Abstract
Background:
Glucose variations are common throughout sleep and wakefulness in people with type 1 diabetes mellitus (T1DM). The objective of this investigation was to characterize the time-varying coupling between glucose and unstructured physical activity over a 60-hr period in young adults with T1DM. The hypothesis was that coupling would differ during sleep versus wakefulness and would exhibit circadian variations.
Method:
Young adults with T1DM treated with an insulin pump participated in the study. Glucose variations were monitored with a continuous glucose monitoring system, and activity was assessed using an activity-monitoring band worn on the nondominant wrist. Simultaneous glucose and physical activity data across a continuous 60-hr period were used for analysis. Wavelet coherence analysis was employed to quantify the coupling between physical activity and glucose. Cosinor analysis was used to assess whether glucose/activity coherence exhibited significant circadian variations.
Results:
Participants comprised 23 adults, aged 18−30 years, with T1DM. Coherence analysis demonstrated substantial coupling between physical activity and glucose variations during both wakefulness and sleep. For rapid (10–30 min) fluctuations, mean coherence was higher during sleep than wakefulness (F = 10.86, p = .003). Rapid glucose variations consistently led to changes in activity (p = .001) during sleep but not during wake. Cosinor analysis revealed significant circadian modulation of glucose/activity coupling, especially for fluctuation periods 2–4 hr.
Conclusions:
Unstructured physical activity and glucose variations demonstrated strong time- and frequency-dependent coupling over a 60-hr period in young adults with T1DM, with sleep/wake differences and circadian modulation evident in this relationship.
Keywords: sleep, circadian, type 1 diabetes, glucose fluctuations
Glucose levels fluctuate widely through both the day and night in individuals with type 1 diabetes mellitus (T1DM). Prior research has shown that insulin sensitivity exhibits a circadian rhythm (Coomans et al., 2013) and that circadian-clock genes are active in a wide range of peripheral tissues (Summa & Turek, 2014). In particular, insulin sensitivity in people with T1DM is lowest during the second half of the night, requiring increased insulin doses (Trümper, Reschke, & Molling, 1995). Further, glucose homeostasis appears to differ between sleep and wake states, independent of the circadian influence (Van Cauter et al., 1991). Poor glucose homeostasis during sleep may contribute to sleep disruption, which, in turn, can negatively impact glucose control, even in healthy individuals (Van Cauter, 1997). However, there has been limited investigation into the circadian and sleep/wake influences on glucose homeostasis in individuals with T1DM.
Physical activity also contributes to glucose variations. Prior research has shown that structured physical activity influences glucose control in both healthy individuals (Houmard et al., 2004) and those with T1DM (Quirk, Blake, Tennyson, Randell, & Glazebrook, 2014). Routine (unstructured) daily activity also may contribute significantly to glucose variations. Routine daily activity also exhibits a sleep/wake pattern, with little movement in the sleep period and increased but variable activity during the waking hours. These variations in routine daily activity between sleep and wake may contribute significantly to state- or circadian-related changes in glucose, but research has not directly investigated this question. In the present study, we hypothesized that the strength of the relationship between glucose and routine activity is different between sleep and wake states and also exhibits a circadian pattern.
It is likely that the relationship between physical activity and glucose variations is bidirectional and changes over the course of a 24-hr day. For example, at times during the day, activity may influence glucose, but at other times glucose may influence activity. Research has shown that structured physical exercise results in changes in glucose levels in those with T1DM and that these changes can occur hours after the structured exercise occurs (Bally, Laimer, & Stettler, 2015). Conversely, research has also shown that rapid variations in glucose are associated with increased awakenings and associated physical activity in children with T1DM (Pillar et al., 2003). We hypothesized that the strength and nature of the relationship (coupling) between glucose and routine activity varies over the course of time and depends upon the speed of fluctuations in the values. To test our hypotheses, we carried out an investigation using wavelet coherence analysis (WCA) to describe the time-varying and frequency-specific relationship between unstructured physical activity and glucose in young adults with T1DM. WCA can be applied to two time series and permits the assessment of both time-varying and frequency-specific coupling (Grinsted, Moore, & Jevrejeva, 2004; Torrence & Compo, 1998).
The specific aims of this investigation were to (1) quantify the coupling between glucose variations and routine unstructured activity over a 60-hr period, (2) determine differences in this coupling between sleep and wakefulness, and (3) identify the strength and timing of circadian variations in glucose/activity coupling in young adults with T1DM. We utilized an observational longitudinal study design in the natural environment.
Method
Participants
We recruited participants from the Chicago metropolitan area through flyers posted in public locations and online and assessed eligibility for the study over the phone prior to the first visit. Young adults (N = 23) with T1DM who wore insulin pumps and did not work rotating or night shifts participated in this longitudinal study in the natural environment. In order to be eligible, participants had to have had diabetes for at least 5 years to ensure that endogenous insulin secretion was minimal or absent. We excluded night- or rotating-shift work, as such work schedules alter normal sleep and wake patterns and would confound interpretation of study findings. We also excluded individuals who were using prescription or nonprescription medication that might alter sleep or wakefulness, those with uncontrolled thyroid disease, and those who reported diabetes complications (the latter two of which may alter normal sleep and wake patterns). The institutional review board of the University of Illinois at Chicago approved all study procedures. We obtained written informed consent from all participants.
