Abstract
Purpose
A proportion of patients with pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) do not achieve treatment goals or experience side effects on their current therapy. In such cases, switching patients to a new drug while discontinuing the first may be a viable and appropriate treatment option. CAPTURE was designed to investigate how physicians manage the switching of patients to riociguat in real-world clinical practice. Observations from the study were used to assess whether recommendations in the riociguat prescribing information are reflected in clinical practice.
Methods
CAPTURE was an international, multicenter, uncontrolled, retrospective chart review that collected data from patients with PAH or inoperable or persistent/recurrent CTEPH who switched to riociguat from another pulmonary hypertension (PH)-targeted medical therapy. The primary objective of the study was to understand the procedure undertaken in real-world clinical practice for patients switching to riociguat.
Results
Of 127 patients screened, 125 were enrolled in CAPTURE. The majority of patients switched from a phosphodiesterase type 5 inhibitor (PDE5i) to riociguat and the most common reason for switching was lack of efficacy. Physicians were already using the recommended treatment-free period when switching patients to riociguat from sildenafil, but a slightly longer period than recommended for tadalafil. In line with the contraindication, the majority of patients did not receive riociguat and PDE5i therapy concomitantly. Physicians also followed the recommended dose-adjustment procedure for riociguat.
Conclusion
Switching to riociguat from another PH-targeted therapy may be feasible in real-world clinical practice in the context of the current recommendations.
Electronic supplementary material
The online version of this article (10.1007/s00408-018-0100-3) contains supplementary material, which is available to authorized users.
Keywords: Chronic thromboembolic pulmonary hypertension, Endothelin receptor antagonists, Phosphodiesterase type 5 inhibitors, Pulmonary arterial hypertension, Real-world evidence, Riociguat
Introduction
Pulmonary hypertension (PH) is a debilitating condition that is classified based on the underlying etiology into five categories, including pulmonary arterial hypertension (PAH; group 1) and chronic thromboembolic pulmonary hypertension (CTEPH; group 4) [1–3].
There are several approved treatments for PAH: endothelin receptor antagonists (ERAs), prostacyclin analogs or prostaglandin I2 (IP) receptor agonists, phosphodiesterase five inhibitors (PDE5i), and the soluble guanylate cyclase (sGC) stimulator riociguat [1, 3, 4]. For patients with CTEPH, the gold standard treatment is the potentially curative pulmonary endarterectomy (PEA) [1, 5, 6]. Medical therapy with riociguat remains the option for patients who are ineligible for surgery, or develop persistent/recurrent CTEPH [1, 6–15]. Balloon pulmonary angioplasty is an emerging, minimally invasive treatment currently under investigation [16].
For patients with PAH who do not achieve treatment goals, current European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines recommend double- or triple-sequential combination therapy [1]. In studies investigating escalation of treatment, the initial drug is routinely continued. However, in cases where patients do not respond to the initial therapy, there are no data on whether the additional clinical effect is based on the drug combination. As such, in some cases it may be better to discontinue the original agent before starting a new therapy [17].
This strategy of switching patients from one PH-targeted therapy to another is largely unexplored in clinical practice. Small studies and case reports have demonstrated positive outcomes after switching, but these have largely involved switching within a drug class, and were mainly due to lack of efficacy [18–20] or safety and tolerability [21, 22] of the former drug.
Riociguat is currently the only medical therapy approved for the treatment of both PAH and inoperable or persistent/recurrent CTEPH [23, 24]. Switching from a PDE5i to riociguat in PAH patients with an insufficient response to treatment has been explored in the RESPITE study. Results from this uncontrolled pilot study indicated that this may be a feasible and effective treatment strategy [25]. Subgroup analysis from a CTEPH early access study and a study of 23 patients switching from PDE5i to riociguat suggest that switching from off-label PH-targeted therapy to riociguat is well tolerated in patients with CTEPH [20, 26].
Despite promising preliminary data, little is known about how switching to riociguat is managed in clinical practice. The CAPTURE study was designed to investigate how and why patients with PAH and inoperable or persistent/recurrent CTEPH are switched from other PH-targeted therapies to riociguat in real-world clinical practice. Data from the study were also used to assess whether recommendations from the riociguat prescribing information were in line with real-world practice [23].
Methods
Study Design
CAPTURE (clinicaltrial.gov: NCT02545465) was an international, multicenter, uncontrolled, retrospective chart review that collected data from patients with PAH or inoperable or persistent/recurrent CTEPH, who switched to riociguat from another PH-targeted therapy.
Patients
Male and female patients with PAH or inoperable or persistent/recurrent CTEPH who were switched to riociguat from another PH-targeted medical therapy and completed a 5-month documentation period were included. All patients were ≥ 18 years and provided written informed consent. Patients who did not switch therapy but received riociguat purely as an add-on to an ERA or prostacyclin analog were not eligible.
