Abstract
Purpose:
The purpose of this substudy of a large randomized controlled trial was to evaluate the efficacy of the Mindfulness-Based Stress Reduction (Breast Cancer) (MBSR[BC]) program compared to usual care (UC) in normalizing blood levels of pro-inflammatory cytokines among breast cancer survivors (BCS).
Method:
A total of 322 BCS were randomized to either a 6-week MBSR(BC) program or a UC. At baseline and 6 and 12 weeks, 10 ml of venous blood and demographic and clinical data were collected and/or updated. Plasma cytokines (interleukin [IL]-1β, IL-6, IL-10, tumor necrosis factor [TNF] α, transforming growth factor [TGF] β1, soluble tumor necrosis factor receptor [sTNFR] 1) were assayed. Linear mixed models were used to assess cytokine levels across three time points (baseline and 6 and 12 weeks) by group (MBSR[BC] vs. UC).
Results:
Of the six measured cytokines, three were nondetectable at rates greater than 50% (IL-10, IL-1β, TGF-β1) and, because of overall low prevalence, were not analyzed further. For the remaining cytokines (TNFα, IL-6, sTNFR1), results showed that TNFα and IL-6 increased during the follow-up period (between 6 and 12 weeks) rather than during the MBSR(BC) training period (between baseline and 6 weeks), while sTNFR1 levels did not change significantly across the 12-week period.
Conclusions:
Study results suggest that MBSR(BC) affects cytokine levels in BCS, mainly with increases in TNFα and IL-6. The data further suggest that B-cell modulation may be a part of immune recovery during breast cancer management and that increases in TNFα and IL-6 may be markers for MBSR(BC)-related recovery.
Keywords: MBSR(BC), Mindfulness-Based Stress Reduction, breast cancer, biomarkers, cytokines
Breast cancer survivors (BCS) comprise the largest group of cancer survivors (41%). Although BCS are living longer due to new treatments, they often suffer late disease or treatment effects, which may affect their quality of life (QOL; Kenyon, Mayer, & Owens, 2014; Wu & Harden, 2014). Symptoms including fatigue, depression, sleep disturbances, and pain are sustained complaints associated with the diagnosis, treatment, and recovery phase of breast cancer (BC; Accortt, Bower, Stanton, & Ganz, 2015; Berger, Gerber, & Mayer, 2012; Bower & Ganz, 2015; Fagundes, LeRoy, & Karuga, 2015; Jones et al., 2015). A variety of studies among BCS have reported associations between these symptoms and levels of systemic cytokines. For example, researchers noted elevated blood levels of interleukin 1 receptor agonist (IL-1RA), IL-6, IL-1β, and tumor necrosis factor (TNF) α during and after radiation therapy (De Sanctis et al., 2014; Saligan & Kim, 2012). Investigators have also reported associations among BC chemotherapy, fatigue, and sleep disturbances with increased TNFα (Bower, Ganz, Irwin, Arevalo, & Cole, 2011), increased IL-6, and decreased IL-1RA (Liu et al., 2012). In addition, researchers found significantly higher IL-6 concentrations among depressed cancer patients compared to healthy controls (Anisman, Kokkinidis, & Merali, 2002) and demonstrated that acute psychological stress leads to short-term upregulation of the immune response (Schedlowski, Engler, & Grigoleit, 2014), with chronic illness associated with decreased IL-2 and interferon γ (Moynihan, 2003). Tumor necrosis factor receptor (TNFR), a protein receptor that binds with TNFα and protects against its potentially harmful effects as a way to regulate inflammation (Aderka, Engelmann, Maor, Brakebusch, & Wallach, 1992) and stress response (Carlsson et al., 2014), has also demonstrated an association with BC- or BC-treatment-related symptoms. Bower, Ganz, Irwin, Kwan, et al. (2011) found that elevated concentrations of soluble TNFR (sTNFR) in the blood following BC treatment were associated with higher levels of fatigue in BCS who received chemotherapy treatment versus those who did not. Although research has not fully elucidated the molecular mechanisms mediating these cytokine effects, authors believe they are associated with the direct effects of cytokines on the brain, hypothalamic–pituitary–adrenal axis, and cancer cells (Hayslip et al., 2015).
Mindfulness-Based Stress Reduction (MBSR) attenuates negative psychological and somatic symptoms of cancer, especially BC. Researchers have reported significant positive effects of this treatment for depressed mood and QOL (Henderson et al., 2013; Hoffman et al., 2012); anxiety, depression, and perceived stress (Lengacher et al., 2014; Wurtzen et al., 2013, 2015); and poor sleep quality and fatigue (Andersen et al., 2013; Lengacher et al., 2012). Investigators have also examined the effects of MBSR on inflammatory markers in BCS, with findings suggesting that treatment with MBSR affects both T-cell activity (Lengacher et al., 2013) and blood cytokine levels (Bower et al., 2015; Carlson, Speca, Faris, & Patel, 2007; Witek-Janusek et al., 2008). Based on these previous reports, we hypothesized in the present study that treatment with MBSR would result in changes in blood cytokine levels in BCS consistent with a stabilization and normalization of the immune response. Furthermore, we hoped to show that changes in cytokine levels could serve as a biomarker for the biological influence of MBSR among BCS following BC treatment. The major aim of this research, therefore, in the context of a large parent randomized controlled trial, was to evaluate the efficacy of the MBSR(BC) program among posttreatment BCS in normalizing blood levels of measures of immune function, namely, pro-inflammatory cytokines and sTNFR1.
