Abstract
Sickness behaviors, adaptive responses to infections, include lethargy, depression, reduced eating and grooming, and concentration problems resulting from interactions between the immune and neuroendocrine systems. Detecting these responses is especially critical in the elderly, as the infections that cause them can lead to cognitive impairment. While deficits in spatial learning, a hippocampal-dependent form of learning, are part of the sickness response, directional heading errors (DHEs; an indicator of spatial-learning deficits) and their time trajectories need further examination. Therefore, we simultaneously investigated the time trajectory of age-dependent sickness responses and spatial learning over 5 days in adult (5–6 months) and aged (22 months) male Brown-Norway rats injected with 250 μg/kg lipopolysaccharide (LPS; experimental group) or 0.9% sodium chloride (control group). LPS administration resulted in pronounced, age-dependent weight loss and decreased food intake that persisted in the aged group. Animals were tested for 5 days (trial) in the Morris water maze. After 7 days of rest, animals were retested for 2 days (retention). Adult and aged LPS-treated animals displayed greater differences in mean DHE than the control groups, indicating that they exhibited more DHE over the trial days. Experimental groups did not show consistent DHE improvement until Day 4 (adult) or 5 (aged). LPS had no effect on probe or retention trials. We conclude that LPS activation of the immune system results in a selective, age-dependent impairment in spatial learning, decreased food intake, and weight loss. All of these results are prolonged in aged animals.
Keywords: aging, illness behavior, intraperitoneal lipopolysaccharide, maze learning/*drug effects, memory, Brown-Norway, time factors
Acute infections, whether viral or bacterial, generate a host of responses ranging from fever to loss of appetite in individuals of all ages. While these responses, collectively called sickness behaviors, are considered adaptive and thought to facilitate recovery, they also can have deleterious effects in some individuals (Kelley et al., 2003). In older adults, an immune challenge such as an infection can lead to impaired cognition (Bucks et al., 2008), delirium (Fong, Tulebaev, & Inouye, 2009), or neurodegenerative disease progression (Perry, Cunningham, & Holmes, 2007). Other changes in the elderly related to infection may include a decline in functional ability or an inability to return to their previous state of functioning related to self-care and independence (Bula, Ghilardi, Wietlisbach, Petignat, & Francioli, 2004; Caljouw et al., 2013; Gozalo, Pop-Vicas, Feng, Gravenstein, & Mor, 2012). Associations between infections and cognitive impairment in the elderly are an area of increased investigation.
To better understand these complex correlations (among infection, infection responses, and cognition), animal models that parallel human behaviors are effective. An immune challenge with intraperitoneal (ip) administration of lipopolysaccharide (LPS), a component of the cell wall of gram-negative bacteria, is used in animals to simulate an infection. In response to an immune challenge, pro-inflammatory cytokines, which mediate sickness responses including decreased food intake and motor activity, are released (Godbout et al., 2005; Huang, Henry, Dantzer, Johnson, & Godbout, 2008; Kelley et al., 2003). In aged animals, research has shown that the cytokine response to an immune challenge is greater and more sustained than in younger animals, resulting in more pronounced responses including a greater decrease in food intake (Huang et al., 2008), increased weight loss (Godbout et al., 2005), cognitive impairment (Barrientos et al., 2006), and recovery delays such as a persistent decrease in activity (Abraham & Johnson, 2009). While studies have shown sickness responses to persist in aged animals, the length of time for which investigators have evaluated animals postimmune challenge has varied. Further, few studies have determined when behaviors return to baseline (Godbout et al., 2008). Clarifying this time trajectory will indicate when adaptive behaviors become harmful and will contribute to the development of interventions designed to prevent negative outcomes.
In the present study, we focused on the effects of an immune challenge with LPS on cognitive function in rats, specifically spatial learning and memory. Spatial learning depends on environmental interactions characterized by exploration and searching in humans as well as animals. The ability to learn and recall places in the environment is essential for survival (Vorhees & Williams, 2014a). Animals need to be able to search for food and return to their homes, and humans need to be able to understand and find their way (navigate) around familiar and new environments (Vorhees & Williams, 2014b). Although spatial navigation is a necessary skill for successful functioning in humans, navigational ability may be diminished in the aged (Moffat, 2009). While animal studies evaluating the effects of immune challenge on spatial learning have revealed deficits based on varied indices (Arai, Matsuki, Ikegaya, & Nishiyama, 2001; Barrientos et al., 2006; Shaw, Commins, & O’Mara, 2001), the results have been contradictory, partly due to variation among protocols. For the present study, we used the Morris water maze (MWM), which involves place learning and navigation and is a well-established test of spatial learning and memory (Vorhees & Williams, 2014b).
Although the accepted standard of study for establishing learning curves using the MWM is 5–6 days, generally the time needed to reach asymptotic performance (Vorhees & Williams, 2006), the period that researchers have used for evaluating spatial learning has varied from 2 to 5 days. The duration of retention studies, during which animals are retested in the MWM after a defined rest period, has varied among studies from 1 to 3 days following rest periods of 0–7 days. Spatial learning measures generally have included swim latency (seconds to reach a hidden platform) or path length. Investigators have found directional heading error (DHE), the difference between the animal’s initial heading position and the most direct path to the platform, to be a sensitive indicator of spatial learning in other paradigms (Hebda-Bauer, Morano, & Therrien, 1999). However, researchers have not reported DHE in animals facing an immune challenge. Probe trials, another measure of spatial learning, have not been consistently included in spatial learning studies and, when they are, the probe trial timing has varied (Cunningham & Sanderson, 2008).
