Abstract
Background:
The role of oxidative stress remains unclear in the multifactorial pathophysiologic mechanism of lung disease in preterm infants.
Aims:
The aim of this study was to examine the associations among chronic lung disease (CLD), oxidative stress, and oxygen requirements in preterm infants.
Design:
Prospective, longitudinal, and correlational design.
Subjects:
Preterm infants born at <32 weeks’ gestation (N = 31), median gestation of 29.0 weeks (range 24.9–31.7).
Measurements:
The diagnosis of CLD was obtained from the medical record. Oxidative stress was measured using 8-hydroxydeoxyguanosine (8-OHdG) in the cord blood at birth and urine on Days 1 and 7. Oxygen requirements were measured using fraction of inspired oxygen (FIO2) recorded in the first hour after birth/admission and the average FIO2 during the first 12 hr and 7 days after birth. Descriptive statistics are presented. Comparison analyses were performed using Kruskal–Wallis and Fisher’s exact tests.
Results:
Infants with CLD (n = 12) had lower gestational age (p = .04) and weight (p = .04) at birth, more days on the ventilator (p = .004), and longer neonatal intensive care unit stay (p = .04) compared to infants without CLD (n = 19). CLD was associated with lower oxidative stress levels (p = .03) and higher oxygen requirements during the first 12 hr (p = .025) and on Day 7 (p = .001). Lower oxidative stress levels on Day 7 were associated with higher oxygen requirements in the first 12 hr (p = .01) and on Day 7 (p = .03).
Conclusion:
Our results linking CLD and higher oxygen requirements with low oxidative stress contradict previous reports. Findings identify a gap in knowledge for postresuscitation oxygen therapy in preterm infants and expose the role of oxidative stress from inflammation and intermittent hypoxia in the etiology of CLD.
Keywords: preterm infant, oxidative stress, chronic lung disease
Neonatal respiratory distress syndrome (RDS) occurs in over half of preterm infants, with the incidence increasing to over 90% in infants born at <28 weeks’ gestation. Clinical management strategies to treat preterm infants diagnosed with RDS appear commonly in the literature. Discussions focus on balancing sufficient respiration with oxygen requirements while decreasing the risk of chronic lung disease (CLD), also referred to as bronchopulmonary dysplasia. Nearly half of the preterm infants diagnosed with RDS develop CLD and require oxygen therapy beyond 36 weeks’ corrected gestational age (CGA; Stoll et al., 2010). The etiology of CLD is multifactorial and is associated with oxidative stress mechanisms, dysregulated systemic inflammatory responses, genetics, infection, and lung injury/mechanical ventilation (McEvoy et al., 2014).
Oxidative stress, one immunologic mechanism of interest in the etiology of CLD, is defined as the irreversible damage caused by the presence of excessive reactive oxygen species and/or inadequate antioxidant defenses (Fellman & Raivio, 1997; Marseglia et al., 2014; Saugstad, 2010). Oxidative stress is frequently measured using biomarkers that detect levels of fragmented proteins (e.g., o-tyrosine), DNA (e.g., 8-hydroxydeoxyguanosine [8-OHdG]), and lipids (e.g., 8-isoprostane). Higher levels of these biomarkers suggest increased permanent damage associated with oxidative stress.
Several conditions expose preterm infants to oxidative stress mechanisms: (1) reperfusion injury and hyperoxia at birth (Fellman & Raivio, 1997; Marseglia et al., 2014; Saugstad, 2010), (2) increased oxygen requirements from hypoxia and increased use of oxygen to treat hypoxia from RDS after birth, and (3) inadequate antioxidant defenses and DNA repair mechanisms due to immaturity of the immune system (Perrone, Negro, Tataranno, & Buonocore, 2010; Vento et al., 2009). Previous studies in preterm infants have identified an association between oxidative stress and CLD (Joung et al., 2011; Saugstad, 2010; Vento et al., 2009). Specifically, preterm infants with CLD have shown significantly higher levels of 8-OHdG 7 days after birth compared to infants without CLD (Joung et al., 2011; Vento et al., 2009).
Our previous report looked at associations between feeding intolerance in preterm infants born at <32 weeks’ gestational age and an oxidative stress biomarker (8-OHdG). We measured biomarker levels at birth in the cord blood and on Days 1 and 7 in urine. The aim of this secondary analysis was to examine the associations among the diagnoses of RDS and CLD, 8-OHdG levels from the samples collected at birth and on Days 1 and 7 after birth, and oxygen requirements on Days 1 and 7 after birth. Based on the previous findings in the literature, we hypothesized that infants with lung disease (RDS and CLD) compared to infants without CLD would have (1) higher 8-OHdG levels in the cord blood at birth and in the urine on Days 1 and 7 and (2) higher oxygen requirement levels. Similarly, we hypothesized that infants with higher oxidative stress levels in the cord blood at birth and in the urine on Days 1 and 7 would have higher oxygen requirements in the first hour and first 12 hr after birth and on Day 7 after birth.
Method
Subjects/Setting
This study had a prospective, longitudinal, and correlational design. We received approval from the institutional review board affiliated with Nebraska Medicine. Eligible preterm infants were <32 weeks’ gestational age at birth in a Level-III neonatal intensive care unit (NICU) at a tertiary medical center in the Midwestern United States. We excluded infants with major congenital anomalies. We invited a parent of the eligible infant to participate within 24 hr after birth and, if the parent agreed, enrolled the infant in the study. Day of enrollment is defined as Day 1 of the study. More specific details of the methods appear in the published description of the parent study (Moore et al., 2013).