Method
Participants
We recruited 322 BCS from April 2009 through March 2013 from the Moffitt Cancer Center, Carol and Frank Morsani Center for Advanced Healthcare, and the Life Hope Medical Group, located in Tampa, Florida. All participants were 21 years or older with a diagnosis of Stage 0–III BC. Additionally, participants were eligible if they had completed treatment (i.e., lumpectomy, mastectomy, radiation, and chemotherapy) within 2 weeks to 2 years of study enrollment. Exclusion criteria were diagnoses of Stage IV cancer, severe psychiatric disorder, and/or BC recurrence. Although the primary purpose of this study was to compare the MBSR(BC) and usual care (UC) conditions, we also enrolled 40 healthy controls as a comparison group to gauge cytokine levels in BCS participants relative to a noncancer population.
Procedures
Study design and randomization
We randomly assigned participants to one of the two conditions: (1) the formal 6-week MBSR(BC) program or (2) a UC wait-listed regimen. We stratified subject randomization by type of surgery (lumpectomy vs. mastectomy), BC treatment (chemotherapy with or without radiation vs. radiation alone), and BC stage (Stage 0/I vs. II/III). We performed this stratification, along with the blocking mechanism, to ensure a balanced distribution of baseline factors between the two study groups. We assessed participants at baseline, at the end of the 6-week program, and at 12 weeks in order to determine the longer term effects of MBSR(BC).
Recruitment and data collection procedures
We invited interested patients who met the inclusion criteria to an orientation session held in the Moffitt Cancer Center Integrative Medicine Program, where all data collection at 6 and 12 weeks occurred. During the orientation session, we provided information about the study, answered any questions, obtained informed consent along with baseline demographic and clinical history, and collected 10 ml of venous blood. We then randomized participants into the study groups.
MBSR(BC) intervention
MBSR(BC) is a 6-week program adapted from Dr. Jon Kabat-Zinn’s original 8-week program (Kabat-Zinn, Lipworth, & Burney, 1985; Kabat-Zinn et al., 1992) to address specific symptomology of BCS. The program consists of formal practice of sitting meditation, body scan, gentle Hatha yoga, and walking meditation as well as informal practice. Participants randomized to MBSR(BC) attended 2-hr weekly sessions for 6 weeks conducted by a psychologist with expertise in MBSR(BC). Participants also received training manuals and compact discs to guide at-home practice, and we asked them to record practice time(s) into daily diaries. A member of the study’s implementation team contacted each participant in the MBSR(BC) group weekly for each of the 12 weeks of the study. During these calls, the study team member addressed any concerns participants had about practices and reminded each participant to continue their daily (i.e., informal and formal) practices of mindfulness for 15–45 min per day and to record these practices in their daily diary. The expected time for practice was standard and did not vary during or postintervention. The reduction in the program duration from 8 to 6 weeks did not reduce the key content or objectives of the MBSR intervention. The structure, methods, and key program characteristics identified for the 8-week program remain the focus for each of the 6 weeks, including the four practice techniques, group processes, and components of self-regulation of attention and nonjudgmental acceptance of the present experience (Kabat-Zinn, 1990).
Usual care
Participants randomized to UC attended standard posttreatment clinic visits and had the opportunity to participate in the MBSR(BC) program following the study period.
Fidelity
A research assistant (RA) used a structured observational method to evaluate the MBSR(BC) instructor’s adherence to the intervention protocol, which included practice components and interactional components of the process (teacher-to-student, student-to-teacher, student-to-student support). For this fidelity component, the RA used the Observational Checklist for Time Analysis of the MBSR Program as a tool to monitor the consistent delivery of content and time specific to each weekly class session for the 6 weeks. As mentioned above, we also asked participants to record their daily informal and formal practices in a daily diary using the Daily Time Monitoring of the MBSR Activity worksheet. This type of monitoring allowed participants to enter data on the specific practice, whether it was informal or formal, and how much time they spent using the preferred practice in their various routine activities.
Measurements
Plasma cytokine analyses
We collected a minimum of 10 ml of venous blood from each participant into vacutainer tubes containing heparin anticoagulant between 10 a.m. and noon at baseline and 6 and 12 weeks. We separated the plasma by centrifugation at 3,000 rpm for 15 min and then stored it at −80°C until analysis. We assayed plasma cytokines using commercial enzyme-linked immunoabsorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN) following the manufacturer’s protocols. For IL-1β, IL-6, IL-10, and TNFα, we used high-sensitivity Quantikine HS kits (cat. # SSLB00C, SS600B, SS100C, and SSTA00D, respectively). For sTNFR1 and transforming growth factor β1 (TGF-β1), we used Duoset ELISA kits (cat. # DY225 and DY240, respectively). We assayed each plasma sample in duplicate. For each plate, we generated a standard curve using four-parameter logistic (4-PL) regression analysis from which we converted the optical density (OD) units from the unknown samples to cytokine protein units. We considered samples with OD values less than the lowest standard to be “nondetects” and assigned them a value of 50% of the detection limit (lowest standard). For samples with values greater than the highest standard, we diluted and reassayed them until the OD values fell within the OD range of the standard curve.
Demographic data and clinical history
We collected standard socioeconomic and demographic data, including age, gender, ethnicity, highest education level completed, marital status, annual income, and employment status. Additionally, we collected standard clinical history including information on cancer diagnosis, type of cancer treatment, and dates of treatment. We initially collected all demographic and clinical history data at baseline and then updated it at 6 and 12 weeks.
