Abstract
Objective
To investigate the epidemiology of medication errors and error-related adverse events in adults in primary care, ambulatory care and patients’ homes.
Design
Systematic review.
Data source
Six international databases were searched for publications between 1 January 2006 and 31 December 2015.
Data extraction and analysis
Two researchers independently extracted data from eligible studies and assessed the quality of these using established instruments. Synthesis of data was informed by an appreciation of the medicines’ management process and the conceptual framework from the International Classification for Patient Safety.
Results
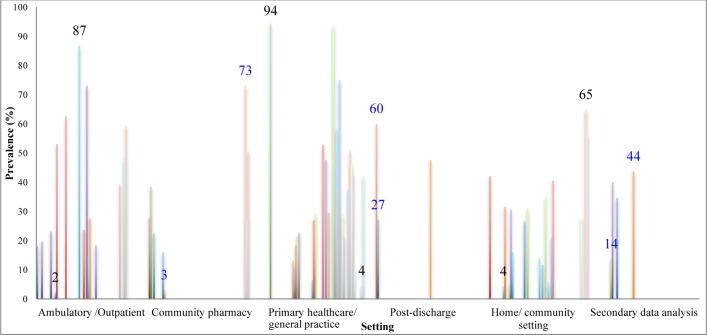
60 studies met the inclusion criteria, of which 53 studies focused on medication errors, 3 on error-related adverse events and 4 on risk factors only. The prevalence of prescribing errors was reported in 46 studies: prevalence estimates ranged widely from 2% to 94%. Inappropriate prescribing was the most common type of error reported. Only one study reported the prevalence of monitoring errors, finding that incomplete therapeutic/safety laboratory-test monitoring occurred in 73% of patients. The incidence of preventable adverse drug events (ADEs) was estimated as 15/1000 person-years, the prevalence of drug–drug interaction-related adverse drug reactions as 7% and the prevalence of preventable ADE as 0.4%. A number of patient, healthcare professional and medication-related risk factors were identified, including the number of medications used by the patient, increased patient age, the number of comorbidities, use of anticoagulants, cases where more than one physician was involved in patients’ care and care being provided by family physicians/general practitioners.
Conclusion
A very wide variation in the medication error and error-related adverse events rates is reported in the studies, this reflecting heterogeneity in the populations studied, study designs employed and outcomes evaluated. This review has identified important limitations and discrepancies in the methodologies used and gaps in the literature on the epidemiology and outcomes of medication errors in community settings.
Keywords: medication errors, adverse drug events, error-related adverse drug events, prevalence, incidence, risk factor
Strengths and limitations of this study.
This is the first systematic review on the epidemiology of medication errors and medication-associated harm in community settings. The use of the International Classification for Patient Safety conceptual framework helped with framing and organising the findings from this systematic review.
A rigorous and transparent process has been employed, which included no language restrictions in undertaking searches, independent screening of titles, abstracts and full-text papers, independent data extraction, and critical appraisal of included studies by two reviewers.
Outcomes have been reported in a variety of ways using different tools and methodology, which made it difficult to undertake any quantitative pooled summary of the results.
Despite the comprehensiveness of the searches, we found no data regarding errors during medication dispensing and administration. This might be due to the lack of ‘dispensing error’ and ‘administration error’ terms in our search strategy, although ‘medication therapy management’ was included as a more overarching search term.
There is at present no agreed, consistently applied set of confounders that should be taken into account when trying to make causal inferences.
Introduction
Patient safety is a public concern in healthcare systems across the world.1 Medication errors and error-related adverse drug events (ADEs) are common and are responsible for considerable patient harm.1 More specifically, ADEs can lead to morbidity, hospitalisation, increased healthcare costs and, in some cases, death.1 It has been estimated that 5%–6% of all hospitalisations are drug-related,2 3 with one estimate suggesting that ADEs causing hospital admission in the UK occur in around 10% of inpatients; approximately half of these ADEs are believed to be preventable.4 The cost of medication errors worldwide has been estimated as US$42 billion/year.5
Since the release of To Err is Human: Building a Safer Health System by the Institute of Medicine (now the National Academy of Medicine),6 which focused on acute care settings, most patient safety research has been conducted in hospital settings.7 8 Given that international and national policy drivers are for patients to be increasingly managed in primary, ambulatory and home settings in order to realise the goals of more accessible, patient-centred and efficient healthcare,9 there is an increased sense of urgency to further focus attention on community care contexts, particularly in relation to medication safety. With an ageing population, particularly in economically developed countries, as well as the use of polypharmacy, there is a need to empower patients, particularly those with chronic diseases, to self-care safely.
The aim of this systematic review was to investigate the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts (ie, primary care, ambulatory and home settings). Box 1 provides definitions of the key terms employed in this review.
Box 1. Key definitions.
Adverse drug event (ADE): Bates et al 84 define ADE as ‘an injury resulting from medical intervention related to a drug’. 84 Some ADEs are caused by underlying medication errors and therefore they are preventable.
Medication error: The National Coordinating Council for Medication Error Reporting and Prevention defines a medication error as ‘any preventable event that may cause or lead to inappropriate medication use or patient harm, while the medication is in the control of the health care professional, patient, or consumer. Such events may be related to professional practice, health care products, procedures, and systems, including prescribing; order communication; product labelling, packaging, and nomenclature; compounding; dispensing; distribution; administration; education; monitoring; and use’. 85 Medication errors can result from any step of the medication-use process: selection and procurement, storage, ordering and transcribing, preparing and dispensing, administration, or monitoring.1
Non-prescription drugs: Medicines that can be sold legally without a drug prescription.
Over-the-counter (OTC) drug: The Food and Drug Administration defines OTC drugs as ‘drugs that have been found to be safe and appropriate for use without the supervision of a health care professional such as a physician, and they can be purchased by consumers without a prescription’.86
Prescription drug: Drugs that cannot be sold legally without a prescription.
Methods
Protocol and reporting
The study protocol was developed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and was registered in PROSPERO.10 11 The detailed systematic review protocol has also been published.12
Eligibility criteria/study selection
Studies conducted in adults (≥18 years) who were looked after in the community and living in their own or family homes without home healthcare or nursing home were eligible for inclusion in this review. The studied patients could have been self-managing, receiving care in primary care or ambulatory care settings, or any combination of the above. Studies were included if they were population-based, cross-sectional or cohort studies, which were suitable to estimate the incidence and prevalence of medication errors or ADEs. These study designs and case–control studies were considered eligible to study risk factors for the development of error-related ADEs. Studies with prescribed and/or over-the-counter (OTC) medications as the exposure of interest were eligible.
Paediatric studies (<18 years) and studies on patients receiving care in hospital at home settings (ie, continuous medical and/or nursing care provided to patients in their own homes), in nursing homes, as hospitalised inpatients or in emergency departments (ED) were excluded. Randomised controlled trials were excluded since these could not be used to reliably assess the incidence and/or prevalence of the outcomes of interest. Existing reviews were also excluded since the focus was on the primary literature. Incompletely reported studies, for example, in the form of abstracts, were not eligible for inclusion. Studies on illegal substance abuse, herbal products and those focusing on particular medications were also excluded.
No restriction on the language of publication was employed.
Data sources and search strategy
Search terms were developed based on the systematic review protocol.12 The search terms and detailed search strategies are presented in online supplementary appendix 1. In summary, these involved identifying search terms (and their synonyms) in relation to medication safety, community care settings and study design, and combining these concepts with the Boolean operator AND to identify studies that intersected all three search concepts of interest. Examples of the search terms used included the following: for the outcome: medication safety, medication error, preventable adverse drug event and patient error; for the setting: ambulatory care, outpatient, self-care, primary healthcare and general practice; and for the study design: cohort study, cross sectional study and observational study. Six biomedical databases were searched, including the Cumulative Index to Nursing and Allied Health Literature, EMBASE, Eastern Mediterranean Regional Office of the WHO, MEDLINE, PsycINFO and Web of Science, between 1 January 2006 and 31 December 2015. Google Scholar was searched for additional studies. An international panel of experts was also contacted to identify unpublished work and research in progress (online supplementary appendix 1). The reference list of all included studies was further reviewed for additional possible eligible studies.
bmjopen-2017-019101supp001.pdf (104.3KB, pdf)
The databases were searched by GAA. The title and abstracts were then independently screened for eligible studies according to the above detailed selection criteria by GAA and a second reviewer, NAS. The corresponding authors of the eligible articles were contacted if additional information was needed. Disagreements were resolved by discussion between the reviewers or by arbitration by a third reviewer, AS, if a decision could not be reached. Full-text articles were retrieved from selected studies and reviewed according to the selection criteria. Each copy of the selected studies was retrieved and the reason for excluding other studies was clearly noted.
Data extraction and risk of bias assessment
Data were independently extracted and recorded onto a customised data extraction sheet by two reviewers (GAA and NAS, or GAA and MAM). Discrepancies were resolved by discussion or by arbitration by an additional reviewer (AS), if necessary.
Key information, such as study design, study type (retrospective, prospective), population of interest, exposure of interest, outcomes of interest and main findings, was extracted.
The risk of bias assessment was independently carried out on each study by two reviewers (GAA and NAS, or GAA and NA) using the Critical Appraisal Skills Programme (CASP) quality assessment tool for cohort and case–control studies,13 and cross-sectional studies were assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for descriptive studies.14 Any disagreements were resolved by consensus or by arbitration by a third reviewer (AS) if a decision could not be reached. Each study was given an overall grading as being at high, medium or low risk of bias.
Data synthesis
Data were summarised in detailed data tables, which included information on the incidence, prevalence, relative risk and ORs, together with 95% CIs, for each study (where available). A descriptive and narrative synthesis of the extracted data was undertaken.
The following is the definition of incidence rate used in this review: ‘the number of patients with one or more [medication error or preventable ADE] (numerator) divided by the total number of patients at risk per time unit (denominator)’. 15 The following is the definition of prevalence rate used in the data extraction: ‘the number of patients experiencing one or more [medication error or preventable ADE] (numerator) divided by the total number of patients in the study population (denominator)’. 16 The prevalence rate per population was either reported and extracted directly from the included study or calculated from data provided in the study.
We worked with the definitions of medication errors and error-related ADEs employed in individual studies. These errors may have occurred anywhere in the medicines’ management process.1 Medication errors were described according to (1) the stage in the medicines’ management process when the error occurred, that is, prescribing, dispensing, administration and monitoring1; and (2) the type of error that occurred in each stage according to the conceptual framework for the International Classification for Patient Safety (ICPS) definitions (box 2).17
Box 2. Classification of definitions used in this systematic review.
Administration error: ‘Any discrepancy between how the medication is given to the patient and the administration directions from the physician or hospital guidelines’.1
Prescribing error: ‘Medication error occurring during the prescription of a medicine that is about writing the drug order or taking the therapeutic decision, appreciated by any non-intentional deviation from standard reference such as: the actual scientific knowledge, the appropriate practices usually recognized, the summary of the characteristics of the medicine product, or the mentions according to the regulations. A prescribing error notably can concern: the choice of the drug (according to the indications, the contraindications, the known allergies and patient characteristics, interactions whatever nature it is with the existing therapeutics, and the other factors), dose, concentration, drug regimen, pharmaceutical form, route of administration, duration of treatment, and instructions of use; but also the failure to prescribe a drug needed to treat an already diagnosed pathology, or to prevent the adverse effects of other drugs’.17
Inappropriate prescribing: ‘The use of medicines that introduce a significant risk of an adverse drug-related event where there is evidence for an equally or more effective but lower-risk alternative therapy available for treating the same condition. Inappropriate prescribing also includes the use of medicines at a higher frequency and for longer than clinically indicated, the use of multiple medicines that have recognized drug–drug interactions and drug–disease interactions, and importantly, the under-use of beneficial medicines that are clinically indicated but not prescribed for ageist or irrational reasons’.87
Monitoring error: ‘Failure to review a prescribed regimen for appropriateness and detection of problems, or failure to use appropriate clinical or laboratory data for adequate assessment of patient response to prescribed theory’.17
Dispensing error: ‘Deviation from the prescriber’s order, made by staff in the pharmacy when distributing medications to nursing units or to patients in an ambulatory pharmacy setting’.17
Other discrepancies:‘Any differences between the medication described by the patient and caregivers with the drugs listed by their general practitioners (GP) or between the medications listed in the discharge letter for the primary care physician with those in the patient discharge medication list’.31 32
Risk factors were categorised as patient, healthcare professional and medication-related risk factors.
Changes from the original protocol
The following changes were made from the plans described in the research protocol12: (1) due to the large quantity of studies found during the initial search and because of medications and practice changes over the years, only studies published in the last 10 years were included: 1 January 2006–31 December 2015; (2) only studies with the incidence or prevalence rate per number of patients were included; and (3) meta-analysis was not possible due to the heterogeneity of outcomes, methods and definitions.
Results
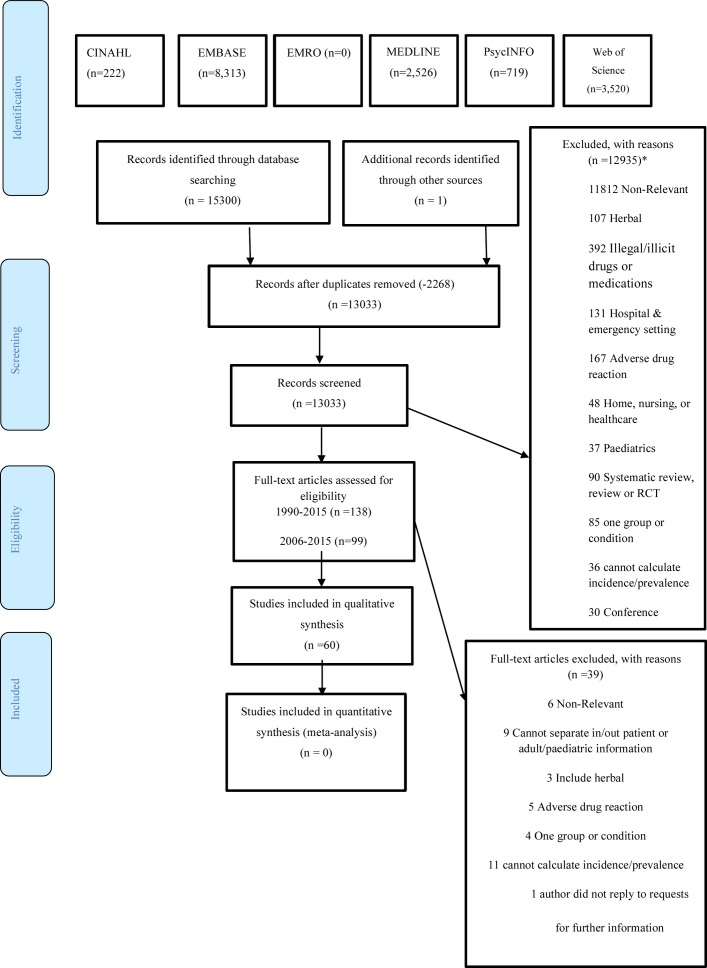
A total of 13 033 potentially eligible studies were identified after removing duplicates, of which 59 studies met the inclusion criteria. One additional study was identified through hand-searching. Therefore, a total of 60 studies were included in the systematic review (figure 1).
Figure 1.
PRISMA flow diagram (from Moher et al 88). CINAHL, Cumulative Index to Nursing and Allied Health Literature; EMRO, Eastern Mediterranean Regional Office; RCT, randomised controlled trial. *Articles may be duplicated between the excluded groups.
One study was available only in German and one in Spanish. Those two papers were retrieved and translated into English by native speakers.18 19
The key characteristics of all included studies are presented in table 1. The quality assessments of these studies are summarised in tables 2A and 2B.
Table 1.
