Abstract
Aims:
To describe correlations and agreement between salivary and serum B-type natriuretic peptide (BNP), C-reactive protein (CRP), interleukin (IL)-6, and IL-10 and determine which biomarkers predict worse functional class in patients with heart failure (HF).
Methods:
Serum and saliva were collected from 75 hospitalized patients with HF (57 ± 12 years, 43% female, New York Heart Association [NYHA] Classes I [4%], II [43%], and III [53%]). Oral inflammation was rated as good, fair, or poor. Spearman’s ρ and Bland–Altman were used to determine correlations and agreement of the salivary and serum forms of each biomarker. Logistic regressions were used to determine which biomarkers predicted worse NYHA functional class, controlling for depression, body mass index, smoking, and oral inflammation.
Results:
Median biomarker concentrations were as follows: BNP (serum 361 pg/ml, saliva 9 pg/ml), CRP (serum 13 ng/ml, saliva 25.6 ng/ml), IL-6 (serum 19.3 pg/ml, saliva 10.5 pg/ml), and IL-10 (serum 64.1 pg/ml, saliva 4.7 pg/ml). There was a moderate-to-strong correlation for serum–salivary CRP, weak correlation for serum–salivary IL-6, and no correlations for serum–salivary BNP and IL-10. The Bland–Altman test showed good salivary–serum agreement for all biomarkers, but as serum concentrations rose, salivary measures underestimated serum levels. Visible oral inflammation was the only predictor of worse NYHA class.
Keywords: cytokines, inflammatory biomarkers, B-type natriuretic peptide, periodontal disease
A number of serum biomarkers are strongly associated with prognosis and disease severity in patients with heart failure (HF). In a review article published in 2010, Bozkurt, Mann, and Deswal described a large body of evidence showing that inflammatory biomarker levels are elevated at early phases of HF (such as in New York Heart Association [NYHA] functional Class II) and continue to rise with worsening NYHA functional class. Although researchers have not been able to determine whether this is a causal or correlational relationship, evidence suggests that inflammation plays a role in the progression of HF disease severity.
Biomarkers strongly associated with HF severity or prognosis such as C-reactive protein (CRP; Tang et al., 2008), B-type natriuretic peptide (BNP; Sachedva, Horwich, & Fonarow, 2010), interleukin (IL)-6 (Jug et al., 2009), and IL-10 (Yamaoka, Yamaguchi, Okuyama, & Tomoike, 1999) have traditionally been measured in serum specimens. In working with an interdisciplinary team that included oral health researchers, we became aware that saliva represents a convenient source of biomarkers that can be collected noninvasively. Moreover, in the setting of clinical research in patients’ homes, patients could collect saliva samples, themselves, for later analysis by point-of-care devices, reducing the inconvenience and discomfort associated with the collection of serial serum samples for biomarker analysis. Consequently, we felt that salivary biomarkers could be a valuable tool for both clinicians and researchers working with patients with HF.
Although prior studies have reported that saliva can be used to measure biomarkers in patients with cardiovascular disease (CVD) and acute myocardial infarction (Christodoulides et al., 2005; Jones et al., 1994; Miller et al., 2010; Mirzaii-Dizgah & Jafari-Sabet, 2011), only one study of HF patients has examined salivary biomarkers. In a study comparing 40 outpatients with HF to 45 healthy controls, Offenbacher and Beck (2014) found that salivary N-terminal pro-BNP (NT-proBNP) had 82.2% sensitivity and 100% specificity for the presence of HF. However, there was a poor correlation between serum and salivary concentrations. The study was limited by its focus on a single biomarker and by the sole use of correlations to evaluate serum–salivary biomarker agreement.
Researchers frequently use correlations to determine whether a new measure agrees with an old measure, but this method can be statistically inappropriate. In 1986, Bland and Altman published a landmark paper on the best ways to compare two methods of clinical measurement. The authors pointed out that two measures can be highly correlated but in poor agreement. They provided an example of how one test estimated the gestational age of a baby to be 35 weeks, and another estimated the gestational age to be anywhere from 34 weeks to 40 weeks; these measures were statistically highly correlated but did not agree with each other when plotted on a graph. Since the publication of Bland and Altman’s original paper, researchers have been using their method to determine agreement between biomarkers (Bland & Altman, 1999). To conduct a Bland–Altman test with measures from saliva and serum, the researcher would calculate the mean difference of all the serum–salivary pairs (i.e., accuracy), the standard deviation (SD) of the difference (precision), and the mean difference ± 2 SDs (the confidence intervals [CIs] or limits of agreement). The mean value of each serum–salivary pair and the mean difference of each pair are plotted on a scatterplot. Agreement between the serum and salivary measures is determined by the percentage of pairs that fall within the predefined CIs (Bland & Altman, 1986).
In addition to calculating agreement as well as correlation when doing research using both salivary and serum markers of inflammation, researchers should take into account whether or not the participants have periodontal disease. Periodontal disease, defined as an inflammatory process in the mouth, affects the soft and hard tissues that support the teeth and can range from early signs of gingivitis to gum destruction and tooth loss (American Academy of Periodontology, n.d.). Because periodontal disease has systemic inflammatory effects (Offenbacher & Beck, 2014), researchers should control for the presence of oral inflammation when studying the link between inflammatory biomarkers and HF functional class and disease severity. However, to our knowledge, only one group of researchers has ever studied the link between periodontal disease and HF. In 2004, Wood and Johnson (2004) conducted a cross-sectional analysis of National Health and Nutrition Examination Survey data and found that self-reported periodontal disease was an independent risk factor for the presence of HF.
