Abstract
Background
Behavioural and psychological symptoms of dementia are distressing for patients and are frequently treated with second-generation antipsychotics. Concerns about the drugs' safety resulted in a Medicines and Healthcare Products Regulatory Agency (MHRA) warning against their use in March 2009.
Methods
Second-generation antipsychotic drug use was determined amongst patients with dementia admitted to the University Hospitals Birmingham National Health Service Foundation Trust, between July 2005 and December 2011. An interrupted time series analysis was carried out to investigate changes in rates of prescribing following the safety warning. Risperidone was analysed separately, in accordance with its limited licence for use in older adults with dementia, granted in October 2008.
Results
Before the safety warning, second-generation antipsychotic use was increasing in patients with dementia. After the MHRA warning, their use fell by 1.9% per month compared with that before. Use of risperidone continued to rise over the same period, often against the terms of its licence.
Conclusions
Drug safety warnings may influence prescribing practice, although continued use of antipsychotics in dementia could reflect a lack of alternative treatment options.
Keywords: mental health
Introduction
In the UK, an estimated 800 000 people are living with dementia.1 Dementia is highly prevalent in the hospital setting, with a reported 42% of emergency admissions fulfilling the Diagnostic and Statistical Manual IV diagnostic criteria: caring for these patients represents a significant challenge to all health care professionals.2,3 Patients with dementia can suffer mood disorders, agitation and psychosis, grouped into the syndrome of Behavioural and Psychological Symptoms of Dementia (BPSD).4 Depending on how it is defined, up to 100% of dementia patients may experience BPSD at some point, which can be distressing for both the patient and their carers, reduce quality-of-life and are associated with poorer clinical outcomes.5,6 It is therefore essential to adequately identify and treat patients suffering with BPSD.
For decades, doctors have turned to antipsychotics to treat BPSD, although not without controversy.7 In the UK, antipsychotics are prescribed to 10% of patients with dementia in the community, and up to a third of dementia patients in care home settings.8,9 Through the 1990s and early 2000s, a growing proportion of patients with dementia were prescribed antipsychotics, driven by the new availability of second-generation (or atypical) drugs such as olanzapine, quetiapine and risperidone.10–12 However, the evidence that antipsychotics are effective in BPSD is somewhat limited: evidence is conflicting, and statistical significance is not always matched with clinical benefit.13–15
The use of antipsychotics in older people is limited by their potentially dangerous safety profile. Of particular concern are the increased risks of cerebrovascular adverse events (CVAE) and death.16 Evidence including data from randomized controlled trials suggests that the risk of CVAE is probably 2 to 3 times higher in patients prescribed an antipsychotic,17 and the risk of death was found to be significantly higher (odds ratio (OR) 1.54) compared with placebo.18 Overall, the adverse effects may completely offset the benefits of second-generation antipsychotics.19
Given the limited efficacy and significant risks, it is not surprising that the continued use of antipsychotics has been controversial. In 2004, the UK Committee on Safety of Medicines issued a warning that risperidone and olanzapine increases the risk of CVAE and death in older patients, and they should not be used to treat BPSD.20 In 2005, the US Food and Drug Administration (FDA) reviewed available evidence and determined that second-generation antipsychotics are associated with an increased risk of death.21 Up to this point, antipsychotics had been prescribed ‘off-label’, that is, outside the terms of its licence (or marketing authorization). However, in October 2008, risperidone was granted a limited licence for BPSD.22 But despite the recommendation that antipsychotics should only be used short term (if at all), the drugs are often inappropriately prescribed for extended periods, even on a repeating basis.8 In March 2009, the Medicines and Healthcare Products Regulatory Agency (MHRA) published a warning in its Drug Safety Update about the risks of all antipsychotics in older people and added risperidone to its Black Triangle (▾) list of medicines following the amendment to its licence, indicating the start of intensive safety monitoring.23
Interrupted time-series analysis with segmented regression analysis is a method developed to investigate changes in prescribing patterns over time, to estimate the effect of an intervention.24 A number of studies have used this approach to evaluate the effect of safety warnings on the prescribing of antipsychotics to patients with dementia. In a study of mixed inpatients and outpatients in the USA, antipsychotic use fell following the 2005 FDA warning, although a Canadian study found that prescribing continued to rise albeit at a reduced rate.25,26
While most studies have looked at antipsychotic prescribing practices in community and care home settings, as many as 40% of these prescriptions were initiated in hospital.27 The aims of this study were to investigate the rate of second-generation antipsychotic prescribing in patients with dementia in a hospital setting and to determine whether prescribing behaviour changed around the time of the MHRA warning about their safety. In addition, risperidone prescriptions were examined, to determine the compliance with the limited terms of its licence.
