Abstract
Introduction:
Cystic fibrosis (CF) is a complex disease that includes both pulmonary and gastrointestinal challenges, resulting in decreased weight. Pulmonary symptoms of CF are extremely variable. Greater body mass at an early age is associated with improved pulmonary function, but it is unknown at what age weight becomes predictive of pulmonary disease severity. The purpose of this study was to investigate the relationship between birth weight and pulmonary function in CF.
Methods:
Birth weight and pulmonary data were obtained. Linear regressions were used to examine the relationship between these two variables. A one-tailed t-test was used to compare birth weights between CF patients and the national average.
Results:
Birth weight was significantly lower in babies with CF and correlated with pulmonary disease at ages 6 and 10 years but not with age at which Pseudomonas aeruginosa colonization was observed.
Discussion:
These data suggest that CF growth deficiency has prenatal origins. Early nutritional intervention for babies with CF and a low birth weight is warranted to maximize pulmonary potential.
Keywords: cystic fibrosis, birth weight, respiratory, genetics, pediatrics
Cystic fibrosis (CF) is an autosomal recessive genetic disorder affecting approximately 1 in every 3,500 newborns (Savage et al., 2014). As it is the most common life-limiting autosomal recessive disease in Caucasians, pediatric and advanced practice nurses are likely to encounter children with CF in the primary care setting. The disease is caused by inherited mutations in the CF transmembrane conductance regulator (CFTR) gene, which lead to defective ion transport. This compromise in function results in the accumulation of viscous mucus throughout the respiratory tract, pancreas, and intestine, ultimately leading to respiratory disease and digestive complications.
An inability to gain weight is a major medical complication for patients with CF. Improving the nutritional health of CF patients at younger ages is therefore an important clinical goal. Moreover, enhancing nutritional status in children with CF, such as weight, height, and vitamin levels, is associated with improved lung function and may lessen the likelihood of comorbid diseases (Forrester, Knox, Smyth, & Fogarty, 2013; Konstan et al., 2003; Steinkamp & Wiedemann, 2002; Stephenson et al., 2013; Yen, Quinton, & Borowitz, 2013; Zemel, Jawad, FitzSimmons, & Stallings, 2000). Previous research has established the positive correlation between weight and pulmonary function of young patients with CF (Konstan et al., 2003; Steinkamp & Wiedemann, 2002; Yen et al., 2013; Zemel et al., 2000). Weight for age was positively correlated with 5-year survivorship in one study of patients with CF aged 5½ years or older (n = 5,820; Liou et al., 2001). In addition, while growth indexes at age 3 were not correlated with signs and symptoms of pulmonary disease at age 3, the growth indexes at age 3 were positively correlated with pulmonary function at age 6 (Konstan et al., 2003). Pulmonary function was highest for the patients who had a weight for age that remained above the 10th percentile from age 3 to age 6 and lowest for those patients who remained below the 10th percentile throughout this period. Therefore, while the relationship between growth parameters and pulmonary function is not fixed, nutritional intervention does have the potential to substantially influence pulmonary function. However, while weight and weight gain are influential in predicting and improving the pulmonary prognosis and overall health of patients with CF in childhood (Kerem et al., 2014; Konstan et al., 2003), the age at which weight becomes predictive is unknown.
Studies have established that children and adults with CF have an increased risk for low weight for age (Lai, Corey, FitzSimmons, Kosorok, & Farrell, 1999; Lai et al., 1998). In addition, infants with CF have significantly lower birth weights when compared to healthy infants (Festini et al., 2005; Haeusler, Frisch, Waldhor, & Gotz, 1994; Marcus et al., 1991; Muller, Thamm, Lietz, Handrick, & Walter, 1999). For example, in one retrospective cohort study in Tuscany, Italy, researchers compared birth weights and gestational ages of newborns diagnosed with CF (n = 70) with those of healthy newborns (n = 290,059; Festini et al., 2005). The mean birth weight of newborns with CF was 246.2 g less than the mean birth weight of unaffected newborns, a significant difference. In addition, 14.4% of the newborns with CF were delivered preterm (36 6/7 weeks or less), whereas only 5.5% of newborns without CF were preterm, again, a significant difference; however, birth weights of preterm affected and unaffected neonates did not differ. In contrast, a significant difference did exist between birth weights of affected and unaffected neonates born at term. This finding suggests that the difference in weight between affected and unaffected newborns develops during the final weeks of gestation and can be observed at birth.
To better characterize the association between birth weight and clinical morbidities, we conducted the present retrospective cohort study of patients with CF who were cared for at the Cystic Fibrosis Center at Rainbow Babies and Children’s Hospital in Cleveland, OH. Specifically, we compared birth weights of patients with CF to national norms in the same time frame and examined the relationship between birth weight and pulmonary phenotype status (forced expiratory volume [FEV] and Pseudomonas aeruginosa infection).