Study Procedures
Data were retrospectively collected from patient medical records for the 12-month period prior to switching and the 5-month period post-switching. For patients who discontinued riociguat, data were still collected for the 5-month post-switch period.
Decisions about clinical management of each patient, including riociguat treatment duration, were determined solely by the treating physician, without influence from the study protocol.
Outcome Measures
Primary Outcome Measures
Primary outcome measures included information on riociguat dose adjustment during switching, vital signs during the dose-adjustment period (systolic and diastolic blood pressure and heart rate), switch medication, reason for switching, and duration of treatment-free periods between previous medication and riociguat.
A treatment-free period was defined as the number of days between the last intake of the switched therapy and the first treatment with riociguat (excluding the last day of pre-switch drug intake and the first day of riociguat treatment). A treatment-free period of 0 indicates that riociguat was started 1 day after the last intake of the switched drug. A negative value for the treatment-free period indicates the switched drug was discontinued after the start of riociguat.
Other Variables
Patient characteristics (including baseline demographics, medical history, and clinical characteristics) and clinical parameters (6-min walking distance [6MWD], Borg Dyspnea Index, World Health Organization function class [WHO FC], and biomarkers) were also collected.
Safety Assessments
Incidences of adverse events (AEs) and serious adverse events (SAEs), including those of special interest (serious hemoptysis and symptomatic hypotension), were assessed.
Statistical Analyses
All variables were analyzed descriptively and all patients who received at least one dose of riociguat were included in the analysis. No imputation of missing information was applied except for partial dates (full rules listed in Supplementary Information).
Results
Patient Population
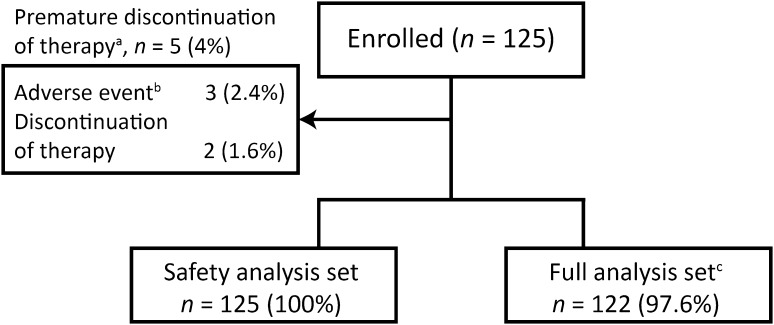
The total documentation period for the study was October 1, 2012 to May 31, 2016. Of the 127 patients screened, 125 were enrolled (Fig. 1). All enrolled patients completed the 5-month post-switch period and were included in the safety analysis set (SAF); 122 (98%) were included in the full analysis set (FAS) due to the exclusion of three patients for violations of the enrollment criteria. FAS data are presented, with the exception of demographic and safety-related data, where SAF data are presented.
Fig. 1.
Patient disposition. aPrimary reason for premature discontinuation of riociguat therapy. bAdverse events reported: Patient 1–dizziness, dyspepsia, and oxygen consumption increased; patient 2–exacerbation of pulmonary hypertension; patient 3–dyspnea and back pain. cThree patients were not included in the full analysis set due to violation of the inclusion or exclusion criteria
At baseline, most patients were in WHO FC III (66%) and had inoperable or persistent/recurrent CTEPH (85%). The mean (standard deviation [SD]) 6MWD and N-terminal prohormone of brain natriuretic peptide at baseline were 354 (110) m and 806 (1041) pg/mL, respectively (Table 1).
Table 1.
Patient and disease characteristics at baseline
| Characteristic | Safety analysis set (n = 125) |
|---|---|
| Age, years | 64 (16) |
| Female, n (%) | 76 (61) |
| BMIa, kg/m2 | 27 (7) |
| Time from initial diagnosis to start of riociguat, months | 55 (54) |
| PAH, n (%) | 40 (32) |
| CTEPH, n (%) | 85 (68) |
| Inoperable | 33 (39) |
| Persistent | 41 (48) |
| Recurrent | 11 (13) |
| BPA performed prior to start of riociguat treatment | 12 (14) |
| 6MWDb, m | 354 (110) |
| WHO FC I/II/III/IVc, % | 2/27/66/5 |
| NT-proBNPd, pg/mL | 806 (1041) |
Data are mean (SD) unless otherwise stated; an = 111, bn = 101, cn = 112, dn = 47
6MWD 6-min walking distance, BMI body mass index, BPA balloon pulmonary angioplasty, CTEPH chronic thromboembolic pulmonary hypertension, NT-proBNP N-terminal prohormone of brain natriuretic peptide, PAH pulmonary arterial hypertension, SD standard deviation, WHO FC World Health Organization functional class
Five patients (4%) prematurely discontinued riociguat therapy (Fig. 1).