Methods
Population and data collection
The study population was drawn from patients admitted to University Hospitals Birmingham National Health Service (NHS) Foundation Trust, a large acute teaching hospital in Birmingham, UK. The study included admissions between 1 July 2005 and 31 December 2011, a period covering the March 2009 MHRA safety warning, in which there were a total of 318 641 inpatient episodes. From these admissions, dementia patients were defined as those with any recorded International Classification of Diseases (ICD)-10 code in the range F00–F03, incorporating Alzheimer's disease, vascular dementia, dementia in other diseases classified elsewhere and unspecified dementia. Additional dementia patients were identified as those prescribed an anti-dementia drug (World Health Organisation (WHO) Anatomical Therapeutic Chemical (ATC) class N06D: donepezil, galantamine, memantine or rivastigmine), even in the absence of an explicit dementia diagnosis. These drugs are only licensed for use in dementia in the UK and have no other clinical indication, so are not likely to identify patients without dementia.
Demographic and clinical data, including age, sex, admission date and duration, and cerebrovascular co-morbidity, were obtained from the electronic patient record. Details of antipsychotic prescriptions, including drug name, dose, frequency and duration, were obtained from the electronic prescribing system. The mode of each prescription was determined as ‘pro re nata’ (PRN)—to be taken at the stated dose as required, ‘regular’—to be given at regular intervals while the patient is an inpatient for the duration indicated and ‘to take out’ (TTO)—to be continued after discharge as a regular medication for a given duration. The second-generation antipsychotics fall in the WHO ATC class N05A and comprise: amisulpride, aripiprazole, clozapine, olanzapine, quetiapine and risperidone. In addition, patients prescribed both risperidone and furosemide were identified.
Statistical analysis
Monthly rates of antipsychotic prescribing are presented as percentages of all dementia patients admitted during the study period. Risperidone was separated from the other second-generation drugs, because as of October 2008, it was licensed for the short-term treatment of aggression in dementia, whereas the remainder were used ‘off-label’. To test whether trends in antipsychotic prescribing practice were associated with the MHRA safety warning, segmented regression analysis was carried out using rates of prescribing as the dependent variable. Incorporated into the model were the continuous variables ‘month of the study period’ and ‘month post-intervention’, and the categorical term ‘intervention’ for the March 2009 warning: respectively, these covariates describe the gradient of antipsychotic prescribing before the intervention, the change in gradient after the intervention and the step-change that occurred at the time of the intervention. An additional categorical covariate of ‘history of cerebrovascular disease’ was included in the models, since an increased risk of stroke and transient ischaemic attack was specifically mentioned in this and previous warnings. There was potential for individual patients to be counted multiple times, if they had been admitted more than once during the study period. To take into account any within-patient correlation, analysis was carried out using generalized estimating equations, incorporating into the model the number of times each patient was admitted.
Results
Increasing incidence of dementia in the hospital population
Between July 2005 and December 2011, there was a steady increase in the number of admissions of patients with dementia each month (from 370 in the first 6 months of the study period to 670 in the last). Because of the variability in monthly admissions, antipsychotic prescriptions are expressed as rates. In addition to the increasing admission numbers, there was also a small but significant increase in the proportion of patients admitted with dementia, compared with the total number of patients admitted during this period (Pearson's R = 0.57, P < 0.001; Fig. 1).
Fig. 1.
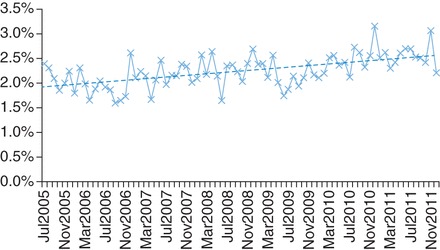
Proportion of patients with dementia admitted each month increased over the study period. Dotted line indicates regression line (Pearson's R = 0.57, P < 0.001).
Over the study period, 4612 patients with dementia were admitted during a total of 7167 episodes. 3143 of these patients were admitted once, 1469 patients were admitted on more than one occasion.