There are a limited number of studies evaluating the effects of an immune challenge on spatial learning concurrently with other behaviors in aged animals. Examining how long cognitive and behavioral effects persist and how recovery is affected is critical to understanding brain–behavior relationships in aged animals. This knowledge also could provide the foundations for early biomarker identification that has the potential to limit spatial impairments and provide safe environments for patients. Such research is especially important for elderly patients who experience increased difficulty with spatial navigation (Davis, Therrien, & West, 2008; Head & Isom, 2010; Moffat, Zonderman, & Resnick, 2001). The aim of the present study was to characterize the profile and time trajectory of sickness responses and their relationships to spatial learning and memory in adult and aged animals after an immune challenge with LPS. Measured outcomes were food intake, weight change, and spatial learning and memory (retention).
Material and Method
We used a repeated-measures four-group experimental design to examine the effects of ip administration of LPS on food intake, weight change, and spatial learning.
Procedures
Animals
We obtained 33 adult (5–6 months, n = 16) and aged (22 months, n = 17) male Brown-Norway rats from Harlan Sprague-Dawley Inc. The Brown-Norway rat, an accepted aging model due to its life span (50% survival is 32 months of age) and lack of pathologies (Turturro et al., 1999), has not been used to examine the effects of LPS on spatial learning. We housed all animals individually in a temperature-controlled room (25°C) on a reverse 12-hr light–dark cycle and allowed them free access to standard rodent food and water. We handled and weighed animals daily 1 hr prior to lights off. The University of Michigan Animal Care and Use Committee approved all procedures.
Treatment protocols
LPS from Escherichia coli (Sigma-Aldrich Corporation, St. Louis, MO; serotype 0111:B4) was dissolved in filtered sterile 0.9% sodium chloride solution. We randomly assigned animals within each age-group to the experimental or control group and gave them a single ip injection of either 250 μg/kg LPS (n = 10/age-group) or saline (0.9%; adult: n = 6; aged: n = 7). We chose this LPS dose because it elicited differences in food intake and weight loss in adult and aged animals during pilot work. We administered injections 3 hr after lights off so that we could conduct behavioral testing during the animals' active period. MWM testing began 1½ hr after injections for the adult and 5 hr after for the aged groups. We chose these times based on evidence showing that the pattern of temperature responses, an indicator of sickness in animals, differs between adult and aged animals (Florez-Duquet, Peloso, & Satinoff, 2001; Foster, Conn, & Kluger, 1992).
Sickness behaviors
An immune challenge results in responses such as decreased activity and food intake (Dantzer, 2004). In this study, we considered the primary indicators of sickness to be reduced food intake and subsequent weight loss. We recorded weight and food intake daily.
Spatial learning
We used the MWM to assess spatial learning, adapting our procedure from Therrien’s (1982) study on hippocampal lesions and place navigation studies (Hebda-Bauer et al., 1999). The tub environment simulates place learning in a large-scale space. The task requires animals to locate a hidden platform using a stationary array of extra maze cues. Rats cannot identify the platform, which is submerged 1 cm under the water’s surface and 15 cm from the tub wall, visually or by sound or smell. The temperature of the water was 35°C ± 1°C. Testing involved four trials a day for 5 days. Researchers examining learning curves generally consider 5−6 days adequate for animals to reach an asymptotic level of learning (Vorhees & Williams, 2006). After 5 testing days, animals rested for 7 days, and then we conducted 2 days of retention testing using the same protocol.
We recorded DHE and swim latency to assess spatial learning. To compute DHE, we determined the degree of difference between the animal’s initial heading position (when it straightened and swam one body length) and the most direct path to the platform. The study’s primary investigator recorded the directional heading. Prior to testing, we established an interrater reliability of .90. Swim latency was the time in seconds the animal took to reach the platform.
We conducted a probe trial at the end of both testing periods, that is, Day 5 of initial testing and Day 2 of retention testing, to determine if animals had learned the platform location. During the probe trials, we removed the platform and allowed the animal to swim freely for 30 s. We recorded the path each animal took, subsequently traced that path to determine the distance the animal traveled in each quadrant of the tub, and finally quantified the percentage of the total distance traveled that the animal completed in the correct (goal) quadrant in comparison to the other quadrants.
Data Analysis
We analyzed data using the SAS statistical software (Version 9.3, Cary, NC). All statistical assumptions underlying the analysis techniques were assessed. Because of nonconstant variance among the groups, we analyzed variables using SAS Proc Mixed to fit a linear mixed model with heterogeneous error variances. Different covariance structures were considered to determine the best-fitting model. We analyzed all outcome data using mixed-model analysis of variance that examined the effects of between-subject factors of group based on age (adult or aged) and experimental status (control or experimental) and a within-subject factor based on time (days postinjection). We examined all variables over a 5-day period after injections to assess trends and determine their persistence over time. To determine whether trends over the 5-day postinjection period were similar between the groups, we also examined two- and three-way interactions between the within-subject factor of time and the between-subject factor of group. Where appropriate, we conducted post hoc comparisons of the means using a Tukey–Kramer adjustment for multiple comparisons. A significance level of α = .05 was considered in all analyses.