Systematic review data extraction table
| Key characteristics of included studies | ||||||||||||
| Author, year | Country/city | Study design/type | Population of interest | Exposure of interest | Outcome of interest | Main finding | Conclusion, n/N (%) | Additional notes | ||||
| Self-reported medication errors | ||||||||||||
| 1. | Adam et al, 200972 | Australia | Cross-sectional | Analysis of data from 3522 adults participating in stage 2 of the North West Adelaide Health Study aged ≥18 years | Unclear | Self-reported adverse event (medication, diagnosis and others). Using survey. |
Of the total 3522 survey participants, 148 (4.2%) reported an adverse event causing harm in the previous 12 months, giving an annual incidence of 4.2% (95% CI 3.4% to 5.0%). Medication error: The main types of adverse events perceived as causing harm were medication error (reported by 46% of the 148 participants reporting adverse events). |
Medication error prevalence: 68/3522=1.9% | Subjective data rather than objective |
|||
| 2. | Lu and Roughead, 201120 | Australia, Canada, New Zealand, UK, USA, Germany and The Netherlands | Cross-sectional (secondary analysis) | 11 910 adult respondents aged ≥18 years. Data from the 2007 Commonwealth Fund International Health Policy Survey. |
Prescribed drug | Self-reported medication error and compare factors associated with medication errors across the seven countries. Using survey. |
Self-reported medication errors prevalence: 752 respondents had medication error (Australia=7.4%; Canada=5.7%; New Zealand=5.9%; UK=5.2%; USA=7%; Germany=5.2%; The Netherlands=8%). Risk factors across countries included seeing multiple specialists, multiple chronic conditions, hospitalisation and multiple emergency room visits. |
Medication error prevalence: 752/11 910=6.3% | Prevalence for medication error alone from table 1, while the risk factors for both medical and medication error. |
|||
| 3. | Sears et al, 201221 | Australia, Canada, France, Germany, the Netherlands, New Zealand, UK and USA | Descriptive (secondary/retrospective analysis) | 9944 adults aged ≥18 years from the community setting |
Taking medication regularly | Patient-related risk factors associated with self-reported medication errors. Using telephone survey. |
Medication error prevalence: 570 respondents with medication errors occurring in the community setting. Approximately 4 out of every 5 self-reported medication errors occurred in the community setting. |
Medication error prevalence: 570/9944=5.7% | Risk factors for both hospital and community setting |
|||
| 4. | Mira et al, 201373 | Alicante, Spain | Cross-sectional | 382 elderly aged ≥65 years from primary care. Patients on polypharmacy (five or more drugs) and with comorbidity: cardiovascular (51.6%); diabetes (34.3%). |
Prescribed and self-medications | Frequency of mistakes in communication between the physician and the patient and their medication error in the last year. Using semistructured interviews. |
Medication error prevalence: 75.1% of the patients reported having made at least one mistake with the medication in the last year. Risk factors: Multiple comorbidities (p=0.006), frequent changes in prescription (p=0.02), not considering the prescriptions of other physicians (p=0.01), inconsistency in the messages (p=0.01), being treated by various different physicians at the same time (p=0.03), a feeling of not being listened to (p<0.001) or loss of trust in the physician (p<0.001). *The error due to drug confusion had very severe consequences, requiring a visit to the emergency service or hospital admission. |
Medication error prevalence: 287/382=75% | Consequence* | |||
| Risk factors | ||||||||||||
| 5. | Sorensen et al, 200676 | 4 states of Australia | Cross-sectional, prospective | 204 general practice patients living in their own home aged 37–99 years | Prescribed drugs | Prevalence and interrelationships of medication-related risk factors for poor patient health outcomes identifiable through ‘in-home’ visit observations. | Risk factors: Prevalence of nominal medication-related risk factors and health outcomes among the sample of 204 patients. 1. Multiple medication storage locations used=17 (8.3%). 2. Expired medication present=40 (19.6%). 3. Discontinued medication repeats retained=43 (21%). 4. Hoarding of medications=43 (21%). 5. Therapeutic duplication present=50 (24.5%). Administration error: 6. No medication administration routine=56 (27.5%). 7. Poor adherence=107 (52.5%). 8. Confused by generic and trade names=114 (55.9%). |
|||||
| 6. | Vuong and Marriott, 200625 | Melbourne, Australia | Descriptive | 142 discharged adults aged ≥55 years who were returning to independent care at home. Patients at risk of medication misadventure. |
Discharge prescribed drugs | Unnecessary medicine stored at home as a risk factor. Using home visit within 5 days of discharge. |
Unnecessary medicine stored at home prevalence: 85/142=60%. 85 (60%) of 142 patients who received a home visit allowed removal of medicines that had expired or no longer required. Prescribing error: drug duplication prevalence: 32 (27%) patients allowed removal of 82 duplicate packs of the same item that was no longer required. A total of 390 medicines were removed with a mean of 4.6 medicines per patient (range 1–21). |
Unnecessary medicine stored at home prevalence: 85/142=60% | No information on how many patients had unnecessary medicine. Information available is on the patient allowed to remove unnecessary medicine. |
|||
| 7. | Pit et al, 200874 | New South Wales, Australia. | Cross-sectional study | 849 elderly aged ≥65 years from general practice | Self-medications | Prevalence of self-reported risk factors for medication misadventures. Tool used: Medication Risk Assessment Form (patient survey) |
Risk factors: 1. Using at least one medication for more than 6 months (95%). 2. More than one doctor involved in their care (59%). 3. Had three or more health conditions (57%). 4. Used five or more medicines (54%). 5. ADRs, in the last month 39% of participants experienced difficulties sleeping, felt drowsy or dizzy (34%), had a skin rash (28%), leaked urine (27%), had stomach problems (22%) or had been constipated (22%). |
*ADR as a risk factor for medication misadventure may not be related to the use of medication in all cases. |
||||
| 8. | Mosher et al, 201275 | Iowa, USA | Cohort prospective | 310 elderly aged ≥65 years who were cognitively intact from a Veterans Administration primary care clinic | Taking five or more non-topical medications | Association of health literacy with medication knowledge, adherence and ADEs. Using interview and chart review. |
Total: 310 patients Prevalence of ADEs: ADEs occurred in 51 patients (16.5%) of the patients within the first 3 months of the study, which increased to 119 patients (38.4%) over the full 12-month follow-up period. Risk factor: Association of health literacy with ADEs: The incidence of ADEs at 3 and 12 months appeared higher among patients with low health literacy, but this was not statistically significant. |
Low health literacy increased the risk of ADEs. | ||||
| Medicines’ management process: | ||||||||||||
| 9. | Koper et al, 201323 | Austria | Descriptive | 169 patients from general practice taking five or more medicines. Mean age: 76.4±8.5 SD years. Of the 169 patients, 158 were elderly aged ≥65 years. |
Prescribed and OTC drug | Medication errors including non-evidence-based medications, dosing errors and potentially dangerous interactions in all patients. Potential interactions were identified using the Lexi-Interact database. PIMs in subgroup of elderly patients according to the PRISCUS list. Using case report form filled by the GPs. |
Prescribing error prevalence: Indication: 158 of the 169 patients (93.5%) had at least one non-evidence-based medication. Dosing error: 74 of the 169 patients (43.8%) had at least one dosing error. DDI prevalence: Category D interactions: 99 patients (58%) had at least one category D interaction. Category X interactions: 4 patients (2.4%) had at least one category X interaction. PIM prevalence: 59 of seniors (37.3%) had at least one medication that was inappropriate. |
Medication error prevalence: 1. Non-evidence-based medications: 158/169=93.5%. 2. Dosing error: 74/169=43.8%. 3. Category D drug interaction: 99/168=58%; category X drug interaction: 4/168=2.4%. 4. PIMs: 59/158=37.3%. |
A medication was classified as non- evidence-based if the indication for use indicated by the GP was not mentioned in any peer-reviewed chapter of UpToDate. |
|||
| 10. | Mand et al, 201433 | Germany | Descriptive retrospective | 24 619 elderly aged ≥65 years from family practice with at least one diagnosis named in the Beers list | Prescribed drug | PDDI frequency and whether there are gender-related or age-related differences. Analysis from electronic patient records. |
Prescribing error: Contraindication or drug–disease interaction prevalence: 10.4% of elderly were exposed to at least one PDDI. Risk factors: 1. Patients over 75 years (OR 1.10, CI 1.05 to 1.15). 2. Number of drugs prescribed (>4 drugs: OR 1.91, CI 1.83 to 2.00). 3. Blood clotting disorders/receiving anticoagulant therapy (OR 2.38, CI 2.15 to 2.64) showed the strongest association with PDDI. |
PDDI prevalence: 2560/24 619=10.4% | ||||
| 11. | Gagne et al, 200836 | Regione Emilia-Romagna, Italy | Cohort retrospective | 4 222 165 regional Emilia-Romagna residents. Outpatient aged from 0 to ≥85 years. |
Prescribed drug | Clinically important potential DDI. Risk factors. Outpatient prescription data from the Regional Emilia-Romagna. DDI screening tool: a list of clinically important potential DDIs included 12 drug pairs that could be captured using the regional Emilia-Romagna database. |
Prescribing error: DDI prevalence: exposed to potential DDI adults (19 to ≥85 years)=7893. Unexposed adult=7013. Total=14 906. |
DDI prevalence: 7893/14 906=53% | Risk factors for all age groups including paediatrics. All age groups included so results should be considered cautiously. |
|||
| 12. | Dallenbach et al, 200724 | Geneva, Switzerland | Descriptive, retrospective file review | 591 outpatients, mean age 39 years |
Prescription drug and drug currently taking | Clinically significant ADI. Prescription review. DDI screening tool: DDI compendia and (ePocrates RX) with clinical decision support. |
Prescribing error: DDI prevalence: In 135 of the consultations, a potentially clinically significant ADI was identified. |
DDI prevalence: 135/591=23% | ||||
| 13. | Obreli Neto et al, 201126 | Brazil | Cross-sectional | 2627 elderly aged 60–88 years from the primary healthcare | Prescribed drug | Potential risks in drug prescriptions: DDI and PIM. Using prescription review. DDI screening tool: (DrugDigest, Medscape and Micromedex). PIM using Beers criteria 2003. |
Prescribing error: DDI prevalence: Using DrugDigest showed that 4.7% and 28.4% of the elderly presented at least one potential DDI classified as major and moderate, respectively. Using Medscape showed that 3.4% and 19.3% of the elderly presented at least one potential DDI classified as major and moderate, respectively. Using Micromedex showed that 3.1% and 29.1% of the elderly presented at least one potential DDI classified as major and moderate, respectively. Prescribing error: PIM prevalence: 26.9% of the patients had prescriptions with at least one PIM. |
DDI prevalence: 3.1%–29.1% PIM prevalence: 26.9% |
||||
| 14. | Secoli et al, 201030 | Sao Paulo, Brazil | Cross-sectional | 2143 community-dwelling elderly aged ≥60 years. Data were obtained from the SABE (Health, Well-Being and Ageing) survey. |
≥2 prescribed drug use | Potential DDIs and identify associated factors. Using home interview. DDI screening tool: Micromedex Healthcare Series. |
Prescribing error: DDI prevalence: 568/2143=26.5%. Risk factors: The use of six or more medications (OR 3.37, 95% CI 2.08to 5.48) or having hypertension (OR 2.56, 95% CI 1.73 to 3.79), diabetes (OR 1.73, 95% CI 1.22to 2.44) or heart problems (OR 3.36, 95% CI 2.11 to 5.34) significantly increased the risk of potential DDI. |
DDI prevalence: 568/2143=26.5% | ||||
| 15. | Obreli Neto et al, 201227 | 5 cities of Brazil | Cross-sectional | 12 343 elderly aged ≥60 years from the primary public health system | Prescription for two or more drugs (prescribed both within and across prescriptions) | Potential DDIs (presence of a minimum of 5 days overlap in supply of an interacting drug pair) and predictor of DDI. Using medical prescriptions and patients’ medical records review. DDI screening tool: DDI checker programmes (DrugDigest, Drugs, Micromedex and Medscape). |
12 343 patients (5855 exposed; 6488 unexposed). Prescribing error: DDI prevalence: 47.4% Risk factors: Female sex (OR=2.49 (95% CI 2.29 to 2.75)), diagnosis of ≥3 diseases (OR=6.43 (95% CI 3.25 to 12.44)) and diagnosis of hypertension (OR=1.68 (95% CI 1.23 to 2.41)) were associated with potential DDIs. Age was associated with an increasing risk of DDIs. Number of prescribers, number of drugs consumed, ATC codes and drugs that act on CYP450 presented positive associations with potential DDIs in univariate and multivariate analyses of drug therapy characteristics. |
DDI prevalence: 5855/12 343=47.4% | ||||
| 16. | Indermitte et al, 200734 | Switzerland | Descriptive | 434 passer-by customers aged ≥18 years from community pharmacies | Prescription-only medicines and OTC drug | Potential drug interactions. 1. Observation of customer contacts and interviews with passer-by customers purchasing selected OTC drugs. 2. Telephone interviews with regular customers treated with selected prescription-only medicines identified in community pharmacies’ databases. DDI screening tool: Pharmavista database. |
Prescribing error: DDI prevalence: Observation of passer-by customers. Of 1183 passer-by customers observed, 164 purchased at least one of the selected OTC drugs. 102 (62.2%) of those subjects were interviewed. 43 (42.2%) mentioned taking prescribed drugs and 3 of them were exposed to potential drug interactions of moderate severity. Telephone interview with regular customers. Out of 592 regular customers selected from the community pharmacy database, 434 (73.3%) could be interviewed. Prevalence of DDI in regular customers: 69 (15.9%) of them were exposed to a potential drug interaction between purchased OTC drug for self-medication and their prescription-only medicines. Furthermore, 116 (26.7%) regular customers were exposed to potential drug interactions within their prescribed drugs and in 28 (6.5%) multiple (>2) potential drug interactions were found. |
DDI prevalence: 3/102=3%, 69/434=16%, 116/434=26.7% | ||||
| 17. | Mahmood et al, 200735 | USA | Cross-sectional, retrospective | 2 795 345 patients who filled prescriptions for medications involved potential DDI from 128 Veterans Affairs medical centres. Ambulatory care clinic. |
Prescribed drug | Clinically important DDI. Database analysis of pharmacy records. DDI screening tool: a list of 25 potential DDI. |
Prescribing error: DDI prevalence: The overall rate of potential DDIs was 21.54 per 1000 veterans exposed to the object or precipitant medications of interest. |
DDI prevalence: 2.15% | Age not mentioned | |||
| 18. | Lapi et al, 200937 | Dicomano, Italy | Cohort, a two-wave, population-based survey | 568 community-dwelling elderly aged ≥65 years | Prescription and non-prescription drugs used at least 1 week before enrolment | Suboptimal prescribing: Inappropriate medication=1991 Beers criteria (13 items out of the original 39 (33.3%) Beers list medications were considered). DDI screening tool: Micromedex Drug-Reax system. Using population-based survey. |
Prescribing error: Potential DDI prevalence was significantly higher in 1999 compared with 1995 (30.5% vs 20.1%; p<0.001). Inappropriate prescriptions were significantly higher in 1995 compared with 1999 (9.1% vs 5.1%; p=0.004). |
Potential DDI prevalence: 30.5%, p<0.001 Inappropriate medication prevalence: 5.1%, p=0.004 |
||||
| 1995 | 1999 | P values | ||||||||||
| Inappropriate medication | 47 (9.1%) | 26 (5.1%) | 0.004 | |||||||||
| DDI | 97 (20.1%) | 147 (30.5%) | <0.001 | |||||||||
| Major DDI | 20 (4.7%) | 24 (5.6%) | 0.585 | |||||||||
| Risk factors: Polypharmacy always predicted a substantial increase in the risk of the PIM and DDI. | ||||||||||||
| 19. | Nobili et al, 200938 | Lecco, Italy | Cross-sectional, retrospective | 58 800 community-dwelling elderly aged ≥65 years registered under the local health authority of Lecco |
Receiving at least two coadministered prescriptions | DDIs and associated risk factors (age, sex and number of prescriptions). DDI screening tool: Italian computerised interaction database. Analysed all prescriptions dispensed from 1 January 2003 to 31 December 2003. |
Prescribing error: DDI prevalence: 9427 elderly people (16%) were exposed to drug combinations with the potential for 13 932 severe DDIs. Mean number of DDI per patient was 0.2 (range 0–9). Risk factors: Age and number of chronic drugs were associated with an increasing risk of DDIs. The adjusted OR increased from 1.07 (95% CI 1.3 to 1.11) in patients aged 70–74 years to 1.52 (95% CI 1.46 to 1.60) in those aged 85 or older. Elderly taking more than five chronic drugs had a statistically significant higher risk of potentially severe DDIs (OR=5.59, 95% CI 5.39 to 5.80) than those receiving less than 3 (reference category) or 3–5 chronic drugs (OR=2.71, 95% CI 2.63 to 2.80). |
Potentially severe DDI prevalence: 9427/58 800=16% | Only the interactions identified as severe were considered in these analyses. | |||
| 20. | Guthrie et al, 201539 | Scotland, UK | Cross-sectional | 311 881 residents aged ≥20 years from the community-dispensed prescribing data (general practice). Living in own home: 308 660. |
Prescribed drugs | Potentially serious DDI. Patient characteristics associated with the presence of potentially serious DDI. DDI screening tool: analysis of community-dispensed prescribing data using British National Formulary 2010. |
Prescribing error: DDI prevalence: 40 689 adults (13%) had potentially serious DDI in 2010 (for both residents living in own home and care home). Number of patient with potentially serous DDI for residence living in their own home in 2010=13 615. |
DDI prevalence: 13 615/308 660=4.4% | Resident living in both care home or own home. Risk factors for own home and care home. |
|||
| 21. | Maio et al, 200640 | Emilia-Romagna, Italy | Cohort retrospective | 849 425 elderly outpatients aged ≥65 years from the Emilia-Romagna outpatient prescription claims database | Prescribed drugs | PIM using the 2002 Beers criteria and factors associated with PIM. Prescription review. |
Prescribing error: PIM prevalence: A total of 152 641 (18%) elderly had one or more occurrences of PIM prescribing. Risk factors: 1. Older age (≥85 years) (OR 1.18, 95% CI 1.16 to 1.2, p<0.05). 2. ≥10 drugs prescribed (OR 7.33, 95% CI 7.15 to 7.51, p<0.05). 3. ≥4 chronic conditions (OR 1.76, 95% CI 1.72 to 1.81, p<0.05). |
PIM prevalence: 152 641/849 425=18% | ||||
