Abstract
For precision health care to be successful, an in-depth understanding of the biological mechanisms for symptom development and severity is essential. Omics-based research approaches facilitate identification of the biological underpinnings of symptoms. We reviewed literature for omics-based approaches and exemplar symptoms (sleep disruption, cognitive impairment, fatigue, gastrointestinal [GI] distress, and pain) to identify genes associated with the symptom or symptoms across disease processes. The review yielded 27 genes associated with more than one symptom. ABCB1 (MDR1), APOE, BDNF, CNR1, COMT, DAT1 (SLC6A3), DRD4, ESR1, HLA-DRB1, IL10, IL1B, IL6, LTA, PTGS2 (COX-2), SLC6A4, and TNF were associated with cognitive impairment and pain, which had the most genes in common. COMT and TNF were related to all symptoms except sleep disruption. IL1B was associated with all symptoms except cognitive impairment. IL10, IL1A, IL1B, IL1RN, IL6, and IL8 (CXCL8) were linked with all the exemplar symptoms in various combinations. ABCB1 (MDR1) and SLC6A4 were associated with cognitive impairment, GI distress, and pain. IL10 and IL6 were linked to cognitive impairment, fatigue, and pain. APOE and BDNF were associated with sleep disruption, cognitive impairment, and pain. The 27 genes were associated with canonical pathways including immune, inflammatory, and cell signaling. The pathway analysis generated a 15-gene model from the 27 as well as 3 networks, which incorporated new candidate genes. The findings support the hypothesis of overlapping biological underpinnings across the exemplar symptoms. Candidate genes may be targeted in future omics research to identify mechanisms of co-occurring symptoms for potential precision treatments.
Keywords: symptom, genomic, epigenomic, transcriptomic, review, pathway
Precision health care requires an understanding of the biological mechanisms underlying the development and severity of symptoms across disease processes. Such knowledge should also provide an explanation as to why some symptoms often co-occur in clusters. Omics approaches (i.e., genomics, epigenomics, and transcriptomics) have shown great promise for deciphering the biological underpinnings of symptoms,
Recently, there has been increasing support for research aimed at identifying those mechanisms underlying the development of symptoms and symptom clusters (Corwin et al., 2014; Miaskowski et al., 2017). Most of these studies have examined symptoms within the context of a chronic condition using an omics-based approach. However, very little has been done to either (a) assess support for associations between specific genes and a symptom in a disease-agnostic manner or (b) assess support for associations of specific genes across multiple symptoms.
The purpose of this manuscript was to review the literature related to omics and selected exemplar symptoms in a disease-agnostic manner, to identify genes associated with these symptoms, and then to determine which genes are associated with multiple symptoms. Our goal is to identify overlapping biological underpinnings across symptoms. We also sought to identify additional gene candidates for future evaluation based on biological relatedness to those genes associated with multiple symptoms.
Methods
We conducted a purposeful search of the literature in PubMed including all indexed publications prior to June 2017. Included articles had to be reporting on primary research with a quantitatively measured phenotype for the symptom of interest, have statistical analyses with the symptom as a dependent variable, and have significant findings (explicated in the manuscript) for the gene of interest, given our goal of identifying genes implicated in symptom phenotypes. We further limited search results to studies published in English and excluded reviews, meta-analyses, commentary, opinions, and non-data-based articles.
We selected symptoms for their relevance to the symptom science portion of the National Institute of Nursing Research (NINR) strategic plan and for their likelihood of co-occurrence across multiple disease processes (NINR, 2016). Thus, we selected the symptoms and search terms of “sleep disruption,” “cognitive impairment,” “fatigue” (excluding chronic fatigue syndrome), and “pain” (excluding acute coronary syndrome). We also wished to include the concept of gastrointestinal (GI) distress, but this entry did not yield results; therefore, we changed terms to reflect particular symptoms that are incorporated in GI distress: “nausea,” “vomiting,” “diarrhea,” and “constipation” and refer to these as “GI distress” in this manuscript.
For each symptom, we queried on three different omic approaches: genomic, epigenomic, and transcriptomic. Search terms for genomics included the symptom search term and also “polymorphism,” “genetic,” OR “genomic.” Similarly, the search terms for the epigenomic approach included the symptom search term and also “DNA methylation,” “histone modification,” “chromatin condensation,” “epigenetic,” OR “epigenomic.” Finally, search terms for the transcriptomic approach included the symptom search term and also “gene expression” OR “transcriptome.”