Prior Therapy
At the time of switching to riociguat, most patients were receiving PDE5i monotherapy (n = 46; 38%) or ERA + PDE5i (n = 43; 35%). Most patients switched to riociguat from a PDE5i (n = 98, 80%), with 67 (55%) switching from sildenafil and 31 (25%) from tadalafil. The mean (SD) treatment durations prior to switching were 43 (32) and 23 (16) months for sildenafil and tadalafil, respectively.
Among patients with PAH, most were receiving ERA + PDE5i (n = 24; 60%) or ERA + PDE5i + prostacyclin (n = 9; 23%) at the time of switching, and 34 patients (85%) switched PDE5i for riociguat. Among patients with CTEPH, the most common prior treatment was a PDE5i in 43 patients (52%) and ERA + PDE5i in 19 patients (23%), and 64 patients (78%) switched PDE5i for riociguat (Table 2).
Table 2.
Duration of treatment-free period during switching (full analysis set)
| Switched drug(s) | Treatment-free perioda, days | |||||
|---|---|---|---|---|---|---|
| PAH (n = 40) | CTEPH (n = 82) | Total population (n = 122) | ||||
| n (%) | Median (range) | n (%) | Median (range) | n (%) | Median (range) | |
| PDE5i | ||||||
| Sildenafil | 22 (55) | 0 (− 12 to 13) | 45 (55) | 0 (− 24 to 2) | 67 (55) | 0 (− 24 to 13) |
| Tadalafil | 12 (30) | 2 (0–5) | 19 (23) | 2 (− 1 to 5) | 31 (25) | 2 (− 1 to 5) |
| ERA | ||||||
| Ambrisentan | 0 | − | 2 (2) | 1 (− 1 to 3) | 2 (2) | 1 (− 1 to 3) |
| Bosentan | 3 (8) | 1 (− 1 to 51) | 6 (7) | − 1 (− 5 to 16) | 9 (7) | − 1 (− 5 to 51) |
| Prostacyclin analog | ||||||
| Beraprost | 0 | − | 2 (2) | 1 (0–2) | 2 (2) | 1 (0–2) |
| Iloprost | 1 (3) | 0 | 3 (4) | 0 (− 1 to 23) | 4 (3) | 0 (− 1 to 23) |
| Double combination therapy | ||||||
| Bosentan + sildenafil | 0 | − | 2 (2) | 6 (− 1 to 13) | 2 (2) | 6 (− 1 to 13) |
| Iloprost + sildenafil | 1 (3) | 3 | 2 (2) | − 1 (− 1 to 0) | 3 (2) | 0 (− 1 to 3) |
| Epoprostenol + sildenafil | 1 (3) | 0 | 0 | − | 1 (1) | 0 |
| Triple combination therapy | ||||||
| Bosentan + iloprost + sildenafil | 0 | − | 1 (1) | − 1 | 1 (1) | − 1 |
CTEPH chronic thromboembolic pulmonary hypertension, ERA endothelin receptor antagonist, PAH pulmonary arterial hypertension, PDE5i phosphodiesterase type 5 inhibitor
aTreatment-free period was the number of days between the day of last intake of switched PH drug(s) and the day of first treatment with riociguat (excluding the last day with pre-switch PH drug intake and the first day with riociguat). If the switched drug was discontinued after the start of riociguat, the treatment-free period is negative. A treatment-free period of 0 indicates riociguat was started 1 day after the last intake of the switched drug
Post-Switch Therapy
The most common post-switch treatment was riociguat monotherapy (n = 64; 52%), or ERA + riociguat (n = 45; 37%).
Among patients with PAH, the most common post-switch therapies were ERA + riociguat (n = 27; 68%), ERA + riociguat + prostacyclin (n = 7; 18%), and riociguat monotherapy (n = 6; 15%). Among patients with CTEPH, the most common post-switch therapies were riociguat monotherapy (n = 58; 71%) and ERA + riociguat (n = 18; 22%).
In line with the contraindication for concomitant use of the two drugs, most patients did not receive riociguat + PDE5i. However, one patient received concomitant riociguat + PDE5i for 12 days. This patient experienced SAEs of worsening right heart failure (two episodes, both of which were resolved and not considered study-drug related); symptomatic hypotension was also reported although not during the time in which concomitant riociguat + PDE5i was administered. The stop date for PDE5i therapy was incomplete for another patient.
Reason for Switch
The most common reason for switching to riociguat was lack of efficacy of the prior therapy (n = 102; 84%). Other reasons included patient request and lack of tolerability (Table 3).