| 22. | de Oliveira Martins et al, 200641 | Lisbon, Portugal | Cross-sectional | 213 elderly aged ≥65 years from 12 community pharmacies | Prescription and home medications | IDU by 1997 Beers and 2003 Beers explicit criteria. Using survey. |
Prescribing error: PIM prevalence: Using the 1997 Beers explicit criteria, 75 occurrences of inappropriate medicines were detected in 59 patients (27.7%). Using the 2003 Beers explicit criteria inappropriate medication was detected in 82 patients (38.5%). Risk factors: The occurrence of inappropriate medicines was significantly associated with the consumption of a high number of drugs. |
IDU prevalence: 59/213=27.7% using 1997 Beers IDU prevalence: 82/213=38.5% using 2003 Beers |
||||
| 23. | Pugh et al, 200642 | Austin, Texas, USA | Cross-sectional, retrospective | 1 096 361 outpatient elderly aged ≥65 years using national data from the Veterans Health Administration | Prescribed drug only | Potentially IP included in the 2006 HEDIS criteria and to determine if patient risk factors are similar to those found using Beers criteria. Using database. |
Prescribing error: IP prevalence: Overall, 19.6% of older veterans were exposed to HEDIS 2006 drugs. Risk factors: 1. Patients receiving ≥10 medications were at greatest risk of exposure in men (OR 8.2, 95% CI 8 to 8.4) and women (OR 9.6, 95% CI 8.2 to 11.2). 2. Patients with more outpatient clinic visits (≥10) were at greater risk regardless of gender (OR 1.4, 95% CI 1.3 to 1.6). 3. Diagnosis with other mental illness (eg, depression, anxiety) alone or in combination with serious mental illness was associated with higher risk of potentially IP for women (OR 1.3, 95% CI 1.1 to 1.5). |
Potentially IP prevalence: 214 887/1 096 361=19.6% | ||||
| 24. | Saab et al, 200643 | Lebanon | Descriptive | 277 elderly aged ≥65 years from 10 community pharmacies | Prescription and/or OTC medications | IDU (Beers criteria, missing doses, inappropriate frequency of administration, poor memory, drug–disease interaction, DDI, inappropriate dose, duplicated therapy, discontinuation of therapy, adverse effect and inappropriate indication). Factors that predict potentially inappropriate drug intake. Review patient profile using community pharmacy data and inperson interviews. |
Prescribing error: PIM prevalence: The prevalence of elderly outpatient with at least one inappropriate medication: 165/277 (59.6%) (include five patients with ADR). Inappropriate medication use was most frequently identified in terms of Beers criteria (22.4%), missing doses (18.8%) and incorrect frequency of administration (13%). Drug–disease interaction in 28 patients (10.1%), DDI 14 (5.1%), duplicate therapy 12 (4.3%). Risk factors: Female sex (65.7% vs 53.3% for male, p=0.03). There were also significant associations between the likelihood of use of an inappropriate drug and (1) increased number of medical illnesses (p<0.00002); (2) consumption of an OTC drug and/or prescription drug (p=0.048 and p=0.0035, respectively); and (3) consumption of both OTC and prescription drugs (p<0.0002). |
IDU prevalence: 62/277=22.4% using Beers criteria | Just extracted the IDU by Beers criteria because the IDU includes 5 cases of ADR and some patients had more than one IDU. Risk factors for all types of IDU. |
|||
| 25. | Zuckerman et al, 200644 | USA | Cohort retrospective | 487 383 community-dweller elderly aged ≥65 years. Data from MarketScan Medicare Supplemental and Coordination of Benefits database. |
Prescribed drug | Inappropriate medication use using Beers criteria | Prescribing error: PIM prevalence: 204 083 elderly used inappropriate medication. Use of inappropriate drugs was associated with a 31% increase in risk of nursing home admission, compared with no use of inappropriate drugs (adjusted relative risk 1.31, 99% CI 1.26 to 1.36). |
Inappropriate medication use prevalence: 204 083/487 383=41.9% | ||||
| 26. | Bregnhøj et al, 200745 | Copenhagen, Denmark | Cross-sectional | 212 elderly aged ≥65 years with polypharmacy (≥5 drugs) patients from primary care | Subsidised and non-subsidised medications prescribed | IP measured by the MAI: 10 criteria are indication, effectiveness, dosage, directions practicality, directions correctness, DDI, drug–disease interaction, duplication, duration and expense). Patients exposed to polypharmacy were identified via the database recording the drug subsidy system of Danish pharmacies and questionnaire. |
Prescribing error: IP prevalence: The majority of the patients, namely 94.3%, had one or more inappropriate ratings among their medications. |
IP prevalence: 200/212=94.3% | ||||
| 27. | Johnell and Fastbom, 200846 | Sweden | Cross-sectional | 731 105 people aged ≥75 years from the Swedish Prescribed Drug Register (secondary data analysis) | Prescribed drug only and multidose drug dispensing | Whether the use of multidose drug dispensing is associated with potential IDU (ie, anticholinergic drugs, long-acting benzodiazepines, concurrent use of ≥3 psychotropic drugs and combinations of drugs that may lead to potentially serious DDIs). Information from the Swedish Prescribed Drug Register. |
Prescribing error: PIM prevalence: Prevalence of potential IDU in multidose dispensing users: 40.3% (women: 41%, men: 38.5%). Prevalence of potential IDU In prescription users: 13.6% (women: 15%, men: 11.5%). The multidose users had higher prevalence of all indicators of potential inappropriate drug than prescription users. 1. The younger elderly (aged 75–79 years) who used multidose drug dispensing had the highest frequency of all indicators of potential IDU. 2. Most indicators of IDU were more common in women than men. 3. Multidose drug dispensing among those aged 75–79 years old was even more strongly associated with any IDU, anticholinergic drugs, three or more psychotropic drugs in both men and women, and long-acting benzodiazepines among men. |
PIM prevalence: Multidose dispensing users: 292 737/731 105=40% Prescription users: 99 430.3/731 105=13.6% |
Multidose drug dispensing means that patients get their drugs machine-dispensed into one unit for each dose occasion and packed in disposable bags. | |||
| 28. | Berdot et al, 200947 | Dijon, Bordeaux, Montpellier, France | Cohort prospective | 6343 community-dwelling elderly aged ≥65 years | Prescribed drug | PIM using 1997 and 2003 Beers criteria, Fick and Laroche. Face-to-face interview using standardised questionnaire. |
Prescribing error: PIM prevalence: One-third (31.6%) of the study participants reported using at least one inappropriate medication at study entry. |
PIM prevalence: 2004/6343=31.6%, p<0.001 | ||||
| 29. | Haider et al, 200948 | Sweden | Cross-sectional, register-based study | 626 258 older people aged 75–89 years from the Swedish Prescribed Drug Register (secondary data analysis) | Prescribed drug only | If low education associated with potential IDU (ie, anticholinergic drugs, long-acting benzodiazepines, concurrent use of ≥3 psychotropic drugs and clinically relevant potential DDI). Information from the Swedish Prescribed Drug Register. |
Prescribing error: PIM prevalence: The proportion of participants reporting use of at least one potential IDU was 34.6%. Risk factors: Subjects with low education had a higher probability of potential IDU (OR 1.09, 95% CI 1.07 to 1.17). Older age, being a woman and higher CCI were associated with the highest frequencies of potential IDU. |
IDU prevalence: 216 685/626 258=34.6% | ||||
| 30. | Lai et al, 200949 | Taiwan | Descriptive | 2 133 864 patients aged ≥65 years between 2001 and 2004 from ambulatory care National Health Insurance claim database | Prescribed drug | PIM prescribing using updated 2003 Beers criteria and the characteristics of and risk factors for such prescribing | Prescribing error: PIM prevalence: A mean of 63.8% of the older population received a PIM at least once a year in 2001–2004. Details: 2001: 1 974 869 patients of whom 1 297 425 had inappropriate prescription (65.7). 2002: 2 026 737 patients of whom 1 312 147 had inappropriate prescription (64.7). 2003: 2 077 677 patients of whom 1 295 227 had inappropriate prescription (62.3). 2004: 2 133 864 patients of whom 1 333 792 had IP (62.5). Risk factors: The only patient characteristic associated with an increased likelihood of the prescribing of PIM was female sex (male sex: OR 0.982 (95% CI 0.980 to 0.983)) (p<0.001) and when ≥4 drugs were prescribed (p<0.001). The following are physician characteristics associated with a greater likelihood of the prescribing of PIM: 1. Male sex (OR 1.206, 95% CI 1.202 to 1.210, p<0.001). 2. Older age (43–50 years: OR 1.021, 95% CI 1.018 to 1.025, p<0.001; ≥51 years: OR 1.238, 95% CI 1.235 to 1.242, p<0.001). 3. Family medicine/general practice (OR 1.267, 95% CI 1.265 to 1.269, p<0.001). |
PIM prevalence: 2001: 65.7% 2002: 64.7% 2003: 62.3% 2004: 1 333 792/2 133 864=62.5% |
||||
| 31. | Ryan et al, 200950 | Ireland | Cohort prospective | 500 patients aged ≥65 years from primary care | Prescribed drug | IP using 2003 Beers criteria and IPET. Screening patients’ medical records (electronic and paper). |
Prescribing error: PIM prevalence: 65 patients (13%) and 52 patients (10.4%) had at least one medicine prescribed inappropriately using 2003 Beers and IPET criteria, respectively. |
IP prevalence: Beers 2003: 65/500=13% IPET: 52/500=10.4% |
||||
| 32. | Ryan et al, 200951 | Cork, Southern Ireland | Descriptive case record review | 1329 elderly aged ≥65 years from primary care | Prescribed drugs | A—1. PIM using 2003 Beers and STOPP criteria. 2. PPO using START criteria. B—Relationship between age and number of prescription drugs and IP. Case record through paper and electronic record review. |
Prescribing error: PIM prevalence: IP rate identified by Beers criteria in 18.3% (243) of patients. IP rate identified by STOPP was 21.4% (284). PPO was identified in 22.7% (302) of patients using the START tool. Risk factors: A significant correlation was found between the occurrence of PIM and the following: 1. The number of medicines prescribed when calculated using Beers criteria (rs=0.270, p<0.01) and STOPP (rs=0.356, p<0.01) using Spearman’s ρ correlation test. 2. Age using Beers criteria (rs=0.068, p<0.01) and STOPP (rs=0.071, p<0.01). 3. Increasing CCI score identified by STOPP (rs=0.210, p<0.01). |
PIM prevalence: Beers: 243/1329=18.3% STOPP: 284/1329=21.4% PPO prevalence: START: 302/1329=22.7% |
Spearman’s ρ correlation test |
|||
| 33. | Akazawa et al, 201052 | Tokyo, Japan | Cohort retrospective | 6628 elderly patients aged ≥65 years from health insurance claim data (secondary data analysis) | Prescribed drugs | PIM using modified Beers criteria in Japan. Drug utilisation review using medical and pharmacy claim from database of Japan Medical Data Center. |
Prescribing error: PIM prevalence: 43.6% (2889/6628) were prescribed at least one PIM. Risk factors: Factors positively associated with PIM prescriptions at a significance level of 5% included the following: hospital admission (OR=3.35, 95% CI 2.43 to 4.62); polypharmacy (OR=5.69, 95% CI 5 to 6.48); prescriptions from a hospital (OR=1.19), general medicine practitioner (OR=1.46) or psychiatrist/neurologist (OR=2.33); and comorbid conditions including peptic ulcer disease without bleeding (OR=4.18, 95% CI 3.52 to 4.97), depression (OR=3.69), cardiac arrhythmias (OR=1.93), other neurological disorders (Parkinson’s disease, multiple sclerosis and epilepsy; OR=1.88) and congestive heart failure (OR=1.46). PIM users had significantly higher hospitalisation risk (1.68-fold), more outpatient visit days (1.18-fold) and higher medical costs (33% increase) than did non-users. |
PIM prevalence: 2889/6628=43.6% | *Consequence | |||
| 34. | Zaveri et al, 201053 | Ahmedabad city, India | Descriptive prospective | 407 geriatric patients aged ≥65 years from medicine outpatient department | Prescribed drug | PIM using 2003 Beers criteria. Using prospective proforma data collection. |
Prescribing error: PIM prevalence: Out of 407 patients, 96 patients (23.6%) received at least one drug that was potentially inappropriate. Risk factors: There was highly significant association between the number of drugs prescribed and frequency of use of PIMs (p<0.0002). |
PIM prevalence: 96/407=23.6% | ||||
| 35. | Barnett et al, 201154 | Tayside, Scotland, UK | Cohort | 65 742 elderly aged 66–99 years living in home | Prescribed drug | PIM using 2003 Beers criteria and the association between exposure to PIM and mortality. Using dispensing and prescribing database and medical record. |
Prescribing error: PIM prevalence: PIM found in 20 304 (30.9%) patients living at home. Risk factors: After adjustment for age, sex and polypharmacy, 1. Patient at increased risk of receiving at least one PIM if they were younger, female and had higher polypharmacy. 2. Receiving at least one PIM was not associated with increased risk of mortality (adjusted OR 0.98, 95% CI 0.92 to 1.05). |
PIM prevalence: 20 304/65 742=30.9% | Risk factors for both care home and home |
|||
| 36. | Chang et al, 201155 | Taipei, Taiwan | Cohort | 193 outpatient elderly patients aged ≥65 years with polypharmacy (≥8 chronic medications) from Medication Safety Review Clinic in Taiwanese Elders (MSRC-Taiwan) study | Prescribed drugs and dietary supplement excluding herbals | PIM using six different criteria and drug-related problem: the 2003 version of the Beers criteria (from the USA), the Rancourt (from Canada), the Laroche (from France), STOPP (from Ireland), the Winit-Watjana (from Thailand) and the NORGEP criteria (from Norway). Analyse baseline data from the MSRC-Taiwan study. Secondary data analysis. |
Prescribing error: PIM prevalence: The proportion of patients who had at least one PIM varied from 24% (the NORGEP criteria) to 73% (the Winit-Watjana criteria). Approximately 31% (the STOPP criteria) to 42% (the NORGEP criteria) of PIMs identified were considered as drug-related problems by the medication review team experts. Risk factors: In the bivariate analysis, the common characteristics associated with having at least one PIM in all criteria were a high number of chronic conditions and a high number of chronic medications. |
PIM prevalence: 24%–73% | ||||
| 37. | Leikola et al, 201156 | Finland | Cross-sectional | 841 509 non-institutionalised elderly patients aged ≥65 years from Finland’s Social Insurance Institution prescription register of all reimbursed drugs for outpatients | Prescribed and OTC medications that are reimbursed | PIM using 2003 Beers criteria | Prescribing error: PIM prevalence: 14.7% (n=123 545) had received PIMs according to the Beers 2003 criteria. |
PIM prevalence: 123 545/841 509=14.7% | ||||
| 38. | Lin et al, 201157 | Taiwan | Cross-sectional, retrospective analysis | 327 elderly patients aged ≥65 years from outpatient clinic of a community health centre | Prescribed drugs | PIM using 2003 Beers criteria and risk factors of PIM use. Using data review. |
Prescribing error: PIM prevalence: The prevalence of patients having at least one PIM was 27.5% (90/327). Risk factors: Independent risk factors for PIMs are older age (OR=1.05, 95% CI 1.00 to 1.09, p=0.046), higher number of prescribed medications (OR=1.06, 95% CI 1.39 to 1.98, p<0.001) and diagnosis of acute diseases (OR=8.98, 95% CI 4.71 to 17.1, p<0.001). |
PIM prevalence: 90/327=27.5% | ||||
| 39. | Woelfel et al, 201170 | California, USA | Cross-sectional | 295 elderly aged ≥65 years from ambulatory population of Medicare beneficiaries | Prescribed drug | PIM using 2003 Beers criteria. Using medication review |
Prescribing error: PIM prevalence: 54 (18.3% beneficiaries were taking at least one PIM. Risk factors: The number of medications was significantly greater in the PIM than the non-PIM group (p<0.001). |
PIM prevalence: 54/295=18.3% | ||||
| 40. | Zhang et al, 201158 | USA | Cohort retrospective | 3570 elderly community-based respondents aged ≥65 from 2007 MEPS, a nationally representative survey of the US community-dwelling population | Prescribed drug | PIM using Zhan criteria and risk factors for PIM use. Information from MEPS database. |
Prescribing error: PIM prevalence: PIM prevalence in 2007: 13.84% (CI 12.52 to 15.17). PIM prevalence in 1996: 21.3% (CI 19.5 to 23.1). Risk factors: Older women, people taking ≥25 prescriptions, people with middle family income, people living in the South census region, and people who said they were in fair or poor health were more likely to have received an inappropriate medication during the year. |
PIM prevalence: 13.84%–21.3% | ||||
| 41. | Haasum et al, 201259 | Sweden | Cross-sectional, retrospective | 1 260 843 home-dwelling elderly aged ≥65 years from the Swedish Prescribed Drug Register | Prescribed drug only | Potentially IDU (use of anticholinergic drugs, long-acting benzodiazepines, concurrent use of ≥3 psychotropics and potentially serious DDIs). Information from the Swedish Prescribed Drug Register. |
Prescribing error: PIM prevalence: 11.6% of the home-dwelling elderly were exposed to potentially IDU. |
Potentially IDU prevalence: 145 749/1 260 843= 11.6% | Information on both institutionalised and home-dwelling. Extracted home-dwelling information only. |
|||
| 42. | Candela Marroquí et al, 201219 | Cáceres, Spain | Descriptive | 471 patients aged ≥65 years from health centres | Consumed medications | Potentially IP using STOPP/START criteria. Using patient interview and medical chart review. |
Prescribing error: PIM prevalence: 249 patients (52.8%, 95% CI 48.3 to 57.3) had potentially IP according to STOPP/START criteria. STOPP: 162 patients (34.3%, 95% CI 30.2% to 38.8%). START: 114 patients (24.2%, 95% CI 20.5% to 28.2%). |
Potentially IP prevalence: 249/471=52.8% (95% CI 48.3 to 57.3) |
||||
| 43. | Nyborg et al, 201260 | Norway | Cross-sectional, retrospective | 445 900 home-dwelling elderly aged ≥70 years from the Norwegian Prescription Database |
Prescribed drug | Prevalence of and predictors for PIM use by the NORGEP criteria. Survey undertaken based on data from the Norwegian Prescription Database. |
Prescribing error: PIM prevalence: 34.8% of the study population was exposed to at least one PIM. Risk factors: The odds of receiving potentially harmful prescriptions increased with the number of doctors involved in prescribing (OR 3.52, 99% CI 3.44 to 3.60 for those with ≥5 compared with those with 1 or two prescribers). Women were at higher risk for PIMs (OR 1.6, 99% CI 1.58 to 1.64). |
PIM prevalence: 155 341/445 900= 34.8% (99% CI 34.7 to 35) |
||||
| 44. | Yasein et al, 201261 | Jordan | Cross-sectional | 400 elderly aged ≥65 years from family practice clinic | Prescribed drug | Polypharmacy (≥5 drugs) and IP using 2003 Beers criteria. Using patient file and patient interview. |
Prescribing error: PIM prevalence: Inappropriate medications as determined by Beers criteria independent of diagnosis accounted for 118 (29.5%) patients. |
IP prevalence: 118/400=29.5% | ||||
| 45. | Blozik et al, 201362 | Helsana, Switzerland | Cohort | 2008: 1 059 495 2009: 1 047 939 2010: 929 791 Community-dwelling adults aged >18 years from claim data of Helsana |
