Abstract
This study examined the effects of prematurity, cumulative medical risk, and proximal and distal social forces on individual differences in the activity of the hypothalamic–pituitary–adrenal (HPA) axis in young adulthood. A prospective sample of 149 infants born healthy preterm (PT; n = 22), sick PT (n = 93, medical illness, neurological illness, small for gestational age), and full term (n = 34) was recruited from a Level III neonatal intensive care unit in southern New England between 1985 and 1989 and followed to age 23 years. Cumulative medical risk was indexed across seven assessment waves (spanning 17 years) using medical and neurological health status at birth, toddlerhood (ages 18 and 30 months), childhood (ages 4 and 8 years), and adolescence (ages 12 and 17 years). Distal risk included socioeconomic status (SES) at birth. Proximal social factors were indexed from assessments of the home environment and measures of child vulnerability and maternal self-esteem, involvement, and control style from birth, 4 years, 8 years, and 12 years. At age 23 years, five saliva samples were collected upon awakening, 45 min after waking, 4 hr after waking, 8 hr after waking, and bedtime (later assayed for cortisol). Results reveal effects of cumulative medical risk on the diurnal pattern of HPA axis activity, with moderating effects of SES and proximal social factors. Findings are discussed in terms of implications for contemporary theories related to developmental sensitivity and susceptibility to context and the developmental origins of health and disease theory.
Keywords: diurnal cortisol, prematurity, cumulative risk, protection, HPA
When adversities are present early in life, environmentally responsive biological systems may become susceptible to them, thus affecting adult health (Barker, 1995). Pre- and perinatal adversities (child abuse, neglect, maltreatment) affect the stress reactivity and/or diurnal regulation of the hypothalamic–pituitary–adrenal (HPA) axis (Cicchetti & Rogosch, 2012). Prematurity often calls for frequent, high-intensity, invasive medical procedures to manage neonatal illness and physiological instability (Holsti, Grunau, Oberlander, & Whitfield, 2005). More than 70% of preterm (PT) infants spend time in neonatal intensive care units (NICUs; March of Dimes Perinatal Data Center, 2011). Frequent medical procedures and maternal separation have the potential to be chronic stressors for premature infants (Holsti et al., 2005). Not surprisingly, numerous studies show that premature infants are more likely than full-term (FT) infants to suffer poor health as adults (Saigal, 2014).
Premature birth, exposure to repeated stressful neonatal procedures, and socioecological adversity over the life course contribute to cumulative stress and, consequently, to allostatic load (Walker, Irving, Andrew, & Belton, 2002). Recent studies show that the nature of these effects may be amplified or attenuated by various proximal (the immediate caregiving environment; Gunnar & Donzella, 2002) and distal (poverty, socioeconomic factors; Blair et al., 2011) social contextual forces. Additionally, among the most compelling findings are observations suggesting that cumulative (in contrast to singular or acute) exposure to adversity has the potential to result in chronic wear and tear on downstream physiological systems (e.g., HPA axis) with long-term implications for health (McEwen & Lasley, 2003). Infants born prematurely are likely to have neonatal illness(es), which affect the degree to which proximal and distal social forces externally buffer the negative consequences of chronic stress. Yet, little attention has been given to the interrelated roles of prematurity, health risks, and developmental context on HPA function.
Stress for premature infants begins prenatally (Lu & Halfon, 2003) and continues postnatally (Holsti et al., 2005). Dysregulation of the HPA axis, which represents one of the main components of the neuroendocrine stress response system (with cortisol as its primary by-product), has significant health impacts for former PT infants. Numerous studies have reported evidence of this stress and its long-term effects in the form of altered cortisol levels. For example, PT infants with bronchopulmonary dysplasia (BPD) had lower cortisol secretion compared to healthy PT infants (Watterberg et al., 2004), while those born with intraventricular hemorrhage (IVH) had high cortisol concentrations (Aucott, Watterberg, Shaffer, & Donohue, 2008). PT infants born small for gestational age (SGA) had a blunted diurnal cortisol pattern compared to FT peers at infancy (Osterholm, Hostinar, & Gunnar, 2012) and increased insulin resistance at adulthood (Dalziel, Parag, Rodgers, & Harding, 2007). PT infants born at less than 28 weeks gestational age had lower cortisol reactivity levels compared to FT infants (Holsti, Weinberg, Whitfield, & Grunau, 2007). Their basal cortisol levels shifted from low levels at 3 months (corrected age) to high levels at 8 months and 18 months (corrected age; Grunau et al., 2007). At school age, former PT infants who had more invasive NICU procedures had lower morning cortisol levels compared to those with fewer procedures (Brummelte et al., 2015). At ages 8–12 years, former PT infants had a higher cortisol awakening response (CAR) compared to FT peers (Buske-Kirschbaum et al., 2007). In adults, low birth weight was associated with high cortisol concentrations, hypertension, and type 2 diabetes in one study (Matthews, 2002) and high blood pressure and poor insulin resistance in another (Dalziel et al., 2007).
Chronic and acute stress from early life adversity, or “toxic stress,” promote HPA dysregulation which, in turn, affects immune, endocrine, and metabolic function (Johnson, Riley, Granger, & Riis, 2013). Chronic exposure to stress is associated with lower morning cortisol levels, higher concentrations of evening cortisol, a flatter diurnal rhythm, and a higher daily volume of cortisol output (Gunnar & Vazquez, 2001; Miller, Chen, & Zhou, 2007). Children exposed to high-risk environments had a blunted CAR (Gunnar & Vazquez, 2001). Adults who experienced chronic stress through ecological risks (e.g., minority racial status, lower education level) had lower morning cortisol levels, higher evening concentration, and a blunted diurnal pattern (Miller et al., 2007). The effect of poverty, or low socioeconomic status (SES), on the HPA axis is evident early in life. Infants 7–24 months of age living in poverty had higher cortisol levels compared to matched infants not living in poverty (Blair et al., 2011). Effects of low SES in childhood on morning cortisol concentrations persisted into adulthood in another study (Gustafsson, Janlert, Theorell, & Hammarstrom, 2010). Collectively, the evidence suggests that the stress associated with PT birth joins with the effects of low SES on HPA dysregulation to further contribute to physical and psychological health deficits over the life course (Erickson, Drevets, & Schulkin, 2003; McEwen & Lasley, 2003).