Thus, the aims of our study were to (1) determine correlations between salivary and serum measures of BNP, CRP, IL-6, and IL-10; (2) use the Bland–Altman method to determine salivary–serum agreement for these measures; and (3) determine whether serum or salivary biomarkers of BNP, CPR, IL-6, or IL-10 are predictors of worse NYHA functional class in hospitalized patients with HF, after controlling for relevant comorbidities and levels of visible inflammation in the mouth.
Methods
Design and Sample
This investigation was an a priori analysis of baseline data from a randomized, controlled trial (N = 90) testing cognitive behavioral therapy for depressive symptoms in patients hospitalized with HF. The sample (N = 75) in this analysis includes those subjects who had matched data on at least one set of salivary and serum biomarkers at baseline.
Patients were eligible for inclusion in the randomized trial if they had been admitted to the hospital with a primary or secondary diagnosis of preserved or nonpreserved systolic function HF and were 21 years of age or older. Patients were excluded for having coexisting terminal illness, end-stage HF (defined as the presence of left ventricular assist device, continuous inotropic infusions, or hospice care), cognitive impairment, active suicidality, history of psychotic or bipolar illness, or current alcohol dependence or other substance abuse or for being non-English speaking or having other communication barriers.
Protocol
The University of Kentucky Institutional Review Board approved the study, and all patients provided written informed consent. Enrollment began in 2011 and ended in 2013. Demographics, clinical characteristics, salivary and serum measures, and an oral assessment were collected at each patient’s bedside at one time point at baseline.
Measurement
Saliva collection
We used a standardized protocol for collection of unstimulated whole saliva based on the work of Navazesh (1993). We provided patients with a 20 ml vial that contained freeze-dried protease inhibitor. We asked them to refrain from eating, drinking, and brushing their teeth 1 hr prior to collection. At approximately 11 a.m., the research nurse instructed patients to expectorate into the vial until 5 ml had been collected. After collection, we froze saliva samples at −20°C for a minimum of 24 hr. We thawed samples slowly at 4°C, centrifuged them, and froze them at −80°C for long-term storage. We thawed salivary samples again in large batches for analysis, which was performed within 6 months of sample acquisition.
Measurement of salivary CRP, IL-6, and IL-10
We used a single-plex kit from Millipore to analyze salivary CRP and a six-plex kit from Millipore (Billerica, MA) to measure salivary IL-6 and IL-10. We ran undiluted samples for IL-6, IL-10, and CRP in duplicate according to the manufacturer’s protocol. The plates were read in a Luminex IS100 (Austin, TX). All data were generated from Milliplex Analyst software (version 5.1) (Vigene Inc., Carlisle, MA) using a 7-point standard calculating unknown values from a five-parameter logistic curve-fitting method. The intra-assay coefficient of variation (CV) average for the six-plex was less than 10% with an interassay value of less than 15%. The intra-assay CV value for CRP was 8% with an interassay CV of 17.5%.
Measurement of salivary BNP
We measured salivary BNP using the Beckman Coulter Access 2 Immunoanalyzer with Biosite Triage reagent, a method traditionally used to analyze serum specimens. We thawed, centrifuged, and pipetted the saliva samples into an instrument sample cup prior to analysis. We incubated the samples in a reaction vessel and added Lumi-Phos* 530 to the vessel. We measured light generated by the reaction with a luminometer and determined the amount of analyte in the sample from a stored, multipoint calibration curve.
Serum biomarkers
We collected venous blood samples at the same time as baseline saliva sample. A nurse or phlebotomist drew the blood using universal precautions. A purple-top was used to collect blood for BNP, and a red-top was used to collect blood for CRP, IL-6, and IL-10. Within 2 hr of the blood draw, we used a portion of the whole blood to measure BNP using the point-of-care Biosite Triage® (San Diego, Triage). We centrifuged, aliquoted, and froze the remaining blood at −80°C. Serum samples for CRP, IL-6, and IL-10 were analyzed in the same manner as salivary samples for these markers.
NYHA class
We used a structured interview to determine patients’ NYHA functional class, a subjective indicator of functional status (Mills & Haught, 1996). Based on the interview, we assigned patients to Class I (ordinary physical activity caused no symptoms of fatigue, dyspnea, angina, or palpitations), II (symptoms occurred with ordinary physical activity), III (symptoms occurred with less than ordinary physical activity), or IV (symptoms occurred at rest).
Oral inflammation
Prior to saliva collection, the research nurse examined the patient’s mouth with a penlight and gloved finger for signs and symptoms of oral inflammation. The nurse rated patients as good (no complaints, no obvious mucosal inflammation, no reported loose teeth or symptoms of disease), fair (may have complaints, localized areas of mucosal inflammation, areas of visible decay, no obvious tooth or gum infection), or poor (generalized areas of mucosal inflammation, multiple broken-down teeth, obvious tooth or gum infection, or reported loose teeth; Burke et al., 2003; Miller et al., 2014). For data analysis, we categorized patients’ degree of oral inflammation as good or fair/poor.
