Abstract
Background
Hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−), metastatic breast cancer (MBC) accounts for 73% of all MBCs. Endocrine therapy (ET) is the basis of first-line (1L) therapy for patients with HR+/HER2− MBC. Novel therapies have demonstrated improvements in progression-free survival (PFS) compared to ET. The clinical relevance of PFS is being debated, as there is no proven direct correlation with overall survival (OS) benefit to date. We reviewed studies of HR+/HER2− MBC to assess PFS and other factors that influence OS and treatment response, and health-related quality of life (HRQoL).
Methods
The Embase®, Medline®, and Cochrane databases were systematically searched to identify studies in adult women with HR+/HER2− MBC, published between January 2006 and January 2017, and written in English. Phase II and III randomized controlled trials (RCTs), observational, and retrospective studies were included.
Results
Seventy-nine RCTs were identified: 58 (73%) in the 1L+ setting and 21 (27%) in second-line or greater settings. PFS hazard ratios (HRs) were reported in 61 (77%) studies; 31 (39%) reported significant PFS improvements. OS was reported in 44 (41%) studies; 12 (15%) reported significant OS improvements. Significant improvements in both PFS and OS were reported in only 6 (8%) studies (1 Phase II; 5 Phase III). Patients with HER2− MBC received, on average, ≥5 lines of therapy, with no consistent treatment pathway. Baseline characteristics, prior therapies, and the type and number of post-progression therapies significantly impacted OS. PFS, response rates, and HRQoL decreased with each line of therapy (EuroQol 5 Dimensions: 0.78 1L vs. 0.70 post-progression).
Conclusion
Few RCTs in HR+/HER2− MBC have demonstrated significant improvements in OS. Factors other than choice of 1L therapy impact OS, including post-progression therapies, which cannot be controlled in RCTs. This study emphasizes the importance of PFS improvement in 1L treatment of HR+/HER2− MBC.
Keywords: breast cancer, overall survival, progression-free survival, health-related quality of life, systematic literature review
Introduction
Breast cancer is currently the most common malignancy diagnosed in women and is associated with the second-highest mortality rates, after lung cancer. In 2016, there were 246,660 confirmed diagnoses of breast cancer and an estimated 40,450 deaths attributed to the disease.1 Approximately 12.4% of women will be diagnosed with breast cancer in their lifetime and,2 though the malignancy is diagnosed at an early stage in 90% of patients, most tumors will progress to advanced or metastatic disease.3,4 Progression to metastatic breast cancer (MBC) is associated with median survival times of 18–24 months, and only 5% of patients are anticipated to be disease-free and alive at 5 years following tumor metastasis.5 Approximately 30%–40% of women diagnosed with invasive breast cancer will eventually develop MBC;6 in the USA, the 5-year survival rate for women with MBC is about 26%.1
The most common neoplasms of the breast, found in 74% of patients, are hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2−). This subset of breast cancer has the most favorable disease prognosis, as HR-negative tumors respond to therapy at a lower rate and HER2+ tumors are more aggressive.7 The standard of care for post-menopausal women with HR+/HER2− breast cancer is endocrine therapy (ET).8 The primary agents used are selective androgen receptor modulators, which include tamoxifen (TAM) and fulvestrant (FUL); and aromatase inhibitors, which include exemestane (EXE), letrozole (LET), and anastrozole (ANA).9 Despite the variety of available therapies, only 20%–40% of patients will respond to these agents and most will develop resistance during their course of therapy.10,11
Management of resistance requires the use of drugs that target the resistance pathway and subsequently improve sensitivity to ET.8 Drugs approved by the US Food and Drug Administration for the treatment of HR+/HER2− advanced breast cancer in combination with aromatase inhibitors are the mammalian target of rapamycin (mTOR) inhibitor everolimus (EVE) and the cyclin-dependent kinase (CDK) 4/6 inhibitors abemaciclib, palbociclib (PALBO), and ribociclib (RIBO). EVE and PALBO have been shown to improve progression-free survival (PFS) compared to ET alone in first-and second-line randomized controlled trials (RCTs).12,13 RIBO, given as first-line (1L) therapy for post-menopausal women with HR+/HER2− advanced breast cancer in combination with LET, has demonstrated improvement in PFS compared with LET alone in a Phase III RCT.14
While the above-mentioned clinical trials reported improvements in PFS, data on overall survival (OS) with the use of some drugs, such as PALBO and RIBO, are limited, and it is uncertain whether improved PFS correlates with benefits in OS. To evaluate the available evidence for PFS and OS across Phase II and III RCTs of patients with HR+/HER2− MBC, we conducted a systematic literature review (SLR). In addition, we conducted a targeted literature search to identify factors that may influence OS in HR+/HER2− MBC and underlie treatment patterns. We also examined efficacy and health-related quality of life (HRQoL) changes relative to the line of therapy in HR+/HER2− MBC to determine if any trends were present.
Methods
SLR protocol
An SLR was performed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.15 The inclusion criteria for the literature search and the methods of analysis were specified in advance and documented in a protocol, details are provided in the following section.
SLR eligibility criteria
The SLR included original reports of Phase II and III RCTs in adult women with HR+/HER2− MBC, written in English and published between January 2006 and January 2017. Trials that enrolled both HER2− and HER2+ patients were included if >80% of the enrolled patients had HER2− disease, requiring that results were provided for the HER2− subgroup. The extracted outcome measures for efficacy included PFS or time-to-progression (TTP), and OS reported as either median survival (in months) or hazard ratios (HRs) vs. comparators. Publications reporting meta-analyses were also retained in the SLR for reference cross-checking.