Just as some external factors such as low SES or neglect contribute to dysfunction of the HPA axis, positive environmental influences across the developmental continuum serve as cumulative protective factors, including warmth and responsivity in the home environment, maternal involvement, and a positive maternal self-esteem. For infants, caregivers are the most proximal source of positive environmental influences. Infants who had longer durations of cosleeping and breastfeeding with mothers had quicker poststressor cortisol recovery (Beijers, Riksen-Walraven, & De Weerth, 2013). In toddlers (ages 7–24 months) born at FT, poor maternal sensitivity was related to higher cortisol levels, while, conversely, positive maternal sensitivity was associated with lower cortisol (Berry et al., 2016). Responsive caregiving and positive family functioning mediated the effects of toxic stress (i.e., socioeconomic disadvantage, poor social support) on health and developmental outcomes, while sensitive parenting (e.g., secure attachment) modulated children’s stress response (Gunnar & Donzella, 2002). This finding suggests that early and long-term positive influences from the caregiving environment are powerful buffers of the effects of acute and cumulative stress on the HPA system across development.
The developmental origins of health and disease (DOHaD) theory provides a conceptual framework from which to consider the combined effects of prematurity, stress, and social forces. The main tenants of DOHaD suggest that prenatal and perinatal stress provokes and permanently programs adaptive changes in endocrine and metabolic systems that affect outcomes into adulthood, such as health, memory, learning, and executive function (Dalziel et al., 2007; Matthews, 2002). Adult health outcomes associated with early stress include hypertension, type 2 diabetes, and coronary heart disease. Research suggests that stress-related neuroendocrine reactivity and regulation are among the mechanisms that translate these effects into health-related disparities (Dalziel et al., 2007; Matthews, 2002). Within the past 25 years, research has replicated the basic tenants of DOHaD, and the contemporary assumption is that the ideas of the basic phenomenon can be generalized across diverse populations. Critics of DOHaD theory fault (a) a predominance of retrospective study designs (De Boo & Harding, 2006; Gluckman, Hanson, & Pinal, 2005) and (b) a focus on a minimalist mechanism with the need to reach beyond endocrine and metabolic systems to include the interplay of genetic, epigenetic, physiological, and social environment across the life course (Lu & Halfon, 2003; Sullivan, Hawes, Winchester, & Miller, 2008).
The present study attempts to address some of these criticisms. It is the only prospective, longitudinal study in the United States with a heterogeneous PT sample and a FT comparison group investigating the effects of medical risk and distal and proximal social forces on developmental outcomes from infancy to young adulthood. Guided by the DOHaD, this study expands upon earlier work by examining the effects of risk (medical, distal socioeconomic) and proximal social factors on HPA (dys)regulation in a medically diverse PT sample at age 23 years. Specifically, we hypothesized the following:
Cumulative medical risk and distal socioeconomic risk will be associated with dysregulation of the diurnal pattern of HPA activity.
Proximal social factors will attenuate the effects of cumulative medical risk and distal socioeconomic risk on the diurnal pattern of HPA activity.
Material and Method
Participants
At recruitment
We recruited 215 infants at birth from a Level III NICU in a specialty hospital in 1985–1989. A priori sample recruitment criteria included birth weight <1,850 g and gestational age <37 weeks for PT infants, absence of maternal mental illness, and English as the mother’s primary language. Within the same period, we recruited FT infants (n = 55) with uncomplicated labor and delivery, absence of neonatal illness, birth weight ≥2,450 g, and gestational age at birth ≥38 weeks from the same medical center. Healthy PT infants (n = 33) had no medical or neurological neonatal illness. Sick PT infants (n = 127) were infants with medical illness (e.g., BPD, necrotizing enterocolitis [NEC], and sepsis), neurological illness (e.g., IVH, meningitis, and shunted hydrocephalus), or who were SGA. Among the SGA PT infants, 20 had neonatal illness (n = 11 medical illness, n = 2 neurological illness, and n = 7 medical and neurological illness) and 11 were without neonatal illness. SES was equally distributed within and across all groups (FT, healthy PT, and sick PT) at recruitment. Fewer than 10% of the parent(s) we approached declined participation in the study.
At age 23 years
We invited all participants from the original sample (N = 215) to participate in the ninth follow-up study at age 23 years. The participants had been assessed in a series of studies and continually tracked between time points (see Scott, Winchester, & Sullivan, 2017). Regular mailings, holiday and birthday cards, a study newsletter, and social media were used to optimize sample retention. Of the original sample, 180 participants completed a comprehensive assessment battery (84% retention) at age 23 years. Those who dropped from the study (n = 35) were 10 FT, 9 healthy PT, and 16 sick PT. We found no differences between those who dropped out at age 23 years and those who participated on neonatal illness (birth weight, gestational age, neonatal risk, total hospitalized days, duration of oxygen, BPD, NEC, sepsis, IVH, meningitis, and hydrocephalus), SES, parent education, marital status, or race. However, more male (n = 23) than female (n = 12) participants dropped from the study at age 23 years, χ2(1) = 4.25, p = .039.
Procedures
At each age point (birth/early infancy, 18 months, 30 months, 4 years, 8 years, 12 years, 17 years, and 23 years), participants and their parent(s) were assessed in a research protocol at the hospital laboratory. Neonatal data were collected from medical records. Cumulative medical risk variables were obtained from medical records, and physical health assessments were completed. SES captured distal risk at each age point. Proximal social variables were collected at birth/infancy, 4 years, 8 years, and 12 years by maternal report, observation in the home, and laboratory paradigms. At age 23 years, a health assessment was performed and instructions were given for providing saliva samples. Informed consent was obtained from parent(s) at each time point prior to 23 years, along with child assent at ages 8, 12, and 17 years and from participants at age 23 years. University and hospital institutional review boards approved each study.
Measures
Saliva collection
We asked participants to collect five saliva specimens on a single day: upon awakening, 45 min after waking, 4 hr after waking, 8 hr after waking, and at bedtime. To maximize adherence to sample collection times, we suggested that participants set their watches or smartphones for timed alarm reminders of their usual waking time and collection times (Hanrahan, McCarthy, Kleiber, Lutgendorf, & Tsalikian, 2006). Specimens were frozen at or below −20°C within 1 hr of collection or stored in a cooler with ice packs if they could not immediately be frozen. We instructed participants not to eat, drink liquids, brush teeth, engage in vigorous exercise, or smoke for 15 min prior to each specimen collection (Granger, Johnson, Szanton, Out, & Lau Schumann, 2012). Participants completed the salivary log form at each specimen collection by recording the date, collection time for each specimen (including wake-up time and bedtime), food, drink, smoking, exercise, menstrual cycle (females only), use of steroids and medications, sleep patterns, and mood (i.e., happy, sad, angry, and relaxed). After we obtained salivary specimens from participants, we stored them at −40°C or below until we shipped them overnight with dry ice for assay.
