Abstract
Introduction:
Cigarette smoking is a known risk factor for postoperative complications. Quitting or cutting down on cigarettes around the time of surgery may reduce these risks. This study aimed to determine the feasibility of using electronic nicotine delivery systems (ENDS) to help patients achieve this goal, regardless of their intent to attempt long-term abstinence.
Methods:
An open-label observational study was performed of cigarette smoking adults scheduled for elective surgery at Mayo Clinic Rochester and seen in the pre-operative evaluation clinic between December 2014 and June 2015. Subjects were given a supply of ENDS to use prior to and 2 weeks after surgery. They were encouraged to use them whenever they craved a cigarette. Daily use of ENDS was recorded, and patients were asked about smoking behavior and ENDS use at baseline, 14 days and 30 days.
Results:
Of the 105 patients approached, 80 (76%) agreed to participate; five of these were later excluded. Among the 75, 67 (87%) tried ENDS during the study period. At 30-day follow-up, 34 (51%) who had used ENDS planned to continue using them. Average cigarette consumption decreased from 15.6 per person/d to 7.6 over the study period ( P < .001). At 30 days, 11/67 (17%) reported abstinence from cigarettes.
Conclusion:
ENDS use is feasible in adult smokers scheduled for elective surgery and is associated with a reduction in perioperative cigarette consumption. These results support further exploration of ENDS as a means to help surgical patients reduce or eliminate their cigarette consumption around the time of surgery.
Implications:
Smoking in the perioperative period increases patients’ risk for surgical complications and healing difficulties, but new strategies are needed to help patients quit or cut down during this stressful time. These pilot data suggest that ENDS use is feasible and well-accepted in surgical patients, and worthy of exploration as a harm reduction strategy in these patients.
Introduction
Cigarette smoking increases the risks for postoperative complications in patients undergoing surgery, including cardiac, respiratory, and wound-related complications, and abstinence from smoking reduces these risks. 1 The duration of abstinence necessary for reduction of these risks is not known, but some evidence suggests that even a brief period of abstinence may be beneficial, 2 , 3 and that abstinence in the postoperative period itself may be helpful. 4 Numerous toxic compounds in cigarettes, including carbon monoxide, may contribute to risk, but available evidence suggests that patients benefit when nicotine replacement therapy (NRT) is used to achieve abstinence. 5 Although there are efficacious interventions available to help smokers quit, 6 including patients scheduled for elective surgery, the implementation of these interventions into clinical practice has proved challenging. For example, despite several years of active tobacco control efforts, at Mayo Clinic Rochester, approximately 40% of cigarette smokers still smoke on the morning of their surgical procedure (unpublished observations). Clearly, new strategies are needed to reduce exposure to cigarette smoke in the perioperative period.
Electronic nicotine delivery systems (ENDS) have recently exploded in popularity. 7 , 8 Also known as “electronic cigarettes” or “E-cigarettes,” these devices vaporize nicotine solutions with some devices mimicking the look and feel of tobacco cigarettes. ENDS have been promoted as potential harm-reduction devices. 9 Although data are limited, some studies (but not all) suggest that at least some cigarette smokers are using ENDS to reduce or eliminate tobacco smoking. 10–14 Given that ENDS produce a nicotine-containing vapor, it is likely that any deleterious effects are less than conventional cigarettes, as many of the harmful constituents in tobacco smoke result from the combustion of tobacco leaf. Although the content of vapors produced by different ENDS varies and their long-term safety is not known, the levels of harmful substances found in ENDS are generally lower than those produced by combustible tobacco products. 15 , 16 ENDS are also available in a range of nicotine concentrations, including nicotine-free. However, the net public health effects of the widespread introduction of ENDS remain almost wholly unknown, and their potential impact (for good or harm) is a subject of considerable debate. 17
NRT is a common component of efficacious interventions to help surgical patients quit smoking. 6 It is possible that ENDS, as a form of NRT, could be useful in helping smokers reduce or eliminate their smoking in the perioperative period, especially given emerging data that smokers may view ENDS more favorably than traditional NRT. 18 In pilot survey work, we have shown that smokers scheduled for elective surgery who are seen in Mayo Clinic Rochester Preoperative Evaluation Center express considerable interest in using ENDS to reduce their tobacco consumption. 19 However, it is not clear whether patients scheduled for surgery, who may have no experience with ENDS and many distractions in the busy perioperative period, would be able to consistently utilize these devices.