Studies with fewer than 10 patients and those that lacked any of the measures of interest were excluded. No limitations were placed on therapies except that they must be systemic. Studies of surgical interventions, radiotherapy/chemoradiation, or adjuvant/neo-adjuvant therapies were excluded.
In cases of duplicate publications or conference abstracts reporting data from a study with an available manuscript, the most recent manuscript was prioritized unless an older manuscript or more recent abstract included data points that were missing in the later manuscript.
The targeted literature search also included a review of post hoc statistical analyses of factors influencing OS, non-randomized studies of treatment patterns, and studies reporting HRQoL in HR+/HER2− MBC. The search strategy was based on similar criteria as mentioned earlier; however, eligible study designs also included observational studies, retrospective chart reviews, and patient surveys, provided that data on outcomes of interest were reported.
Information sources
Studies were identified based on searches of the Embase, Medline, Daily Medline, and Medline In-Process electronic databases, Cochrane Central Register of Controlled Trials, Cochrane Database of Reviews of Effect, and Cochrane Database of Systematic Reviews. Additionally, congress abstracts from the American Society of Clinical Oncology, European Society of Medical Oncology, European Breast Cancer Conference, and San Antonio Breast Cancer Symposium libraries were identified. The literature search was conducted in January 2017 and included studies from January 2006 up to the date of the search. The full search strategy is available in Tables S1–S3.
Study selection
Following the database searches, two independent analysts reviewed and selected studies based on abstracts and titles. The eligibility of abstracts and full-text articles was then independently assessed by the two reviewers in a standardized manner. Discrepancies were resolved by consensus between both reviewers.
Data collection process
A data extraction sheet was developed to tabulate the studies’ characteristics (detailed below). One reviewer extracted the data and a second checked the extracted data for accuracy. As above, discrepancies were resolved by consensus between the two reviewers.
Data items
The following data elements were extracted from each study: 1) trial characteristics included reference, name of trial, study design, phase of study, line of therapy (1L or second and later [2L+]), study interventions, class of intervention drug, and treatment arms; 2) population characteristics included total number of randomized participants, median age, number of patients with Eastern Cooperative Oncology Group performance status of 0 and 1, number of HER2− patients, number of HR+ patients, number of PR+ patients, endocrine status (resistant, sensitive, or mixed), number of premenopausal participants, number of patients with visceral metastases, number of patients with 1, 2, or ≥3 metastatic sites, number of patients with prior ET, and number of patients with prior adjuvant/neo-adjuvant/metastatic-setting chemotherapy; 3) trial outcomes included type of primary endpoint, whether PFS/TTP and/or OS were reported, PFS/TTP and OS results (median months of survival, HRs, confidence intervals, and p-values).
For the targeted literature search, additional data elements extracted included any HRQoL outcomes, patient burden (humanistic), number of lines of therapies including duration, sequence and efficacy in each line, and factors associated with OS.
Results
Study selection
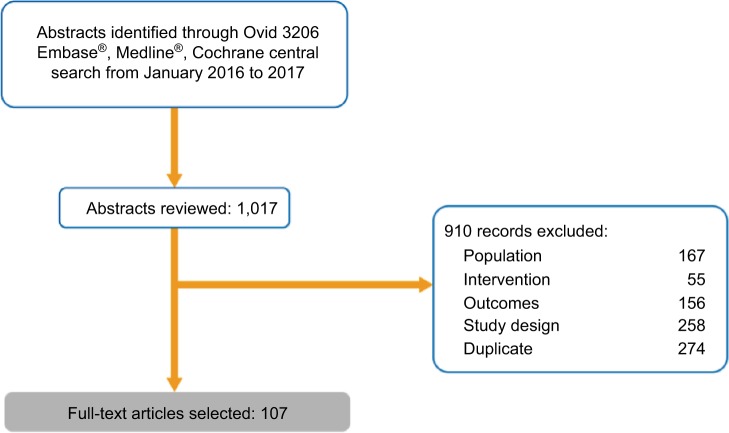
The database search returned 1017 records (Embase: 501, Cochrane: 272, Medline: 244). Of these, 636 were excluded due to failure to meet the inclusion criteria of population, intervention, outcomes, or study design. A further 274 duplicates were excluded, leaving a total of 107 full-text articles that were extracted and included in this SLR, 79 of which represent unique studies (58 1L, 21 2L+; Figure 1). The remaining 28 articles presented updated or interim data, the results of subgroup analyses, or were meta-analyses.
Figure 1.
Randomized controlled trial evidence flow for systematic literature review of clinical evidence.
Study characteristics
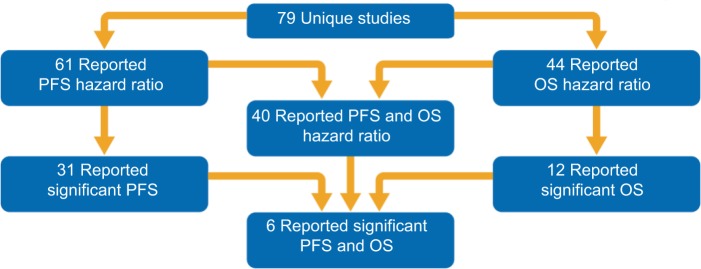
The breakdown of study treatments included in this SLR is presented in Table 1 and Figure 2. In total, 58 1L studies and 21 2L+ studies were included. PFS HR data were reported in 61 of the 79 unique studies (77%); of these, 31 (51%) reported significant PFS improvements. OS HR data were reported in 44 of the 79 studies (56%); of these, only 12 (27%) reported a significant OS improvement. Significant improvements in both PFS and OS were reported in only 6 (8%) studies (1 Phase II; 5 Phase III).