Method
Participants
This study was conducted at the Cystic Fibrosis Center at Rainbow Babies and Children’s Hospital and was approved by the institutional review board at University Hospitals Case Medical Center. Of the 127 patients with CF who were cared for at this center during the 6-month recruitment period, 79 met inclusion criteria for this study. The study cohort included CF patients who were born between 1975 and 2005, had been full-term deliveries, and had pancreatic insufficiency. Patients were deemed to have pancreatic insufficiency if their fecal elastase levels measured <100 μg/g of stool or, in older patients, if pancreatic enzyme therapy was beneficial. Patients who received lung transplants were included in the study sample up until the age of their transplant. We excluded CF patients who had been premature at birth or were a twin, as both are risk factors for low birth weight (Alexander, Kogan, Martin, & Papiernik, 1998; Olsen, Groveman, Lawson, Clark, & Zemel, 2010). We also excluded patients with pancreatic sufficiency, as these patients tend to have less acute clinical manifestations of CF (Kerem et al., 2014). In addition, we excluded patients born prior to 1975 to control for the introduction of new treatments for CF, such as aerosolized TOBI® or enteric-coated pancreatic enzymes (Carroccio et al., 1988; Ramsey et al., 1993) and patients born after 2005 due to a lack of accumulated clinical data.
Data Collection
We obtained clinical information from the electronic medical records that collate each patient’s clinical data generated during inpatient and outpatient visits. Birth weight of the CF patients was not recorded in the medical records database; therefore, we placed a survey in every patient’s chart to obtain the patient’s birth weight and gestational age via patient and/or parent self-report at the next scheduled clinic visit.
Clinical Symptom Assessment
We collected two categories of information from the electronic medical records: (1) demographic information (year of birth, gender, CFTR genotype, and presence/absence of meconium ileus) and (2) pulmonary phenotype (FEV1%, age at first documented P. aeruginosa colonization). Pulmonary phenotype included both pulmonary function and pulmonary infection. Pulmonary function can be characterized by the amount of air a person can forcibly exhale in 1 s (FEV1). FEV1% is the percentage of the predicted FEV1 based on findings in healthy controls matched for age, gender, height, and weight. We examined FEV1% across four time periods: ages 5–7, 9–11, 14–16, and 19–21 years. In this article, we refer to these time points by the median age: ages 6, 10, 15, and 20, respectively. We selected the patient’s best FEV1% measurement during each of those time periods for analysis, excluding FEV1 measurements obtained during acute exacerbations. We analyzed susceptibility to pulmonary infection using the age at which P. aeruginosa colonization was first identified in each patient. Criteria for P. aeruginosa colonization included three consecutive clinic visits at which cultures were positive for P. aeruginosa.
Statistical Analysis
We summarized demographic information using means, standard deviations, and frequencies. To compare mean birth weight of individuals with CF to the national average birth weight published by the Centers for Disease Control, matched for gender, we used one-tailed t-tests. To examine relationships between birth weight and FEV1%, at ages 6, 10, 15, and 20 years, we used linear regressions. We performed data analysis using IBM SPSS Statistics, Version 21, for Mac.
Results
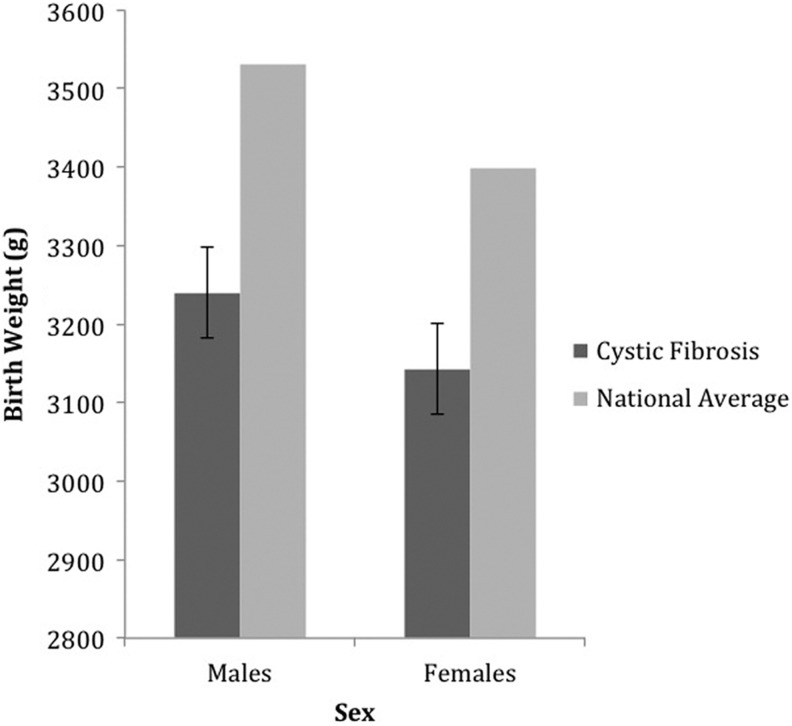
Table 1 shows demographic characteristics for study participants. Gender-matched comparisons between birth weights of patients with CF and national birth-weight averages indicated that the mean birth weights for males (3,239.80 g, SD = 367.83 g, n = 40) and females (3,142.94 g, SD = 422.75 g, n = 39) with CF were significantly lower than the national averages of 3,530.20 g for males, t(39) = −5.00, p < .01, and 3,399.19 g for females, t(39) = −3.79, p = .01 (Figure 1). We found no significant difference in birth weight between males and females with CF (p = .28), therefore, we grouped males and females together for the remaining analyses.