Procedure
We met participants in a private room in the college of nursing at University of Illinois at Chicago. After participants provided informed consent, we placed a subcutaneous abdominal sensor on each for the continuous glucose monitoring system (CGMS; Guardian-RT™, Medtronic) and applied an activity monitor (Actiwatch2™, Respironics) to each participant’s nondominant wrist. We performed a brief health history and physical exam and drew a blood sample to measure hemoglobin A1c (HbA1c), which we did not use as an exclusion criterion but rather as a clinically relevant biomarker of diabetes control (higher values indicate reduced glucose control). Participants also completed the Epworth Sleepiness Scale (ESS), a validated questionnaire to measure subjective sleepiness (Johns, 1991; Spira et al., 2011). Scores for the ESS range from 0 to 24, with scores ≥10 indicating clinically significant sleepiness.
Participants then wore the CGMS and actigraphy monitor for 3 days and nights in their natural environments. We analyzed glucose and activity data collected from 6 p.m. on the first day through 6 a.m. on the fourth day of the study. The CGMS recorded average interstitial glucose every 5 min. Prior research has shown that the system provides a reliable and valid estimate of glucose concentrations when compared with capillary blood glucose measurements (Gross & Mastrototaro, 2000). Additionally, we trained and instructed all participants to recalibrate the CGMS every 12 hr with a capillary blood glucose measurement. The activity monitor logged activity count totals every 30 s and smoothed (5-min moving average) and resampled them every 5 min to allow for alignment with glucose values. For missing data, we carried the last nonmissing value forward until the next nonmissing point (maximum cumulative missing data were 120 min [two subjects]). We determined the sleep using Actiware software Version 6.0 (Respironics) on the recommended medium threshold setting, which research has validated for objective measurement of sleep (Shin, Swan, & Chow, 2015, p. 2).
Statistical Methods
Wavelet coherence
WCA identifies time-varying and frequency-specific coupling between two processes (Grinsted et al., 2004). Wavelet theory has been reviewed elsewhere (Islam, 2011), but briefly, wavelets are time-limited mathematical functions useful for decomposing recorded physical activity and glucose waveforms into different frequency components and then computing the time-varying coherence between them at each underlying frequency. We used the Morlet wavelet function, performing computations with the wavelet coherence toolbox (Grinsted et al., 2004). This function yielded updated coherence values every 5 min for each of 84 underlying oscillations, with periods ranging from 10 to 1,248 min. For the present analyses, we excluded all coherence values potentially influenced by “end effects,” as described by Grinsted, Moore, and Jevrejeva (2004). To facilitate interpretation of the 60-hr recordings, we collapsed the 84 wavelet “scales” into six bands with differing period ranges (Band 1, 10–30 min; Band 2, 30−60 min; Band 3, 60−120 min; Band 4, 120−240 min; Band 5, 240−480 min; and Band 6, 480−960 min). We also segmented each recording according to sleep and wake periods, with each wake period being bisected into equal halves, yielding three approximately 8-hr segments for each circadian day. Due to their shorter length, we assessed each of these segments by WCA using only the first four bands (periods of 10−240 min). Because analysis of variance (ANOVA) demonstrated no significant differences (p > .05 for each) among the three sleep intervals or among the six wake subintervals, we averaged these intervals to provide separate single values for mean coherence and mean phase during sleep and during wake for each band in each subject.
To identify which computed coherence values were statistically significant (p < .05), we used Monte Carlo simulations (N = 500; Grinsted et al., 2004). We tabulated the mean coherence, number of intervals of significant coherence, and mean duration of these intervals for each band of each recording. To allow more intuitive interpretation, we converted the phase relationship between glucose and activity to an equivalent delay (in min) as a function of time in each band. We used ANOVA to identify differences in coherence parameters among the bands and between sleep/wake intervals using each of these factors as a repeated measure (STATA 14; StataCorp). We determined pairwise differences between bands and sleep/wake intervals by post hoc comparisons controlled by Scheffe’s test.
Circadian analysis
To characterize circadian variations in coherence, we used cosinor analysis. For each band of each recording, we fitted (least squares regression) a cosine wave with a period of 1,440 min (24 hr) to the coherence data as follows:
We report the average amplitude, acrophase (clock time of peak coherence), and Pearson correlation (r 2) to the raw coherence data of the best fit cosine wave for each band.
Results
Participants
A total of 25 young adults met inclusion criteria; two had incomplete glucose and actigraphy data and thus were not included in the final analysis. Table 1 provides detailed demographic and clinical characteristics of the 23 participants who completed the study. As highlighted in Table 1, participants’ mean HbA1c was slightly higher than the 7.0% target recommended by the American Diabetes Association, and participants did not report subjectively high levels of sleepiness. All participants wore their own insulin pumps (various manufacturers) during the study, and no changes were made to their usual insulin dosing regimens or diabetes care.
Table 1.