Demographics, clinical variables, and depressive symptoms
We collected demographic and clinical data by patient interview and chart review. We used the admission height and weight to calculate body mass index (BMI) and the Charlson Comorbidity Index (Charlson, Pompei, Ales, & MacKenzie, 1987; Katz, Chang, Sangha, Fossel, & Bates, 1996) to collect data on comorbidities by chart review and patient interview. Because depressive symptoms are related to functional status, we measured the presence of depressive symptoms using the Patient Health Questionnaire 9 (PHQ-9; Kroenke, Spitzer, & Williams, 2001). The PHQ-9 contains 9 items that are rated on a scale from 0 (not at all) to 3 (nearly every day). The instrument is scored by summing the item ratings. Total scores range from 0 to 27; higher scores indicate worse depressive symptoms. The PHQ has evidence of reliability and validity, and researchers have previously used it in patients with HF (Ackermann et al., 2005; Kroenke et al., 2001).
Data Analysis
We conducted data analysis using SPSS v.19 (SPSS Inc., Chicago). Due to the non-normality of the distribution of biomarkers, we conducted transformations using log (x + 5) or 1/(x + 5). Salivary CRP and IL-10 could not be transformed, so we categorized these biomarkers into dichotomous variables: Salivary CRP values were categorized as low (<51) or high (≥51), and salivary IL-10 levels were categorized as not detectable (<0.13) or detectable (≥0.13).
We used descriptive statistics to describe the sample characteristics, Spearman’s ρ correlations to determine correlations between serum and salivary biomarkers, and the Bland and Altman statistic method to assess serum–salivary agreement (Bland & Altman, 1986).
To determine whether salivary and serum biomarkers were an independent predictor of NYHA functional class, we conducted a series of logistic regressions with NYHA Class I/II or III as the dichotomous dependent variable and PHQ-9, smoking status, BMI, a single biomarker (either serum or salivary), and inflammation of the mouth entered as independent variables.
Results
Sample Characteristics
Of the 90 participants in the clinical trial, 75 had at least one pair of serum–salivary biomarkers at baseline and were included in data analysis. The average age of the participants was 57.4 ± 12.2 years, 43% were female, 20% were African American, 80% were Caucasian, and 53% had NYHA Class III HF; no patients had NYHA Class IV HF (Table 1). The most common comorbidities were diabetes, a self-reported history of depression, and prior myocardial infarction. The average BMI was 36.1 kg/m2 (±10.4). For oral inflammation assessments, 31% of patients were rated as good, 39% as fair, and 29.7% as poor.
Table 1.
Demographic and Clinical Characteristics of the Overall Sample.
| Characteristics | Overall Sample |
|---|---|
| % (n), Mean ± SD, or Median (25th Percentile, 75th Percentile) | |
| Female | 42.7% (32) |
| Age, years | 57.4 ± 12.2 |
| African American | 20% (15) |
| Body mass index, kg/m2 | 36.1 ± 10.4 |
| New York Heart Association functional class | |
| Class I | 4% (3) |
| Class II | 42.7% (32) |
| Class III | 53.3% (40) |
| Left ventricular ejection fraction | 36.2 ± 14.8 |
| Etiology of HF | |
| Ischemic | 40% (30) |
| Hypertensive | 18.7% (14) |
| Idiopathic | 13% (10) |
| Other | 28.3% (21) |
| Smoking status | |
| Never smoked | 48% (36) |
| Former smoker | 30.7% (23) |
| Current smoker | 17.3% (13) |
| Years since diagnosed with HF | 3 (1, 7.3) |
| Newly diagnosed with HF in the past year | 18.7% (14) |
| Comorbidities | |
| Prior myocardial infarction | 32% (24) |
| Atrial fibrillation | 21.3% (16) |
| CABG surgery | 20% (15) |
| Biventricular pacemaker | 12% (9) |
| Implanted cardiac defibrillator | 18.7% (14) |
| Prior stroke | 16% (12) |
| Chronic obstructive pulmonary disease | 28% (21) |
| Diabetes | 61.3% (46) |
| Self-reported history of depression | 40% (30) |
| Asthma | 9.3% (7) |
| Lupus or rheumatoid arthritis | 0% (0) |
| Home medications prior to hospital admission | |
| ACE inhibitor | 50.7% (38) |
| Angiotensin receptor blocker | 13.3% (10) |
| Beta-blocker | 69.3% (52) |
| Digoxin | 12% (9) |
| Diuretic | 66.7% (50) |
| Antidepressant | 20% (15) |
Note. N = 75. ACE = angiotensin converting enzyme; CABG = coronary artery bypass grafting; HF = heart failure.
Biomarker Concentrations
All patients had detectable salivary and serum CRP concentrations, with 28 of them having salivary CRP concentrations above the maximum level of detection (>51 ng/ml) and seven having serum CRP levels above >51 ng/ml; we coded these as 52 ng/ml. For IL-10, 18 patients had saliva levels below the level of detection (<0.13 pg/ml) and were coded as 0.12 pg/ml, while 0 patients had serum IL-10 levels below the level of detection. All of the salivary and serum measures for BNP and IL-6 fell within the detectable range. There were several high-end outliers for serum BNP (n = 2), salivary BNP (n = 3), salivary IL-6 (n = 2), serum IL-10 (n = 3), and salivary IL-10 (n = 3). For the remainder of data analysis, we recoded these outliers (all above the 95th percentile) to match the 95th percentile value. Table 2 contains a summary of descriptive data for salivary and serum analytes.