Prior to these analyses, we obtained descriptive statistics on all main study variables. We examined group differences in food intake, actual weight, and DHE using independent sample t tests with IBM SPSS 22.0. The residual errors in the dependent variables of DHE and swim latency were not normally distributed; therefore, we transformed the variables using a natural log transformation and used the transformed variables in all analyses. However, we used the actual data in all charts and refer to the variables by actual variable names. We averaged the DHE and swim-time latency data by trial day and, for each probe trial, we calculated the total percentage of time spent in the quadrant where the platform had been located.
Results
Food Intake
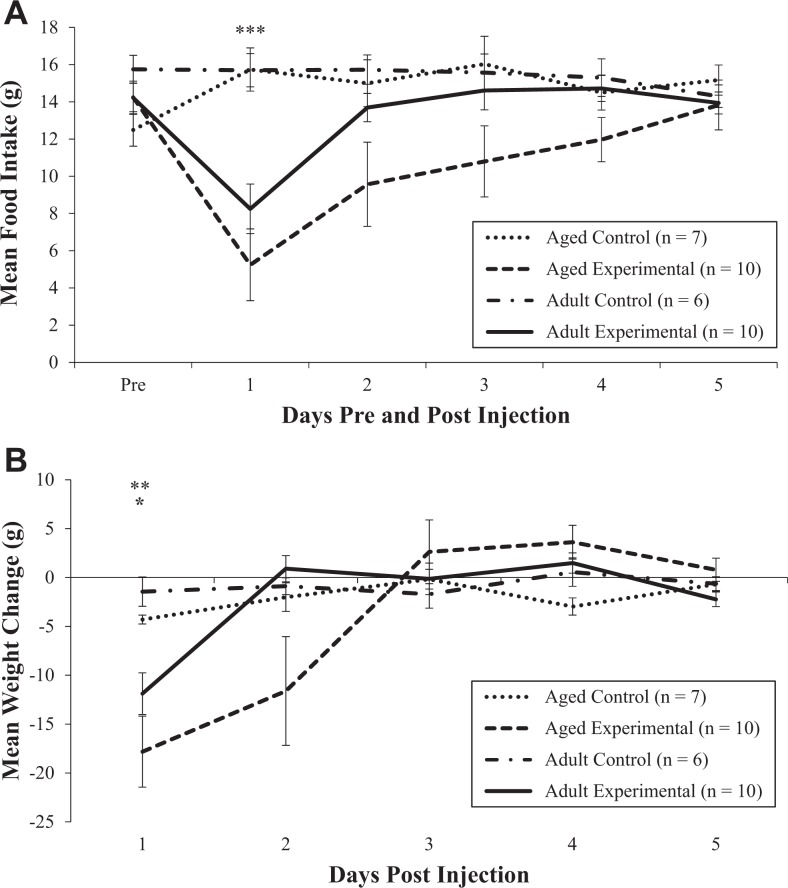
Analysis of average daily food intake prior to the injections revealed no differences between the adult and aged groups (p = .89). Within the first 24 hr following LPS injections, food intake was markedly decreased for experimental animals in both age-groups. On Day 2, food intake in the aged experimental animals continued to be significantly reduced, while adult animals began to increase their intake. Although the food intake in both groups was near baseline levels by Day 5, the increase in intake was more gradual in the aged animals than in the adult animals, as shown in Figure 1A. A repeated-measures analysis of food intake showed a main effect of experimental group, F(1, 30) = 15.26, p = .0005, and day, F(4, 118) = 4.97, p = .001, and a significant interaction between experimental group and day, F(4, 118) = 7.43, p < .0001. There was not a main effect of age (p = .11). Post hoc analysis confirmed significant differences in food intake between the control and experimental groups overall on Day 1 (p < .0001).
Figure 1.
Effect of lipopolysaccharide on mean food intake (A) and weight change (B) in adult and aged rats. Error bars represent ± standard error of the mean (SE). *p < .05 for change in weight for adult control and experimental groups on Day 1 (B); **p < .01 for change in weight for aged control and experimental groups on Day 1 (B); ***p < .001 for food intake for control and experimental groups (age-groups combined) on Day 1 (A).
Weight Change
Baseline weight did not differ between control and experimental animals within the adult (p = .56) or aged (p = .39) groups. Following LPS administration, both experimental groups lost a significant amount of weight on the first day. While the decrease in weight was similar between the adult and aged experimental groups on Day 1 post-LPS (3.4% and 3.9%, respectively), only the aged experimental group continued to lose weight on Day 2 (2.6% decrease). By Day 3, animals in the aged experimental group began to gain weight, as shown in Figure 1B. A repeated-measures analysis revealed significant differences in mean weight loss across all testing days among the groups based on experimental status, F(1, 30) = 7.40, p = .01, and age, F(1, 30) = 5.11, p = .031, and across the days for all groups, F(4, 114) = 13.76, p < .0001. There also were significant interactions between experimental status and day, F(4, 114) = 8.80, p < .0001, and age-group and day, F(4, 114) = 3.26, p = .01. There was a trend toward significance in the three-way interaction between experimental group, age-group, and day, F(5, 114) = 2.05, p = .076. Post hoc analysis confirmed a significant difference in weight loss between the experimental groups and control groups on Day 1 in the aged (p = .001) and adult (p = .05) animals. There were also significant differences in weight loss between the aged and adult groups overall on Day 2 (p = .033). None of the four groups had returned to baseline weight by Day 5 after the injections. However, the decrease in weight from baseline was greatest in the adult (p = .038) and aged experimental groups (3.4% and 5.2%, respectively). After 7 days of rest, the weight of the aged control and experimental groups remained decreased in comparison to baseline weight, while the weight of both adult groups had increased to more than baseline weight. Although the difference was not significant, the changes in weight were slightly larger in the experimental groups.