This study aimed to determine the feasibility and acceptability of ENDS in the perioperative period among cigarette smokers scheduled for elective surgery. A secondary objective was to determine how access to ENDS was associated with changes in cigarette consumption both preoperatively and up to 2 weeks following discharge from the surgical facility.
Methods
This study was approved by the Mayo Clinic Institutional Review Board, Rochester, Minnesota. Written informed consent was obtained.
Recruitment
Subjects were recruited from patients scheduled for elective surgery who were evaluated in the Mayo Clinic Preoperative Evaluation Center (POE), where approximately 15% of elective surgical patients at Mayo Clinic Rochester are seen. Patients undergoing a wide variety of elective procedures, including orthopedic, plastic and reconstructive, and oncologic procedures, are evaluated in this center. Inclusion criteria included age at least 18 years and current smoking (defined as >100 cigarettes lifetime consumption and self-report of smoking either every day or some days) prior to evaluation. For women of child-bearing potential, a negative pregnancy test was required. Exclusion criteria included current use of END (past use was not an exclusion), current use of pharmacotherapy for nicotine dependence, pregnancy or lactation, and those whose surgeons specifically directed them not to use NRT prior to surgery. Eligible subjects were approached on a convenience basis and invited to participate, regardless of any intent to modify smoking behavior in the perioperative period; that is, subjects were not selected based on their willingness to quit or cut down smoking.
Study Procedures
After enrollment, study personnel delivered a brief intervention emphasizing the importance of quitting or cutting down on smoking in the perioperative period ( Supplementary Appendix ). The intervention also introduced the concept of ENDS, and provided instructions for their use. They were encouraged to use ENDS instead of cigarettes when they desired to smoke.
Study subjects were then given a supply of NJOY ENDS sufficient for use in the preoperative period and up to 2 weeks postoperatively in one of three varieties depending on patient preference and baseline cigarette consumption: NJOY KingsTraditional Gold (2.4% nicotine), NJOY Kings Traditional Bold (4.5% nicotine, offered to subjects smoking ≥15 cigarettes/d) and NJOY Kings Menthol (3% nicotine). The NJOY Traditional Gold product was selected because it is a single-use, disposable product that requires minimal training, and because there were published investigation of its pharmacokinetics at the time of study design. 20 According to the product label, each NJOY device delivers the equivalent of approximately one pack of tobacco cigarettes (20 cigarettes), although there is considerable variability in use patterns and recent data suggest that actual delivery does not achieve nicotine levels comparable to a cigarette. 10 The cost per device is $4.75, which is less expensive or comparable to purchasing regular cigarettes, depending on the pattern of ENDS use. Study subjects were supplied a sufficient number of devices to completely replace their use of tobacco cigarettes from the time of POE evaluation until 2 weeks after anticipated discharge from the surgical facility (median length of stay 1 day, IQR 0–2), along with an additional four devices to account for variability in use patterns. For example, the median time from POE evaluation to surgery is 1 day. Thus, a typical subject who smokes 20 cigarettes per day would have been given 15 NJOY ENDS, plus an additional 4 to account for subject variability. Study subjects scheduled for surgery more than 1 week from the time of POE evaluation were given sufficient supply to support 1 week of preoperative and 2 weeks of postoperative ENDS use.
Study Measurements
Assessments were performed at baseline in the POE clinic, and at 14 and 30 days post discharge from the surgical facility. In addition, patients were asked to keep a daily diary of ENDS use for 1 week before surgery and 14 days after discharge.