Table 1.
Demographic Characteristics of Participating Patients With Cystic Fibrosis.
| Characteristic | n (%) |
|---|---|
| Sex, male | 40 (51) |
| Age (years) | |
| ≤4 | 11 (14) |
| 5–9 | 13 (16) |
| 10–14 | 15 (19) |
| 15–19 | 22 (28) |
| 20–24 | 11 (14) |
| ≥25 | 7 (9) |
| Meconium ileus at birth, present | 28 (35) |
| Genotype | |
| F508del homozygous | 48 (61) |
| F508del heterozygous | 31 (39) |
| Race | |
| Caucasian | 77 (97) |
| African American | 2 (3) |
| Pseudomonas aeruginosa colonization, present | 42 (53) |
Note. N = 79.
Figure 1.
The average birth weight of participating males (n = 40) and females (n = 39) with cystic fibrosis compared with national averages.
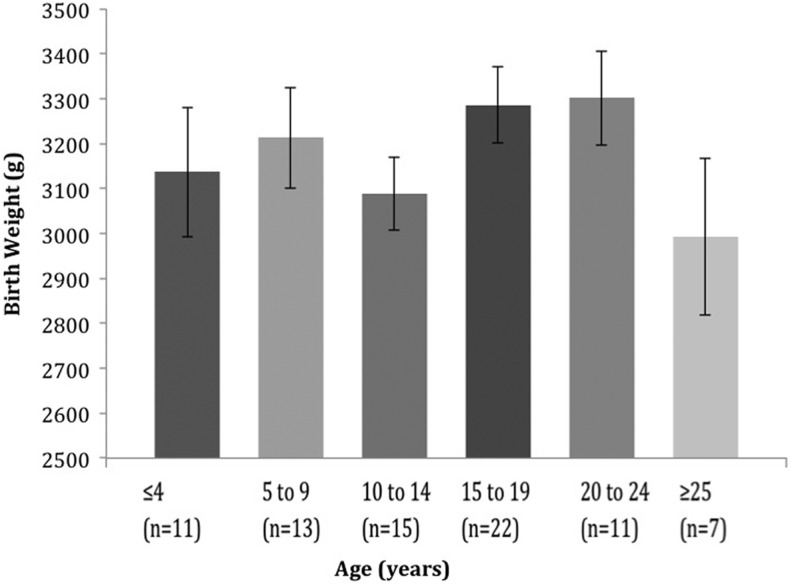
To test for differences in birth weight by year of birth, we used a one-way analysis of variance. We grouped participants by current age—≤4 years, 5–9 years, 10–14 years, 15–19 years, 20–24 years, and ≥25 years—and found that birth year had no effect upon birth weight, F(5, 78) = 0.414, p = .42 (Figure 2). This finding suggests that no significant change in average birth weight has occurred among infants with CF over the past 25 years. Therefore, we did not stratify further analyses based on birth cohort.
Figure 2.
The average birth weight of patients with cystic fibrosis grouped by age on December 31, 2013, by one-way analysis of variance, p = .42.
We also examined the presence of meconium ileus and CFTR genotype (F508del homozygotes vs. compound heterozygotes) for a relationship with birth weight. Birth weight did not differ significantly by the presence or absence of meconium ileus or CFTR genotype (data not shown). Therefore, we grouped these patients together for the remaining analyses.