Statistical Methods
We only performed data analyses on cytokines that had less than a 50% nondetectable rate. At higher rates, important sample statistics (e.g., median) are impossible to determine (Helsel, 1990). For initial tests comparing cytokine levels in healthy controls with levels in our participant group of BCS, we used the Wilcoxon rank sum test. For comparisons between our experimental groups (UC vs. MBSR[BC]), we used the “intent-to-treat” principle.
Our study design, coupled with initial baseline assessment, resulted in three separate assessment time points and hence the opportunity for repeated measures analysis. We used linear mixed models to assess the interaction between participant assignment (MBSR[BC] vs. UC) and time (baseline and 6 and 12 weeks) in relationship to cytokine levels. That is, these models tested whether the rate of cytokine change varied by study assignment. The mixed-model analysis also allowed for inclusion and analysis of subjects with some missing data (which was minimal). The linear mixed models assumed an unstructured correlation pattern, and we used a two-sided p value of <.05 to define statistical significance for all analyses. We conducted model estimation in Mplus v. 7.1 (Muthén & Muthén, 1998–2003) using full information maximum likelihood estimation to benefit from all available information in the data. That is, we included participants with missing information in the analyses under the assumption of missing at random (i.e., “missingness” conditional on observed variables).
Results
We included a total of 322 participants (n = 152 MBSR[BC]; n = 147 UC) from the parent study in the present analyses. Differences between MBSR(BC) and UC participants on baseline demographic or clinical characteristics were not statistically significant. We asked MBSR(BC) participants to practice mindfulness techniques 15–45 min per day at least 6 days a week. We established compliance a priori as completing at least 75% of the assigned practice. By this definition, 95% of MBSR(BC) participants complied with treatment. Demographic and clinical characteristics for the participants and a Consolidated Standards for Reporting Trials (CONSORT) flowchart showing participant progress through the phases of the parent study are available in the study by Lengacher et al. (2016).
Of the six cytokines that we measured, three were nondetectable at rates greater than 50% (IL-10, IL-1β, and TGF-β1) and we did not analyze them further (Table 1). We did conduct analyses on the remaining three cytokines (TNFα, IL-6, and sTNFR1).
Table 1.
Prevalence of Nondetectable Amounts in Cytokine Assays.
| Group | Used in Analyses |
Not Used in Analyses |
||||
|---|---|---|---|---|---|---|
| TNFα (%) | IL- 6 (%) | sTNFR1 (%) | IL-1β (%) | IL-10 (%) | TGFβ1 (%) | |
| Healthy controls | 29 | 0 | 0 | 44 | 71 | 60 |
| Usual care | 39 | 2 | 0 | 68 | 68 | 54 |
| MBSR(BC) | 30 | 0 | 0 | 72 | 71 | 50 |
Note. Cytokines with a nondetectable rate >50% were not used in further analyses. MBSR(BC) = Mindfulness-Based Stress Reduction for Breast Cancer.
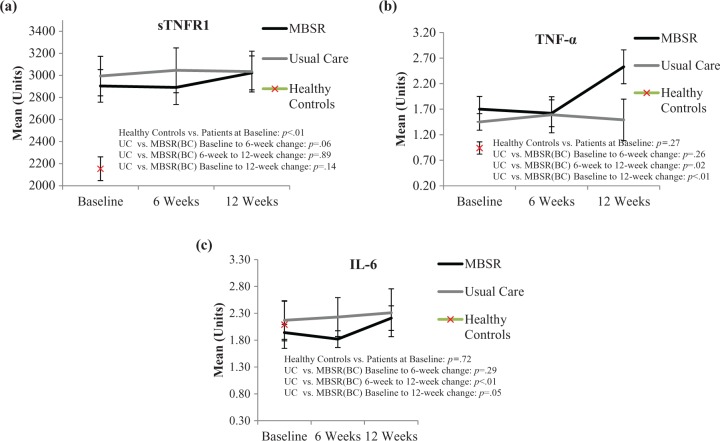
We first compared cytokine levels between the patient samples and healthy controls (n = 40). BCS demonstrated significantly higher levels of sTNFR1 than the controls throughout the length of the study (Figure 1a, p < .01). The magnitude of this difference had a Cohen’s d effect size of .44. Differences between the healthy controls and BCS were not statistically significant for TNFα or IL-6 (p = .27 and p = .72, respectively). Because the healthy controls tended to be younger, with a mean (M) age of 51, than the BCS (M = 57 years; p < .05), we ran the cytokine analyses as analysis of covariance (ANCOVA), adjusting for age, but the results for TNFα and IL-6 remained nonsignificant.
Figure 1.
Means (and standard error of the mean [SEM]) for (a) soluble tumor necrosis factor receptor (sTNFR) 1, (b) tumor necrosis factor (TNF) α, and (c) interleukin (IL) 6 at each time point by randomization assignment and at baseline for healthy controls. p Value for the baseline comparison between healthy controls and patients represents Wilcoxon rank sum test. The other p values represent the linear slope parameter of the mixed model (Assignment × Time Point interaction).
Across patients, cytokine levels varied according to some subgroups (see Table 2). These correlations were small (Spearman’s ρ < .20) but consistent across time points. Correlations were most evident with IL-6, where mastectomy (vs. lumpectomy) and chemotherapy treatment were associated with lower levels of IL-6, while radiation treatment and self-identifying as Black were associated with higher levels of IL-6. Treatment with mastectomy was associated with higher levels of TNFα, while this cytokine was consistently lower in patients who had received radiation treatment. Age and Hispanic ethnicity were associated with sTNFR1, with older patients having higher levels of sTNFR1 than younger patients and participants self-identifying as Hispanic having lower levels of sTNFR1 than those who did not.
Table 2.
