia, Ivr1 mRNA levels remained at approximately 25% of those observed for aerobic controls in each line tested, whereas those of Ivr2 had dropped to near the limits of detection within 12 h. When expression of these genes in the inbreds was compared with that in the hybrid, mRNA levels in the more vigorous hybrid line (NK508) indicated reduced sensitivity of Ivr2 to hypoxia and reduced sensitivity of Ivr1 to anoxia.
Rapid, Transient Decreases in Suc Synthase Activity Accompanied the Invertase Responses of Oh43
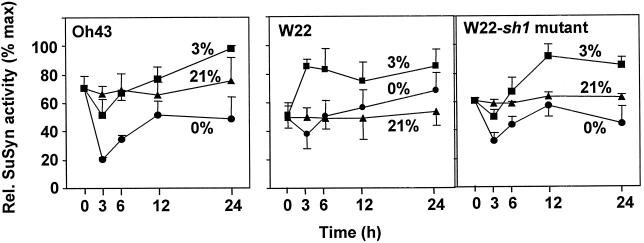
The speed of change for Suc synthase activity was tested under low oxygen in the Oh43 inbred to determine if shifts occurred as rapidly as they did for invertase in this line. Figure 4 shows significant, abrupt, unexpected declines in the Suc synthase activity of Oh43 root tips under 0% (v/v) O2 (P = 0.01 for 3 h), followed by recovery to near aerobic levels within 12 h of treatment. The same degree of response was not observed for the other inbred (W22, Fig. 4, and Zeng et al., 1998) (P = 0.1 for 3 h) or in the NK508 hybrid (not shown). However, this pattern of Suc synthase activity in the Oh43 inbred was also strongly apparent in a sh1-null mutant (Fig. 4, P = 0.03 for 3 h under 0% [v/v] O2). Responses in the latter were the result of the SUS1 Suc synthase acting alone. Both patterns were consistent with the possibility that rapid decreases in the SUS1 enzyme activity might contribute to the first phases of low-oxygen adjustment, when SH1 activity is otherwise prominent (Fig. 4 and Zeng et al., 1998). Levels of Sus1 and Sh1 mRNA (not shown) increased rather than decreased during the sudden, transient dip in Suc synthase activity observed in the Oh43 material. This discrepancy suggested post-transcriptional control at the translational level (Fennoy and Bailey-Serres, 1995; Fennoy et al., 1998) and/or via enzyme regulation (Huber et al., 1996; Zeng et al., 1998).
Figure 4.
Time course of the effects of low oxygen on Suc synthase activity from root tips of intact maize seedlings under 0% (v/v) O2 (anoxic), 3% (v/v) O2 (hypoxic), or 21% (v/v) O2 (aerobic) conditions for the Oh43 inbred, with data from the W22 inbred and an isogenic W22-sh1 mutant (null for the Sh1 gene) shown for comparison (the latter from Zeng et al., 1998). Treatments and samples were as in Figure 1.
Low Oxygen Decreased Both Invertase/Suc Synthase Balance and the Capacity for Suc Cleavage
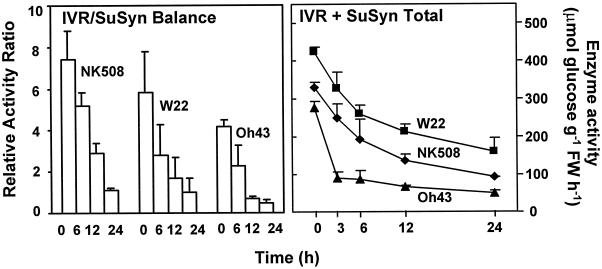
Invertase activity consistently decreased to a greater degree under low oxygen than did that of Suc synthase in all lines tested. Figure 5 shows that the invertase/Suc synthase balance changed to an essentially similar degree in the inbred and hybrid, favoring Suc cleavage via Suc synthase in each instance. A marked change in this balance occurred within 6 h of anoxic treatment for both NK508 and W22 root tips, and within 12 h for Oh43 seedlings.
Figure 5.
Estimated capacity for Suc flux through Suc synthase versus invertase during the progression of low-oxygen events under anoxia. The relative invertase (IVR)/Suc synthase (SuSyn) balance and the estimated capacity for Suc cleavage (invertase + Suc synthase activities) were calculated from activity data not shown plus those presented in Figure 1 and those in Zeng et al. (1998). Data represent ranges.