Table 1.
Systematic literature review results according to OS and PFS reporting
| N | All studies | First-line studies | Second-line studies and beyond |
|---|---|---|---|
| Reported PFS months | 67 | 47 | 20 |
| Reported PFS hazard ratio | 61 | 45 | 16 |
| Reported significant PFS | 31 | 19 | 12 |
| Reported OS months | 46 | 35 | 11 |
| Reported OS hazard ratio | 44 | 27 | 17 |
| Reported significant OS | 12 | 9 | 3 |
| Reported PFS and OS months | 39 | 29 | 10 |
| Reported PFS and OS hazard ratio | 40 | 31 | 9 |
| Reported significant PFS and OS | 6 | 5 | 1 |
| Total unique studies | 79 | 58 | 21 |
Abbreviations: OS, overall survival; PFS, progression-free survival.
Figure 2.
Systematic literature review results according to OS and PFS reporting.
Abbreviations: OS, overall survival; PFS, progression-free survival.
PFS
More Phase III than Phase II (15 vs. 4) RCTs reported statistically significant improvements in PFS in 1L therapy. Among the 19 1L studies that reported significant PFS improvement, 9 were of tyrosine kinase inhibitors (TKI) plus chemotherapy (CHEMO) and 3 were of CDK 4/6 inhibitor plus ET treatments.
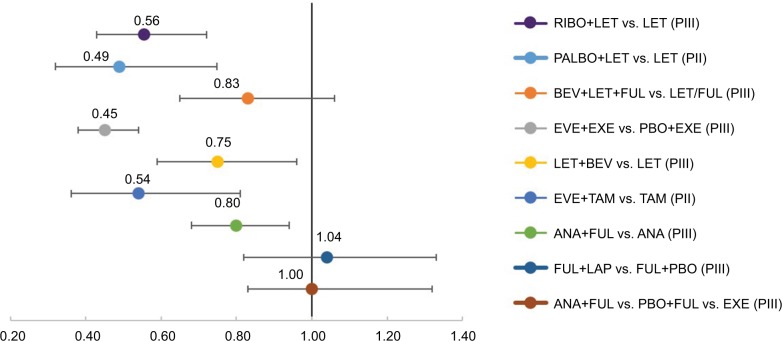
We further evaluated PFS among RCTs that included ET as a control arm. Here, the greatest difference in PFS among arms was seen with the addition of a CDK4/6 inhibitor (PALBO or RIBO) to LET (HRs of 0.49 and 0.56, respectively) or mTOR inhibitor (EVE) to either EXE or TAM (HRs of 0.45 and 0.54, respectively). Of these, the PALBO+LET and EVE+TAM trials were 1L studies, while EVE+EXE included mostly 2L+ patients.13–15 Statistically significant improvements in PFS were also seen with the addition of bevacizumab (BEV) to LET vs. LET alone (HR =0.75) and BEV to LET or FUL (vs. either LET or FUL, HR =0.83).16,17 Although the addition of ANA to FUL (vs. FUL alone) led to significant improvements in PFS (HR =0.80) in one study, this finding was not supported by another study (ANA+FUL vs. placebo + FUL, HR =1.0; Figure 3).17
Figure 3.
PFS HRs in selected randomized controlled trials
Notes: <1 favors experimental arm, >1 favors control arm.
Abbreviations: ANA, anastrole; BEV, bevacizumab; EXE, exemestance; EVE, everolimus; FUL, fulvestrant; HR, hazard ratio; LAP, lapatinib; LET, letrozole; PII, Phase II; PIII, Phase III; PALBO, palbociclib; PBO, placebo; PFS, progression-free survival; RIBO, ribociclib; TAM, tamoxifen.
Overall survival
Among the 79 RCTs, 46 (58%) reported incremental OS months, and 44 (56%) presented HRs for OS. However, only 12 studies (15%) reported statistically significant improvements in OS: 9 were 1L and 3 were 2L+.
Six Phase III RCTs reported statistically significant improvements in OS in 1L therapy compared to 3 Phase II trials. Among the 9 1L studies that reported significant OS improvement, 3 were of ET and 2 were of TKI+CHEMO treatments.
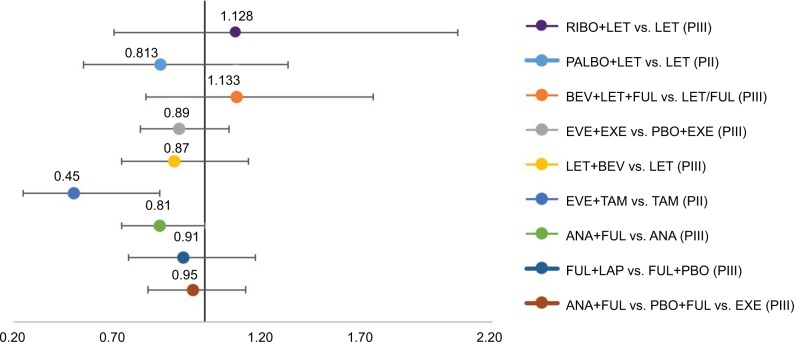
Among RCTs with ET as a control arm, only 1 of 9 studies reported statistically significant improvements in OS. The addition of EVE to TAM (vs. TAM alone) yielded a 55% reduction in risk of death, though this was reported in a small (n=111) Phase II study of patients receiving 1L therapy.16 The addition of PALBO to LET vs. LET led to a 19% risk reduction (HR=0.81).13 In 2 RCTs, the addition of ANA to FUL yielded HRs of 0.81 and 0.95, respectively.18,19 The addition of BEV to either LET or LET+FUL did not yield consistent results with HRs of 0.87 and 1.13,20,17 respectively (Figure 4).