Table 3.
Reasons for switching to riociguat (full analysis set)
| Reasons for switching to riociguat, n (%) | PAH (n = 40) | CTEPH (n = 82) | Total population (n = 122) |
|---|---|---|---|
| Lack of efficacy of prior PH-targeted therapy | 32 (80) | 70 (85) | 102 (84) |
| Patient request | 5 (13) | 2 (2) | 7 (6) |
| Lack of tolerability | 2 (5) | 3 (4) | 5 (4) |
| Cost/reimbursement issues | 0 (0) | 3 (4) | 3 (2) |
| Physician’s decision | 1 (3) | 2 (2) | 3 (2) |
| Availability of targeted medication | 0 (0) | 2 (2) | 2 (2) |
CTEPH chronic thromboembolic pulmonary hypertension, PAH pulmonary arterial hypertension, PH pulmonary hypertension
Duration of Treatment-Free Period
The median (range) treatment-free period prior to commencing riociguat was 0 (− 24 to 51) days. The median treatment-free period was longer in patients who switched from tadalafil, recorded as 2 (− 1 to 5) days, than in those who switched from sildenafil, recorded as 0 (− 24 to 13) days, where 0 days indicated riociguat was started the day following the last intake of switched drug (Table 2). Due to the study imputation rules for partial or missing dates (see Supplementary Information), the patient with the incomplete stop date (day missing) led to a reported overlap of 24 days, affecting the calculation of the mean treatment-free period for switching from sildenafil to riociguat.
Dose-Adjustment Procedure
Riociguat was most frequently initiated at 3.0 mg/day (n = 79; 65%), followed by 1.5 mg/day (n = 20; 16%) and 7.5 mg/day (n = 16; 13%); two patients (2%) were started on 1 mg/day. The mean (SD) initial dose was 3.3 (1.8) mg/day.
The mean (SD) number of dose-adjustment steps was 2.4 (1.5), with the majority of patients receiving three or four dose adjustments (n = 66; 54% and n = 12; 10%, respectively). For 27 patients (22%), only the initial dose was documented. Vital signs were assessed at each dose-adjustment step; mean systolic blood pressure remained above 100 mmHg during each increase in riociguat (Supplementary Table 1).
The mean (SD) maintenance dose of riociguat was 6.7 (1.7) mg/day with 77% of patients achieving a maintenance dose of 7.5 mg/day within 8 weeks.
Safety
Treatment-Free Period
Two patients (2%), both with CTEPH, experienced an AE during the treatment-free period (liver disorder and hyperlipidemia).
Dose-Adjustment Period
Most AEs occurred during the 8-week dose-adjustment period (up to Day 56), with 51 patients (41%) experiencing AEs during this time (Table 4, Supplementary Table 2, and Supplementary Fig. 1). The most common AEs were dizziness (n = 11; 9%), dyspepsia (n = 10; 8%), and headache (n = 6; 5%). Hypotension was experienced by five patients (4%).
Table 4.
Patients experiencing AEs during the dose-adjustment period (safety analysis set)
| n (%) | Dose-adjustment perioda (n = 125) |
|---|---|
| AEs | 51 (41) |
| Drug-related AEs | 32 (26) |
| SAEs | 6 (5) |
| Drug-related SAEs | 2 (2) |
| Discontinuations | 1 (1) |
| AEs resulting in dose reduction or interruption | 11 (9) |
AE adverse event, SAE serious adverse event
aData are shown for AEs that start within the dose-adjustment period only, defined as any event arising or worsening on the day of or after start of riociguat where the start date ≤ the date of maintenance dose of riociguat or missing
SAEs occurred in six patients (5%); in two patients (2%) the events were considered study-drug related: one patient experienced palpitations, a viral infection, and cardiac catheterization, and one patient experienced right ventricular failure. One patient (1%) discontinued riociguat during the dose-adjustment period and 11 patients (9%) experienced AEs that resulted in a dose reduction or interruption.
Efficacy
6MWD, N-terminal pro-brain natriuretic peptide, and WHO FC at baseline and last follow-up visit are shown in Supplementary Figs. 2, 3, and 4, respectively.
Discussion
CAPTURE was a retrospective chart review designed to understand how patients with PAH or CTEPH switched to riociguat from other PH-targeted therapies in real-world clinical practice.
The main reason for switching to riociguat was a lack of efficacy of previous PH-targeted therapies. This is in contrast to previously published studies where patients mainly switched due to comfort, safety, and tolerability [22, 27–36]. However, these studies focused on switching from intravenous or subcutaneous prostacyclins to a second prostacyclin (mainly treprostinil) or an ERA, whereas in CAPTURE, 80% of patients switched from an oral PDE5i. Therefore, as well as the difference in mechanism of action between PH therapies leading to varying side effect profiles, administration procedure may also play a role in the reason for switch. It has also been reported that patients switch from sildenafil due to AEs [21], and in CAPTURE, 4% of the population switched due to lack of tolerability of prior therapy.