Symptoms were divided, so that each author screened abstracts for one symptom’s relevant articles. If needed, they read the article to extract the necessary information or exclude the article. Many eliminated reports did not describe measurement of a symptom or analysis of the symptom directly. Many studies had a symptom listed as part of a larger phenotype, and several studies assessed a drug as treatment for the symptom rather than the symptom, itself.
We analyzed those genes that were significantly associated with more than one symptom using Ingenuity Pathway Analysis (IPA, QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis). IPA software allows for a variety of analyses and data interpretations by harnessing knowledge about biological pathways and interaction networks. We conducted the analyses using the Ingenuity Knowledge Base (genes only) as reference, considering both direct and indirect relationships. Network interactions included endogenous chemicals and were limited to 35 molecules and 25 networks. Node type excluded non-mammalians, and the data source excluded the Mouse Genome Database. Species was limited to humans, and no tissue lines were chosen. All mutations were considered. IPA produced an output with an interaction model of the 27 genes only, the most significant canonical pathways associated with the 27 genes, and the networks of molecules from the Ingenuity Knowledge Base that interact with the 27 genes.
Results
Cognitive impairment and pain symptoms were associated with the same genes more frequently than any other two symptoms (Table 1): ABCB1 (MDR1), APOE, BDNF, CNR1, COMT, DAT1 (SLC6A3), DRD4, ESR1, HLA-DRB1, IL10, IL1B, IL6, LTA, PTGS2 (COX-2), SLC6A4, and TNF. COMT and TNF were related to all symptoms except sleep disruption. IL1B was associated with all symptoms except cognitive impairment. All of the symptoms investigated were associated with one or more interleukin genes (IL10, IL1A, IL1B, IL1RN, IL6, and IL8/CXCL8) in various combinations. Six genes had three symptoms associated with them: ABCB1 (MDR1) and SLC6A4 (cognitive impairment, GI distress, and pain); IL10 and IL6 (cognitive impairment, fatigue, and pain); APOE and BDNF (sleep disruption, cognitive impairment, and pain); and NTRK2 (sleep disruption, cognitive impairment, and fatigue). We present symptom-specific findings in Supplemental Tables A1–A5 along with table-specific references.
Table 1.
Associations in the Literature Between Genes and Selected Symptoms, with Methodological Approach.
| Gene | Symptom | Approach | ||||||
|---|---|---|---|---|---|---|---|---|
| Sleep Disruption | Cognitive Impairment | Fatigue | GI Distressa | Pain | Genomic | Transcriptomic | Epigenomic | |
| ABCB1 (MDR1) | √ | √ | √ | GI, P | C | |||
| APOE | √ | √ | √ | S, C, P | C | |||
| BACE1 | √ | C | C | |||||
| BDNF | √ | √ | √ | S, C, P | C | |||
| CHRNA7 | √ | C | C | |||||
| CLOCK | √ | S | S | S | ||||
| CNR1 | √ | √ | C, P | |||||
| COMT | √ | √ | √ | √ | C, F, GI, P | |||
| CRP | √ | √ | C | F | ||||
| DAT1 (SLC6A3) | √ | √ | C, P | |||||
| DMN2 | √ | C | C | |||||
| DRD2 | √ | √ | C, GI | |||||
| DRD3 | √ | √ | GI, P | |||||
| DRD4 | √ | √ | C, P | |||||
| ESR1 | √ | √ | C, P | |||||
| FMR1 | √ | C | C | C | ||||
| GHRL | √ | C | C | |||||
| HAT Tip60 | √ | S | S | |||||
| HLA-DRB1 | √ | √ | P | C | ||||
| HTR1A | √ | √ | GI, P | |||||
| HTR3B | √ | √ | GI, P | |||||
| IL10 | √ | √ | √ | C, F, P | P | |||
| IL1a | √ | √ | GI, P | |||||
| IL1B | √ | √ | √ | √ | S, F, GI, P | P | ||
| IL1RN | √ | √ | GI, P | |||||
| IL6 | √ | √ | √ | C, P | F, P | |||
| IL8 (CXCL8) | √ | √ | F, P | |||||
| KLOTHO | √ | C | C | |||||
| LTA | √ | √ | C, P | P | ||||
| MAPT | √ | C | C | |||||
| NTRK2 | √ | √ | √ | S, F | C | |||
| OPRM1 | √ | √ | GI, P | P | ||||
| PER2 | √ | S | S | |||||
| PER3 | √ | S | S | |||||
| PTGS2 (COX-2) | √ | √ | C, P | |||||
| SIRT1 | √ | √ | P | S | ||||
| SLC6A4 (5-HTTLPR) | √ | √ | √ | C, GI, P | C, P | |||
| SORL1 | √ | C | C | |||||
| TNF | √ | √ | √ | √ | F, P | C, GI, P | C | |
| TNFRSF1A | √ | C | C | |||||
| TRPV1 | √ | P | P | P | ||||
Note. Bold text indicates that the gene has been associated in the literature with two or more symptoms. C = cognitive impairment; F = fatigue; GI = gastrointestinal; P = pain; S = sleep disruption.
aPlease refer to text for terms used in GI distress search.