Unit Practice for Resuscitation and Oxygen Administration
Resuscitation practice in the NICU at the time of the study was consistent with the 2010 American Heart Association (AHA) guidelines (Kattwinkel et al., 2010). Infants of less than 32 weeks’ gestational age were given blended supplemental oxygen at 30% with adjustments as needed based on the use of pulse oximetry to target saturation levels to 85–95% in the delivery room, as described in Escrig et al. (2008). Supplemental oxygen administration following that provided in the delivery room was titrated to keep saturation levels between 85% and 95%. The NICU staff used oxygen saturation alarms at the infants’ bedside to maintain targeted levels.
Theoretical Rationale
The model of allostatic load in premature infants (Moore, Berger, & Wilson, 2014) served as the theoretical rationale for this study. Briefly, the theory posits that the general stress of prematurity induces a state of allostasis (physiologic adaptations during change). The infant’s physiologic response may be adaptive or maladaptive (dysregulation), with the latter eventually leading to allostatic load (damage caused by dysregulation) represented as complications of prematurity, specifically CLD in this study. This model is appropriate for the current study because it proposes a reciprocal association in preterm infants between CLD and the physiologic dysregulation of oxidative stress.
Measures
We obtained a number of demographic and medical variables from the infant’s medical record, including sex, gestational age at birth (weeks), race, premature rupture of membranes (no/yes), antenatal steroids (no/yes), birth weight (g), Apgar scores at 1 and 5 min, first recorded pH of the infant’s blood, number of days the infant had required ventilator support by Day 7, and the administration of surfactant therapy (no/yes).
Lung disease
We obtained status regarding RDS and CLD from the medical diagnosis coding at discharge in the infant’s medical record. The research team verified the diagnoses using progress notes and clinical data in the medical record. The definition of RDS this NICU used is consistent with the Vermont Oxford Network’s (2013) definition. The diagnosis of CLD was defined as the need for supplemental oxygen at 36 weeks’ CGA.
Oxidative stress
Oxidative stress was measured using plasma levels of the biomarker 8-OHdG collected from cord blood at birth and urinary levels collected on Days 1 and 7. We labeled all samples with the study ID and sample number and stored them at −80°C to await batch processing. Cayman Assay Services assayed all samples using the 8-hydroxy-2-deoxy Guanosine enzyme immune assay (EIA) kit (#589320; Cayman Chemical, Ann Arbor, MI). 8-OHdG assays are specific and sensitive methods of measurement that reflect the water-soluble fragments of cellular DNA believed to have been damaged by oxidative stress to cellular DNA believed to be caused from oxidative stress (Cooke, Evans, Herbert, & Lunec, 2000; Saito et al., 2000). We obtained extra cord blood collected at birth per hospital protocol to use for this study. Cord blood levels of 8-OHdG are expressed as nanogram/milliliter. We collected urine as a spot sample on Days 1 and 7 by placing a sterile cotton ball inside the infant’s diaper during daytime hours (08:00–20:00 hr). We obtained creatinine levels for each urine specimen in a 1:20 dilution based on a standard dilution test to normalize for variances in urinary output. Urinary 8-OHdG levels are expressed as a ratio of microgram per milligram creatinine. Further details of the assay appear in the published description of the parent study (Moore et al., 2013).
Oxygen requirements
We defined and calculated oxygen requirements as (a) the average fraction of inspired oxygen (FIO2) recorded in the first hour after admission to the NICU, (b) the average FIO2 recorded during the first 12 hr after birth (Hr 1–12), and (c) the average FIO2 recorded on Day 7 (24-hr average).
Data Analyses
Descriptive statistics and comparative analyses were conducted using SPSS, Version 22 (SPSS Inc., Chicago, IL) and SAS (SAS Institute Inc., Cary, NC). Lung diseases (RDS and CLD) were dichotomized into yes/no categories. The oxidative stress biomarker (8-OHdG) was distributed into interquartiles for analyses (lower 25%, middle 50%, and upper 25%). The median oxygen requirement was compared between lung disease and oxidative stress distributions. Nonparametric tests were used to compare the demographic and medical variables and associations between groups due to the small sample sizes in each group. Mann–Whitney U and Fisher’s exact tests were used to compare the infants without/with the diagnoses of RDS and CLD and oxidative stress distributions. Kruskal–Wallis tests were used to compare the oxidative stress distributions to median oxygen requirements. Pairwise deletions were used for missing data.
Results
A total of 31 infants born at <32 weeks’ gestation were enrolled in the study between September 2011 and July 2012, and 1 infant died on Day 8. Table 1 displays the demographic and medical variables for the entire sample and for infants without/with RDS and CLD. Within the first week of life, 90% (n = 28) of the 31 infants were diagnosed with RDS. We found no significant differences in demographic or medical variables between infants without and with RDS. Although the difference was not significant, infants with RDS had slightly higher oxygen requirements upon admission to the NICU (p = .08) and during the first 12 hr after birth (p = .07) compared to infants without RDS. At 36 weeks’ CGA, 40% (n = 12) of the 30 infants were diagnosed with CLD. Infants diagnosed with CLD had a significantly lower gestational age (p = .04) and weight (p = .04) at birth with more days on the ventilator (p = .004) by Day 7 and a longer length of stay (p = .04) compared to infants without CLD. Infants with CLD also had higher levels of FIO2 during the first 12 hr after birth (p = .025) and on Day 7 (p = .001) compared to infants without CLD.
Table 1.