Table 2.
Analyte Levels.
| Biomarker | N | Minimum | Maximum | Median |
|---|---|---|---|---|
| Serum BNP (pg/ml) | 71 | 17 | 1,440 | 361 |
| Salivary BNP (pg/ml) | 62 | 1 | 335 | 9 |
| Serum CRP (ng/ml) | 62 | 0.89 | 51 | 12.97 |
| Salivary CRP (ng/ml) | 72 | 0.03 | 51 | 25.56 |
| Serum IL-6 (pg/ml) | 56 | 0.45 | 275 | 19.32 |
| Salivary IL-6 (pg/ml) | 72 | 0.12 | 251 | 10.49 |
| Serum IL-10 (pg/ml) | 56 | 8.18 | 574 | 64.07 |
| Salivary IL-10 (pg/ml) | 72 | 0.12 | 146 | 4.47 |
Note. BNP = b-type natriuretic peptide; CRP = c-reactive protein; IL = interleukin.
Correlations and Scatterplots
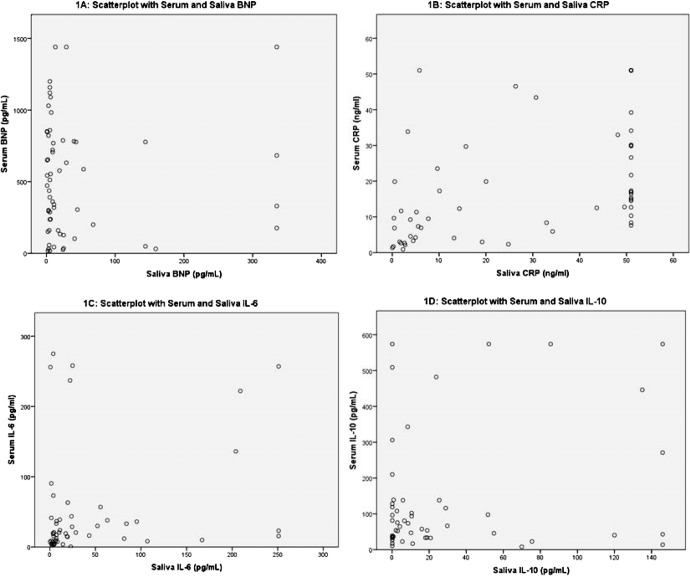
There was a moderate-to-strong correlation between serum and salivary CRP (rs = .594, p < .001), a weak correlation between serum and salivary IL-6 (rs = .288, p = .037), and no significant correlations between serum and salivary BNP (rs = −.064, p = .628) or serum and salivary IL-10 (rs = .068, p = .629). See Figure 1 for scatterplots comparing serum and salivary biomarker levels.
Figure 1.
Scatterplots of serum and salivary B-type natriuretic peptide, C-reactive protein, interleukin (IL)-6, and IL-10.
Results of Bland–Altman Testing
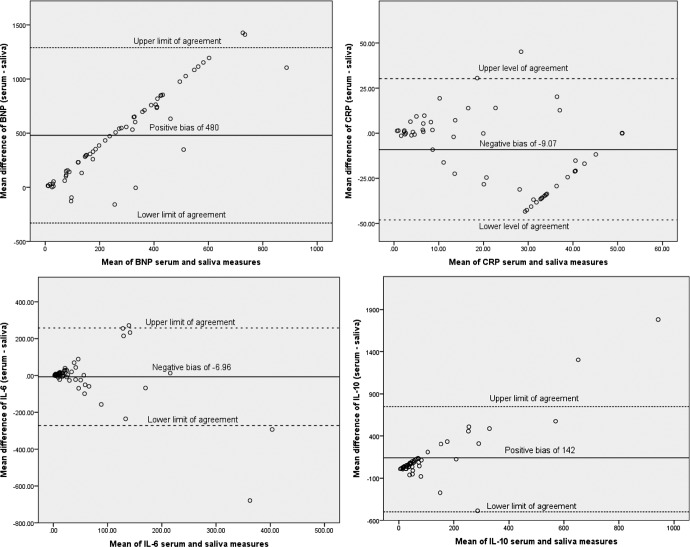
Figure 2 depicts the level of agreement between salivary and serum BNP, CRP, IL-6, and IL-10. For serum and salivary BNP, there was a large positive bias of 480 pg/ml, indicating that serum concentrations of BNP were much higher than the salivary concentrations (Figure 1). All but two mean differences fell within the limits of agreement. As serum BNP levels increased, the difference between the serum and saliva levels became larger. This indicates that saliva was an accurate measure of serum BNP at low concentrations but underestimated serum BNP when serum concentrations were elevated—a Type I measurement error.
Figure 2.
Bland–Altman tests for serum and salivary B-type natriuretic peptide, C-reactive protein, interleukin (IL)-6, and IL-10.