Prescribed drug submitted for reimbursement | Prevalence of polypharmacy and PIM using 2003 Beers criteria or the PRISCUS list. Using analysis of data based on claim data from Switzerland health insurance. |
Prescribing error: PIM prevalence: According to 2003 Beers criteria, 10.3% of the community-dwelling population aged >65 years received at least one medication which is PIM, and according to the PRISCUS list 1, 16.0% of persons had a PIM. When using both Beers and PRISCUS criteria, 21.1% of the population received at least one PIM. Of those persons older than 65 years asking for reimbursement of medications, 12.9% received at least one PIM according to 2003 Beers, 20.2% according to PRISCUS and 26.6% of either definition. Risk factors: Women were more likely to receive a PIM: 25.5% of women as compared with 15.4% of men when both Beers and PRISCUS definitions were used. |
PIM prevalence: 21.1% | There are huge discrepancies in estimating the prevalence of PIM depending on the definition used. | |||
| 46. | Cahir et al, 201363 | Ireland | Cohort retrospective | 931 community-dwelling elderly aged ≥70 years from 15 general practices | Prescribed drug and OTC | The association between potentially IP using STOPP and health-related outcomes (ADEs, HRQOL, and hospital accident and ED). Using patient self-report and medical record. |
Prescribing error: PIM prevalence: Prevalence of potentially IP was 40.5% (n=377). ADE prevalence: In total, 674 of 859 participants (78%) were classified as having at least one ADE during the study period. Risk factors: Patients with ≥2 potentially IP indicators were: 1. Twice as likely to have an ADE (adjusted OR 2.21, 95% CI 1.02 to 4.83, p<0.05). 2. Significantly lower mean HRQOL utility (adjusted coefficient −0.09, SE 0.02, p<0.001). 3. A twofold increased risk in the expected rate of ED visits (adjusted incidence rate ratio 1.85, 95% CI 1.32 to 2.58, p<0.001). |
Potentially IP prevalence: 377/931=40.5% ADE prevalence: 674/859=78% |
*Consequence. Type of ADE was not mentioned. |
|||
| 47. | Weng et al, 201364 | Taiwan | Cross-sectional, retrospective | 780 older patients aged ≥65 years from the outpatient geriatric clinic | Long-term prescribed drugs (≥28 days) for chronic diseases, not OTC |
Impact of number of drugs prescribed on the risk of PIM using STOPP criteria. Patient medical chart review. |
Prescribing error: PIM prevalence: 302 patients (39%) had at least one PIM. Risk factors: Multivariate analysis revealed that PIM risk was associated with the number of medications prescribed (p<0.001) and the presence of cardiovascular (p<0.001) or gastrointestinal disease (p=0.003). Patients prescribed ≥5 drugs (adjusted OR=5.4) had significantly higher PIM risk than those prescribed ≤2 drugs. |
PIM prevalence: 302/780=39% | ||||
| 48. | Zimmermann et al, 201318 | German | Cohort longitudinal analysis | Follow-up 3: n=1942 Baseline: n=3214 1855 elderly aged ≥75 years from primary care. Data from the prospective, multicentre, observational study ‘German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe)’. |
Prescribed drug | PIM using Beers, PRISCUS list. By checking medications during visits to patients’ homes. |
Prescribing error: PIM prevalence: At baseline, PIM prevalence is 29% (848) (according to the PRISCUS list, which decreased to 25.0% (464) 4.5 years later (χ2: 7.87, p=0.004). The Beers list yielded a prevalence of 21% (620) at baseline, decreasing after 4.5 years to 17.1% (317) (χ2: 10.77, p=0.000). Risk factors: By PRISCUS list: The risk for PIM increase with: 1. Increasing age of the patients (OR: 1.06, CI 1.00 to 1.13, p=0.037). 2. The presence of depression (OR: 2.42, CI 1.65 to 3.57, p=0.000). 3. Increasing number of prescription drugs (OR: 1.99, CI 1.80 to 2.18, p=0.000). By contrast, the risk of taking PIM decrease by using PRISCUS list with the number of present illness (OR: 0.88, CI 0.80 to 0.97, p=0.012). As the growing number of ingested prescription drugs increased the risk for the ingestion of PIM from the Beers list (OR: 1.66, CI 1.50 to 1.84; p=0.000). |
Prescribing error: PIM prevalence: 17%–29% |
||||
| 49. | Baldoni et al, 201429 | Ribeirao Preto, Brazil | Cross-sectional | 1000 elderly aged ≥60 years from outpatient pharmacy | Prescribed drug, self-medication (309 users) and OTC (802 users) | Prevalence and factors associated with PIM using 2003 and 2012 Beers criteria. Using structured interview questionnaire. |
Prescribing error: PIM prevalence: According to Beers criteria 2003, 480 (48.0%) participants used at least one PIM, the mean being 1.38 (SD=0.65) PIMs/person, ranging from 1 to 5. According to Beers criteria 2012, 592 (59.2%) participants used at least one PIM, the mean being 1.56 (SD=0.81) PIMs/person, ranging from 1 to 6. ADE: During the interview 45.5% of participants reported complaints related to ADEs; 94.5% of these were caused by prescribed medication. Risk factors: Factors that are associated with PIMs use were female gender, self-medication, use of OTC medications, complaints related to ADEs, psychotropic medication and more than five medications. *Ten medications with the highest prevalence of self-reported ADEs complaints are clonidine, amitriptyline, metformin, fluoxetine, dexchlorpheniramine, diclofenac, captopril, acetylsalicylic acid, simvastatin and hydrochlorothiazide. Among them, five were considered PIMs according to Beers criteria, of which clonidine, amitriptyline and dexchlorpheniramine are listed in both criteria, while fluoxetine is listed only in Beers criteria 2003 and diclofenac is listed only in Beers criteria 2012. |
PIM prevalence by Beers criteria 2003: 480/1000= 48.0% PIM prevalence by Beers criteria 2012: 592/1000= 59.2% |
*Error-related adverse event | |||
| 50. | Castillo-Páramo et al, 201465 | Spain | Cross-sectional | 272 electronic records of elderly aged ≥65 years from primary healthcare | Prescribed drugs | PIM using STOPP/START criteria and version adapted to Spanish primary healthcare and factors may modulate PIM onset. Using electronic health record and paper clinical record. |
Prescribing error: PIM prevalence: The prevalence of PIM (misprescribing and overprescribing) using the STOPP original criteria was 37.5% (95% CI 31.7 to 43.2), and 50.7% (95% CI 44.7 to 56.6) using the STOPP Spanish AP2012 version. The prevalence of underprescribing was 45.9% (95% CI 40.0 to 51.8) with the START original criteria, and 43.0% (95% CI 37.1 to 48.9) with the START AP2012 version. Risk factors: A significant correlation was found between the number of STOPP PIM and age or number of prescriptions, and between the number of START PIM with age, CCI and number of prescriptions. |
PIM prevalence: 102/272 (STOPP)=37.5% (95% CI 31.7 to 43.2), 138/272 (STOPP AP2012)=50.7% (95% CI 44.7 to 56.6), 125/272 (START)=45.9%, 117/272 (START AP2012)=43% | ||||
| 51. | Vezmar Kovačević et al, 201466 | Serbia Belgrade | Cross-sectional, prospective | 509 elderly aged ≥65 years from five community pharmacies | Prescribed drug | PIM and PPO using STOPP/START criteria. Using patient interview and medical, biomedical record. |
Prescribing error: PIM prevalence: There were 164 PIMs identified in 139 patients (27.3%) by STOPP and 439 PPOs identified in 257 patients (50.5%) by START. Risk factors: Patients with more than four prescriptions had a higher risk for PIM (OR 2.85, 95% CI 1.97 to 4.14, p<0.001) and ≥9 medications (OR 7.43, 95% CI 3.20 to 17.23, p<0.001). Patients older than 74 years were more likely to have a PPO (75–84 years: OR 1.47, 95% CI 1.01 to 2.13, p=0.041; and ≥85 years: OR 1.79, 95% CI 1.19 to 2.83, p=0.009). |
PIM prevalence: 139/509=27.3% PPO prevalence: 257/509=50.5% |
||||
| 52. | Amos et al, 201567 | Emilia-Romagna, Italy | Cohort retrospective | 865 354 elderly aged ≥65 years community-dwelling from administrative care data | Prescribed drug only | PIM using updated Maio criteria and patient characteristics related to IP. Using regional Emilia-Romagna administrative healthcare database. |
Prescribing error: PIM prevalence: A total of 240 310 (28%) older adults were exposed to at least one PIM. Risk factors: The oldest group (≥85) followed by patients aged 75–84 had 53% and 25% greater odds of receiving PIM than patients 65–75 years old, respectively (OR=1.53, 95% CI 1.50 to 1.55; OR=1.25, 95% CI 1.23 to 1.26, respectively). These odds of exposure to any PIM were slightly lower among men than women (OR=0.98, 95% CI 0.97 to 1.00). An increase in the number of medications prescribed to the patient corresponded with higher odds of PIM exposure. Older GPs (≥56), male GPs and solo practice GPs were more likely to prescribe PIMs to their older patients. |
PIM prevalence: 240 310/865 354=28% | ||||
| 53. | Hedna et al, 201568 | Sweden | Cohort retrospective | 542 elderly aged ≥65 years from the Swedish Total Population Register (primary care) |
Prescribed drug | Prevalence of potentially IPs using STOPP criteria and to investigate the association between potentially IPs and occurrence of ADRs. Using the Swedish Prescribed Drug Register, medical records and health administrative data. |
Prescribing error: PIM prevalence: 226 patients using primary healthcare had potentially IP. Risk factors: Persons prescribed potentially IP had more than twofold increased odds to experience ADRs (OR 2.47, 95 % CI (1.65 to 3.69), p<0.001), compared with that in persons without potentially IP. |
Potentially IP prevalence: 226/542=42% | *Error-related adverse event. The association between PIPs and occurrence of ADRs was for primary care, outpatient or inpatient and hospitalised patients. |
|||
| 54. | Moriarty et al, 201569 | Ireland | Cohort prospective | 2051 elderly aged ≥65 years from The Irish Longitudinal Study on ageing. Community-dwelling elderly. |
Prescribed drug only | PIM and PPO using STOPP, Beers criteria, ACOVE indicators and START. Using face-to-face interview, then follow-up after 1 and 2 years. |
Prescribing error: PIM prevalence |
PIM: 36.7%–64.8% | ||||
| Baseline N (%) (95% CI) |
Follow-up N (%) (95% CI) |
|||||||||||
| Any PIM using STOPP, Beers, ACOVE | 1259 (61.4%) (CI 59.3 to 63.5) |
1330 (64.8%) (CI 62.8 to 66.9) |
||||||||||
| Any PPO using START, ACOVE | 1094 (53.3%) (CI 51.2 to 55.5) |
1161 (56.6%) (CI 54.5 to 58.8) |
||||||||||
| Both PIM and PPO | 753 (36.7%) | 843 (41.1%) | ||||||||||
| Risk factors: Female sex, age and higher number of medicines were significantly associated with change in PIM prevalence. Age and higher numbers of medicines and chronic conditions were found to be significantly associated with change in PPO prevalence. | ||||||||||||
| 55. | Ramia and Zeenny, 201471 | Lebanon | Cross-sectional | 284 outpatients aged ≥18 years visiting community pharmacy | Patients on ≥1 of the chronic medications mentioned in the study | The completion of therapeutic/safety monitoring tests. Patients were subjected to a questionnaire assessing the appropriateness of their laboratory-test monitoring. |
Monitoring error: 1. 185 of the patients (65%) were found to complete some, but not all, of the recommended therapeutic/safety monitoring tests. 2. 76 of the patients (27%) completed all recommended therapeutic/safety monitoring. 3. 23 of the patients (8%) did not complete any of the recommended monitoring tests. |
Incomplete therapeutic/safety laboratory-test monitoring prevalence: 208/284=73% | ||||
| Other: discrepancies | ||||||||||||
| 56. | Tulner et al, 200931 | Amsterdam, The Netherlands | Descriptive prospective | 120 elderly aged >65 years from Dutch geriatric outpatient | Using more than one prescribed or OTC medications | 1. Frequency and relevancy of discrepancies in drug use. 2. Frequency of MDAPEs. 3. Contributing factors such as increasing age, cognitive status and depressive symptoms, the number of medications used, and the number of physicians visited by the patient. By comparing the medication described by the patient and caregivers with the drugs listed by their GP. |
Other: discrepancies prevalence: At least one discrepancy (deletion, addition or difference in dosage) between the medication lists from the patient, the GP or the pharmacy was present in 104 patients (86.7%) involving 386 drugs. MDAPES: MDAPES were identified in 29 patients (24.2%). 7 patients had undertreatment due to deletions. 9 patients had ADR due to additions. 13 patient had DDI. Risk factors: Patients with ≥1 discrepancy reported using a higher mean number of drugs (5.9 vs 4.0; p<0.05) and had more prescribing physicians in addition to their GP (1.1 vs 0.43; p<0.05). Both the presence of discrepancies (Pearson’s r’, 0.293; p=0.05) and MDAPEs (Pearson’s r’, 0.230; p=0.012) were significantly correlated with the number of medications reported by the patient. *The highest rates of discrepancies were seen for acetaminophen (86.7%), laxatives (82.9%) and formulations for dermatological or ophthalmological diseases (81.3%). |
Discrepancies prevalence: 104/120=86.7% *Error-related adverse event: MDAPEs: 29/120=24.2% |
*Error-related adverse event | |||
| 57. | Cornu et al, 201232 | Brussels, Belgium | Cohort retrospective | 189 elderly aged ≥65 years discharged from acute geriatric department of a Belgian university hospital | Prescribed drug | Incidence and type of discrepancies between the discharge letter for the primary care physician and the patient discharge medication and identify possible patient-related determinants for experiencing discrepancies. Discrepancies were categorised as omitted drug, unintended continuation (discontinued home medication documented as home medication), discrepant dose, missing dose, and discrepant brand, omission of a brand name, discrepant frequency, missing frequency or an incorrect pharmaceutical form. By comparing the medications listed in the discharge letter for the primary care physician with those in the patient discharge medication list. |
Other: discrepancies prevalence: Almost half of these patients (n=90, 47.6%) (95% CI 40.5 to 54.7) had one or more discrepancies in medication information at discharge. *Two discrepancies (1.2%) were categorised as having the potential to cause severe patient harm. These discrepancies consisted of a wrong dose (doubled the prescribed dose) of digoxin in the patient discharge medication list and the listing of a low-molecular-weight heparin in the patient discharge medication list that was intentionally omitted in the discharge letter because of the development of heparin-induced thrombocytopaenia during hospitalisation. Risk factors: The explorative multivariate model adjusted for age, sex, length of hospital stay and residential situation showed that when the discharge letter contained more than five drugs, the likelihood of experiencing one or more drug discrepancies was 3.22 (95% CI 1.40 to 7.42, p=0.006) times higher than when five or fewer drugs were mentioned. Increasing numbers of drugs in the discharge medication list (OR 1.19, 95% CI 1.07 to 1.32, p=0.001) and discharge letter (OR 1.18, 95% CI 1.07 to 1.32, p=0.001) were associated with a higher risk for discrepancies. |
Discrepancies prevalence: 90/189=47.6% (95% CI 40.5 to 54.7) | *Error-related adverse event | |||
| Preventable ADEs | ||||||||||||
| 58. | Field et al, 200777 | USA | Cohort | 30 000 elderly ≥65 years from ambulatory care | Prescribed drug | ADE resulting from patients error and risk factors. By electronic tracking of administrative data, review of medical records, reports from clinicians, hospital discharge summaries and ED visit. |
Preventable ADE: ADE resulting from patients’ error prevalence: 113 individuals experienced ADE and potential ADE. Risk factor: In a multivariate analysis, there was a dose–response association between patient errors leading to ADEs and potential ADEs and regularly scheduled medications; compared with zero to two medications, the OR for three to four medications was 2.0 (95% CI 0.9 to 4.2); for five to six medications was 3.1 (95% CI 1.5 to 7.0); and for seven or more medications was 3.3 (95% CI 1.5 to 7.0). The strongest association was with the CCI; compared with a score of 0, the OR for a score of 1–2 was 3.8 (95% CI 2.1 to 7.0); for a score of 3–4 was 8.6 (95% CI 4.3 to 17.0); and for a score of 5 or more was 15.0 (95% CI 6.5 to 34.5). |
ADE resulting from patients’ error prevalence: 113/30 000=0.38% | *ADE resulting from patients’ error | |||
| 59. | Gandhi et al, 201022 | Boston and Indianapolis, USA | Cross-sectional | 68 013 outpatients, mean age 48 and 47 years | Prescribed drug | ADE. Using electronic health record screening, chart review and ADE monitor. |
Preventable ADE incidence: The overall rate was 138 ADEs/1000 person-years across the two sites. Preventable ADEs rate 15/1000 person-years across two sites. *Most common drugs associated with preventable ADE were ACE inhibitors and beta-blockers. |
Preventable ADEs rate 15/1000 person-years across two sites | *Preventable ADE | |||
| 60. | Obreli-Neto et al, 201228 | Ourinhos microregion, Brazil |
Cohort prospective | 433 elderly aged ≥60 years from the primary public health system | Prescribed drugs both within and across prescriptions | DDI-related ADR incidence and factors. Using phone or face-to-face structured interview. DDI screening tool: DDI checker programmes (DrugDigest, Drugs, Micromedex and Medscape). |
Preventable ADE: DDI-related ADR incidence: occurred in 30 patients (6.9%). Gastrointestinal bleeding occurred in 37% of the DDI-related ADR cases, followed by hyperkalaemia (17%) and myopathy (13%). Seventeen DDI-related ADRs were classified as severity level 2, and hospital admission was necessary in 11 cases. *Warfarin was the most commonly involved drug (37% of cases), followed by acetylsalicylic acid (17%), digoxin (17%) and spironolactone (17%). Risk factors: The multiple logistic regression showed that the following were associated with the occurrence of DDI-related ADRs: 1. Age ≥80 years (OR 4.4, 95% CI 3.0 to 6.1, p<0.01). 2. CCI ≥4 (OR 1.3, 95% CI 1.1 to 1.8, p<0.01). 3. Consumption of five or more drugs (OR 2.7, 95% CI 1.9 to 3.1, p<0.01). 4. Use of warfarin (OR 1.7, 95% CI 1.1 to 1.9, p<0.01). |
Incidence of DDI-related ADR: 30/433=6.9% | *Error-related adverse event | |||
ACOVE, Assessing Care of Vulnerable Elders; ADE, adverse drug event; ADI, adverse drug interaction; ADR, adverse drug reaction; CCI, Charlson Comorbidity Index; DDI, drug–drug interaction; ED, emergency department; GP, general practitioners; HEDIS, Health Plan Employer Data and Information Set; HRQOL, health-related quality of life; IDU, inappropriate drug use; IP, inappropriate prescribing; IPET, improved prescribing in the elderly tool; MAI, Medication Appropriate Index; MDAPE, medication discrepancy adverse patient event; MEPS, Medical Expenditure Panel Survey; NORGEP, Norwegian General Practice; OTC, over-the-counter; PDDI, potential drug–disease interaction; PIM, potentially inappropriate medicine; PPO, potential prescribing omissions; START, Screening Tool to Alert doctors to Right Treatment; STOPP, Screening Tool of Older Person’s Prescriptions.