Demographic and Medical Variables for Entire Sample, Infants Without/With Respiratory Distress Syndrome (RDS), and Infants Without/With Chronic Lung Disease (CLD).
| Variable | Sample | Without RDS | With RDS | Without CLD | With CLD |
|---|---|---|---|---|---|
| N = 31 | n = 3 | n = 28 | n = 18a | n = 12a | |
| Gender, female | 11 (35.5) | 1 (33.3) | 10 (35.7) | 8 (44.4) | 3 (25) |
| Race | |||||
| Caucasian | 26 (83.8) | 2 (66.7) | 24 (85.7) | 15 (83.3) | 10 (83.4) |
| African American | 3 (9.7) | 0 (0) | 3 (10.7) | 2 (11.1) | 1 (8.3) |
| Other | 2 (6.5) | 1 (33.3) | 1 (3.6) | 1 (5.6) | 1 (8.3) |
| Premature rupture of membranes | |||||
| No/unknown | 19 (61.3) | 1 (33.3) | 18 (64.3) | 12 (66.7) | 6 (50) |
| Yes | 12 (38.7) | 2 (66.7) | 10 (35.7) | 6 (33.3) | 6 (50) |
| Antenatal steroids‡ | |||||
| No/unknown | 1 (3.2) | 0 (0) | 1 (3.6) | 0 (0) | 1 (8.3) |
| Yes | 30 (96.8) | 3 (100) | 27 (96.4) | 18 (100) | 11 (91.7) |
| Gestational age at birth (weeks) | 29.0 ± 1.9 | 29.4 ± 4.0 | 29.0 ± 1.7 | 29.5 ± 1.9 | 28.2 ± 1.6* |
| Birth weight (g) | 1,192 ± 376 | 1,569 ± 863 | 1,152 ± 290 | 1,323 ± 397 | 1,022 ± 271* |
| Apgar score | |||||
| 1 min | 4.7 ± 2.3 | 6.7 ± 3.2 | 4.4 ± 2.2 | 5.0 ± 2.4 | 3.8 ± 2.0 |
| 5 min | 6.9 ± 1.4 | 7.7 ± 1.5 | 6.8 ± 1.4 | 6.8 ± 1.4 | 6.8 ± 1.5 |
| First pHb | 7.31 ± 0.07 | 7.29 ± 0.1 | 7.31 ± 0.1 | 7.31 ± 0.1 | 7.30 ± 0.1 |
| No. of days of ventilator support by Day 7 | 3.0 ± 3.0 | 2.7 ± 4.6 | 3.0 ± 2.9 | 1.6 ± 1.9 | 5.2 ± 3.2** |
| Length of stay (days) | 83.5 ± 28.7 | 83.0 ± 36.0 | 83.5 ± 28.6 | 73.9 ± 23.4 | 97.8 ± 30.9* |
| Surfactant | |||||
| No | 13 (41.9) | 2 (66.7) | 11 (39.3) | 8 (44.4) | 4 (33.3) |
| Yes | 18 (58.1) | 1 (33.3) | 17 (60.7) | 10 (55.6) | 8 (66.7) |
| FIO2 c | |||||
| Admission | 0.32 ± 0.13 | 0.23 ± 0.03 | 0.33 ± 0.13 | 0.32 ± 0.12 | 0.34 ± 0.15 |
| 12 hr | 0.26 ± 0.09 | 0.21 ± 0.01 | 0.27 ± 0.09 | 0.24 ± 0.03 | 0.30 ± 0.13* |
| Day 7 | 0.25 ± 0.06 | 0.21 ± 0.01 | 0.25 ± 0.06 | 0.23 ± 0.04 | 0.28 ± 0.07** |
Note. Values are provided as mean ± SD or n (%). Mann–Whitney U and Fisher’s exact tests were used for comparison between infants without/with RDS and infants without/with CLD. Pairwise deletions were used for missing data.
aTotal infants without/with CLD are 30 because of an infant passing prior to the diagnosis of CLD, which occurs at 36 weeks’ gestation. bFirst recorded pH in medical record from venous, capillary, and arterial samples. cThe average fraction of inspired oxygen (FIO2) recorded in the first hour after admission to the neonatal intensive care unit, the average FIO2 recorded during the first 12 hr after birth (Hr 1–12), and the average FIO2 recorded on Day 7 (24-hr average).
*p < .05 and **p < .01 between infants without/with CLD.
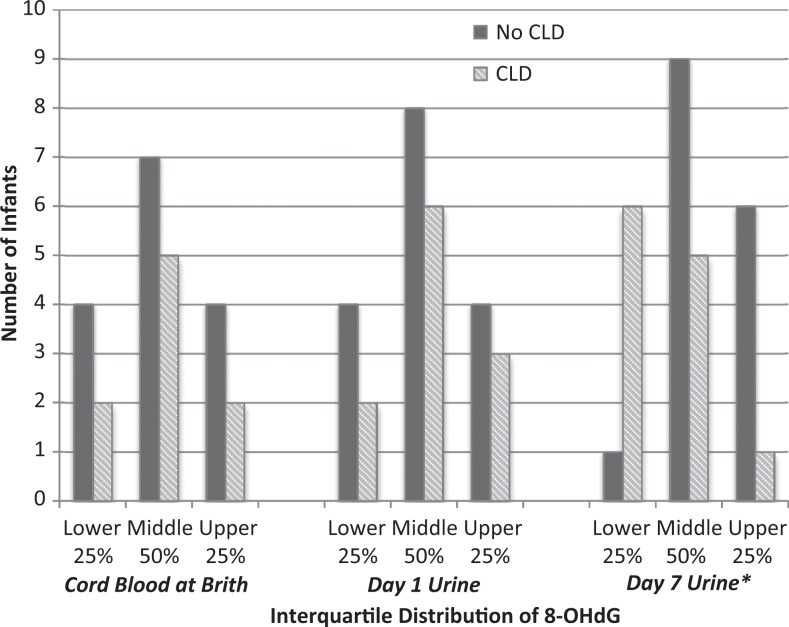
Of the 93 specimens possible for this analysis, 14% (13 of 93) were missing: six (6.5%) cord blood specimens were missing because the specimens were unavailable after birth and seven (6.5%) urine specimens were missing on Days 1 (n = 3) and 7 (n = 4) due to procedural errors or inadequate volume. Table 2 illustrates the levels and ranges of 8-OHdG. A diagnosis of CLD was associated with being in the lower quartile of urinary 8-OHdG levels on Day 7 (p = .03) as presented in Figure 1. Details regarding the association between the distribution of 8-OHdG levels and the oxygen requirements are shown in Table 2. The infants distributed in the lower quartile of urinary 8-OHdG levels on Day 7 required higher levels of FIO2 during the first 12 hr (p = .01) and on Day 7 (p = .03) as displayed in Figure 2.