For CRP, there was a slight negative bias (−9.07 ng/ml), meaning that serum levels were lower than the corresponding salivary levels. Differences between serum and salivary measures increased as CRP levels increased, although on a lesser scale compared to the BNP measures. All but two outliers of the difference between the serum and saliva measures fell within the limits of agreement.
With regard to IL-6, there was a slight negative bias (−6.96 pg/ml), meaning that serum levels were lower than the corresponding salivary levels. Similar to the CRP results, differences between serum and salivary IL-6 measures increased as serum levels increased. All but three outliers of the mean differences between the serum and saliva measures fell within the limits of agreement.
For serum and salivary IL-10, there was a large positive bias (141.79 pg/ml), with serum levels much higher on average than salivary levels. As serum levels of IL-10 increased, salivary measures progressively underestimated the serum concentration. All but two of the differences between the serum and saliva measures fell within the limits of agreement.
Prediction of NYHA Class
In logistic regressions to determine whether any salivary or serum biomarkers were independent predictors of worse NYHA functional class (Table 3), none of the biomarkers were significant. The presence of oral inflammation was a significant predictor of worse NYHA functional class in six of the eight regression models, after controlling for depressive symptoms, smoking status, BMI, and individual salivary or serum biomarkers, with a trend toward significance in the other two models. Current smoking was associated with higher odds of worse NYHA functional class in only one model, with a trend toward significance in three additional models.
Table 3.
Logistic Regression Models to Predict Worse NYHA Functional Class.
| Regression Models | Serum Measures |
Salivary Measures |
||||||
|---|---|---|---|---|---|---|---|---|
| β | p Value | Exp (β) | 95% CI | β | p Value | Exp (β) | 95% CI | |
| CRP | ||||||||
| PHQ-9 | 0.096 | .209 | 1.100 | [0.948, 1.277 | 0.076 | .257 | 1.079 | [0.946, 1.229] |
| Smoking | –2.061 | .046* | 0.127 | [0.017, .960] | –1.676 | .084 | 0.187 | [0.028, 1.251] |
| BMI | 0.030 | .404 | 1.031 | [0.960, 1.107] | 0.051 | .157 | 1.052 | [0.981, 1.129] |
| Serum/salivarya CRP | 0.444 | .695 | 1.559 | [0.170, 14.322] | 0.193 | .756 | 1.213 | [0.359, 4.098] |
| Oral inflammation | 1.302 | .055 | 3.675 | [0.975, 13.852] | 1.417 | .024* | 4.123 | [1.207, 14.084] |
| IL-6 | ||||||||
| PHQ-9 | 0.029 | .705 | 1.029 | [0.886, 1.195] | 0.078 | .249 | 1.081 | [0.947, 1.235] |
| Smoking | –1.528 | .145 | 0.217 | [0.028, 1.696] | –1.651 | .077 | 0.192 | [0.031, 1.197] |
| BMI | 0.042 | .297 | 1.043 | [0.964, 1.129] | 0.049 | .167 | 1.051 | [0.980, 1.127] |
| Serum/salivarya IL-6 | 1.159 | .172 | 3.187 | [0.605, 16.790] | –3.931 | .498 | 0.020 | [0.000, 1,714.25] |
| Oral inflammation | 1.538 | .053 | 4.658 | [0.981, 22.119] | 1.385 | .027* | 3.993 | [1.169, 13.642] |
| IL-10 | ||||||||
| PHQ-9 | 0.050 | .494 | 1.051 | [0.912, 1.212] | 0.072 | .280 | 1.075 | [0.943, 1.225] |
| Smoking | –1.549 | .130 | 0.212 | [0.029, 1.581] | –1.816 | .054 | 0.163 | [0.026, 1.031] |
| BMI | 0.039 | .322 | 1.040 | [0.962, 1.124] | 0.051 | .154 | 1.052 | [0.981, 1.129] |
| Serum/salivarya IL-10 | 0.123 | .873 | 1.130 | [0.251, 5.083] | –0.426 | .483 | 0.653 | [0.199, 2.144] |
| Oral inflammation | 1.737 | .026* | 5.681 | [1.226, 26.319] | 1.468 | .021* | 4.342 | [1.243, 15.172] |
| BNP | ||||||||
| PHQ-9 | 0.046 | .482 | 1.047 | [0.921, 1.189] | 0.087 | .232 | 1.091 | [0.946, 1.257] |
| Smoking | –0.1771 | .057 | 0.170 | [0.027, 1.055] | –1.781 | .061 | 0.168 | [0.026, 1.086] |
| BMI | 0.048 | .185 | 1.049 | [0.977, 1.125] | 0.021 | .556 | 1.021 | [0.952, 1.096] |
| Serum/salivary BNP | –0.487 | .430 | 0.614 | [0.183, 2.059] | –6.371 | .341 | 0.002 | [0.000, 845.865] |
| Oral inflammation | 1.485 | .023* | 4.415 | [1.230, 15.852] | 1.456 | .030* | 4.288 | [1.155, 15.920] |
Note. BMI = body mass index; BNP = B-type natriuretic peptide; CRP = C-reactive protein; IL = interleukin; NYHA = New York Heart Association; PHQ-9 = Patient Health Questionnaire 9.
aSerum biomarker for the serum biomarker model and salivary biomarker for the salivary biomarker model.
*Significant independent predictor at p < .05.