IPA
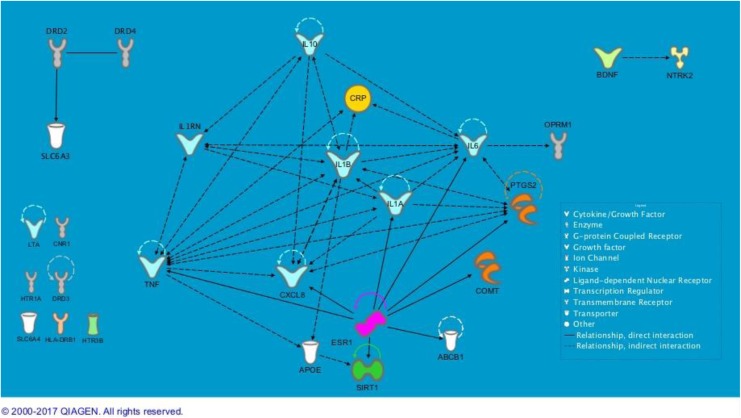
Relationships among the 27 genes that were associated with two or more symptoms are presented in Figure 1. Among these genes, seven had no known direct or indirect biological relationships with any other genes within the group: CNR1, DRD3, HLA-DRB1, HTR1A, HTR3B, LTA, and SLC6A4. The remaining 20 genes showed a direct or indirect biological relationship with at least one other gene to make three groupings: (1) the smallest interaction involved BDNF and NTRK2; (2) a three-gene interaction was noted for DRD2, DRD4, and DAT1 (SLC6A3); (3) the main relationship model included the remaining 15 genes, mostly cytokines: IL1A, IL1B, IL1RN, IL6, IL8 (CXCL8), IL10, TNF, PTGS2 (COX-2), CRP, OPRM1, COMT, ABCB1 (MDR1), SIRT1, APOE, and ESR1. All exemplar symptoms were represented in the main relationship model in various combinations.
Figure 1.
Relationships among genes associated with two or more symptoms. Ingenuity Pathway Analysis did not find relationships to others for the seven genes in the bottom left corner. Semicircles above genes indicate the gene’s relationship with itself. For example, ESR1 (estrogen receptor 1) inhibits and acts on itself, as indicated by the looped arrow, through many interactions. ABCB1 = ATP-binding cassette subfamily B member 1 gene; APOE = apolipoprotein E gene; BDNF = brain-derived neurotrophic factor gene; CNR1 = cannabinoid receptor 1 gene; COMT = catechol-O-methyltransferase gene; CRP = C-reactive protein gene; CXCL8 = C-X-C motif chemokine ligand 8 gene; DRD2 = dopamine receptor 2 gene; DRD3 = dopamine receptor 3 gene; DRD4 = dopamine receptor 4 gene; ESR1 = estrogen receptor 1; HLA-DRB1 = major histocompatibility complex, class II, DR beta 1 gene; HTR1A = 5-hydroxytryptamine receptor 1 alpha gene; HTR3B = 5-hydroxytryptamine receptor 3 beta gene; IL1A = interleukin 1 alpha gene; IL1B = interleukin 1 beta gene; IL1RN = interleukin 1 receptor antagonist gene; IL6 = interleukin 6 gene; IL10 = interleukin 10 gene; LTA = lymphotoxin alpha gene; NTRK2 = neurotrophic receptor tyrosine kinase 2 gene; OPRM1 = opioid receptor mu 1 gene; PTGS2 = prostaglandin-endoperoxide synthase 2 gene; SIRT1 = sirtuin 1 gene; SLC6A3 = solute carrier family 6 member 3 gene; SLC6A4 = solute carrier family 6 member 4 gene; TNF = tumor necrosis factor gene. Figures produced from IPA are available under an open-access CC-BY license for purposes of publication.