Spatial Learning
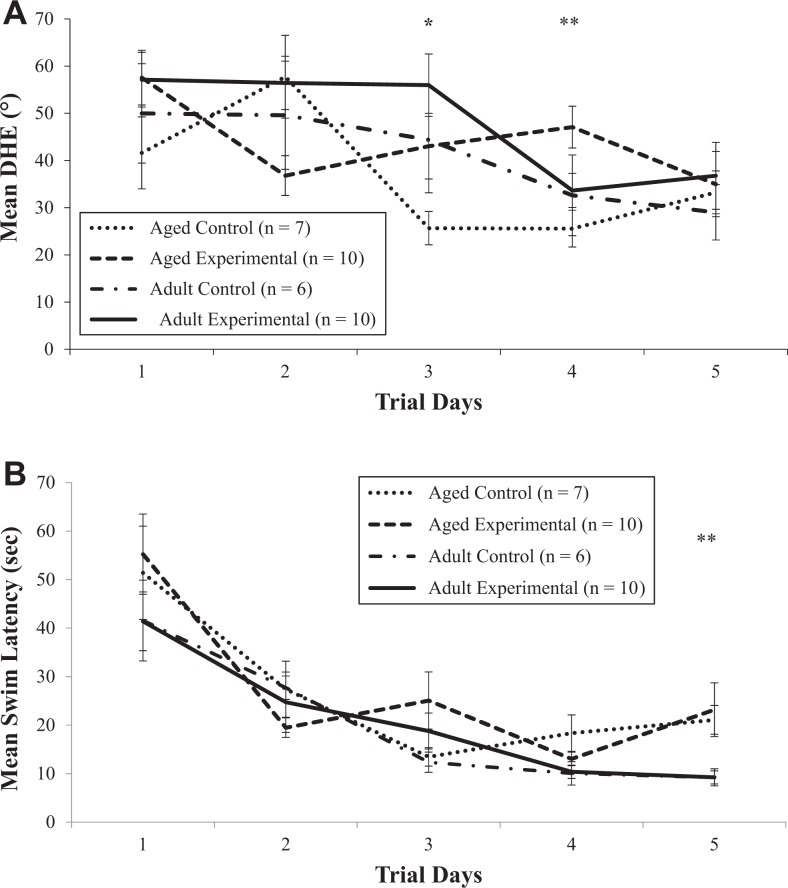
Following injections, the DHE learning curves of the adult and aged control groups improved over time. However, the adult experimental group did not display an improvement in DHE until Day 4. In the aged experimental group, while DHE improved on Day 2, the learning curve was not sustained as DHE increased on Day 3 and did not improve again until Day 5. Figure 2A shows the mean DHE for all four groups for each day. Repeated-measures analysis revealed significant differences in mean DHE across the 5 days postinjections for all groups, F(4, 120) = 8.63, p < .0001, indicating DHE improved over time, and in the two-way interaction between trial day and experimental status, F(4, 120) = 2.40, p = .053, suggesting that the pattern of DHE varied between the control and experimental groups across the postinjection days. There were trends toward significance between the control and experimental groups averaged across the days, F(1, 30) = 3.72, p = .063, indicating that LPS-treated animals displayed greater DHE over the trial days, and in the two-way interaction between trial day and age-group, F(4, 120) = 2.36, p = .057, suggesting different trends over time in the two age-groups. Post hoc comparisons revealed significant differences in mean DHE in the experimental group (age-groups combined) between Days 1 and 5 (57.34 ± 3.98 degrees vs. 35.87 ± 4.81 degrees, respectively, p = .0009) and in the control group (age-groups combined) between Days 2 and 4 (53.99 ± 6.88 degrees vs. 28.82 ± 4.38 degrees, respectively, p = .002). These results indicate that the control animals displayed an improvement in DHE earlier than did experimental animals. Further, t tests revealed significant differences in DHE between the control and experimental groups, within the aged animals only, on Days 3 (p = .044) and 4 (p = .004), suggesting that errors in directional heading persisted in the aged experimental animals. Retesting DHE for retention revealed no differences between the groups based on experimental status (p = .48), suggesting that animals had learned the platform location.
Figure 2.
Effect of lipopolysaccharide on mean directional heading error (DHE) (A) and swim latency (B) in adult and aged rats. Error bars represent ± standard error of the mean (SE). *p < .05 and **p < .01 for DHEs for aged control and experimental groups on Days 3 and 4 (A). **p < .01 for swim latency for adult and aged groups (control and experimental groups combined) on Day 5 (B).