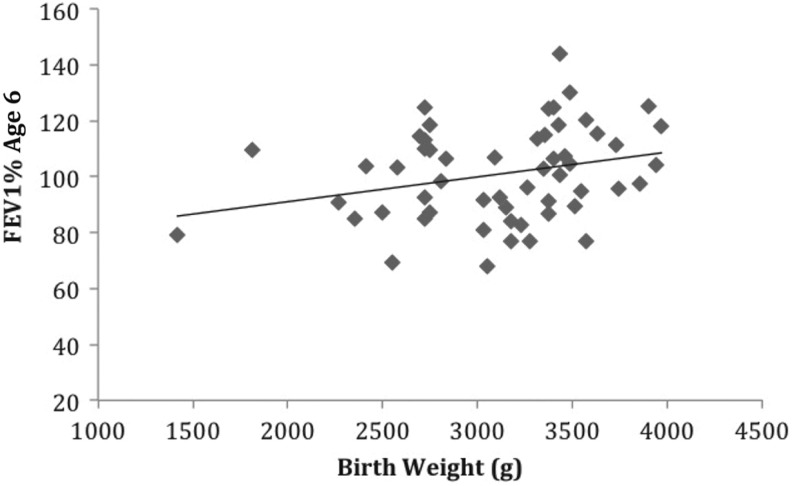
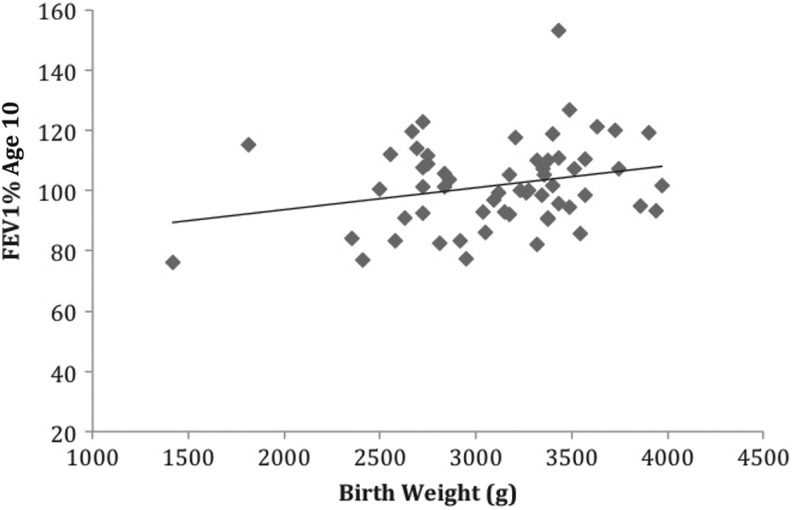
Birth weight predicted FEV1% at two time points. For every 100 g that birth weight increased, FEV1% at age 6 increased by 1%, b = 0.01, t(54) = 2.104, p = .04. Birth weight also explained 8% of the variance in FEV1% at age 6, adj. R 2 = .08, F(1, 54) = 4.43, p = .04 (Figure 3). Birth weight also predicted FEV1% at age 10 years, b = 0.01, t(58) = 1.98, p = .05, as well as explaining 7% of the variance in FEV1% at age 10, adj. R 2 = .07, F(1, 58) = 3.94, p = .05 (Figure 4). However, we found no correlations between FEV1% and birth weight at ages 15, b = −0.001, t(44) = −0.013, p = .93; or 20 years, b = 0.006, t(16) = 0.417, p = .683.
Figure 3.
The relationship between birth weight and percentage of expected forced expiratory volume in 1 s (FEV1%) at 6 years of age (n = 55), R 2 = .08, p = .04.
Figure 4.
The relationship between birth weight and percentage of expected forced expiratory volume in 1 s at 10 years of age (n = 59), R 2 = .07, p = .05.
We found documentation of P. aeruginosa colonization for 42 patients. There was no significant difference in the age of colonization between males and females (p = .94); therefore, we grouped males and females together for analysis. Mean age at time of first P. aeruginosa colonization was 8.36 years (SD = 4.88) among these 42 patients. We assessed the association between birth weight and P. aeruginosa colonization at an early age (first occurring in patients younger than 10 years) by comparing mean birth weight for patients 10 years and older who were not colonized (n = 22, 3,100.25 ± 76.44 g) to that of patients who were first colonized prior to age 9 (n = 26, 3,088.26 ± 112.59 g) and found no statistically significant difference in mean birth weight between the groups (p = .93).
Discussion
In the present study, we assessed birth weights of newborns with CF and identified a relationship between birth weight and later childhood pulmonary function. We found significantly lower birth weights in both males and females with CF compared to the national average birth weights matched for gender (290.6 g and 256.3 g lower, respectively). Our findings on birth weight are similar to those of previous studies in which investigators observed significantly lower birth weights in newborns with CF compared to those without CF (Festini et al., 2005; Haeusler et al., 1994; Marcus et al., 1991; Muller et al., 1999).