Table 2A.
Systematic review quality assessment: Joanna Briggs Institute Critical Appraisal Checklist for descriptive/case series and cross-sectional
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Overall appraised | |||
| 1 | Ramia and Zeenny, 201471
Adult |
Y | Y | N | N | NA | NA | Y | Y | Y | High | Patients were subjected to a questionnaire assessing the appropriateness of their laboratory-test monitoring, may cause recall bias. |
| 2 | Sorensen et al, 200676
Adult |
Y | Y | N, risk factors related to patient not studied | Y | NA | NA | Y | Y | Y | High | |
| 3 | Vuong and Marriott, 200625
Adult |
U | Y | N | Y | NA | NA | N | Y | Y, percentage was used but statistics was not described in the full text. | High | Unclear sampling strategy. |
| 4 | Adams et al, 200972
Adult |
Y | Y | Y (but for all types of adverse event) | N (self-reported adverse events) | NA | NA | N | Y | Y | High | Risk of recall bias and attribution with self-reported adverse events. |
| 5 | Gandhi et al, 201022
Adult |
U | Y | N | Y | Y | NA | NA | Y | Y | High | |
| 6 | Lu and Roughead, 201120
Adult |
Y | Y | Y | N (subjective patient-reported medication error) | Y | NA | NA (secondary analysis) | N (telephone survey, self-reported) | Y | High | Risk of recall bias with patient-reported medication error. |
| 7 | Sears et al, 201221
Adult |
Y | Y | Y | N (subjective self-reported medication error) | Y | NA | NA (secondary analysis) | N (telephone survey, self-reported) | Y | High | Risk of recall bias with patient self-reported medication error. |
| 8 | Koper et al, 201323
Adult |
N (convenience) | Y | N | Y | NA | NA | NA (100% participants) | Y | Y | High | Selection bias. |
| 9 | Dallenbach et al, 200724
Adult-DDI |
N (consecutive) | N | N | Y | NA | NA | NA (retrospective) | Y | Y | Moderate | |
| 10 | Indermitte et al, 200734
Adult-DDI |
Y (pharmacy choose); N (first 12 customers) | Y | N | Y | NA | NA | Y | Y | Y | High | |
| 11 | Mahmood et al, 200735
Adult-DDI |
Y | Y | N | Y | NA | NA | NA (retrospective) | Y | Y | High | Patients may actually be on other drugs so may not catch all the DDI. |
| 12 | Guthrie et al, 201539
Adult-DDI |
Y | Y | Y (but for both own home and care home) | Y | Y | NA | NA (secondary analysis) | Y | Y | High | Risk factors for both own home and care home. |
| 13 | de Oliveira Martins et al, 200641
Elderly-PIM |
N (first came to pharmacy carrying prescription for two or more drugs) | Y | Y, but not all | Y | Y | NA | N | Y | Y | High | Self-reported data from elderly concerning drug use may lead to information bias. |
| 14 | Pugh et al, 200642
Elderly-PIM |
Y | Y | Y | Y | Y | NA | NA (secondary data analysis) | Y | Y | High | May underestimate the exposure because they do not account for OTC. |
| 15 | Saab et al, 200643
Elderly-PIM |
Y | Y | Y | Y | NA | NA | Y | Y | Y | High | Self-reported data from elderly concerning drug use may decrease accuracy. |
| 16 | Bregnhøj et al, 200745
Elderly-PIM |
N (each GP was asked to recruit six patients who were randomly selected) | Y | N | Y | NA | NA | Y | Y | Y | High | Selection bias. |
| 17 | Johnell and Fastbom, 200846
Elderly-PIM |
Y | Y | Y | Y | Y | NA | Y | Y | Y | High | Did not look for comorbidity as a risk factor because data were from Swedish Prescribing Drug Register. |
| 18 | Haider et al, 200948
Elderly-PIM |
Y | Y | Y | Y | NA | NA | NA | Y | Y | High | |
| 19 | Lai et al, 200949
Elderly-PIM |
Y | Y | Y | Y | NA | NA | NA (secondary analysis) | Y | Y | High | Did not address comorbidity as a risk factor. |
| 20 | Ryan et al, 200951
Elderly-PIM |
Y | Y | Y | Y | NA | NA | N | Y | Y | High | May underestimate the outcome because they do not account for OTC. |
| 21 | Zaveri et al, 201053
Elderly-PIM |
U | Y | Y | Y | NA | NA | N | Y | Y | High | Not enough information in the article. |
| 22 | Leikola et al, 201156
Elderly-PIM |
Y | Y | N | Y | NA | NA | NA | Y | Y | High | May underestimate the outcome because database lacks diagnostic patient data, therefore used the Beers 2003 criteria independent of diagnoses and the data provide no information on the use of PIMs that are not reimbursable. Nine PIMs that were not reimbursable in Finland in 2007: triazolam, belladonna alkaloids, diphenhydramine, hydroxyzine, ferrous sulfate, bisacodyl, nitrofurantoin and clonidine. |
| 23 | Lin et al, 201157
Elderly-PIM |
U | Y | Y | Y | NA | NA | NA | Y | Y | High | |
| 24 | Woelfel et al, 201170
Elderly-PIM |
Y | Y | Y | Y | NA | NA | NA | Y | Y | High | |
| 25 | Haasum et al, 201259
Elderly-PIM |
Y | Y | N | Y | Y | NA | NA (secondary data analysis) | Y | Y | High | |
| 26 | Nyborg et al, 201260
Elderly-PIM |
Y | Y | Y | Y | Y | NA | NA (secondary data analysis) | Y | Y | High | |
| 27 | Yasein et al, 201261
Elderly-PIM |
N | Y | N | Y | Y | NA | N | Y | Y | Moderate | |
| 28 | Candela Marroquín et al, 201219
Elderly-PIM |
N (convenience sample) | Y | N | Y | NA | NA | N | Y | Y | Moderate | Sampling strategy. Subjective information on socioeconomic and clinical variables may decrease accuracy. |
| 29 | Weng et al, 201364
Elderly-PIM |
Y | Y | Y | Y | Y | NA | N | Y | Y | High | Sampling strategy. |
| 30 | Baldoni et al, 201429
Elderly-PIM |
U | Y | Y | Y | Y | NA | Y | Y | Y | High | |
| 31 | Castillo-Páramo et al, 201465
Elderly-PIM |
Y | Y | Y | Y | Y | NA | Y | Y | Y | High | Electronic health record use limitations (incomplete record and quality of data). |
| 32 | Vezmar Kovačević et al, 201466
Elderly-PIM |
Y | Y | Y | Y | NA | NA | N | Y | Y | High | |
| 33 | Nobili et al, 200938
Elderly-DDI |
Y | Y | Y | Y | NA | NA | NA (administrative database) | Y | Y | High | The use of administrative database limits looking for comorbidity as a confounder. |
| 34 | Secoli et al, 201030
Elderly-DDI |
U | Y | Y | Y | NA | NA | NA | Y | Y | High | May underestimate the true DDI prevalence because they do not account for OTC. |
| 35 | Obreli Neto et al, 201227
Elderly-DDI |
Y | Y | Y | Y | NA | NA | NA (data from primary healthcare system) | Y | Y | High | May underestimate the DDI prevalence because (1) most instruments available for assessing DDIs consider only pairs of drugs and do not account for interactions involving combinations of three or more drugs so (2) did not account for OTC. |
| 36 | Pit et al, 200874
Elderly |
Y | Y | Y | Y | NA | NA | Y | Y | Y | High | |
| 37 | Tulner et al, 200931
Elderly |
N (consecutive) | Y | Y | Y | NA | NA | Y | Y | Y | High | Information on medication described by the patient and caregivers may not always be accurate. |
| 38 | Obreli Neto et al, 201126
Elderly-DDI |
Y | Y | N | Y | NA | NA | NA | Y | Y | High | |
| 39 | Mira et al, 201373
Elderly |
Y | Y | Y | Y | NA | NA | Y | Y | Y | High | Self-reported medication error from elderly concerning drug use may have recall bias. |
| 40 | Mand et al, 201433
Elderly |
Y | Y | Y | Y | NA | NA | NA | Y | Y | High |
1 Was study based on a random or pseudo-random sample?
2 Were the criteria for inclusion in the sample clearly defined?
3 Were confounding factors identified and strategies to deal with them stated?
4 Were outcomes assessed using objective criteria?
5 If comparisons are being made, was there sufficient descriptions of the groups?
6 Was follow-up carried out over a sufficient time period?
7 Were the outcomes of people who withdrew described and included in the analysis?
8 Were outcomes measured in a reliable way?
9 Was appropriate statistical analysis used?
DDI, drug-drug interaction; GP, general practitioner; N, no; NA, not applicable; OTC, over-the-counter; PIM, potentially inappropriate medication; U, unclear; Y, yes.
Table 2B.
Systematic review quality assessment: Critical Appraisal Skills Programme for cohort study
| Study design: cohort | ||||||||||||||||
| Reference | Quality domains | |||||||||||||||
| 1 | 2 | 3 | 4 | 5(a) | 5(b) | 6(a) | 6(b) | 7 | 8 | 9 | 10 | 11 | 12 | Overall quality | ||
| Are the results of the study valid? | What are the results? | Will the results help locally? | ||||||||||||||
| 1 | Maio et al, 200640
PIM |
Y | Y | Y | Y | Y, age, gender, geographical location, number of medication, number of chronic condition and income | N | Y | Y (1 year) retrospective | PIM prevalence: 18%. Older age, polypharmacy and greater number of chronic conditions were significant predictors of PIM use. |
P<0.05, 95% CI | Y | Y | Y | – | Moderate |
| None | ||||||||||||||||
| 2 | Zuckerman et al, 200644
PIM |
Y | Y | Y | Y | Y, but used for irrelevant outcome | Y | Y | Y (2 years) | Inappropriate medication use prevalence: 41.9% | P=0.01, 99% CI | Y | Cannot tell (generalisability) | Y | Limited information from the database. Confounding factors were for the nursing home admission rather than for PIM. |
Moderate |
| - | ||||||||||||||||
| 3 | Field et al, 200777
Elderly |
Y | Y | Y | Y | Y, age, gender, comorbidity, number of medications | Y | Y | Y (1 year) | ADE resulting from patients’ error prevalence: 0.38% | P<0.05 | Y | Y | Y | Possible drug-related incidence for which necessary information was not documented in the medical record was not considered. | High |
| None | ||||||||||||||||
| 4 | Gagne et al, 200836
DDI |
Y | Y | Y | Y | Y, age, gender, geographical location, comorbidity, number of medication prescribed | Y | Y | Y (1 year) | DDI: prevalence: 53% | 95% CI | Y | Y | Y | Applying the US list of clinically important DDI to Italy may underestimate the prevalence as it captured only 12 out of the 25 DDI original list. Unable to extract risk factors data as it is for all age groups. | High |
| None | ||||||||||||||||
| 5 | Berdot et al, 200947
Elderly-PIM |
Y | Y | Y | Y | Y, but for irrelevant outcome | Y | Y | Y (4 years) | PMI prevalence: 31.6% | 95% CI, p<0.05 | Y | Y | Y | Self-report and data from healthcare insurance plan are not perfect for actual drug consumption. Recall bias. Confounding factors were for the risk of falls rather than for PIM. |
High |
| – | ||||||||||||||||
| 6 | Lapi et al, 200937
Elderly-PIM |
Y | Y | Y | Y | Y, comorbidity, polypharmacy, stroke, heart failure | Y | Y | Y (1 year) | 1999: IP prevalence: 5.1% Potential DDI prevalence: 30.5% Potential major DDI: 5.6% Polypharmacy was a predictor of PIM use. |
P<0.05, 95% CI | Y | N | Y | Self-reported diagnosis and medication use may cause recall bias. Beers list cannot be fully applied to Italy; it most reflects US drug market. |
Moderate |
| Age, gender | ||||||||||||||||
| 7 | Ryan et al, 200950
Elderly-PIM |
Y | Y | Y | Y | N | Cannot tell | Y | Y (6 months) | Medicine prescribed inappropriately. Beers 2003: 13% IPET: 10.4% |
Cannot tell | Y | Y | Y | – | Low |
| – | ||||||||||||||||
| 8 | Akazawa et al, 201052
Elderly-PIM |
Y | Y | Y | Y | Y, age, gender, polypharmacy (>5 drugs), hospitalisation, comorbidities | Y | Y | Y (1 year) | Prevalence of PIM: 43.6%. Inpatient service use, polypharmacy and comorbidities were significant predictors of PIM use. |
95% CI, p<0.05 | Y | Y | Y | Medical information cannot be taken from claim data, unobserved confounder. PIM not associated with age as several other studies. |
High |
| None | ||||||||||||||||
| 9 | Barnett et al, 201154
Elderly-PIM |
Y | Y | Y | Y | Y, age, sex, polypharmacy and place of residence | Y | Y | Y (2 years) | PIM prevalence: 30.9%. Patients at increased risk of receiving at least one PIM if they were younger, female and had higher polypharmacy. |
95% CI | Y | Y | Y | Comorbidity not accounted for. Risk factors for both care home and home. |
High |
| Comorbidity | ||||||||||||||||
| 10 | Chang et al, 201155
Elderly-PIM |
Y | Y | Y | Y | Y, age, sex, education, number of chronic medication, number of chronic conditions and number of ED visits | Y | Y | Y (12, 24 weeks) | PIM: 24%–73% Number of chronic drugs and number of chronic conditions were a common risk factor in all criteria. |
P<0.05 | Y | Y | Y | May underestimate the prevalence because several drugs in Taiwan were not available in the sex criteria. | High |
| None | ||||||||||||||||
| 11 | Zhang et al, 201158
Elderly-PIM |
Y | Y | Y | Y | Y, race, gender, family income, educational level, census region, number of prescription, self-rated health status | Y | Y | Cannot tell | Prevalence of PIM was from 13.84% (95% CI 12.52 to 15.17) to 21.3% (95% CI 19.5 to 23.1). | 95% CI, p<0.05 | Y | Y | Y | Recall bias due to self-reported survey. Did not assess DDI and underuse so may underestimate the prevalence. | Moderate |
| None | ||||||||||||||||
| 12 | Cornu et al, 201232
Elderly |
Y | Y | Y | Y | Y, age, gender, residential situation before admission, residential situation after discharge, number of drugs in the discharge letter or list | Y | Y | Y (from admission to discharge) | Almost half of these patients (47.6% (95% CI 40.5 to 54.7)) had one or more discrepancies in medication information at discharge. | 95% CI, p<0.05 | Y | Cannot tell | Y | Was done in one centre that may have different procedure of discharge. | Moderate |
| Comorbidity | ||||||||||||||||
| 13 | Mosher et al, 201275
Elderly |
Y | Y | Y | Y | Y, health literacy | Y | Y | Y (3 and 12 months) | ADEs occurred in 51 patients (16.5%) of the patients within the first 3 months of the study, which increased to 119 patients (38.4%) over the full 12-month follow-up period. |
P<0.05 | Y | Cannot tell | Y | Results may be biased due to sampling strategy. | Moderate |
| Age, number of medications, comorbidity | ||||||||||||||||
| 14 | Obreli-Neto et al, 201228
DDI |
Y | Y | Y | Y | Y | Y | Y | Y (4 months) | Incidence of DDI-related ADR (6.9%) | 95% CI, p<0.05 | Y | Y | N | Recall bias from weekly meeting with patient. Most instruments available for assessing DDIs consider only pairs of drugs and do not account for interaction involving combinations of three or more drugs so the risk of DDI may be underestimated. |
Moderate |
| None | ||||||||||||||||
| 15 | Blozik et al, 201362
Adult |
Y | Y | Y | Y | Y, gender | Y | Y | Y (3 years) | Prevalence of PIM: 21.1% | 95% CI | Y | Y | Y | – | High |
| Age, number of medications, number of disease | ||||||||||||||||
| 16 | Cahir et al, 201463
Elderly-PIM |
Y | Y | Y | Y | Y, age, gender, socioeconomic status, private health insurance, comorbidity, number of repeat drug, social support and network, adherence | Y | Y | Y (6 months) retrospective study | Prevalence of potentially IP was 40.5%. | 95% CI | Y | N | Y | Recall bias due to self-reported ADE | Moderate |
| None | ||||||||||||||||
| 17 | Zimmermann et al, 201318 Elderly-PIM |
Y | Y | Y | Y | Y, gender age, number of medications, number of disease, depression, education | Y | Y | Y (4.5 years) | At baseline PIM prevalence is 29% (848) according to the PRISCUS list, which decreased to 25.0% (464) 4.5 years later and 21% according to the Beers list decreasing after 4.5 years to 17.1% (317). |
95% CI, p<0.05, OR and CI for risk factors | Y | Y | Y | – | High |
| None | ||||||||||||||||
| 18 | Amos et al, 201567
Elderly-PIM |
Y | Y | Y | Y | Y, age, gender, geographical location, number of medication | Y | Y | Y (1 year) retrospective study | PIM prevalence 28%, and older age, female, number of medications increase risk of PIM | 95% CI, p<0.05 | Y | Cannot tell | Y | May underestimate the true PIM prevalence because they do not account for OTC. | Moderate |
| Number of chronic conditions | ||||||||||||||||
| 19 | Hedna et al, 201568
Elderly-PIM |
Y | Y | Y | Y | N | Y | Y | Y (3 months) retrospective | Potentially IP prevalence: 42% ADR caused by potentially IP. |
95% CI, p<0.05 | Y | Cannot tell | Y | Undetected confounders | Moderate |
| Age, gender, number of medication, number of chronic condition | ||||||||||||||||
| 20 | Moriarty et al, 201569
Elderly-PIM |
Y | Y | Y | Y | Y, age, gender, number of medication, number of chronic condition, level of education | Y | Y | Y (1 year) | PIM prevalence: 36.7%–64.8%. Female, age and higher number of medicines were associated with change in PIM prevalence. Age and higher numbers of medicines and chronic conditions were found to be associated with change in PPO prevalence. |
95% CI | Y | Y | Y | Lack of information on OTC from the pharmacy claim data. | High |