Analysis of swim latency showed that while all four groups improved over time, the learning curves were not equal. Both adult groups showed typical learning curves with an asymptote reached by Day 4. In contrast, both aged groups showed inconsistent learning curves (Figure 2B). Repeated-measures analysis revealed significant differences in mean swim-time latency among the groups across the days based on age, F(1, 30) = 9.08, p = .005, indicating that the swim latency varied over trial days 1–5 based on age and across the 5 days postinjection for all the groups, F(4, 124) = 39.70, p < .0001. These results indicate that swim latency improved over time. There also was a significant interaction between trial day and age, F(4, 124) = 2.85, p = .026, suggesting that the swim latency varied between the age-groups. Post hoc comparisons revealed significant differences between the first day of testing and the remaining 4 days in both adult (p = .01) and aged (p < .0001) groups (control and experimental combined), indicating that swim time improved in all groups after the first trial day. Further, there were significant differences in mean swim latency between the adult and aged groups overall on Day 5, as the swim latency of the aged group was twice that of the adult group (22.33 ± 3.40 s vs. 9.23 ± 1.17 s, respectively, p = .002). Retesting swim latency for retention revealed that LPS did not affect retention (p = .59).
LPS had no effect on the percentage of distance traveled that was in the correct quadrant. There was a significant effect of time across groups, F(1, 30) = 12.01, p = .0016, indicating that the distance traveled in the correct quadrant improved from the first to the second probe trial and among the groups based on age, F(1, 28) = 4.97, p = .034. While both age-groups improved the distance traveled in the correct quadrant from the first to the second probe trial, the adult groups traveled a greater distance in the correct quadrant overall.
Discussion
Our results show that an LPS immune challenge produced more severe sickness behaviors in aged animals than in adult animals. These responses were characterized primarily by decreased food intake and subsequent weight loss. Although food intake improved by Day 5 postinjection, which marked the end of MWM testing, the increased intake was not sufficient for weight to return to baseline levels in any of the four groups. After 7 days of rest following the end of MWM testing, weight had returned to baseline levels in both adult groups but not in the aged groups. While this finding may be explained partly by the exercise the animals performed in the MWM, as these animals were generally sedentary, we do not know how quickly the aged animals returned to baseline weight following the injections. Interestingly, after 7 days of rest, the aged experimental group’s weight had decreased from baseline more than that of the aged control group, while the adult experimental group’s weight had increased from baseline more than that of the adult control group. Using a different paradigm, others have shown that it may take 2 weeks or longer for aged animals’ weight to return to baseline after challenges (Wolden-Hanson, 2010).
The effects of an immune challenge on food intake and weight are an important consideration when caring for patients with infections. Illness anorexia, or a suppression in food intake, is common during infections and likely mediated by pro-inflammatory cytokines (Asarian & Langhans, 2010). While anorexia is a part of the acute-phase response to infection and considered to be adaptive, it can be detrimental if prolonged (Langhans, 2007). A sustained decrease in food intake can affect outcomes by altering nutritional reserves (Konsman & Dantzer, 2001) and lead to cachexia, which could result in death (Plata-Salaman, 2001). It is important to determine when illness anorexia and weight loss in response to infection (or immune challenge) become maladaptive and whether sustained weight loss affects cognitive performance, recovery, or susceptibility to future infections. In the elderly, timing is critical, and addressing these issues is important for developing interventions aimed at the prevention and/or treatment of malnutrition and weight loss following an infection.
Considering that human responses may be similar to the responses we observed in this animal study, our findings emphasize the importance of recognizing early indicators of infections, particularly decreases in food intake, especially in aged individuals, in order to identify and treat infections earlier. Indeed, among the factors that Gavazzi and Krause (2002) note lead to worse outcomes in elderly individuals with an infection is that of delayed diagnosis and treatment due to lack of recognition that infection may be present even in the absence of fever, potentially leaving anorexia or general weakness as the only sign.
We have reported here that aged animals developed impairment in spatial learning from LPS activation of the immune system. Yet aging alone did not contribute to spatial-learning deficits. Examination of the learning curves in both control groups showed decreased DHE over time, indicating that the aged animals did learn. The increased DHE in the LPS groups suggests that, as immune activation occurs, brain areas needed for learning, most likely hippocampus, are affected. Barrientos and colleagues (2009) reported increased hippocampal Interleukin-1β (IL-1β) levels in both young (3 months of age) and aged rats up to 24 hr after an immune challenge. Previous researchers have also noted age-related differences in response to an immune challenge in the hippocampus, which plays an important role in spatial learning and memory. Indeed, Barrientos and colleagues (2009) reported that 4 and 8 days after an immune challenge, hippocampal IL-1β levels remained increased in only the aged animals. Similarly, age-related effects of an immune challenge in humans might include cognitive difficulties such as not being able to learn about new places or environments, an inability to grasp or remember new information, and becoming lost in new or familiar places.
Importantly, our results show that one dose of LPS impaired learning for several days postadministration in both experimental groups. While these serious effects occurred with a single LPS dose, infections produce the equivalent of multiple doses (prolonged dose repeated for days) as a live organism replicates over time.