At the laboratory visit, we screened female participants for pregnancy test using the OSOM hCG Combo Pregnancy Kit (Sekisui Diagnostics LLC, 2012). If the pregnancy test was positive, we contacted female participants at least 3 months postpregnancy to collect salivary specimens (Granger, Hibel, Fortunato, & Kapelewski, 2009).
Determination of salivary cortisol
On the day of assay, samples were thawed, centrifuged to remove mucins, and tested in duplicate for cortisol using a commercially available enzyme immunoassay without modification to the manufacturer’s protocol (Salimetrics LLC, Carlsbad, CA). The test volume was 25 µl, range of sensitivity from 0.007 to 3.0 ug/dl, and on average, inter- and intraassay coefficients of variation were less than 15% and 10%, respectively.
Cortisol values below the lower limit of sensitivity were recoded to the value of the lowest calibrator (0.012 ug/dl, n = 3) and those greater than 4.0 ug/dl (n = 1) were recoded to missing since these values are physiologically implausible (Jacobson, Bihun, & Chiodo, 1999). The one participant with a cortisol value between 3.0 and 4.0 ug/dl was included in the analysis as that value was physiologically plausible. We dropped five participants (n = 2 FT, n = 3 sick PT) from analysis due to potentially confounding effects (use of prescription methadone, postpartum mother, rotating work schedule; Granger et al., 2009). Raw cortisol values were skewed, so we improved them for analysis with a natural log transformation. We screened and winsorized outliers greater than 3 standard deviations from the mean to reduce outliers (Tukey, 1977).
Computation of index scores
Prior to the computation of all index scores, we conducted multiple imputation procedures within AMOS, utilizing maximum likelihood estimation at the scale composite level for the distal socioeconomic index and proximal social factors. For the medical risk index, we handled missing data by carrying the last value forward due to ordinal-level data.
Cumulative medical risk index
To formulate cumulative medical risk, we conducted a higher order confirmatory factor analysis (CFA) using SPSS AMOS, Version 6.0 (IBM). The model fit was acceptable, χ2(1) = 0.25, p = .62; Root Mean Square Error of Approximation (RMSEA) < .01, 90% CI [0.00, 0.14], Normed Fit Index (NFI) = .99, and the standardized factor loadings ranged from 0.25 to 0.97. Each participant received a single cumulative medical risk index score. Cumulative medical risk included variables from general neonatal factors, medical, and neurological factors at birth as well as medical and neurological health status at toddlerhood and childhood/adolescence from physical assessment (Winchester, Sullivan, & Msall, 2014). Higher values on the cumulative medical risk index indicate overall greater medical risk from birth to age 17.
Birth factor
The birth factor included general neonatal information and medical and neurological neonatal variables. General neonatal information was collected at birth, including the Hobel neonatal illness acuity score (Hobel, Hyvarinen, Okada, & Oh, 1973), length of hospital stay, birth weight, and gestational age. Medical neonatal variables included medical health status, NEC, BPD, and total hours of supplemental oxygen. Neurological neonatal factors included neurological health status, IVH, and shunted hydrocephalus.
Toddler factor
The medical risk factor at toddlerhood included medical and neurological health status variables at 18 and 30 months. Medical health status was classified as normal (no abnormalities), suspect (continued chronic respiratory problems, cardiac murmurs, referral for hearing, and orthopedic issues), or abnormal (asthma, allergies, diabetes, and/or autoimmune deficiencies). Neurological health status was classified as normal (no abnormalities), suspect (fine motor weakness, unilateral sensorineural hearing loss, uncorrected vision problems, atypical neurologic findings in tone, reflexes, gait, or movement with no specific diagnosis), or abnormal (cerebral palsy, blindness, deafness, shunted hydrocephalus, uncontrolled seizures, or attention deficit/hyperactivity disorder; Niswander & Gordon, 1972; Prechtel & Beitema, 1967).
Child/adolescent factor
The medical risk factor at child/adolescence included medical health status variables (e.g., normal, suspect, and abnormal) at ages 4, 8, 12, and 17 years and neurological health status variables (e.g., normal, suspect, and abnormal) at ages 4, 8, 12, and 17 years.
Distal socioeconomic risk index
Socioeconomic risk was assessed at each time point using the Hollingshead Four-Factor Index (Hollingshead, 1975). This measure of SES comprises maternal and paternal educational and occupational levels, with lower scores indicative of greater socioeconomic risk. Since SES did not change over time, we used the value at the birth time point for distal socioeconomic risk.
Proximal social factors index
We employed a higher order CFA to create a cumulative proximal social factors index, again using SPSS AMOS, Version 6.0. The model fit was acceptable, χ2(1) = 0.59, p = .44; RMSEA < .01, 90% CI [0.00, 0.16], NFI = .99. Standardized factor loadings ranged from 0.30 to 0.84, with one factor loading below 0.30. Each participant received a single proximal social factors index score that was derived from variables from home environment stimulation, maternal perception of child vulnerability, maternal self-esteem, maternal involvement, and maternal control style at birth and 4, 8, and 12 years (Winchester et al., 2014), with higher values on the index indicative of greater cumulative proximal social factors.
Home environment
Home environment stimulation was measured using the Home Observation for Measurement of the Environment inventory (HOME; Caldwell & Bradley, 2001) during a home visit at birth and ages 4, 8, and 12 years. The HOME was designed to capture social and emotional aspects of the home environment by direct observation and a semistructured interview with the mother. Subscales include emotional and verbal responsivity, encouragement of maturity, emotional climate, growth-fostering materials and experiences, provision for active stimulation, family participation in developmentally stimulating experiences, paternal involvement, and aspects of the physical environment. Items are scored as yes/no (yes = 1, no = 0) and summed for a total score.
Maternal perception of child vulnerability
Mothers reported on their perceptions of child vulnerability at birth and 4, 8, and 12 years using a 14-item instrument with items designed as Likert-type scales ranging from 1, completely disagree, to 5, completely agree (McGrath, 1997). Items were summed and yielded a total score, with higher scores indicative of greater concerns for the child’s health.
Maternal self-esteem
Mothers reported on their belief in themselves as mother at birth and when their children were 4, 8, and 12 years of age using a 25-item questionnaire (McGrath, 1997). Responses are provided on a 5-point Likert-type scale and range from 1, completely disagree, to 5, completely agree. Items on this questionnaire ascertain the mother’s attitudes toward six components of mothering: caretaking ability, ability as a mother, acceptance of the child, expected relationship with the child, complications during pregnancy, parental influence and body image, and maternal health. Items are summed to yield a total score, with higher scores indicative of greater maternal self-esteem.