Discussion
We expected to find that serum and/or salivary biomarkers would predict worse NYHA functional class in hospitalized patients with HF. Instead, we found that visible oral inflammation was a significant predictor of worse functional class. Although this result may seem surprising, our findings are consistent with a large body of evidence showing that periodontal disease is an independent predictor of CVD-related adverse events and mortality (DeStefano, Anda, Kahn, Williamson, & Russell, 1993; Offenbacher & Beck, 2014).
In the 1980s and 1990s, researchers began publishing evidence that periodontal disease was related to major CVD-related adverse events and mortality (DeStefano et al., 1993; Mattila et al., 1989). Eventually, other health conditions such as stroke, diabetes, preterm birth, and osteoporosis were also linked to periodontal disease (Offenbacher & Beck, 2014). Initially, researchers thought that the relationship between periodontal disease and CVD was due to shared risk factors. However, prospective studies controlling for risk factors demonstrated that periodontal disease was indeed an independent predictor for the development of CVD (Beck, Garcia, Heiss, Vokonas, & Offenbacher, 1996; DeStefano et al., 1993; Holmlund, Holm, & Lind, 2010). Oral bacteria can live throughout the body and have “stealth-like” properties that allow them to evade the immune system and stay in the body long term. Beck, Garcia, Heiss, Vokonas, and Offenbacher (1996) proposed that the relationship between periodontal disease and CVD was due to this systemic bacterial dissemination altering the blood vessels and triggering an acute phase response in the liver, leading to systemic inflammation. This persistent systemic inflammation then contributes to the development of CVD. Subsequent research findings supported this theory and added the knowledge that affected individuals may carry an underlying genetic risk for hyperinflammatory conditions (Offenbacher & Beck, 2014).
Although the link between periodontal disease and CVD has been well studied, to our knowledge, very little research has been conducted on the link between oral health and disease progression in patients with HF. Our findings suggest that researchers should conduct prospective studies on the link between oral health and HF morbidity. At the very least, we believe that our results demonstrate the importance of assessing and controlling for oral inflammation when measuring serum or salivary inflammatory biomarkers in this population. The protocol that we used to measure oral inflammation was simple and could be administered by a nurse or trained research assistant in less than a minute.
Our secondary findings were that BNP, CRP, IL-6, and IL-10 can be detected in the whole saliva of patients with HF and that the serum and salivary concentrations of CRP and IL-6 were correlated. Furthermore, in Bland–Altman tests, the majority of salivary concentrations fell within the limits of agreement, although we also found limitations of using the salivary measurements. In particular, salivary BNP and IL-10 displayed strong, systematic Type I errors and were more accurate at lower serum levels. Serum levels were also higher than salivary levels for BNP and IL-10. Therefore, if researchers use saliva to measure BNP and IL-10, they can expect that salivary results will be lower than serum results and that salivary values may underestimate high serum values.
Foo et al. (2012) reported that salivary NT-proBNP was 100% specific inidentifying patients with HF in comparison to healthy controls. Our study differed from Foo’s in that we did not have healthy controls in our study, we measured BNP rather than NT-proBNP, and we used the Bland–Altman test in addition to correlation testing. Both serum BNP and serum NT-proBNP have demonstrated excellent sensitivity for HF, and these two measures have equivalent accuracy (Nayer, Aggarwal, & Galwanker, 2014). Our use of the Bland–Altman test gave more detailed information about the relationship between serum and salivary BNP than could be found using correlations alone. Although salivary BNP had a Type I measurement error, BNP was detectable in the saliva of all patients with HF in our study, and most salivary measures fell within the limits of agreement. Furthermore, there was a trend toward salivary BNP levels predicting worse functional class. Thus, we believe that future research with a larger sample of patients, including a sample of healthy controls, is needed to determine cutoff values of salivary BNP or salivary NT-proBNP that can independently predict HF presence and severity.
The scatterplots for CRP and IL-6 also displayed Type I measurement errors, though to a lesser extent than BNP and IL-10. Meanwhile, in contrast to BNP and IL-10, measures of CRP and IL-6 tended to be higher in saliva compared to serum. The higher levels of salivary CRP are not surprising given that two thirds of the sample (68.7%) had visible inflammation in the mouth, which has been shown to increase salivary levels of CRP and other inflammatory biomarkers (Al Moharib et al., 2014). On the other hand, in a systematic review, researchers pointed out that there have been conflicting results with regard to a link between salivary IL-6 and periodontal disease: Some have found higher rates of IL-6 with periodontal disease and others have not (Jaedicke, Preshaw, & Taylor, 2000).
The use of whole saliva to measure biomarkers is a promising method that, if accurate, could afford benefits for researchers, clinicians, and patients. Potential benefits include safety for the handler (Pfaffe, 2011), noninvasiveness, and ease of collection with no need for a phlebotomist (Foo et al., 2012). Saliva does not clot like blood (Mohamed, Campbell, Cooper-White, Dimeski, & Punyadeera, 2012; Punyadeera, 2011), so preserving the specimen is less complicated. Furthermore, if providers have difficulty obtaining a venous sample, the present research has shown that it is possible to measure several important biomarkers via the saliva. Finally, from a research perspective, the use of salivary measures rather than serum could improve feasibility and possibly retention for longitudinal studies. Assuming that the concentrations of biomarkers do not deteriorate in saliva over time, it might be feasible for patients to collect their own saliva samples and store them in a home freezer rather than having to go to a site for collection or requiring the research personnel to make repeated home visits for serial collections of serum samples.
