Abstract
Introduction:
We examined the cost-effectiveness of smoking cessation integrated with treatment for post-traumatic stress disorder (PTSD).
Methods:
Smoking veterans receiving care for PTSD ( N = 943) were randomized to care integrated with smoking cessation versus referral to a smoking cessation clinic. Smoking cessation services, health care cost and utilization, quality of life, and biochemically-verified abstinence from cigarettes were assessed over 18-months of follow-up. Clinical outcomes were combined with literature on changes in smoking status and the effect of smoking on health care cost, mortality, and quality of life in a Markov model of cost-effectiveness over a lifetime horizon. We discounted cost and outcomes at 3% per year and report costs in 2010 US dollars.
Results:
The mean of smoking cessation services cost was $1286 in those randomized to integrated care and $551 in those receiving standard care ( P < .001). There were no significant differences in the cost of mental health services or other care. After 12 months, prolonged biochemically verified abstinence was observed in 8.9% of those randomized to integrated care and 4.5% of those randomized to standard care ( P = .004). The model projected that Integrated Care added $836 in lifetime cost and generated 0.0259 quality adjusted life years (QALYs), an incremental cost-effectiveness ratio of $32 257 per QALY. It was 86.0% likely to be cost-effective compared to a threshold of $100 000/QALY.
Conclusions:
Smoking cessation integrated with treatment for PTSD was cost-effective, within a broad confidence region, but less cost-effective than most other smoking cessation programs reported in the literature.
Introduction
Post-traumatic stress disorder (PTSD) is associated with high smoking prevalence 1 and more intensive use of tobacco. 2 Smoking contributes to elevated mortality rates in PTSD. 3
Smoking cessation programs have proven effective for smokers with serious mental illnesses. 4–7 There is little information on the cost-effectiveness of these treatments. 8 Cessation interventions for smokers identified during psychiatric hospitalization 9 and for smokers seeking outpatient psychiatric care for depression 10 have been found cost-effective, with a cost-effectiveness ratio well below the $100 000 per quality adjusted life year (QALY) threshold often used in the United States.
Smoking interventions may be less cost-effective in those with PTSD and other serious mental illnesses. Quit rates for individuals with mental illness are lower than in other smokers 11 , 12 and more resources may be needed for successful treatment. The incremental value of quitting is attenuated by the lower quality of life and higher nonsmoking mortality rates in persons with mental illness.
A randomized clinical trial tested the integration of smoking cessation services in smokers seeking care for PTSD from the Veterans Health Administration. This trial found that the integrated program resulted in significantly greater prolonged abstinence from tobacco after 12 months of follow-up. 13 We now assess the treatment’s cost-effectiveness.
Methods
Smokers receiving treatment for PTSD at Veterans Affairs (VA) medical centers were randomized to receive smoking cessation services integrated with their mental health treatment or referral to a smoking cessation clinic (SCC). Participants randomized to Integrated Care (IC) were provided with smoking cessation services including five weekly sessions, pharmacotherapy for those attempting to quit, three booster sessions, and monthly follow-up sessions. These services were delivered by the provider of their PTSD therapy. Participants randomized to SCC were referred to a specialized outpatient smoking program. Smoking status was assessed by self-report and exhaled carbon monoxide of 8 ppm or less, and verification by urine cotinine of <100ng/mL in those reporting 7-day abstinence. Study design, subject inclusion criteria, treatment methods, and outcomes measurement are described more fully in the previous paper. 13
Study inclusion criteria included a diagnosis of PTSD resulting from military-related trauma, verified according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Other inclusion criteria were regular cigarette use (≥10 cigarettes/d for at least half of the days in the past month without use of other tobacco products), motivation to quit smoking, and completion of at least four treatment sessions within 1 month at a specialized VA outpatient treatment program for PTSD. Exclusion criteria included diagnosis of any psychotic disorder, bipolar disorder, substance dependence not in remission, an imminent risk of suicide or violence, or gross impairment from an organic condition. Written informed consent was obtained according to procedures approved by the Institutional Review Boards of each of the 10 enrolling sites.
Cost of Smoking Cessation Services
Utilization of smoking cessation services was recorded on a case-report form. Study participants were asked to report services and medications received outside the study using the time follow-back method. The cost of counseling services was estimated using the hourly cost of employing a provider of PTSD services as reported in the VA general ledger, including benefits and overhead. The amount of VA-supplied tobacco pharmacotherapy was obtained by the VA Decision Support System database, an activity-based costing system implemented at all VA medical centers. 14 Pharmacotherapy costs included the cost of initial prescribing visit, the supply cost of the medication itself, and the VA dispensing cost. We report all bupropion utilization and cost regardless of indication.
Health Services Utilization and Cost
We estimated the cost of VA care for study participants, including outside care funded by VA. The estimated cost of each VA encounter, including mental health treatment for PTSD, was drawn from Decision Support System. We also obtained payments for non-VA care that was sponsored by VA from the purchased care data system. Other hospital stays and outpatient visits not sponsored by VA were obtained via self-report. We used the amount of the VA paid claim for inpatient stays sponsored by VA. For other inpatient stays, we obtained the bill for the hospital stay and adjusted charges by the hospital’s cost-to-charge ratio. In a few cases, the bill could not be obtained and cost was estimated based on the length of stay. We categorized costs as being related or unrelated to mental health on the basis of clinic type, inpatient unit type, or for purchased care, the diagnosis. The cost of smoking cessation services were added to the other costs obtained from the VA costing system, including the cost of treatment of PTSD in mental health settings.