Previous authors have attributed growth deficiency (reduced height and weight) in children and adults with CF to lower caloric intake, higher energy expenditure, and maldigestion (Groleau et al., 2014; Zemel et al., 1996). Low caloric intake is unlikely to fully explain the reduced birth weight because maternal nutrition and placental functioning supply fetal nutrition. However, authors have offered several other hypotheses to explain the decreased birth weight observed in infants with CF. One hypothesis relates to the absence of CFTR in the placenta (Faller, Egan, & Ryan, 1995; Mylona, Glazier, Greenwood, Sides, & Sibley, 1996). CFTR is thought to play a role in placental ion exchange (Davis, Shennan, & Boyd, 1985). If placental CFTR is missing, reduced fetal nutrition could be a possible result (Festini et al., 2005). Another hypothesis is that decreased exocrine pancreatic function in utero could reduce intrauterine nutrition (Festini et al., 2005). A third hypothesis involves energy expenditure. Researchers have observed higher energy expenditure in patients with CF (Vaisman, Pencharz, Corey, Canny, & Hahn, 1987). Fetal energy expenditure in CF has yet to be determined and remains a possible explanation for the decreased birth weight. Finally, authors have also hypothesized involvement of the endocrine system in lower birth weight in CF. A study in both newborn pigs and humans with CF identified significantly decreased neonatal insulin-like growth factor 1 (IGF1) levels in newborn pigs (n = 9) and newborn infants (n = 23) with CF compared to control populations (n = 11 and n = 41, respectively; Rogan et al., 2010). Researchers have previously identified a positive correlation between IGF1 levels and birth weight in newborns without CF (n = 53; Giudice et al., 1995); thus perhaps IGF1 levels are a contributing factor to lower birth weight in newborns with CF. While this and previous studies have identified a significantly reduced birth weight in infants with CF, its origin requires additional research.
The effect of birth weight on lung function in later life has been studied extensively in non-CF populations. For instance, researchers found that very low birth weight was associated with an increased risk for lung function abnormalities by school age (Cazzato, Ridolfi, Bernardi, Faldella, & Bertelli, 2013; Lista et al., 2014). However, the majority of infants in these studies were preterm; the association of worsened lung function with very low birth weight may thus be due to inadequate lung development. There are conflicting reports on the association between birth weight and later lung function in individuals who were born at full term (>37 weeks) and were in the normal range of weight. While some studies have found no association between birth weight and later lung function (Cheung, Karlberg, Low, & Ip, 2001; Laerum et al., 2004; Matthes, Lewis, Davies, & Bethel, 1995; Shaheen, Sterne, Tucker, & Florey, 1998), others have identified a positive association (Barker et al., 1991; Canoy et al., 2007; Lawlor, Ebrahim, & Davey Smith, 2005; Pei et al., 2010; Stein et al., 1997). The conflicts in these reports may be due to differences in population characteristics, methods of data collection, or age at lung function characterization. To our knowledge, the present study is the first designed to investigate the association between birth weight and later pulmonary phenotypes in the CF population. Our findings support and extend findings of prior studies of children and adults with CF in which weight at a certain age was positively correlated to FEV1% at later ages (Forrester et al., 2013; Konstan et al., 2003; Steinkamp & Wiedemann, 2002; Stephenson et al., 2013; Yen et al., 2013; Zemel et al., 2000). Evidence from studies such as these support the current standard of care for clinical efforts directed at improving weight gain in CF patients. We believe that identifying CF patients with birth weights in the lower range of normal is beneficial, as they have a higher risk of developing more severe pulmonary manifestations of CF, such as lower FEV1%. More aggressive efforts at fostering weight gain should begin at younger ages for those newborns with CF at higher risk.
In the present study, we found, specifically, that birth weight was correlated with FEV1% at 6 and 10 years of age. At age 6, for every 100 g increase in birth weight, FEV1% increased by 1%. Previous studies have suggested that even a small increase in FEV1% in childhood may have a significant clinical impact later in life, including increases in longevity (Corey, Edwards, Levison, & Knowles, 1997; Schluchter, Konstan, & Davis, 2002; Schluchter, Konstan, Drumm, Yankaskas, & Knowles, 2006). In addition, the 7–8% of the variance we observed in FEV1% at ages 6 and 10 that could be attributed to birth weight indicates that, while birth weight contributes to later lung function, it does not explain the vast majority of variation in FEV1% in CF patients at those ages.
The absence of a correlation between birth weight and FEV1% at ages 15 and 20 suggests that, as patients age, factors other than birth weight are more likely to contribute to pulmonary function. Previous studies have found that decline in pulmonary function was associated with body mass index, P. aeruginosa colonization, pancreatic status, and CF-related diabetes (Kerem et al., 2014). As we did not identify any association between birth weight and P. aeruginosa colonization, we suspect that birth weight loses some of its predictive value once these environmental factors and disease phenotypes become more prevalent and influential on the patient’s FEV1%. For example, the average age of P. aeruginosa colonization in this study was 8.36 years. Since birth weight was not predictive of age of P. aeruginosa colonization, the onset of P. aeruginosa would influence FEV1% independently from birth weight. Thus, these findings concur with previous findings demonstrating that weight and weight gain in childhood is important for later FEV1% (Zemel et al., 2000). Since birth weight does not seem to influence pulmonary function in young adults with CF, nutritional support during early childhood remains a very important component of increasing FEV1%.