Maternal involvement and control style
Maternal involvement and maternal control-style variables were collected during videotaped paradigms at the hospital laboratory when children were 4, 8, and 12 years of age. Maternal involvement was assessed at age 4 years by having children and their mothers engaged in a spontaneous free-play session with age-appropriate toys. Examiners coded the videotape of the session using the Parent/Caregiver Involvement Scale, in which higher scores indicate greater parent involvement (Farran et al., 1987). At ages 8 and 12 years, maternal involvement was assessed in a session using Tangoes Puzzle™ pieces, where investigators asked the mother to “teach” her child to assemble a series of five puzzles. Examiners later coded the videotape of the session for behaviors of involvement, teaching, pacing, and appropriateness on a 5-point Likert-type scale, with higher scores indicative of greater maternal involvement (Sullivan & McGrath, 1999).
Maternal control style was collected at ages 4 and 8 years using Playskool Pipeworks™ pieces in a problem-solving task. The mother and child were sequentially presented two completed models and enough Pipeworks pieces to replicate a table and wagon. The mother was asked to provide as much assistance as needed for her child to complete both tasks. Examiners later coded the videotape of the problem-solving session (Seifer & McGrath, 1991). At age 12 years, maternal control style was collected during the previously mentioned Tangoes Puzzle teaching segment. Coders rated supportive presence, quality of assistance, and maternal affect on 5-point Likert-type scales, with the higher summed score indicative of greater maternal control style (Sullivan & McGrath, 1999). Examiners were blind to prematurity status of participants and maintained an interrater reliability of 95% and above on measurement coding for both maternal involvement and maternal control assessments.
Analytic Strategy
We examined descriptive statistics for the complete sample of 215 participants (included vs. excluded in the analysis) and for the cortisol analysis sample (n = 149). We compared participant characteristics between those who were included in the cortisol analysis and those who were not. Analyses conducted for sample characteristics included χ2 for the comparison of categorical variables between FT, healthy PT, and sick PT groups and analysis of variance for continuous variables. We conducted preliminary analyses to compare the diurnal patterns of cortisol among the participants. Next, we used generalized linear mixed models (GLMMs) to examine the effects of cumulative medical risk index, distal SES risk, and the proximal social factor index on diurnal cortisol pattern. We conducted all analyses using SAS Version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Descriptive Statistics of Participants at Age 23 Years
Of the 180 participants who completed the comprehensive assessment at a mean age of 23.1 years (SD = 1.01), 149 (83%) had diurnal cortisol samples and were included in the present analyses. Of these 149 participants, 115 had been PT infants (healthy PT n = 22, sick PT n = 93) and 34 FT infants, slightly more than half were female (54%), 86% were White, 10% were African American, 1% were Native American, 1% were Asian, and 2% identified as more than one race. SES categories were equally represented across all groups. The majority of participants had completed high school or the equivalent (95%, n = 134), 80% (n = 117) were single, and 69% (n = 102) employed. As expected, the FT group had the highest birth weight and gestational age compared with the PT groups. The sick PT group had the highest neonatal risk score; longest hospital stay; most use of supplemental oxygen; and highest occurrences of BPD, NEC, sepsis, IVH grades III/IV, hydrocephalus, and meningitis of the three groups (see Table 1).
Table 1.
Neonatal Demographic Characteristics of Study Sample at Age 23 Years (N = 149) by Study Group.
| Characteristic | Full Term |
Healthy PT |
Sick PT |
F | df | p |
|---|---|---|---|---|---|---|
| (n = 34) |
(n = 22) |
(n = 93) |
||||
| M (SD) | M (SD) | M (SD) | ||||
| Birth weight (g) | 3,390.41 (436.16)a | 1,493.64 (189.65)b | 1,208.89 (327.25)c | 519.51 | 2, 146 | <.01 |
| Gestational age (weeks) | 39.85 (0.82)a | 31.14 (1.64)b | 29.81 (2.57)c | 270.56 | 2, 146 | <.01 |
| Hobel neonatal risk score | 1.53 (3.59)c | 56.59 (20.69)b | 91.18 (29.48)a | 165.15 | 2, 146 | <.01 |
| Total days hospitalized | 3.06 (0.42)c | 32.14 (9.16)b | 55.97 (26.38)a | 79.28 | 2, 146 | <.01 |
| Hours of received oxygen | 0.00 (0.00)b | 14.46 (19.15)b | 447.41 (656.13)a | 12.57 | 2, 146 | <.01 |
| n (%) | n (%) | n (%) | χ2 | df | p | |
| Gender, female | 17 (50.0) | 12 (54.5) | 51 (54.8) | 0.24 | 2 | .89 |
| Bronchopulmonary dysplasia | 0 (0)b | 0 (0)b | 20 (21.5)a | 13.91 | 2 | <.01 |
| Necrotizing enterocolitis | 0 (0)b | 0 (0)b | 13 (13.9)a | 8.58 | 2 | .01 |
| Sepsis | 0 (0)b | 0 (0)b | 10 (10.7)a | 6.46 | 2 | .04 |
| Intraventricular hemorrhage Grades III/IV | 0 (0) | 0 (0) | 8 (8.6) | 9.56 | 4 | .05 |
| Meningitis | 0 (0) | 0 (0) | 1 (1.1) | 0.61 | 2 | .74 |
| Hydrocephalus | 0 (0) | 0 (0) | 2 (2.2) | 1.22 | 2 | .54 |
Note. df = degrees of freedom; PT = preterm.
a,b,cSignificant differences between groups, a > b > c.
Participants who did not have diurnal cortisol samples (n = 31) included 4 former PT participants with developmental compromise, 22 participants (n = 9 FT, n = 2 healthy PT, and n = 11 sick PT) with scheduling conflicts, and 5 participants (n = 2 FT and n = 3 sick PT) who were dropped due to confounding variables (i.e., medication, estrogen-based birth control, and rotating sleep–wake schedule). We found no significant differences between participants who had diurnal cortisol samples at 23 years compared to those who did not on gender, neonatal illness, SES, race, level of education, employment, or occupational status. There were, however, differences between those who did and those who did not complete diurnal saliva collection: Of those who did not complete the collection, seven (23%) had sepsis, χ2(1) = 7.56, p < .01; three (10%) had meningitis, χ2(1) = 9.58, p < .01; and four (13%) had hydrocephalus, χ2(1) = 10.64, p < .01.