Statistical Methods
Differences in health care utilization between treatment groups were compared using a negative binomial regression. Costs were compared using a gamma regression with log link function.
Quality of Life
Health related quality of life (health utility) was assessed at 3, 6, 12, 15 and 18-months using the self-administered version of the Quality of Well Being Scale. This scale measures quality of life in four domains: symptom/problem complex, mobility, physical activity, and social activity. 15 , 16 Scores represents preference-based utilities on a scale that ranges from 0 (representing death) to 1 (representing perfect health).
We divided the utility value in the final follow-up assessment by the age and smoking-status matched utility weights from a population of primary care patients 17 to estimate the effect of nonsmoking factors, including mental illness, on trial participants’ quality of life.
Model
The cost of smoking cessation is incurred in the short-term, while most of the benefit is realized in the future, after the end of the trial. We modeled the effect of smoking cessation on health care cost and QALYs, the standard measure of outcomes used in cost-effectiveness analysis. Our Markov model had two nonabsorbing states, smoker and former smoker. Parameters used in the model are presented in Table 1 . Our review of the literature found the spontaneous cessation rate of smokers to be 4.3% per year, 18 the relapse rate among quitters be 15.0% in the first year after a sustained 1-year quit 19 and diminishing in subsequent years. 19–22 We did not find any studies on the effect of PTSD on quit or relapse rates. Because PTSD is associated with more intense smoking behavior, we assumed that spontaneous quit rates of smokers with PTSD was 75% of other smokers and that relapse rates from former smokers with PTSD were 150% of the rates of other former smokers. One-way sensitivity analyses were used to evaluate these assumptions.
Table 1.
Model Parameters for Changes in Smoking Status, Mortality, Quality of Life and Cost Obtained From Literature Review
| Parameter | Parameter value | Source |
|---|---|---|
| Quit rate among current smokers (% per year) | 4.3% | Ref. 18,23 |
| Relapse rate among former smokers after 1 year of abstinence (% per year) | Ref. 19–22 | |
| Year 2 after initial quit | 15% | |
| Year 3–5 after initial quit | 5% | |
| Year 6–9 after initial quit | 2% | |
| Year ≥10 after initial quit | 1% | |
| Excess mortality relative to never smokers (relative hazard) | Ref. 24–27 | |
| Female current smokers age 24–54 | 1.369 | |
| Female current smokers age 55–74 | 2.533 | |
| Female current smokers age ≥75 | 1.411 | |
| Female former smokers age 24–54 | 1.214 | |
| Female former smokers age 55–74 | 1.666 | |
| Female former smokers age ≥75 | 1.111 | |
| Male current smokers age 24–54 | 2.486 | |
| Male current smokers age 55–74 | 2.550 | |
| Male current smokers age ≥75 | 1.326 | |
| Male former smokers age 24–54 | 1.074 | |
| Male former smokers age 55–74 | 1.992 | |
| Male former smokers age ≥75 | 1.074 | |
| All-cause mortality hazard in PTSD | 2.1 | Ref. 28,29 |
| Quality of life (preference-based utilities) | Ref. 17 | |
| Female moderate smokers age 55–64 | 0.7648 | |
| Female moderate smokers age 65–74 | 0.7520 | |
| Female moderate smokers age ≥75 | 0.6778 | |
| Female former smokers age 55–64 | 0.7827 | |
| Female former smokers age 65–74 | 0.7709 | |
| Female former smokers age ≥75 | 0.6987 | |
| Male moderate smokers age 55–64 | 0.7815 | |
| Male moderate smokers age 65–74 | 0.7575 | |
| Male moderate smokers age ≥75 | 0.7112 | |
| Male former smokers age 55–64 | 0.8020 | |
| Male former smokers age 65–74 | 0.7802 | |
| Male former smokers age ≥75 | 0.7358 | |
| Health care charges incurred by smokers and former smokers relative to the general population (relative charges) | ||
| Smokers | 1.1881 | |
| Recent quitters (<5 years) | 1.2476 | |
| Long-term quitters (≥5 years) | 0.9595 | Ref. 30 |
| Annual health care cost (US $2010) | Ref. 31 | |
| Female age 18–24 | 2235 | |
| Female age 25–44 | 3347 | |
| Female age 45–64 | 6229 | |
| Female age 65–90 | 9623 | |
| Male age 18–24 | 1072 | |
| Male age 25–44 | 2158 | |
| Male age 45–64 | 5217 | |
| Male age 65–90 | 10 249 |
PTSD = post-traumatic stress disorder.
Mortality rates were estimated by applying published hazard ratios for smokers and former smokers to age-specific US mortality rates of never smokers. 24–27 We estimated the extra mortality risk associated with PTSD from causes other than smoking by comparing rates of the all-cause mortality of PTSD 28 , 29 to our estimate of the smoking related mortality risk based on smoking prevalence and age in these cohorts.
The model used a 3-month cycle, the minimum interval between study follow-up assessments. It was calibrated against published mortality rates among smokers and former smokers 32 , 33 and reports of the expected years of life for smokers at the time of a permanent quit. 33 , 34
The model was constructed using trial data on participant age, the effect of mental health on quality of life, and the incremental effect of the experimental intervention on the cost of smoking cessation services and initial smoking outcomes.