Table 2.
Oxygen Requirements (FIO2) of Preterm Infants by Quartile of 8-OHdG Levels in Cord Blood at Birth and in Urine on Days 1 (12 hr) and 7.
| 8-OHdG-Level Quartile | FIO2 Admission Mean (SD) | FIO2 12 hr Mean (SD) | FIO2 Day 7 Mean (SD) |
|---|---|---|---|
| Cord blood (ng/ml) | |||
| Lower 25% (n = 6) | 0.35 (0.14) | 0.25 (0.04) | 0.25 (0.05) |
| Middle 50% (n = 13) | 0.33 (0.10) | 0.27 (0.12) | 0.26 (0.08) |
| Upper 25% (n = 6) | 0.34 (0.20) | 0.25 (0.04) | 0.23 (0.03) |
| p Value | .74 | .94 | .64 |
| Day 1 urine (μg/mg creatinine) | |||
| Lower 25% (n = 7) | 0.34 (0.19) | 0.25 (0.04) | 0.24 (0.05) |
| Middle 50% (n = 14) | 0.31 (0.12) | 0.27 (0.12) | 0.24 (0.04) |
| Upper 25% (n = 7) | 0.34 (1.0) | 0.27 (0.08) | 0.26 (0.09) |
| p Value | .59 | .78 | .87 |
| Day 7 urine (μg/mg creatinine) | |||
| Lower 25% (n = 7) | 0.32 (0.09) | 0.35 (0.16) | 0.27 (0.04) |
| Middle 50% (n = 14) | 0.32 (0.16) | 0.23/0.22 (0.03) | 0.24/0.22 (0.06) |
| Upper 25% (n = 7) | 0.34 (0.13) | 0.24 (0.02) | 0.24 (0.07) |
| p Value | .58 | .012* | .028* |
Note. Oxygen requirements were defined as fraction of inspired oxygen (FIO2) recorded in the first hour after admission to the neonatal intensive care unit, the average FIO2 required during the first 12 hr after admission, and the average FIO2 required on Day 7. Kruskal–Wallis tests were used to compare oxygen requirements between the 8-OHdG quartiles. Pairwise deletions were used for missing data. 8-OHdG = 8-hydroxydeoxyguanasine.
*p < .05 between quartiles.
Figure 1.
The incidence of chronic lung disease (CLD) in preterm infants per interquartile distributions of 8-hydroxydeoxyguanosine (8-OHdG) in the cord blood at birth and in the urine on Days 1 and 7. Pairwise deletions were used for missing data. Fisher’s exact tests were used to compare distributions between infants without/with CLD in the cord blood (p = 1.0), the urine on Day 1 (p = .89), and the urine on Day 7 (p = .03). *p < .05.
Figure 2.
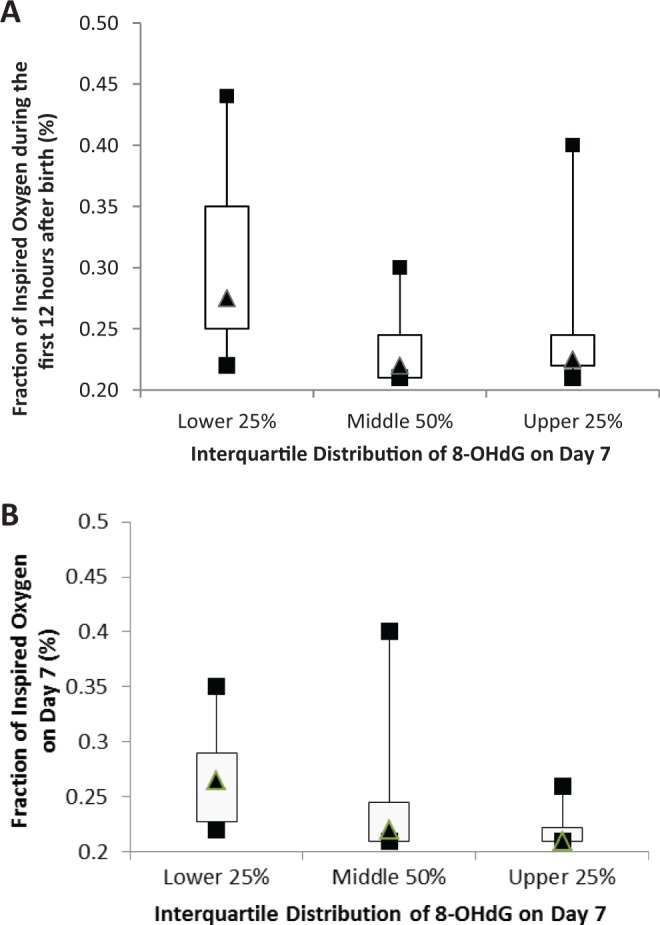
Descriptive statistics for the fraction of inspired oxygen (FIO2) infants required (A) during the first 12 hr after birth and (B) on Day 7 by interquartile distribution of 8-hydroxydeoxyguanosine (8-OHdG) levels on Day 7. Pairwise deletions were used for missing data. The infants in the lower quartile of urinary 8-OHdG levels on Day 7 required higher levels of FIO2 during the first 12 hr (p = .01) and on Day 7 (p = .03).