Baseline
A survey administered via iPad (REDCap Survey, a secure, web-based electronic data capture tool hosted at Mayo Clinic) 21 queried demographic information, baseline measures of smoking history, and Surgical Risk and Health Concerns Indices assessing knowledge of how smoking affects surgical risk and health in general, respectively. 22 If subjects had used ENDS, additional items queried the reasons they used ENDS and their perceived benefits. Finally, the survey included items used in our prior work regarding interest in using ENDS to maintain perioperative abstinence (four items), perceived benefits in using ENDS to maintain perioperative abstinence (four items), and perceived barriers to using ENDS to maintain perioperative abstinence (five items). 19 The factor structure of ENDS-related indices, including internal consistency of scales and factor loading of each indicator was previously analyzed and found acceptable. 19
Daily Diary Up to 14 Days Post Discharge
At the time of enrollment, subjects were given a paper diary in which to record their episodes of use of either ENDS or tobacco cigarettes over this period, as well as the number of ENDS finished each day. The diary also included binary response items (agree/disagree) to be completed at 14 days regarding their experience in using ENDS. Subjects were asked to return the diary via mail, and received $40 remuneration if they did so. Study personnel contacted participants by phone at 14 days to remind them to send the diary and survey. Study personnel first attempted to contact the subject on day 14, and for up to 1 week after that time. If the patient reported losing the diary or not recording their use, study personnel verbally completed the 14-day survey with the patient during this phone call.
30 Days Post Discharge
Subjects were contacted by telephone to determine smoking behavior since surgery, ENDS utilization and a summary of ENDS use.
Statistical Analyses
The primary endpoints of this pilot study were the proportion of subjects who utilized ENDS before and after surgery and the number of times it was utilized. The secondary endpoint of this study was cigarette consumption. With a sample size of 80, this study was designed to have a power of 0.90 to detect a 20% decrease in cigarettes per day compared with baseline values. Descriptive statistics were used to characterize each of the primary and secondary endpoints listed above, with 95% CI used to present variability for proportions and standard deviation for continuous variables. Survey information was entered into REDCap directly by the participant (for enrollment survey) or indirectly by study personnel (for 14- and 30-day follow-up), which allowed for the automated export of data to statistical packages for analysis. Indices including the Surgical Risk Index, the Health Concerns Index, and three ENDS-related indices assessing interest in, perceived benefits of, and barriers to perioperative use, were scored and reported as mean ± standard deviation. The Surgical Risk Index was scored by summing the number of “yes responses.” For the Health Concerns Index, each response was assigned a numerical value, with higher values indicating greater concern. For the ENDS-related indices, a score was calculated by averaging the numerical values assigned to each Likert response (1 = strongly disagree, 5 = strongly agree). Thirty-day outcomes were compared to baseline using Wilcoxon sign rank tests.
Results
A flow diagram of the recruitment process is illustrated in Figure 1 . Enrollment among patients who were eligible and approached for consent was high (76% of eligible patients enrolled). Of the 80 patients enrolled, five were excluded after enrollment (reasons shown in Figure 1 ). Of the 75 remaining participants, 53 (71%) returned the daily diaries; 63 (84%) and 67 (89%) were contacted at days 14 and 30, respectively. The median time from enrollment to surgery was 1 day [IQR 1–3.25].
Figure 1.
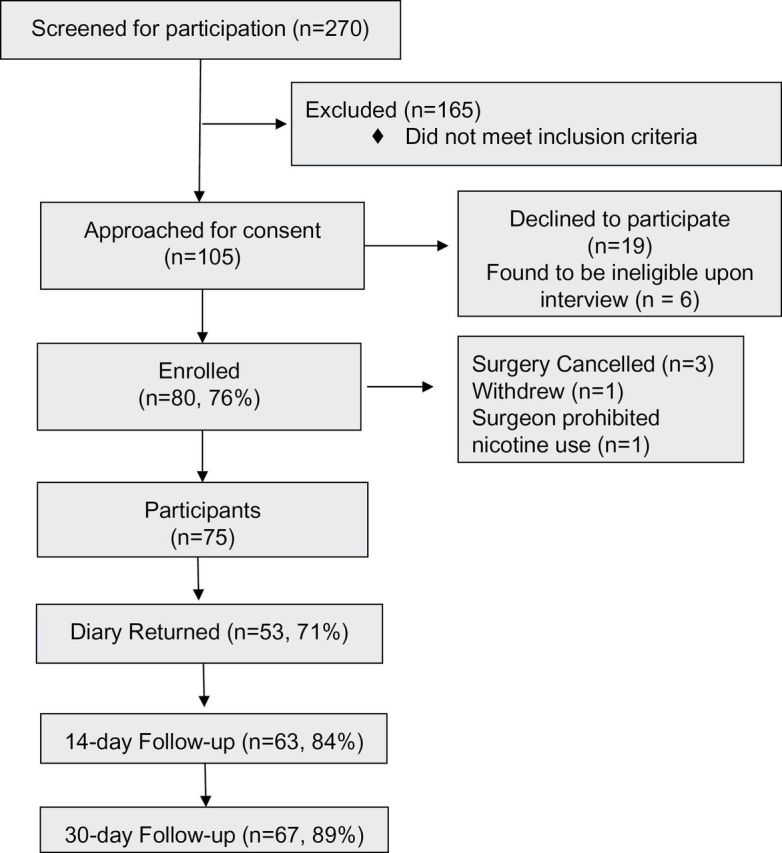
Flow of patient recruitment, participation, and follow-up.