We used data from a large study of health care claims to determine the effect of smoking status on health care charges relative to the entire population. 30 This study found former smokers incurred higher costs than continuing smokers in the first 5 years after quitting, and lower costs thereafter. We applied these estimates to age and gender specific health care cost from the US Medical Expenditure Panel Survey. 31
Published estimates were used for age and gender specific estimates of quality of life 17 of former smokers and current smokers. We made the simplifying assumption that, survival, and quality of life in former smokers is unaffected by the length of time since quitting.
We used a lifelong time horizon and the perspective of the health care payer. All future LYs, costs, and QALYs were discounted at 3% per annum. Costs were expressed in 2010 US dollars. The statistical significance of the cost-effectiveness finding was determined via a Monte Carlo probabilistic sensitivity analysis, randomly sampling 1000 sets of parameters from their estimated probability distributions. The analysis accounts for uncertainty of both trial findings and the model parameters. An incremental cost-effectiveness ratio (ICER) was determined from each random draw. The percentage of ICERs that failed to meet the criterion for cost-effectiveness represents the P value of the test of the statistical hypothesis that the intervention was cost-effective at a particular cost-effectiveness threshold. 35 The model was constructed using commercially available software (TreeAge 2012). A Supplementary Appendix with a more complete description of the model, input parameters, and sensitivity analyses is available on online.
Results
The study enrolled 943 Veterans and randomized 472 to IC and 471 to SCC. Cost data were available on 938 participants, as authorization to access medical records was rescinded by three participants and identifiers could not be matched to the VA data system for two additional participants. Most participants (94%) were men, consistent with gender distribution among those enrolled in VHA. At randomization, trial participants were a mean age of 54.6 years (range 21.8 to 80.1 years, SD 8.68 years). Average daily cigarette intake was slightly above one pack (mean = 21.6, SD = 10.5). At least one in six participants reported chest pain (29%), cardiovascular diagnoses (including angina and heart attack) (17%), respiratory diagnoses (including asthma, chronic bronchitis, and emphysema) (29%), or diabetes (23%). Eight percent reported a current diagnosis of cancer. Twenty-six percent of participants reported none of these medical conditions.
The study population had an elevated level of comorbid mental health problems. These included current alcohol abuse (18%), past alcohol dependence (60%), substance use disorders (52%), and major depressive disorder (73%). Baseline CAPS scores (mean = 75.2, SD = 18.4) indicated a clinically severe sample. 36 There were no significant differences between study arms in baseline characteristics.
Table 2 reports the cost and quantity of smoking cessation treatment services used by each treatment group. Participants randomized to IC incurred $1286 in smoking cessation services cost, $735 more than the smoking cessation services cost incurred by those randomized to SCC ( P < .01). Participants randomized to IC incurred more smoking cessation counseling cost and had more counseling visits than those randomized to SCC ( P < .01). There was substantial use of tobacco pharmacotherapy in both groups, but significantly greater total cost for tobacco pharmacotherapy among those randomized to IC ($483 vs. $334 in SCC, P < .01).
Table 2.
Utilization and Cost of Smoking Cessation Services During the Trial, by Treatment Group
| Integrated care, N = 470 | Smoking cessation clinic, N = 468 | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Costs | |||||
| Bupropion | 129.10 | 210.46 | 94.89 | 194.37 | ** |
| Nicotine gum and lozenge | 253.29 | 634.01 | 147.28 | 434.53 | * |
| Nicotine patch | 59.97 | 108.92 | 56.94 | 119.74 | |
| Varenicline | 40.95 | 134.40 | 34.94 | 121.25 | |
| Total tobacco pharmacotherapy cost | 483.31 | 713.41 | 334.05 | 507.56 | * |
| Counseling visits | 802.78 | 598.97 | 217.39 | 294.72 | * |
| Total cost of smoking cessation services | 1286.09 | 1045.51 | 551.43 | 621.92 | * |
| Days of pharmacotherapy | |||||
| Bupropion | 72.5 | 134.2 | 53.0 | 122.1 | |
| Nicotine gum and lozenge | 31.7 | 79.3 | 18.4 | 54.3 | * |
| Nicotine patch | 25.6 | 51.8 | 24.3 | 57.0 | |
| Varenicline | 9.7 | 35.6 | 7.7 | 31.9 | |
| Counseling visits | 8.6 | 6.5 | 2.3 | 3.2 | * |
*Significantly different at P < .01.
**Significantly different at P < .05.
Table 3 reports health care utilization and cost incurred during 18 months of follow-up. Participants randomized to IC incurred less inpatient mental health costs ( P < .05) and more outpatient mental health costs ( P < .01), but there were no significant difference between groups in total mental health care costs ( P = .82). There were no significant differences between groups in the total costs exclusive of smoking cessation services incurred. Participants randomized to IC incurred $24 171 in health care cost compared to $25 305 incurred by those randomized to SCC. Since this difference was not significant, we modeled cost-effectiveness assuming that the intervention had no effect on the short-term cost of health services. We tested the effect of this exclusion with sensitivity analysis.
Table 3.