Associations Between Patient Subgroup and Levels of Cytokines Across All Three Time Points (Spearman’s ρ).
| Subgroup Category | TNFα |
IL-6 |
sTNFR1 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Week | 12 Week | Baseline | 6 Week | 12 Week | Baseline | 6 Week | 12 Week | |
| Stage of disease | .08 | −.07 | .04 | .03 | .01 | −.04 | −.01 | .00 | −.02 |
| Mastectomy (vs. lumpectomy) | .15** | .11 | .13* | −.11* | −.13* | −.16* | .03 | .03 | .00 |
| Chemotherapy treatment | .07 | −.06 | .01 | −.16** | −.03 | −.11* | .05 | .03 | .04 |
| Radiation treatment | −.10 | −.09 | −.14* | .13* | .18** | .19** | −.01 | .03 | .05 |
| Endocrine treatment | −.04 | .04 | .06 | −.07 | −.07 | −.07 | −.01 | .01 | .00 |
| Time since treatment | −.03 | .01 | .04 | −.12* | −.08 | −.05 | −.11 | −.08 | −.04 |
| Age | .02 | .13* | .03 | .04 | .07 | .12* | .14* | .13* | .19** |
| Black | −.01 | −.03 | −.04 | .11 | .12* | .10 | −.04 | −.06 | −.06 |
| Hispanic | −.03 | −.13* | −.07 | −.03 | −.06 | −.10 | −.14* | −.17* | −.15* |
Note. Categorical variables include mastectomy, chemotherapy treatment, radiation treatment, endocrine treatment, Black race, and Hispanic ethnicity. Continuous variables include stage of disease, time since treatment, and age. Positive correlations indicate higher cytokine levels associated with the subgroup. Negative correlations indicate lower cytokine levels associated with the subgroup. Highlighting indicates consistent correlations (with at least one statistically significant value and at least two values with a magnitude greater than .10) across multiple time points. IL-6 = interleukin-6; sTNFR1 = soluble tumor necrosis factor receptor 1; TNFα = tumor necrosis factor α.
*p < .05. **p < .01.
Further analyses using linear mixed models to compare cytokine levels in the UC and MBSR(BC) over the three time points showed that TNFα (p < .01) and IL-6 (p = .05) increased over the 12 weeks at a higher rate for the MBSR(BC) patients than for the UC patients (see Figure 1a–c), while there was no significant between-group difference for sTNFR1 (p = .14). When examining the means in Figure 1a–c, we noted that the effects appeared not to be linear across the three time points. To explore this nonlinearity, we evaluated the change in cytokines during the MBSR(BC) training period (baseline to 6 weeks) and the change during the follow-up period (6–12 weeks). Results of this analysis showed that the change in TNFα in the MBSR(BC) group occurred mainly during the follow-up period (p = .02) rather than the training period (p = .26). We observed the same delayed pattern for IL-6 (p = .29 and p < .01, respectively). For sTNFR1, results suggested a dip in values for MBSR(BC) during the training period (p = .06) but little change during the follow-up period (p = .89).
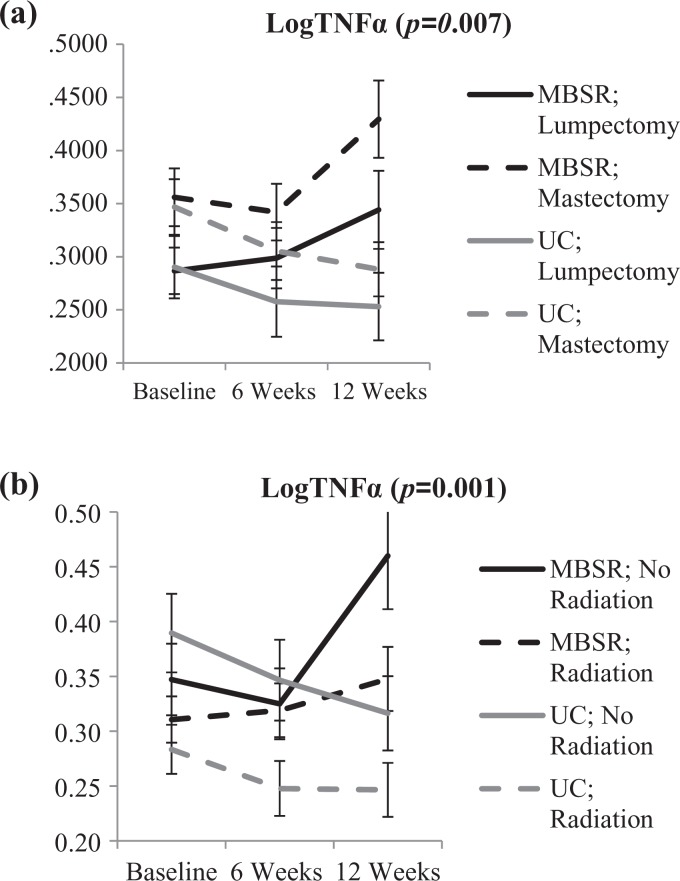
To control for the observed patient subgroup effects (see Table 2), we completed a follow-up analysis of the TNFα and IL-6 effects including the subgroups as covariates across all three time points. For TNFα, when type of surgery or radiation was a covariate, the MBSR(BC) treatment effect remained statistically significant for both subgroups (p = .007 and p = .001, respectively). Figure 2 shows an initial separation between those who had mastectomy (higher TNFα levels) and those who had lumpectomy and those who had radiation and those who did not (lower TNFα levels). Figure 2 also shows MBSR(BC)-related increases in TNFα levels for both of these patient groups relative to the UC group. IL-6 levels varied significantly among the patient subgroups for surgery type, chemotherapy, radiation, and Black ethnicity. When we used these subgroups as covariates for the effect of MBSR(BC) across the three time points, MBSR(BC)-related changes remained statistically significant (surgery type, p = .03; chemotherapy, p = .04; radiation, p = .04; and identifying as Black, p = .03).