Summary of Demographic and Clinical Characteristics of Participants.
| Characteristic | Mean + SD or n (%) |
|---|---|
| Age (years) | 24 ± 4.0 |
| Sex, male | 9 (39.1) |
| Current smoker, yes | 1 (4.35) |
| Body mass index (kg/m2) | 25.9 ± 3.6 |
| HbA1c %; mmol/mol | 7.6 ± 1.0; 60 ± 10.9 |
| Diabetes duration (years) | 12.2 ± 4.9 |
| ESS | 4.5 ± 2.7 |
Note. N = 23. ESS = Epworth Sleepiness Scale; HbA1c = hemoglobin A1c.
Time-Varying Characteristics of Coupling Between Glucose and Activity Over the 60-hr Period
Mean coherence by fluctuation band for 60-hr recording
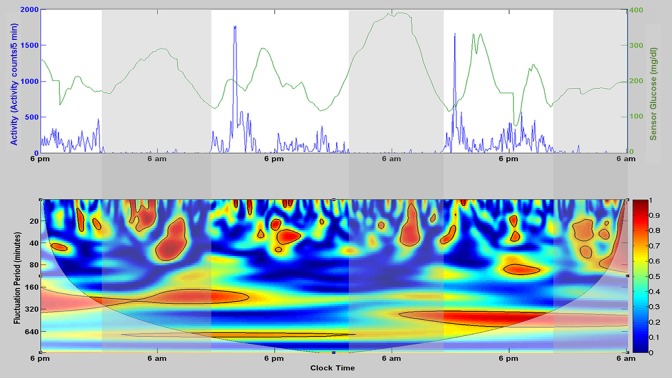
Figure 1 depicts the characteristic patterns of physical activity and glucose associated with time of day and sleep/wake intervals for a single subject (top panel). The lower panel displays the time- and frequency-dependent coherence between these two processes as a heat map. Globally, the group mean coherence over the entire 60-hr recording interval and overall fluctuation periods (10−960 min) was 0.39 ± 0.14 (SD). Mean coherence by band was as follows: Band 1, 0.38 ± 0.02; Band 2, 0.35 ± 0.04; Band 3, 0.34 ± 0.06; Band 4, 0.38 ± 0.08; Band 5, 0.39 ± 0.10; Band 6, 0.46 ± 0.19. Mean coherence in Bands 2 and 3 was significantly lower than that for Band 6 (p ≤ .01 for each).
Figure 1.
Characteristic patterns of physical activity and glucose associated with time of day and sleep/wake intervals for a single subject (top) and the time- and frequency-dependent coherence between these two processes as a heat map (bottom). The top panel illustrates raw activity (total activity counts per 5 min [blue]) and glucose values (mg/dl [green]) over a single 60-hr recording period, with clear daily (circadian) variations in both activity and glucose. Vertical shaded regions denote the recorded sleep periods for this subject. The bottom panel illustrates the time-varying and frequency-specific coherence between activity and glucose. The color bar at the right of this panel provides the scale for coherence (ranging from 0 to 1), with intervals of statistically significant coherence shaded red and enclosed by a black border.
Intervals of significant coherence
It is evident (Figure 1) that even within each band the coupling (coherence) between glucose and activity was time varying, with multiple discrete intervals of statistically significant coherence exhibited during the 60-hr recording. Table 2 summarizes the average number and duration of these statistically significant intervals.
Table 2.
Summary of Discrete Intervals of Significance and Mean Duration of Intervals With Significant Coherence.
| Variable | Band |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 60 hr | ||||||
| N | 23 | 23 | 23 | 22 | 20 | 14 |
| No. of intervals, mean (SD) | 20.5 (2.5) | 9.5 (2.5) | 4.6 (1.3) | 2.3 (0.9) | 1.7 (0.6) | 1.1 (0.3) |
| Duration (min), mean (SD) | 37.6 (8.0) | 91.0 (22.3) | 181.1 (82.7) | 428.2 (214.2) | 626.3 (301.2) | 872.1 (477.7) |
| Wake | ||||||
| N | 23 | 23 | 23 | 15 | — | — |
| No. of intervals, mean (SD) | 9.3 (2.2) | 4.5 (1.9) | 2.3 (1.2) | 1.5 (0.5) | — | — |
| Duration (min), mean (SD) | 35.5 (9.0) | 79.5 (29.6) | 139.0 (138.5) | 311.1 (211.7) | ||
| Sleep | ||||||
| N | 23 | 23 | 23 | 18 | — | — |
| No. of intervals, mean (SD) | 8.3 (2.3) | 3.5 (1.3) | 2.2 (1.1) | 1.4 (0.6) | — | — |
| Duration (min), mean (SD) | 33.1 (11.7) | 70.1 (32.3) | 97.5 (66.9) | 128.9 (84.9) | — | — |
Note. N indicates the number of subjects that had at least one discrete interval of significant coherence.