Canonical Pathways
Next, IPA generated canonical pathways most significant to this list of 27 genes. From this query, we identified five different canonical pathways that overlapped with our gene list. Each of these pathways incorporated various interleukins, TNF, and additional genes from our model. The first pathway, Altered T- and B-Cell Signaling in Rheumatoid Arthritis, contained eight molecules from our model with major histocompatibility complexes. Communication Between Innate and Adaptive Immune Cells included eight molecules with HLA-DR. Hepatic Cholestasis contained nine molecules with ESR1 and ABCB1 (MDR1). Role of Cytokines in Mediating Communication Between Immune Cells included seven molecules, and IL6 Signaling consisted of eight molecules from our model with ABCB1 (MDR1) and CRP (refer to Supplemental Figures A1–A5 for canonical pathways).
Networks
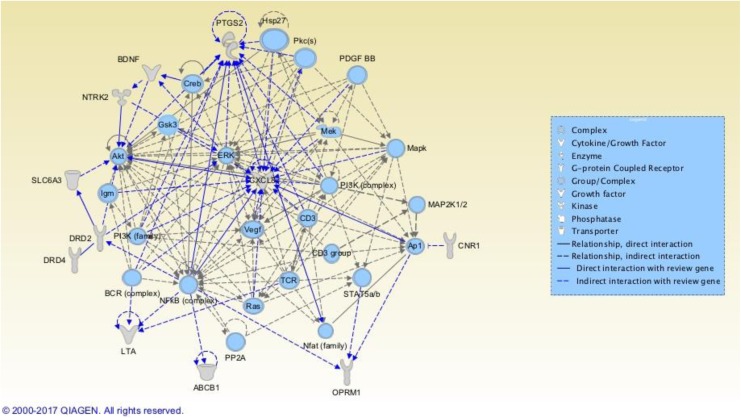
IPA generated networks from the 27 genes of interest by comparing them with a global molecular network from the Ingenuity Knowledge Base to identify connectivity among the molecules. We queried IPA with our list of 27 genes, and the resulting three networks each contained more than 3 of the 27 genes. The first network, Organismal Injury and Abnormalities, Cardiovascular Disease, Nutritional Disease, depicted in Figure 2, included 11 of the 27 genes of interest: ABCB1 (MDR1), BDNF, CNR1, IL8 (CXCL8), DRD2, DRD4, LTA, NTRK2, OPRM1, PTGS2 (COX-2), and DAT1 (SLC6A3). IPA scored this network at 22, indicating a high level of fit between our list of genes submitted and this network of molecules in IPA (Fisher’s exact test of 1 × 10−22) and a low probability of identifying this network by chance. Higher network scores indicate higher level of fit and are used to rank the networks of interest. All exemplar symptoms were represented in this network, and most of the molecules are involved in the MAPK signaling pathway.
Figure 2.
Organismal Injury and Abnormalities, Cardiovascular Disease, Nutritional Disease Ingenuity Pathway Analysis (IPA) Network. Genes colored gray are among the 27 genes we are exploring in this review; those colored blue have potential for future research. Bright blue relationships are drawn to or from the review genes to highlight relationships. (Readers viewing the paper version of this article are referred to the online version to view the figure in color.) Reviewed genes included in this network are ABCB1 = ATP-binding cassette subfamily B member 1 gene; BDNF = brain-derived neurotrophic factor gene; CNR1 = cannabinoid receptor 1 gene; CXCL8 = C-X-C motif chemokine ligand 8 gene; DRD2 = dopamine receptor 2 gene; DRD4 = dopamine receptor 4 gene; LTA = lymphotoxin alpha gene; NTRK2 = neurotrophic receptor tyrosine kinase 2 gene; OPRM1 = opioid receptor mu 1 gene; PTGS2 = prostaglandin-endoperoxide synthase 2 gene; SLC6A3 = solute carrier family 6 member 3 gene. Figures produced from IPA are available under an open-access CC-BY license for purposes of publication.
Interestingly, some other genes that surfaced during our review but were not linked to more than one symptom appeared in this network. For instance, AKT1, CREB1, and RAS group (RAB5, RAB7; Supplemental Table A2, Cognitive Impairment); ERK (MAPK1) and MAPK (Supplemental Table A5, Pain); and NFkB (complex; Supplemental Table A3, Fatigue). These findings indicate some additional biological support for these genes within the context of our exemplar symptoms, though associations between these genes and our chosen symptoms may not yet appear in the literature. Figure 2 also shows other genes/molecules that were included in this network but that we did not find to be associated with the exemplar symptoms in our review.