Baseline Characteristics
Most participants were older, male, at least high-school educated, and white ( Table 1 ). Most also had a long history of cigarette consumption and had made at least one prior quit attempt, with about one-third making an attempt within the past year. Approximately half stated that they intended to remain abstinent after surgery, and approximately one in four felt that they were likely or very likely to succeed in doing so. Values of the Surgical Risk Index and Health Concerns Index were consistent with a strong appreciation of the risks of smoking to health.
Table 1.
Demographics and Baseline Data a
| Age | 60±9 |
| Female gender | 31 (42) |
| Education of high school/GED and beyond | 71 (96) |
| Caucasian | 106 (95) |
| Cigarettes/d | 16±9.7 |
| Prefer menthol cigarettes | 4 (5) |
| Number of year of smoking | 36±13.6 |
| At least one quit attempt previously | 63 (84) |
| Tried to quit within last year | 28 (37) |
| No plan to quit smoking | 9 (12) |
| Nicotine dependence (FTND score) | 4.3±2.0 |
| Surgical risk index (four items, max score = 4) b | 2.9±1.4 |
| Health concern index (three items, max score = 9) b | 7.0±1.1 |
| Plan to stay off cigarettes after surgery | 52 (69) |
| Interest index (four items, max score = 20) b | 17.6±2.1 |
| Perceived benefits (four items, max score = 20) b | 16.9±2.5 |
| Barriers index (four items, max score = 20) b | 9.9±2.6 |
| Likely to stay off cigarettes after surgery | |
| Very likely | 2 (3) |
| Likely | 24 (32) |
| Neither likely nor unlikely | 33 (44) |
| Unlikely | 13 (17) |
| Very unlikely | 3 (4) |
| Succeed at quitting smoking | |
| Extremely sure | 1 (1.3) |
| Very sure | 16 (21.3) |
| Somewhat sure | 35 (48) |
| Not at all sure | 22 (29.3) |
a For proportions, values are given as n (%) for the 75 patients included in the analysis. Values for continuous variables are presented as mean ± standard deviation. FTND = Fagerstrom test for nicotine dependence; GED= general educational development, a marker of high school completion equivalence.
b Indices calculated as described in the methods.
Approximately two-thirds of participants had heard of ENDS, but had never tried them; most of the remainder had tried them, but no longer used them. Among those who had tried ENDS in the past ( n = 24), the most common reason was to attempt abstinence from cigarettes. However, most of these individuals did not find them useful for this purpose. Table 2 lists the interest in, perceived benefits of, and perceived barriers to using ENDS in the perioperative period for all participants. High proportions agreed or strongly agreed that they would be willing to use ENDS to help them eliminate or reduce regular cigarette use around the time of surgery, and similar proportions perceived health benefits of doing so. The corresponding values of the indices calculated from these responses regarding interest, perceived benefits, and perceived barriers were consistent with favorable perceptions of perioperative ENDS use ( Table 1 ).
Table 2.