Utilization and Cost of Health Services During the Trial, by Treatment Group
| Integrated care, N = 470 | Smoking cessation clinic, N = 468 | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Inpatient costs | |||||
| Mental health settings | 1319 | 5763 | 2620 | 10 263 | ** |
| Other settings | 4738 | 21 417 | 4965 | 19 024 | |
| Sub-total | 6057 | 22 176 | 7585 | 22 279 | |
| Outpatient costs | |||||
| Mental health settings | 7164 | 7463 | 5690 | 5792 | * |
| Other settings | 8549 | 9337 | 9528 | 10 545 | |
| Sub-total | 15 713 | 12 944 | 15 218 | 13 207 | |
| Pharmacy cost | 2401 | 2518 | 2502 | 3333 | |
| Total cost exclusive smoking cessation services | 24 171 | 29 568 | 25 305 | 30 276 | |
| Smoking cessation costs | 1286 | 1046 | 551 | 622 | * |
| Total cost inclusive smoking cessation services | 25 457 | 29 747 | 25 857 | 30 268 | |
| Inpatient stays | |||||
| Mental health | 0.13 | 0.46 | 0.19 | 0.60 | |
| Other | 0.35 | 1.01 | 0.40 | 1.00 | |
| Total stays | 0.48 | 1.12 | 0.59 | 1.23 | |
| Inpatient days of stay | |||||
| Mental health | 2.45 | 15.5 | 4.11 | 16.4 | |
| Other | 3.14 | 20.9 | 4.31 | 22.5 | |
| Total days | 5.59 | 25.9 | 8.42 | 30.4 | |
| Outpatient visits | |||||
| Mental health | 44.7 | 44.4 | 39.5 | 37.7 | |
| Other | 35.4 | 31.9 | 39.2 | 34.7 | |
| Total visits | 80.1 | 62.0 | 78.7 | 58.0 | |
*Significantly different at P < .01.
**Significantly different at P < .05.
Table 4 presents trial findings used in the model. After 12 months, biochemically verified prolonged abstinence was present in 42/472 (8.9%) in those randomized to IC and in 21/471 (4.5%) in those randomized to standard smoking clinic. There were 4.4% more quitters with IC (8.9% with IC less 4.5% in SCC). It thus cost $16 697 per additional quit with integrated case (the incremental cost of $735 in additional smoking cessation services divided by the incremental effectiveness of 4.4%).
Table 4.
Cost, Outcomes, and Participant Characteristics From Trial Used in Smoking Cessation Model
| Variable | Base case | Standard deviation |
|---|---|---|
| 12-month prolonged biochemical-verified abstinence | ||
| Integrated care | 8.9% | — |
| Standard smoking clinic | 4.5% | — |
| Cost of intervention (US $2010) | ||
| Sustained abstinence | ||
| Integrated care | 1656 | 1116 |
| Standard smoking clinic | 763 | 676 |
| No sustained abstinence | ||
| Integrated care | 1248 | 1031 |
| Standard smoking clinic | 540 | 617 |
| Population characteristics | ||
| Male (percent) | 93.6 | — |
| Age at randomization (years) | 54.6 | 8.68 |
| Utility adjustment | ||
| Utility relative to general population of same age, gender, and smoking status | 0.652 | 0.188 |
We divided each utility value obtained at the end of follow-up by age and smoking status matched utility weights from a population of primary care patients. 17 Participant responses to Quality of Well Being resulted in a preference based utility weight that was 0.652 of the expected value given their smoking status and age. This represents the effect of nonsmoking factors, including PTSD, on quality of life.
The base-case model estimated that a smoker with the average characteristics of a trial participant who quit using tobacco would realize a discounted gain of 0.979 LYs or 0.584 QALYs.
Modeling results are presented in Table 5 . Discounted life time cost with the IC intervention was $146 645, or $836 greater than the $145 809 lifetime cost of standard care. Persons receiving IC were expected to live 14.006 LYs, or 0.043 LYs more than the 13.963 LYs realized with standard care. The ICER was $19 240/LY (the additional $836 cost divided by 0.043 additional in LYs).
Table 5.
Cost, Outcomes, and Cost-Effectiveness From Lifetime Markov Model
| Strategy | Integrated care | Standard smoking clinic | Difference |
|---|---|---|---|
| Cost | |||
| Cost of cessation treatment in trial | 1286 | 551 | 735 |
| Discounted cost of follow-up health services | 145 359 | 145 258 | 101 |
| Total discounted cost | 146 645 | 145 809 | 836 |
| Outcomes | |||
| Discounted life years | 14.006 | 13.963 | 0.043 |
| Discounted quality adjusted life years (QALY) | 7.054 | 7.028 | 0.026 |
| Incremental cost-effectiveness ratio | |||
| $/LY | 19 240 | ||
| $/QALY | 32 257 | ||
Persons receiving IC were expected to realize 7.0542 QALYs, 0.0259 QALYs more than the 7.0282 QALYs realized with standard care. The ICER was $32 257/QALY ($836 extra cost divided by an additional 0.0259 QALYs).
One-way sensitivity analyses were used to test the effect of modeling assumptions. Including health costs incurred during the trial resulted in a finding that IC had $298 lower lifetime costs ($170 816 vs. $171 114 in SCC) and was thus dominant; IC cost less and was more effective than SCC. The great variance in within-trial health care costs makes for a very large confidence region around this estimate, however.
We considered the effect of excluding “unrelated” health care costs, a sensitivity analysis recommended by US cost-effectiveness guidelines. 37 Health care costs incurred during the extended life span resulting from IC are considered “unrelated” to the cost of the intervention. We estimated that $446 of the total discounted life-time costs of IC were unrelated. Excluding these costs reduces the ICER to $14 990/QALY.
We conducted a sensitivity analysis using parameters from a study that found former smokers incur greater health care costs than continuing smokers. 38 This sensitivity analysis found total discounted life-time costs were $131 240 in the IC group and $129 580 in those randomized to SCC, an incremental cost of $1660, and an ICER of $64 015/QALY.