The total relative capacity for Suc cleavage was estimated as invertase plus Suc synthase activity (Fig. 5). This consistently decreased in each of the lines tested, but did so with particular speed in the Oh43 inbred. Resource conservation would be favored, but Suc import would not. Decreases in the capacity for sucrolysis also paralleled those indicated to occur for carbon flux through glycolysis under low oxygen (Bouny and Saglio, 1996; Drew, 1997).
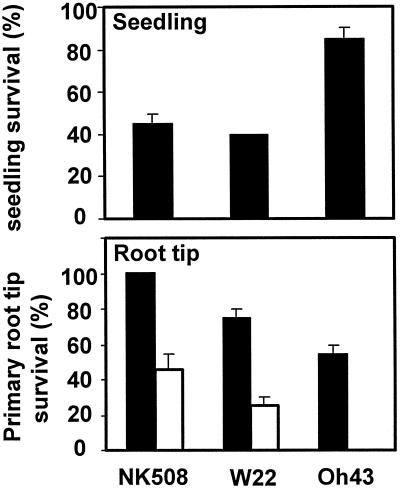
Root Tip Viability Was Minimal But Seedling Survival Maximal in the Oh43 Inbred
To determine whether the pronounced effect of low oxygen on invertase activity might be associated with a survival advantage, the capacity for Oh43 seedlings to recover from extended low oxygen was compared with that of NK508 and W22 (Fig. 6). Seedlings were subjected to anoxia for a 7-d period (which was probably preceded by 1–2 h of initial hypoxia in the system utilized), then allowed to recover. Regrowth of the Oh43 seedlings was associated with the initiation of new adventitious roots rather than with continued elongation by pre-existing roots.
Figure 6.
Percentage seedling survival after 7 d (black bars) and root-tip survival after 12 and 24 h (black and white bars, respectively) of whole-seedling exposure to 0% (v/v) O2 (anoxia) imposed as N2 gas flow in darkness for hybrid NK508, and inbreds W22 and Oh43. Treatment was initiated 5 to 7 d after germination, when primary roots had reached approximately 5 cm. Capacity for regrowth was tested by replacing the N2 flow with ambient air (21% [v/v] O2) for an additional 6 d. Viability was appraised by the formation of new adventitious roots in addition to the maintenance of a turgid, growing shoot. Data represent means ± se of three separate experiments. Twenty-four hours after excision (not shown), less than 50% of NK508 root tips survived and all primary root tips from Oh43 had died.
In contrast, individual root tips proved considerably more vulnerable to low oxygen than intact seedlings (Fig. 6). A 24-h fully anoxic treatment was a severe, often lethal stress for root tips, even when preceded by short periods of hypoxia (Sachs et al., 1980; Drew, 1997). At the seedling level, long-term survival rates were less affected. In fact, the greatest sensitivity of root tips to low-oxygen stress was accompanied by the strongest capacity for long-term seedling survival.
DISCUSSION
The significance of this work is the demonstration that a rapid shift from invertase to Suc synthase balance occurs early during low-oxygen adjustment. The extent and relative speed of this response is consistent with the importance of Suc as the central carbon source for glycolysis, and the differences between the cleavage paths in terms of the potential to affect sugar signaling. Second, the mechanism of this change is shown here to include rapid alterations in the activities of both invertases and Suc synthases. Invertase repression involves marked drops in activity as well as transcript levels, whereas enzyme level regulation is implicated for sharp changes in Suc synthase activity. Finally, the total capacity for Suc cleavage is also shown here to fall during low-oxygen stress, and in some instances may drop below the limits for maintenance of root tip viability and/or stress recovery. Whole-seedling analyses indicated that the early mortality of non-essential sinks is associated with long-term survival under extreme stress, and may provide an additional means of reducing sink activity. These observations underscore the importance of resource conservation for low-oxygen survival and also the potential for Suc cleavage to differentially affect sugar sensing.