Ours is the first study to examine DHE as an indicator of spatial learning following an immune challenge. DHE is considered a sensitive measure of spatial learning (Hebda-Bauer et al., 1999; Vorhees & Williams, 2006). While we did not observe an age effect on DHE, aged animals in the experimental group did display greater DHE for longer than did the adult animals in the experimental group. Hebda-Bauer, Morano, and Therrien (1999), who measured DHE using a different paradigm, found that aged animals took longer to show improvement in DHE. This finding suggests that DHE in aged animals following an immune challenge requires further study. Shaw, Commins, and O’Mara (2001) reported that adult animals administered one LPS dose performed worse on the last trial day in a direct swim to the hidden platform compared to the control group and the group receiving LPS for 5 consecutive days. However, their results may not be comparable to ours because they measured the time spent in direct route to the platform instead of DHE, used a fixed-start instead of a random-start protocol, and administered a lower LPS dose. Our results, however, do parallel findings from human work. Older adults tested on a maze-learning task in a virtual environment similar to the MWM had more DHE and took longer to find the target than did younger adults (Davis et al., 2008; Moffat et al., 2001). Taken together, these results indicate the need for further work on spatial-learning deficits associated with an immune challenge in both adult and aged animals. Of particular interest would be studies that examined dose-dependent effects, prolonged cytokine activation, and repeated immune challenges and those that used a wider array of spatial learning and memory measures.
It is important to note that the effect of LPS on spatial learning was restricted to DHE in the present study. While others have found that LPS administration resulted in increased swim latencies in adult animals (Arai et al., 2001; Shaw et al., 2001), swim latency was increased only in the aged animals in the present study, and this effect was not due to the LPS administration. These results suggest an age effect rather than an experimental effect, which is consistent with the findings of others following an immune challenge (Barrientos et al., 2006). The discrepancy in the findings may be partially explained by differences in protocols. Arai, Matsuki, Ikegaya, and Nishiyama (2001) administered a larger dose of LPS on 2 days rather than on 1 day and assessed latencies for 2 days in comparison to 5. While Shaw and colleagues (2001) studied the effects of a single, lower dose of LPS on swim latency over 5 days, they did not find an increase until Day 4 as opposed to Day 1 in the present study.
We observed no differences between the experimental and control animals in either age-group during the probe or retention trials, indicating that the LPS effects were short lived. The learning that had occurred by Day 4 (adult) or 5 (aged) appeared to be robust and carried over to both the probe and retention trials. It is not clear why the animals in the present study improved their learning curves and carried over these improvements to both the probe and retention tests. Once the hippocampus was more functional (less inhibited), learning apparently occurred quickly. Another viable explanation is that some environmental learning was occurring during the first 4 test days, but it was not sufficient to direct heading accurately. In contrast to our probe and retention results, others have reported conflicting results that may be related to protocol variability. Differences in probe or retention trial protocols included the timing, length, or number of indicators assessed (Arai et al., 2001; Barrientos et al., 2006; Shaw et al., 2001). Probe trials performed earlier, for example, at a point where impairment was still evident might result in different findings (Cunningham & Sanderson, 2008).
Cunningham and Sanderson (2008) offer another plausible explanation of differences across studies. They suggest that, if animals were no longer experiencing sickness responses, differences in day-to-day results could be partially explained by differences in the animals’ condition. We examined other sickness behaviors simultaneously with spatial learning in the present study and found that the increase in DHE in LPS-treated animals persisted in both the presence and absence of other sickness responses. Measures such as route and distance traveled might provide evidence that could be more useful when examining animals experiencing sickness following LPS administration. However, the serious errors in directional heading that we reported here show that adult and aged animals exposed to an immune challenge had spatial-learning deficits. While LPS also affects the amount of locomotor activity, which can confound an assessment of spatial learning and memory, in the present study a change in activity level likely did not account for the LPS effects on DHE as the strain of rat we used was sedentary. Although we did measure spontaneous locomotor activity via hourly activity counts in this study, we have not reported the results here.
The mechanism by which an immune challenge affects spatial learning and hence cognition has been the subject of considerable discussion. Administration of LPS leads to peripheral immune system and microglia activation resulting in the release of pro-inflammatory cytokines, which mediate sickness behaviors (Kelley et al., 2003). Further, LPS has been shown to inhibit long-term potentiation, a type of synaptic plasticity important for spatial memory (Hennigan, Trotter, & Kelly, 2007), and decrease levels of brain-derived neurotrophic factor, which contributes to neuronal growth and survival and is also involved in learning and memory (Kim et al., 2013).
Of particular interest from our results is the fact that the aged experimental animals’ deficit in spatial learning was prolonged, as they took longest of all groups to show improvement in DHE. Understanding why aged animals might experience prolonged spatial-learning deficits following an immune challenge has important implications for the care of elderly patients experiencing an infection. As indicated previously, researchers have noted age-related differences in the hippocampus in response to an immune challenge, with IL-1β levels being sustained for longer in aged animals than in younger animals (Barrientos et al., 2009). The deficits we observed in the aged experimental animals in the present study were still present 4 days post-LPS. Microglia appear to transition to a primed state in normal aging and may be the source of exaggerated cytokine levels in response to an immune challenge (Barrientos, Frank, Watkins, & Maier, 2012). Research has also shown that increased pro-inflammatory cytokine levels inhibit long-term potentiation or decrease brain-derived neurotrophic factor (Barrientos, Kitt, Watkins, & Maier, 2015).