The ICER was only slightly affected by alternate assumptions about the natural cessation rate of current smokers and the relapse rate in former smokers. If the long-term cessation rate in continuing smokers with PTSD was 40% of the value for the general population (rather than the 75% assumed in the base case model), the ICER would have been or $29 781/QALY, or 7.7% less. If the long-term relapse rates in former smokers with PTSD was the same as in the general population (rather than the 150% assumed in the base case model), the ICER would have been 13.3% less, or $27 949/QALY.
The quality of life (utility adjustment) for trial participants had a more significant effect. The ICER would have been $21 031/QALY if trial participants had the same quality of life as smokers and former smokers of the same age in the general population. Age also had a more significant effect; entering the model at 45 years of age lowered the ICER to $23 037/QALY. Entering the model at age 65 increased the ICER to $40 724/QALY.
We also conducted a one-way sensitivity analysis using other definitions of abstinence. The ICER was lower when less restrictive definitions of abstinence were employed. Using prolonged self-report as the definition of abstinence (with 15.5% abstinent in IC and 7.0% in SCC) resulted in an ICER of $19 226/QALY. Using biochemically verified 30-day point prevalence estimate at 18 months (with 16.9% abstinent in IC and 9.3% abstinent in SCC) yielded an ICER of $20 958/QALY. Using biochemically verified 7-day point prevalence estimate at 18 months (with 18.2% abstinent in IC and 10.8% abstinent in SCC) yielded an ICER of $21 497/QALY.
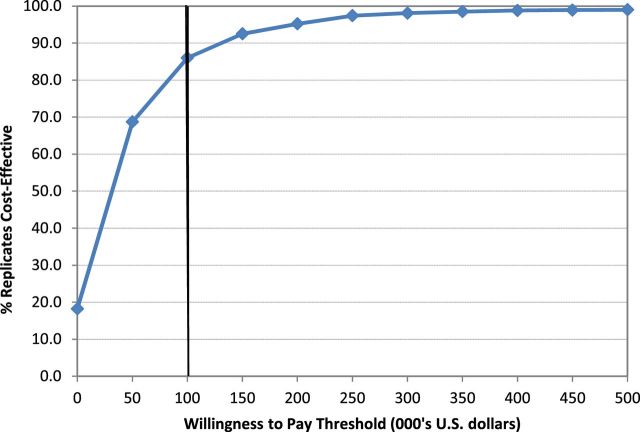
A probabilistic sensitivity analysis was used to test the significance of the cost-effectiveness finding. Figure 1 is a plot of the cost-effectiveness acceptability curve, showing the percentage of replicates found to be cost-effective at different willingness to pay thresholds. At the conventional willingness to pay threshold of $100 000/QALY, IC was 86.0% likely to be cost-effective. At willingness to pay threshold of $200 000/QALY or greater, IC was more than 95.0% likely to be cost-effective.
Figure 1.
Cost-effectiveness acceptability curve.
Discussion
This randomized clinical trial determined that the integration of smoking cessation services with treatment for PTSD was cost-effective relative to standard care.
IC cost $16 697 per additional quit. This was considerably more costly than the median incremental cost effectiveness of about $3000 per quit found in a systematic review of 14 smoking cessation studies of individuals without mental illness. 39 The cost per quit was also greater that other trials in mental health settings: including $11 496 per quit in smokers receiving treatment for depression in a psychiatric clinic 10 and $1272 per quit among smokers identified during psychiatric hospitalization. 9
The incremental cost effectiveness of $32 257 QALY was high (less efficient) than the ratios found for other smoking cessation interventions, typically not limited to smokers with mental illness. Brief advice provided during office visits had an ICER of $1240–$3620/QALY (in $2010). 40 Addition of pharmacotherapies to counseling had an incremental cost effectiveness of $1133–$1774/QALY. 41 Varenicline for prevention of relapse in recent quitters had an incremental cost-effectiveness of $3413/QALY. 42
The incremental cost effectiveness ratio of IC was also greater than the ratios found in other smoking cessation trials conducted in mental health settings. The intervention for smokers receiving depression treatment in a psychiatric clinic had an ICER of $9580 per life-year. 10 The intervention for smokers identified during psychiatric hospitalization had an ICER of $428 per QALY. 9
Like other smoking cessation interventions, smoking cessation integrated with PTSD treatment was cost-effective, with an ICER well below the commonly used threshold for judging cost-effectiveness ($100 000/QALY in the United States).
Our model estimated that trial participants who quit smoking will realize an additional 0.584 QALYs. This is less than the two QALYs per quit benefit estimated for other smokers. 40 , 43–46 The difference reflects the higher nonsmoking mortality hazard and lower health related quality of life associated with PTSD, and the older age of trial participants.
This study used a strict definition of abstinence. Patients were considered nonabstinent if they failed to attend 18-month follow-up or if they did not have biochemically verified abstinence at 18 months. Many smoking cessation trials have used less stringent definitions of abstinence, including point prevalence or repeated point prevalence, self-report without biochemical verification, or a shorter follow-up period. 47 Had we used a less-restrictive definition of abstinence used in other trials, IC would be regarded as more cost-effective.
Reviews of economic evaluation of smoking cessation programs have noted a wide variation in the methodologies employed. 39 , 41 , 48 The effect of variations in follow-up time and definitions of abstinence on cost-effectiveness findings have not been evaluated, however. A less restrictive definition of what constitutes a quit may mischaracterize short-term quit attempts as prolonged abstinence, resulting in a lower cost-effectiveness ratio.