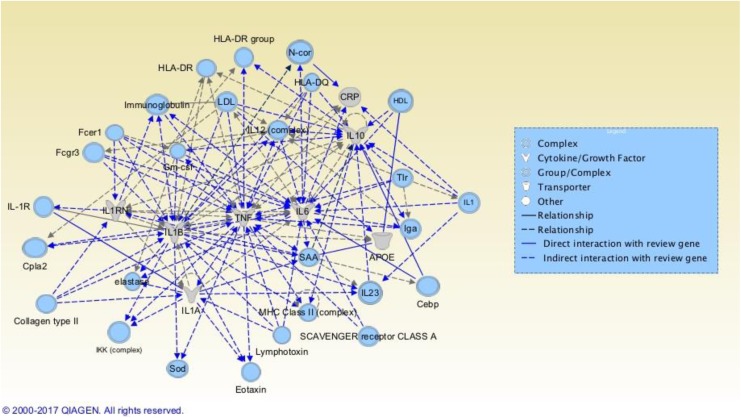
The second network, Hereditary Disorder, Organismal Injury and Abnormalities, Respiratory Disease, contained 8 of the 27 genes of interest: APOE, CRP, IL6, IL10, IL1A, IL1B, IL1RN, and TNF. In addition to the genes discussed in this review, this model includes HLA-DR as a group. Again, all symptoms of interest were included in this network (see Figure 3). The IPA network score was 14. Sod (SOD1) was linked to cognitive impairment but no other exemplar symptom (Supplemental Table A2, Cognitive Impairment). See Figure 3 for other prospective genes/molecules within this network that did not surface during our review.
Figure 3.
Hereditary Disorder, Organismal Injury and Abnormalities, Respiratory Disease Ingenuity Pathway Analysis (IPA) Network. Genes colored gray are among the 27 genes we are exploring in this review; those colored blue have potential for future research. Bright blue relationships are drawn to or from the review genes to highlight relationships. (Readers viewing the paper version of this article are referred to the online version to view the figure in color.) Reviewed genes included in this network are APOE = apolipoprotein E gene; CRP = C-reactive protein gene; IL6 = interleukin 6 gene; IL10 = interleukin 10 gene; IL1A = interleukin 1 alpha gene; IL1B = interleukin 1 beta gene; IL1RN = interleukin 1 receptor antagonist gene; TNF = tumor necrosis factor gene. Figures produced from IPA are available under an open-access CC-BY license for purposes of publication.
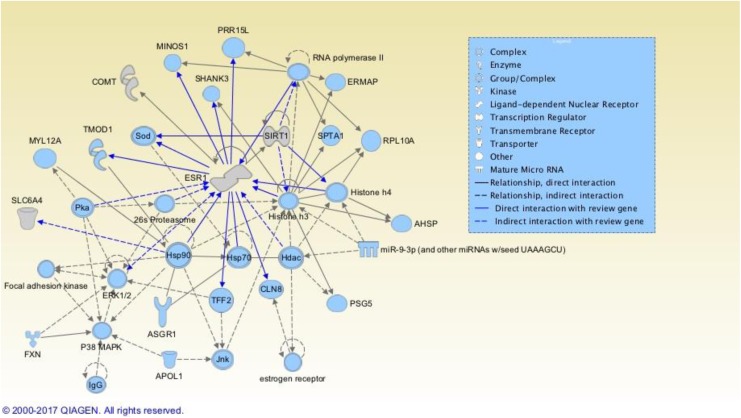
The third network, Hematological System Development and Function, Tissue Morphology, Cell-to-Cell Signaling and Interaction, incorporated 4 of the 27 genes we found to be in common among the symptoms we investigated (see Figure 4): COMT, ESR1, SIRT1, and SLC6A4. The IPA score was 6 for this network. Other genes in this network that we found in our literature search to be associated with only one of the exemplar symptoms included ERK 1/2 and P38 MAPK (MAPK1) in the pain review and ESR2, Hsp70 (HSPA8) SHANK, and SOD1 genes from the cognitive impairment search. See Figure 4 for genes/substances not found in our review but included in the network.
Figure 4.
Hematological System Development and Function, Tissue Morphology, Cell-to-Cell Signaling and Interaction Ingenuity Pathway Analysis (IPA) Network. Genes colored gray are among the 27 genes we are exploring in this review; those colored blue have potential for future research. Bright blue relationships are drawn to or from the review genes to highlight relationships. (Readers viewing the paper version of this article are referred to the online version to view the figure in color.) Reviewed genes included in this network are COMT = catechol-O-methyltransferase gene; ESR1 = estrogen receptor 1; SIRT1 = sirtuin 1 gene; SLC6A4 = solute carrier family 6 member 4 gene. Figures produced from IPA are available under an open-access CC-BY license for purposes of publication.