In summary, both adult and aged animals injected with LPS in the present study became sick, and there was a trend toward the effects on weight loss being more pronounced and sustained in the aged group. The short-term effects of one LPS dose created not only sickness but also problems with learning and memory. Other researchers have used animal models of disease to address clinical questions and study physiologic phenomena such as aging and spatial learning (Kasper, 2013). Since cognitive changes are an issue with elderly individuals who are experiencing acute infections, our findings in the present study among rats parallel the cognitive events observed in humans and can guide future research in both animal and human studies. We suggest that the existing constellation of sickness behaviors used by clinicians and researchers be expanded to include a broader and more comprehensive assessment of cognitive deficits. Further, as we found that a single dose of LPS can produce cognitive and metabolic problems, there is a need to explore effects on spatial learning of increased, longer lasting, or repeated immune challenges as well as the effects of these challenges on other cognitive functions. From a clinical perspective, our findings suggest that infections, especially in the elderly, should be considered a risk for cognitive decline such as deficits in learning and memory, changes that seriously affect independence and likely increase the cost of hospitalization and recovery. Thus, early recognition of the array of sickness behaviors associated with infections is critical to the development and administration of timely interventions, especially among the aged.
Footnotes
Author Contribution: B. J. Kupferschmid contributed to conception and design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. B. A. Therrien contributed to conception and design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this research was supported in part by the following institutions: National Institutes of Health (NIH), National Institute of Nursing Research (NINR), T32 NR07074; The University of Michigan: Claude D. Pepper Older American Independence Center and the School of Nursing New Investigator Award and Nursing Fellowship Funds; Sigma Theta Tau, Rho Chapter; Neuroscience Nursing Foundation.
ORCID iD: Barbara J. Kupferschmid, PhD, RN http://orcid.org/0000-0001-9524-8997
References
- Abraham J., Johnson R. W. (2009). Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain, Behavior, and Immunity, 23, 396–401. doi:10.1016/j.bbi.2008.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Matsuki N., Ikegaya Y., Nishiyama N. (2001). Deterioration of spatial learning performances in lipopolysaccharide-treated mice. Japanese Journal of Pharmacology, 87, 195–201. [DOI] [PubMed] [Google Scholar]
- Asarian L., Langhans W. (2010). A new look on brain mechanisms of acute illness anorexia. Physiology and Behavior, 100, 464–471. doi:10.1016/j.physbeh.2010.04.009 [DOI] [PubMed] [Google Scholar]
- Barrientos R. M., Frank M. G., Hein A. M., Higgins E. A., Watkins L. R., Rudy J. W., Maier S. F. (2009). Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain, Behavior, and Immunity, 23, 46–54. doi:10.1016/j.bbi.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R. M., Frank M. G., Watkins L. R., Maier S. F. (2012). Aging-related changes in neuroimmune-endocrine function: Implications for hippocampal-dependent cognition. Hormones and Behavior, 62, 219–227. doi:10.1016/j.yhbeh.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R. M., Higgins E. A., Biedenkapp J. C., Sprunger D. B., Wright-Hardesty K. J., Watkins L. R.…Maier S. F. (2006). Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiology of Aging, 27, 723–732. doi:10.1016/j.neurobiolaging.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Barrientos R. M., Kitt M. M., Watkins L. R., Maier S. F. (2015). Neuroinflammation in the normal aging hippocampus. Neuroscience, 309, 84–99. doi:10.1016/j.neuroscience.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucks R. S., Gidron Y., Harris P., Teeling J., Wesnes K. A., Perry V. H. (2008). Selective effects of upper respiratory tract infection on cognition, mood and emotion processing: A prospective study. Brain, Behavior, and Immunity, 22, 399–407. doi:10.1016/j.bbi.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Bula C. J., Ghilardi G., Wietlisbach V., Petignat C., Francioli P. (2004). Infections and functional impairment in nursing home residents: A reciprocal relationship. Journal of the American Geriatrics Society, 52, 700–706. doi:10.1111/j.1532-5415.2004.52205.x [DOI] [PubMed] [Google Scholar]
- Caljouw M. A., Kruijdenberg S. J., de Craen A. J., Cools H. J., den Elzen W. P., Gussekloo J. (2013). Clinically diagnosed infections predict disability in activities of daily living among the oldest-old in the general population: The Leiden 85-plus Study. Age and Ageing, 42, 482–488. doi:10.1093/ageing/aft033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C., Sanderson D. J. (2008). Malaise in the water maze: Untangling the effects of LPS and IL-1beta on learning and memory. Brain, Behavior, and Immunity, 22, 1117–1127. doi:10.1016/j.bbi.2008.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. (2004). Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. European Journal of Pharmacology, 500, 399–411. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Therrien B. A., West B. T. (2008). Cue conditions and wayfinding in older and younger women. Research in Gerontological Nursing, 1, 252–263. doi:10.3928/19404921-20081001-06 [DOI] [PubMed] [Google Scholar]