Recognition that infants with a diagnosis of CF and a birth weight at the lower end of normal are at risk for poorer pulmonary outcomes should prompt careful clinical follow-up for slowing growth parameters. At a minimum, health-care providers should perform serial measurements of head circumference, weight, and height at each well-baby/well-child visit, with recumbent length measured for the initial 24–36 months of age. Use of calibrated high-quality measuring equipment is essential, as is accurate plotting on appropriate growth curves. Providers can incorporate several strategies for monitoring growth into primary care settings, including use of customized algorithms in the electronic health record incorporating consensus guidelines for assessment of history and growth parameters, training of clinicians to accurately obtain anthropometric measures, and increased visit frequency if growth slows (Savant, Britton, Petren, McColley, & Gutierrez, 2014). Comprehensive care of children with CF in primary care settings requires knowledge of current recommendations for clinical management, which are listed in the Cystic Fibrosis Foundation’s Clinical Care Guidelines for Caregivers (www.cff.org/For-Caregivers/CF-Clinical-Care-Guidelines/).
The prenatal lack of CFTR is detrimental to infants with CF, as evidenced by the occurrence of meconium ileus and pancreatic destruction at birth in these infants. This study provides further support that lack of CFTR prenatally is detrimental, as lower birth weights correlated with poorer pulmonary function in later childhood in the patients with CF. These findings indicate that initiation of aggressive treatments earlier in childhood for patients with lower birth weights may be beneficial. Ivacaftor is a medication used for treating CF that corrects CFTR function in patients with specific mutations and is approved for patients as young as 2 years of age. While the feasibility and efficacy of correcting CFTR function in utero with such corrector or potentiator drugs remains to be seen, these data suggest that improvement in birth weight in infants with CF may be beneficial to their long-term overall health.
In conclusion, this study confirmed previous findings of decreased birth weights in infants with CF but also identified for the first time a correlation between birth weight and later childhood lung function. Given the limitations of this study, which include small sample size, self-report data, and range of birth cohorts, a similar study should be completed using the national CF patient registry. Unfortunately, the national CF registry currently contains very few birth weights (Zhang, Shoff, & Lai, 2015). However, with new electronic medical record systems in place that record such data as birth weight, such a study will be possible in the future. Finally, as the cause of low birth weight in CF is still unknown, additional research to identify the etiology is warranted. It is likely that increased understanding of prenatal growth deficiency could lead to more information about postnatal growth and development in CF and identify future therapeutic targets.
Acknowledgments
We thank the Clinical Studies Core in the Department of Pediatrics and Rainbow Babies and Children’s Hospital for assistance in patient data collection.
Authors’ Note: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contribution: Rebecca Darrah contributed to conception, design, data acquisition, data analysis, and interpretation; drafted and critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy. Rebecca Nelson contributed to conception, design, data acquisition, data analysis, and interpretation; drafted and critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy. Elizabeth G. Damato contributed to data analysis and interpretation, drafted and critically revised the manuscript, gave final approval, and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy. Michael Decker contributed to interpretation, critically revised the manuscript, gave final approval, and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy. Anne Matthews contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy. Craig A. Hodges contributed to conception, design, data acquisition, data analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work, ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Clinical Studies Core is supported by NIH P30 DK27651. Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under award no. P30NR015326. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Alexander G. R., Kogan M., Martin J., Papiernik E. (1998). What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clinical Obstetrics and Gynecology, 41, 114–125. [DOI] [PubMed] [Google Scholar]
- Barker D. J., Godfrey K. M., Fall C., Osmond C., Winter P. D., Shaheen S. O. (1991). Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. British Medical Journal, 303, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoy D., Pekkanen J., Elliott P., Pouta A., Laitinen J., Hartikainen A. L.…Jarvelin M. R. (2007). Early growth and adult respiratory function in men and women followed from the fetal period to adulthood. Thorax, 62, 396–402. doi:10.1136/thx.2006.066241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroccio A., Pardo F., Montalto G., Japichino L., Iacono G., Collura M., Notarbartolo A. (1988). Effectiveness of enteric-coated preparations on nutritional parameters in cystic fibrosis. A long-term study. Digestion, 41, 201–206. [DOI] [PubMed] [Google Scholar]