Preliminary Analysis of Diurnal Cortisol Patterns
As expected, there was a significant effect of sampling time of day on cortisol levels, F(4, 570) = 154.84, p < .01. Consistent with a typical diurnal pattern of HPA-axis activity, cortisol levels peaked within the first hour after awakening and reached the lowest levels at bedtime. The unstandardized slope estimate for cortisol levels between awakening and 45-min postawakening for participants as a whole was .28 (SE = .06), t(437) = 4.67, p < .01, and the unstandardized slope estimate for levels between 45-min and 4-hr postawakening was −.14 (SE = .06), t(437) = −2.30, p = .02.
Effects of Cumulative Medical Risk, Distal Socioeconomic Risk, and Proximal Social Factors on Diurnal Cortisol Pattern
Utilizing a GLMM, which accounts for repeated measures, we examined the effects of cumulative medical risk index, distal socioeconomic risk, and cumulative proximal social factors index on the diurnal cortisol pattern. This model included the main effects for each of the predictors of interest, saliva sampling time of day, two-way and three-way interactions of predictors (e.g., Cumulative Medical Risk Index × Distal Socioeconomic Risk × Cumulative Proximal Factors Index), and the three-way interaction of predictor index variables by time. The model revealed a significant three-way interaction of cumulative medical risk index, distal socioeconomic risk, and cumulative proximal social factors index on diurnal cortisol by time, F(5, 553) = 2.31, p = .04.
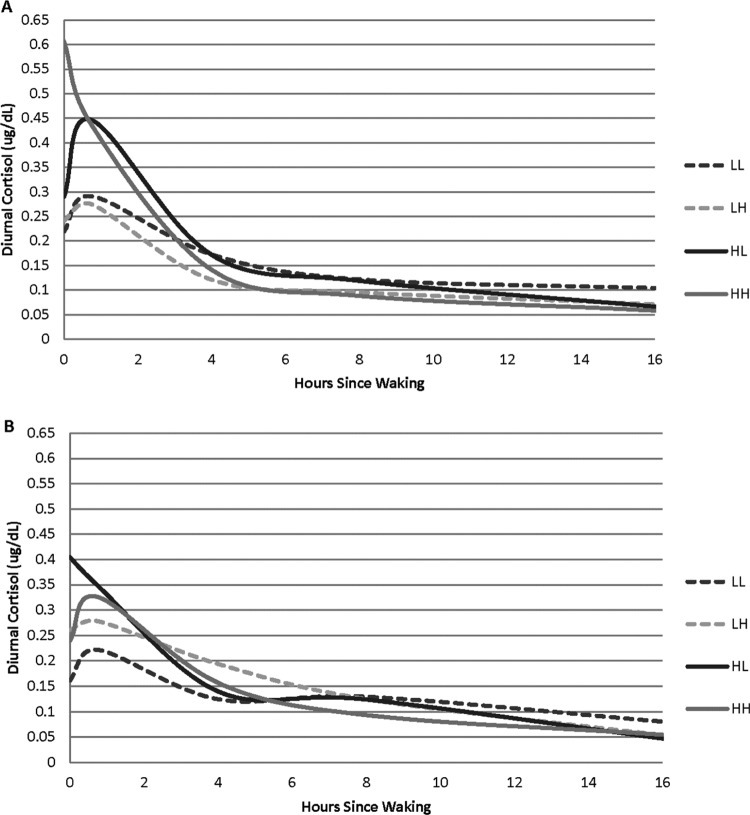
To further probe the interaction terms, we computed predicted cortisol levels for high and low levels of the predictors using parameter estimates from the initial analyses (Table 2). Specifically, this approach allowed for examination of different estimations for groups based on above average (1 SD above the mean) or below average (1 SD below the mean) proximal social factors, distal socioeconomic risk, and cumulative medical risk. To ease interpretation of the graphs, we graphed high proximal social factors (1 SD above the mean) and low proximal social factors (1 SD below the mean) separately. Within each of these graphs, we present four subgroups: high socioeconomic risk/high cumulative medical risk (HH group), high socioeconomic risk/low cumulative medical risk (HL group), low socioeconomic risk/low cumulative medical risk (LL group), and low socioeconomic risk/high cumulative medical risk (LH group).
Table 2.
Parameter Estimates From Final Model.
| Intercept | Regression Effects Models |
||||
|---|---|---|---|---|---|
| Fixed-Effect Intercept Model | Waking | 45 min After Waking | 4 hr After Waking | 8 hr After Waking | |
| b (SE) | b (SE) | b (SE) | b (SE) | b (SE) | |
| −2.74 (.07) | 1.46 (.08) | 1.60 (.08) | .84 (.08) | .53 (.08) | |
| Main effects | |||||
| Medical risk | −0.10 (.07) | 0.19 (.08) | 0.11 (.08) | .10 (.08) | −.01 (.08) |
| Distal SES risk | −0.15 (.08) | 0.40 (.09) | 0.33 (.09) | .15 (.09) | .09 (.09) |
| Proximal factors | −0.12 (.08) | 0.02 (.10) | 0.02 (.10) | .12 (.10) | .17 (.10) |
| Interaction effects | |||||
| Cumulative Medical Risk × Proximal Factors | 0.03 (.09) | −0.14 (.11) | 0.00 (.11) | .11 (.11) | .00 (.11) |
| Distal SES Risk × Proximal Factors | 0.02 (.06) | −0.06 (.07) | −0.05 (.07) | −.05 (.07) | −.05 (.07) |
| Cumulative Medical Risk × Distal SES Risk | 0.10 (.07) | −0.15 (.08) | −0.13 (.08) | −.12 (.08) | −.14 (.08) |
| Cumulative Medical Risk × Distal SES Risk × Proximal Factors | 0.04 (.07) | −0.24 (.08) | −0.08 (.08) | −.10 (.08) | −.06 (.08) |
Note. Unstandardized estimates are followed by standard errors in parentheses. Parameter estimates were derived from standardized (z-score) variables. Bedtime cortisol values were used as the reference category for the time variable. SE = standard error; SES = socioeconomic status.
Figure 1A illustrates the predicted values for individuals classified with low proximal social factors. Predicted values for the HH group seem to indicate no CAR, a sharp decline in diurnal cortisol 2-hr postawakening, a continued low diurnal pattern, and the lowest levels at bedtime. Predicted values for the HL group show a markedly high CAR. For the LH and LL groups, predicted values for the diurnal cortisol pattern appear regulated and show a typical postawakening response.
Figure 1.