- Florez-Duquet M., Peloso E., Satinoff E. (2001). Fever and behavioral thermoregulation in young and old rats. American Journal of Physiology—Regulatory Integrative & Comparative Physiology, 280, R1457–R1461. [DOI] [PubMed] [Google Scholar]
- Fong T. G., Tulebaev S. R., Inouye S. K. (2009). Delirium in elderly adults: Diagnosis, prevention and treatment. Nature Reviews Neurology, 5, 210–220. doi:10.1038/nrneurol.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K. D., Conn C. A., Kluger M. J. (1992). Fever, tumor necrosis factor, and interleukin-6 in young, mature, and aged Fischer 344 rats. American Journal of Physiology, 262, R211–R215. [DOI] [PubMed] [Google Scholar]
- Gavazzi G., Krause K. H. (2002). Ageing and infection. Lancet Infectious Diseases, 2, 659–666. [DOI] [PubMed] [Google Scholar]
- Godbout J. P., Chen J., Abraham J., Richwine A. F., Berg B. M., Kelley K. W., Johnson R. W. (2005). Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB Journal, 19, 1329–1331. doi:10.1096/fj.05-3776fje [DOI] [PubMed] [Google Scholar]
- Godbout J. P., Moreau M., Lestage J., Chen J., Sparkman N. L., O’Connor J.…Johnson R. W. (2008). Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology, 33, 2341–2351. doi:10.1038/sj.npp.1301649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozalo P. L., Pop-Vicas A., Feng Z., Gravenstein S., Mor V. (2012). Effect of influenza on functional decline. Journal of the American Geriatrics Society, 60, 1260–1267. doi:10.1111/j.1532-5415.2012.04048.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D., Isom M. (2010). Age effects on wayfinding and route learning skills. Behavioural Brain Research, 209, 49–58. doi:10.1016/j.bbr.2010.01.012 [DOI] [PubMed] [Google Scholar]
- Hebda-Bauer E. K., Morano M. I., Therrien B. (1999). Aging and corticosterone injections affect spatial learning in Fischer-344 X Brown Norway rats. Brain Research, 827, 93–103. [DOI] [PubMed] [Google Scholar]
- Hennigan A., Trotter C., Kelly A. M. (2007). Lipopolysaccharide impairs long-term potentiation and recognition memory and increases p75NTR expression in the rat dentate gyrus. Brain Research, 1130, 158–166. doi:10.1016/j.brainres.2006.10.066 [DOI] [PubMed] [Google Scholar]
- Huang Y., Henry C. J., Dantzer R., Johnson R. W., Godbout J. P. (2008). Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiology of Aging, 29, 1744–1753. doi:10.1016/j.neurobiolaging.2007.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper C. E. (2013). Animal models of exercise and obesity. Annual Review of Nursing Research, 31, 1–17. doi:10.1891/0739-6686.31.1 [DOI] [PubMed] [Google Scholar]
- Kelley K. W., Bluthe R. M., Dantzer R., Zhou J. H., Shen W. H., Johnson R. W., Broussard S. R. (2003). Cytokine-induced sickness behavior. Brain, Behavior, and Immunity, 17, S112–S118. [DOI] [PubMed] [Google Scholar]
- Kim S. E., Ko I. G., Shin M. S., Kim C. J., Jin B. K., Hong H. P., Jee Y. S. (2013). Treadmill exercise and wheel exercise enhance expressions of neutrophic factors in the hippocampus of lipopolysaccharide-injected rats. Neuroscience Letters, 538, 54–59. doi:10.1016/j.neulet.2013.01.039 [DOI] [PubMed] [Google Scholar]
- Konsman J. P., Dantzer R. (2001). How the immune and nervous systems interact during disease-associated anorexia. Nutrition, 17, 664–668. [DOI] [PubMed] [Google Scholar]
- Langhans W. (2007). Signals generating anorexia during acute illness. Proceedings of the Nutrition Society, 66, 321–330. doi:10.1017/S0029665107005587 [DOI] [PubMed] [Google Scholar]
- Moffat S. D. (2009). Aging and spatial navigation: What do we know and where do we go? Neuropsychology Review, 19, 478–489. doi:10.1007/s11065-009-9120-3 [DOI] [PubMed] [Google Scholar]
- Moffat S. D., Zonderman A. B., Resnick S. M. (2001). Age differences in spatial memory in a virtual environment navigation task. Neurobiology of Aging, 22, 787–796. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Cunningham C., Holmes C. (2007). Systemic infections and inflammation affect chronic neurodegeneration. Nature Reviews Immunology, 7, 161–167. doi:10.1038/nri2015 [DOI] [PubMed] [Google Scholar]
- Plata-Salaman C. R. (2001). Cytokines and feeding. International Journal of Obesity and Related Metabolic Disorders, 25, S48–S52. doi:10.1038/sj.ijo.0801911 [DOI] [PubMed] [Google Scholar]
- Shaw K. N., Commins S., O’Mara S. M. (2001). Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behavioural Brain Research, 124, 47–54. [DOI] [PubMed] [Google Scholar]
- Therrien B. A. (1982). Sex differences in the effects of hippocampal lesions on place navigation (Unpublished doctoral dissertation). University of Michigan, Ann Arbor, MI. [Google Scholar]
- Turturro A., Witt W. W., Lewis S., Hass B. S., Lipman R. D., Hart R. W. (1999). Growth curves and survival characteristics of the animals used in the biomarkers of aging program. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 54, B492–B501. [DOI] [PubMed] [Google Scholar]
- Vorhees C. V., Williams M. T. (2006). Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nature Protocols, 1, 848–858. doi:10.1038/nprot.2006.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C. V., Williams M. T. (2014. a). Assessing spatial learning and memory in rodents. ILAR Journal, 55, 310–332. doi:10.1093/ilar/ilu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C. V., Williams M. T. (2014. b). Value of water mazes for assessing spatial and egocentric learning and memory in rodent basic research and regulatory studies. Neurotoxicology and Teratology, 45, 75–90. doi:10.1016/j.ntt.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Wolden-Hanson T. (2010). Changes in body composition in response to challenges during aging in rats. Interdisciplinary Topics in Gerontology, 37, 64–83. doi:10.1159/000319995 [DOI] [PubMed] [Google Scholar]