We acknowledge several limitations of this study. Our simplified model did not consider the effect of smoking on the development of specific smoking-related diseases. These more complex models may offer a false sense of precision, however, because there is little information on quit and relapse rates in groups defined by smoking-related diseases. We adjusted information on the relapse rates of former smokers and the future quitting among smokers to reflect the smoking behavior in PTSD. Although this adjustment was based on simple assumptions, our findings were robust across a wide range of assumptions regarding relapse and spontaneous quit rates. We used available data on the relative health care cost of current smokers and former smokers; neither this nor other available estimates avoid the confounding between illness and quitting. The pre-existing illnesses that lead to cessation are associated with high health care cost; these extra costs are not the result of smoking cessation. Our model relied on quality of life estimates for current and former developed in the United Kingdom as no US estimates were available.
With the success of smoking cessation efforts, those who continue to use tobacco are more likely to have co-occurring mental illness and substance use disorders. Public health efforts to reduce tobacco use will increasingly need to focus on these historically hidden populations. 49
An effective treatment directed at smokers with PTSD was found to be cost-effective, but less cost-effective than other smoking cessation interventions typically deployed in samples of smokers without mental illness. This was partly attributable to the more rigorous definition of abstinence used in the trial. It was also attributable to the cost of the intensive behavioral counseling interventions that were part of the IC intervention. Other factors contributing to the high cost-effectiveness ratio were the lower quality of life and high nonsmoking morality in PTSD, and the older age of trial participants.
Funding
This research was supported by the Cooperative Studies Program and grant P50 DA09253 from the National Institute on Drug Abuse. Trial registration: http://ClinicalTrials.gov identifier: NCT00118534.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The views expressed herein are those of the authors and not necessarily those of the US Department of Veterans Affairs (VA). We gratefully acknowledge the assistance of Shuo Chen and Andrew Siroka of the Health Economics Resource Center. MWS contributed to this research as a VA employee prior to joining Truven Health Analytics.
References
- 1. Fu SS, McFall M, Saxon AJ, et al. . Post-traumatic stress disorder and smoking: a systematic review . Nicotine Tob Res . 2007. ; 9 ( 11 ): 1071 – 1084 . doi: 10.1080/14622200701488418 . [DOI] [PubMed] [Google Scholar]
- 2. Beckham JC, Kirby AC, Feldman ME, et al. . Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder . Addict Behav . 1997. ; 22 ( 5 ): 637 – 647 . doi: 10.1016/S0306-4603(96)00071-8 . [DOI] [PubMed] [Google Scholar]
- 3. Boscarino JA . A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention . Psychosom Med . 2008. ; 70 ( 6 ): 668 – 676 . doi: 10.1097/PSY.0b013e31817bccaf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Evins AE . Review: bupropion increases abstinence from smoking without affecting mental state in people with schizophrenia . Evid Based Ment Health . 2010. ; 13 ( 4 ): 120 . doi: 10.1136/ebmh.13.4.120 . [DOI] [PubMed] [Google Scholar]
- 5. Hall SM, Prochaska JJ . Treatment of smokers with co-occurring disorders: emphasis on integration in mental health and addiction treatment settings . Annu Rev Clin Psychol . 2009. ; 5 : 409 – 431 . doi: 10.1146/annurev.clinpsy.032408.153614 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsoi DT, Porwal M, Webster AC . Interventions for smoking cessation and reduction in individuals with schizophrenia . Cochrane Database Syst Rev . 2013. ;(2):CD007253. doi:10.1002/14651858.CD007253.pub3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams KE, Reeves KR, Billing CB, Jr, Pennington AM, Gong J . A double-blind study evaluating the long-term safety of varenicline for smoking cessation . Curr Med Res Opin . 2007. ; 23 ( 4 ): 793 – 801 . doi: 10.1185/030079907X182185 . [DOI] [PubMed] [Google Scholar]
- 8. Banham L, Gilbody S . Smoking cessation in severe mental illness: what works? Addiction . 2010. ; 105 ( 7 ): 1176 – 1189 . doi: 10.1111/j.1360-0443.2010.02946.x . [DOI] [PubMed] [Google Scholar]
- 9. Barnett PG, Wong W, Jeffers A, Hall SM, Prochaska JJ . Cost effectiveness of smoking cessation treatment initiated during psychiatric hospitalization . J Clin Psych . In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnett PG, Wong W, Hall S . The cost-effectiveness of a smoking cessation program for out-patients in treatment for depression . Addiction . 2008. ; 103 ( 5 ): 834 – 840 . doi: 10.1111/j.1360-0443.2008.02167.x . [DOI] [PubMed] [Google Scholar]