Differences in number of intervals of significant coherence
Repeated-measures ANOVA revealed significant independent effects of band (p < .00005) and sleep/wake state (p < .04) on the number of intervals with significant coherence, with no interaction. The number of intervals of significant coherence decreased progressively from Band 1 to Band 6 (Table 2). Post hoc contrasts (Scheffe) revealed that the numbers of significant intervals in Bands 1−3 were significantly different from each other as well as from the numbers in Bands 4−6 (p ≤ .0005 for each comparison); the numbers in Bands 4−6, however, did not differ from one another. When stratifying for sleep and wake intervals, we found that Bands 3 and 4 were significantly different from Bands 1 and 2 during both sleep (p ≤ .037 for each comparison) and wake (p ≤ .0005 for each comparison), but Bands 3 and 4 did not differ from each other during either sleep or wake.
Difference in duration of intervals of significant coherence
The mean duration of the intervals progressively increased with increasing band for the 60-hr recordings. One-way ANOVA with post hoc contrasts revealed that mean durations in Bands 1−3 were significantly shorter than those in Bands 4−6 (p < .02 for each), and mean duration in Band 4 was significantly shorter than that in Band 6 (p ≤ .0005). Repeated-measures ANOVA revealed significant effects of band, sleep/wake, state and their interaction on the mean duration of coherent intervals (p ≤ .0003 for each). Stratified one-way ANOVA demonstrated (Table 2) that the mean duration of intervals of significant coherence in Band 4 was significantly shorter during sleep than during wake intervals (F = 9.76, p = .004). During sleep and wake, the mean duration of intervals in Band 1 was less than those in Bands 3 and 4 (p ≤ .001 for each during sleep; p ≤ .04 for each during wake). During sleep, Band 2 mean duration was significantly less than that of Band 4 (p = .02), and during wake, mean durations in Bands 2 and 3 were significantly less than that in Band 4 (p < .0005 for each).
Differences in Coherence Between Sleep and Wake States
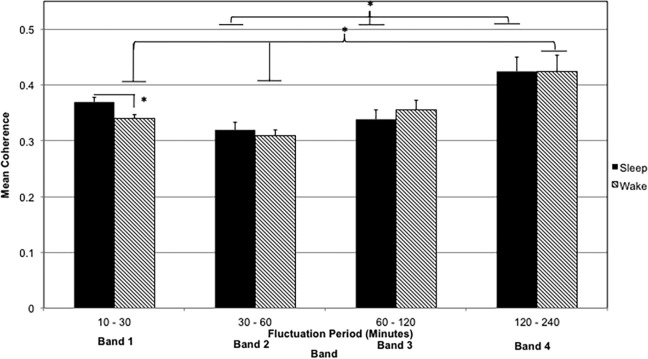
Mean coherence during sleep and wake
Considering the temporal variations in coherence we observed (Figure 1; Table 2), we hypothesized that coupling (mean coherence) would differ during sleep versus wake. Figure 2 illustrates the mean coherence within each band for sleep and wake intervals. Repeated-measures ANOVA using band and sleep/wake state as within-subject repeated factors revealed a significant effect of band (F = 17.7, p < .00005) on mean coherence, but the effects of sleep/wake state and its interaction with band were not significant. One-way ANOVA with post hoc comparisons revealed that mean coherence was highest in Band 4 (2- to 4-hr fluctuation periods) during both sleep and wake intervals (F = 6.7, p = .0004 and F = 7.17, p = .0002, respectively). The mean coherence in Band 4 differed significantly from those of Bands 1 and 2 during wake intervals (p ≤ .01 for each) and from those of Bands 2 and 3 during sleep intervals (p ≤ .013 for each). In addition, in Band 1 (10- to 30-min fluctuations), the mean coherence was significantly higher during sleep (0.37 ± 0.04) than during wake (0.34 ± 0.04) intervals (F = 4.34, p = .04).
Figure 2.
Mean coherence within each band for sleep and wake intervals. Error bars denote SD. *p < .05.
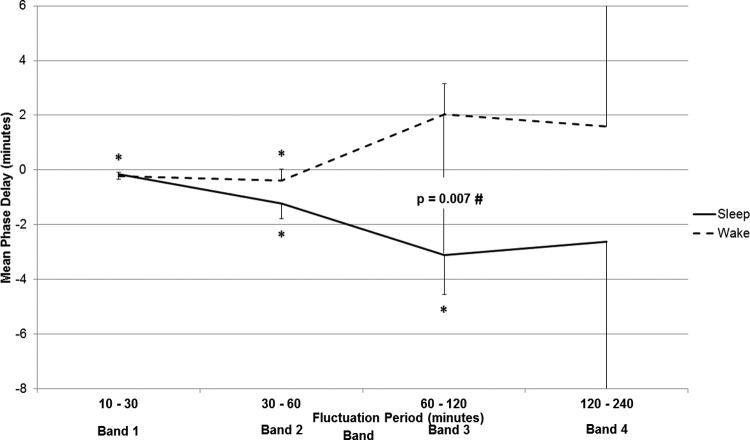
Phase delay during sleep and wake
The temporal alignment between coherent fluctuations in glucose and activity is indicated by the phase calculation, which we converted to an equivalent delay, in minutes. Mean phase delay in Band 2 was significantly negative (changes in glucose leading changes in activity) for the 60-hr recordings (−1.5 ± 1.9 min, p = .0014); delays for all other bands were not significantly different from 0. Figure 3 presents the mean phase delay for each band (1–4) stratified according to sleep and wake intervals. During sleep intervals, the mean phase delay was negative in all bands, indicating that changes in glucose were consistently leading changes in activity. This negative delay was significantly different from 0 only for fluctuation periods of 30–120 min (Bands 2 and 3; p < .02 for each; Figure 3). During wakefulness, the phase delay was significantly negative in Bands 1 and 2 (p < .05 for each) but was much more variable and, on average, positive (changes in activity leading changes in glucose) in Bands 3 and 4. One-way ANOVA revealed that mean phase in Band 3 was significantly more negative during sleep versus wake (−4.4 ± 7.1 min during sleep vs. 1.6 ± 4.9 min during wake; F = 11.13, p = .0017). The same sleep/wake trend was seen among Bands 2–4 (Figure 3).