Discussion
In the present study, we explored possible associations among diagnosis of lung disease (RDS and CLD) in preterm infants, oxidative stress measured as 8-OHdG level, and oxygen requirements during the first 7 days after birth. We found no significant differences between infants without and with RDS in oxidative stress levels or oxygen requirements. Infants with CLD, however, were more likely to have a lower gestational age and weight at birth and to require more days on the ventilator than infants without CLD. The presence of a CLD diagnosis was also associated with decreased oxidative stress levels and increased oxygen requirements during the first week after birth. The infants with higher oxidative stress levels on Day 7 required lower levels of oxygen on Day 7. The findings linking higher levels of oxidative stress with both CLD and lower oxygen requirements were unexpected. Thus, we will focus this discussion on interpretation of the oxidative stress results.
Because hyperoxia is one of the mechanisms associated with oxidative stress, minimizing supplemental oxygen during resuscitation at birth is one change that practice researchers believed would decrease oxidative stress and thereby reduce the risk of CLD. In 2010, the AHA guidelines for cardiopulmonary resuscitation and emergency cardiovascular care during neonatal resuscitation suggested administering blended oxygen to obtain specific, targeted preductal saturations during resuscitation (Kattwinkel et al., 2010). Studies in preterm infants using resuscitation protocols consistent with the 2010 AHA guidelines have shown favorable results for reducing hyperoxia and oxidative stress (Kapadia et al., 2013; Rook et al., 2014).
We compared our results with those of two major reports published after the AHA guidelines were released that used oxidative stress biomarkers at similar collection times (Kapadia et al., 2013; Rook et al., 2014). The studies compared levels of an oxidative stress biomarker between a high-oxygen–initiated resuscitation group and a low-oxygen–initiated resuscitation group. In both studies, however, the high-oxygen–initiated and the low-oxygen–initiated groups were receiving similar FiO2 levels within minutes after birth. Kapadia et al. (2013) reported lower plasma oxidative stress levels at 1 hr after birth and less CLD in the group receiving low-oxygen–initiated resuscitation. Rook et al. (2014) did not find associations between urinary oxidative stress levels and oxygen resuscitation level on Day 1 or 6, and they did not report on CLD. Similar to Rook’s study, in the present study we did not find associations between urinary oxidative stress levels on Day 1 or 7, with oxygen requirements during the first hour after birth. However, we did find that infants distributed in the upper quartile of urinary oxidative stress levels on Day 7 were less likely to have CLD and had lower oxygen requirements on Days 1 and 7 compared to infants in the middle and lower quartiles.
Several differences in the methods we used versus those used in the above studies may explain the conflicting results. Both Rook et al. (2014) and Kapadia et al. (2013) compared oxidative stress levels with oxygen-initiated resuscitation protocol groups, whereas we looked at oxidative stress levels within one group of infants who received similar levels of oxygen-initiated resuscitation. Other major differences between Kapadia’s study and Rook’s and the present studies are that (1) Kapadia compared oxidative stress biomarker levels an hour after birth with CLD, while we compared the biomarker levels 7 days after birth with CLD; (2) Kapadia used an oxidative stress biomarker that measured lipid damage, whereas Rook’s study and our study used 8-OHdG, which is used to measure DNA damage; and (3) Kapadia used plasma samples, which are a better reflection of acute changes compared to the urinary samples used in Rook’s study and our study. Even with these variations in methods, the differences in findings may suggest that hyperoxia damage is specific to particular cellular sites. The differences also suggest that damage from hyperoxia during resuscitation is associated with an increase in plasma oxidative stress levels 1 hr after birth but that this effect may be short lived. These findings highlight a gap in the knowledge regarding the effects of postresuscitation oxygen delivery on oxidative stress levels and CLD.
Oxidative stress is associated, not only with reperfusion injury and hyperoxia as discussed earlier but also with inflammation (Marseglia et al., 2014; Saugstad, 2010). Scientists are beginning to understand the cyclical association between inflammation and oxidative stress in various adult lung diseases (Rawdin et al., 2013; Reuter, Gupta, Chaturvedi, & Aggarwal, 2010; Rosanna & Salvatore, 2012). An exaggerated inflammatory response in preterm infants has been implicated in the etiology of CLD (Aghai et al., 2010; Bose et al., 2011; Shima, Nishimaki, Nakajima, Kumasaka, & Migita, 2011; Stichel et al., 2011). Researchers have found increased levels of inflammatory markers during the first week after birth in the blood (Bose et al., 2011), urine (Shima et al., 2011), gastric fluid (Stichel et al., 2011), and tracheal aspirates (Aghai et al., 2010) of preterm infants who later develop CLD. Increased time exposed to mechanical ventilation also causes injury to and inflammation in the lungs of preterm infants (Jobe et al., 2008). We did not measure inflammatory biomarkers in the present study, but the infants who later developed CLD did require more days on the ventilator compared to infants who did not develop CLD. Our results support the role of lung injury and suggest the role of inflammation in the etiology of CLD. It remains unclear, however, how inflammation and oxidative stress interact to play a role in CLD. Further understanding of the cyclic association between inflammatory and oxidative stress mechanisms is essential to advance the science.
A major factor to consider when interpreting our results is that infants with CLD were born earlier compared to the infants without CLD. The prematurity of physiologic mechanisms and the fragility of immature organ systems may be major causes of dysregulation. Interpretation of the potential physiologic mechanisms for lower levels of 8-OHdG remains inconclusive based on the many confounding factors, including prematurity. The level of 8-OHdG in the blood or urine is dependent on the DNA repair enzymes recognizing and removing the oxidized lesions (Cooke et al., 2000). The infants with lower levels of 8-OHdG may not necessarily have had less oxidative stress compared to infants with higher levels of 8-OHdG. Instead, they may have had inadequate reparation of the DNA, which would equate to lower amounts of the oxidized lesion, or 8-OHdG, to be excised and secreted into the urine. This inadequate physiologic response may be directly related to an immature immune system. Therefore, our findings emphasize the association between oxidative stress and inflammation and de-emphasize the role of hyperoxia in the etiology of CLD.