Figure 4.
OS HRs in selected studies.
Notes: <1 favors experimental arm, >1 favors control arm.
Abbreviations: ANA, anastrole; BEV, bevacizumab; FUL, fulvestrant; EXE, exemestance; EVE, everolimus; HR, hazard ratio; LAP, lapatinib; LET, letrozole; OS, overall survival; PII, Phase II; PIII, Phase III; PALBO, palbociclib; TAM, tamoxifen; PBO, placebo; RIBO, ribociclib.
PFS and OS by study phase and line of therapy
Among the 79 unique RCTs, only 6 studies (8%) reported statistically significant differences in both PFS and OS. Among these studies, the majority (5) were investigations of 1L therapies, and Phase III RCTs (also 5). Among 4 Phase III 1L studies that reported statistical significance in PFS and OS, 2 were of TKI+CHEMO, 1 of TKI+ET, and 1 of ET combination.
Factors influencing overall survival
Multiple studies conducted subgroup analyses to identify clinical factors with an impact on OS. Park et al reported that median PFS of at least 7.6 months was associated with significantly longer OS (HR=0.34, confidence intervals: 0.25:0.46, p<0.001).21 This and other studies also reported that demographic factors, baseline characteristics, prior adjuvant/neoadjuvant therapy as well as post-progression therapy, and the total number of lines of therapy have significant impact on final OS.22–30
Although RCTs can balance study populations on baseline characteristics and prior therapies, post-progression therapies or metastases cannot be controlled for in 1L studies. Factors demonstrated to affect OS are summarized in Table 2.
Table 2.
Factors impacting OS in MBC
| Demographics | Disease characteristics | Prior therapy | Post-progression therapy |
|---|---|---|---|
| Age ≥65 years | Measurable disease | Prior endocrine therapy | Type of post-progression |
| Region | ECOG (1–2 vs. 0) Number of organs involved Number of metastatic sites Visceral involvement CNS metastases Liver metastases Disease-free interval |
Prior chemotherapy (adjust/neoadjuvant) | Lines of post-progression therapy |
Abbreviations: CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; MBC, metastatic breast cancer, OS, overall survival.
Treatment patterns and HRQoL
Our targeted search found significant variability in the treatments used for 1L and 2L+ therapies, with no preferred treatment pathway and many patients receiving up to 6 lines of therapy.31 The median length of PFS and response to treatment decreased with subsequent lines of therapy.21 Park et al further reported that median PFS of >7.6 months in 1L treatment was an important predictor of longer PFS in 2L, and PFS of >5.1 months in 2L was associated with longer PFS in 3L.21
There were very limited data available on HRQoL associated with PFS. One abstract reported EuroQol 5 Dimensions (EQ-5D) data from a study of S1 (tegafur, gimeracil, and oteracil) vs. taxanes (paclitaxel or docetaxel), showing decline in EQ-5D scores from 1L therapy to post-progression (0.81 vs. 0.72). Additionally, a recent abstract presenting data from the MONALEESA-1 trial on RIBO and LET treatment in HR+/HER2− MBC reported that HRQoL declined post-progression.32
Discussion
OS, as a direct measurement of clinical benefit to a patient, has been a preferred measurement of efficacy in MBC RCTs. While no measurement can definitively gauge treatment efficacy, OS conforms to the standards of evidence-based medicine in that it is easily measured, and is considered unbiased and objective. However, our research has demonstrated that, overall, a minority of studies have reported significant OS improvements: among the 79 identified RCTs in HR+/HER2− MBC, only 12 reported improvements in OS. Besides the choice of treatment in 1L therapy, many factors influence OS. Multiple studies reported that patient demographics, baseline characteristics, prior adjuvant/neoadjuvant therapy as well as post-progression therapy, and the total number of lines of therapy have significant impacts on final OS. Though further removed from objectivity, PFS is commonly reported because it can be observed while respecting the time constraints that often impinge on clinical trials. Most importantly, PFS in 1L was documented as a significant factor in OS.33
There is significant heterogeneity in MBC treatment, with many lines of therapies and no defined pathway. Macalalad et al documented variability in treatment patterns and over 5 lines of therapies among MBC patients.31 Kantar Health CancerMpact 2016 also reported on variability of treatment choices and observed that a significant proportion of patients who progress on a previous line of therapy subsequently utilize the next line of treatment for up to 6 lines.34 With the considerable variability in treatment patterns and many contributing factors to OS, there are concerns that the efficacy of 1L therapies measured by OS may be diluted or biased in clinical trials, thereby underestimating their true clinical benefit.
Both PFS and response rates decrease as MBC progresses. Park et al reported a decline in months of PFS and response rates by line of therapy among MBC patients.21 Quality of life also decreases from 1L therapy to post-progression, as reported by Fukuda et al.32 As such, improvement in PFS with maintained HRQoL may be a more suitable and robust endpoint in 1L RCTs of patients with HR+/HER2− MBC.
Conclusion
The goal of treatment in MBC is to prolong life while maintaining the quality of survival. As such, RCTs of MBC treatments ideally measure OS and HRQoL. In clinical trials of 1L therapies, OS is affected by multiple factors that cannot be controlled. This study examined important characteristics of RCTs in MBC and their relevance to OS. In addition to demographics, baseline characteristics, and prior adjuvant/neoadjuvant therapy, final OS is influenced by post-progression therapy and the total number of lines of therapy. This SLR found that ultimately very few 1L RCTs report OS improvement. PFS improvement is more often reported and its significance is perhaps understated. PFS, response rates, and QoL decrease as the disease progresses and with each line of therapy. PFS in 1L is an important predictor of PFS in further lines of therapy. In 1L treatments for MBC, PFS improvement coupled with maintained HRQoL provides patients with more meaningful time and may be considered the best possible outcome.