Interest, Perceived Benefits, and Barriers to E-Cigarette (E-Cig) Use
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Willing to try e-cigs to help me stay off or cut down regular cigarette around the time of surgery | 44 (59) | 30 (40) | 1 (1) | 0 (0) | 0 (0) |
| If they were available free of charge, I would try to use them to help stay off or cut down regular cigarette use around the time of surgery | 43 (57) | 30 (40) | 2 (3) | 0 (0) | 0 (0) |
| Even if I needed to buy them myself, it would be worth to try e-cigs to stay off or cut down regular cigarettes around the time of surgery | 28 (37) | 35 (47) | 10 (13) | 2 (3) | 0 (0) |
| I think that e-cigarettes could help me stay off or cut down regular cigarette use around the time of surgery. | 30 (40) | 35 (47) | 10 (13) | 0 (0) | 0 (0) |
| Using e-cigarettes instead of smoking regular cigarettes could help me do better after my surgery | 27 (36) | 37 (49) | 11 (15) | 0 (0) | 0 (0) |
| E-cigarettes could help me cope with not being able to smoke regular cigarettes while in the hospital for my surgery | 25 (33) | 36 (48) | 13 (17) | 0 (0) | 1 (1) |
| It would be better for my health if I could use e-cigarettes around the time of surgery rather than smoking regular cigarettes | 30 (40) | 36 (48) | 9 (12) | 0 (0) | 0 (0) |
| Using e-cigarettes could help me improve my health around the time of surgery | 29 (39) | 35 (47) | 11 (15) | 0 (0) | 0 (0) |
| Nicotine could cause problems for my surgery whether I get it by smoking or through e-cigarettes | 15 (20) | 33 (44) | 24 (32) | 2 (3) | 1 (1) |
| It would be hard for me to learn how to use e-cigarettes around the time of my surgery | 4 (5) | 6 (8) | 19 (25) | 36 (48) | 10 (13) |
| I have too many other things to worry about other than to try e-cigarettes around the time of surgery | 3 (4) | 6 (8) | 19 (25) | 36 (48) | 11 (15) |
| E-cigarettes would be too expensive for me to use | 3 (4) | 3 (4) | 36 (48) | 28 (37) | 5 (7) |
| I am concerned that e-cigarettes are not safe | 2 (3) | 5 (7) | 28 (37) | 32 (43) | 8 (10) |
Values given as n (%) for the 75 participants. 1 = strongly agree; 2 = agree; 3 = neither agree nor disagree; 4 = disagree; 5 = strongly disagree.
ENDS Utilization (Primary Outcome)
For the 67 participants contacted on day 30, 58 (87%) reported at least one use of ENDS during the study period; 21 (32%) used ENDS before their surgery, and 58 (87%) used them afterward. At day 30, 34 (51%) of the 58 participants who had used the ENDS reported planning to continue using them in the future. Nine (16%) reported having finished their given ENDS supply and having already purchased additional ENDS for continued use.
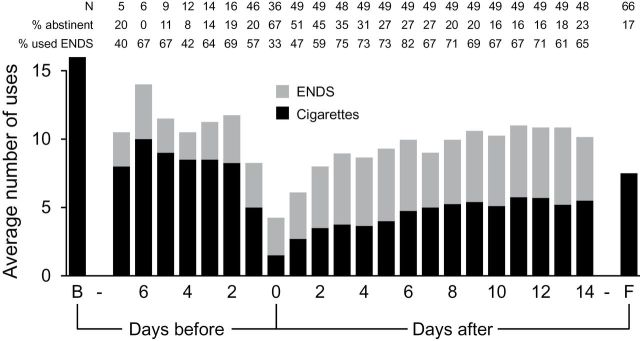
ENDS use in the 53 participants who returned their daily diaries is presented in Figure 2 . The number of ENDS uses (defined as “e-times,” or number of episodes of ENDS use per day) was relatively stable from 1 week prior to surgery until 14 days after surgery (the period over which free ENDS were provided), as was the proportion of participants using ENDS. The relatively low absolute number of ENDS users from 2 to 7 days prior to surgery reflects the fact that most subjects were enrolled the day prior to surgery; regardless of when enrolled, approximately two-thirds of patients used ENDS preoperatively. On the day of surgery, 12 (33%) of participants used ENDS.
Figure 2.
Cigarette and electronic nicotine delivery systems (ENDS) usage for study participants at the following timepoints: baseline (B, at enrollment), the 7 days before surgery (days −7 to −1), the day of surgery (day 0), the 14 days after surgery (days 1–14), and 30-day follow-up (F). The number of subjects reporting data each day ( N ) appears at the top of the figure, along with the proportion of subjects reporting who were abstinent from cigarettes on that day, and the proportions of subjects reporting who used ENDS at least once that day.