In line with ESC/ERS guidelines [1], most patients with PAH were receiving combination therapy before switching; the majority of these switched from a PDE5i to riociguat. However, patients with inoperable or persistent/recurrent CTEPH were receiving off-label PH-targeted therapies, as riociguat is the only medical therapy approved in CTEPH [1, 23, 24]. Following switching, many patients with PAH received double or triple combination therapy in conjunction with riociguat, while most patients with CTEPH switched to riociguat monotherapy.
Of note, due to concomitant riociguat and PDE5i being contraindicated [23, 24], most patients in CAPTURE did not receive riociguat + PDE5i in combination. One patient had overlapping treatment with a PDE5i and riociguat; however, although the patient experienced right heart failure and hypotension after PDE5i had stopped, these events resolved and riociguat treatment was completed.
The US prescribing information recommends 24- and 48-h treatment-free periods for patients switching from sildenafil and tadalafil, respectively [23], based on the drop in systemic blood pressure caused by concomitant administration of PDE5i and nitrates [37, 38], which have a similar mechanism of action to riociguat. The treatment-free periods observed for sildenafil and tadalafil in CAPTURE suggest that clinicians may be in line with the recommendations for sildenafil, but use a longer than recommended treatment-free period for tadalafil.
Riociguat is administered using an 8-week individual dose-adjustment scheme, starting at 1.0 mg three times a day (tid) and is increased every 2 weeks in the absence of hypotension, to a 2.5 mg tid–maximum [23, 24, 39]. CAPTURE showed that physicians tended to adhere to this protocol, with most patients initiating treatment of riociguat at 3 mg/day. However, 24 patients were started at lower than recommended doses of riociguat. This may be due to over-cautiousness on the part of the physician regarding the contraindication and risk of hypotension with PDE5i + riociguat, as 18 of these patients switched from PDE5i therapy. Additionally, 16 patients initiated riociguat at the maximal dose of 7.5 mg/day, which may be due to physician concern for clinical worsening with lower doses.
The percentage of patients receiving a 7.5 mg/day (2.5 mg tid) maintenance dose was similar in CAPTURE to the percentages in the PATENT-1 and CHEST-1 Phase III clinical trials. In CAPTURE, 77% of patients reached the maximum dose by Week 8, while in PATENT-1 and CHEST-1, the maximum dose was reached by 75% (at Week 12) [40] and 77% (at Week 16) [41], respectively.
The data from CAPTURE suggest that switching to riociguat from other PH-targeted therapies in clinical practice may be carried out safely and is well tolerated. AEs were rarely reported during the treatment-free period, and during the dose-adjustment period, only one patient discontinued riociguat, and 11 patients experienced AEs that resulted in dose reduction or interruption. Although it is important to interpret these observations with caution based on the inherent selection bias of a retrospective chart review (discussed below), they are in line with the results of published case studies and retrospective analyses of both PAH and CTEPH patients switching from other PH-targeted therapies (mainly PDE5i) to riociguat in real-world clinical practice [18, 20, 42–45]. The results from CAPTURE also support preliminary data from the RESPITE clinical trial, which indicated that switching from sildenafil or tadalafil to riociguat in patients with PAH not reaching treatment goals was safe and well tolerated [25].
A key limitation of CAPTURE is that, as a retrospective chart review, it had an inherent selection bias. Patients who died after switching but before giving informed consent for the study were not included. Although this bias applies to all chart reviews, it is important to exercise caution when interpreting the data from CAPTURE, as exclusion of patients who died means that the most unwell patients were not included in the safety analyses. Another study limitation, also owing to its retrospective nature, is the limited efficacy data, with only a small proportion of patients having post-baseline measurements for 6MWD, Borg dyspnea index, WHO FC, and biomarkers. These low patient numbers may be reflective of efficacy parameters primarily being assessed in patients who are not responding well to treatment, resulting in biased data. Moreover, clinical parameters were analyzed by original visit number and an artificial visit window scheme, meaning that the data available at each visit were highly variable. These low and variable patient numbers mean that it is almost impossible to interpret the data.
In conclusion, most patients in CAPTURE were initiated and uptitrated on riociguat in line with recommendations in the label, with a similar percentage of patients achieving the maximum maintenance dose in real-world clinical practice as in the PATENT and CHEST clinical trials. No new safety signals were observed. These data suggest that switching may be feasible in the context of current recommendations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding was provided by Bayer AG and Merck Sharp and Dohme. The authors wish to acknowledge the study nurse Sina Heuser for her contribution to the study.