Supplementary materials
Search strategy
Table S1.
Ovid Medline® Epub ahead of print, in-process, and other non-indexed citations. Ovid Medline® daily and Ovid Medline® 1946 to present database
| Date: January 2017 | ||
| 1 | Exp breast neoplasms/ | 250,773 |
| 2 | (Breast adj6 cancer$).af. | 246,948 |
| 3 | (Breast adj6 neoplas$).af. | 252,542 |
| 4 | (breast adj6 carcinoma$).af. | 63,807 |
| 5 | (Breast adj6 tumor$).af. | 7401 |
| 6 | (Breast adj6 tumor$).af. | 48,416 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 336,672 |
| 8 | Metasta$.mp. or exp neoplasm metastasis/ | 477,568 |
| 9 | 7 and 8 | 74,656 |
| 10 | (“Metastatic breast cancer” or “metastatic breast neoplasms”).af. | 11,972 |
| 11 | 9 or 10 | 74,661 |
| 12 | “Hormone receptor positive”.af. | 2300 |
| 13 | “Hormone receptor-positive”.af. | 2300 |
| 14 | (“Estrogen receptor-positive” or “oestrogen receptor-positive”).af. | 4218 |
| 15 | “Progesterone receptor-positive”.af. | 732 |
| 16 | “Hormone sensitive”.af. | 3719 |
| 17 | 12 or 13 or 14 or 15 or 16 | 10,513 |
| 18 | 11 and 17 | 1955 |
| 19 | Exp randomized controlled trials/ | 111,704 |
| 20 | Randomized controlled trial.pt. | 448,501 |
| 21 | Exp random allocation/or exp randomization/ | 89,826 |
| 22 | Exp placebos/ | 34,191 |
| 23 | Exp double-blind method/or double-blind$.af. | 180,225 |
| 24 | Exp multicenter study/or Multicent$.af. | 279,405 |
| 25 | Random$.ti,ab,kw,sh. | 1,124,651 |
| 26 | Blind$.ti,ab,kw,sh. | 263,750 |
| 27 | Placebo$.ti,ab,kw,sh. | 203,823 |
| 28 | Parallel$.ti,ab,kw,sh. | 266,233 |
| 29 | Exp clinical trial, phase 3/ | 13,116 |
| 30 | Exp clinical trial, phase 2/ | 29,002 |
| 31 | (“Phase III” or “phase 2” or (“phase III” or “phase II”)).af. | 112,861 |
| 32 | 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 | 1,752,319 |
| 33 | 18 and 32 | 610 |
| 34 | Limit 33 to yr=“2006–Current” | 422 |
| 35 | Limit 34 to “review articles” | 74 |
| 36 | 34 not 35 | 348 |
| 37 | Limit 36 to humans | 297 |
| 38 | Remove duplicates from 37 | 244 |
Notes: Bold font indicates the total number of articles collect in each search.
Table S2.
EBM Reviews, Cochrane Database of Systematic Reviews 2005 to June 9, 2017; Database Info Icon EBM Reviews, ACP Journal Club 1991 to May 2017; Database Info Icon EBM Reviews, Database of Abstracts of Reviews of Effects 1st Quarter 2016; Database Info Icon EBM Reviews, Cochrane Central Register of Controlled Trials April 2017; Database Info Icon EBM Reviews, Cochrane Methodology Register 3rd Quarter 2012; Database Info Icon EBM Reviews, Health Technology Assessment 4th Quarter 2016; Database Info Icon EBM Reviews, NHS Economic Evaluation Database 1st Quarter 2016
| Date: January 2017 | ||
| 1 | Exp breast neoplasms | 9184 |
| 2 | Breast adj6 cancer$ | 21,934 |
| 3 | Breast adj6 neoplas$ | 10,989 |
| 4 | Breast adj6 carcinoma$ | 2112 |
| 5 | Breast adj6 tumor$ | 573 |
| 6 | Breast adj6 tumor$ | 1851 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 23,744 |
| 8 | Metasta$ | 21,265 |
| 9 | Neoplasm Metastasis.sh. | 2226 |
| 10 | 8 or 9 | 21,265 |
| 11 | 7 and 10 | 6121 |
| 12 | “Hormone receptor positive” | 607 |
| 13 | Hormone receptor-positive’ 588 | 607 |
| 14 | Estrogen receptor-positive’ or “oestrogen receptor-positive” 654 | 522 |
| 15 | “Progesterone receptor-positive” 250 | 109 |
| 16 | Hormone sensitive 986 | 210 |
| 17 | 12 or 13 or 14 or 15 or 16 2325 | 1318 |
| 18 | 11 and 17 | 453 |
| 19 | Limit 18 to yr=“2006–Current” | 329 |
| 20 | Limit 19 to english language | 284 |
| 21 | Limit 20 to humans | 278 |
| 22 | Remove duplicates from 21 | 272 |
Notes: Bold font indicates the total number of articles collect in each search.
Table S3.