1 Did the study address a clearly focused issue?
2 Was the cohort recruited in an acceptable way?
3 Was the exposure accurately measured to minimise bias?
4 Was the outcome accurately measured to minimise bias?
5(a) Have the authors identified all important confounding factors? List the ones you think might be important, that the author missed.
5(b) Have they taken account of the confounding factors in the design and/or analysis?
6(a) Was the follow-up of subjects complete enough?
6(b) Was the follow-up of subjects long enough?
7 What are the results of this study?
8 How precise are the results?
9 Do you believe the results?
10 Can the results be applied to the local population?
11 Do the results of this study fit with other available evidence?
12 What are the implications of this study for practice?
ADE, adverse drug event; ADR, adverse drug reaction; ATC, Anatomical Therapeutic Chemical; DDI, drug–drug interaction; ED, emergency department; IP, inappropriate prescribing; IPET, improved prescribing in the elderly tool; N, no; OTC, over-the-counter; PIM, potentially inappropriate medication; PPO, potential prescribing omission, U, unclear;Y, yes.
Nine studies were conducted in Asia, 4 in Australia, 32 in Europe, 8 in North America, 5 in South America and 2 were conducted across continents (one study covering two Australian countries, three European countries, one North American country and one South American country,20 and one study across two Australian countries, four European countries, one North American country and one South American country).21 Nineteen studies were conducted in primary healthcare or general practice contexts, 15 studies in home or community settings, 16 studies in ambulatory care or outpatient settings, 5 studies in community pharmacies and 2 studies in postdischarge settings, while 3 studies used secondary data analysis.
Eleven studies enrolled adults in all age groups (>18 years), three studies reported the mean age only,22–24 one enrolled those 55 years or older,25 five enrolled those aged 60 years or older,26–30 and the majority of studies (n=40 studies, 67%) enrolled patients 65 years or older. If the study included adult and paediatric data, only relevant adult data were extracted.
The quality of the cross-sectional or descriptive studies using the JBI Critical Appraisal Checklist was high for nine studies, moderate for ten studies and low for one study. The quality of the cohort studies using the CASP quality assessment tool was high for 37 studies and moderate for 3 studies.
Different methods of medication errors and error-related adverse events identification were used in the studies, including data review (electronic/paper-based medical record review, lab review, prescription review), database analysis, patient survey (face-to-face or telephone interview and survey or questionnaire), patient self-report and home visits.
Medication errors
Incidence and/or prevalence
We found no study reporting data on the incidence of medication errors. Estimates of community setting medication error prevalence were available from 53 studies.18–21 23 24 26 27 29–73
Self-reported medication errors
The period prevalence of self-reported medication errors was measured in four cross-sectional studies by Adams et al, Lu and Roughead, Sears et al 21 and Mira et al.20 21 72 73 In the first three studies, the period prevalence was reported as 2%, 6% and 6%, respectively,20 21 72 while in Mira et al’s study 75% of elderly patients with multiple comorbidities and polypharmacy (five or more drugs) reported having made at least one mistake with their medication (including errors related to dose, similar appearance of medications and lack of understanding of the physician’s instructions).73 In this study, in 5% of cases, errors due to drug confusion had very severe consequences, requiring a visit to the emergency services or hospital admission.73 That wide differences in prevalence were seen between the first three studies and the last may be due to population factors. Mira et al’s study population comprised older polymedicated patients with multiple comorbidities. This elderly group had a greater risk of error, while the first three studies had populations including any patient over 18 years.
Medication error according to medicines’ management process
Prescribing errors
The point or period prevalence of prescribing errors was reported in 46 studies. In these studies, prescribing errors included errors in drug indications, drug–disease interactions, drug–drug interactions (DDI) and dosing error, as well as inappropriate prescribing, which was the most common error reported.
Indication
Koper et al 23 found that, on average, 2.7 medications per patient were not indicated, with a total of 94% of patients having medications prescribed by the general practitioner (GP), but not mentioned in the indication of the UpToDate.23
Drug–disease interactions or contraindications
Drug–disease interactions were measured in one study by Mand et al 33 with a prevalence of 10%.33
Drug–drug interactions
The prevalence of DDIs was measured in 11 studies and ranged from 2% to 58%.23 24 26 27 30 34–39 This could in part have been due to the fact that different DDI screening tools were used, namely DDI compendia and ePocrates RX, Thomson Micromedex program, Pharmavista database, BotPlus program of the General Council of Pharmacists’ Official Colleges, British National Formulary 2010, Italian computerised interaction database, DrugDigest, Drugs, Micromedex and Medscape.
Inappropriate prescribing
The prevalence of potentially inappropriate medication (PIM) was measured in 37 studies in the elderly age group only (≥65 years) and ranged from 5% to 94%.18 19 23 26 29 37 40–70 This extremely wide range of inappropriate prescribing prevalence estimates is likely to be, at least in part, due to the different detection tools used, namely Beers 2003, the 2006 Health Plan Employer Data and Information Set, improved prescribing in the elderly tool, Medication Appropriate Index, PRISCUS and Screening Tool of Older Person’s Prescriptions criteria. Johnell and Fastbom46 and Haider et al mentioned two other specific criteria.46 48
The prevalence of potential prescribing omission (PPO) was measured in five studies for the elderly age group only (≥65 years), ranging from 23% to 57%.19 51 65 66 69 PPO was detected by the Screening Tool to Alert doctors to Right Treatment and Assessing Care of Vulnerable Elders.
Dosing errors
Koper et al 23 found that overdosing and/or underdosing was found in 44% of patients.23
Monitoring errors
Monitoring errors were measured in one study by Ramia and Zeenny,71 who found that 73% of patients had incomplete therapeutic/safety laboratory-test monitoring tests.71
Other errors: discrepancy
One study found that at least one discrepancy between the medication lists from the pharmacy, the GP or the patient was present in 86.7% of patients.31 In another study, almost half of the patients (47.6%; 95% CI 40.5 to 54.7) had one or more discrepancies in medication information at discharge.32
The reported point or period prevalence of medication errors in the community settings, including self-reported medication errors, prescribing errors (indication, drug–disease interaction, DDI, dosing error and inappropriate prescribing), monitoring error and discrepancies, had a very wide range from 2% to 94%. Figure 2 shows the medication errors prevalence estimates stratified according to the settings. The highest prevalence was in primary healthcare or general practice (94%).
Figure 2.
Medication errors prevalence estimates according to settings.
Risk factors
Risk factors for medication errors were either related to patients, healthcare professionals and/or medications.
Patient-related risk factors
Patient-related risk factors for the development of medication errors were discussed in 33 studies.18 20 27 29–33 37 38 40–43 48 49 51–53 55 57 58 60 62 64–67 69 70 73–75
Seven risk factors related to patients were addressed in the included studies: polypharmacy, increased age, number of diseases or comorbidities, female, low level of education, hospital admission and middle family income (table 3).
Table 3.
Medication errors patient-related risk factors
| Risk factor | Studies with positive association (n) | Controlled studies (n) | Controlled for | Specific information | OR or RR (95% or 99% CI) p values |
| Age ≥75 years | 13 (24, 33, 37, 42, 44, 52, 53, 55, 61, 69–71, 73) |
10 | NA | ≥80 years | OR 1.021 (95% CI 1.018 to 1.023) p<0.00149 |
| Adjusted for age, sex, number of regular medicine and diagnosed chronic condition | Older age | OR 1.03 (95% CI 1.02 to 1.04) p<0.0569 | |||
| NA | Older age | OR 1.05 (95% CI 1 to 1.09) p=0.04657 | |||
| NA | Older age | OR 1.06 (95% CI 1.0 to 1.13) p=0.03718 | |||
| NA | ≥75 years | OR 1.10 (95% CI 1.05 to 1.15) p<0.00133 | |||
| NA | ≥85 years | OR 1.18 (95% CI 1.16 to 1.20) p<0.0540 | |||
| Adjusted for sex, age and number of chronic drugs | ≥85 years | OR 1.52 (95% CI 1.46 to 1.6)38 | |||
| NA | ≥85 years | OR 1.53 (95% CI 1.5 to 1.55) p<0.0167 | |||
| NA | ≥85 years | OR 1.79 (95% CI 1.19 to 2.83) p=0.00966 | |||
| Adjusted for sex and age | ≥75 years | OR 4.03 (95% CI 3.79 to 4.28) p<0.00127 | |||
| Comorbidity or number of disease or chronic condition drug group (CCDG) score ≥4 |
10 (24, 26, 33, 44, 47, 56, 59, 73, 77, 78) | 3 | Adjusted for age, sex, number of regular medicines and diagnosed chronic condition | Higher number of chronic conditions | PPO: OR 1.47 (95% CI 1.39 to 1.56) p<0.0569 |
| NA | CCDG score ≥4 | OR 1.76 (95% CI 1.72 to 1.81) p<0.0540 | |||
| Adjusted for age and sex | Diagnosed disease ≥3 | OR 6.43 (95% CI 3.25 to 12.44) p<0.00127 | |||
| CCI | 3 (52, 55, 69) | 1 | NA | CCI <2 | RR 2.885 (95% CI 1.972 to 4.22) p=065 |
| Female gender | 10 (33, 35, 47, 52, 53, 62, 64, 66, 71, 73) | 4 | Adjusted for age, sex, number of regular medicines and diagnosed chronic condition | PIM: OR 1.27 (95% CI 1.07 to 1.5) p<0.0569 | |
| Adjusted | OR 1.6 (99% CI 1.58 to 1.64)60 | ||||
| Adjusted for age, sex, education level, partnership, per capita income and occupation | Beers 2003: OR 2.5 (95% CI 1.9 to 3.5) Beers 2012: OR 1.8 (95% CI 1.3 to 2.5)29 |
||||
| Adjusted for sex and age | OR 2.49 (95% CI 2.29 to 2.75) p<0.00127 | ||||
| Health literacy or low education | 2 (52, 79) | 1 | Adjusted for age, sex, type of residential area and comorbidity | OR 1.09 (95% CI 1.07 to 1.17)48 | |
| Hospital admission | 2 (26, 56) | 1 | NA | OR 3.35 (95% CI 2.43 to 4.62) p<0.0552 | |
| Middle family income | 1 (62) | NA | NA | ||
| Polypharmacy | 26 (22–24, 33, 35–37, 41, 42, 44–46, 53, 55–57, 59, 61, 62, 68–71, 73, 74, 78) | 18 | NA | Higher number of prescribed medications | OR 1.06 (95% CI 1.39 to 1.98) p<0.00157 |
| Adjusted for age, sex, number of regular medicines and diagnosed chronic condition | Higher number of prescribed medications | PIM: OR 1.2 (95% CI 1.17 to 1.24) p<0.05 PPO: OR 1.04 (95% CI 1.01 to 1.07) p<0.0569 |
|||
| NA | ≥4 medications | OR 1.91 (95% CI 1.83 to 2.0) p<0.00133 | |||
| NA | Higher number of prescribed medications | OR 1.99 (95% CI 1.80 to 2.18) p=0.00018 | |||
| Adjusted for age, sex, education level, partnership, per capita income and occupation | ≥5 medications | Beers 2003: OR 2.9 (95% CI 2.1 to 3.8) Beers 2012: OR 2.7 (95% CI 2 to 3.6)29 |
|||
| Adjusted for disability, coronary artery disease, heart failure and other comorbidities | ≥5 medications | IP: OR 2.9 (95% CI 1.5 to 5.8) Potential major DDI: 3.8 (95% CI 1.7 to 8.2)37 |
|||
| Adjusted for age, sex, number of chronic conditions and number or drug consumed | ≥3 medications | OR 3.21 (95% CI 2.78 to 3.59) p<0.00127 | |||
| Adjusted for age, sex, length of hospital stay and residential situation | ≥5 medications | OR 3.22 (95% CI 1.40 to 7.42) p=0.00632 | |||
| NA | ≥6 medications | OR 3.37 (95% CI 2.08 to 5.48) p<0.00130 | |||
| NA | ≥7 medications | OR 4.528 (95% CI 4.52 to 4.54) p<0.00149 | |||
| Adjusted for age, sex, CCI, history of cardiovascular disorder and history of digestive disorder | ≥5 medications | OR 5.4 (95% CI 3 to 9.7) p<0.00164 | |||
| Adjusted for sex, age and number of chronic drugs | ≥6 medications | OR 5.59 (95% CI 5.39 to 5.80)38 | |||
| NA | ≥5 medications | OR 5.69 (95% CI 5.0 to 6.48) p<0.0552 | |||
| NA | ≥6 medications | STOPP: RR 6.837 (95% CI 4.155 to 11.247) START: RR 2.051 (95% CI 1.25 to 3.367)65 |
|||
| NA | ≥10 medications | OR 7.33 (95% CI 7.15 to 7.51) p<0.0540 | |||
| NA | ≥9 medications | OR 7.43 (95% CI 3.20 to 17.23) p<0.00166 | |||
| NA | ≥10 medications | Male: OR 8.2 (95% CI 8 to 8.4) Female: OR 9.6 (95% CI 8.2 to 11.2)42 |
|||
| NA | ≥10 medications | OR 11.45 (95% CI 11.2 to 11.7) p<0.0167 |
Several definitions of polypharmacy existed, ranging from prescription of at least three to six medications concurrently. Twenty-six studies showed a positive association between medication error and polypharmacy,18 27 29–33 37 38 40–42 49 51–53 55 57 58 64–67 69 70 74 of which 18 mentioned the estimated OR ranging from 1.06 to 11.45.18 27 29 30 32 33 37 38 40 42 49 52 57 64–67 69
Older age (≥75 years) was associated with medication errors in 13 studies,18 27 33 38 40 48 49 51 57 65–67 69 of which 10 mentioned the OR ranging from 1.02 to 4.03.18 27 33 38 40 49 57 66 67 69
Healthcare professional-related risk factors
Nine risk factors related to healthcare professionals for the development of medication errors were identified: more than one physician involved in their care, family medicine/GP specialty, age ≥51 years, male GP, frequent changes in prescription, not considering the prescription of other physicians, inconsistency in the information and outpatient clinic visits (see table 4).27 31 42 49 52 60 67 73 74
Table 4.