Figure 2.
Means (and SEM) for logTNFα at each time point by randomization assignment and (a) surgery type and (b) radiation. The p values represent the Time × Treatment interaction when controlling for surgery type or radiation, respectively. Although there were main effects of surgery type (p < .01) and radiation (p < .01), interactions with surgery type (in the first) and radiation (in the second) were not statistically significant.
Discussion
The major aim of this study was to evaluate the efficacy of the MBSR(BC) program among posttreatment BCS in normalizing blood levels of pro-inflammatory cytokines. We hypothesized that MBSR(BC) participants would experience greater improvements than UC participants in biological stress markers (pro-inflammatory immune cytokines and sTNFR1) at 6 weeks, with sustained improvements at 12 weeks.
The women in our study group of BCS were relatively healthy, being an average of 7–8 months past the end of cancer treatment and reporting an incidence of fever and sickness during the study of <15%. This state of health probably accounted for the low detection rate of IL-β, IL-10, and TGF-β (see Table 1), all of which previous research has shown to have low detection levels in nonsymptomatic patients, such as participants at baseline in cancer studies (Pusztai et al., 2004). The relatively asymptomatic nature of participants in the present study might also have contributed to our findings of only one cytokine (sTNFR1; see Figure 1) that demonstrated higher levels in the BCS group than in the healthy control group at baseline and the relatively low magnitude of cytokine levels correlating with certain patient characteristics (see Table 2).
sTNFR1 is released from cells when they are stimulated with TNFα; therefore, the soluble receptor serves as a marker for TNFα activity (Diez-Ruiz et al., 1995). Previous researchers have reported that sTNFR reaches elevated concentrations in the blood following BC treatment (Bower, Ganz, Irwin, Kwan, et al., 2011); thus, the elevated levels in the patients in the present study could be a residual “after glow.” These patients also had slightly, though not significantly, elevated TNFα levels (see Figure 1b), supporting the notion of an “after glow” response to cancer treatment.
Participant characteristics, such as age, ethnicity, time since treatment, and type of cancer treatment, are potential confounders in studies of blood cytokine levels in posttreatment BCS. For example, Brouwers et al. (2015) reported that IL-6 and monocyte chemoattractant protein 1 increased with advancing age, and Pine et al. (2016) and Boggs et al. (2014) showed that several cytokines, including IL-6 and TNFα, were increased in European American compared to African American lung cancer patients. Furthermore, in other studies, these cytokines were increased to a varying extent following either radiation (De Sanctis et al., 2014) or chemotherapy (Bower, Ganz, Irwin, Kwan, et al., 2011; Liu et al., 2012). Our study is the first to report a comprehensive analysis of the associations between various cancer treatments, stage of disease, patient age, and ethnicity and the levels of three different cytokines over a 12-week period. For example, mastectomy was related to an increase in TNFα and a decrease in IL-6 compared to lumpectomy (see Table 2). On the other hand, chemotherapy was associated with a decreased level of IL-6, while radiation was associated with a decreased level of TNFα and an increased level of IL-6. We also observed that increasing age was associated with an increase in sTNFR1 only, while Black women showed an increase in IL-6 and Hispanic women a decrease in sTNFR1. The mechanisms of these changes are beyond the scope of this study, but the results importantly demonstrate the relationships that various patient factors have with the day-to-day levels of specific cytokines and also show that these factors do not necessarily lead to a global increase or decrease in levels of cytokines in the blood. Researchers should take this variability in effects into account when planning and interpreting future studies in this area.
A major goal of the study was to determine the influence of MBSR(BC) on blood cytokine levels as measures of immune function and as possible blood markers indicative of the effects of MBSR(BC) therapy. Previously, we reported a positive correlation between MBSR(BC) and T helper 1 (Th1) activity (Lengacher et al., 2013) and also showed that B cells and natural killer cells recovered in the blood to a greater degree than T cells in BCS following cancer therapy. Stimulated human B cells readily produce TNFα and IL-6 (Bao & Cao, 2014; Nielsen, Bornsen, Sellebjerg, & Brimnes, 2016); IL-6 increases T-helper activity and TNFα promotes B cell–derived plasma cell development in animals and humans (Bao & Cao, 2014; Langkjaer et al., 2012). Of the three consistently measurable proteins in patients in the present study, TNFα and IL-6 significantly increased in the blood of patients in the MBSR(BC) group relative to those in the UC, while there was no change in the sTNFR1 level. Even when we used patient subgroups as covariates, the MBSR(BC) treatment effect remained statistically significant.
From the present results and those of our previous report (Lengacher et al., 2013), it appears that, in our patient population, during the 6-week practice phase of the intervention, MBSR(BC) promoted the production of TNFα and IL-6, possibly from B cells, and that these cytokines might have contributed to the increased Th1 activity we observed in these patients. Bower and colleagues (2015) reported that younger women with BC had significant reductions in pro-inflammatory gene expression (p = .009) and inflammatory signaling (p = .001) after participating in a mindful awareness practices study. In another MBSR study, Carlson, Speca, Faris, and Patel (2007) found a slight but nonsignificant increase in TNFα during the practice period, while Witek-Janusek et al. (2008) reported a significant increase in IL-4, IL-10, and IL-6 post-MBSR intervention.