Characteristics of dementia patients receiving an antipsychotic
Patient and admission characteristics were analysed to explore whether patients who were prescribed a second-generation antipsychotic differed from those given neither first- nor second-generation drugs. Dementia patients prescribed an antipsychotic had a median age of 83 (interquartile range (IQR) 79–87), younger than those not prescribed the drugs (median age 85, IQR 79–89, P < 0.001, Mann–Whitney U test). The average duration of admission was also different: patients prescribed an antipsychotic had a median admission length of 10 days (IQR 3–29 days), staying longer in hospital than patients not prescribed antipsychotics (median 7 days, IQR 2–22 days, P < 0.001, Mann–Whitney U test).
Antipsychotic prescribing
Of the 7167 admissions identified during the study period, second-generation antipsychotics were prescribed in 1085 (15.1%, 95% CI 14.3–16.0%). During most of these admissions, just one antipsychotic was prescribed (876 of 1085 admissions). However, in 25 admissions, 2 different second-generation antipsychotics were prescribed, and in 184 admissions, different combinations of first- and second-generation drugs were prescribed, to a maximum of 4 different antipsychotics. The majority of admissions (1055 of 1085) saw second-generation antipsychotics prescribed for use in hospital, of which 639 (61%) also received drugs on discharge. Only 30 admissions resulted in a TTO prescription without prior use during the admission.
The rates of second-generation antipsychotic prescribing were examined using generalized estimating equations with a binary logistic model, incorporating the March 2009 intervention date and a history of cerebrovascular disease. Risperidone was analysed separately, since it was licensed for the treatment of BPSD from October 2008. The rates of second-generation antipsychotic prescribing are shown in Fig. 2a. After the MHRA warning, the rate of prescribing declined compared with before (1.9% per month, 95% CI 0.4–3.5%, P = 0.015; Table 1). There was no statistically significant step-change in prescribing rates (OR 0.76, 95% CI 0.56–1.05, P = 0.091), and a diagnosis of cerebrovascular disease was not statistically significantly associated with antipsychotic prescribing (OR 1.19, 95% CI 0.92–1.54, P = 0.18).
Fig. 2.
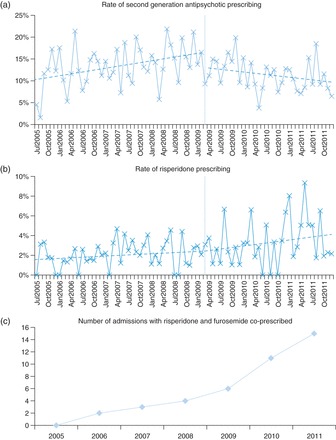
Trends in antipsychotic prescribing. (a) The prescription rate of second-generation antipsychotics (excluding risperidone) was rising before the MHRA safety warning, after which their use declined (1.9% per month, P = 0.015). (b) Risperidone use continued to rise throughout the study period, with a marginal increase in prescribing after its addition to the Blue Triangle list of medicines (0.3% per month, P = 0.86). Blue dotted lines indicate regression before and after the March 2009 warning, the warning indicated by the vertical blue line. (c) Concomitant use of risperidone and furosemide increased throughout the study period.
Table 1.
Binary logistic regression model, using generalized estimation equations, for second-generation antipsychotic prescribing (excluding risperidone)
| OR (95% CI) | P -value | |
|---|---|---|
| Gradient (per month) pre-intervention | 1.01 (1.01–1.02) | 0.002 |
| Step-change after MHRA warning | 0.76 (0.56–1.04) | 0.091 |
| Change in gradient (per month) post-intervention | 0.98 (0.97–1.00) | 0.015 |
| Effect of a recorded cerebrovascular disease | 1.19 (0.92–1.54) | 0.18 |
Risperidone prescribing
After being granted a licence for the short-term treatment of BPSD in Alzheimer's disease, March 2009 saw risperidone added to the Black Triangle list of medicines. However, analysis of risperidone prescription rates did not reveal any change in practice after this intervention (0.3% increase in monthly prescriptions, 95% CI −2.6–3.1%, P = 0.86; Fig. 2b).