Rapid Repression of Maize Invertases by Low Oxygen: Both Enzyme and mRNA Levels Drop Sharply
In contrast to other genes for Suc cleavage and fermentative metabolism (Sachs et al., 1996; Dolferus et al., 1997; Drew, 1997), invertase genes are strongly repressed by low oxygen. Unlike the maintenance or up-regulation observed at the protein and activity levels for other glycolytic and sucrolytic reactions (Ricard et al., 1994; Sachs et al., 1996; Dolferus et al., 1997; Drew, 1997), pronounced decreases were observed for invertase activity within 3 h of anoxia and 6 h of hypoxia (Fig. 1). In addition, the speed and extent of this response varied with genotype. In each instance, the eventual longer-term decreases in activity were similar to those reported after overnight anoxic treatment of maize roots (Ricard et al., 1998) and from whole-seedling submergence studies (Bertani et al., 1981; Guglielminetti et al., 1995, 1997; Perata et al., 1997). However, the rapid responses reported here put the timing of invertase repression early enough to participate in the transition period (Sachs et al., 1996), to enhance early energy savings (Dieuaide-Noubhani et al., 1995), and to alter intermediate signals from the carbon flux through sugar signaling systems to downstream genes (Koch, 1996).
Contributing mechanisms for invertase repression by low oxygen are shown here to include marked reductions in steady-state levels of both Ivr1 and Ivr2 invertase mRNAs (Figs. 2 and 3, respectively). These dropped within 3 h of anoxia for all genotypes, and also under hypoxia for the inbreds tested. Although changes in transcript abundance were less prominent in hypoxic responses by the hybrid, both inbreds had completed a strong, stable, downward readjustment of invertase mRNA levels within 3 h of hypoxia. The speed of this drop was compatible with its capacity to effect ensuing changes at the enzyme level in both inbreds (compare Fig. 1 with Figs. 2 and 3) and with the potential for short-term hypoxia to alter subsequent anoxic responses (Andrews et al., 1994; Drew, 1997). The rapid drops in invertase mRNA abundance may have been due to either changes in transcription and/or message stability (Sachs et al., 1980; Bailey-Serres and Freeling, 1990; Crosby and Vayda, 1991; Fennoy and Bailey-Serres, 1995; Hochachka et al., 1996; Drew, 1997). However, the latter is highlighted by evidence indicating that transcription can continue without interruption under low oxygen, even for many non-anaerobic genes (Fennoy et al., 1998).
Proteolysis is probably an additional effector of the reductions in invertase activity and can occur rapidly in the tips of more mature roots (Andrews et al., 1994). Nonetheless, rates of invertase activity loss reported here are still greater than those of general proteolysis under low oxygen, and indicate that some degree of specificity may be involved, possibly akin to that mediated by the SCF system in yeast (Johnston, 1999).
Differential Sugar-Responsiveness of Invertase Genes Was Not Evident in Their Low-Oxygen Regulation
A key point of contrast between invertase responses reported here (Figs. 1–3) and those described previously for the two Suc synthases (Zeng et al., 1998) is the relationship between their sugar and oxygen regulation. For Suc synthases, the two genes show differential responses to both sugars and oxygen. Sh1 is generally induced by either anoxia or sugar deprivation, whereas Sus1 is induced by hypoxia or sugar abundance (Koch et al., 1992; Xu et al., 1996; Zeng et al., 1998). Such parallels between sugar responses and oxygen responses were not evident for the invertase genes, however. The similarity between sugar and oxygen regulation of Suc synthase genes is compatible with current evidence for a hexokinase-based system for sugar signal transduction in which flux through this first step in glycolysis is linked to a carbohydrate-responsive gene expression as well as metabolism (Koch, 1996; Jang et al., 1997; Smeekens, 1998). Because low oxygen can so markedly perturb carbon flux through this step in glycolysis (Saglio et al., 1980; Hole et al., 1992; Xia and Saglio, 1992; Bouny and Saglio, 1996; Drew, 1997), hypoxia and anoxia have the potential to differentially alter the expression of many sugar-modulated genes. Nonetheless, this pattern of commonality between sugar and oxygen responses (Zeng et al., 1998) was not evident for the Ivr1 or Ivr2 invertases.