- Cazzato S., Ridolfi L., Bernardi F., Faldella G., Bertelli L. (2013). Lung function outcome at school age in very low birth weight children. Pediatric Pulmonology, 48, 830–837. doi:10.1002/ppul.22676 [DOI] [PubMed] [Google Scholar]
- Cheung Y. B., Karlberg J. P., Low L., Ip M. (2001). Birth weight and adult lung function in China. Thorax, 56, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey M., Edwards L., Levison H., Knowles M. (1997). Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. Journal of Pediatrics, 131, 809–814. [DOI] [PubMed] [Google Scholar]
- Davis B., Shennan D. B., Boyd C. A. (1985). Chloride transport in cystic fibrosis placenta. Lancet, 1, 392–393. [DOI] [PubMed] [Google Scholar]
- Faller D. P., Egan D. A., Ryan M. P. (1995). Evidence for location of the CFTR in human placental apical membrane vesicles. American Journal of Physiology, 269, C148−C155. [DOI] [PubMed] [Google Scholar]
- Festini F., Taccetti G., Repetto T., Reali M. F., Campana S., Mergni G.…de Martino M. (2005). Gestational and neonatal characteristics of children with cystic fibrosis: A cohort study. Journal of Pediatrics, 147, 316–320. doi:10.1016/j.jpeds.2005.04.031 [DOI] [PubMed] [Google Scholar]
- Forrester D. L., Knox A. J., Smyth A. R., Fogarty A. W. (2013). Measures of body habitus are associated with lung function in adults with cystic fibrosis: A population-based study. Journal of Cystic Fibrosis, 12, 284–289. doi:10.1016/j.jcf.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice L. C., de Zegher F., Gargosky S. E., Dsupin B. A., de las Fuentes L., Crystal R. A.…Rosenfeld R. G. (1995). Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. Journal of Clinical Endocrinology and Metabolism, 80, 1548–1555. doi:10.1210/jcem.80.5.7538146 [DOI] [PubMed] [Google Scholar]
- Groleau V., Schall J. I., Dougherty K. A., Latham N. E., Maqbool A., Mascarenhas M. R., Stallings V. A. (2014). Effect of a dietary intervention on growth and energy expenditure in children with cystic fibrosis. Journal of Cystic Fibrosis, 13, 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler G., Frisch H., Waldhor T., Gotz M. (1994). Perspectives of longitudinal growth in cystic fibrosis from birth to adult age. European Journal of Pediatrics, 153, 158–163. [DOI] [PubMed] [Google Scholar]
- Kerem E., Viviani L., Zolin A., MacNeill S., Hatziagorou E., Ellemunter H.…Group E. P. R. S. (2014). Factors associated with FEV1 decline in cystic fibrosis: Analysis of the ECFS patient registry. European Respiratory Journal, 43, 125–133. doi:10.1183/09031936.00166412 [DOI] [PubMed] [Google Scholar]
- Konstan M. W., Butler S. M., Wohl M. E., Stoddard M., Matousek R., Wagener J. S.…Morgan W. J. (2003). Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. Journal of Pediatrics, 142, 624–630. doi:10.1067/mpd.2003.152 [DOI] [PubMed] [Google Scholar]
- Laerum B. N., Svanes C., Gulsvik A., Iversen M., Thorarinsdottir H. R., Gislason T.…Omenaas E. (2004). Is birth weight related to lung function and asthma symptoms in Nordic-Baltic adults? Respiratory Medicine, 98, 611–618. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Corey M., FitzSimmons S., Kosorok M. R., Farrell P. M. (1999). Comparison of growth status of patients with cystic fibrosis between the United States and Canada. American Journal of Clinical Nutrition, 69, 531–538. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Kosorok M. R., Sondel S. A., Chen S. T., FitzSimmons S. C., Green C. G.…Farrell P. M. (1998). Growth status in children with cystic fibrosis based on the National Cystic Fibrosis Patient Registry data: Evaluation of various criteria used to identify malnutrition. Journal of Pediatrics, 132, 478–485. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Ebrahim S., Davey Smith G. (2005). Association of birth weight with adult lung function: Findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax, 60, 851–858. doi:10.1136/thx.2005.042408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou T. G., Adler F. R., Fitzsimmons S. C., Cahill B. C., Hibbs J. R., Marshall B. C. (2001). Predictive 5-year survivorship model of cystic fibrosis. American Journal of Epidemiology, 153, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lista G., Castoldi F., Bianchi S., Lupo E., Cavigioli F., Farolfi A.…Ferrerio E. (2014). Lung function and respiratory health at school age in ventilated very low birth weight infants. Indian Journal of Pediatrics, 81, 275–278. doi:10.1007/s12098-013-1129-1 [DOI] [PubMed] [Google Scholar]
- Marcus M. S., Sondel S. A., Farrell P. M., Laxova A., Carey P. M., Langhough R., Mischler E. H. (1991). Nutritional status of infants with cystic fibrosis associated with early diagnosis and intervention. American Journal of Clinical Nutrition, 54, 578–585. [DOI] [PubMed] [Google Scholar]