These networks identified many additional genes that are known to be biologically related to the genes found during our review but that have not been investigated within the context of our exemplar symptoms to date.
Discussion
This literature review and the IPA analyses provide a glance into the burgeoning area of symptom science, which focuses on increasing our understanding of the biological underpinnings of symptoms using omics approaches. They also provide evidence that certain genes and biological pathways may underlie the biology of multiple symptoms, offering biological evidence explaining why some symptoms co-occur. Using the data generated from the review and a functional omics analysis tool (IPA), we could identify the biological pathways and networks associated with these genes that are currently supported by the literature as well as identify additional candidate genes for future investigations.
We found 27 genes in the literature that were significantly associated with development or severity of more than one symptom. Using IPA software, we found three groupings among the 27 genes. The first was a two-gene relationship between BDNF and NTRK2. (Descriptions of genes in this section were drawn from the Genetics Home Reference [U.S. National Library of Medicine, 2013] and Online Mendelian Inheritance in Man, 2017.) BDNF is a nerve growth factor thought to play a role in neuroplasticity and regulation of synapse transmission. NTRK2 is involved in the MAPK pathway, plays a role in memory and learning, and is associated with obesity and mood disorders ( Online Mendelian Inheritance in Man OMIM®, 2017; U.S. National Library of Medicine, 2013).
The second grouping was a three-gene interaction for DRD2, DRD4, and DAT1 (SLC6A3). DRD2 and DRD4 have a direct interaction and inhibit adenylyl cyclase, which is needed to convert ATP to cAMP for energy production. DRD2 is a G-coupling protein associated with locomotion, reward, reinforcement, and learning, and DRD4 regulates emotion and behavior. DRD2 also activates DAT1 (SLC6A3), which encodes a dopamine transporter responsible for dopamine clearance.
In the third grouping, we found that 15 of the 27 genes have known direct or indirect biological relationships with each other. This grouping included genes representing all five of our symptoms of interest, further supporting common underlying biological processes across symptoms. IL1A and IL1B are proinflammatory cytokines, and the inhibitor of these genes is IL1RN. IL6 promotes B-cell maturation and inflammation. IL8 (CXCL8) is a chemokine that attracts neutrophils, basophils, and T cells. IL10 regulates immunity and inflammation by inhibiting the synthesis of certain cytokines synthesis, including TNF. TNF is proinflammatory, secreted by macrophages, and involved in many processes including apoptosis, cell differentiation and proliferation, lipid metabolism, and coagulation. It is implicated in many inflammatory diseases. PTGS2 (COX-2) is a proinflammatory enzyme used to produce prostaglandins and its upregulation is linked to cancer, especially apoptosis resistance. HLA-DRB1, major histocompatibility complex II, binds with peptides from extracellular proteins and presents them on the surface of the cell for immune system recognition. It also plays a role in autoimmune disease. C-reactive protein (CRP) is part of the humoral immune response and reacts to foreign pathogens and damaged cells. OPRM1 is an opioid receptor with roles in dopamine modulation. COMT inactivates catecholamines, including dopamine. ABCB1 (MDR1) transports molecules across cell membranes and the blood–brain barrier. SIRT1’s function is not clearly known, but the gene is thought to be involved in the stress response. APOE clears very-low-density lipoproteins and is known for its relation to Alzheimer’s disease. Finally, ESR1 encodes a receptor for estrogen. Estrogen and estrogen receptors are involved in sexual development, bone health, breast and endometrial cancers, and cardiovascular disease. In our model, ESR1 appears in the center of several genes, which it directly influences TNF, IL8 (CXCL8), IL1A, IL6, PTGS2 (COX-2), COMT, ABCB1 (MDR1), and SIRT1. These findings suggest a commonality among genes, symptoms, and disease processes.
Canonical pathways identified through IPA using common genes from our review primarily included immune, inflammatory, and cell-signaling pathways. From a disease perspective, it makes sense that these types of pathways would be significant. Most disease processes involve some degree of immune, inflammatory, and cell-signaling response, so the sharing of symptoms across these disease processes may indeed be linked to these mechanisms, which are common regardless of the disease specifics. For instance, heart disease (leading to myocardial infarction) and cancer have vastly different mechanisms of disease creation. But as both diseases progress, they create changes in the immune system, which trigger an inflammatory response and alterations in cell signaling (Deftereos et al., 2014; Hung et al., 2015). It is these changes that may help create those symptoms that are common to both (as in our exemplar symptoms here). Future work to decrease the impact of these common symptoms should focus on regulating the changes that occur within these pathways that account for the common omic changes in the genetic pathways and networks presented here.