The role of hypoxia/ischemia is another source of oxidative stress to consider in this population. One possible interpretation of lower levels of oxidative stress in our study is that these infants were under oxygenation even though the oxygen-initiated resuscitation followed the 2010 AHA resuscitation guidelines. Under oxygenation at birth may have initiated further oxidative stress from inflammation and hypoxia (Eltzschig & Carmeliet, 2011). Because infants in our study received resuscitation and postresuscitation practices similar to the low-oxygen–initiated resuscitation group in Kapadia et al.’s (2013) study, we are surprised that their results for CLD incidence within the low-oxygen–initiated resuscitation group were not more similar to ours. However, we realize that other factors including maternal infection, neonatal nutrition and diet, as well as genetic and epigenetic influences all play a role in this complication.
Another possible explanation for the inconsistency in findings between our study and previous studies may be related to physiologic conditions in utero. Authors have posited that inflammatory mechanisms associated with CLD begin before birth (Kunzmann, Collins, Kuypers, & Kramer, 2013; McEvoy et al., 2014). The presence of these mechanisms in utero would exacerbate the oxidative stress and inflammatory mechanisms associated with reperfusion, hypoxia, and hyperoxia in the neonatal period. Interestingly, we found no associations between the oxidative stress levels measured in the cord blood at birth and the incidence of CLD in the present study. However, we measured only one biomarker of oxidative stress in the cord blood. Future studies would provide better evidence for determining the effect of intrauterine oxidative stress and inflammation on CLD if researchers were to measure a comprehensive panel of oxidative stress biomarkers and inflammatory cytokines in the prenatal period and in the cord blood.
Finally, the disparity in findings might also be explained by the intermittent hypoxia that occurs postresuscitation. Research conducted since 2010 to determine the specific protocols for the use of blended oxygen percentages (i.e., 30% vs. 90%) and postresuscitation use of supplemental oxygen (i.e., maintaining saturations within a specific range) have shown mixed results, and, thus, these questions remain hot topics in neonatal care (Boost II, 2013; Cherian, Morris, Evans, & Kotecha, 2014; Finer et al., 2010; Gandhi, Rich, & Finer, 2013; Lim et al., 2014; Saugstad & Aune, 2014). The most effective saturation range during postresuscitation care for preterm infants remains unknown (Cherian et al., 2014), but evidence suggests that a higher range (91–95%) is preferred over a lower range (85–89%; BOOST II, 2013). Lim et al. (2014) examined the actual amount of time preterm infants (n = 45) spent within a predefined specific saturation range (88–92%). The authors found that the infants spent more time outside than inside the targeted range, concluding that they were exposed to excessive hyperoxic and hypoxic conditions. A similar finding in a review of several large studies comparing targeted ranges for postresuscitation oxygen therapy (BOOST II, 2013) exposed discrepancies in saturation monitoring. Regardless of the specific oxygen-saturation target range used, many preterm infants still encounter periods of oxygen titration because of the number of desaturations and saturation changes that commonly occur in this population. In adults with obstructive sleep apnea, oxidative stress from intermittent hypoxia is believed to play a significant role in poor outcomes (e.g., hypertension) from dysregulation of the inflammatory and sympathetic nervous systems (Kasai & Bradley, 2011; Makarenko et al., 2014). Infants exposed to fluctuating levels of oxygen saturation and supplemental oxygen may experience episodes of intermittent hypoxia similar to those caused by obstructive sleep apnea in adults that may increase inflammation. Over time, inflammatory and oxidative stress mechanisms within a preterm infant’s immature immune system are likely to become dysregulated, leading to an exacerbation of CLD pathophysiologic mechanisms.
A number of characteristics of the present study limit its comparability with the previous reports discussed above. Our study was not an interventional study and therefore was not blinded. It was also a smaller study with infants who had a higher mean gestational age at birth compared to the previous reports. Finally, because ours was a secondary analysis, we did not collect specific resuscitation data and strict oxygen supplementation protocols were not followed. Nevertheless, our findings add to the discussion regarding the general concept of oxidative stress in this population.
Our study has several limitations. Our sample and interquartile ranges were small and recruitment occurred only in one NICU. Our oxidative stress biomarker provided minimal, nonspecific data. The study lacked any additional corroborating or refuting analyses, such as other biomarkers of acute damage with no reparation, because we used only one marker. Measuring oxidative bases of DNA using an enzyme-linked immunosorbent assay (ELISA) kit instead of using a more accurate and reproducible method such as liquid chromatography has several limitations. These include decreased specificity and sensitivity, multiple interferences, and the possible effect of catabolism on urine concentrations of 8-OHdG. However, this assay is an affordable noninvasive alternative that investigators have used in previous studies. Because the entire sample received the same nutrition delivery protocols, variations in catabolism would likely originate from patient differences. The variability in timing of urine collection, especially on Day 1, may have impacted the results; however, the consent and the Day 1 urine were collected within 24 hr of delivery for all infants. We did not include other factors affecting oxidative stress and oxygenation in our data collection (e.g., inflammatory markers). As the aims of this secondary analysis were not the primary aim of the study, some comparisons may be underpowered.