All of these reports suggest a complex response to MBSR, where some cytokine levels increase while others decrease. Furthermore, the data suggest that B-cell modulation may be a part of immune recovery during BC management and that increases in TNFα and IL-6 may be markers not only for MBSR-related immune restoration but also for psychological efficacy, since the patients who completed the MBSR(BC) program showed improvements in various measures of psychological well-being (Lengacher et al., 2016).
Clinical Implications
The foundation that previous MBSR(BC) research established demonstrated that health-care providers can implement this patient-centered therapy as part of a complementary approach to traditional medicine during cancer survivorship. The present findings provide a rationale for MBSR(BC) as an evidence-based therapy that may help normalize pro-inflammatory cytokine levels after cancer treatment. Since BC treatment results in decreased efficiency of the immune system and acute psychological stress leads to short-term upregulation of the immune response (Schedlowski et al., 2014), BCS are often at an increased risk of infection (Campbell, Scott, Maecker, Park, & Esserman, 2005). MBSR(BC) may facilitate restoration of the immune response, which may ultimately lead to better health. Additionally, BC patients usually express interest in continually monitoring their health posttreatment. Clinicians might consider offering this program to survivors who want to stay healthy but are anxious about recurrence and often suffer from other psychological and physical symptoms.
Limitations
As mentioned previously, the relatively healthy nature of our sample and the time since cancer treatment may have attenuated the results. Whether MBSR(BC) would increase or decrease levels of the cytokines we explored in a sicker (or more recently treated) sample is unclear and requires future study. Additionally, as Robins et al. (2013) pointed out, it is difficult to accurately observe biological changes following psychosocial interventions due to the influence of numerous other psychological and physical factors. This challenge suggests the need for studies with greater statistical power to detect small effects. Another limitation of the present study is that there was no long-term follow-up of participants beyond the 12-week study period. Unlike medication, MBSR(BC) is designed to be a learned skill for patients to take with them and continue practicing after training. Future work is required to determine whether long-term MBSR(BC) practice further influences cytokine activity.
Conclusion
In summary, our results show that MBSR(BC) affects cytokine levels, specifically by increases in TNFα and IL-6. TNFα, sTNFR1, and IL-6 blood levels varied according to the patient subgroup of BCS across multiple clinical and demographic factors. Women in the MBSR(BC) group demonstrated an increase in blood levels of TNFα and IL-6, and these cytokine levels did not differ significantly between the healthy control group at baseline and the UC group across the 12 weeks. These data suggest that B-cell modulation may be a part of immune recovery during BC management and that increases in TNFα and IL-6 may be markers for MBSR(BC)-related immune restoration. Future studies should evaluate the biological stress response and MBSR(BC)-mediated improvements in BCS over a longer follow-up period.
Footnotes
Authors’ Note: Inquiries on the methods used in the study and other research-related information can be obtained by contacting the corresponding author. Access to data and samples will be made available in accordance with the formal data-sharing plan that was included in the parent grant submission to the National Cancer Institute. Name and URL of Registry: ClinicalTrials.gov, www.ClinicalTrials.gov Registration Number: NCT01177124. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The study protocol was approved by the institutional review board at the University of South Florida to ensure the ethical treatment of participants.
Author Contribution: R. Reich contributed to conception and design, contributed to analysis and interpretation, drafted the manuscript, critically revised the manuscript, gave final approval, and agree to be accountable for all aspects of work ensuring integrity and accuracy. C. Lengacher contributed to conception and design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. K. Klein contributed to conception and design, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. C. Newton contributed to interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. S. Shivers contributed to acquisition, analysis, and interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. S. Ramesar contributed to acquisition, critically revised the manuscript, gave final approval, and agree to be accountable for all aspects of work ensuring integrity and accuracy. C. Alinat contributed to interpretation, drafted the manuscript, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. C. Paterson contributed to acquisition, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. A. Le contributed to interpretation, critically revised the manuscript, gave final approval, and agree to be accountable for all aspects of work ensuring integrity and accuracy. J. Park contributed to analysis and interpretation, critically revised the manuscript, gave final approval, and agree to be accountable for all aspects of work ensuring integrity and accuracy. V. Johnson-Mallard contributed to interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. M. Elias contributed to interpretation, drafted the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. M. Moscoso contributed to interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. M. Goodman contributed to acquisition, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. K. Kip contributed to analysis and interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by a grant from the National Cancer Institute, award no. 1R01CA131080-01A2. The work contained within this publication was supported in part by the Survey Methods Core Facility at the H. Lee Moffitt Cancer Center & Research Institute.