Compliance with Ethical Standards
Conflict of interest
The CAPTURE study was supported by Bayer AG and Merck Sharp and Dohme. Editorial support was provided by Adelphi Communications Ltd, supported by Bayer AG. HG reports personal fees from Actelion, AstraZeneca, Bayer AG, GSK, Janssen Cilag, Lilly, Pfizer, and United Therapeutics. J-LV reports grants from Actelion, Bayer AG, GSK, Lilly, and Merck, and holding the Actelion Chair for research in PH within his institution. NT reports lecture honoraria from Actelion, Bayer Yakuhin, Daiichi-Sankyo, and being a member of an endowed department with Actelion. MH reports board membership for Actelion, Bayer AG, GSK, and Novartis; lecture fees from Actelion, AstraZeneca, Bayer AG, GSK, Lilly, MSD, Novartis, and Pfizer; and personal fees from Actelion, Bayer AG, GSK, Lilly, and Novartis. MO-L has nothing to disclose. LM reports personal fees from Actelion and Bayer AG, and consulting, speaker fees, and research funding from Actelion and Bayer AG. MC reports employment by Bayer AG. KV reports employment by Bayer AG. EG reports grants from Actelion, Bayer AG, GSK, Lilly, and Pfizer; personal fees from Bayer AG, Milteny, Novartis, and United Therapeutics; and non-financial support from Alexion.
Ethical Approval
The study was carried out within an approved indication in accordance with guidelines and regulations of EMA, FDA and applicable local laws and regulations. ICH-GCP guidelines were followed wherever possible. In all countries where reference to an IEC/IRB is required, documented approval for appropriate IEC/IRB will be obtained for all participating centers prior to study start.
Informed Consent
Informed consent was obtained from all individual participants included in this study.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00408-018-0100-3) contains supplementary material, which is available to authorized users.
A correction to this article is available online at https://doi.org/10.1007/s00408-018-0112-z.
Change history
4/3/2018
The original version of this article unfortunately contained a mistake. In the “Results” section, the percentage of patients with inoperable or persistent/recurrent CTEPH included in the study was reported as 85%. This has been corrected to 68% with this erratum.
References
- 1.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk NA, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, McLaughlin VV, Dalaan AM, Satoh T, Galiè N. Treatment of pulmonary hypertension. Lancet Respir Med. 2016;4(4):323–336. doi: 10.1016/S2213-2600(15)00542-1. [DOI] [PubMed] [Google Scholar]
- 4.Ghofrani HA, Humbert M, Langleben D, Schermuly R, Stasch JP, Wilkins MR, Klinger JR. Riociguat: mode of action and clinical development in pulmonary hypertension. Chest. 2017;151(2):468–480. doi: 10.1016/j.chest.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins D. Pulmonary endarterectomy: the potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2015;24(136):263–271. doi: 10.1183/16000617.00000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, Lang I, Madani MM, Ogino H, Pengo V, Mayer E. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D92–D99. doi: 10.1016/j.jacc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Bonderman D, Wilkens H, Wakounig S, Schafers HJ, Jansa P, Lindner J, Simkova I, Martischnig AM, Dudczak J, Sadushi R, Skoro-Sajer N, Klepetko W, Lang IM. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33(2):325–331. doi: 10.1183/09031936.00087608. [DOI] [PubMed] [Google Scholar]
- 8.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, Hodgkins D, Goldsmith K, Hughes RJ, Sheares K, Tsui SS, Armstrong IJ, Torpy C, Crackett R, Carlin CM, Das C, Coghlan JG, Pepke-Zaba J. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177(10):1122–1127. doi: 10.1164/rccm.200712-1841OC. [DOI] [PubMed] [Google Scholar]
- 9.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, Goldsmith K, Coghlan JG, Pepke-Zaba J. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33(2):332–338. doi: 10.1183/09031936.00092008. [DOI] [PubMed] [Google Scholar]
- 10.Freed DH, Thomson BM, Berman M, Tsui SS, Dunning J, Sheares KK, Pepke-Zaba J, Jenkins DP. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141(2):383–387. doi: 10.1016/j.jtcvs.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 11.Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, Capener D, Sephton P, Hamilton N, Armstrong IJ, Billings C, Lawrie A, Sabroe I, Akil M, O’Toole L, Kiely DG. ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J. 2012;39(4):945–955. doi: 10.1183/09031936.00078411. [DOI] [PubMed] [Google Scholar]