Many questions remain regarding CLD and oxidative stress in preterm infants. The threshold for when high or low levels of oxidative stress cause irreversible damage to the immature lungs remains unclear and needs to be examined in terms of hyperoxia, hypoxia, and supplemental oxygen. Different methods of measuring oxidative stress, especially the use of biomarkers in blood versus in urine, need to be explored. For example, while plasma levels reflect more acute damage, urine levels may reflect damage over a period of hours, as seen with other stress biomarkers (Moore, Schmid, & French, 2015). Likewise, variability in oxygen therapy and intermittent hypoxia need to be examined to guide practice guidelines for postresuscitation care of RDS in the NICU. Further research is needed on the role of oxidative stress and inflammation occurring in utero, which could lead to preventive interventions during the prenatal period. Future research also should incorporate a comprehensive panel of plasma and urinary biomarkers of permanent damage, such as isofurans or isoprostanes; antioxidant panels, such as superoxide dismutase; and inflammatory cytokines. Such a panel would provide more specific information about effects. Translational research to identify interactions among RDS and CLD, specific free radicals, antioxidants, exaggerated inflammatory response, and supplemental oxygen will be essential to advance the science.
Findings from this study positively reflect the adoption of the 2010 AHA practice guidelines for cardiopulmonary resuscitation and emergency cardiovascular care during neonatal resuscitation to reduce hyperoxia and oxidative stress during resuscitation. The association that we found between low levels of oxidative stress and high oxygen requirements is contradictory to previous reports. Our findings identify a gap in knowledge for postresuscitation oxygen therapy in preterm infants and suggest a potential role for oxidative stress from inflammation and intermittent hypoxia in the etiology of CLD during the neonatal period.
Footnotes
Author Contribution: TAM contributed to conception and design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work, ensuring integrity and accuracy. KKS contributed to design, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. AAB contributed to design, acquisition, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. AMB contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Authors’ Note: Dr. Moore has participated sufficiently in the conception and design of this article to take public responsibility for it.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Moore is currently funded by NIH 1K01NR014474-01. Funding for the research project was provided by the University of Nebraska Medical Center Clinical Research Center, Foundation for Neonatal Research and Education, Sigma Theta Tau International—Gamma Pi Chapter.
References
- Aghai Z. H., Saslow J. G., Meniru C., Porter C., Eydelman R., Bhat V.…Bhandari V. (2010). High-mobility group box-1 protein in tracheal aspirates from premature infants: Relationship with bronchopulmonary dysplasia and steroid therapy. Journal of Perinatology, 30, 610–615. doi:10.1038/jp.2010.16 [DOI] [PubMed] [Google Scholar]
- BOOST II United Kingdom, Australia, and New Zealand Collaborative Groups. (2013). Oxygen saturation and outcomes in preterm infants. New England Journal of Medicine, 368, 2094–104. [DOI] [PubMed] [Google Scholar]
- Bose C., Laughon M., Allred E. N., Van Marter L. J., O’Shea T. M., Ehrenkranz R. A.…Leviton A. (2011). Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatric Research, 69, 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian S., Morris I., Evans J., Kotecha S. (2014). Oxygen therapy in preterm infants. Paediatric Respiratory Reviews, 15, 135–141. doi:10.1016/j.prrv.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Cooke M. S., Evans M. D., Herbert K. E., Lunec J. (2000). Urinary 8-oxo-2’-deoxyguanosine-source, significance and supplements. Free Radical Research, 32, 381–397. [DOI] [PubMed] [Google Scholar]
- Eltzschig H. K., Carmeliet P. (2011). Hypoxia and inflammation. New England Journal of Medicine, 364, 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrig R., Arruza L., Izquierdo I., Villar G., Sáenz P., Gimeno A.…Vento M. (2008). Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: A prospective, randomized trial. Pediatrics, 121, 875–881. [DOI] [PubMed] [Google Scholar]
- Fellman V., Raivio K. O. (1997). Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatric Research, 41, 599–606. doi:10.1203/00006450-199705000-00001 [DOI] [PubMed] [Google Scholar]
- Finer N., Saugstad O., Vento M., Barrington K., Davis P., Duara S.…Rich W. (2010). Use of oxygen for resuscitation of the extremely low birth weight infant. Pediatrics, 125, 389–391. doi:10.1542/peds.2009-1247 [DOI] [PubMed] [Google Scholar]
- Gandhi B., Rich W., Finer N. (2013). Achieving targeted pulse oximetry values in preterm infants in the delivery room. Journal of Pediatrics, 163, 412–415. doi:10.1016/j.jpeds.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Jobe A. H., Hillman N., Polglase G., Kramer B. W., Kallapur S., Pillow J. (2008). Injury and inflammation from resuscitation of the preterm infant. Neonatology, 94, 190–196. doi:10.1159/000143721 [DOI] [PubMed] [Google Scholar]
- Joung K. E., Kim H., Lee J., Shim G. H., Choi C. W., Kim E.…Choi J. (2011). Correlation of urinary inflammatory and oxidative stress markers in very low birth weight infants with subsequent development of bronchopulmonary dysplasia. Free Radical Research, 45, 1024–1032. [DOI] [PubMed] [Google Scholar]