Risperidone use was investigated further to compare prescribing practices in this study to those recommended in the product licence. The recommended starting dose for the treatment of aggression in dementia is 0.25 mg twice daily, up to a maximum of 1 mg twice daily. In this study, patients with PRN risperidone prescriptions had a median daily dose of 0.5 mg (IQR 0.5–1 mg), similar to regular and TTO prescriptions, both with median daily doses of 1 mg (IQR both 0.5–1.5 mg) (P = 0.11, Kruskal–Wallis test). While none of the PRN daily doses exceeded the 2 mg recommended limit, 16 regular prescriptions (6.3%) and 6 TTO prescriptions (5.7%) were found to exceed this dose. Risperidone is licensed to treat aggression in Alzheimer's dementia for a maximum of 6 weeks (42 days). In this study, the median duration of PRN prescriptions was 3.5 days (IQR 1–21 days) and for regular prescriptions was 4 days (IQR 1–13 days). For TTO prescriptions, no prescription duration was recorded in 54 cases (51%). For those prescriptions with a duration indicated, the median was 68.5 days (IQR 13–236.5 days). This is considerably longer than the 42-day limit given in the product licence. In total, 10 (3.9%) of the PRN and regular prescriptions had a duration exceeding 42 days, whereas 29 (56%) of recorded TTO prescriptions were for ∼42 days (P < 0.001, χ2 test). Finally, the revised risperidone product licence includes a warning that co-treatment with furosemide was associated with an increase in mortality and that prescribers should exercise caution when using the drugs together.22 In this study, only 41 patients were prescribed both risperidone and furosemide; however, there was a clear rising trend in their concomitant use (Fig. 2c).
Discussion
Main findings
Between July 2005 and December 2011, an increasing number of patients with dementia were admitted to the University Hospitals Birmingham NHS Foundation Trust, reflecting a growing prevalence of dementia in the inpatient setting. The ageing population is likely to explain some of the increase, as might improving recognition of dementia as an important co-morbidity and therefore its inclusion in coded admission notes.
In this study, patients prescribed a second-generation antipsychotic were on average younger than those not given the drugs and tended to stay in hospital for longer. Over the entire study period, an average of 15.1% of admissions of dementia patients resulted in an antipsychotic prescription. Segmented regression analysis of second-generation antipsychotic use (excluding risperidone) revealed a reduction in the rate of prescribing after the March 2009 MHRA safety warning. Despite addition of risperidone to the Black Triangle list of medicines in March 2009, there was no change in the trend of its use, which continued to rise as other second-generation drugs were used less. While the licensed dose was rarely exceeded in these patients, other limits of the licence were often ignored: patients were frequently prescribed risperidone for prolonged periods, and its concomitant use with furosemide increased over the study period.
What is already known
In care home settings, antipsychotic use in patients with dementia has been estimated at between 18 and 30%, with patients frequently receiving multiple prescriptions over relatively short time periods.8,28 Additionally, as care home patients get older, they are less likely to be prescribed the drugs.29,30
In the USA in the late 1980s, there were concerns that conventional antipsychotic drugs were being over-used in patients with dementia, and change in the law resulted in a decrease in their use.31 In the wake of the more recent warnings, similar drops in antipsychotic prescribing have been seen in community settings.25,32
What this study adds
This study looked for the first time at second-generation antipsychotic use in hospital inpatients with dementia. A similar proportion of inpatients were prescribed the drugs as patients in community settings. Moreover, when the information was recorded, TTO prescriptions for risperidone were often written for several weeks. It has been noted previously that many dementia patients in the community have antipsychotic treatment initiated in secondary care, and prescriptions are often continued on a repeating basis.8,27 Therefore, the true extent of long-term antipsychotic use is likely to have been under-estimated in this study. It is imperative that any attempt to reduce their use is targeted at both primary and secondary care settings.
After the MHRA warning against their use, rates of antipsychotic prescribing reduced. This is consistent with direct health care professional communications of this sort influencing practice in a hospital setting. However, the relatively modest change in prescribing rates suggests that there is room for improvement. It is important to ensure that the warnings reach those doctors writing the prescriptions. For example, with the arrival of electronic prescribing with clinical decision support, reminders of the warnings could appear before each prescription is issued. A more fundamental limitation to the impact of warning against antipsychotic use in patients with dementia is the lack of alternative, evidence-based strategies to treat BPSD. Risperidone remains the only antipsychotic licensed for use in BPSD, and although it was added to the Black Triangle list of medicines in March 2009, there was no change in prescribing practice, and its use continued to rise at the same rate as before. This emphasizes the need for alternative treatments, before warnings are likely to be fully adhered to.