Predicted diurnal cortisol pattern for medical risk and distal socioeconomic risk by (A) low and (B) high proximal social factor index. LL = low distal socioeconomic risk/low cumulative medical risk; LH = low distal socioeconomic risk/high cumulative medical risk; HL = high distal socioeconomic risk/low cumulative medical risk; HH = high distal socioeconomic risk/high cumulative medical risk.
Figure 1B illustrates the predicted values for individuals classified with high proximal social factors. Unlike predicted values for low proximal social factors, predicted values for those with high proximal social factors in the HH group do appear to show a CAR and typical pattern for diurnal cortisol. Conversely, the HL predicted values do not show a CAR. Within the LH and LL groups, the predicted values show a seemingly regulated diurnal cortisol pattern and typical postawakening response.
Discussion
This report contributes to our understanding of the influences of developmental context on HPA function in formerly premature infants. The findings reveal that cumulative medical risk and SES at birth were associated with individual differences in diurnal cortisol patterns at age 23 years. Importantly, proximal social factors (i.e., home environment and maternal perception of child vulnerability, self-esteem, control, and involvement) had protective/buffering effects, suggesting a possible mechanism for targeted interventions aimed at reducing the effects of early adversity on young adult outcomes of infants born prematurely.
Former infants most at risk of an atypical diurnal cortisol pattern included those with low cumulative proximal social factors and high cumulative medical risk. Participants with high medical risk included former PT infants with high neonatal acuity, respiratory illness (i.e., BPD), neurological illness (i.e., IVH Grades III and IV, hydrocephalus), and immune dysregulation (i.e., NEC, sepsis). Our cumulative medical risk index went beyond the neonatal period and captured new as well as ongoing medical and/or neurological problems. Consistent with the notion of toxic stress, we speculate that high cumulative medical risk increases vulnerabilities that may include downregulated immune responsiveness and increased infections due to neonatal illnesses and iatrogenic effects of medical treatments and that these effects may be compounded by frequent painful postnatal medical procedures. This, in turn, may make these individuals more sensitive to environmental factors, thus placing them at risk for poor immune, cardiovascular, endocrine, and metabolic function; increased infections due to high inflammatory response (Blalock, 2005); and consequences for psychological health (Johnson et al., 2013; Rhen & Cidlowski, 2005).
To our knowledge, ours is the first study investigating the effects of cumulative proximal social factors on regulation of diurnal cortisol patterns in PT infants at young adulthood. In the present sample, cumulative proximal social factors significantly contributed to the adult diurnal pattern for former PT infants. This cumulative index included aspects of positive stimulation within the home environment, maternal perception of child vulnerability, maternal self-esteem, maternal control style, and maternal involvement from birth to age 12 years. Numerous studies report findings consistent with the notion that the developing HPA axis is socially regulated and that features of the proximal caregiving environment, in particular, have profound effects on the HPA-axis stress response system. Prevention science is focused on designing interventions to modify the caregiving environment as a means to reduce the effects of early adversity on later adult outcomes. The present findings underscore that these programs and techniques may be especially important to develop and apply in the context of the early caregiving environments of infants born prematurely.
While the diurnal patterns shown in Figure 1A make sense due to high socioeconomic risk and low proximal social factors, the pattern for the HL group in Figure 1B is unexpected, given the high proximal social factors. There are several potential mechanisms that could be responsible for altering the diurnal pattern of cortisol production. Cortisol secretion is affected by a range of feedbacks, both positive and negative, as well as the anatomical and functional pathways connecting the hippocampus to the suprachiasmatic nucleus (SCN). Generally speaking, the SCN in the hypothalamus regulates the diurnal activity of the HPA axis (Clow, Hucklebridge, Stadler, Evans, & Thorn, 2010). The hippocampus plays a central role in the regulation of the CAR, which is illustrated by the sharp rise and fall of cortisol in the first waking hour (e.g., Figure 1B, LL group). The SCN and HPA axis are not fully developed at birth. Numerous aspects of the circumstances surrounding premature birth and associated neurological complications have the potential to affect the developmental trajectory of the immature SCN and HPA axis. Studies have shown that hypoactivity of the HPA axis may be due to an increased negative feedback sensitivity to glucocorticoids (Fries, 2008) and hypersecretion of cortisol may be due to an unresponsive negative glucocorticoid feedback in the HPA-axis loop. Studies that explore individual differences in cortisol reactivity and regulation in response to the Trier Social Stress test, administration of low-dose dexamethasone, and characterization of glucocorticoid receptor sensitivity in peripheral blood mononuclear cells in adults who were born premature would be worthwhile next steps for beginning to disentangle the sources of atypical cortisol production.
Strengths and Limitations
Although this study had many strengths, one limitation is our restriction of saliva sampling to a single day: We collected diurnal salivary cortisol 5 times on one weekday. The full study protocol at the 23-year time point involved more than 10 hr of data collection over 2–3 days per participant. Given the primary study aims, we were sensitive to the participant burden that would be added by including multiple days of saliva collection. While studies suggest that there is considerable day-to-day variation in the diurnal pattern of cortisol production (Doane & Adam, 2010), recent reviews have suggested that in large epidemiological research, the “minimal protocol” needed for reliable measurement of the diurnal rhythm is three saliva samples in 1 day (Adam et al., 2010).
We carefully considered and addressed issues of potential confounders. The sample size precluded our ability to categorize individuals when probing the interactions, thus we utilized predicted group values from the model estimates. Future research using larger samples should examine the different combinations of medical, socioeconomic, and proximal social factors using observed data.
Conclusion
To our knowledge, this is the only prospective, longitudinal study in the United States following participants from birth to age 23 years with a heterogeneous PT sample and a FT comparison group that investigated the effects of cumulative medical and socioeconomic risks, in conjunction with cumulative proximal social factors, on the HPA stress response system. The PT birth rate in the United States remains steady at 11.4% (March of Dimes Foundation, 2014). The incorporation of multiple indicators of cumulative risk and proximal social factors offers predictive models of health and developmental outcomes for premature infants (Halfon, Larson, Lu, Tullis, & Russ, 2014) as well as a powerful tool in nursing practice. Variables within risk indexes offer identification markers of those at risk for poor HPA-axis regulation, while the promotive effects of family-based protective factors serve as informative building blocks for the development of intervention programs. The predictive utility of these indexes illustrates how positive proximal influences alter the effects of toxic stress and subsequent health and developmental outcomes and suggests modifiable contexts in which to ameliorate the effects of risk and stress on diurnal patterns of cortisol and, subsequently, the HPA system.