- 11. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH . Smoking and mental illness: a population-based prevalence study . JAMA . 2000. ; 284 ( 20 ): 2606 – 2610 . doi: 10.1001/jama.284.20.2606 . [DOI] [PubMed] [Google Scholar]
- 12. Sung HY, Prochaska JJ, Ong MK, Shi Y, Max W . Cigarette smoking and serious psychological distress: a population-based study of California adults . Nicotine Tob Res . 2011. ; 13 ( 12 ): 1183 – 1192 . doi: 10.1093/ntr/ntr148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McFall M, Saxon AJ, Malte CA, et al. . Integrating tobacco cessation into mental health care for posttraumatic stress disorder: a randomized controlled trial . JAMA . 2010. ; 304 ( 22 ): 2485 – 2493 . doi: 10.1001/jama.2010.1769 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith MW, Chen S, Siroka AM, Hamlett-Berry K . Using policy to increase prescribing of smoking cessation medications in the VA healthcare system . Tob Control . 2010. ; 19 ( 6 ): 507 – 511 . doi: 10.1136/tc.2009.035147 . [DOI] [PubMed] [Google Scholar]
- 15. Kaplan RM, Ganiats TG, Sieber WJ, Anderson JP . The Quality of Well-Being Scale: critical similarities and differences with SF-36 . Int J Qual Health Care . 1998. ; 10 ( 6 ): 509 – 520 . doi: 10.1093/intqhc/10.6.509 . [DOI] [PubMed] [Google Scholar]
- 16. Pyne JM, Sieber WJ, David K, Kaplan RM, Hyman Rapaport M, Keith Williams D . Use of the quality of well-being self-administered version (QWB-SA) in assessing health-related quality of life in depressed patients . J Affect Disord . 2003. ; 76 ( 1–3 ): 237 – 247 . doi: 10.1016/S0165-0327(03)00106-X . [DOI] [PubMed] [Google Scholar]
- 17. Vogl M, Wenig CM, Leidl R, Pokhrel S . Smoking and health-related quality of life in English general population: implications for economic evaluations . BMC Public Health . 2012. ; 12 : 203 . doi: 10.1186/1471-2458-12-203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levy DT, Graham AL, Mabry PL, Abrams DB, Orleans CT . Modeling the impact of smoking-cessation treatment policies on quit rates . Am J Prev Med . 2010. ; 38 ( suppl 3 ): S364 – 372 . doi: 10.1016/j.amepre.2009.11.016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawkins J, Hollingworth W, Campbell R . Long-term smoking relapse: a study using the british household panel survey . Nicotine Tob Res . 2010. ; 12 ( 12 ): 1228 – 1235 . doi: 10.1093/ntr/ntq175 . [DOI] [PubMed] [Google Scholar]
- 20. Gilpin EA, Pierce JP, Farkas AJ . Duration of smoking abstinence and success in quitting . J Natl Cancer Inst . 1997. ; 89 ( 8 ): 572 – 576 . doi: 10.1093/jnci/89.8.572 . [DOI] [PubMed] [Google Scholar]
- 21. Wetter DW, Cofta-Gunn L, Fouladi RT, Cinciripini PM, Sui D, Gritz ER . Late relapse/sustained abstinence among former smokers: a longitudinal study . Prev Med . 2004. ; 39 ( 6 ): 1156 – 1163 . doi: 10.1016/j.ypmed.2004.04.028 . [DOI] [PubMed] [Google Scholar]
- 22. Krall EA, Garvey AJ, Garcia RI . Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study . Nicotine Tob Res . 2002. ; 4 ( 1 ): 95 – 100 . doi: 10.1080/14622200110098428 . [DOI] [PubMed] [Google Scholar]
- 23. Hyland A, Li Q, Bauer JE, Giovino GA, Steger C, Cummings KM . Predictors of cessation in a cohort of current and former smokers followed over 13 years . Nicotine Tob Res . 2004. ; 6 ( suppl 3 ): S363 – 369 . doi: 10.1080/14622200412331320761 . [DOI] [PubMed] [Google Scholar]
- 24. Friedman GD, Tekawa I, Salder M, Sidney S . Smoking and mortality: the Kaiser Permanente experience. Changes in cigarette-related disease risks and their implications for prevention and control . Smoking and Tobacco Control Monographs, National Cancer Institute . 1997. ; 8 : 472 – 499 . doi: http://cancercontrol.cancer.gov/brp/tcrb/monographs/8/m8_6.pdf . Accessed April 8, 2015 . [Google Scholar]
- 25. LaCroix AZ, Lang J, Scherr P, et al. . Smoking and mortality among older men and women in three communities . N Engl J Med . 1991. ; 324 ( 23 ): 1619 – 1625 . doi: 10.1056/NEJM199106063242303 . [DOI] [PubMed] [Google Scholar]
- 26. Prescott E, Osler M, Andersen PK, et al. . Mortality in women and men in relation to smoking . Int J Epidemiol . 1998. ; 27 ( 1 ): 27 – 32 . doi: 10.1093/ije/27.1.27 . [DOI] [PubMed] [Google Scholar]
- 27. Taylor DH, Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA . Benefits of smoking cessation for longevity . Am J Public Health . 2002. ; 92 ( 6 ): 990 – 996 . doi: 10.2105/AJPH.92.6.990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boscarino JA . Posttraumatic stress disorder and mortality among U.S. Army veterans 30 years after military service . Ann Epidemiol . 2006. ; 16 ( 4 ): 248 – 256 . doi: 10.1016/j.annepidem.2005.03.009 . [DOI] [PubMed] [Google Scholar]
- 29. Bullman TA, Kang HK . Posttraumatic stress disorder and the risk of traumatic deaths among Vietnam veterans . J Nerv Ment Dis . 1994. ; 182 ( 11 ): 604 – 610 . doi: 10.1097/00005053-199411000-00002 . [DOI] [PubMed] [Google Scholar]