Of participants returning diaries, 46 answered items regarding their experiences using ENDS. Of these, 39 (85%) would be willing to try ENDS again for future surgeries, 29 (63%) felt that ENDS helped them cope with not smoking regular cigarettes, 33 (72%) felt that ENDS helped them quit or cut down on regular cigarettes, and 35 (76%) felt that their health was benefitted by their ENDS use.
Tobacco Use (Secondary Outcome)
At 30 days after discharge, 11 of the 67 participants contacted (17%) self-reported 7-day point prevalence abstinence from smoking. For these 67 participants, cigarette consumption at 30 days decreased significantly compared with baseline consumption (from 15.6 to 7.6 cigarettes per day, P < .001). Figure 2 presents cigarette use for the 53 participants who returned their daily diaries. The proportion of respondents who were abstinent on a given day in the preoperative period ranged from 0% to 20%. Two-thirds of those who reported their smoking behavior on the day of surgery maintained abstinence, with the proportion of abstainers ranging from 16% to 51% in the subsequent 14 days. Over this postoperative period, on average approximately half of the instances of nicotine self-administration by subjects were via cigarettes, and half via ENDS.
Discussion
The major finding of this feasibility study was that when cigarette smokers scheduled for elective surgery were offered free ENDS at the time of pre-anesthesia evaluation, a high proportion utilized them in the perioperative period, with an associated reduction in cigarette consumption.
Consistent with our prior formative work, 19 interest in ENDS utilization was high in this pre-surgical population, as indicated by the baseline survey, the high enrollment rate and the high rate of utilization. This occurred despite the considerable life disruptions that surround the surgical experience, relatively high level of nicotine dependence, relatively low self-efficacy for maintaining abstinence, and no prior experience with ENDS for most patients. Approximately half of patients were sufficiently satisfied with their experience that they planned to continue ENDS use, and most would be willing to use ENDS again for future surgeries. These findings suggest that ENDS are potentially feasible and well-accepted in surgical patients who smoke. To our knowledge, there are no prior comparable studies reporting uptake of ENDS when their use is encouraged by healthcare professionals in a medical population.
This study also provides evidence that the use of ENDS in surgical patients was associated with a reduction in cigarette consumption. The potential of ENDS to impact smoking behavior has led to exploration of whether they could be effective tools to reduce or eliminate cigarette consumption, with variable results. 11 The current findings are consistent with prior observations in different settings that ENDS use is associated with modification of tobacco use. However, with this observational study design it is not possible to determine whether their use actually changed smoking behavior. Surgery itself serves as a “teachable moment” for changes in smoking behavior, 23 and patients may spontaneously reduce or eliminate consumption in the absence of any intervention. Whether ENDS are efficacious in modifying smoking behavior in this setting requires further investigation using a control group not given ENDS. Nonetheless, these preliminary data are at least consistent with the concept that ENDS use could facilitate a reduction in cigarette consumption.
Given the apparent feasibility of ENDS use in surgical patients, several questions need to be explored before their use could be recommended in the perioperative period. Perioperative abstinence clearly reduces the risk for pulmonary and wound-related complications; whether reduced consumption would also be beneficial is unknown. Initial evidence suggests that dual use can reduce exposure to toxicants in cigarettes in the short term. 16 However, it is not clear, for patients unwilling to abstain, whether advocating a harm reduction strategy of replacing some portion of regular cigarette consumption with ENDS would be beneficial to surgical outcomes. Tobacco interventions incorporating approved NRT are efficacious to achieve sustained postoperative abstinence in the surgical population 6 ; the efficacy of ENDS remains to be determined. If efficacy were equivalent, ENDS would have the potential to be more effective in practice, given the high level of interest expressed in this and our prior study. As a further indication of potential interest, in a prior study of patch NRT in the same study setting (in which the intent to abstain also was not an inclusion criterion), 24 approximately 10% of those approached enrolled, compared with 76% in the present study. Given the relatively low nicotine delivery of the ENDS product used in the present study, it is possible that newer ENDS products that deliver nicotine at levels comparable to smoking could have an even greater impact on reducing or eliminating tobacco use.