IPA generated three major networks of molecules from the Ingenuity Knowledge Base that interact with the 27 genes. The IPA scoring indicated that the networks have a very good fit. The lowest score (network 3) from the Fisher’s exact test result was 1 × 10−6, giving this network a one in a million chance of containing at least the same number of network-eligible molecules by chance when randomly choosing 35 molecules from the Ingenuity Knowledge Base. The other two networks are even less likely to have occurred by chance, at 1 × 10−22 (Network 1) and 1 × 10−11 (Network 2).
The first network, Organismal Injury and Abnormalities, Cardiovascular Disease, Nutritional Disease, contained 11 of the 27 genes. It also contained all exemplar symptoms, further supporting our hypothesis. Most of the molecules in this network are involved in the MAPK-signaling pathway. Genes in this network that had surfaced during our review but were not linked with more than one symptom were AKT1 and CREB1, which affect cognition and memory; RAS (RAB5, RAB7), a proto-oncogene group linked with cognitive impairment (see Supplemental Table A2, Cognitive Impairment); ERK (MAPK1) and MAPK, which are associated with pain (see Supplemental Table A5, Pain); and NFkB (complex), which is linked with fatigue (see Supplemental Table A3, Fatigue). These findings provide some additional support for exploring these genes within the context of specific symptoms. Other genes included in the network but not found at all in our review are AP-1, activator protein-1, a transcription regulator; BCR (complex), a transmembrane receptor in the MAPK pathway; CD3 group, signal transducers for T cells; MEK, mitogen-associated extracellular signal-related kinase in the MAPK pathway; GSK3, involved in glycogen metabolism; Hsp27, heat shock protein family B (small) member 1, part of the stress response; IgM, an immunoglobulin; Nfat (family), a group of transcription factors important for T cells; PDGF BB, platelet-derived growth factors; PI3K (family), lipid kinases; Pkc(s), a group of protein kinases; PP2A, protein phosphatase for negative control of cell growth and division; STAT5a/b, transcription factors; TCR, T-cell transmembrane receptor; and VEGF, a growth factor involved in angiogenesis.
The second network, Hereditary Disorder, Organismal Injury and Abnormalities, Respiratory Disease, included 8 of the 27 genes. Again, all exemplar symptoms were contained in this network. IL1R, which was only associated with pain in our review, came up in this network, as did Sod (SOD1), which destroys free superoxide radicals and showed up in our cognitive impairment review. Other prospective genes/molecules within this network that did not surface during our review included CEBPA, a transcription factor that controls expression; collagen type II (COL2A1), important for connective tissues; Cpla2 (PLA2G4A), involved in hydrolysis of phospholipids; elastase, an enzyme; eotaxin, a chemokine; Fcer1, an IgE receptor; Fcgr3 (FCGR3B), an IgG receptor; Gm-csf (CSF2), a cytokine; IL23 (IL23A), which creates part of IL23 with IL12; IL12 (complex); immunoglobulin; LDL; N-cor (NCOR1); SAA, the serum amyloid apolipoproteins; scavenger receptor Class A, a group involved with lipoprotein uptake; and TLR, toll-like receptors that identify pathogens for the immune system ( Online Mendelian Inheritance in Man OMIM®, 2017; U.S. National Library of Medicine, 2013).
Finally, the third network, Hematological System Development and Function, Tissue Morphology, Cell-to-Cell Signaling and Interaction, included 4 of the 27 genes. Genes/substances not found in our review but included in the network were 26S proteasome, a complex that removes damaged proteins; AHSP, a protein binder; APOL1, a high-density lipid involved in lipid exchange and transport; ASGR1, a transmembrane receptor; CLN8, a transmembrane protein in the endoplasmic reticulum; ERMAP, a transmembrane protein; focal adhesion kinase, which is involved in cell adhesion and motility; FXN, a mitochondrial protein; Hdac, the histone deacetylase family; histone h3 and histone h4; Hsp90, which assists with protein folding; IgG, an immunoglobulin; Jnk (MAPK8); MINOS1, a protein binder; miR-9-3p, a microRNA that regulates post-transcription gene expression; MYL12A, which regulates smooth muscle and non-smooth muscle contraction; Pka group, protein kinases implicated in several cancers; PRR15L, part of the ATP family; PSG5, an immunoglobulin; RNA polymerase II, which is essential for transcription; RPL10A, a ribosomal protein; SPTA1, a scaffold postsynaptic density protein implicated in autism; TFF2, a scaffold protein of erythrocyte plasma membranes; and TMOD1, which also plays a role in shaping the erythrocyte membrane skeleton. While this network contained the fewest number of genes found in our review, the IPA score was still substantial, indicating a good fit for the network.