Embase database 1974 to 2017 June 09
| Date: January 2017 | ||
| 1 | Breast cancer’.af. | 404,912 |
| 2 | Exp breast tumor/ | 456,726 |
| 3 | (Breast adj6 tumor*).mp | 10,358 |
| 4 | (Breast adj6 tumor*).mp | 132,238 |
| 5 | (Breast adj6 neoplas*).mp | 22,949 |
| 6 | (Breast adj6 cancer*).mp | 446,177 |
| 7 | (Breast adj6 carcinoma*).mp | 95,202 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 513,111 |
| 9 | Metastasis/ | 310,169 |
| 10 | Metasta* | 697,570 |
| 11 | 9 or 10 | 697,570 |
| 12 | 8 and 11 | 123,533 |
| 13 | (“metastatic breast neoplasms” or “metastatic breast neoplasm” or “metastatic breast cancer”).mp | 19,097 |
| 14 | 12 or 13 | 123,533 |
| 15 | Hormone receptor positive’ OR “hormone receptor-positive” | 3917 |
| 16 | Progesterone receptor-positive’ OR “progesterone receptor positive” | 1185 |
| 17 | Estrogen receptor-positive’ or “oestrogen receptor-positive” | 7549 |
| 18 | Hormone sensitive’ | 4924 |
| 19 | Hormone adj3 positive | 5460 |
| 20 | 15 or 16 or 17 or 18 or 19 | 17,999 |
| 21 | 14 and 20 | 4258 |
| 22 | Exp “randomized controlled trial”/ | 481,221 |
| 23 | Randomization/ | 84,943 |
| 24 | Random*.ti,ab. | 1,181,594 |
| 25 | Parallel*.ti,ab | 303,245 |
| 26 | ([Single or double or triple] adj3 [blind* or mask* or dummy]).ti,ab. | 201,720 |
| 27 | Double-blind’ or “double-blinded” | 219,339 |
| 28 | Multicenter study’ or multicent* | 277,968 |
| 29 | Blind*.ti,ab. | 341,487 |
| 30 | Placebo*.ti,ab | 254,042 |
| 31 | (“Phase III” OR “phase II”).ti,ab | 40,098 |
| 32 | (“Phase III” OR “phase II”).ti,ab | 111,214 |
| 33 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 2,028,041 |
| 34 | 21 and 33 | 1173 |
| 35 | Limit 34 to human | 1094 |
| 36 | Limit 35 to english language | 1075 |
| 37 | Limit 36 to yr=“2006 -Current” | 898 |
| 38 | Limit 37 to embase | 562 |
| 39 | Limit 38 to (article or conference abstract) | 501 |
Notes: Bold font indicates the total number of articles collect in each search.
Acknowledgments
This paper was presented at the 2017 European Society for Medical Oncology as a poster presentation with interim findings. The poster’s abstract was published in the Annals of Oncology (2017) 28 (suppl_5): v74-v108. We thank Jaclyn Hearnden for her assistance in preparing the manuscript. This study was sponsored by Novartis.
Footnotes
Author contributions
A Forsythe and G Tremblay made substantial contributions to the conception and design, analyzed and interpreted the data, and helped to draft the article. A Shor acquired, analyzed, and interpreted data. D Chandiwana, J Barth, M Thabane, and J Baeck contributed to conception and development of this study and evaluation and interpretation of the data. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
D Chandiwana, J Barth, M Thabane and J Baeck are employees of Novartis. The authors report no other conflicts of interest in this work.
References
- 1.National Cancer Institute SEER Cancer Stat Facts: Female Breast Cancer. [Accessed March 29, 2018]. Available from: http://seer.cancer.gov/statfacts/html/breast.html.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Clemons M, Danson S, Hamilton T, Goss P. Locoregionally recurrent breast cancer: incidence, risk factors and survival. Cancer Treat Rev. 2001;27(2):67–82. doi: 10.1053/ctrv.2000.0204. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Martino M, Ballestrero A, Zambelli A, et al. Long-term survival in patients with metastatic breast cancer receiving intensified chemotherapy and stem cell rescue: data from the Italian registry. Bone Marrow Transplant. 2013;48(3):414–418. doi: 10.1038/bmt.2012.149. [DOI] [PubMed] [Google Scholar]
- 6.Visovsky C. Treatment considerations for the management of patients with hormone receptor-positive metastatic breast cancer. J Adv Pract Oncol. 2014;5(5):321–330. [PMC free article] [PubMed] [Google Scholar]
- 7.American Cancer Society . Breast Cancer Facts & Figures. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 8.Reinert T, Barrios CH. Definition of first-line endocrine therapy for hormone receptor-positive advanced breast cancer. J Clin Oncol. 2016;34(16):1959–1560. doi: 10.1200/JCO.2015.66.0803. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute Breast Cancer Treatment (PDQ®) – Health Professional Version. [Accessed March 29, 2018]. Available from: https://www.cancer.gov/types/breast/hp/breast-treatment-pdq.