References
- Accortt E. E., Bower J. E., Stanton A. L., Ganz P. A. (2015). Depression and vasomotor symptoms in young breast cancer survivors: The mediating role of sleep disturbance. Archives of Women’s Mental Health, 18, 565–568. [DOI] [PubMed] [Google Scholar]
- Aderka D., Engelmann H., Maor Y., Brakebusch C., Wallach D. (1992). Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. Journal of Experimental Medicine, 175, 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S. R., Wurtzen H., Steding-Jessen M., Christensen J., Andersen K. K., Flyger H.…Dalton S. O. (2013). Effect of mindfulness-based stress reduction on sleep quality: Results of a randomized trial among Danish breast cancer patients. Acta Oncologica, 52, 336–344. doi:10.3109/0284186x.2012.745948 [DOI] [PubMed] [Google Scholar]
- Anisman H., Kokkinidis L., Merali Z. (2002). Further evidence for the depressive effects of cytokines: Anhedonia and neurochemical changes. Brain, Behavior, and Immunity, 16, 544–556. [DOI] [PubMed] [Google Scholar]
- Bao Y., Cao X. (2014). The immune potential and immunopathology of cytokine-producing B cell subsets: A comprehensive review. Journal of Autoimmunity, 55, 10–23. doi:10.1016/j.jaut.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Berger A. M., Gerber L. H., Mayer D. K. (2012). Cancer-related fatigue: Implications for breast cancer survivors. Cancer, 118, 2261–2269. doi:10.1002/cncr.27475 [DOI] [PubMed] [Google Scholar]
- Boggs J. M., Beck A., Felder J. N., Dimidjian S., Metcalf C. A., Segal Z. V. (2014). Web-based intervention in mindfulness meditation for reducing residual depressive symptoms and relapse prophylaxis: A qualitative study. Journal of Medical Internet Research, 16, e87 doi:10.2196/jmir.3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J. E., Crosswell A. D., Stanton A. L., Crespi C. M., Winston D., Arevalo J.…Ganz P. A. (2015). Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer, 121, 1231–1240. doi:10.1002/cncr.29194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A. (2015). Improving outcomes for breast cancer survivors: Perspectives on research challenges and opportunities. Chan, Switzerland: Springer International Publishing. [Google Scholar]
- Bower J. E., Ganz P. A., Irwin M. R., Arevalo J. M., Cole S. W. (2011). Fatigue and gene expression in human leukocytes: Increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain, Behavior, and Immunity, 25, 147–150. doi:10.1016/j.bbi.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Irwin M. R., Kwan L., Breen E. C., Cole S. W. (2011). Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology, 29, 3517–3522. doi:10.1200/jco.2011.36.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers B., Dalmasso B., Hatse S., Laenen A., Kenis C., Swerts E.…Wildiers H. (2015). Biological ageing and frailty markers in breast cancer patients. Aging, 7, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. J., Scott J., Maecker H. T., Park J. W., Esserman L. J. (2005). Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Research and Treatment, 91, 163–171. [DOI] [PubMed] [Google Scholar]
- Carlson L. E., Speca M., Faris P., Patel K. D. (2007). One year pre–post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain, Behavior, and Immunity, 21, 1038–1049. doi:10.1016/j.bbi.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Carlsson A. C., Juhlin C. C., Larsson T. E., Larsson A., Ingelsson E., Sundstrom J.…Arnlov J. (2014). Soluble tumor necrosis factor receptor 1 (sTNFR1) is associated with increased total mortality due to cancer and cardiovascular causes—Findings from two community based cohorts of elderly. Atherosclerosis, 237, 236–242. doi:10.1016/j.atherosclerosis.2014.09.005 [DOI] [PubMed] [Google Scholar]
- De Sanctis V., Agolli L., Visco V., Monaco F., Muni R., Spagnoli A.…Enrici R. M. (2014). Cytokines, fatigue, and cutaneous erythema in early stage breast cancer patients receiving adjuvant radiation therapy. Biomed Research International, 2014, 523568 doi:10.1155/2014/523568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Ruiz A., Tilz G. P., Zangerle R., Baier-Bitterlich G., Wachter H., Fuchs D. (1995). Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. European Journal of Haematology, 54, 1–8. [DOI] [PubMed] [Google Scholar]
- Fagundes C., LeRoy A., Karuga M. (2015). Behavioral symptoms after breast cancer treatment: A biobehavioral approach. Journal of Personalized Medicine, 5, 280–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayslip J., Dressler E. V., Weiss H., Taylor T. J., Chambers M., Noel T.…Moscow J. A. (2015). Plasma TNF-alpha and soluble TNF receptor levels after doxorubicin with or without co-administration of mesna—A randomized, cross-over clinical study. PLoS One, 10, e0124988 doi:10.1371/journal.pone.0124988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsel D. R. (1990). Less than obvious—Statistical treatment of data below the detection limit. Environmental Science & Technology, 24, 1766–1774. [Google Scholar]
- Henderson V. P., Massion A. O., Clemow L., Hurley T. G., Druker S., Hebert J. R. (2013). A randomized controlled trial of mindfulness-based stress reduction for women with early-stage breast cancer receiving radiotherapy. Integrative Cancer Therapies, 12, 404–413. doi:10.1177/1534735412473640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C. J., Ersser S. J., Hopkinson J. B., Nicholls P. G., Harrington J. E., Thomas P. W. (2012). Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in Stage 0 to III breast cancer: A randomized, controlled trial. Journal of Clinical Oncology, 30, 1335–1342. doi:10.1200/jco.2010.34.0331 [DOI] [PubMed] [Google Scholar]
- Jones S. M. W., LaCroix A. Z., Li W., Zaslavsky O., Wassertheil-Smoller S., Weitlauf J.…Caire-Juvera G. (2015). Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. Journal of Cancer Survivorship, 9, 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. (1990). Full catastrophe living: Using the wisdom of your body and mind to face stress. New York, NY: Bantam Books. [Google Scholar]