Rapid Effects of Low Oxygen Were Implicated for Suc Synthase at the Enzyme Level
Suc synthase responses were found to change in an atypical manner when examined as a counterpart to those of the especially rapid invertase changes in Oh43 root tips (Fig. 4). The unusual, transient drop in Suc synthase activity also showed a marked similarity to that observed earlier in a sh1-null mutant (Zeng et al., 1998 and Fig. 4). In this mutant, the SH1 Suc synthase protein was absent, so it is tempting to speculate that the similar pattern of Suc synthase activity in Oh43 may have arisen from the action of the SUS1 rather than SH1 Suc synthase under low oxygen. Comparison with the rising levels of mRNA for both Suc synthases (not shown) implicates a translational or enzyme-level mechanism of regulation in some lines, which could involve changes in mRNA stability and/or protein elongation (Fennoy and Bailey-Serres, 1995; Fennoy et al., 1998), as well as effects on Suc synthase phosphorylation (Huber et al., 1996). Conditions affecting both processes appear to change under low-oxygen conditions (S. Chalivendra and M. Sachs, personal communication; Zhang and Chollet., 1997).
Sharp Shifts in Invertase/Suc Synthase Balance Favored ATP Conservation and Reduced Sugar Signaling
The invertase/Suc synthase ratio dropped significantly in only 6 h for both NK508 and W22 root tips, and more slowly for Oh43 (Fig. 5). Mean ratios fell to half their original values during these first 6 h. Such shifts have been indicated at the protein level in longer-term studies of maize root tips (Springer et al., 1986; Bailey-Serres et al., 1988; Guglielminetti et al., 1997; Ricard et al., 1998; Zeng et al., 1998), and also in seedlings and embryos of other species (Ricard et al., 1991; Guglielminetti et al., 1995, 1997). Decreased invertase activities were evident after long-term flooding of rice seedlings (Bertani et al., 1981), overnight anoxia treatment of maize roots (Ricard et al., 1998), and after 1 or more d of submergence for whole seedlings of maize (Guglielminetti et al., 1997), wheat, rice, and barley (Perata et al., 1997). Eventual changes in the invertase/Suc synthase ratio were recently indicated to favor the Suc synthase path of Suc cleavage by 30-fold (Perata et al., 1997). However, a sharp shift toward this pathway could have important, immediate advantages to cellular adjustment at both the metabolic and gene expression levels.
The first of these advantages could be ascribed to any change in ATP conservation resulting from a shift toward the Suc synthase reaction. The anaerobic energy budget is generally considered to be a central feature of low-oxygen acclimation (Hochachka et al., 1996; Drew, 1997; Perata et al., 1997; Vartapetian and Jackson, 1997) and would be affected early by the rapid invertase repression described here. One possibility is that energy conservation could be realized if invertase reduction decreased Suc cycling. This process of continued Suc synthesis and breakdown has been appraised in vivo, and is estimated to consume a substantial portion of the ATP in aerobic maize root tips (Dieuaide-Noubhani et al., 1995). Another possibility is that an invertase-to-Suc synthase shift could theoretically double the immediate ATP savings associated with the entry of Suc cleavage products into glycolysis under low oxygen. Unlike invertase, only half of the Suc synthase products are free hexoses that need subsequent phosphorylation. Metabolism of the other Suc synthase product, UDP-Glc, depends on PPi (diagrammed by Guglielminetti et al. [1995] and Perata et al. [1997]). The positive energetics of this mechanism were initially proposed for starved aerobic cells by Huber and Akazawa (1986) and in other systems by Xu et al. (1989), but its importance under anaerobic conditions has been called into question by the limited supplies of PPi and their apparent lack of turnover (Drew, 1997).
A second point of significance for the invertase/Suc synthase shift, and particularly its relative speed, is the potential effect this can have on sugar signaling. As noted above, invertase has a distinct disadvantage in the realm of sugar signal transduction under low oxygen, since both its hexose products are theoretically “sensible” by membrane-based and hexokinase-linked sensing systems. The extent of carbon metabolism would be the same, but it could have a proportionally greater impact on genes for costly biosynthetic processes. Effects of invertase repression could be especially beneficial if there were corresponding reductions in specific hexokinases (as in humans [Bell et al., 1996]). This is a distinct possibility in higher plants, where there are multiple forms of gluco- and fructokinases having as-yet-undefined roles in respiration versus sugar sensing (Renz and Stitt, 1993; Galina et al., 1995; Bouny and Saglio, 1996; Drew, 1997). Invertase reductions could also decrease the hexoses available to membrane-based sensors (Lalonde et al., 1999). Finally, metabolic studies have indicated that Glc is significantly less effective as a respiratory substrate under low oxygen than are Fru or Suc (Bouny and Saglio, 1996). Collectively, this evidence supports the potential for down-regulation of invertase under low oxygen to be advantageous in the limitation of carbon flux through free Glc and subsequent reduction of Glc-based signals of sugar abundance.