Figure 3.
Phase delay (mean ± SD) for each band during sleep and wake periods. Negative phase delay indicates that changes in glucose occurred prior to (led) changes in activity, while positive phase delay indicates that changes in activity led changes in glucose. In Band 3, the mean phase delay was significantly negative during sleep with respect to during wake (p = .007). *p < .05, indicating that the mean phase delay was significantly different from zero; #p < .05, indicating that the mean phase delay was significantly different between sleep and wake.
Circadian Analysis
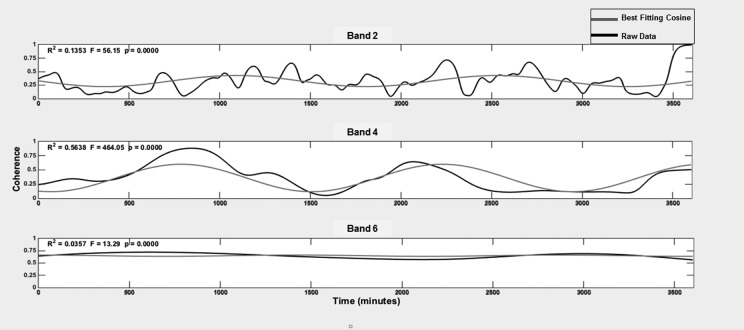
We found support that the coupling between glucose and activity exhibits a circadian pattern. Figure 4 illustrates the best fit circadian cosine waves overlaid on the time-varying coherence data for one 60-hr recording. For clarity, only Bands 2, 4, and 6 are presented; overall characteristics and fit for Bands 1, 3, and 5 were similar. The fit (r 2) for coherence tended to improve from Band 1 through 4 and then decreased for Band 6. Table 3 provides the mean r 2, amplitude, and acrophase of the best fit cosine waves for each band. One-way ANOVA (F = 6.69, p ≤ .0001) with pairwise contrasts (Scheffe) revealed that the means of r 2 for Bands 4 and 5 were significantly greater than those for Bands 1–3 (p ≤ .02 for each) and 6 (p > .0001). ANOVA also demonstrated (F = 4.43, p = .002) that the amplitudes of circadian variations in coherence for Bands 1–3 and 5 were significantly lower than the amplitude for Band 4 (p ≤ .03 for each comparison), and the amplitudes for Bands 2–5 were significantly higher than the amplitude for Band 6 (p ≤ .01 for each comparison). The acrophase (time associated with peak coherence) ranged from 5 p.m. to 7 p.m. and was equivalent across all bands.
Figure 4.
Circadian cosine wave fit for one subject. In each subpanel, the black line represents the actual time-varying coherence data for one band, and the gray line depicts the best fit cosine wave for that band. For clarity, only Bands 2, 4, and 6 are depicted.
Table 3.
Parameters of Circadian Cosinor Regression for Coherence.
| Band | Mean r 2 | Mean Amplitude | Acrophase (SD in min) |
|---|---|---|---|
| 1 | .04 (.03) | .05 (.02) | 5:16 p.m. (210.6) |
| 2 | .09 (.05) | .07 (.03) | 5:36 p.m. (172.7) |
| 3 | .09 (.08) | .07 (.04) | 5:50 p.m. (185.7) |
| 4 | .23 (.17)* | .11 (.06)† | 6:38 p.m. (201.5) |
| 5 | .21 (.18)* | .09 (.04) | 6:14 p.m. (203.4) |
| 6 | .05 (.05) | .03 (.02) | 6:25 p.m. (199.5) |
*Significantly higher than Bands 1, 2, 3, and 6 (p ≤ .01). †Significantly higher than Bands 1–3 and 6 (p ≤ .03).
Discussion
In the present study, we provide a systematic evaluation of the relationship between routine, unstructured physical activity, and glucose variations across wake and sleep periods for multiple days in young adults with T1DM in their natural home/work environment. Coherence analysis demonstrated substantial coupling between activity and glucose variations with one third to one half of their variances being shared during both wakefulness and sleep. Moreover, our findings suggest multiple modes of activity/glucose coupling with differing characteristic time scales and potentially different physiological mechanisms of control. For example, coherence demonstrated significant circadian variations over a 60-hr time span, and this circadian rhythm in coupling was distinctly strongest for activity/glucose fluctuations with periods between 2 and 4 hr. Conversely, only the most rapid fluctuations (periods of 10–30 min) demonstrated significant differences between wake and sleep states, with mean activity/glucose coherence being higher during sleep than wakefulness. It is also noteworthy that for fluctuations with periods from 10 to 60 min, changes in glucose consistently preceded coherent changes in activity. For slower fluctuations, this timing relationship was much more variable, with glucose changes tending to lead to activity changes during sleep periods and vice versa during wakefulness.