Medication errors healthcare professional-related risk factors
| Risk factor | Studies with positive association (n) | Controlled studies (n) | Adjusted for | OR or RR or beta (95% or 99% CI) p values |
| Age ≥51 years | 2 (53, 71) | 2 | NA | OR 1.03 (95% CI 1.01 to 1.06) p<0.0167 |
| NA | OR 1.238 (95% CI 1.235 to 1.242) p<0.00149 | |||
| More than one physician involved in their care | 5 (22, 33, 64, 77, 78) | 3 | NA | Beta 0.7 (95% CI 0.5 to 1.0) p=0.03473 |
| Adjusted for age, sex, number of chronic conditions and number or drug consumed | OR 1.39 (95% CI 1.17 to 1.67) p<0.00127 | |||
| Adjusted for age and number of prescriber | OR 3.52 (99% CI 3.44 to 3.60)60 | |||
| Male general practitioner | 2 (53, 71) | 2 | NA | OR 1.07 (95% CI 1.05 to 1.10) p<0.0167 |
| NA | OR 1.206 (95% CI 1.202 to 1.210) p<0.00149 | |||
| Frequent changes in prescription | 1 (77) | 1 | NA | Beta 0.4 (95% CI 0.2 to 0.9) p=0.01973 |
| Not considering the prescription of other physicians | 1 (77) | 1 | NA | Beta 1.9 (95% CI 1.1 to 3.2) p=0.01373 |
| Inconsistency in the information | 1 (77) | 1 | NA | Beta 4.4 (95% CI 1.3 to 14.8) p=0.01373 |
| Outpatient clinic visit | 1 (46) | 1 | NA | 1.4 (male 95% CI 1.3 to 1.4) (female 95% CI 1.3 to 1.6)42 |
| Family medicine/general practice specialty | 3 (53, 56, 71) | 3 | NA | OR 1.06 (95% CI 1.03 to 1.10) p<0.0167 |
| NA | OR 1.267 (95% CI 1.265 to 1.269) p<0.00149 | |||
| NA | OR 1.46 (95% CI 1.28 to 1.65) p<0.0552 |
CCI, Charlson Comorbidity Index; IP, inappropriate prescribing; NA, not applicable; PIM, potentially inappropriate medication; PPO, potential prescribing omission; START, Screening Tool to Alert doctors to Right Treatment; STOPP, Screening Tool of Older Person’s Prescriptions.
Medication-related risk factors
Medication-related risk factors for the development of medication error were multiple medication storage locations used, expired medication present, discontinued medication repeats retained, hoarding of medications, therapeutic duplication,25 no medication administration routine, poor adherence and patients confused by generic and trade names.76 In one study by Johnell and Fastbom,46 multidose drug dispensing users (ie, medicines machine-packed into unit-dose bags for each time of administration) were more exposed to all indicators of potentially inappropriate drug.46
Receiving anticoagulant therapy (OR 2.38, 95% CI 2.15 to 2.64) was strongly associated in one study to potential drug–disease interactions.33
The use of OTC and/or prescribed drugs was a risk factor in two additional studies.29 43 The use of OTC medications was associated with PIM; the OR after adjusting for age, sex, education level, partnership, per capita income and occupation was 2.5 (95% CI 1.7 to 3.6) using Beers 2003 and 1.8 (95% CI 1.2 to 2.5) using Beers 2012.29
Error-related adverse events
Error-related adverse events or preventable ADEs were mentioned in six studies.22 28 29 31 32 77 The most frequently reported consequences were ED visits and hospitalisation.
Two methods for detecting ADE were applied: an ADE monitor (ie, using computerised programs composed of rules that identified incidents suggesting that an ADE might be present)22 and using trigger tools to detect ADEs.77
Incidence and/or prevalence
One study estimated preventable ADE incidence as 15/1000 person-years.22 ACE inhibitors and beta-blockers were the most common medications associated with preventable ADE.22 The estimate of the prevalence of preventable ADE was calculated from five studies as detailed below.28 29 31 32 77
All stages of medicines’ management process
Field et al found the prevalence of error caused by patients leading to an adverse event to be 0.38%, that is, less than 1% of the overall population experienced a medication-related adverse event. They found that the majority of patient errors-related adverse events (n=129) occurred in modifying the medication regimen (42%), administering the medication (32%) or not following clinical advice about medication use (22%).77 The medications associated with more than 10 preventable ADEs were anticoagulants/antiplatelets, cardiovascular drugs, diuretics, hypoglycaemics and non-opioid analgesics.77
Error-related adverse events according to medicines’ management process
Prescribing errors
Drug–drug interaction
Obreli-Neto et al 28 found that DDI-related adverse drug reaction (ADR) occurred in 7% of patients. Warfarin, digoxin, spironolactone and acetylsalicylic acid were the drugs most commonly associated with DDI-related ADRs.28
Potentially inappropriate medication
Forty-six per cent of participants reported complaints related to ADEs by interview; 95% of these were caused by prescribed medications.29
Use of inappropriate drugs was associated with an increased risk of nursing home admission, hospitalisation, more outpatient visit days, ED visits and having ADEs or ADRs.44 52 63 68
Other errors
Adverse events (undertreatment due to deletions, ADR due to additions and DDI) related to discrepancy between the medication lists from the patient, the GP or the pharmacy were identified in 24% of patients.31 Two discrepancies were categorised as having the potential to cause severe patient harm.32
Risk factors
Risk factors for the error-related adverse events were discussed in three studies only.28 31 77
Patient-related risk factors
Field et al found that the number of regularly scheduled medications (seven or more medications) (OR 3.3, 95% CI 1.5 to 7.0) and a Charlson Comorbidity Index (CCI) score of 5 or more (OR 15.0, 95% CI 6.5 to 34.5) were both associated with higher risk of patient error leading to preventable ADE.77 Obreli-Neto et al 28 found that an age of 80 years or more (OR 4.4, 95 % CI 3.0 to 6.1, p<0.01), a CCI of 4 or more (OR 1.3, 95% CI 1.1 to 1.8, p<0.01) and consumption of five or more medications (OR 2.7, 95% CI 1.9 to 3.1, p<0.01) were associated with the occurrence of DDI-related ADRs. In addition, Tulner et al 31 found that the number of medications was significantly positively correlated with medication discrepancy adverse patient events.
Medication-related risk factors
The use of medication with narrow therapeutic indices such as warfarin was associated with an increased risk of DDI-related ADRs (OR 1.7, 95% CI 1.1 to 1.9, p<0.01).28
Discussion
Summary of main findings
We sought to critically review previous studies conducted in the community of the incidence/prevalence of medication errors and associated adverse events and to identify the main risk factors. We identified 60 studies carried out in various countries providing a comprehensive assessment of the available evidence on the epidemiology of medication errors and error-related ADEs in community settings.
No relevant studies on the incidence of medication errors in these settings were found. The reported point or period prevalence of medication errors in community settings had a very wide range (ie, 2%–94%). This wide range appears, at least in part, to be due to the inconsistency in the definitions of the medication errors used in the studies, differences in populations studied, methodologies employed for error detection and different outcome measures. More than half (37 studies) of the resulting studies were regarding the prescription of inappropriate drugs within the prescribing error stage in an elderly age group using different criteria. The comparison of those criteria is challenging due to the difference in medication use, consumption and availability of those medications to patients between countries. Further work is needed to review errors occurring at administration and dispensing stages of the medicines’ management process.
As for preventable ADEs, which may in some cases occur as a result of medication errors, only one study reported error-related adverse events incidence, measured as 15/1000 person-years.22 The prevalence of preventable ADE was further reported in five other studies and varied according to the medication error type that resulted in the adverse event.
The most common patient-related risk factors for both medication errors and preventable ADEs mentioned were the number of medications used by the patient and the increased age of patients.
Strengths and limitations
The main strength of this systematic review is that a rigorous and transparent process has been employed, which included no language restrictions, an independent screening of titles and abstracts, independent data extraction and critical appraisal of included studies by two reviewers. It is the first review undertaken within community settings. The use of the ICPS conceptual framework,17 which provides a comprehensive definition of each concept and type of error in the medicines’ management process, is a further strength.
However, several limitations need to be considered. First, despite the thorough process, no data were found regarding the dispensing error stage. This might be due to the lack of a ‘dispensing error’ key term in our search strategy, although ‘medication therapy management’ as a key term was included. However, 10 studies on dispensing errors were excluded because they failed to satisfy the inclusion criteria on one or more counts. Second, no data were found regarding the administration error stage. However, 14 studies on administration errors were also excluded for the same previous reason. Third, this systematic review had different outcomes reported in a variety of ways using different tools and methodology, which made combining results in one meta-analysis difficult. Lastly, the studies addressed risk factors adjusted for different confounders, which makes it difficult to generate comparable estimates and/or make causal inferences about whether the harm resulted from the medication error.
Comparison of the findings with previous studies
The definitional variation issue is supported by another two reviews.78 79 Other systematic reviews focusing on the safety of primary care contexts only have identified studies with vastly different prevalence estimates of the rates of medication errors. These reflect differences in definitions, sampling strategy and populations studied; none have investigated the risk factors for medication errors.80 81
Implications for research, policy and practice
There is a need for (1) improvement in the quality of research in this area—it is important that all researchers provide a standardised set of outcome measures of medication errors or internationally accepted terminology and definitions of key concepts; (2) training and monitoring of healthcare professionals with the involvement of medication safety pharmacists in the community; (3) empowering and educating the patients and the public, particularly those with chronic diseases and polypharmacy, to increase their knowledge of medication safety with a record of the current medication list for each patient; (4) patient use of tools and technology particularly for monitoring and follow-up; and (5) encourage the reporting of medication errors, administration errors and dispensing errors.82 This would strengthen the quality of research, improve the development of strategies to detect and prevent these errors, and provide a safer environment for the community to self-care safely.
Conclusions
We found a very wide variation in the medication error and error-related adverse events rate between studies, which, at least in part, reflects differences in their definitions, methodologies employed for error detection or clinical heterogeneity, that is, differences in populations studied and different outcome measures. Most of the studies were conducted on elderly populations in economically developed countries. There is therefore clearly a need to extend this work to low-income and middle-income countries, particularly give the WHO’s recent launch of a Global Medication Safety Challenge.82 83 Furthermore, most studies focused only on inappropriate prescribing with relatively little attention to other stages such as administration and dispensing. The most common patient and medication-related risk factors for both medication errors and preventable ADEs were the number of medications used by the patient, increased age and receiving anticoagulant therapy. The most common healthcare professional-related risk factor for medication error was when more than one practitioner was involved in the care of patients and care provision by family medicine and GP specialities.
This study has identified important limitations and discrepancies in the methodology used to study medication errors and error-related ADEs in community settings. These findings need to be considered in the context of designing future research related to medication safety. More research is needed in the areas of incidence of medication errors, administration error and dispensing errors and reporting. Researchers should use a more consistent set of definitions and outcomes in order to facilitate collation and synthesis of data.
Ethics and dissemination
The systematic review protocol was published in BMJ Open on 31 August 2016 and is registered with PROSPERO, an international prospective register of systematic reviews.11 12 It is reported using PRISMA. Trial registration number: CRD42016048126.
Supplementary Material
Acknowledgments
We are grateful to Marshall Dozier for her help with formulating the search strategy; Kathrin Cresswell and Andrea Fuentes Pacheco for non-English studies' translation; and the experts in the field for unpublished and in progress work and experts within the Farr Institute.
Footnotes
Contributors: GAA conceived the idea for this review, conducted the systematic literature search, study inclusion, data extraction and quality assessment. NAS participated in the study inclusion, data extraction and quality assessment. MAM participated in data extraction. NA participated in data extraction and quality assessment. GAA led the writing and drafting of the manuscript, and this was commented on critically by AS, EG, HA and NAS.
Funding: The systematic review protocol is part of GAA’s PhD study at The University of Edinburgh. King Saud University, College of Pharmacy funded the scholarship. AS is supported by the Farr Institute. The project was financially supported through Prince Abdullah bin Khalid Celiac Disease Research Chair, Vice Deanship of Research Chairs, King Saud University and The University of Edinburgh.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All available data can be obtained by contacting the corresponding author.