- 10.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 11.Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16(7):1979–1987. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 12.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole vs. letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 14.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30(22):2718–2724. doi: 10.1200/JCO.2011.39.0708. [DOI] [PubMed] [Google Scholar]
- 17.Martin M, Loibl S, von Minckwitz G, et al. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the letrozole/fulvestrant and avastin (LEA) study. J Clin Oncol. 2015;33(9):1045–1052. doi: 10.1200/JCO.2014.57.2388. [DOI] [PubMed] [Google Scholar]
- 18.Mehta RS, Barlow WE, Albain KS, et al. Combination and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367(5):435–344. doi: 10.1056/NEJMoa1201622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston SR, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013;14(10):989–998. doi: 10.1016/S1470-2045(13)70322-X. [DOI] [PubMed] [Google Scholar]
- 20.Dickler MN, Barry WT, Cirrincione CT, et al. Phase III trial evaluating letrozole as first-line endocrine therapy with or without bevacizumab for the treatment of postmenopausal women with hormone receptor-positive advanced-stage breast cancer: CALGB 40503 (Alliance) J Clin Oncol. 2016;34(22):2602–2609. doi: 10.1200/JCO.2015.66.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park IH, Lee KS, Ro J. Effects of second and subsequent lines of chemotherapy for metastatic breast cancer. Clin Breast Cancer. 2015;15(1):e55–e62. doi: 10.1016/j.clbc.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol. 2015;33(32):3781–3787. doi: 10.1200/JCO.2015.61.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gligorov J, Doval D, Bines J, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(12):1351–1360. doi: 10.1016/S1470-2045(14)70444-9. [DOI] [PubMed] [Google Scholar]
- 24.Lam SW, de Groot SM, Honkoop AH, et al. Paclitaxel and bevacizumab with or without capecitabine as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a multicentre, open-label, randomised phase 2 trial. Eur J Cancer. 2014;50(18):3077–3088. doi: 10.1016/j.ejca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen DL, Bjerre KD, Jakobsen EH, et al. Gemcitabine plus docetaxel versus docetaxel in patients with predominantly human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: a randomized, phase III study by the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2011;29(36):4748–4754. doi: 10.1200/JCO.2010.33.9507. [DOI] [PubMed] [Google Scholar]
- 26.Twelves C, Awada A, Cortes J, et al. Subgroup analyses from a phase 3, open-label, randomized study of eribulin mesylate versus capecitabine in pretreated patients with advanced or metastatic breast cancer. Breast Cancer (Auckl) 2016;10:77–84. doi: 10.4137/BCBCR.S39615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twelves C, Cortes J, Vahdat L, et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res Treat. 2014;148(3):553–561. doi: 10.1007/s10549-014-3144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welt A, Marschner N, Lerchenmueller C, et al. Capecitabine and bevacizumab with or without vinorelbine in first-line treatment of HER2/neu-negative metastatic or locally advanced breast cancer: final efficacy and safety data of the randomised, open-label superiority phase 3 CARIN trial. Breast Cancer Res Treat. 2016;156(1):97–107. doi: 10.1007/s10549-016-3727-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zielinski C, Lang I, Inbar M, et al. Bevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer (TURANDOT): primary endpoint results of a randomised, open-label, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(9):1230–1239. doi: 10.1016/S1470-2045(16)30154-1. [DOI] [PubMed] [Google Scholar]
- 30.Luck HJ, Lubbe K, Reinisch M, et al. Phase III study on efficacy of taxanes plus bevacizumab with or without capecitabine as first-line chemotherapy in metastatic breast cancer. Breast Cancer Res Treat. 2015;149(1):141–149. doi: 10.1007/s10549-014-3217-y. [DOI] [PubMed] [Google Scholar]
- 31.Macalalad AR, Hao Y, Lin PL, et al. Treatment patterns and duration in post-menopausal women with HR+/HER2-metastatic breast cancer in the US: a retrospective chart review in community oncology practices (2004-2010) Curr Med Res Opin. 2015;31(2):263–273. doi: 10.1185/03007995.2014.980885. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda T, Shiroiwa T, Shimozuma K, et al. Long-term Eq-5d score for patients with metastatic breast cancer; comparison of first-line oral S-1 and taxane therapies in the randomized “select” trial. Value Health. 2015;18(7):A467. doi: 10.1007/s11136-016-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma S, O’Shaughnessy J, Burris HA, et al. Health-related quality of life of postmenopausal women with hormone receptor–positive, HER2-advanced breast cancer treated with ribociclib + letrozole: Results from MONALEESA-2. J Clin Oncol. 2017;35(31 Suppl):133. [Google Scholar]
- 34.CANCERMPACT . More Than Just Numbers. New York City, NY, USA: Kantar Health; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Ovid Medline® Epub ahead of print, in-process, and other non-indexed citations. Ovid Medline® daily and Ovid Medline® 1946 to present database
| Date: January 2017 | ||
| 1 | Exp breast neoplasms/ | 250,773 |
| 2 | (Breast adj6 cancer$).af. | 246,948 |
| 3 | (Breast adj6 neoplas$).af. | 252,542 |
| 4 | (breast adj6 carcinoma$).af. | 63,807 |
| 5 | (Breast adj6 tumor$).af. | 7401 |
| 6 | (Breast adj6 tumor$).af. | 48,416 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 336,672 |
| 8 | Metasta$.mp. or exp neoplasm metastasis/ | 477,568 |
| 9 | 7 and 8 | 74,656 |
| 10 | (“Metastatic breast cancer” or “metastatic breast neoplasms”).af. | 11,972 |
| 11 | 9 or 10 | 74,661 |
| 12 | “Hormone receptor positive”.af. | 2300 |
| 13 | “Hormone receptor-positive”.af. | 2300 |
| 14 | (“Estrogen receptor-positive” or “oestrogen receptor-positive”).af. | 4218 |
| 15 | “Progesterone receptor-positive”.af. | 732 |
| 16 | “Hormone sensitive”.af. | 3719 |
| 17 | 12 or 13 or 14 or 15 or 16 | 10,513 |
| 18 | 11 and 17 | 1955 |
| 19 | Exp randomized controlled trials/ | 111,704 |
| 20 | Randomized controlled trial.pt. | 448,501 |
| 21 | Exp random allocation/or exp randomization/ | 89,826 |
| 22 | Exp placebos/ | 34,191 |
| 23 | Exp double-blind method/or double-blind$.af. | 180,225 |
| 24 | Exp multicenter study/or Multicent$.af. | 279,405 |
| 25 | Random$.ti,ab,kw,sh. | 1,124,651 |
| 26 | Blind$.ti,ab,kw,sh. | 263,750 |
| 27 | Placebo$.ti,ab,kw,sh. | 203,823 |
| 28 | Parallel$.ti,ab,kw,sh. | 266,233 |
| 29 | Exp clinical trial, phase 3/ | 13,116 |
| 30 | Exp clinical trial, phase 2/ | 29,002 |
| 31 | (“Phase III” or “phase 2” or (“phase III” or “phase II”)).af. | 112,861 |
| 32 | 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 | 1,752,319 |
| 33 | 18 and 32 | 610 |
| 34 | Limit 33 to yr=“2006–Current” | 422 |
| 35 | Limit 34 to “review articles” | 74 |
| 36 | 34 not 35 | 348 |
| 37 | Limit 36 to humans | 297 |
| 38 | Remove duplicates from 37 | 244 |
Notes: Bold font indicates the total number of articles collect in each search.