Loss of Non-Essential Sink Organs and Seedling Survival under Long-Term Stress
At an organ level, the rapid decreases in total sucrolytic capacity under low oxygen (Fig. 5) could be advantageous for resource conservation as long as a threshold level of Suc metabolism is preserved. Such reductions may be especially important, since glycolytic rate under low oxygen “corresponds precisely to the depletion of Suc” (Bouny and Saglio, 1996), and would complement regulation of glycolytic flux by hexokinase under low oxygen (Renz and Stitt, 1993; Bouny and Saglio, 1996; Drew, 1997). The further importance of Suc under anaerobic conditions is implicated by the reversal of Suc/Glc ratios during hypoxic pretreatments, such that Suc predominates after a short period of acclimation to sublethal stress (Bouny and Saglio, 1996). Finally, although phloem function and Suc unloading are impaired under anoxia (Saglio, 1985), measurements of G-6-P levels (sustained by glycolysis) indicated that at least some Suc continued to be delivered to root tips (Bouny and Saglio, 1996). Intact root tips consistently fared as well, typically better in this regard than sugar-supplemented, excised tips. Even a much reduced Suc supply could be a critical resource under low oxygen, and factors affecting its initial cleavage could influence not only carbon use, but also translocation from seeds or leaves to roots.
At the seedling level, however, allocation of vital carbon resources to non-essential sinks could be detrimental under severe low-oxygen stress. In fact, seedling survival under long-term oxygen deprivation was significantly greater for the Oh43 inbred, despite the level of root tip mortality observed after short-term anoxia in this line (Fig. 6). This loss of carbon sinks could theoretically minimize the loss of carbon supply critical to the survival of severe low-oxygen stress. Interestingly, similar overall responses occur in a number of more flood-adapted species (Vartapetian and Jackson, 1997), which show a higher rather than a lower level of oxygen sensitivity in some organs (Vartapetian and Jackson, 1997).
In conclusion, the speed and extent of change for invertase expression, together with rapid shifts in Suc synthase regulation, move control of Suc metabolism into the early phases of low-oxygen acclimation. The rapid down-regulation of invertases also contrasts markedly to other genes for Suc use and energy metabolism under low oxygen (Hochachka et al., 1996; Drew, 1997; Perata et al., 1997; Vartapetian and Jackson, 1997). This has potentially immediate advantages for the conservation of Suc and possibly ATP, as well as for sugar signaling (less Glc for hexose sensing systems). Finally, the whole-seedling responses observed here suggest that multiple levels and mechanisms may contribute to the conservation of vital carbon supplies under low-oxygen stress.
Footnotes
This research was supported by a grant from the National Science Foundation and by the University of Florida Experiment Station (journal series no. R–07084).