The combined evidence from the literature reviews, the IPA model, the linkage with canonical pathways, and the creation of IPA networks supports the hypothesis of overlapping biological underpinnings across exemplar symptoms. But there were some genes identified within these networks that were only associated with one symptom. There were also genes identified that have never been studied in relation to our selected exemplar symptoms. We believe these pathways and networks identify gaps in current literature and potential loci for future symptom omics research, which may help move symptom science forward.
While our results do have the potential for significant impact, our review and findings do also have some notable limitations. The first is the limitation of our exemplar symptoms. While we chose these specific exemplar symptoms on which to focus our review, other symptoms may have also added to the support. Our literature search was not exhaustive and was limited to PubMed. While PubMed includes most literature, there may be items published elsewhere that were not captured. Our findings may reflect publication bias because many of the omics approaches used were candidate approaches, therefore reflecting biases toward specific biological pathways thought to be involved in the development or severity of symptoms, as well bias introduced by limiting inclusion to only those articles with significant findings.
Conclusion
Nursing practice focused on symptom management should be based on evidence, including evidence supporting precision care for symptoms. Knowledge about the biological underpinnings for a symptom or co-occurring symptoms could aid in the identification of individuals at risk for symptom development or more severe symptom presentation as well as in treatment decisions and development of treatment interventions. Additionally, biological links between symptoms may indicate that treatment strategies could also be linked. In this article, we present current evidence provided by omics-based investigations that supports a role for biological pathways in symptom development and co-occurrence in a disease-agnostic manner.
Supplementary Material
Footnotes
Authors’ Contribution: M. K. McCall contributed to conception and design; contributed to acquisition, analysis, and interpretation of the data; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. A. G. Stanfill contributed to conception and design; contributed to acquisition, analysis, and interpretation of the data; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. E. Skrovanek contributed to conception and design; contributed to acquisition, analysis, and interpretation of the data; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. J. R. Pforr contributed to conception and design; contributed to acquisition, analysis, and interpretation of the data; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. S. W. Wesmiller contributed to conception and design; contributed to acquisition, analysis, and interpretation of the data; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Y. P. Conley contributed to conception and design; contributed to acquisition, analysis, and interpretation of the data; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclose receipt of the following financial support for the research, authorship, and/or publication of this article: National Institutes of Health (T32NR009759).
ORCID iD: Maura K. McCall, MSN, RN  http://orcid.org/0000-0002-8155-6793
http://orcid.org/0000-0002-8155-6793
Ansley Grimes Stanfill, PhD, RN  http://orcid.org/0000-0001-7620-1130
http://orcid.org/0000-0001-7620-1130
Supplemental Material: Supplementary material is available for this article online.
References
- Corwin E. J., Berg J. A., Armstrong T. S., DeVito Dabbs A., Lee K. A., Meek P., Redeker N. (2014). Envisioning the future in symptom science. Nursing Outlook, 62, 346–351. doi:10.1016/j.outlook.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Deftereos S., Angelidis C., Bouras G., Raisakis K., Gerckens U., Cleman M. W., Giannopoulos G. (2014). Innate immune inflammatory response in the acutely ischemic myocardium. Journal of Medicinal Chemistry, 10, 653–662. [DOI] [PubMed] [Google Scholar]
- Hung R. J., Ulrich C. M., Goode E. L., Brhane Y., Muir K., Chan A. T.…Henderson B. (2015). Cross cancer genomic investigation of inflammation pathway for five common cancers: Lung, ovary, prostate, breast, and colorectal cancer. Journal of the National Cancer Institute, 107 doi:10.1093/jnci/djv246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C., Barsevick A., Berger A., Casagrande R., Grady P. A., Jacobsen P.…Marden S. (2017). Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. Journal of the National Cancer Institute, 109, djw253 doi:10.1093/jnci/djw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Nursing Research. (2016). The NINR strategic plan: Advancing science, improving lives. A vision for nursing science. Retrieved from https://www.ninr.nih.gov/sites/www.ninr.nih.gov/files/NINR_StratPlan2016_reduced.pdf
- Online Mendelian Inheritance in Man OMIM®. (2017). Baltimore, MD: Johns Hopkins University; Retrieved from https://www.omim.org/ [Google Scholar]
- U.S. National Library of Medicine. (2013). Genetics Home Reference. Retrieved from https://ghr.nlm.nih.gov/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.