Limitations
The segmented regression analysis employed in this study offers a powerful quasi-experimental method for associating changes in clinical practice with safety warnings.24 However, because of the observational nature of the study, it is only possible to state that the change in prescribing occurred at the same time as the warning, not because of it. It is possible that an additional, unknown factor, coincident with the MHRA warning, also influenced practice.
It is possible that the reduction in use of second-generation antipsychotics seen here was accompanied by an increase in first-generation drug use, with doctors potentially reverting back to the older drugs. However, it has been shown previously that this is unlikely to be the case.33 Indeed, analysis of first-generation antipsychotics confirms that, after March 2009, the rate of prescribing fell significantly compared with before (2.7% per month, 95% CI 1.3–4.1%, P < 0.001). Therefore, this study reflects a true overall reduction in antipsychotic drug prescribing.
The University Hospitals Birmingham NHS Foundation Trust is a large hospital with a high throughput of older patients with medical problems. However, since the study was conducted on only one site, it may not be fully representative of other hospitals.
Finally, this study only looked at rates of prescribing, and not of medicines actually administered. Also, all types of prescription were considered together (regular, PRN and TTO). Therefore, these results may not reflect the true level of antipsychotic consumption. However, 96% of TTO and 98% of PRN prescriptions occurred in admissions where antipsychotics were also prescribed as regular medication. Most importantly, the purpose of this study was to examine doctors' prescribing habits; therefore, the analysis of prescriptions issued remains valid.
Funding
This work was supported by the National Institute for Health Research (NIHR) through the Collaborations for Leadership in Applied Health Research and Care for Birmingham and Black Country (CLAHRC-BBC) programme.
Acknowledgements
We thank the data analysts, David Westwood and Mariam Afzal, for obtaining the information from the electronic patient databases; and James Hodson, for expertise on statistical analyses.
References
- 1. Lakey L, Chandaria K, Quince C, et al. Dementia 2012: A National Challenge. London: Alzheimer's Society, 2012. [Google Scholar]
- 2. Sampson EL, Blanchard MR, Jones L, et al. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry 2009;195(1):61–6. [DOI] [PubMed] [Google Scholar]
- 3. Harwood RH. Dementia for hospital physicians. Clin Med 2012;12(1):35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballard C, Day S, Sharp S, et al. Neuropsychiatric symptoms in dementia: importance and treatment considerations. Int Rev Psychiatry 2008;20(4):396–404. [DOI] [PubMed] [Google Scholar]
- 5. Steinberg M, Shao H, Zandi P, et al. , Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry 2008;23(2):170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ballard C, Corbett A, Chitramohan R, et al. Management of agitation and aggression associated with Alzheimer's disease: controversies and possible solutions. Curr Opin Psychiatry 2009;22(6):532–40. [DOI] [PubMed] [Google Scholar]
- 7. Barton R, Hurst L. Unnecessary use of tranquilizers in elderly patients. Br J Psychiatry 1966;112(491):989–90. [DOI] [PubMed] [Google Scholar]
- 8. Shah SM, Carey IM, Harris T, et al. Antipsychotic prescribing to older people living in care homes and the community in England and Wales. Int J Geriatr Psychiatry 2011;26(4):423–34. [DOI] [PubMed] [Google Scholar]
- 9. Alldred A, Petty D, Bowie P, et al. Antipsychotic prescribing patterns in care homes and relationship with dementia. Psychiatric Bull 2007;31:329–32. [Google Scholar]
- 10. Rapoport M, Mamdani M, Shulman KI, et al. Antipsychotic use in the elderly: shifting trends and increasing costs. Int J Geriatr Psychiatry 2005;20(8):749–53. [DOI] [PubMed] [Google Scholar]
- 11. Lövheim H, Sandman PO, Karlsson S, et al. Changes between 1982 and 2000 in the prevalence of behavioral symptoms and psychotropic drug treatment among old people with cognitive impairment in geriatric care. Int Psychogeriatr 2009;21(5):941–8. [DOI] [PubMed] [Google Scholar]