However, despite these potential benefits and our finding that salivary measures of BNP, CRP, IL-6, and IL-10 are detectable in patients with HF, more research is needed. Questions that were not answered in this study include the following: Is there a cutoff of salivary levels that can predict future morbidity and mortality in patients with HF? Can interventions designed to reduce HF severity result in corresponding reductions in salivary biomarker concentrations, and are these measures appropriate indicators of improved prognosis? Can these biomarkers be detected through use of swab devices, an easier but more expensive method of collecting saliva? Does oral inflammation independently contribute to worsening functional status over time?
Limitations
Our study was limited by its cross-sectional design, small sample size, and the fact that some patients were not able to contribute salivary or serum samples. As mentioned in the methods, 15 of 90 patients in the clinical trial did not have matching serum–salivary samples, partially due to difficulty obtaining venous access. Furthermore, some patients with HF, who were undergoing intravenous diuresis, complained of a dry mouth and had difficulty providing 5 ml of saliva. However, we were still able to obtain matching saliva–serum samples from the majority (75 of 90) of patients in the larger study, indicating that whole saliva is a feasible collection method for most patients with HF. Finally, 28 patients had salivary CRP concentrations above the maximum level of detection, and we did not dilute and rerun those samples. Instead, we coded these samples as 52 ng/ml for data analysis, which likely underestimated the concentrations of salivary CRP.
Conclusions
Salivary CRP and IL-6 concentrations correlated with serum measures in patients with HF; however, only visible levels of oral inflammation, not biomarkers, predicted worse NYHA functional class. Maintaining and promoting oral health is an important component of nursing practice, and this study highlights the importance of assessing the mouth in patients with HF. Prospective studies are needed to explore the relationship between oral health and poor outcomes in patients with HF, as this may be an important target for screening and nursing intervention in this population.
Footnotes
Author Contributions: Rebecca L. Dekker contributed to conception, design, acquisition, analysis, and interpretation; drafted the manuscript critically; revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Terry A. Lennie contributed to conception, design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Debra K. Moser contributed to conception, design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Craig S. Miller contributed to conception, design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Jeffrey L. Ebersole contributed to conception, design, interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Misook L. Chung contributed to conception, design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Charles L. Campbell contributed to conception, design, acquisition; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Alison Bailey contributed to conception, design, acquisition; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Elizabeth G. Tovar contributed to conception, design, acquisition, and interpretation; drafted the manuscript critically; revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health, National Institute of Nursing Research (K23 NR013480 to RLD); the University of Kentucky College of Nursing Center for Biobehavioral Research on Self-Management (NINR, P20 NR 010679 to DKM, TAL, MLC, RLD, EGT); and a University of Kentucky Faculty Research Support Grant (EGT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
References
- Ackermann R. T., Rosenman M. B., Downs S. M., Holmes A. M., Katz B. P., Li J.…Inui T. S. (2005). Telephonic case-finding of major depression in a Medicaid chronic disease management program for diabetes and heart failure. General Hospital Psychiatry, 27, 338–343. [DOI] [PubMed] [Google Scholar]
- Al Moharib H. S., Al Mubarak A., Al Rowis R., Geevarghese A., Preethanath R. S., Anil S. (2014). Oral fluid based biomarkers in periodontal disease: Part 1. Saliva. Journal of International Oral Health, 6, 95–103. [PMC free article] [PubMed] [Google Scholar]
- American Academy of Periodontology. (n.d.). Periodontal disease fact sheet. Retrieved from http://www.perio.org/newsroom/periodontal-disease-fact-sheet.