In the present study, rapid fluctuations (10–30 min) in glucose and activity were characterized by somewhat lower mean coherence but a larger number of brief intervals of significant coherence as compared to slower fluctuations. This mode of coupling may be of particular importance during sleep, as the mean coherence of rapid fluctuations was higher during sleep than wakefulness (Figure 2). Further, phase calculations demonstrated that rapid glucose variations consistently led to activity changes (Figure 3), suggesting the possibility that rapid changes of glucose during sleep led to awakenings and attendant physical movements (activity). Multiple awakenings from sleep are typically short in duration but result in poorer sleep quality. Children with T1DM exhibit more awakenings than healthy controls (Matyka, Crawford, Wiggs, Dunger, & Stores, 2000). Also in these children, rapid decreases in glucose have been associated with more frequent awakenings from sleep (Pillar et al., 2003). In addition, motor activity, assessed by actigraphy, was increased during periods of nocturnal hypoglycemia in children with T1DM (Radan et al., 2004). Our findings indicate that brief movements during sleep, likely due to awakening, are strongly coupled to rapid glucose fluctuations in young adults with T1DM. This observation is important, as it may suggest that glucose fluctuations play a role in sleep disruption in people with T1DM. Sleep fragmentation (due to multiple arousals) results in decreased insulin sensitivity in healthy individuals (Stamatakis & Punjabi, 2009). Therefore, to the extent that glucose fluctuations result in sleep disruption in people with T1DM, they may ultimately result in decreased insulin sensitivity, which has deleterious effects on glycemic control. Poor sleep quality also has deleterious effects on cardiovascular health (Cappuccio, Cooper, D’Elia, Strazzullo, & Miller, 2011); thus, sleep disruption due to glucose fluctuations in people with T1DM may result in accelerating development of cardiovascular disease, a leading cause of death in this population (de Ferranti et al., 2014). Understanding how changes in glucose are related to increased activity during sleep is important; maintenance of normal sleep plays a critical role in maintenance of metabolic and cardiovascular health (Hanlon & Van Cauter, 2011).
During wakefulness, mean coherence for rapid fluctuations was lower than during sleep in the present study, with glucose fluctuations consistently leading changes in physical activity. However, for slower fluctuations the delay differed significantly across sleep/wake intervals, with activity changes tending to lead glucose changes during wakefulness and the opposite during sleep. Interpretation of the delay helps us understand how to maximize coherence between the two signals, but we cannot determine true cause and effect from it. However, these findings do suggest that the relationship between glucose and physical activity is likely both bidirectional and operating on multiple time scales, especially during wakefulness. This proposition is not surprising because during the daytime many factors, including caloric intake (Neu et al., 2015); aerobic and resistance exercise (Davey et al., 2013; McMahon et al., 2007; Silveira et al., 2014; Sonnenberg, Kemmer, & Berger, 1990); and work, school, and family demands impact daytime glucose control in people with T1DM.
In addition to physical activity, other factors influence glucose metabolism. Of particular relevance to the present data, hormones secreted during sleep and wake can impact glucose levels. For example, growth hormone, secreted during deep sleep (Davidson, Moldofsky, & Lue, 1991), reduces insulin sensitivity (Rizza, Mandarino, & Gerich, 1982), and increased glucose levels are seen when the levels of growth hormone are elevated. In addition, diet and insulin-dosing regimen have a major influence on time-varying glucose levels. Despite not controlling for these other factors in the natural setting of the present study, our findings are novel in demonstrating that normal routine activity accounts for more than one third of the total variance in glucose level throughout a 60-hr period. Future studies that control for neurohumoral, dietary, and insulin treatment effects will help to define the mechanisms behind the coupling we report here.
We showed, for the first time, that circadian influences also impact the coupling between glucose variations and physical activity. Coherence of 2- to 4-hr activity/glucose fluctuations demonstrated the greatest circadian modulation (Figure 4 and Table 3), and we observed peak coupling during the early evening hours (Table 3). Multiple biological processes exhibit circadian patterns, including insulin sensitivity in individuals with T1DM (Hinshaw et al., 2013; Tatò, Tatò, Beyer, & Schrezenmeir, 1991). Further, insulin sensitivity is lowest during the early morning hours in people with T1DM (Trümper et al., 1995). These factors may contribute to the circadian pattern of coherence we have reported here. In addition, we allowed our participants to engage in their normal exercise routines. Moderate physical exercise during the day increases the likelihood of nocturnal hypoglycemia (Davey et al., 2013; McMahon et al., 2007). In adolescents with T1DM, glucose needs remained elevated for as long as 7–11 hr after completion of moderate daytime exercise (McMahon et al., 2007). To the extent that glucose utilization rate impacts glucose/activity coherence, the above findings suggest that exercise may have contributed to a circadian variation in coherence in our participants. Circadian modulation of the relationship between glucose and activity is important to understand as it may help to optimize management strategies, such as timing and dosage of insulin administration, in individuals with T1DM.