- Kapadia V. S., Chalak L. F., Sparks J. E., Allen J. R., Savani R. C., Wyckoff M. H. (2013). Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics, 132, e1488–e1496. doi:10.1542/peds.2013-0978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T., Bradley T. D. (2011). Obstructive sleep apnea and heart failure: Pathophysiologic and therapeutic implications. Journal of the American College of Cardiology, 57, 119–127. doi:10.1016/j.jacc.2010.08.627 [DOI] [PubMed] [Google Scholar]
- Kattwinkel J., Perlman J. M., Aziz K., Colby C., Fairchild K., Gallagher J.…Zaichkin J. (2010). Neonatal resuscitation: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics, 126, e1400–e1413. [DOI] [PubMed] [Google Scholar]
- Kunzmann S., Collins J. J. P., Kuypers E., Kramer B. W. (2013). Thrown off balance: The effect of antenatal inflammation on the developing lung and immune system. American Journal of Obstetrics and Gynecology, 208, 429–437. doi:10.1016/j.ajog.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Lim K., Wheeler K. I., Gale T. J., Jackson H. D., Kihlstrand J. F., Sand C.…Dargaville P. A. (2014). Oxygen saturation targeting in preterm infants receiving continuous positive airway pressure. Journal of Pediatrics, 164, 730–736.e1 doi:10.1016/j.jpeds.2013.11.072 [DOI] [PubMed] [Google Scholar]
- Makarenko V. V., Usatyuk P. V., Yuan G., Lee M. M., Nanduri J., Natarajan V.…Prabhakar N. R. (2014). Intermittent hypoxia-induced endothelial barrier dysfunction requires ROS-dependent MAP kinase activation. American Journal of Physiology—Cell Physiology, 306, C745–C752. doi:10.1152/ajpcell.00313.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseglia L., D’Angelo G., Manti S., Arrigo T., Barberi I., Reiter R. J., Gitto E. (2014). Oxidative stress-mediated aging during fetal and perinatal periods. Oxidative Medicine and Cellular Longevity, 2014, 1–8. Retrieved from http://dx.doi.org/10.1155/2014/358375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy C. T., Jain L., Schmidt B., Abman S., Bancalari E., Aschner J. L. (2014). Bronchopulmonary dysplasia: NHLBI workshop on the primary prevention of chronic lung diseases. Annals of the American Thoracic Society, 11, S146–S153. doi:10.1513/AnnalsATS.201312-424LD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. A., Berger A. M., Wilson M. E. (2014). A new way of thinking about complications of prematurity. Biological Research for Nursing, 16, 72–82. doi:10.1177/1099800412461563 [DOI] [PubMed] [Google Scholar]
- Moore T. A., Schmid K. K., French J. A. (2015). Comparison of cortisol samples in the first two weeks of life in preterm infants. Journal of Pediatric Endocrinology and Metabolism, 28, 415–420. [DOI] [PubMed] [Google Scholar]
- Moore T. A., Wilson M. E., Schmid K. K., Anderson-Berry A., French J. A., Berger A. M. (2013). Relations between feeding intolerance and stress biomarkers in preterm infants. Journal of Pediatric Gastroenterology and Nutrition, 57, 356–362. doi:10.1097/MPG.0b013e3182953093 [DOI] [PubMed] [Google Scholar]
- Perrone S., Negro S., Tataranno M. L., Buonocore G. (2010). Oxidative stress and antioxidant strategies in newborns. Journal of Maternal-Fetal & Neonatal Medicine, 23, 63–65. doi:10.3109/14767058.2010.509940 [DOI] [PubMed] [Google Scholar]
- Rawdin B. J., Mellon S. H., Dhabhar F. S., Epel E. S., Puterman E., Su Y.…Wolkowitz O. M. (2013). Dysregulated relationship of inflammation and oxidative stress in major depression. Brain, Behavior, and Immunity, 31, 143–152. doi:10.1016/j.bbi.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S., Gupta S. C., Chaturvedi M. M., Aggarwal B. B. (2010). Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biology and Medicine, 49, 1603–1616. doi:10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook D., Schierbeek H., Vento M., Vlaardingerbroek H., van der Eijk A. C., Longini M.…Vermeulen M. J. (2014). Resuscitation of preterm infants with different inspired oxygen fractions. Journal of Pediatrics, 164, 1322–1326.e3 doi:10.1016/j.jpeds.2014.02.019 [DOI] [PubMed] [Google Scholar]
- Rosanna D. P., Salvatore C. (2012). Reactive oxygen species, inflammation, and lung diseases. Current Pharmaceutical Design, 18, 3889–3900. [DOI] [PubMed] [Google Scholar]
- Saito S., Yamauchi H., Hasui Y., Kurashige J., Ochi H., Yoshida K. (2000). Quantitative determination of urinary 8-hydroxydeoxyguanosine (8-OHdG) by using ELISA. Research Communications in Molecular Pathology and Pharmacology, 107, 39–44. [PubMed] [Google Scholar]
- Saugstad O. D. (2010). Oxygen and oxidative stress in bronchopulmonary dysplasia. Journal of Perinatal Medicine, 38, 571–577. [DOI] [PubMed] [Google Scholar]
- Saugstad O. D., Aune D. (2014). Optimal oxygenation of extremely low birth weight infants: A meta-analysis and systematic review of the oxygen saturation target studies. Neonatology, 105, 55–63. [DOI] [PubMed] [Google Scholar]
- Shima Y., Nishimaki S., Nakajima M., Kumasaka S., Migita M. (2011). Urinary β-2-microglobulin as an alternative marker for fetal inflammatory response and development of bronchopulmonary dysplasia in premature infants. Journal of Perinatology, 31, 330–334. doi:10.1038/jp.2010.129 [DOI] [PubMed] [Google Scholar]
- Stichel H., Bäckström E., Hafström O., Nilsson S., Lappalainen U., Bry K. (2011). Inflammatory cytokines in gastric fluid at birth and the development of bronchopulmonary dysplasia. Acta Paediatrica, 100, 1206–1212. doi:10.1111/j.1651-2227.2011.02286.x [DOI] [PubMed] [Google Scholar]
- Stoll B. J., Hansen N. I., Bell E. F., Shankaran S., Laptook A. R., Walsh M. C.…Higgins R. D. (2010). Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics, 126, 443–456. doi:10.1542/peds.2009-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento M., Moro M., Escrig R., Arruza L., Villar G., Izquierdo I.…Asensi M. A. (2009). Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics, 124, e439–449. [DOI] [PubMed] [Google Scholar]
- Vermont Oxford Network. (2013). 2014 manual of operations: Part 2: Data definitions & infant data forms (No. 18.0). Burlington, VT: Author. [Google Scholar]