- 12.Mayer E, Jenkins D, Lindner J, D’Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke-Zaba J, Simonneau G, Dartevelle P. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, D’Armini AM, Morsolini M, Snijder R, Bresser P, Torbicki A, Kristensen B, Lewczuk J, Simkova I, Barbera JA, de Perrot M, Hoeper MM, Gaine S, Speich R, Gomez-Sanchez MA, Kovacs G, Hamid AM, Jais X, Simonneau G. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18):1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 14.Cannon JE, Su L, Kiely DG, Page K, Toshner M, Swietlik E, Treacy C, Ponnaberanam A, Condliffe R, Sheares K, Taboada D, Dunning J, Tsui S, Ng C, Gopalan D, Screaton N, Elliot C, Gibbs S, Howard L, Corris P, Lordan J, Johnson M, Peacock A, Mackenzie RR, Coghlan G, Schreiber B, Dimopoulos K, Wort J, Gaine S, Moledina S, Jenkins DP, Pepke-Zaba J. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the UK national cohort. Circulation. 2016;133:1761–1771. doi: 10.1161/CIRCULATIONAHA.115.019470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepke-Zaba J, Ghofrani HA, Hoeper M. Medical management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):pii: 160107. doi: 10.1183/16000617.0107-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang I, Meyer B, Ogo T, Matsubara H, Kurzyna M, Ghofrani HA. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143):pii: 160119. doi: 10.1183/16000617.0119-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taichman DB, Ornelas J, Chung L, Klinger J, Lewis S, Mandel J, Palevsky H, Rich S, Sood N, Rosenzweig EB, Trow TK, Yung R, Elliott CG, Badesch D. Pharmacological therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146(2):449–475. doi: 10.1378/chest.14-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen A, Korsholm K, Mellemkjaer S, Nielsen-Kudsk JE. Switching from sildenafil to riociguat for the treatment of PAH and inoperable CTEPH: real-life experiences. Respir Med Case Rep. 2017;22:39–43. doi: 10.1016/j.rmcr.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davey RA, Benza RL, Murali S, Raina A. Phosphodiesterase type 5 inhibitor to riociguat transition is associated with hemodynamic and symptomatic improvement in pulmonary hypertension. Pulm Circ. 2017;7(2):539–542. doi: 10.1177/2045893217708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto K, Tanabe N, Suda R, Sasaki A, Matsumura A, Ema R, Kasai H, Kato F, Sekine A, Nishimura R, Jujo T, Sugiura T, Shigeta A, Sakao S, Tatsumi K. Riociguat for patients with chronic thromboembolic pulmonary hypertension: usefulness of transitioning from phosphodiesterase type 5 inhibitor. Respir Invest. 2017;55(4):270–275. doi: 10.1016/j.resinv.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Lichtblau M, Harzheim D, Ehlken N, Marra A, Pinado FP, Grunig E, Egenlauf B. Safety and long-term efficacy of transition from sildenafil to tadalafil due to side effects in patients with pulmonary arterial hypertension. Lung. 2015;193(1):105–112. doi: 10.1007/s00408-014-9657-7. [DOI] [PubMed] [Google Scholar]
- 22.Vachiery JL, Hill N, Zwicke D, Barst R, Blackburn S, Naeije R. Transitioning from i.v. epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension. Chest. 2002;121(5):1561–1565. doi: 10.1378/chest.121.5.1561. [DOI] [PubMed] [Google Scholar]
- 23.Bayer AG (2017) Adempas: US prescribing information. http://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf. Accessed 6 Oct 2017
- 24.Bayer AG (2017) Adempas (riociguat tablets): EU summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034.pdf. Accessed 6 Oct 2017
- 25.Hoeper MM, Simonneau G, Corris PA, Ghofrani H-A, Klinger JR, Langleben D, Naeije R, Jansa P, Rosenkranz S, Scelsi L, Grünig E, Vizza CD, Chang M, Colorado P, Meier C, Busse D, Benza RL (2017) RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. Eur Respir J 50(3). 10.1183/13993003.02425-2016 [DOI] [PMC free article] [PubMed]
- 26.McLaughlin V, Jansa P, Nielsen-Kudsk JE, Halank M, Simonneau G, Grünig E, Ulrich S, Rosenkranz S, Gomez Sanchez MA, Pulido T, Pepke-Zaba J, Barbera JA, Hoeper MM, Vachiery JL, Lang I, Carvalho F, Meier C, Mueller K, Nikkho S, D’Armini A. Riociguat in patients with chronic thromboembolic pulmonary hypertension: results from an early access study. BMC Pulm Med. 2017;17:216. doi: 10.1186/s12890-017-0563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubenfire M, McLaughlin VV, Allen RP, Elliott G, Park MH, Wade M, Schilz R. Transition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trial. Chest. 2007;132(3):757–763. doi: 10.1378/chest.06-2118. [DOI] [PubMed] [Google Scholar]