This study is limited by the lack of a control group, and future studies should incorporate a healthy control group to determine to what extent the reported findings are unique to people with T1DM. Further, we did not control exercise in the present study, and as discussed, structured physical exercise can have effects on glucose levels. We did not find any differences in the mean coherence within any band during sleep or wake between those participants who self-reported exercise during the study (n = 14) and those who reported no exercise (n = 9, data not shown). Thus, we do not believe that controlling physical activity would have substantially influenced the findings.
In summary, in the present study we demonstrated, for the first time to our knowledge, that the relationship between glucose and activity exhibits circadian modulation and that the strength and nature of coupling differ between sleep and wake. This study provides the motivation for larger scale trials aimed at determining the mechanisms behind these reported relationships. Understanding circadian patterns and sleep/wake differences in coupling between glucose and routine unstructured physical activity may allow for improved nursing management strategies for patients with diabetes.
Footnotes
Authors’ Contribution: Sarah S. Farabi contributed to conception, design, data acquisition, analysis, and interpretation; drafted the manuscript, critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work ensuring integrity and accuracy. David W. Carley contributed to conception, design, data analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work ensuring integrity and accuracy. Lauretta Quinn contributed to conception, design, data analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be held accountable for all aspects of work ensuring integrity and accuracy.
Authors’ Note: The corresponding author can be contacted for information on retrieving the underlying data.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded in part by a grant from the American Association of Diabetes Educators Foundation/Sigma Theta Tau International and an NIH TL-1 Institutional Fellowship, 5TL1TR000049-05.
References
- Bally L., Laimer M., Stettler C. (2015). Exercise-associated glucose metabolism in individuals with type 1 diabetes mellitus. Current Opinion in Clinical Nutrition and Metabolic Care, 18, 428–433. doi:10.1097/MCO.0000000000000185 [DOI] [PubMed] [Google Scholar]
- Cappuccio F. P., Cooper D., D’Elia L., Strazzullo P., Miller M. A. (2011). Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. European Heart Journal, 32, 1484–1492. doi:10.1093/eurheartj/ehr007 [DOI] [PubMed] [Google Scholar]
- Coomans C. P., van den Berg S. A. A., Lucassen E. A., Houben T., Pronk A. C. M., van der Spek R. D.…Meijer J. H. (2013). The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes, 62, 1102–1108. doi:10.2337/db12-0507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey R. J., Howe W., Paramalingam N., Ferreira L. D., Davis E. A., Fournier P. A., Jones T. W. (2013). The effect of midday moderate-intensity exercise on postexercise hypoglycemia risk in individuals with type 1 diabetes. Journal of Clinical Endocrinology and Metabolism, 98, 2908–2914. doi:10.1210/jc.2013-1169 [DOI] [PubMed] [Google Scholar]
- Davidson J. R., Moldofsky H., Lue F. A. (1991). Growth hormone and cortisol secretion in relation to sleep and wakefulness. Journal of Psychiatry & Neuroscience, 16, 96–102. [PMC free article] [PubMed] [Google Scholar]
- de Ferranti S. D., de Boer I. H., de Fonseca V., Fox C. S., Golden S. H., Lavie C. J.…Eckel R. H. (2014). Type 1 diabetes mellitus and cardiovascular disease: A scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care, 37, 2843–2863. doi:10.2337/dc14-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted A., Moore J., Jevrejeva S. (2004). Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Processes in Geophysics, 11, 561–566. [Google Scholar]
- Gross T. M., Mastrototaro J. J. (2000). Efficacy and reliability of the continuous glucose monitoring system. Diabetes Technology & Therapeutics, 2, 19–26. doi:10.1089/15209150050214087 [DOI] [PubMed] [Google Scholar]
- Hanlon E. C., Van Cauter E. (2011). Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proceedings of the National Academy of Sciences of the United States of America, 108, 15609–15616. doi:10.1073/pnas.1101338108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw L., Dalla Man C., Nandy D. K., Saad A., Bharucha A. E., Levine J. A.…Basu A. (2013). Diurnal pattern of insulin action in type 1 diabetes: Implications for a closed-loop system. Diabetes, 62, 2223–2229. doi:10.2337/db12-1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J. A., Tanner C. J., Slentz C. A., Duscha B. D., McCartney J. S., Kraus W. E. (2004). Effect of the volume and intensity of exercise training on insulin sensitivity. Journal of Applied Physiology, 96, 101–106. doi:10.1152/japplphysiol.00707.2003 [DOI] [PubMed] [Google Scholar]