References
- 1. Mark S, Little J, Geller S, et al. Principles and practices of medication safety : DiPiro JT TR, Yee GC, Matzke GR, Wells BG, Posey L, Pharmacotherapy: a pathophysiologic approach. New York: McGraw-Hill, 2011. [Google Scholar]
- 2. Einarson TR. Drug-related hospital admissions. Ann Pharmacother 1993;27(7-8):832–40. 10.1177/106002809302700702 [DOI] [PubMed] [Google Scholar]
- 3. Krähenbühl-Melcher A, Schlienger R, Lampert M, et al. Drug-related problems in hospitals: a review of the recent literature. Drug Saf 2007;30:379–407. [DOI] [PubMed] [Google Scholar]
- 4. Kongkaew C, Hann M, Mandal J, et al. Risk factors for hospital admissions associated with adverse drug events. Pharmacotherapy 2013;33:827–37. 10.1002/phar.1287 [DOI] [PubMed] [Google Scholar]
- 5. Aitken M, Gorokhovich L. Advancing the responsible use of medicines: applying levers for change. SSRN Electronic Journal 2012. 10.2139/ssrn.2222541 [DOI] [Google Scholar]
- 6. : Kohn LT, Corrigan JM, Donaldson MS, To err is human: building a safer health system. Washington (DC): National Academies Press, 2000. [PubMed] [Google Scholar]
- 7. Sheikh A, Panesar SS, Larizgoitia I, et al. Safer primary care for all: a global imperative. Lancet Glob Health 2013;1:e182–e183. 10.1016/S2214-109X(13)70030-5 [DOI] [PubMed] [Google Scholar]
- 8. Cresswell KM, Panesar SS, Salvilla SA, et al. Global research priorities to better understand the burden of iatrogenic harm in primary care: an international delphi exercise. PLoS Med 2013;10:e1001554 10.1371/journal.pmed.1001554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monitor. Moving healthcare closer to home: Literature review of clinical impacts. 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/459268/Moving_healthcare_closer_to_home_clinical_review.pdf
- 10. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 11. Assiri GA, Grant L, Aljadhey H, et al. Investigating the epidemiology of medication errors and error-related adverse drug events (ADEs) in primary care, ambulatory care and home settings: a systematic review protocol. BMJ Open 2016;6:e010675 10.1136/bmjopen-2015-010675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Assiri GA, Grant L, Aljadhey H, et al. Investigating the epidemiology of medication errors and error-related adverse drug events (ADEs) in primary care, ambulatory care and home settings: a systematic review protocol. BMJ Open 2016;6:e010675–8. 10.1136/bmjopen-2015-010675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Citical Appraisal Skills Programme checklist for cohort studies. 2015. http://www.casp&uk.net/wp&content/uploads/2011/11/CASP_Cohort_Appraisal_Checklist_14oct10.pdfwebcite
- 14. Joanna Briggs Institute. Checklist for critical appraisal of descriptive studies. 2015. http://joannabriggs.org/assets/docs/jbc/operations/criticalAppraisalForms/JBC_Form_CritAp_DescCase.pdf
- 15. Thomsen LA, Winterstein AG, S⊘ndergaard B, et al. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother 2007;41:1411–26. 10.1345/aph.1H658 [DOI] [PubMed] [Google Scholar]
- 16. Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother 2011;45(7-8):977–89. 10.1345/aph.1P627 [DOI] [PubMed] [Google Scholar]
- 17. Wolrd Health Organization. The conceptual framework for the international classification for patient safety. Final Technical report. Geneva: World Health Organization, 2009. [Google Scholar]
- 18. Zimmermann T, Kaduszkiewicz H, Van Den Bussche H, et al. Potentially inappropriate medication in elderly primary care patients: A retrospective, longitudinal analysis. [German]. Bundesgesundheitsbl 2013;56:941–9. [DOI] [PubMed] [Google Scholar]
- 19. Candela Marroquín E, Mateos Iglesia N, Palomo Cobos L. [Adequacy of medication in patients 65 years or older in teaching health centers in Cáceres, Spain]. Rev Esp Salud Publica 2012;86:419–34. 10.4321/S1135-57272012000400009 [DOI] [PubMed] [Google Scholar]
- 20. Lu CY, Roughead E. Determinants of patient-reported medication errors: a comparison among seven countries. Int J Clin Pract 2011;65:733–40. 10.1111/j.1742-1241.2011.02671.x [DOI] [PubMed] [Google Scholar]
- 21. Sears K, Scobie A, Mackinnon NJ. Patient-related risk factors for self-reported medication errors in hospital and community settings in 8 countries. Can Pharm J 2012;145:88–93. 10.3821/145.2.cpj88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gandhi TK, Seger AC, Overhage JM, et al. Outpatient adverse drug events identified by screening electronic health records. J Patient Saf 2010;6:91–6. 10.1097/PTS.0b013e3181dcae06 [DOI] [PubMed] [Google Scholar]
- 23. Koper D, Kamenski G, Flamm M, et al. Frequency of medication errors in primary care patients with polypharmacy. Fam Pract 2013;30:313–9. 10.1093/fampra/cms070 [DOI] [PubMed] [Google Scholar]
- 24. Dallenbach MF, Bovier PA, Desmeules J. Detecting drug interactions using personal digital assistants in an out-patient clinic. QJM 2007;100:691–7. 10.1093/qjmed/hcm088 [DOI] [PubMed] [Google Scholar]
- 25. Vuong T, Marriott JL. Unnecessary medicines stored in homes of patients at risk of medication misadventure. Journal of Pharmacy Practice and Research 2006;36:16–20. 10.1002/j.2055-2335.2006.tb00879.x [DOI] [Google Scholar]
- 26. Obreli Neto PR, Vieira JC, Teixeira DRA, et al. Potential risks in drug prescriptions to elderly: A cross-sectional study in the public primary health care system of Ourinhos micro-region, Brazil. Latin American Journal of Pharmacy 2011;30:629. [Google Scholar]
- 27. Obreli Neto PR, Nobili A, Marusic S, et al. Prevalence and predictors of potential drug-drug interactions in the elderly: a cross-sectional study in the brazilian primary public health system. Journal of Pharmacy & Pharmaceutical Sciences 2012;15:344–54. 10.18433/J37K5W [DOI] [PubMed] [Google Scholar]
- 28. Obreli-Neto PR, Nobili A, de Oliveira Baldoni A, et al. Adverse drug reactions caused by drug–drug interactions in elderly outpatients: a prospective cohort study. Eur J Clin Pharmacol 2012;68:1667–76. 10.1007/s00228-012-1309-3 [DOI] [PubMed] [Google Scholar]
- 29. Baldoni AdeO, Ayres LR, Martinez EZ, et al. Factors associated with potentially inappropriate medications use by the elderly according to Beers criteria 2003 and 2012. Int J Clin Pharm 2014;36:316–24. 10.1007/s11096-013-9880-y [DOI] [PubMed] [Google Scholar]
- 30. Secoli SR, Figueras A, Lebrão ML, et al. Risk of potential drug-drug interactions among Brazilian elderly: a population-based, cross-sectional study. Drugs Aging 2010;27:759–70. 10.2165/11538460-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 31. Tulner LR, Kuper IMJA, Frankfort SV, et al. Discrepancies in reported drug use in geriatric outpatients: Relevance to adverse events and drug-drug interactions. Am J Geriatr Pharmacother 2009;7:93–104. 10.1016/j.amjopharm.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 32. Cornu P, Steurbaut S, Leysen T, et al. Discrepancies in medication information for the primary care physician and the geriatric patient at discharge. Ann Pharmacother 2012;46(7-8):983–91. 10.1345/aph.1R022 [DOI] [PubMed] [Google Scholar]
- 33. Mand P, Roth K, Biertz F, et al. Drug-disease interaction in elderly patients in family practice. Int. Journal of Clinical Pharmacology and Therapeutics 2014;52:337–45. 10.5414/CP202003 [DOI] [PubMed] [Google Scholar]
- 34. Indermitte J, Reber D, Beutler M, et al. Prevalence and patient awareness of selected potential drug interactions with self-medication. J Clin Pharm Ther 2007;32:149–59. 10.1111/j.1365-2710.2007.00809.x [DOI] [PubMed] [Google Scholar]
- 35. Mahmood M, Malone DC, Skrepnek GH, et al. Potential drug-drug interactions within Veterans Affairs medical centers. Am J Health Syst Pharm 2007;64:1500–5. 10.2146/ajhp060548 [DOI] [PubMed] [Google Scholar]
- 36. Gagne JJ, Maio V, Rabinowitz C. Prevalence and predictors of potential drug–drug interactions in Regione Emilia-Romagna, Italy. J Clin Pharm Ther 2008;33:141–51. 10.1111/j.1365-2710.2007.00891.x [DOI] [PubMed] [Google Scholar]
- 37. Lapi F, Pozzi C, Mazzaglia G, et al. Epidemiology of suboptimal prescribing in older, community dwellers: a two-wave, population-based survey in Dicomano, Italy. Drugs Aging 2009;26:1029–38. 10.2165/11319390-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 38. Nobili A, Pasina L, Tettamanti M, et al. Potentially severe drug interactions in elderly outpatients: results of an observational study of an administrative prescription database. J Clin Pharm Ther 2009;34:377–86. 10.1111/j.1365-2710.2009.01021.x [DOI] [PubMed] [Google Scholar]
- 39. Guthrie B, Makubate B, Hernandez-Santiago V, et al. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995–2010. BMC Med 2015;13:74 10.1186/s12916-015-0322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maio V, Yuen EJ, Novielli K, et al. Potentially inappropriate medication prescribing for elderly outpatients in emilia romagna, Italy. Drugs Aging 2006;23:915–24. 10.2165/00002512-200623110-00006 [DOI] [PubMed] [Google Scholar]
- 41. de Oliveira Martins S, Soares MA, Foppe van Mil JW, et al. Inappropriate drug use by Portuguese elderly outpatients--effect of the Beers criteria update. Pharm World Sci 2006;28:296–301. 10.1007/s11096-006-9046-2 [DOI] [PubMed] [Google Scholar]
- 42. Pugh MJ, Hanlon JT, Zeber JE, et al. Assessing potentially inappropriate prescribing in the elderly Veterans Affairs population using the HEDIS 2006 quality measure. J Manag Care Pharm 2006;12:537–45. 10.18553/jmcp.2006.12.7.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saab YB, Hachem A, Sinno S, et al. Inappropriate medication use in elderly lebanese outpatients: prevalence and risk factors. Drugs Aging 2006;23:743–52. [DOI] [PubMed] [Google Scholar]
- 44. Zuckerman IH, Langenberg P, Baumgarten M, et al. Inappropriate drug use and risk of transition to nursing homes among community-dwelling older adults. Med Care 2006;44:722–30. 10.1097/01.mlr.0000215849.15769.be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bregnhøj L, Thirstrup S, Kristensen MB, et al. Prevalence of inappropriate prescribing in primary care. Pharmacy World & Science 2007;29:109–15. 10.1007/s11096-007-9108-0 [DOI] [PubMed] [Google Scholar]
- 46. Johnell K, Fastbom J. Multi-dose drug dispensing and inappropriate drug use: A nationwide register-based study of over 700 000 elderly. Scand J Prim Health Care 2008;26:86–91. 10.1080/02813430802022196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berdot S, Bertrand M, Dartigues J-F, et al. Inappropriate medication use and risk of falls – A prospective study in a large community-dwelling elderly cohort. BMC Geriatr 2009;9:30 10.1186/1471-2318-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haider SI, Johnell K, Weitoft GR, et al. The influence of educational level on polypharmacy and inappropriate drug use: a register-based study of more than 600,000 older people. J Am Geriatr Soc 2009;57:62–9. 10.1111/j.1532-5415.2008.02040.x [DOI] [PubMed] [Google Scholar]
- 49. Lai H-Y, Hwang S-J, Chen Y-C, et al. Prevalence of the prescribing of potentially inappropriate medications at ambulatory care visits by elderly patients covered by the Taiwanese National Health Insurance program. Clin Ther 2009;31:1859–70. 10.1016/j.clinthera.2009.08.023 [DOI] [PubMed] [Google Scholar]
- 50. Ryan C, O’Mahony D, Kennedy J, et al. Appropriate prescribing in the elderly: an investigation of two screening tools, Beers criteria considering diagnosis and independent of diagnosis and improved prescribing in the elderly tool to identify inappropriate use of medicines in the elderly in primary care in Ireland. J Clin Pharm Ther 2009;34:369–76. 10.1111/j.1365-2710.2008.01007.x [DOI] [PubMed] [Google Scholar]
- 51. Ryan CristÃn, O’Mahony D, Kennedy J, et al. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol 2009;68:936–47. 10.1111/j.1365-2125.2009.03531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Akazawa M, Imai H, Igarashi A, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother 2010;8:146–60. 10.1016/j.amjopharm.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 53. Zaveri HG, Mansuri SM, Patel VJ. Use of potentially inappropriate medicines in elderly: A prospective study in medicine out-patient department of a tertiary care teaching hospital. Indian J Pharmacol 2010;42:94–8. 10.4103/0253-7613.64499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barnett K, McCowan C, Evans JMM, et al. Prevalence and outcomes of use of potentially inappropriate medicines in older people: cohort study stratified by residence in nursing home or in the community. BMJ Qual Saf 2011;20:275–81. 10.1136/bmjqs.2009.039818 [DOI] [PubMed] [Google Scholar]
- 55. Chang C-B, Chen J-H, Wen C-J, et al. Potentially inappropriate medications in geriatric outpatients with polypharmacy: application of six sets of published explicit criteria. Br J Clin Pharmacol 2011;72:482–9. 10.1111/j.1365-2125.2011.04010.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leikola S, Dimitrow M, Lyles A, et al. Potentially inappropriate medication use among Finnish non-institutionalized people aged ≥65 years: a register-based, cross-sectional, national study. Drugs Aging 2011;28:227–36. 10.2165/11586890-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 57. Lin Y-J, Peng L-N, Chen L-K, et al. Risk factors of potentially inappropriate medications among older patients visiting the community health center in rural Taiwan. Arch Gerontol Geriatr 2011;53:225–8. 10.1016/j.archger.2010.11.017 [DOI] [PubMed] [Google Scholar]
- 58. Zhang Y-J, Liu W-W, Wang J-B, et al. Potentially inappropriate medication use among older adults in the USA in 2007. Age Ageing 2011;40:398–401. 10.1093/ageing/afr012 [DOI] [PubMed] [Google Scholar]
- 59. Haasum Y, Fastbom J, Johnell K. Institutionalization as a risk factor for inappropriate drug use in the elderly: a swedish nationwide register-based study. Ann Pharmacother 2012;46:339–46. 10.1345/aph.1Q597 [DOI] [PubMed] [Google Scholar]
- 60. Nyborg G, Straand J, Brekke M. Inappropriate prescribing for the elderly—a modern epidemic? Eur J Clin Pharmacol 2012;68:1085–94. 10.1007/s00228-012-1223-8 [DOI] [PubMed] [Google Scholar]
- 61. Yasein NA, Barghouti FF, Irshaid YM, et al. Elderly patients in family practice: poly pharmacy and inappropriate prescribing - Jordan. International Medical Journal 2012;19:302–6. [Google Scholar]
- 62. Blozik E, Rapold R, von Overbeck J, et al. Polypharmacy and potentially inappropriate medication in the adult, community-dwelling population in switzerland. Drugs Aging 2013;30:561–8. 10.1007/s40266-013-0073-0 [DOI] [PubMed] [Google Scholar]
- 63. Cahir C, Bennett K, Teljeur C, et al. Potentially inappropriate prescribing and adverse health outcomes in community dwelling older patients. Br J Clin Pharmacol 2014;77:201–10. 10.1111/bcp.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weng M-C, Tsai C-F, Sheu K-L, et al. The impact of number of drugs prescribed on the risk of potentially inappropriate medication among outpatient older adults with chronic diseases. QJM: An International Journal of Medicine 2013;106:1009–15. 10.1093/qjmed/hct141 [DOI] [PubMed] [Google Scholar]
- 65. Castillo-Páramo A, Clavería A, Verdejo González A, et al. Inappropriate prescribing according to the STOPP/START criteria in older people from a primary care setting. Eur J Gen Pract 2014;20:281–9. 10.3109/13814788.2014.899349 [DOI] [PubMed] [Google Scholar]
- 66. Vezmar Kovačević S, Simišić M, Stojkov Rudinski S, et al. Potentially inappropriate prescribing in older primary care patients. PLoS One 2014;9:e95536 10.1371/journal.pone.0095536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Amos TB, Keith SW, Del Canale S, et al. Inappropriate prescribing in a large community-dwelling older population: a focus on prevalence and how it relates to patient and physician characteristics. J Clin Pharm Ther 2015;40:7–13. 10.1111/jcpt.12212 [DOI] [PubMed] [Google Scholar]
- 68. Hedna K, Hakkarainen KM, Gyllensten H, et al. Potentially inappropriate prescribing and adverse drug reactions in the elderly: a population-based study. Eur J Clin Pharmacol 2015;71:1525–33. 10.1007/s00228-015-1950-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moriarty F, Bennett K, Fahey T, et al. Longitudinal prevalence of potentially inappropriate medicines and potential prescribing omissions in a cohort of community-dwelling older people. Eur J Clin Pharmacol 2015;71:473–82. 10.1007/s00228-015-1815-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Woelfel JA, Patel RA, Walberg MP, et al. Use of potentially inappropriate medications in an ambulatory medicare population. The Consultant Pharmacist 2011;26:913–9. 10.4140/TCP.n.2011.913 [DOI] [PubMed] [Google Scholar]
- 71. Ramia E, Zeenny R. Completion of therapeutic and safety monitoring tests in Lebanese outpatients on chronic medications: a cross-sectional study. Patient Prefer Adherence 2014;8:1195–204. 10.2147/PPA.S69250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adams RJ, Tucker G, Price K, et al. Self-reported adverse events in health care that cause harm: a population-based survey. Med J Aust 2009;190:484–8. [DOI] [PubMed] [Google Scholar]
- 73. Mira JJ, Orozco-Beltran D, Perez-Jover V, et al. Physician patient communication failure facilitates medication errors in older polymedicated patients with multiple comorbidities. Fam Pract 2013;30:56–63. 10.1093/fampra/cms046 [DOI] [PubMed] [Google Scholar]
- 74. Pit SW, Byles JE, Cockburn J. Prevalence of self-reported risk factors for medication misadventure among older people in general practice. J Eval Clin Pract 2008;14:203–8. 10.1111/j.1365-2753.2007.00833.x [DOI] [PubMed] [Google Scholar]
- 75. Mosher HJ, Lund BC, Kripalani S, et al. Association of health literacy with medication knowledge, adherence, and adverse drug events among elderly veterans. J Health Commun 2012;17:241–51. 10.1080/10810730.2012.712611 [DOI] [PubMed] [Google Scholar]
- 76. Sorensen L, Stokes JA, Purdie DM, et al. Medication management at home: medication risk factor prevalence and inter-relationships. J Clin Pharm Ther 2006;31:485–91. 10.1111/j.1365-2710.2006.00768.x [DOI] [PubMed] [Google Scholar]
- 77. Field TS, Mazor KM, Briesacher B, et al. Adverse drug events resulting from patient errors in older adults. J Am Geriatr Soc 2007;55:271–6. 10.1111/j.1532-5415.2007.01047.x [DOI] [PubMed] [Google Scholar]
- 78. Alsulami Z, Conroy S, Choonara I. Medication errors in the middle east countries: a systematic review of the literature. Eur J Clin Pharmacol 2013;69:995–1008. 10.1007/s00228-012-1435-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Karthikeyan MBT, Khaleel MI, Sahl M, et al. A systematic review on medication errors. International Journal of Drug Development and Research 2015:7–4. [Google Scholar]
- 80. Olaniyan JO, Ghaleb M, Dhillon S, et al. Safety of medication use in primary care. Int J Pharm Pract 2015;23:3–20. 10.1111/ijpp.12120 [DOI] [PubMed] [Google Scholar]
- 81. Panesar SS, deSilva D, Carson-Stevens A, et al. How safe is primary care? A systematic review. British Medical Journal Quality and Safety 2015;0:1–10. [DOI] [PubMed] [Google Scholar]
- 82. Donaldson LJ, Kelley ET, Dhingra-Kumar N, et al. Medication without harm: who’s third global patient safety challenge. Lancet 2017;389:1680–1. 10.1016/S0140-6736(17)31047-4 [DOI] [PubMed] [Google Scholar]
- 83. Sheikh A, Dhingra-Kumar N, Kelley E, Kieny MP, et al. The third global patient safety challenge: tackling medication-related harm. Bull World Health Organ 2017;95:546–546A. 10.2471/BLT.17.198002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE prevention study group. JAMA 1995;274:29–34. [PubMed] [Google Scholar]
- 85. What is a Medication Error? National coordinating council for medication error reporting and prevention. http://www.nccmerp.org/about-medication-errors
- 86. U.S. Food and Drug Administration. What are over-the-counter (OTC) drugs and how are they approved? http://www.fda.gov/AboutFDA/Transparency/Basics/ucm194951.htm
- 87. Gallagher P, Barry P, O’Mahony D. Inappropriate prescribing in the elderly. J Clin Pharm Ther 2007;32:113–21. 10.1111/j.1365-2710.2007.00793.x [DOI] [PubMed] [Google Scholar]
- 88. Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-019101supp001.pdf (104.3KB, pdf)