- Beck J., Garcia R., Heiss G., Vokonas P. S., Offenbacher S. (1996). Periodontal disease and cardiovascular disease. Journal of Periodontology, 67, 1123–1137. [DOI] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. (1986). Statistical methods for assessing agreement between two methods of clinical measurement. Lancet, 1, 307–310. [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. (1999). Measuring agreement in method comparison studies. Statistical Methods in Medical Research, 8, 135–160. [DOI] [PubMed] [Google Scholar]
- Bozkurt B., Mann D. L., Deswal A. (2010). Biomarkers of inflammation in heart failure. Heart Failure Reviews, 15, 331–341. [DOI] [PubMed] [Google Scholar]
- Burke F. J., Busby M., McHugh S., Delargy S., Mullins A., Matthews R. (2003). Evaluation of an oral health scoring system by dentists in general dental practice. British Dental Journal, 194, 215–218. [DOI] [PubMed] [Google Scholar]
- Charlson M. E., Pompei P., Ales K. L., MacKenzie C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40, 373–383. [DOI] [PubMed] [Google Scholar]
- Christodoulides N., Mohanty S., Miller C. S., Langub M. C., Floriano P. N., Dharshan P.…Anslyn E. (2005). Application of microchip assay system for the measurement of C-reactive protein in human saliva. Lab on a Chip, 5, 261–269. [DOI] [PubMed] [Google Scholar]
- DeStefano F., Anda R. F., Kahn H. S., Williamson D. F., Russell C. M. (1993). Dental disease and risk of coronary heart disease and mortality. British Medical Journal, 306, 688–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo J. Y., Wan Y., Kostner K., Arivalagan A., Atherton J., Cooper-White J.…Punyadeera C. (2012). NT-ProBNP levels in saliva and its clinical relevance to heart failure. PLoS ONE, 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmlund A., Holm G., Lind L. (2010). Number of teeth as a predictor of cardiovascular mortality in a cohort of 7,674 subjects followed for 12 years. Journal of Periodontology, 81, 870–876. [DOI] [PubMed] [Google Scholar]
- Jaedicke K. M., Preshaw P. M., Taylor J. (2000). Salivary cytokines as biomarkers of periodontal diseases. Periodontology, 70, 164–183. [DOI] [PubMed] [Google Scholar]
- Jones K., Reynolds S., Gray M., Hughes K., Rolf S., Davies B. (1994). Salivary PAF in acute myocardial infarction and angina: Changes during hospital treatment and relationship to cardiac enzymes. Thrombosis Research, 75, 503–511. [DOI] [PubMed] [Google Scholar]
- Jug B., Salobir B. G., Vene N., Sebestjen M., Sabovic M., Keber I. (2009). Interleukin-6 is a stronger prognostic predictor than high-sensitive C-reactive protein in patients with chronic stable heart failure. Heart and Vessels, 24, 271–276. [DOI] [PubMed] [Google Scholar]
- Katz J. N., Chang L. C., Sangha O., Fossel A. H., Bates D. W. (1996). Can comorbidity be measured by questionnaire rather than medical record review? Medical Care, 34, 73–84. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R. L., Williams J. B. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila K. J., Nieminen M. S., Valtonen V. V., Rasi V. P., Kesaniemi Y. A., Syrjala L.…Jokinen M. J. (1989). Association between dental health and acute myocardial infarction. British Medical Journal, 306, 688–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. S., Foley J. D., Campell C. L., Christodoulides N., Bhagwandin B., Redding S. W. (2010). Current developments in salivary diagnostics. Biomarkers in Medicine, 4, 171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. S., Foley J. D., 3rd, Floriano P. N., Christodoulides N., Ebersole J. L., Campbell C.…Kryscio R. J. (2014). Utility of salivary biomarkers for demonstrating acute myocardial infarction. Journal of Dental Research, 93, 72S–79S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R. M., Jr, Haught W. H. (1996). Evaluation of heart failure patients: Objective parameters to assess functional capacity. Clinical Cardiology, 19, 455–460. [DOI] [PubMed] [Google Scholar]
- Mirzaii-Dizgah I., Jafari-Sabet M. (2011). Unstimulated whole saliva creatine phosphokinase in acute myocardial infarction. Oral Diseases, 17, 597–600. [DOI] [PubMed] [Google Scholar]
- Mohamed R., Campbell J.-L., Cooper-White J., Dimeski G., Punyadeera C. (2012). The impact of saliva collection and processing methods on CRP, IgE, and myoglobin immunoassays. Clinical and Translational Medicine, 1, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazesh M. (1993). Methods for collecting saliva. Annals of the New York Academy of Sciences, 694, 72–77. [DOI] [PubMed] [Google Scholar]
- Nayer J., Aggarwal P., Galwankar S. (2014). Utility of point-of-care testing of natriuretic peptids (brain natriuretic peptide and n-terminal pro-brain natriuretic peptide) in the emergency department. International Journal of Critical Illness and Injury Science, 4, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S., Beck J. D. (2014). Commentary: Changing paradigms in the oral disease–systemic disease relationship. Journal of Periodontology, 85, 761–764. [DOI] [PubMed] [Google Scholar]
- Pfaffe T. (2011). Diagnostic potential of saliva: Current state and future applications. Clinical Chemistry, 57, 675–687. [DOI] [PubMed] [Google Scholar]
- Punyadeera C. (2011). One-step homogeneous C-reactive protein assay for saliva. Journal of Immunological Methods, 373, 19–25. [DOI] [PubMed] [Google Scholar]
- Sachdeva A., Horwich T. B., Fonarow G. C. (2010). Comparison of usefulness of each of five predictors of mortality and urgent transplantation in patients with advanced heart failure. American Journal of Cardiology, 106, 830–835. [DOI] [PubMed] [Google Scholar]
- Tang W. H., Shrestha K., Van Lente F., Troughton R. W., Martin M. G., Borowski A. G.…Klein A. L. (2008). Usefulness of C-reactive protein and left ventricular diastolic performance for prognosis in patients with left ventricular systolic heart failure. American Journal of Cardiology, 101, 370–373. [DOI] [PubMed] [Google Scholar]
- Wood N., Johnson R. B. (2004). The relationship between tomato intake and congestive heart failure risk in periodontitis subjects. Journal of Clinical Periodontology, 31, 574–580. [DOI] [PubMed] [Google Scholar]
- Yamaoka M., Yamaguchi S., Okuyama M., Tomoike H. (1999). Anti-inflammatory cytokine profile in human heart failure: Behavior of interleukin-10 in association with tumor necrosis factor-alpha. Japanese Circulation Journal, 63, 951–956. [DOI] [PubMed] [Google Scholar]