- Islam M. (2011). Wavelets, its application and technique in signal and image processing. Global Journal of Computer Science & Technology, 11 Retrieved from http://globaljournals.org/GJCST_Volume11/7-Wavelets-its-Application-and-Technique-in-signal-and-image-processing.pdf [Google Scholar]
- Johns M. W. (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14, 540–545. [DOI] [PubMed] [Google Scholar]
- Matyka K. A., Crawford C., Wiggs L., Dunger D. B., Stores G. (2000). Alterations in sleep physiology in young children with insulin-dependent diabetes mellitus: Relationship to nocturnal hypoglycemia. Journal of Pediatrics, 137, 233–238. doi:10.1067/mpd.2000.107186 [DOI] [PubMed] [Google Scholar]
- McMahon S. K., Ferreira L. D., Ratnam N., Davey R. J., Youngs L. M., Davis E. A.…Jones T. W. (2007). Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. Journal of Clinical Endocrinology and Metabolism, 92, 963–968. doi:10.1210/jc.2006-2263 [DOI] [PubMed] [Google Scholar]
- Neu A., Behret F., Braun R., Herrlich S., Liebrich F., Loesch-Binder M.…Schweizer R. (2015). Higher glucose concentrations following protein- and fat-rich meals—The Tuebingen Grill study: A pilot study in adolescents with type 1 diabetes. Pediatric Diabetes, 16, 587–591. doi:10.1111/pedi.12224 [DOI] [PubMed] [Google Scholar]
- Pillar G., Schuscheim G., Weiss R., Malhotra A., McCowen K. C., Shlitner A.…Shehadeh N. (2003). Interactions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitus. Journal of Pediatrics, 142, 163–168. doi:10.1067/mpd.2003.66 [DOI] [PubMed] [Google Scholar]
- Quirk H., Blake H., Tennyson R., Randell T. L., Glazebrook C. (2014). Physical activity interventions in children and young people with type 1 diabetes mellitus: A systematic review with meta-analysis. Diabetic Medicine, 31, 1163–1173. doi:10.1111/dme.12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radan I., Rajer E., Ursic Bratina N., Neubauer D., Krzisnik C., Battelino T. (2004). Motor activity during asymptomatic nocturnal hypoglycemia in adolescents with Type 1 diabetes mellitus. Acta Diabetologica, 41, 33–37. doi:10.1007/s00592-004-0141-3 [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. (1982). Effects of growth hormone on insulin action in man: Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes, 31, 663–669. doi:10.2337/diab.31.8.663 [DOI] [PubMed] [Google Scholar]
- Shin M., Swan P., Chow C. M. (2015). The validity of Actiwatch2 and SenseWear armband compared against polysomnography at different ambient temperature conditions. Sleep Science, 8, 9–15. doi:10.1016/j.slsci.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira A. P. S., Bentes C. M., Costa P. B., Simão R., Silva F. C., Silva R. P., Novaes J. S. (2014). Acute effects of different intensities of resistance training on glycemic fluctuations in patients with type 1 diabetes mellitus. Research in Sports Medicine, 22, 75–87. doi:10.1080/15438627.2013.852096 [DOI] [PubMed] [Google Scholar]
- Sonnenberg G. E., Kemmer F. W., Berger M. (1990). Exercise in type 1 (insulin-dependent) diabetic patients treated with continuous subcutaneous insulin infusion. Prevention of exercise induced hypoglycaemia. Diabetologia, 33, 696–703. [DOI] [PubMed] [Google Scholar]
- Spira A. P., Beaudreau S. A., Stone K. L., Kezirian E. J., Lui L.-Y., Redline S.…Stewart A. (2011). Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 67A, 433–439. doi:10.1093/gerona/glr172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis K. A., Punjabi N. M. (2009). Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest, 137, 95–101. doi:10.1378/chest.09-0791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summa K. C., Turek F. W. (2014). Chronobiology and obesity: Interactions between circadian rhythms and energy regulation. Advances in Nutrition, 5, 312S–319S. doi:10.3945/an.113.005132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatò F., Tatò S., Beyer J., Schrezenmeir J. (1991). Circadian variation of basal and postprandial insulin sensitivity in healthy individuals and patients with type-1 diabetes. Diabetes Research, 17, 13–24. [PubMed] [Google Scholar]
- Torrence C., Compo G. P. (1998). A practical guide to wavelet analysis. Bulletin of the American Meteorological Society, 79, 61. [Google Scholar]
- Trümper B. G., Reschke K., Molling J. (1995). Circadian variation of insulin requirement in insulin dependent diabetes mellitus: The relationship between circadian change in insulin demand and diurnal patterns of growth hormone, cortisol and glucagon during euglycemia. Hormone and Metabolic Research, 27, 141–147. doi:10.1055/s-2007-979926 [DOI] [PubMed] [Google Scholar]
- Van Cauter E. (1997). Roles of circadian rhythmicity and sleep in human glucose regulation. Endocrine Reviews, 18, 716–738. doi:10.1210/er.18.5.716 [DOI] [PubMed] [Google Scholar]
- Van Cauter E., Blackman J. D., Roland D., Spire J. P., Refetoff S., Polonsky K. S. (1991). Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. Journal of Clinical Investigation, 88, 934–942. doi:10.1172/JCI115396 [DOI] [PMC free article] [PubMed] [Google Scholar]