Acknowledgments
This study would not have been possible without the commitment of the participating families. We would also like to acknowledge the dedication of our research team: Robin Miller, Manuela Barcelos, Erica Oliveira, Abbie Popa, and Matthew Straub as well as Tracey Hand, Jessica Parker, and Jessica Bayer for technical support with salivary assays.
Footnotes
Authors’ Contribution: Suzy B. Winchester contributed to data acquisition, data analysis, and interpretation; drafted the manuscript; gave final approval; and agreed to be held accountable for all aspects of work, ensuring integrity and accuracy. Mary C. Sullivan contributed to conception, design, data acquisition, data analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be held accountable for all aspects of work, ensuring integrity and accuracy. Mary B. Roberts contributed to data analysis and interpretation; critically revised the manuscript; gave final approval; and agreed to be held accountable for all aspects of work, ensuring integrity and accuracy. Crystal I. Bryce contributed to data analysis and interpretation; critically revised the manuscript; gave final approval; and agreed to be held accountable for all aspects of work, ensuring integrity and accuracy. Douglas A. Granger contributed to data acquisition, data analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be held accountable for all aspects of work, ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Douglas A. Granger is the founder and Chief Strategy and Scientific Advisor at Salimetrics LLC and SalivaBio LLC, and these relationships are managed by the policies of the committees on conflict of interest at Johns Hopkins University School of Medicine and University of California, Irvine. Suzy B. Winchester, Mary C. Sullivan, Mary B. Roberts, and Crystal I. Bryce declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Institutes of Health and National Institute of Nursing Research (R01 NR003695).
References
- Adam E. K., Doane L. D., Zinbarg R. E., Mineka S., Craske M. G., Griffith J. W. (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35, 921–931. doi:10.1016/j.psyneuen.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aucott S. W., Watterberg K. L., Shaffer M. L., Donohue P. K. (2008). Do cortisol concentrations predict short-term outcomes in extremely low birth weight infants? Pediatrics, 122, 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. (1995). The fetal and infant origins of disease. European Journal of Clinical Investigations, 25, 457–463. [DOI] [PubMed] [Google Scholar]
- Beijers R., Riksen-Walraven J. M., De Weerth C. (2013). Cortisol regulation in 12-month-old human infants: Associations with the infants’ early history of breastfeeding and co-sleeping. Stress, 6, 267–277. doi:10.3109/10253890.2012.742057 [DOI] [PubMed] [Google Scholar]
- Berry D., Blair C., Willoughby M., Granger D. A., Mills-Koonce W. R., & Family Life Project Key Investigators. (2016). Maternal sensitivity and adrenocortical functioning across infancy and toddlerhood: Physiological adaptation to context? Developmental Psychopathology, 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Granger D. A., Willoughy M., Mills-Koonce R., Cox M., Greenberg M. T.,…the FLP Investigators. (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82, 1970–1984. doi:10.1111/j.1467-8624.2011.01643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock J. E. (2005). The immune system as the sixth sense. Journal of Internal Medicine, 257, 126–138. doi:10.1111/j.1365-2796.2004.01441.x [DOI] [PubMed] [Google Scholar]
- Brummelte S., Chau C. M. Y., Cepeda I. L., Degenhardt A., Weinberg J., Synnes A. R., Grunau R. E. (2015). Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology, 51, 151–163. doi:10.1016/j.psyneuen.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A., Krieger S., Wilkes C., Rauh W., Weiss S., Hellhammer D. H. (2007). Hypothalamic-pituitary-adrenal axis function and the cellular immune response in former preterm children. Journal of Clinical Endocrinology and Metabolism, 92, 3429–3435. doi:10.1210/jc.2006-2223 [DOI] [PubMed] [Google Scholar]
- Caldwell B. M., Bradley R. H. (2001). Home inventory administration manual (3rd ed). Little Rock, AR: University of Arkansas at Little Rock. [Google Scholar]
- Cicchetti D., Rogosch F. A. (2012). Neuroendocrine regulation and emotional adaptation in the context of child maltreatment. Monographs of the Society for Research in Child Development, 77, 87–95. doi:10.1111/j.1540-5834.2011.00666.x [Google Scholar]
- Clow A., Hucklebridge F., Stadler T., Evans P., Thorn L. (2010). The cortisol awakening response: More than a measure of HPA axis function. Neuroscience and Biobehavioral Reviews, 35, 97–103. doi:10.1016/j.neubiorev.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Dalziel S. R., Parag V., Rodgers A., Harding J. E. (2007). Cardiovascular risk factors at age 30 following pre-term birth. International Journal of Epidemiology, 36, 907–915. doi:10.1093/ije/dym067 [DOI] [PubMed] [Google Scholar]
- De Boo H. A., Harding J. E. (2006). The developmental origins of adult disease (Barker) hypothesis. Australian and New Zealand Journal of Obstetrics and Gynecology, 46, 4–14. doi:10.1111/j.1479-828X.2006.00506.x [DOI] [PubMed] [Google Scholar]
- Doane L. D., Adam E. K. (2010). Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology, 35, 430–441. doi:10.1016/j.psyneuen.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K., Drevets W., Schulkin J. (2003). Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience and Biobehavioral Reviews, 27, 233–246. doi:10.1016/S0149-7634(03)00033-2 [DOI] [PubMed] [Google Scholar]
- Farran D. C., Kasari C., Yoder P., Harber L., Huntington G., Comfort-Smith M. (1987). Rating mother-child interaction in handicapped and at risk infants In Tamir D., Brazelton T. B., Russell A. (Eds.), Stimulation and intervention in infant development (pp. 297–312). London, England: Freunds Publishing House, Ltd. [Google Scholar]
- Fries E. (2008). Hypocortisolemic disorders (Vol. 174). Basel, Switzerland: Karger. [Google Scholar]
- Gluckman P. D., Hanson M. A., Pinal C. (2005). The developmental origins of adult disease. Maternal and Child Nutrition, 1, 130–141. doi:10.1111/j.1740-8709.2005.00020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. A., Hibel L. C., Fortunato C. K., Kapelewski C. H. (2009). Medication effects on salivary cortisol: Tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34, 1437–1448. doi:10.1016/j.psyneuen.2009.06.017 [DOI] [PubMed] [Google Scholar]