- Kabat-Zinn J., Lipworth L., Burney R. (1985). The clinical use of mindfulness meditation for the self-regulation of chronic pain. Journal of Behavioral Medicine, 8, 163–190. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J., Massion A. O., Kristeller J., Peterson L. G., Fletcher K. E., Pbert L.…Santorelli S. F. (1992). Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. American Journal of Psychiatry, 149, 936–943. [DOI] [PubMed] [Google Scholar]
- Kenyon M., Mayer D. K., Owens A. K. (2014). Late and long-term effects of breast cancer treatment and surveillance management for the general practitioner. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 43, 382–398. doi:10.1111/1552-6909.12300 [DOI] [PubMed] [Google Scholar]
- Langkjaer A., Kristensen B., Hansen B. E., Schultz H., Hegedus L., Nielsen C. H. (2012). B-cell exposure to self-antigen induces IL-10 producing B cells as well as IL-6- and TNF-alpha-producing B-cell subsets in healthy humans. Clinical Immunology, 145, 1–10. doi:10.1016/j.clim.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Lengacher C. A., Kip K. E., Post-White J., Fitzgerald S., Newton C., Barta M.…Klein T. W. (2013). Lymphocyte recovery after breast cancer treatment and mindfulness-based stress reduction (MBSR) therapy. Biological Research for Nursing, 15, 37–47. doi:10.1177/1099800411419245 [DOI] [PubMed] [Google Scholar]
- Lengacher C. A., Reich R. R., Paterson C. L., Ramesar S., Park J. Y., Alinat C. B.…Kip K. E. (2016). Examination of broad symptom improvement due to mindfulness-based stress reduction for Breast Cancer Survivors: A randomized controlled trial. Journal of Clinical Oncology, 34, 2827–2834. doi:10.1200/JCO.2015.65.7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengacher C., Reich R., Post-White J., Moscoso M., Shelton M., Barta M.…Budhrani P. (2012). Mindfulness based stress reduction in post-treatment breast cancer patients: An examination of symptoms and symptom clusters. Journal of Behavioral Medicine, 35, 86–94. doi:10.1007/s10865-011-9346-4 [DOI] [PubMed] [Google Scholar]
- Lengacher C. A., Shelton M. M., Reich R. R., Barta M. K., Johnson-Mallard V., Moscoso M. S.…Kip K. E. (2014). Mindfulness based stress reduction (MBSR(BC)) in breast cancer: Evaluating fear of recurrence (FOR) as a mediator of psychological and physical symptoms in a randomized control trial (RCT). Journal of Behavioral Medicine, 37, 185–195. doi:10.1007/s10865-012-9473-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Mills P. J., Rissling M., Fiorentino L., Natarajan L., Dimsdale J. E.…Ancoli-Israel S. (2012). Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain, Behavior, and Immunity, 26, 706–713. doi:10.1016/j.bbi.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynihan J. A. (2003). Mechanisms of stress-induced modulation of immunity. Brain, Behavior, and Immunity, 17, S11–S16. [DOI] [PubMed] [Google Scholar]
- Muthén L. K., Muthén B. O. (1998. –2003). Mplus user’s guide (7th ed). Los Angeles, CA: Authors. [Google Scholar]
- Nielsen C. H., Bornsen L., Sellebjerg F., Brimnes M. K. (2016). Myelin basic protein-induced production of tumor necrosis factor-alpha and interleukin-6, and presentation of the immunodominant peptide MBP85-99 by B cells from patients with relapsing–remitting multiple sclerosis. PLoS One, 11, e0146971 doi:10.1371/journal.pone.0146971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine S. R., Mechanic L. E., Enewold L., Bowman E. D., Ryan B. M., Cote M. L.…Caporaso N. E. (2016). Differential serum cytokine levels and risk of lung cancer between African and European Americans. Cancer Epidemiology Biomarkers & Prevention, 25, 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai L., Mendoza T. R., Reuben J. M., Martinez M. M., Willey J. S., Lara J.…Valero V. (2004). Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine, 25, 94–102. [DOI] [PubMed] [Google Scholar]
- Robins J. L. W., McCain N. L., Elswick R. K., Walter J. M., Gray D. P., Tuck I. (2013). Psychoneuroimmunology-based stress management during adjuvant chemotherapy for early breast cancer. Evidence-Based Complementary and Alternative Medicine, 2013, 372908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan L. N., Kim H. S. (2012). A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain, Behavior, and Immunity, 26, 830–848. doi:10.1016/j.bbi.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlowski M., Engler H., Grigoleit J. S. (2014). Endotoxin-induced experimental systemic inflammation in humans: A model to disentangle immune-to-brain communication. Brain, Behavior, and Immunity, 35, 1–8. doi:10.1016/j.bbi.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Witek-Janusek L., Albuquerque K., Chroniak K. R., Chroniak C., Durazo-Arvizu R., Mathews H. L. (2008). Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain, Behavior, and Immunity, 22, 969–981. doi:10.1016/j.bbi.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. S., Harden J. K. (2014). Symptom burden and quality of life in survivorship: A review of the literature. Cancer Nursing, 38, E29–E54. [DOI] [PubMed] [Google Scholar]
- Wurtzen H., Dalton S. O., Christensen J., Andersen K. K., Elsass P., Flyger H. L.…Johansen C. (2015). Effect of mindfulness-based stress reduction on somatic symptoms, distress, mindfulness and spiritual wellbeing in women with breast cancer: Results of a randomized controlled trial. Acta Oncologica, 54, 712–719. doi:10.3109/0284186x.2014.997371 [DOI] [PubMed] [Google Scholar]
- Wurtzen H., Dalton S. O., Elsass P., Sumbundu A. D., Steding-Jensen M., Karlsen R. V.…Johansen C. (2013). Mindfulness significantly reduces self-reported levels of anxiety and depression: Results of a randomised controlled trial among 336 Danish women treated for Stage I−III breast cancer. European Journal of Cancer, 49, 1365–1373. doi:10.1016/j.ejca.2012.10.030 [DOI] [PubMed] [Google Scholar]