LITERATURE CITED
- Andrews DL, Drew MC, Johnson JR, Cobb BG. The response of maize seedlings of different ages to hypoxic and anoxic stress: changes in induction of Adh1 mRNA, ADH activity, and survival of anoxia. Plant Physiol. 1994;105:53–60. doi: 10.1104/pp.105.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Freeling M. Hypoxic stress-induced changes in ribosomes of maize seedlings. Plant Physiol. 1990;94:1237–1243. doi: 10.1104/pp.94.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Serres J, Kloeckner-Gruissem B, Freeling M. Genetic and molecular approaches to the study of the anaerobic responses and tissue specific gene expression in maize. Plant Cell Environ. 1988;11:351–357. [Google Scholar]
- Bell GI, Pilkis SJ, Weber IT, Polonsky KS. Glucokinase mutations, insulin secretion, and Diabetes mellitus. Annu Rev Physiol. 1996;58:171–186. doi: 10.1146/annurev.ph.58.030196.001131. [DOI] [PubMed] [Google Scholar]
- Bertani A, Brambilla I, Menegus F. Effect of anaerobiosis on carbohydrate content in rice roots. Biochem Physiol Pflanz. 1981;176:835–840. [Google Scholar]
- Bouny JM, Saglio P. Glycolytic flux and hexokinase activities in anoxic maize root tips acclimated by hypoxic pretreatment. Plant Physiol. 1996;111:187–194. doi: 10.1104/pp.111.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby JS, Vayda ME. Stress-induced translational control in potato tubers may be mediated by polysome-associated proteins. Plant Cell. 1991;3:1013–1023. doi: 10.1105/tpc.3.9.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide-Noubhani M, Raffard G, Canioni P, Pradet A, Raymond P. Quantification of compartmented metabolic fluxes in maize root tips using isotope distribution from 13C- or 14C-labeled glucose. J Biol Chem. 1995;270:13147–13159. doi: 10.1074/jbc.270.22.13147. [DOI] [PubMed] [Google Scholar]
- Dolferus R, Ellis M, Bruxelles GD, Trevaskis B, Hoeren F, Dennis ES, Peacock WJ. Strategies of gene action in Arabidopsis during hypoxia. Ann Bot. 1997;79:21–31. [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Duke ER, McCarty DR, Koch KE. Organ-specific invertase deficiency in the primary root of an inbred maize line. Plant Physiol. 1991;97:523–527. doi: 10.1104/pp.97.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennoy SL, Bailey-Serres J. Post-transcriptional regulation of gene expression in oxygen-deprived roots of maize. Plant J. 1995;7:287–295. doi: 10.1046/j.1365-313X.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Fennoy SL, Nong T, Bailey-Serres J. Post-transcriptional processes override transcription in oxygen-deprived roots of maize. Plant J. 1998;15:727–735. doi: 10.1046/j.1365-313X.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Galina A, Reis M, Albuquerque MC, Puyou AG, Puyou MTG. Different properties of the mitochondrial and cytosolic hexokinases in maize roots. Biochem J. 1995;309:105–112. doi: 10.1042/bj3090105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Perata P, Alpi A. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiol. 1995;108:735–741. doi: 10.1104/pp.108.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielminetti L, Wu Y, Boschi E, Yamaguchi J, Favati A, Vergara M, Perata P, Alpi A. Effects of anoxia on sucrose degrading enzymes in cereal seeds. J Plant Physiol. 1997;150:251–258. [Google Scholar]
- Herbers K, Meuwly P, Frommer W, Metraux J-P, Sonnewald U. Systematic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell. 1996;8:793–803. doi: 10.1105/tpc.8.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hole DJ, Cobb BG, Hole P, Drew MC. Enhancement of anaerobic respiration in root tips of Zea mays following low oxygen (hypoxic) acclimation. Plant Physiol. 1992;99:213–218. doi: 10.1104/pp.99.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986;81:1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Huber JL, Liao PC, Gage DA, McMichael RW, Chourey PS, Hannah LC, Koch KE. Phosphorylation of serine-15 of maize leaf sucrose synthase. Plant Physiol. 1996;112:793–802. doi: 10.1104/pp.112.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Leon P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. Feasting, fasting and fermenting: glucose sensing in yeast and other cells. Trends Genet. 1999;15:29–33. doi: 10.1016/s0168-9525(98)01637-0. [DOI] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Koch KE. Molecular crosstalk and the regulation of C- and N-responsive genes. In: Foyer C, Quick P, editors. A Molecular Approach to Primary Metabolism in Plants. London: Taylor and Francis; 1997. pp. 105–124. [Google Scholar]