Table S2.
EBM Reviews, Cochrane Database of Systematic Reviews 2005 to June 9, 2017; Database Info Icon EBM Reviews, ACP Journal Club 1991 to May 2017; Database Info Icon EBM Reviews, Database of Abstracts of Reviews of Effects 1st Quarter 2016; Database Info Icon EBM Reviews, Cochrane Central Register of Controlled Trials April 2017; Database Info Icon EBM Reviews, Cochrane Methodology Register 3rd Quarter 2012; Database Info Icon EBM Reviews, Health Technology Assessment 4th Quarter 2016; Database Info Icon EBM Reviews, NHS Economic Evaluation Database 1st Quarter 2016
| Date: January 2017 | ||
| 1 | Exp breast neoplasms | 9184 |
| 2 | Breast adj6 cancer$ | 21,934 |
| 3 | Breast adj6 neoplas$ | 10,989 |
| 4 | Breast adj6 carcinoma$ | 2112 |
| 5 | Breast adj6 tumor$ | 573 |
| 6 | Breast adj6 tumor$ | 1851 |
| 7 | 1 or 2 or 3 or 4 or 5 or 6 | 23,744 |
| 8 | Metasta$ | 21,265 |
| 9 | Neoplasm Metastasis.sh. | 2226 |
| 10 | 8 or 9 | 21,265 |
| 11 | 7 and 10 | 6121 |
| 12 | “Hormone receptor positive” | 607 |
| 13 | Hormone receptor-positive’ 588 | 607 |
| 14 | Estrogen receptor-positive’ or “oestrogen receptor-positive” 654 | 522 |
| 15 | “Progesterone receptor-positive” 250 | 109 |
| 16 | Hormone sensitive 986 | 210 |
| 17 | 12 or 13 or 14 or 15 or 16 2325 | 1318 |
| 18 | 11 and 17 | 453 |
| 19 | Limit 18 to yr=“2006–Current” | 329 |
| 20 | Limit 19 to english language | 284 |
| 21 | Limit 20 to humans | 278 |
| 22 | Remove duplicates from 21 | 272 |
Notes: Bold font indicates the total number of articles collect in each search.
Table S3.
Embase database 1974 to 2017 June 09
| Date: January 2017 | ||
| 1 | Breast cancer’.af. | 404,912 |
| 2 | Exp breast tumor/ | 456,726 |
| 3 | (Breast adj6 tumor*).mp | 10,358 |
| 4 | (Breast adj6 tumor*).mp | 132,238 |
| 5 | (Breast adj6 neoplas*).mp | 22,949 |
| 6 | (Breast adj6 cancer*).mp | 446,177 |
| 7 | (Breast adj6 carcinoma*).mp | 95,202 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 513,111 |
| 9 | Metastasis/ | 310,169 |
| 10 | Metasta* | 697,570 |
| 11 | 9 or 10 | 697,570 |
| 12 | 8 and 11 | 123,533 |
| 13 | (“metastatic breast neoplasms” or “metastatic breast neoplasm” or “metastatic breast cancer”).mp | 19,097 |
| 14 | 12 or 13 | 123,533 |
| 15 | Hormone receptor positive’ OR “hormone receptor-positive” | 3917 |
| 16 | Progesterone receptor-positive’ OR “progesterone receptor positive” | 1185 |
| 17 | Estrogen receptor-positive’ or “oestrogen receptor-positive” | 7549 |
| 18 | Hormone sensitive’ | 4924 |
| 19 | Hormone adj3 positive | 5460 |
| 20 | 15 or 16 or 17 or 18 or 19 | 17,999 |
| 21 | 14 and 20 | 4258 |
| 22 | Exp “randomized controlled trial”/ | 481,221 |
| 23 | Randomization/ | 84,943 |
| 24 | Random*.ti,ab. | 1,181,594 |
| 25 | Parallel*.ti,ab | 303,245 |
| 26 | ([Single or double or triple] adj3 [blind* or mask* or dummy]).ti,ab. | 201,720 |
| 27 | Double-blind’ or “double-blinded” | 219,339 |
| 28 | Multicenter study’ or multicent* | 277,968 |
| 29 | Blind*.ti,ab. | 341,487 |
| 30 | Placebo*.ti,ab | 254,042 |
| 31 | (“Phase III” OR “phase II”).ti,ab | 40,098 |
| 32 | (“Phase III” OR “phase II”).ti,ab | 111,214 |
| 33 | 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 2,028,041 |
| 34 | 21 and 33 | 1173 |
| 35 | Limit 34 to human | 1094 |
| 36 | Limit 35 to english language | 1075 |
| 37 | Limit 36 to yr=“2006 -Current” | 898 |
| 38 | Limit 37 to embase | 562 |
| 39 | Limit 38 to (article or conference abstract) | 501 |
Notes: Bold font indicates the total number of articles collect in each search.