- Granger D. A., Johnson S. B., Szanton S. L., Out D., Lau Schumann L. (2012). Incorporating salivary biomarkers into nursing research: An overview and review of best practices. Biological Research in Nursing, 14, 347–356. doi:10.1177/1099800412443892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau R. E., Haley D. W., Whitfield M. F., Weinberg J., Yu W., Thiessen P. (2007). Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. Journal of Pediatrics, 150, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M., Donzella B. (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27, 199–220. doi:10.1016/S0306-4530(01)00045-2 [DOI] [PubMed] [Google Scholar]
- Gunnar M., Vazquez D. M. (2001). Low cortisol and a flattening of expected daily rhythm: Potential indices of risk in human development. Development and Psychopathology, 13, 515–538. [DOI] [PubMed] [Google Scholar]
- Gustafsson P. E., Janlert U., Theorell T., Hammarstrom A. (2010). Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology, 35, 613–623. doi:10.1016/j.psyneuen.2009.09.019 [DOI] [PubMed] [Google Scholar]
- Halfon N., Larson K., Lu M., Tullis E., Russ S. (2014). Lifecourse health development: Past, present, future. Maternal Child Health, 18, 344–365. doi:10.1007/s10995-013-1346-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan K., McCarthy A. M., Kleiber C., Lutgendorf S., Tsalikian E. (2006). Strategies for salivary cortisol collection and analysis in research with children. Applied Nursing Research, 19, 95–101. doi:10.1016/j.apnr.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Hobel C. J., Hyvarinen M. A., Okada D. M., Oh W. (1973). Prenatal and intrapartum high-risk screening: I. Prediction of the high-risk neonate. American Journal of Obstetrics and Gynecology, 117, 1–9. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. B. (1975). Four factor index of social status. New Haven, CT: Yale University. [Google Scholar]
- Holsti L., Grunau R. E., Oberlander T. F., Whitfield M. F. (2005). Prior pain induces heightened motor responses during clustered care in preterm infants in the NICU. Early Human Development, 81, 293–302. doi:10.1016/j.earlhumdev.2004.08.002 [DOI] [PubMed] [Google Scholar]
- Holsti L., Weinberg J., Whitfield M. F., Grunau R. E. (2007). Relationships between andrenocorticotropic hormone and cortisol are altered during clustered nursing care in preterm infants born at extremely low gestational age. Early Human Development, 83, 341–348. doi:10.1016/j.earlhumdev.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Jacobson S. W., Bihun J. T., Chiodo L. M. (1999). Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Development and Psychopathology, 11, 195–208. [DOI] [PubMed] [Google Scholar]
- Johnson S. B., Riley A. W., Granger D. A., Riis J. (2013). The science of early life toxic stress for pediatric practice and advocacy. Pediatrics, 131, 319–327. doi:10.1542/peds.2012-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Halfon N. (2003). Racial and ethnic disparities in birth outcomes: A life-course perspective. Maternal and Child Health Journal, 7, 13–30. [DOI] [PubMed] [Google Scholar]
- March of Dimes Foundation. (2014). March of Dimes 2014 premature birth report card. White Plains, NY: Author. [Google Scholar]
- March of Dimes Perinatal Data Center. (2011). Special care nursery admissions. White Plains, NY: National Perinatal Information System/Quality Analytic Services. [Google Scholar]
- Matthews S. G. (2002). Early programming of the hypothalamo-pituitary-adrenal axis. Trends in Endocrinology & Metabolism, 13, 373–380. doi:10.1016/S1043-2760(02)00690-2 [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Lasley E. N. (2003). Allostatic load: When protection gives way to damage. Advances in Mind-Body Medicine, 19, 28–33. [PubMed] [Google Scholar]
- McGrath M. M. (1997). Estimating risk and protect indexes in high-risk children. Clinical Effectiveness in Nursing, 1, 92–104. [Google Scholar]
- Miller G. E., Chen E., Zhou E. S. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi:10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Niswander K. R., Gordon M. (1972). The collaborative perinatal study: The women and their pregnancies. Philadelphia, PA: W. B. Saunders. [Google Scholar]
- Osterholm E. A., Hostinar C. E., Gunnar M. (2012). Alterations in stress responses of the hypothalamic-pituitary-adrenal axis in small for gestational age infants. Psychoneuroendocrinology, 37, 1719–1725. doi:10.1016/j.psyneuen.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Prechtel H., Beitema D. (1967). The neurological examination of the full-term newborn infant. London, England: Heinemann. [Google Scholar]
- Rhen T., Cidlowski J. A. (2005). Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. New England Journal of Medicine, 353, 1711–1723. doi:10.1056/NEJMra050541 [DOI] [PubMed] [Google Scholar]
- Saigal S. (2014). Functional outcomes of very premature infants into adulthood. Seminars in Fetal & Neonatal Medicine, 19, 125–130. doi:10.1016/j.siny.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Scott A., Winchester S. B., Sullivan M. C. (2017). Trajectories of problem behaviors from 4 to 23 years in former preterm infants. International Journal of Behavioral Development. Advance online publication. doi:10.1177/0165025417692899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifer R., McGrath M. M. (1991). Problem solving task scoring manual. Providence, RI: Brown University and Bradley Hospital. [Google Scholar]
- Sekisui Diagnostics LLC. (2012). OSOM® hCG urine control set. Catalog #134. San Diego, CA: Author. [Google Scholar]
- Sullivan M. C., Hawes K., Winchester S. B., Miller R. J. (2008). Developmental origins theory from prematurity to adult disease. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 37, 158–164. doi:10.1111/j.1552-6909.2008.00216.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M. C., McGrath M. M. (1999). Proximal and distal correlates of maternal control style. Western Journal of Nursing Research, 21, 313–334. [DOI] [PubMed] [Google Scholar]
- Tukey J. W. (1977). Exploratory data analysis. Reading, MA: Addison-Wesley. [Google Scholar]
- Walker B. R., Irving R. J., Andrew R., Belton N. R. (2002). Contrasting effects of intrauterine growth retardation and premature delivery on adult cortisol secretion and metabolism in man. Clinical Endocrinology, 57, 351–355. doi:10.1046/j.1365-2265.2002.01606.x [DOI] [PubMed] [Google Scholar]
- Watterberg K. L., Gerdes J. S., Cole C. H., Aucott S. W., Thilo E. H., Mammel M. C.…Shaffer M. L. (2004). Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: A multicenter trial. Pediatrics, 14, 1649–1657. doi:10.1542/peds.2004-1159 [DOI] [PubMed] [Google Scholar]
- Winchester S. B., Sullivan M. C., Msall M. E. (2014). Executive function in infants born preterm with varying birth weights and morbidities at emerging adulthood In Bennett K. P. (Ed.), Executive functioning: Role in early learning processes, impairments in neurological disorders and impact of cognitive behavior therapy (CBT) (pp. 81–114). Hauppauge, NY: Nova Science. [Google Scholar]