- Koch KE, Nolte KD, Duke ER, McCarty DR, Avigne WT. Sugar levels modulate differential expression of maize sucrose synthase genes. Plant Cell. 1992;4:59–69. doi: 10.1105/tpc.4.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE, Wu Y, Xu J. Sugar and metabolic regulation of genes for sucrose metabolism: potential influence of maize sucrose synthase and soluble invertase responses on carbon partitioning and sugar sensing. J Exp Bot. 1996;47:1179–1185. doi: 10.1093/jxb/47.Special_Issue.1179. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, Ward JM. The dual function of sugar carriers: transport and sugar sensing. Plant Cell. 1999;11:707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DR. A rapid and simple method for extracting RNA from maize tissues. Maize Gen Coop News Lett. 1986;60:61. [Google Scholar]
- Perata P, Guglielminetti L, Alpi A. Mobilization of endosperm reserves in cereal seeds under anoxia. Ann Bot. 1997;79:49–56. [Google Scholar]
- Renz A, Stitt M. Substrate specificity and product inhibition of different forms of fructokinases in developing potato tubers. Planta. 1993;190:166–175. [Google Scholar]
- Ricard B, Couee I, Raymond P, Saglio PH, Saint-Ges V, Pradet A. Plant metabolism under hypoxia and anoxia. Plant Physiol Biochem. 1994;32:1–10. [Google Scholar]
- Ricard B, Rivoal J, Spiteri A, Pradet A. Anaerobic stress induces the transcription and translation of sucrose synthase in rice. Plant Physiol. 1991;95:669–674. doi: 10.1104/pp.95.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard B, VanToai T, Chourey P, Saglio P. Evidence for the critical role of sucrose synthase for anoxic tolerance of maize roots using a double mutant. Plant Physiol. 1998;116:1323–1331. doi: 10.1104/pp.116.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, Bittner M, Godt DE. Induction for apoplastic invertase of Chenopodium rubrum by D-glucose and a glucose analog and tissue-specific expression suggest a role in sink-source regulation. Plant Physiol. 1995;108:285–294. doi: 10.1104/pp.108.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MM, Freeling M, Okimoto R. The anaerobic proteins of maize. Cell. 1980;20:761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Sachs MM, Subbaiah CC, Saab IN. Anaerobic gene expression and flooding tolerance in maize. J Exp Bot. 1996;47:1–15. [Google Scholar]
- Saglio PH. Effect of path or sink anoxia on sugar translocation in roots of maize seedlings. Plant Physiol. 1985;77:285–290. doi: 10.1104/pp.77.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio PH, Raymond P, Pradet A. Metabolic activity and energy charge of excised maize root tips under anoxia. Plant Physiol. 1980;66:1053–1057. doi: 10.1104/pp.66.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. Sugar regulation of gene expression in plants. Plant Biol. 1998;1:230–234. doi: 10.1016/s1369-5266(98)80109-x. [DOI] [PubMed] [Google Scholar]
- Smeekens S, Rook F. Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol. 1997;115:7–13. doi: 10.1104/pp.115.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B, Werr W, Starlinger P, Bennett CD, Zokolica M, Freeling M. The shrunken gene on chromosome 9 of Zea mays L is expressed in various plant tissues and encodes an anaerobic protein. Mol Gen Genet. 1986;205:461–468. doi: 10.1007/BF00338083. [DOI] [PubMed] [Google Scholar]
- Stitt M, Sonnewald U. Regulation of metabolism in transgenic plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:341–368. [Google Scholar]
- Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Ann Bot. 1997;79:3–20. [Google Scholar]
- Xia JH, Saglio PH. Lactic acid efflux as a mechanism of hypoxic acclimation of maize toot tips to anoxia. Plant Physiol. 1992;100:40–46. doi: 10.1104/pp.100.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DP, Sung SJS, Loboda T, Kormanik PP, Black CC. Characterization of sucrolysis via the uridine diphosphate and pyrophosphate-dependent sucrose synthase pathway. Plant Physiol. 1989;90:635–642. doi: 10.1104/pp.90.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Avigne WT, McCarty DR, Koch KE. A similar dichotomy of sugar modulation and developmental expression affects both paths of sucrose metabolism: evidence from a maize invertase gene family. Plant Cell. 1996;8:1209–1220. doi: 10.1105/tpc.8.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE. Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional responses. Plant Physiol. 1998;116:1573–1583. doi: 10.1104/pp.116.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Chollet R. Seryl-phosphorylation of soybean nodule sucrose synthase (nodulin-100) by a Ca2+-dependent protein kinase. FEBS Lett. 1997;410:126–130. doi: 10.1016/s0014-5793(97)00537-1. [DOI] [PubMed] [Google Scholar]