- 30. Musich S, Faruzzi SD, Lu C, McDonald T, Hirschland D, Edington DW . Pattern of medical charges after quitting smoking among those with and without arthritis, allergies, or back pain . Am J Health Promot . 2003. ; 18 ( 2 ): 133 – 142 . doi: 10.4278/0890-1171-18.2.133 . [DOI] [PubMed] [Google Scholar]
- 31. Agency for Healthcare Research and Quality . Medical Expenditure Panel Survey . http://meps.ahrq.gov/mepsweb/ . Accessed August 12, 2014 . [PubMed]
- 32. Sloan FA. The Price of Smoking . Cambridge, MA: : MIT Press; ; 2004. . [Google Scholar]
- 33. Bronnum-Hansen H, Juel K . Estimating mortality due to cigarette smoking: two methods, same result . Epidemiology . 2000. ; 11 ( 4 ): 422 – 426 . doi: 10.1097/00001648-200007000-00010 . [DOI] [PubMed] [Google Scholar]
- 34. Howard P, Knight C, Boler A, Baker C . Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO Simulation model: application to a population of US adult smokers . Pharmacoeconomics . 2008. ; 26 ( 6 ): 497 – 511 . doi: 10.2165/00019053-200826060-00004 . [DOI] [PubMed] [Google Scholar]
- 35. van Hout BA, Al MJ, Gordon GS, Rutten FF . Costs, effects and C/E-ratios alongside a clinical trial . Health Econ . 1994. ; 3 ( 5 ): 309 – 319 . doi: 10.1002/hec.4730030505 . [DOI] [PubMed] [Google Scholar]
- 36. Weathers FW, Keane TM, Davidson JR . Clinician-administered PTSD scale: a review of the first ten years of research . Depress Anxiety . 2001. ; 13 ( 3 ): 132 – 156 . doi: 10.1002/da.1029 . [DOI] [PubMed] [Google Scholar]
- 37. Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine . New York, NY: : Oxford University Press; ; 1996. . [Google Scholar]
- 38. U.S. Congressional Budget Office . Raising the Tax on Cigarettes: Effects on Health and the Federal Budget . Washington, DC: : Congress of the United States; ; 2012. . www.cbo.gov/publication/43319 . Accessed March 25, 2015. [Google Scholar]
- 39. Ronckers ET, Groot W, Ament AJ . Systematic review of economic evaluations of smoking cessation: standardizing the cost-effectiveness . Med Decis Making . 2005. ; 25 ( 4 ): 437 – 448 . doi: 10.1177/0272989X05278431 . [DOI] [PubMed] [Google Scholar]
- 40. Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T . Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research . JAMA . 1997. ; 278 ( 21 ): 1759 – 1766 . doi: 10.1001/jama.1997.03550210057039 . [PubMed] [Google Scholar]
- 41. Song F, Raftery J, Aveyard P, Hyde C, Barton P, Woolacott N . Cost-effectiveness of pharmacological interventions for smoking cessation: a literature review and a decision analytic analysis . Med Decis Making . 2002. ; 22 ( suppl 5 ): S26 – 37 . doi: 10.1177/027298902237708 . [DOI] [PubMed] [Google Scholar]
- 42. Taylor M, Leonardi-Bee J, Agboola S, McNeill A, Coleman T . Cost effectiveness of interventions to reduce relapse to smoking following smoking cessation . Addiction . 2011. ; 106 ( 10 ): 1819 – 1826 . doi: 10.1111/j.1360-0443.2011.03493.x . [DOI] [PubMed] [Google Scholar]
- 43. Chirikos TN, Herzog TA, Meade CD, Webb MS, Brandon TH . Cost-effectiveness analysis of a complementary health intervention: the case of smoking relapse prevention . Int J Technol Assess Health Care . 2004. ; 20 ( 4 ): 475 – 480 . doi: 10.1017/S0266462304001382 . [DOI] [PubMed] [Google Scholar]
- 44. Fiscella K, Franks P . Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling . JAMA . 1996. ; 275 ( 16 ): 1247 – 1251 . doi: 10.1001/jama.1996.03530400035035 . [PubMed] [Google Scholar]
- 45. Godfrey C, Parrott S, Coleman T, Pound E . The cost-effectiveness of the English smoking treatment services: evidence from practice . Addiction . 2005. ; 100 ( suppl 2 ): 70 – 83 . doi: 10.1111/j.1360-0443.2005.01071.x . [DOI] [PubMed] [Google Scholar]
- 46. Javitz HS, Swan GE, Zbikowski SM, et al. . Cost-effectiveness of different combinations of bupropion SR dose and behavioral treatment for smoking cessation: a societal perspective . Am J Manag Care . 2004. ; 10 ( 3 ): 217 – 226 . doi: www.ajmc.com/publications/issue/2004/2004-03-vol10-n3/Mar04-1720p217-226/ . Accessed April 8, 2015 . [PubMed] [Google Scholar]
- 47. Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE . Measures of abstinence in clinical trials: issues and recommendations . Nicotine Tob Res . 2003. ; 5 ( 1 ): 13 – 25 . doi: 10.1093/ntr/5.1.13 . [PubMed] [Google Scholar]
- 48. Ruger JP, Lazar CM . Economic evaluation of pharmaco- and behavioral therapies for smoking cessation: a critical and systematic review of empirical research . Annu Rev Public Health . 2012. ; 33 : 279 – 305 . doi: 10.1146/annurev-publhealth-031811-124553 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williams JM, Steinberg ML, Griffiths KG, Cooperman N . Smokers with behavioral health comorbidity should be designated a tobacco use disparity group . Am J Public Health . 2013. ; 103 ( 9 ): 1549 – 1555 . doi: 10.2105/AJPH.2013.301232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.