- Matthes J. W., Lewis P. A., Davies D. P., Bethel J. A. (1995). Birth weight at term and lung function in adolescence: No evidence for a programmed effect. Archives of Disease in Childhood, 73, 231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A. E., Thamm B., Lietz T., Handrick W., Walter S. (1999). Cystic fibrosis: A cause of reduced birth weight? European Journal of Pediatrics, 158, 264. [DOI] [PubMed] [Google Scholar]
- Mylona P., Glazier J. D., Greenwood S. L., Sides M. K., Sibley C. P. (1996). Expression of the cystic fibrosis (CF) and multidrug resistance (MDR1) genes during development and differentiation in the human placenta. Molecular Human Reproduction, 2, 693–698. [DOI] [PubMed] [Google Scholar]
- Olsen I. E., Groveman S. A., Lawson M. L., Clark R. H., Zemel B. S. (2010). New intrauterine growth curves based on United States data. Pediatrics, 125, e214−e224. doi:10.1542/peds.2009-0913 [DOI] [PubMed] [Google Scholar]
- Pei L., Chen G., Mi J., Zhang T., Song X., Chen J.…Zheng X. (2010). Low birth weight and lung function in adulthood: Retrospective cohort study in China, 1948-1996. Pediatrics, 125, e899−e905. doi:10.1542/peds.2008-3086 [DOI] [PubMed] [Google Scholar]
- Ramsey B. W., Dorkin H. L., Eisenberg J. D., Gibson R. L., Harwood I. R., Kravitz R. M.…Smith A. L. (1993). Efficacy of aerosolized tobramycin in patients with cystic fibrosis. New England Journal of Medicine, 328, 1740–1746. doi:10.1056/NEJM199306173282403 [DOI] [PubMed] [Google Scholar]
- Rogan M. P., Reznikov L. R., Pezzulo A. A., Gansemer N. D., Samuel M., Prather R. S.…Stoltz D. A. (2010). Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proceedings of the National Academy of Sciences, USA, 107, 20571–20575. doi:10.1073/pnas.1015281107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage E., Beirne P. V., Ni Chroinin M., Duff A., Fitzgerald T., Farrell D. (2014). Self-management education for cystic fibrosis. Cochrane Database of Systematic Reviews, 9, CD007641 doi:10.1002/14651858.CD007641.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savant A. P., Britton L. J., Petren K., McColley S. A., Gutierrez H. H. (2014). Sustained improvement in nutritional outcomes at two paediatric cystic fibrosis centres after quality improvement collaboratives. BMJ Quality and Safety, 23, i81−i89. doi:10.1136/bmjqs-2013-002314 [DOI] [PubMed] [Google Scholar]
- Schluchter M. D., Konstan M. W., Davis P. B. (2002). Jointly modelling the relationship between survival and pulmonary function in cystic fibrosis patients. Statistic in Medicine, 21, 1271–1287. doi:10.1002/sim.1104 [DOI] [PubMed] [Google Scholar]
- Schluchter M. D., Konstan M. W., Drumm M. L., Yankaskas J. R., Knowles M. R. (2006). Classifying severity of cystic fibrosis lung disease using longitudinal pulmonary function data. American Journal of Respiratory Critical Care Medicine, 174, 780–786. doi:10.1164/rccm.200512-1919OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen S. O., Sterne J. A., Tucker J. S., Florey C. D. (1998). Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax, 53, 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. E., Kumaran K., Fall C. H., Shaheen S. O., Osmond C., Barker D. J. (1997). Relation of fetal growth to adult lung function in south India. Thorax, 52, 895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkamp G., Wiedemann B. (2002). Relationship between nutritional status and lung function in cystic fibrosis: Cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax, 57, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson A. L., Mannik L. A., Walsh S., Brotherwood M., Robert R., Darling P. B.…Stanojevic S. (2013). Longitudinal trends in nutritional status and the relation between lung function and BMI in cystic fibrosis: A population-based cohort study. American Journal of Clinical Nutrition, 97, 872–877. doi:10.3945/ajcn.112.051409 [DOI] [PubMed] [Google Scholar]
- Vaisman N., Pencharz P. B., Corey M., Canny G. J., Hahn E. (1987). Energy expenditure of patients with cystic fibrosis. Journal of Pediatrics, 111, 496–500. [DOI] [PubMed] [Google Scholar]
- Yen E. H., Quinton H., Borowitz D. (2013). Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. Journal of Pediatrics, 162, 530–535. doi:10.1016/j.jpeds.2012.08.040 [DOI] [PubMed] [Google Scholar]
- Zemel B. S., Jawad A. F., FitzSimmons S., Stallings V. A. (2000). Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: Analysis of the Cystic Fibrosis Foundation National CF Patient Registry. Journal of Pediatrics, 137, 374–380. doi:10.1067/mpd.2000.107891 [DOI] [PubMed] [Google Scholar]
- Zemel B. S., Kawchak D. A., Cnaan A., Zhao H., Scanlin T. F., Stallings V. A. (1996). Prospective evaluation of resting energy expenditure, nutritional status, pulmonary function, and genotype in children with cystic fibrosis. Pediatric Research, 40, 578–586. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Shoff S. M., Lai H. J. (2015). Comparing the use of Centers for Disease Control and Prevention and World Health Organization growth charts in children with cystic fibrosis through 2 years of age. Journal of Pediatrics, 167, 1089–1095. doi:10.1016/j.jpeds.2015.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]