- 12. Lövheim H, Gustafson Y, Karlsson S, et al. Comparison of behavioral and psychological symptoms of dementia and psychotropic drug treatments among old people in geriatric care in 2000 and 2007. Int Psychogeriatr 2011;23(10):1616–22. [DOI] [PubMed] [Google Scholar]
- 13. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14(3):191–210. [DOI] [PubMed] [Google Scholar]
- 14. Ballard CG, Waite J, Birks J. Atypical antipsychotics for aggression and psychosis in Alzheimer's disease. Cochrane Database Syst Rev 2006;(1):Art. No.: CD003476. DOI: 10.1002/14651858.CD003476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yury CA, Fisher JE. Meta-analysis of the effectiveness of atypical antipsychotics for the treatment of behavioural problems in persons with dementia. Psychother Psychosom 2007;76(4):213–8. [DOI] [PubMed] [Google Scholar]
- 16. Mittal V, Kurup L, Williamson D, et al. Risk of cerebrovascular adverse events and death in elderly patients with dementia when treated with antipsychotic medications: a literature review of evidence. Am J Alzheimers Dis Other Demen 2011;26(1):10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sacchetti E, Turrina C, Valsecchi P. Cerebrovascular accidents in elderly people treated with antipsychotic drugs: a systematic review. Drug Saf 2010;33(4):273–88. [DOI] [PubMed] [Google Scholar]
- 18. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294(15):1934–43. [DOI] [PubMed] [Google Scholar]
- 19. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med 2006;355(15):1525–38. [DOI] [PubMed] [Google Scholar]
- 20. CSM. Atypical antipsychotic drugs and stroke. http://www.mhra.gov.uk/Safetyinformation/Safetywarningsalertsandrecalls/Safetywarningsandmessagesformedicines/CON10042982004 (5 May 2012, date last accessed).
- 21. FDA. Public health advisory: Deaths with antipsychotics in elderly patients with behavioural disturbances. http://www.fda.gov/forconsumers/consumerupdates/ucm053171.htm2005 [(5 May 2012, date last accessed).
- 22. CHMP. Opinion following an article 30 referral for Risperdal and associated names. European Medicines Agency, Doc. Ref. EMEA/CHMP/384877/2008 2008.
- 23. MHRA. Antipsychotics: use in elderly people with dementia. MHRA Drug Safety Update 2009; 2(8) 2009.
- 24. Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27(4):299–309. [DOI] [PubMed] [Google Scholar]
- 25. Kales HC, Zivin K, Kim HM, et al. Trends in antipsychotic use in dementia 1999–2007. Arch Gen Psychiatry 2011;68(2):190–7. [DOI] [PubMed] [Google Scholar]
- 26. Valiyeva E, Herrmann N, Rochon PA, et al. Effect of regulatory warnings on antipsychotic prescription rates among elderly patients with dementia: a population-based time-series analysis. CMAJ 2008;179(5):438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bowman CE. Antipsychotics for dementia: antipsychotic prescribing in care homes. BMJ 2008;337:a895. [DOI] [PubMed] [Google Scholar]
- 28. Guthrie B, Clark SA, McCowan C. The burden of psychotropic drug prescribing in people with dementia: a population database study. Age Ageing 2010;39(5):637–42. [DOI] [PubMed] [Google Scholar]
- 29. Bronskill SE, Anderson GM, Sykora K, et al. Neuroleptic drug therapy in older adults newly admitted to nursing homes: incidence, dose, and specialist contact. J Am Geriatr Soc 2004;52(5):749–55. [DOI] [PubMed] [Google Scholar]
- 30. Larrayadieu A, Abellan van Kan G, Piau A, et al. Associated factors with antipsychotic use in assisted living facilities: a cross-sectional study of 4367 residents. Age Ageing 2011;40(3):368–75. [DOI] [PubMed] [Google Scholar]
- 31. Shorr RI, Fought RL, Ray WA. Changes in antipsychotic drug use in nursing homes during implementation of the OBRA-87 regulations. JAMA 1994;271(5):358–62. [PubMed] [Google Scholar]
- 32. Sanfélix-Gimeno G, Cervera-Casino P, Peiró S, et al. Effectiveness of safety warnings in atypical antipsychotic drugs: an interrupted time-series analysis in Spain. Drug Saf 2009;32(11):1075–87. [DOI] [PubMed] [Google Scholar]
- 33. Thomas SK, Hodson J, McIlroy G, et al. The impact of direct healthcare professional communication on prescribing practice in the UK hospital setting: an interrupted time series analysis. Drug Saf 2013;36(7):557–64. [DOI] [PubMed] [Google Scholar]