In addition, the consequences of dual use beyond the immediate postoperative period would need to be considered, including the question of who could provide ongoing smoking cessation services and support to dual users, and if such use would potentially interfere with the “teachable moment” effect of surgery to promote spontaneous abstinence. 23 On the other hand, attempts to reduce consumption using NRT in smokers with an intention to quit significantly increases cessation rates, 10 raising the potential that ENDS could serve as an attractive means to initiate pharmacotherapy in this population who might otherwise not be willing to do so.
Finally, promotion of these devices by healthcare professionals given the rapidly evolving state of ENDS development and regulation would be problematic. There is a wide array of available products, with potentially differing safety profiles (which themselves remain to be determined), and the FDA has not approved these devices for any type of smoking cessation or reduction. If ENDS were found in future studies to be effective in reducing perioperative risk, clinicians would likely insist upon a well-characterized, standardized ENDS product approved for this purpose.
Limitations of this study include the likelihood that those most interested in ENDS were more likely to enroll (although consent rates were high) and that results from this specialty practice in the upper Midwest, with a high proportion of Caucasian patients and many with greater than a high school education, may not apply to all practice settings. Also, as mentioned above, this pilot observational study did not have a control group, limiting our ability to determine any effect of ENDS on smoking behavior.
These results support further exploration of ENDS as a means to help surgical patients reduce or eliminate their cigarette consumption around the time of surgery.
Funding
This study was funded internally, including the purchase of the ENDS devices. Use of REDCap software was supported by the National Center for Advancing Translational Sciences (NCATS; grant UL1 TR000135).
Declaration of Interests
SL has been funded by McNeil and GSK to test a nicotine delivery system for tobacco treatment. DOW has been funded by Pfizer to develop a tobacco control curriculum for anesthesiology and surgery residents. IC, MN, SK, DS, and AH have nothing to disclose.
Supplementary Material
Acknowledgments
The authors would like to thank all study staff for their tireless efforts, including Brenda Anderson, Sue Weise and Katherine Kelsey.
References
- 1. Theadom A, Cropley M . Effects of preoperative smoking cessation on the incidence and risk of intraoperative and postoperative complications in adult smokers: a systematic review . Tob Control . 2006. ; 15 ( 5 ): 352 – 358 . doi: 10.1136/tc.2005.015263 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Warner DO . Perioperative abstinence from cigarettes: physiologic and clinical consequences . Anesthesiology . 2006. ; 104 ( 2 ): 356 – 367 . [DOI] [PubMed] [Google Scholar]
- 3. Anderson ME, Belani KG . Short-term preoperative smoking abstinence . Am Fam Physician . 1990. ; 41 : 1191 – 1194 . [PubMed] [Google Scholar]
- 4. Nasell H, Adami J, Samnegard E, Tonnesen H, Ponzer S . Effect of smoking cessation intervention on results of acute fracture surgery: a randomized controlled trial . J Bone Joint Surg Am . 2010. ; 92 ( 6 ): 1335 – 1342 . doi: 10.2106/JBJS.I.00627 . [DOI] [PubMed] [Google Scholar]
- 5. Nolan MB, Warner DO . Safety and efficacy of nicotine replacement therapy in the perioperative period: a narrative review . Mayo Clin Proc . 2015. ; 90 ( 11 ): 1553 – 1561 . doi: 10.1016/j.mayocp.2015.08.003 . [DOI] [PubMed] [Google Scholar]
- 6. Thomsen T, Villebro N, Moller AM . Interventions for preoperative smoking cessation . Cochrane Database Syst Rev . 2014. ; 3 : CD002294 . doi: 10.1002/14651858.CD002294.pub4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stimson GV, Thom B, Costall P . Disruptive innovations: the rise of the electronic cigarette . Int J Drug Policy . 2014. ; 25 ( 4 ): 653 – 655 . doi: 10.1016/j.drugpo.2014.05.003 . [DOI] [PubMed] [Google Scholar]