- 28.Gomberg-Maitland M, Tapson VF, Benza RL, McLaughlin VV, Krichman A, Widlitz AC, Barst RJ. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med. 2005;172(12):1586–1589. doi: 10.1164/rccm.200505-766OC. [DOI] [PubMed] [Google Scholar]
- 29.Sitbon O, Manes A, Jais X, Pallazini M, Humbert M, Presotto L, Paillette L, Zaccardelli D, Davis G, Jeffs R, Simonneau G, Galie N. Rapid switch from intravenous epoprostenol to intravenous treprostinil in patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol. 2007;49(1):1–5. doi: 10.1097/FJC.0b013e31802b3184. [DOI] [PubMed] [Google Scholar]
- 30.de Jesus Perez VA, Rosenzweig E, Rubin LJ, Poch D, Bajwa A, Park M, Jain M, Bourge RC, Kudelko K, Spiekerkoetter E, Liu J, Hsi A, Zamanian RT. Safety and efficacy of transition from systemic prostanoids to inhaled treprostinil in pulmonary arterial hypertension. Am J Cardiol. 2012;110(10):1546–1550. doi: 10.1016/j.amjcard.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Keogh AM, Jabbour A, Weintraub R, Brown K, Hayward CS, Macdonald PS. Safety and efficacy of transition from subcutaneous treprostinil to oral sildenafil in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2007;26(11):1079–1083. doi: 10.1016/j.healun.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Diaz-Guzman E, Heresi GA, Dweik RA, Minai OA. Long-term experience after transition from parenteral prostanoids to oral agents in patients with pulmonary hypertension. Respir Med. 2008;102(5):681–689. doi: 10.1016/j.rmed.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Escolar E, Pineda AM, Correal B, Ahmed T. Transition from prostacyclin analogue infusion to oral therapy in patients with pulmonary arterial hypertension: a 5-year follow-up. Pulm Circ. 2013;3(4):880–888. doi: 10.1086/674761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner MK, Preston IR, Klinger JR, Criner GJ, Waxman AB, Farber HW, Hill NS. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471–1480. doi: 10.1378/chest.130.5.1471. [DOI] [PubMed] [Google Scholar]
- 35.Bourge RC, Tapson VF, Safdar Z, Benza RL, Channick RN, Rosenzweig EB, Shapiro S, White RJ, McSwain CS, Gotzkowsky SK, Nelsen AC, Rubin LJ. Rapid transition from inhaled iloprost to inhaled treprostinil in patients with pulmonary arterial hypertension. Cardiovasc Ther. 2013;31(1):38–44. doi: 10.1111/1755-5922.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preston IR, Feldman J, White J, Franco V, Ishizawar D, Burger C, Waxman AB, Hill NS. Safety and efficacy of transition from inhaled treprostinil to parenteral treprostinil in selected patients with pulmonary arterial hypertension. Pulm Circ. 2014;4(3):456–461. doi: 10.1086/677360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eli Lily (2015) Adcirca: US prescribing information. http://pi.lilly.com/us/adcirca-pi.pdf. Accessed 6 Oct 2017
- 38.Pfizer (2014) Revatio: US prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021845s011,022473s004,0203109s002lbl.pdf. Accessed 6 Oct 2017
- 39.Hill NS, Rahaghi FF, Sood N, Frey R, Ghofrani HA. Individual dose adjustment of riociguat in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respir Med. 2017;129:124–129. doi: 10.1016/j.rmed.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Ghofrani HA, Galiè N, Grimminger F, Grunig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 41.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, Simonneau G, Wilkins MR, Fritsch A, Neuser D, Weimann G, Wang C. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 42.Poch DS. Case report: a patient with pulmonary arterial hypertension transitioning from a PDE-5 inhibitor to Riociguat. BMC Pulm Med. 2016;16(1):82. doi: 10.1186/s12890-016-0229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sulica R, Fenton R, Cefali F. Early observations on the use of riociguat in a large, metropolitan pulmonary arterial hypertension/chronic thromboembolic pulmonary hypertension treatment center. Cardiol Ther. 2015;4(2):209–218. doi: 10.1007/s40119-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weir N, Brown AW, DelaSantine J, King C, Lewis D, Franco-Palacios D, Nathan SD, Shlobin OA (2017) Transition from PDE-5 inhibitors to riociguat in pulmonary hypertension. Am J Respir Crit Care Med 195:A2282
- 45.Raina A, Benza RL, Farber HW. Replacing a phosphodiesterase-5 inhibitor with riociguat in patients with connective tissue disease-associated pulmonary arterial hypertension: a case series. Pulm Circ. 2017;7(3):741–746. doi: 10.1177/2045893217721694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.