- 8. King BA, Alam S, Promoff G, Arrazola R, Dube SR . Awareness and ever-use of electronic cigarettes among U.S. adults, 2010–2011 . Nicotine Tob Res . 2013. ; 15 ( 9 ): 1623 – 1627 . doi: 10.1093/ntr/ntt013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cahn Z, Siegel M . Electronic cigarettes as a harm reduction strategy for tobacco control: a step forward or a repeat of past mistakes? J Public Health Policy . 2011. ; 32 ( 1 ): 16 – 31 . doi: 10.1057/jphp.2010.41 . [DOI] [PubMed] [Google Scholar]
- 10. Wu L, Sun S, He Y, Zeng J . Effect of smoking reduction therapy on smoking cessation for smokers without an intention to quit: an updated systematic review and meta-analysis of randomized controlled . Int J Environ Res . 2015. ; 12 ( 9 ): 10235 – 10253 . doi: 10.3390/ijerph120910235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rahman MA, Hann N, Wilson A, Mnatzaganian G, Worrall-Carter L . E-cigarettes and smoking cessation: evidence from a systematic review and meta-analysis . PloS One . 2015. ; 10 ( 3 ): e0122544 . doi: 10.1371/journal.pone.0122544 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P . Electronic cigarettes for smoking cessation and reduction . Cochrane Database Syst Rev . 2014. ; 12 : CD010216 . doi: 10.1002/14651858.CD010216.pub2 . [DOI] [PubMed] [Google Scholar]
- 13. Polosa R, Caponnetto P, Maglia M, Morjaria JB, Russo C . Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit . BMC Public Health . 2014. ; 14 : 1159 . doi: 10.1186/1471-2458-14-1159 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bullen C, Howe C, Laugesen M, et al. . Electronic cigarettes for smoking cessation: a randomised controlled trial . Lancet . 2013. ; 382 ( 9905 ): 1629 – 1637 . doi: 10.1016/S0140-6736(13)61842-5 . [DOI] [PubMed] [Google Scholar]
- 15. Goniewicz ML, Hajek P, McRobbie H . Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications . Addiction . 2014. ; 109 ( 3 ): 500 – 507 . doi: 10.1111/add.12410 . [DOI] [PubMed] [Google Scholar]
- 16. McRobbie H, Phillips A, Goniewicz ML, et al. . Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein . Cancer Prev Res . 2015. ; 8 ( 9 ): 873 – 878 . doi: 10.1158/1940-6207.CAPR-15-0058 . [DOI] [PubMed] [Google Scholar]
- 17. Palazzolo DL . Electronic cigarettes and vaping: a new challenge in clinical medicine and public health. A literature review . Front Public Health . 2013. ; 1 : 56 . doi: 10.3389/fpubh.2013.00056 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steinberg MB, Zimmermann MH, Delnevo CD, et al. . E-cigarette versus nicotine inhaler: comparing the perceptions and experiences of inhaled nicotine devices . J Gen Intern Med . 2014. ; 29 ( 11 ): 1444 – 1450 . doi: 10.1007/s11606-014-2889-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kadimpati S, Nolan M, Warner DO . Attitudes, beliefs, and practices regarding electronic nicotine delivery systems in patients scheduled for elective surgery . Mayo Clin Proc . 2015. ; 90 ( 1 ): 71 – 76 . doi: 10.1016/j.mayocp.2014.11.005 . [DOI] [PubMed] [Google Scholar]
- 20. Nides MA, Leischow SJ, Bhatter M, Simmons M . Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system . Am J Health Behav . 2014. ; 38 ( 2 ): 265 – 274 . doi: 10.5993/AJHB.38.2.12 . [DOI] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG . Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support . J Biomed Inform . 2009. ; 42 ( 2 ): 377 – 381 . doi: 10.1016/j.jbi.2008.08.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu C, Shi Y, Kadimpati S, et al. . Perioperative smoking behavior of Chinese surgical patients . Anesth Analg . 2013. ; 116 ( 6 ): 1238 – 1246 . doi: 10.1213/ANE.0b013e31828e5cf0 . [DOI] [PubMed] [Google Scholar]
- 23. Shi Y, Warner DO . Surgery as a teachable moment for smoking cessation . Anesthesiology . 2010. ; 112 ( 1 ): 102 – 107 . doi: 10.1097/ALN.0b013e3181c61cf9 . [DOI] [PubMed] [Google Scholar]
- 24. Warner DO, Patten CA, Ames SC, Offord KP, Schroeder DR . Effect of nicotine replacement therapy on stress and smoking behavior in surgical patients . Anesthesiology . 2005. ; 102 : 1138 – 1146 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.